Mobilizing Parkinson’s Disease: The Future of Exercise
Abstract
Exercise is increasingly recognized as an important element in the treatment of Parkinson’s disease but what is exercise targeting? What accounts for the benefits observed in Parkinson’s disease? Is exercise disease modifying? Several modes of exercise have been studied in various doses across a heterogeneous Parkinson’s population. Yet more clarity is needed as to who benefits most and when, from what type of exercise and at which intensity. In this paper, we briefly review the state of the art in key areas and speculate on the likely state of research in each area in the next 20 years. Key areas relate to: (1) the physiological benefits of exercise with respect to disease modification; (2) the best type of exercise; (3) the optimal intensity of exercise; and (4) implementation strategies to increase exercise uptake. A better understanding of these concepts would allow for a more effective, personalized approach, rather than the current “one size fits all” and could most likely confer greater benefits.
Exercise has been hailed as the new medicine in Parkinson’s disease (PD), but is it? What supports this view and what underpins it in terms of the mechanisms at play. Are we really able to target the underlying pathophysiology of PD in all its complexity? Or are we targeting ageing mechanisms per se and does it in fact matter? And is it new—or is it fashionable? Exercise has always formed the backbone of physical rehabilitation therapies. Research suggesting the possibility of neuroprotective effects of exercise has catapulted exercise to the forefront. Given this increase in attention, the importance of exercise as a key element in the treatment of PD is more widely recognized. This is a positive outcome but certainly not a new approach. Below we briefly review the state of the art in key areas and speculate on the likely state of research in each area in the next 20 years. Key areas relate to: (1) the physiological benefits of exercise with respect to disease modification; (2) the best type of exercise; (3) the optimal intensity of exercise; and (4) implementation strategies for increasing exercise/physical activity uptake.
EXERCISE AND DISEASE MODIFICATION
Exercise is defined as a subcategory of physical activity and includes those activities that are planned, structured, repetitive, purposive in nature, and intended to improve one or more components of physical fitness [1]. Exercise is increasingly recognized as an important element in the treatment of PD but what is exercise targeting? What accounts for the benefits observed in PD? Are the effects general and/or specific? Central and/or peripheral? Is it in fact disease modifying? What constitutes a disease modifying effect? A better understanding of this would allow for a more personalized approach rather than the current ‘one size fits all’ and could most likely confer greater benefits. Terms such as disease modifying, neuroprotective and neuroplasticity are often used interchangeable and can be confusing. For the purposes of this review we define disease modifying as delays in underlying pathological or pathophysiological disease processes accompanied by improvement in clinical signs and symptoms; neuroprotective as recovery or regeneration of the nervous system, its cells, structure and function; and, neuroplasticity as the ability of the CNS to remodel and adapt in response to change (e.g. disease, intervention, aging, etc.).
Exercise in animal models of PD reveal activity dependent structural and functional changes in the brain that are general and diffuse in nature, such as altered synpatogenesis, increased endogenous brain neurotrophic factors (e.g., BDNF, GDNF), greater angiogenesis and reduced neuroinflammation [2, 3]. Evidence for targeted effects of exercise on the nigrostriatal pathway include an increase in dopamine release, increased extracellular dopamine through downregulation of dopamine transporter expression, reduced striatal dopamine loss, partial preservation of midbrain dopaminergic neurons and preserved or restored dopamine terminals [2, 3]. This direct impact on the underlying disease pathology resulting in slowing of disease progression suggests a neuroprotective role of exercise in animal models of PD. However, in humans with PD, there is a paucity of evidence suggesting a neuroprotective effect of exercise [4]. Does this represent an absence of disease mitigating effects or an inability to measure changes in the neuropathology with sufficient precision? This brings us back to the question: does it really matter? Are there other important paths to disease modification?
There are a number of small exercise studies in PD that reveal increased gray matter volume, increased serum levels of brain derived neurotrophic factor, cortical motor excitability, elevated striatal dopamine D2 receptor binding and weakening of the overactive indirect striatal pathway DA-D2R expression [4, 5]. These changes have been associated with improvements at the behavioral level. While it is not clear whether these central neuroplastic changes alter the neurodegenerative process, they represent positive central consequences associated with exercise [6]. Whether the benefits confer to other pathophysiological mechanisms in PD is unclear, however would form an important question for future work.
Exercise is associated with a number of structural, vascular and neuromolecular changes in the brain, which contribute to improved physical, cognitive and behavioral function in the aging brain [7]. Exercise does not mitigate the aging process, but it attenuates many of the deleterious systemic and cellular effects leading to improved function of most of the mechanisms involved in aging resulting in substantial therapeutic benefits [8]. Physiological changes are present with exercise, irrespective of PD. It may be that the effect builds resilience to better cope with the burden of disease rather than specific neuroprotective effects. This in and of itself can present as disease modifying in so much as it may attenuate progression of the motor UPDRS by counteracting secondary deconditioning associated with aging or an increasing sedentary lifestyle. Physical activity or an active lifestyle (termed non-exercise physical activity, NEPA) has also been reported to confer significant benefits. Physical activity refers to any bodily movement produced by skeletal muscles that results in expenditure of energy and includes a gamut of behaviors that may encompass household, work, leisure and sports-related activities [1]. Snider and colleagues reported on the positive effects of NEPA on functional performance and attenuation of the UPDRS III motor scores, independent of nigrostrial degeneration [9]. Interesting the same could not be said of formal exercise. NEPA may be far more achievable and accessible for PD given the fact that the mean age is over 60 years. That is not to say we don’t encourage all forms of exercise—rather that we consider a more comprehensive and inclusive approach to the advice we provide to patients in the clinic.
Multiple paths to disease modification are plausible and clinically important. Future exercise trials could systematically measure changes across several pathways including physiological changes (aerobic fitness, strength), functional changes (e.g., gait, falls), clinical changes (motor and non-motor symptoms), central neuroplastic changes (e.g., cortical motor excitability, BDNF levels, accumulation of amyloid plaques and function of cholinergic pathways) and surrogate markers of degeneration (i.e., functional neuroimaging) to more clearly delineate the targeted effects of exercise. Assuming identification of a biomarker or cluster of biomarkers in the near future, examination of the true neuroprotective effects of exercise could be investigated. However, regardless of the outcome of a true neuroprotection study, disease modification via these other pathways remains clinically relevant.
TYPE OF EXERCISE
There has been a rapid accumulation of randomized controlled trials and meta-analytic studies over the past 10–15 years revealing the benefits of exercise in persons with PD at the behavioral level [5, 10]. Multiple forms of exercise have been shown to be beneficial. Exercise programs are typically comprised of four core ingredients: aerobic/endurance training, strength training, balance, flexibility training. Functional/task specific training forms part of a more specialized application of exercise that aims to target functional mobility more specifically (utilizing enhanced strength, endurance, balance and flexibility as core components to support efficient performance of specific tasks); and may facilitate transfer of benefits from core exercise components to better overall functional ability. We know that different forms of exercise are specific but effective and when combined likely support functional gains including improved gait, mobility, activities of daily living and reduced fall rate. Given the task and context specific benefits of exercise in persons with PD, functional training (e.g., gait training, sit to stand, fine motor dexterity) are also important elements of a rehabilitation/exercise program to improve targeted aspects of mobility. Exercise programs such as boxing, dance and tai chi have come in and out of fashion at various times but they are not new or unique. These types of programs contain varying amounts of the core ingredients of exercise (i.e., aerobic, balance, strength and flexibility training) known to be effective in improving targeted systems and overall function and have the added benefit of providing social interaction and comradery. Thus far, it seems clear that physical activity and/or a multimodal approach to exercise are effective in improving targeted physiological systems and physical function, thereby reducing disability. Exercise has primarily targeted the motor and mobility related aspects of the disease. Although some exercise trials in PD have included non-motor signs as outcomes, most often they have been secondary outcomes, rather than the primary target of the intervention. Exercise, with its broad-based effects, has the potential to have disease-modifying effects on non-motor signs (e.g., cognition, sleep, depression) and should be a focus of future trials. Although a complete understanding of the mechanisms that underlie the benefits of exercise in persons with PD is lacking, it is clear that persons with PD who exercise and/or are physically active have better outcomes than those who do not [11].
For the most part, the approach to exercise has been a “one size fits all.” Participants in exercise trials typically constitute a very heterogeneous group (i.e., H&Y 1–3). “Lumping” these patients together reduces the potential effect and limits our understanding of who benefits most from a particular type of exercise intervention. Are we ready to “drill down” to identify the “best” exercise components for a given phenotype? Enhanced understanding of different Parkinson’s phenotypes, possibly linked to genetics and risk, would allow more targeted approach and earlier implementation of clinical trials. Identifying who would benefit most from what type of exercise/activity would foster a more tailored exercise prescription for a given profile. Exercise algorithms could be derived to improve the outcome of particular subgroups. Although physical therapists typically tailor exercise interventions to meet the needs of a given patient, there is little evidence to guide these decisions. Given the vast variability in disease characteristics and subsequent functional consequences among persons with PD, a precision medicine approach is needed to optimize the disease modifying effect of exercise.
DOSING AND TIMING OF EXERCISE
Few trials in PD have examined optimal dose/ response effects of exercise. Some reveal that higher intensity aerobic and strengthening exercise have greater disease modifying effects (improvements in UPDRS motor scores) than moderate/low intensity [12, 13]. Similarly, more challenging higher dosed balance exercise programs have revealed better outcomes compared to less challenging, lower dosed programs. Given the challenges associated with engaging in high intensity exercise, particularly among more sedentary persons with PD, perhaps there is value in identifying the minimum dose of exercise and physical activity necessary to confer benefits. Isn’t some exercise better than none? Nonetheless, for adaptation and benefits to occur, the volume and relative difficulty of the exercise must overload the body in some way. The amount of this challenge or overload will vary according to the capabilities of the individual. Though more studies are needed to identify the optimal dose of various forms of exercise, the characteristics of the individual must be considered. The optimal mode and dose of exercise will be dependent on the profile of a particular individual. A better understanding of the progression of the disease among various phenotypes is necessary in order to carry out more personalized clinical trials.
Given the disease modifying effects of exercise and physical activity in PD, it seems early exercise is better. However, most exercise studies include those with mild to moderate disease, several years after diagnosis, potentially bypassing the most critical period. A more recent phase II clinical trial in de novo PD revealed less worsening of motor symptoms in the high intensity aerobic exercise compared to a wait-list control condition over 6 months [12]. By the same token, a recent meta-analysis examining the dose-response association between physical activity and the risk of PD revealed that the highest levels of total physical activity or moderate to vigorous activity was associated with a significantly reduced risk of PD. In contrast, there was no association between light physical activity and PD risk [14]. To assess disease modifying effects of exercise, future exercise trials should target those most at risk for developing PD (e.g., those with genetic mutations or with anosmia and REM sleep behavior disorder) with high intensity exercise and physical activity. The effect of early exercise on delaying the initiation of PD medications is also worth exploring given the side effects associated with prolonged use of levodopa.
IMPLEMENTATION STRATEGIES: INCREASING PHYSICAL ACTIVITY/EXERCISE UPTAKE
Despite the rapid accumulation of evidence revealing the benefits of exercise and physical activity for persons with PD, many remain sedentary. It is interesting to note that at diagnosis—activity levels in even those with the most mild disease (H&Y I) are extremely low compared to age matched controls [15] with only 30% achieving 30 minutes of walking per day—far less than the levels recommended by guidelines [16]. Perhaps a better place to start a conversation is to address the sedentary behaviors as a starting point and build on these. Given the very positive literature on lifestyle changes and healthy aging, this may be a very effective way to mitigate the burden of PD.
Although there are more community-based exercise programs available now than ever before for persons with PD, widespread dissemination and implementation of exercise programs shown to be effective in randomized controlled trials has not occurred. Perhaps more emphasis on implementation trials is needed to scale up evidence-based programs, improving access to greater numbers of people with PD.
Numerous physical, behavioral, and psychosocial barriers limit adequate uptake of exercise and engagement in physical activity. Low self-efficacy and poor outcome expectation are associated with limited engagement in exercise among those with PD [17]; however, barriers may differ among individuals suggesting a personalized, tailored approach is necessary to overcome relevant barriers in order to increase engagement in physical activity and exercise. In the U.S., a study of Medicare beneficiaries with PD revealed low utilization of physical therapy services (14%), suggesting an untapped resource to prescribe personalized exercise programs, foster an active lifestyle and to provide the necessary supports needed to promote behavioral change [18]. It is essential, but not sufficient, for neurologists/physicians to recommend exercise/physical activity as part of the standard treatment for PD. However, simply suggesting to patients that they should exercise does not provide the necessary guidance for most to be successful.
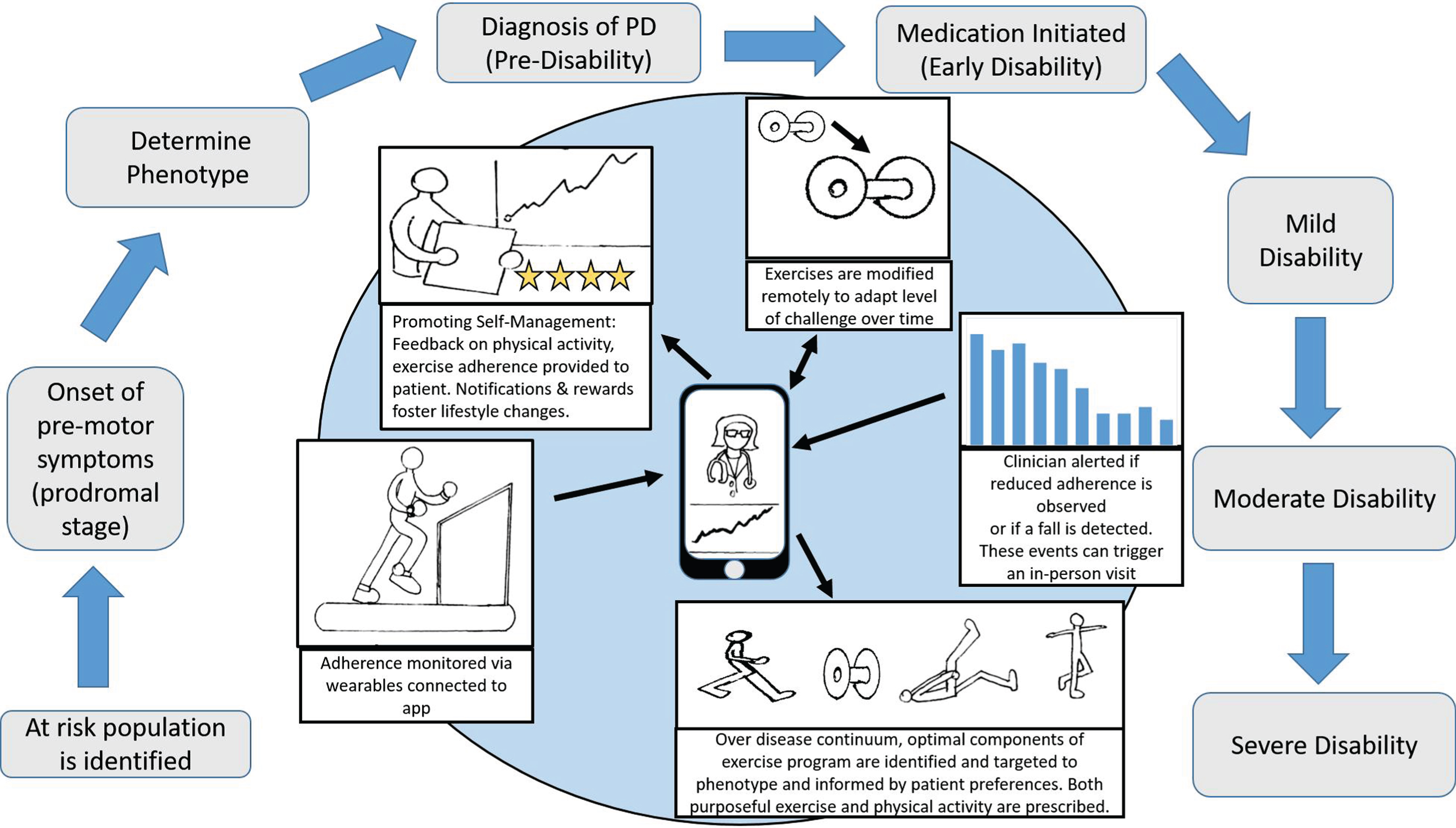
Physical therapists with expertise in PD are uniquely positioned to prescribe the type and dosage of exercise tailored to the needs of individual patients [19]. Given the evidence revealing the prevalence of inactivity early in the course of the disease and the benefits of exercise in de novo PD, it seems early initiation of physical therapy has the potential to have the most impact. Exercise, like medications, must be adapted on a regular basis over the course of the disease to optimize the benefit. Advances in mobile health (mHealth) technologies offer a potential solution to help people with PD engage in physical activity and exercise successfully while staying connected to a physiotherapist or healthcare professional [20]. For example, a set of home-based Rehabilitation Internet-of-Things (RIoT) has the potential to optimize exercise uptake and increase physical activity [21]. A RIoT could include wearable, activity recognition sensors and instrumented rehabilitation devices (i.e., virtual reality systems) that transmit information to a smart phone or tablet regarding the quantity and quality of exercise. Using telerehabilitation approaches, data could be monitored by a healthcare professional and interpreted regularly allowing tailored and timely adaptations to an exercise program. Exercises could be uploaded through smartphone applications (“apps”) to provide the necessary “just right” challenge for each patient based on the data received. Strategies to facilitate behavioral change (i.e., goal setting, feedback, rewards) could be provided through apps to foster a more active lifestyle. Persons with PD could be also connected to others from around the world to provide support, comradery or friendly competitions to increase engagement, both socially and physically. These types of approaches will likely be more mainstream in the future with the goal of improving exercise/physical activity uptake.
Does exercise have significant promise to mitigate the burden and possibly the course of PD? We think so. But to answer that question we will need to design trials that account for the multisystem nature of PD, identify the specific effects of exercise and target the underlying pathophysiology/mechanisms. A better understanding of this would allow for a more personalized approach rather than the current ‘one size fits all’ and could most likely confer greater benefits. It’s no good of course if you don’t do it! So embedding exercise in an individual’s lifestyle, whilst accounting for their preferences and ability will most likely enhance uptake and compliance. As we move further into the digital age, supporting technologies will supplement and facilitate delivery from the earliest stage (prodromal) through to later stages of PD adapting to individuals needs on the way (see Fig. 1). We hope that future research will target these aspects and bring exercise and an active lifestyle as one of the primary tools for clinical management and patient benefit.
Fig.1
Home-based Rehabilitation Internet-of-Things to optimize exercise uptake and increase physical activity over the continuum of Parkinson’s disease (PD).
REFERENCES
[1] | President’s Council on Physical Fitness and Sports ((2001) ) Physical fitness research digest. President’s Council on Physical Fitness and Sports, Washington. |
[2] | Ahlskog JE ((2018) ) Aerobic exercise: Evidence for a direct brain effect to slow Parkinson disease progression. Mayo Clin Proc 93: , 360–372. |
[3] | Petzinger GM , Holschneider DP , Fisher BE , McEwen S , Kintz N , Halliday M , Toy W , Walsh JW , Beeler J , Jakowec MW ((2015) ) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: Targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1: , 29–39. |
[4] | Hirsch MA , Iyer SS , Sanjak M ((2016) ) Exercise-induced neuroplasticity in human Parkinson’s disease: What is the evidence telling us? Parkinsonism Relat Disord 22: (Suppl 1), S78–S81. |
[5] | Mak MK , Wong-Yu IS , Shen X , Chung CL ((2017) ) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13: , 689–703. |
[6] | Lang AE , Espay AJ ((2018) ) Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov Disord 33: , 660–677. |
[7] | Jackson PA , Pialoux V , Corbett D , Drogos L , Erickson KI , Eskes GA , Poulin MJ ((2016) ) Promoting brain health through exercise and diet in older adults: A physiological perspective. J Physiol 594: , 4485–4498. |
[8] | Fiuza-Luces C , Garatachea N , Berger NA , Lucia A ((2013) ) Exercise is the real polypill. Physiology (Bethesda) 28: , 330–358. |
[9] | Snider J , Muller M , Kotagal V , Koeppe RA , Scott PJH , Frey KA , Albin RL , Bohnen NI ((2015) ) Non-exercise physical activity attenuates motor symptoms in Parkinson disease independent from nigrostriatal degeneration. Parkinsonism Relat Disord 21: , 1227–1231. |
[10] | Tomlinson CL , Patel S , Meek C , Herd CP , Clarke CE , Stowe R , Shah L , Sackley CM , Deane KH , Wheatley K , Ives N ((2013) ) Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev 9: , CD002817. |
[11] | Oguh O , Eisenstein A , Kwasny M , Simuni T ((2014) ) Back to the basics: Regular exercise matters in Parkinson’s disease: Results from the National Parkinson Foundation QII registry study. Parkinsonism Relat Disord 20: , 1221–1225. |
[12] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[13] | Corcos DM , Robichaud JA , David FJ , Leurgans SE , Vaillancourt DE , Poon C , Rafferty MR , Kohrt WM , Comella CL ((2013) ) A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 28: , 1230–1240. |
[14] | Fang X , Han D , Cheng Q , Zhang P , Zhao C , Min J , Wang F ((2018) ) Association of levels of physical activity with risk of Parkinson disease. A systematic review and meta-analysis. JAMA Netw Open 1: , e182421. |
[15] | Lord S , Godfrey A , Galna B , Mhiripiri D , Burn D , Rochester L ((2013) ) Ambulatory activity in incident Parkinson’s: More than meets the eye? J Neurol 206: , 2964–2972. |
[16] | American College of Sports Medicine ((2018) ) Guidelines for Exercise Testing and Prescription, 10th Edition. Lippincott, Williams & Wilkins, Philadelphia. |
[17] | Ellis T , Boudreau JK , DeAngelis TR , Brown LE , Cavanaugh JT , Earhart GM , Ford MP , Foreman KB , Dibble LE ((2013) ) Barriers to exercise in people with Parkinson disease. Phys Ther 93: , 628–636. |
[18] | Fullard ME , Thibault DP , Hill A , Fox J , Bhatti DE , Burack MA , Dahodwala N , Haberfeld E , Kern DS , Klepitskava OS , Urrea-Mendoza E , Myers P , Nutt J , Rafferty MR , Schwalb JM , Shulman LM , Willis AW , Parkinson Study Group Healthcare Outcomes and Disparities Working Group ((2017) ) Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89: , 1162–1169. |
[19] | Keus SHJ , Munneke M , Graziano M , Paltamaa J , Pelosin E , Domingos J , Brühlmann S , Ramaswamy B , Prins J , Struiksma C , Rochester L , Nieuwboer A , Bloem B ((2014) ) European physiotherapy guideline for Parkinson’s disease. KNGF/ParkinsonNet, the Netherlands. |
[20] | Ellis TD , Cavanaugh JT , DeAngelis TR , Hendron K , Thomas CA , Saint-Hilaire M , Latham N ((2018) ) Comparative effectiveness of mHealth supported exercise compared to exercise alone for people with Parkinson disease: Randomized controlled pilot study. Phys Ther. in press. |
[21] | Dobkin BH ((2017) ) A rehabilitation-internet-of-things in the home to augment motor skills and exercise training. Neurorehabil Neural Repair 31: , 217–227. |




