Significance and Translational Value of High-Frequency Cortico-Basal Ganglia Oscillations in Parkinson’s Disease
Abstract
The mechanisms and significance of basal ganglia oscillations is a fundamental research question engaging both clinical and basic investigators. In Parkinson’s disease (PD), neural activity in basal ganglia nuclei is characterized by oscillatory patterns that are believed to disrupt the dynamic processing of movement-related information and thus generate motor symptoms. Beta-band oscillations associated with hypokinetic states have been reviewed in several excellent previous articles. Here we focus on faster oscillatory phenomena that have been reported in association with a diverse range of motor states. We review the occurrence of different types of fast oscillations and the evidence supporting their pathophysiological role. We also provide a general discussion on the definition, possible mechanisms, and translational value of synchronized oscillations of different frequencies in cortico-basal ganglia structures. Revealing how oscillatory phenomena are caused and spread in cortico-basal ganglia-thalamocortical networks will offer a key to unlock the neural codes of both motor and non-motor symptoms in PD. In preclinical therapeutic research, recording of oscillatory neural activities holds the promise to unravel mechanisms of action of current and future treatments.
INTRODUCTION
There are several clinico-pathological subtypes of Parkinson’s disease (PD), but all cases share the typical motor symptoms that lead to diagnosis (poverty and slowness of movement, resting tremor, muscle rigidity, postural problems). These symptoms are mainly caused by dopamine (DA) deficiency in the striatum, which in turn depends on the degeneration of nigrostriatal DA projections. Parkinsonian motor symptoms are greatly ameliorated by L-DOPA, a DA precursor that can cross the blood-brain barrier. Unfortunately, L-DOPA pharmacotherapy causes complications that limit its utility. Already within five years of treatment, 30–50% of the patients develop L-DOPA-induced dyskinesia (LID), abnormal involuntary movements that are often debilitating [1]. Moreover, L-DOPA (and other dopaminergic treatments for PD) can induce non-motor complications, such as psychosis (hallucinations, delusion, excitement), a complication particularly common in older PD patients and often associated with cognitive deterioration (reviewed in Cenci and Odin [2]). While LID is elicited by dysregulated DA transmission in the motor striatum [3], and possibly also in the sensorimotor cortex [4], the emergence of psychosis in PD has been linked to altered neurotransmission in limbic brain regions [5].
There is vast consensus that both the primary symptoms of PD and the complications of L-DOPA therapy depend on altered information processing in cortico-basal ganglia-thalamocortical pathways [3–6], but the underlying neural mechanisms remain to be elucidated. Clues of major importance came from the discovery of powerful oscillations of the local field potential (LFP) within deep basal ganglia nuclei in PD patients undergoing functional neurosurgery [7, 8]. In particular, LFP recordings in patients “off” dopaminergic medications revealed prominent oscillations in a broad beta band (8–30 Hz; reviewed in Hammond et al. [9]). Similar oscillations were detected also in dopamine-denervated animals (reviewed in [6, 9, 10]). The exaggerated activity in the beta band was found to be suppressed by dopaminergic drugs, and the degree of drug-induced improvement in bradykinesia and rigidity was found to correlate with the degree of suppression of beta band oscillations in both the STN and cortex (reviewed in [9, 10]). Based on these observations it was hypothesized that, in the untreated parkinsonian state, the basal ganglia engage in abnormally synchronised oscillatory activities in the beta band. Exaggerated oscillatory synchronisation of neuronal activity may disturb information processing in cortico-basal ganglia loops and therefore contribute to both motor and cognitive problems in PD [11].
By now, basal ganglia beta band oscillations in PD (and in animal models thereof) have been the subject of a vast scientific literature (e.g., [8–18]). The present review article primarily focuses on faster oscillations with frequencies spanning the higher end of the gamma band, in particular we discuss the high-frequency oscillations found within a narrow frequency interval of the gamma band that have recently been associated with hyperkinetic states. Our interest in this area has been fuelled by a serendipitous observation made in 6-OHDA-lesioned rats treated with L-DOPA. When L-DOPA elicits dyskinesia in this animal model, the expression of abnormal involuntary movements (AIMs) coincides with the appearance of gamma oscillations in a narrow frequency-band around 80 Hz within motor cortical and basal ganglia circuits [19, 20]. Importantly, the same type of oscillations have now been detected in motor cortex and subthalamic nucleus in PD patients affected by LID [21] (Fig. 1). Notably, these narrowband gamma oscillations are quite distinct from the increased gamma activities detected during normal voluntary movement [22]. Indeed, the latter type of gamma-activity cannot be regarded as proper oscillations at a well-defined frequency, but rather appear to be non-rhythmic activities manifesting in a much broader range of frequencies [19–21, 23–25].
Fig.1
Levodopa-induced dyskinesia is strongly associated with 80 Hz cortical oscillations in both Parkinson’s disease patients and in rodent models of the disease. This gives unique opportunities to search for the network mechanism underlying motor symptoms. (a-b) Rat data (ON/OFF levodopa denoted in red/black). (c-d) Human data (ON and OFF levodopa represented in (c) and (d), respectively). Note the striking similarity of the high-frequency oscillations associated with dyskinesia in rats/humans and the difference of these narrowband resonant oscillations compared to physiological broadband gamma. Figures adapted from Halje et al. and Swann et al. [19, 21]).
![Levodopa-induced dyskinesia is strongly associated with 80 Hz cortical oscillations in both Parkinson’s disease patients and in rodent models of the disease. This gives unique opportunities to search for the network mechanism underlying motor symptoms. (a-b) Rat data (ON/OFF levodopa denoted in red/black). (c-d) Human data (ON and OFF levodopa represented in (c) and (d), respectively). Note the striking similarity of the high-frequency oscillations associated with dyskinesia in rats/humans and the difference of these narrowband resonant oscillations compared to physiological broadband gamma. Figures adapted from Halje et al. and Swann et al. [19, 21]).](https://content.iospress.com:443/media/jpd/2019/9-1/jpd-9-1-jpd181480/jpd-9-jpd181480-g001.jpg)
In addition to the LID-associated oscillations, narrowband high-frequency oscillations in the 100–200 Hz range have recently been observed in limbic nodes of the cortico-basal ganglia system upon pharmacologic treatment with psychotomimetic drugs and in disturbed cognitive states [26, 27]. These recent data suggest a more general pathophysiological role of high-frequency oscillations in conditions involving cortico-basal ganglia dysfunction [23]. In addition to these two types of high-frequency oscillations, dopamine-dependent oscillatory activity of even higher frequencies (above 200 Hz) have been reported in the STN [28] and the GPi [29] in PD patients.
Mechanistically, narrowband oscillations of the LFP are thought to reflect rhythmic synchronizations of transmembrane currents among a local population of neurons. Synchronization appears spontaneously in neural networks even without rhythmic external input. This is a consequence of resonances that naturally appear in any dynamic system endowed with feedback mechanisms when the feedback tends to amplify certain frequencies and suppress others. Feedback mechanisms exist at the level of individual neurons (e.g., voltage-dependent ion channels), at the microcircuit level (e.g., reciprocally connected interneurons), in the interaction between cell populations (e.g., the excitation-inhibition balance between glutamatergic and GABA-ergic cells), and in the interaction between connected brain structures (e.g., the cortico-basal ganglia-thalamic loop). Since resonances are readily and spontaneously appearing in any feedback-controlled system, it is possible that some LFP oscillations are merely epiphenomena without significant consequences to neural information processing, even when they are strongly correlated to specific behavioral states. However, there is considerable and growing support for the notion that LFP oscillations play an important role in both normal brain function and brain pathologies [23, 30–33].
In this review article, we discuss cortico-basal ganglia high-frequency narrowband oscillations from the following perspectives: 1) methodological aspects and taxonomy, 2) principal experimental findings, 3) possible underlying mechanisms, and 4) translational importance.
On how to measure oscillatory activities in the brain
Measures of oscillatory neuronal activities can be obtained using a range of techniques offering different spatiotemporal resolutions and sensitivities.
Scalp electroencephalography (EEG) is the most widely used method to probe oscillatory electrical activities in the human brain. For steady-state oscillatory activity in the cortex/thalamocortical system, EEG can be regarded as a spatially and temporally smoothed version of the local field potential [34]. This smoothing arises mainly because the signal in each EEG electrode correspond to the integrated LFPs over several cm2, but also because of the electrical filtering properties of the tissues located between the electrodes and the brain [34].
Non-invasive recordings of brain electrical activity can also be obtained using magnetoencephalography (MEG), which records magnetic fields generated by the electrical currents in the brain. By measuring the magnetic rather than the electric component of the field created by neuronal currents, MEG is expected to improve both the spatial and temporal resolution of the recorded signals, although this is not always the case [35].
Electrocorticography (ECoG) utilizes high-density subdural electrodes to record electrical activity directly from the surface of the cerebral cortex. This technique can enable recordings of higher frequency components with a high spatial resolution and a significantly improved signal-to-noise, essentially resembling the signal of cortical LFPs [36]. Compared to non-invasive approaches, ECoG recordings are also less susceptible to muscle artefacts.
To record from deep brain nuclei, invasive recording techniques are however required. The shape and size of the recording probe, as well as the electronics used for signal acquisition, can be optimized for the study of single unit activity or LFPs, and trade-off solutions are needed to record both phenomena [37]. Local field potentials (LFPs) are often the preferred measure to assess the degree of neuronal synchronization in a certain volume of brain tissue. Despite being a scalar quantity, the LFP is the potential difference between two points in space, and therefore depends on the length and direction of the vector going between the two measuring points. Hence, the measured LFP crucially depends on the spatial arrangement of the electrodes. The LFP is thought to mainly reflect the local synchronization of dendritic currents induced by excitatory and inhibitory synaptic inputs to the population of cells surrounding each electrode [38]. Spiking events in the nearby cell population are considered to give only a minor contribution to the recorded LFP signal in most situations, but because action potentials of cells are in many cases entrained to the LFP, field potentials are nevertheless frequently used as proxy for local cell activity [39]. However, this approximation may not always be valid because LFPs can also be generated by very large networks of sparsely connected neurons [23, 40]. Because all neuronal activity that influences the potential difference between the electrode pair will contribute to the signal, it is of key importance to use electrode pairs that are arranged in a meaningful way with respect to the cytoarchitecture (for a detailed discussion see [40]). A good strategy is to implant multiple electrodes into the structure of interest, and then create differential measures between all possible pairs of electrodes. With this approach, one can make sure that the current sources creating the field are indeed local (in which case, different pairs of electrodes would show different signals) [41]. Even when LFPs are recorded with the best possible methods, it will be difficult to interpret the significance of differences in LFP amplitudes between structures, since differences in the cytoarchitectonic arrangement of neurons in relation to the recording electrodes, and properties of the extracellular space, will ultimately define the recorded signal [40].
When the same microelectrodes are used to record both unit activity and LFP, the recorded voltage signal is split into a high-frequency and low-frequency part to separate the two signals. Note however, that this separation is not perfect and for higher frequencies of LFP-oscillations a spectral leakage of spiking activity may occur [42]. Although less explored in the context of dyskinesia, unit activity has been shown to provide an independent state description even when restricting the analysis to firing rate changes [20]. Large differences in the physical size of the electrode (e.g., macroelectrodes used for deep-brain stimulation vs. microelectrodes) could influence the capacity to detect certain high-frequency oscillations that are spatially more confined. This could for example explain why 110–160 Hz oscillations are a common finding in micro- but not macroelectrode recordings. For a more extensive discussion on extracellular recording methods we refer the reader to more specific reviews on this subject (e.g., [39, 43]).
On the taxonomy of high-frequency oscillations
The classical EEG nomenclature was established in the 1930 s to describe the dominant, slow waves below ∼35 Hz that are directly visible in unprocessed EEG traces (delta, theta, alpha, and beta). The term gamma was instead applied to indistinct fluctuations faster than 35 Hz [44]. Gamma oscillations are orders of magnitude weaker than the lower-frequency oscillations and their existence in the neocortex was established first in the 1990 s, with the discovery of 40 Hz oscillations in visual cortex. In the last two decades, a plethora of even faster oscillations have been found in EEG and LFP recordings, and these are often collectively called high-frequency oscillations (HFOs) or fast/high gamma oscillations. There is unfortunately no consensus on how to classify and name these phenomena either within or beyond the classical gamma band, or where the border to the “high” gamma range lies. Thus, for the purpose of this review, we need to clarify the definitions and classifications applied to different types of HFOs. First, we exclude phenomena that are not proper oscillations. A proper oscillation has a well-defined frequency, like an auditory tone, as opposed to a non-rhythmic signal, which is more comparable to a hissing sound (there are methods that can reliably distinguish between the two cases, see for example Wen et al. [45]). Second, we distinguish between different HFO phenomena based on the following questions, which we suggest may serve as a good basis for an HFO taxonomy in this context: (1) Is the oscillation transient or is it continuous? (2) In which anatomical structures does it occur? (3) Is it related to or modulated by a specific behavioral, pathological or pharmacological state? (4) In what frequency range does it occur?
In this review, we will also consider any oscillation faster than the classical 40 Hz gamma oscillation to be an HFO. Note that this definition deliberately includes so called finely-tuned gamma (FTG) below 100 Hz as an HFO phenomenon (cf. [46]).
EXPERIMENTAL FINDINGS
In conjunction with neuromodulatory treatment applying deep-brain stimulation (DBS), recordings of LFPs have been obtained from implanted brain structures in human patients during the early postoperative period. This procedure offers an unusual opportunity to obtain invasive recordings from deep structures in the human brain. Under such conditions, a number of studies involving different patient groups have characterized neuronal activity, e.g., in GPi [8, 29, 47–49], STN [8, 28, 48–52], pedunculopontine nucleus (PPN) [53], and thalamus [54, 55]. HFOs were observed in several of these recordings, and broadly, the observed HFOs can be placed in three categories based on their frequency: 60–90 Hz, 120–160 Hz and >200 Hz.
60–90 Hz oscillations
Basal ganglia LFP oscillations in the 60–90 Hz range was first reported by Brown et al. [8], who found a ∼70 Hz peak in STN and GPi of PD patients after levodopa treatment. This phenomenon was further investigated in follow-up studies (see e.g., [49, 50–56]). Interestingly, in a limited number of studies, recordings from deep structures were also combined with EEG/MEG. These recordings suggested the presence of cortical oscillations in this frequency range that emerges after L-DOPA treatment, and which may be functionally coupled to oscillations in deeper structures since the recorded LFP signals were reported to be coherent and displaying relatively constant phase differences over prolonged time periods [48, 49, 57, 58]. From these studies in patients, it has however been difficult to establish whether 60–90 Hz oscillations are associated with the beneficial pro-kinetic effect of L-DOPA therapy or if they instead indicate the transition to a pathological hyperkinetic state, manifesting as dyskinetic movements [56]. In general, it has been concluded that increased HFO amplitude – at least in the deep basal ganglia nuclei – is primarily associated with increased motor activity and/or a state of arousal that may enable motor activity (for a review on this subject see [46]).
In motor cortex, on the other hand, evidence for a physiological role of HFOs in this frequency range is not as convincing. While investigations using non-invasive recording technologies, such as electroencephalography and magnetoencephalography (EEG/MEG; [59–61]), or intracranial electrocorticography (ECoG) recordings in epilepsy patients [62–64] have shown that high-frequency oscillations in the 60–90 Hz frequency band can indeed be found in the motor cortex in association with movements, these findings relate only to brief episodes of movement-related increases in gamma power rather than sustained oscillatory activity. Thus, in healthy individuals, activity in this band does not seem to be characterized by clear sustained rhythms with a well-defined frequency but rather by a transient gamma band power increase that occur during movement onset.
In an alternative view, a pathological role of this HFO in motor cortex was instead first proposed by Halje et al. [19], based on experiments using microwire recordings from motor cortex and dorsal striatum in unilaterally 6-OHDA lesioned rats. The authors found prominent HFOs around 80 Hz that were only present in the lesioned hemisphere during levodopa-induced dyskinesia (LID) [19]. In this seminal study, it was also found that the topical application of an antagonist to dopamine type 1 receptor (D1R) onto the cortical surface was sufficient to break the oscillation and concomitantly suppress dyskinesia.
The relevance of this finding to PD was recently demonstrated by Swann and collegues as a result of the first long-term recordings performed in dyskinetic patients using a combined DBS-electrocorticography (ECoG) device [21]. Importantly, chronically implantable bidirectional electrodes help circumvent experimental caveats associated with the early postoperative phase following DBS-electrode implantation. This phase is not ideally suited for brain recordings, as symptoms are often significantly reduced following electrode implantation (i.e., even when no current is passed through the electrode) –indicating that the symptomatic relief in this case is primarily related to the lesion inflicted by the electrode [65]. By recording neuronal activity over motor cortical areas and in the STN for 12 months, Swann and colleagues could present convincing evidence that, in PD patients, dyskinesia goes hand in hand with the same type of motor cortical HFOs observed in the rat model of LID. A detailed analysis of HFOs was also performed in the STN, prompting the conclusion that this narrow-band HFO is principally pathological rather than pro-kinetic. More specifically, the oscillation was found to be minimally affected by voluntary movements while its presence proved to be a reliable biomarker of dyskinesia. This result is at variance with previous studies that have associated gamma oscillations with the beneficial pro-kinetic effect of levodopa therapy [50, 56]. Such an interpretation likely stems from the increased oscillation amplitude often observed in the STN following levodopa-treatment, and sometimes also in other parts of the cortico-basal ganglia-thalamic loop (also in non-PD subjects) in direct association with motor actions, taking the form of a transient event-related synchronization of neuronal activity (reviewed in Jenkinson et al. 2013, [46]; see also [66]). However, these movement-modulated oscillations may not be functionally equivalent to the long-lasting oscillations found in dyskinetic states, even if the two phenomena may have similar spectral contents. Indeed, such a distinction has been proposed also for beta-band oscillations by a recent study in parkinsonian monkeys, showing that the duration of oscillatory episodes in the beta-band is critical to predict pathological motor states [18].
To further clarify the neurophysiological role of narrow-band HFOs in LID, several studies have further explored this phenomenon in rodents [20, 24, 67, 68]. A study by Dupre and colleagues showed that the oscillations develop together with dyskinetic symptoms with daily levodopa administration during a one-week priming period [24]. During this period, the abnormal involuntary movements became gradually more severe. They also showed that similar oscillations can be induced in L-DOPA-primed rats by independently stimulating dopamine receptors of the D1 or D2 type [24]. At the level of LFPs, these oscillations are particularly strong in corticostriatal circuits and are also observed both in the globus pallidus (corresponding to the external pallidal segment in primates) and in motor nuclei of the thalamus, but are typically somewhat less pronounced in the STN compared to the findings obtained in patients [21]. Overall, LFP oscillatory activities, including HFOs around 80 Hz, can be used as very robust electrophysiological biomarkers to classify parkinsonian and dyskinetic states in the 6-OHDA rodent model, as shown in Tamte et al. [20]. In this study the authors quantitatively compared the spectral components in eight different brain structures to assess which components most reliably predicted brain states associated with untreated parkinsonism versus dyskinesia, with or without additional pharmacological treatment. Classification performance (as estimated by fitting of a Gaussian mixture model to the data [20]) improved steadily with inclusion of a broader spectral content and/or addition of brain structures. Interestingly, HFOs around 80 Hz in the rostral forelimb area (a premotor/supplementary motor area in rodents) were a particularly useful physiological marker of LID [20] (which also appears consistent with the findings by Swann et al. [21]).
110–160 Hz oscillations
HFOs in the 110–160 Hz range have been studied intensely in the hippocampus of healthy animals (for a review, see [69]). Recently, similar HFOs were shown by Brys et al. to be widespread in the basal ganglia and motor cortex of unilaterally 6–OHDA lesioned animals, being present on both sides of the brain (and with relatively increase in power following L-DOPA treatment) [70]. A general feature of these oscillations is that their amplitude is modulated by the phase of a much slower oscillation in the theta range (5–10 Hz) as indicated by measurements of phase-amplitude coupling. In the hippocampus literature, an increase of theta-HFO coupling has been shown to correlate with higher cognitive demands and brain states associated to memory processing [42, 71]. The role of theta-HFO coupling in this PD model is currently unknown, but both L-DOPA and antidyskinetic treatment with 5-HT1A agonists alter the amplitude and frequency of this type of HFO, as well as its coupling to lower frequencies [70].
Intriguingly, a separate line of research has shown that acute administration of psychotomimetic/psychedelic drugs to healthy animals often induce similar HFOs in the prefrontal cortex and the nucleus accumbens [26, 27], structures belonging to the limbic part of the cortico-basal ganglia-thalamic loop. It is therefore tempting to speculate that psychotic symptoms can arise as a consequence of aberrant HFOs (or the brain state associated with these oscillations) in the cognitive and limbic loops of the cortico-basal ganglia network in a similar way as motor symptoms may result from oscillations in sensorimotor loops. This could also explain why some PD patients experience episodes of psychotic symptoms as a side effect of levodopa treatment (reviewed [2]). Therefore, these HFOs may be important to further study in the context of non-motor PD symptoms and neuropsychiatric side effects of current medications [70].
Oscillations above 200 hz
LFP oscillations above 200 Hz were first observed in the parkinsonian brain by Foffani et al. [28], who reported distinctive peaks at 319±33 Hz in the STN of levodopa-treated PD patients. Moreover, Wang et al. [72] detected HFOs above 200 Hz in part of the STN in PD patients off medications, reporting that the power of such oscillations was negatively correlated with akinesia and rigidity scores [54]. Importantly, although HFOs above 200 Hz are often disregarded as leaked spiking activity of a few neurons, Wang et al. [72] were able to show that this oscillation was not directly caused by spiking activity (since the high-frequency power increase remained also after subtraction of all visible unit activity) and could thus be regarded as a proper LFP phenomenon generated by a large population of neurons.
The frequency of these HFOs is strongly modulated by dopamine, with a slowing by about 60 Hz in the dopamine-depleted state (265±33 Hz; [73]). The amplitude is positively correlated to dopamine tone and it is also enhanced during voluntary movement, especially in the L-DOPA-treated state [28, 73]. This pattern of amplitude modulation is very similar to the modulation pattern of the 70–80 Hz HFOs observed in the STN of PD patients [48]. Several studies have also reported modulation of the HFO amplitude by the phase of beta oscillations (phase-amplitude coupling; [15, 73, 74]) and to other frequencies below the beta band [72]. An oscillation with very similar characteristics has also been reported in GPi [29].
With respect to motor signs, it has been reported that UPDRS scores of akinesia/rigidity were negatively correlated with HFO amplitude, i.e., the more impaired patients had weaker HFOs [72]. It is however not clear if HFOs may have a direct impact on the genesis of parkinsonian motor features or are, instead, modulated by them.
Recently, this very high frequency HFO has been proposed as a marker for resting tremor. In particular, Hirschmann et al. [75], showed that HFOs above 200 Hz are positively correlated with tremor at rest (i.e., HFOs are stronger during tremor), although in this study it was not possible to reliably distinguish between resting tremor and voluntary movement.
POSSIBLE MECHANISMS UNDERLYING THE GENERATION OF HIGH-FREQUENCY OSCILLATIONS
In the parkinsonian brain, dopaminergic denervation results in a multitude of neurochemical, physiological, and cellular changes that could potentially make both cortex and striatum prone to produce oscillations at a network-level, leading to the emergence of different types of HFOs. Oscillations can however emerge in a wide range of highly interconnected networks and it remains to be explored which cellular components are predominantly responsible for the tuning of network oscillatory properties. Thus, while it is at present not possible to pinpoint the key drivers of HFOs, some general candidate mechanisms appear worth mentioning.
First, the excitability of certain groups of neurons may change because of altered expression levels of voltage-sensitive or shunting ion channels, or other changes in intrinsic membrane properties [76], which can in turn alter oscillatory properties (akin to what has been reported for lower oscillation frequencies [17]). Second, the synaptic weight of critical connections may change [77, 78]. This increased coupling can facilitate synchronization of independently oscillating neurons, similar to how coupled oscillators synchronize in mechanical systems. Third, the electrical coupling between neurons, in particular fast-spiking interneurons, may change because of altered gap-junction protein composition and/or gap-junction density [13, 79, 80]. Fourth, the interaction between principal cells and interneurons and/or the balance of excitatory/inhibitory activity may alter the resonance properties of the network [81–83].
For HFOs in the 110–160 Hz range, information regarding the underlying mechanisms may also be obtained from characterizing the pharmacological profiles of different drugs known to induce HFOs of this type. A particularly interesting aspect is that psychotomimetic drugs known to act on different receptor systems—for example on either 5-HT2A or NMDA receptors have been found to induce very similar HFO activities in animals [26, 84]. It has been proposed that a partial depression of NMDA-receptor function could be a common underlying mechanism [85]. According to some reports based on local pharmacological manipulations, it is sufficient to interfere with NMDA signaling in one node of the network to induce high-frequency oscillations throughout the limbic loop. Thus, applying an NMDA-antagonist in PFC, hippocampus or nucleus accumbens [86, 87] induces the same LFP oscillations as does a systemic administration of the same drug [84, 88]. Interestingly the extended amygdala network has also been shown to have an intrinsic propensity to oscillate at these frequencies (typically 130–160 Hz), indicating that broadly distributed limbic circuits may be involved in the phenomenon [89]. Relatively few recordings of LFP oscillations in this frequency range have been reported involving non-limbic circuits following pharmacological NMDA-depression, but at least primary motor cortex and parts of the basal ganglia circuitry have been shown to also display this type of activity [70, 90], whereas, for example visual cortex, does not seem to share this propensity (at least in rodents) [91]. For a more extensive overview on the effects of NMDA-antagonist treatment on oscillatory activity, we would like to refer the reader to the review by Hunt and Kasicki [92].
POSSIBLE MECHANISMS UNDERLYING THE PROPAGATION OF HIGH-FREQUENCY OSCILLATIONS
While intrinsic network changes induced by the disease and its pharmacotherapy, may contribute to pushing the network towards a state that can uphold HFOs in certain brain structures, changes in inter-structure connectivity could, in parallel, facilitate the transmission of oscillations of certain frequencies between different brain structures, [68, 93, 94]. It has, however, proven difficult to establish if oscillatory activity can indeed spread from one structure to another and which structures would be the principle drivers in this scenario. We here limit our discussion to three alternative models. These conceptual viewpoints are not entirely mutually exclusive but each emphasize somewhat different mechanistic components.
The flowchart model
It is often presumed that the physiological signaling within the basal ganglia can be directly deduced from the anatomical connectivity in the sense that activity in one structure will directly influence the next downstream brain structure via synaptic excitation or inhibition. According to this view, oscillatory activity is expected to be passed on from one structure to the next in a step-wise chain of events (Fig. 2a). Indeed, in studies of gamma oscillations in cortico-basal ganglia circuits, cross-structure interactions have often been inferred based on this assumption (see e.g., [46, 49]; however, see also [58]). The pattern of gamma oscillations observed in the rodent model of LID do not however comply with connectivity rules in a straightforward manner. For example, narrowband gamma oscillations associated with LID are detected in motor cortex and striatum in the lesioned hemisphere, but they are never observed in the contralateral striatum, despite the bilaterality of corticostriatal projections [95]. Thus, the existence of direct anatomical connections does not seem to be sufficient for cortical gamma oscillations to be transferred to the contralateral striatum.
Fig.2
Hypothetical models for the propagation of high-frequency cortico-basal ganglia oscillations in Parkinson’s disease. a) Stepwise feed-forward propagation of oscillatory activity. b) A system of independent oscillators that are weakly coupled via direct or indirect anatomical links. c) Thalamus (marked ‘A’ in diagram) acting as a central pacemaker.
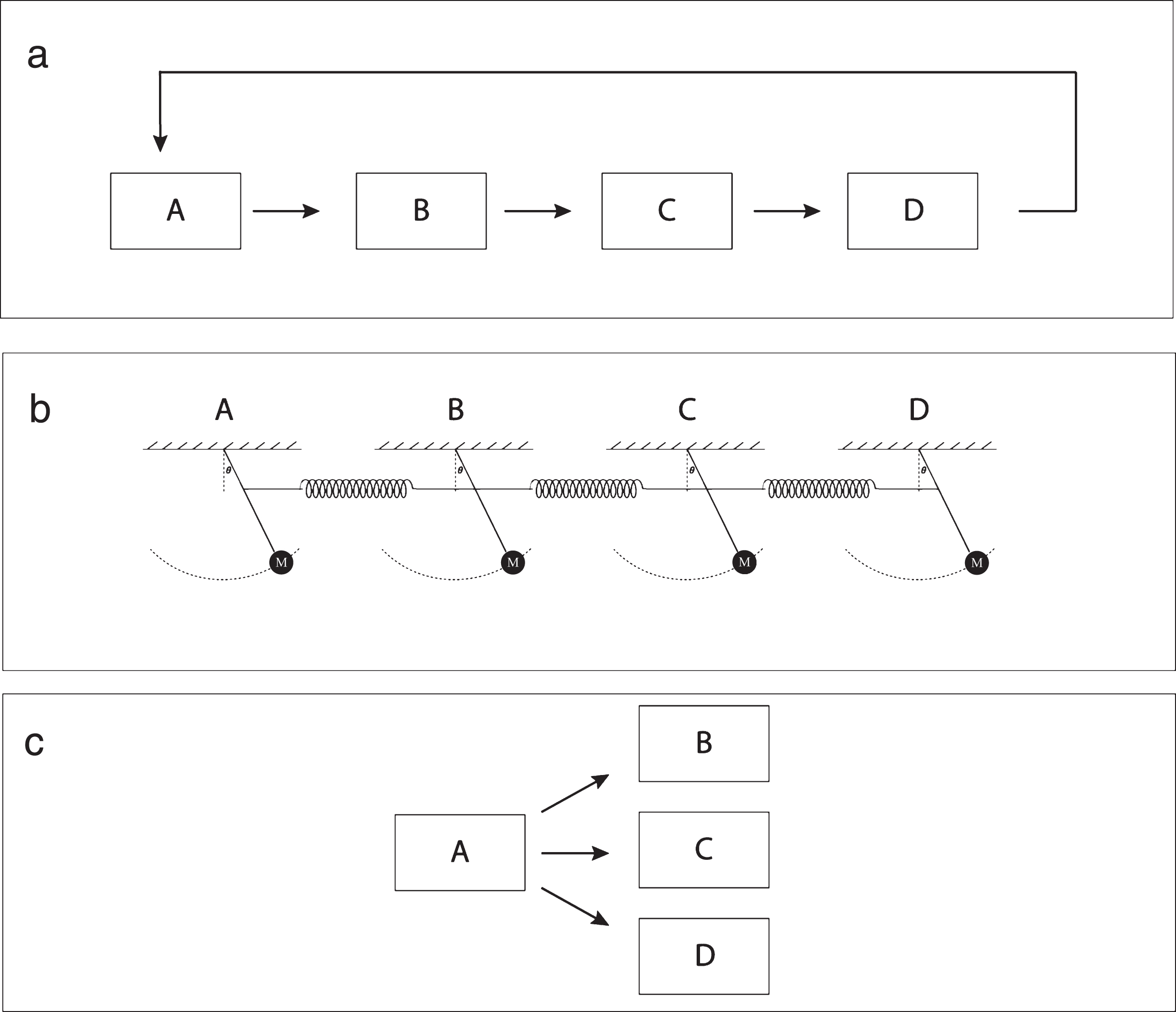
Coupled oscillators
A related but somewhat different view is to consider the interconnected brain structures as a system of coupled oscillators (Fig. 2b). It is possible that several brain structures could have an independent propensity to oscillate in a relatively narrow part of the frequency range under certain conditions, such as during LID [20, 70]. In this situation, even a relatively weak coupling of the oscillators could result in prominent coherent oscillations in multiple structures [96, 97]. On the other hand, structures that do not share the network properties needed to sustain oscillations of the same type would be relatively resistant to rhythmic input of this frequency from upstream structures. This could for example explain why narrowband gamma is not observed in the intact striatum, if the striatal network of an intact brain has different electrophysiological properties that do not uphold narrowband gamma oscillations. It is, however, not clear why brain structures with significant differences in neuronal microcircuitry would become tuned to similar resonance frequencies. In future studies it will therefore be important to clarify the conditions needed for the cortical and striatal microcircuitry to maintain oscillatory activities of high frequencies over extended time periods.
Thalamus as a pacemaker
Because cortex and the basal ganglia are under strong influence of thalamic input, rhythmic thalamic activity has the potential to directly drive fast oscillations in several parts of the circuit, in parallel, via a first-order synaptic connection (Fig. 2c). Indeed, in the visual system, the thalamocortical system has been shown to be a key driver of narrowband cortical gamma oscillations (∼60 Hz) [98]. If narrowband gamma oscillations in cortico-basal ganglia circuits are induced by a similar mechanism, they should be observed only in brain structures that have direct input from thalamic nuclei of the lesioned hemisphere. This notion is supported by preliminary experimental findings suggesting that diffusely projecting nuclei in thalamus, which are known to affect cortical states [99], do in fact display coherent oscillations with cortex during dyskinesia [100]. A pharmacological suppression of this thalamic activity will eliminate high-frequency oscillations also in motor cortex (although this suppression was reported to be insufficient to alleviate LID in preliminary experiments; [100]). Clearly, the role of thalamus in the induction of narrowband gamma oscillations represents quite an important topic for future studies.
Ultimately, because changes at many different levels (ranging from cellular to systems) likely act together, computer modelling will become an indispensable approach to help us understand how cellular processes can induce network dysfunctions, reflected in aberrant oscillatory phenomena and altered effective connectivity between the involved structures [68, 101–104]. At present, however, more detailed experimental data are needed to allow for meaningful modelling, highlighting the importance of joint interdisciplinary efforts by experimentalists and theoreticians.
TRANSLATIONAL IMPORTANCE OF HIGH-FREQUENCY OSCILLATIONS AND FUTURE OUTLOOK
A window into circuit dysfunctions in basal ganglia disorders
Elucidating pathophysiological phenomena at both cellular and systems levels requires studying animal models that mimic central aspects of the disease of interest. Consequently, the generation of valid animal models of basal ganglia disease remains a high research priority [105, 106]. However, even for conditions where reliable and well-characterized models exist, the development of new treatments for neurologic and psychiatric conditions has often been hampered by an insufficient understanding of how to link molecular and pathophysiological processes that ultimately lead to disabilities. In this perspective, high-frequency oscillations in cortico-basal ganglia circuits in PD could provide valuable cues. The striking similarities between rats and humans in the characteristics of certain HFOs strongly suggests that shared underlying mechanisms are at work in both species. This opens up unique opportunities to test hypotheses and investigate candidate mechanisms in valid models that are amenable to multiple levels of experimental investigation. Moreover, the differential manifestations of HFOs in cognitive and limbic circuits as compared to motor circuits observed in animals may provide important clues to the multi-faceted symptomatology of PD.
A biomarker for developing novel therapies
Importantly, even without a full understanding of the underlying mechanisms, HFOs can be used as reliable biomarkers of pathological brain states with which candidate pharmacological treatments can be evaluated and benchmarked against each other. Because of the great similarity of several of these phenomena across species, the effects produced by therapeutic interventions on HFOs are mostly likely to be translationally relevant. In this way, behavioral and neurochemical assessment techniques that are already in use can be complemented with electrophysiological readouts of changes in the brain, providing not only a much richer description [107] but also a biomarker of drug action that is directly translatable. The added value of electrophysiological recordings is exemplified by a couple of recent studies aimed at evaluating a number of putative antidyskinetic compounds in hemiparkinsonian rats [20, 70]. In some cases, neurophysiological signals can be the only available readout since not all pharmacological treatments affecting brain states give rise to detectable changes in motor behavior [70]. In particular, HFOs in limbic circuits (which are associated with psychotic-like states) could offer an exciting new opportunity to evaluate new antipsychotic treatments in animals.
A feedback signal for closed-loop neuromodulation
DBS is a well-established neuromodulation therapy for the advanced stages of PD, although it is an invasive method with several contraindications and some unwanted side-effects [108]. The widespread use of DBS for the symptomatic treatment of PD is therefore prompting a quest for technological developments that can improve efficacy while avoiding troublesome side-effect. In particular, it is argued that adjusting the stimulation parameters to the moment-to-moment needs of the patient (‘adaptive stimulation’) could greatly improve the therapeutic application of DBS [18, 109–113]. Adaptive neuromodulatory stimulation is also referred to as ‘closed-loop’ stimulation because an adaptive system needs to encompass a physiological read-out of selected biomarkers which are used in the feedback control of the stimulation electrodes thus closing the control-loop. To this end, different electrophysiological signals are being considered, derived from LFPs, electrocorticograms (ECoG), EEG, MEG, or indirect measures of brain activity reflected in blood-flow changes (as detected using near-infrared spectroscopy or functional magnetic resonance imaging). Alternatively, one may simply use physical movement parameters, detected using wearable sensors. Despite this wide variety of options, electrophysiological measurements will most likely be needed for more detailed characterizations of brain states. For this reason, most studies have so far been designed to improve electrical stimulation paradigms based on simultaneously recorded neuronal activity using invasive techniques [14, 110, 111]. With respect to HFOs as a potential biomarker to be used in closed-loop DBS to treat LID, the study by Swann and co-authors provided several important insights on how neurophysiological recordings in a closed-loop arrangement could be used to adjust the stimulation parameters to the patients’ symptom fluctuations [21]. In particular, the authors present data that indicate how problems with stimulation-induced dyskinesia could potentially be overcome by feedback control of the stimulator based on the ECoG/STN on-line recordings [21], and have also subsequently confirmed in two patients that significant energy savings can be achieved using this type of adaptive DBS approach [114].
In conclusion, the high-frequency cortico-basal ganglia oscillations discussed in this article have significant translational and scientific implications that deserve to be thoroughly explored. Further research efforts in this area are clearly worthwhile, both for gaining a deeper understanding of basal ganglia disorders and for developing improved therapies.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The authors’ work in this area is supported by grants from Multipark (Multidisciplinary research on Parkinson’s Disease), the Swedish Research Council, the Swedish Governmental Funding for Clinical Research, the Swedish Parkinson Foundation, the Swedish Society for Medical Research, the Swedish Brain Foundation, the Olle Engkvist Foundation, the Parkinson Research Foundation, the Bergvall Foundation, the Royal Physiographic Society, the Crafoord Foundation, the Greta and Johan Kocks Foundation, and the Foundation Sven-Olof Jansons livsverk.
REFERENCES
[1] | Manson A , Stirpe P , Schrag A ((2012) ) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2: , 189–198. |
[2] | Cenci MA , Odin P ((2009) ) Dopamine replacement therapy in Parkinson’s disease: Past, present and future. In Cortico-Subcortical Dynamics in Parkinsons Disease, Humana Press, Springer, pp. 309–334. |
[3] | Cenci MA ((2007) ) Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci 30: , 236–243. |
[4] | Jourdain VA , Tang CC , Holtbernd F , Dresel C , Choi YY , Ma Y , Dhawan V , Eidelberg D ((2016) ) Flow-metabolism dissociation in the pathogenesis of levodopa-induced dyskinesia. JCI insight 1: , e86615. |
[5] | Carey RJ , Pinheiro-Carrera M , Dai H , Tomaz C , Huston JP ((1995) ) L-DOPA and psychosis: Evidence for L-DOPA-induced increases in prefrontal cortex dopamine and in serum corticosterone. Biol Psychiatry 38: , 669–676. |
[6] | Cenci MA , Jorntell H , Petersson P ((2018) ) On the neuronal circuitry mediating L-DOPA-induced dyskinesia. J Neural Transm 125: , 1157–1169. |
[7] | Levy R , Hutchison WD , Lozano AM , Dostrovsky JO ((2000) ) High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20: , 7766–7775. |
[8] | Brown P , Oliviero A , Mazzone P , Insola A , Tonali P , Di Lazzaro V ((2001) ) Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 21: , 1033–1038. |
[9] | Hammond C , Bergman H , Brown P ((2007) ) Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci 30: , 357–364. |
[10] | Brown P ((2007) ) Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol 17: , 656–664. |
[11] | Oswal A , Brown P , Litvak V ((2013) ) Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr Opin Neurol 26: , 662–670. |
[12] | Tinkhauser G , Pogosyan A , Little S , Beudel M , Herz DM , Tan H , Brown P ((2017) ) The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140: , 1053–1067. |
[13] | Little S , Pogosyan A , Neal S , Zavala B , Zrinzo L , Hariz M , Foltynie T , Limousin P , Ashkan K , FitzGerald J , Green AL , Aziz TZ , Brown P ((2013) ) Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: , 449–457. |
[14] | Phookan S , Sutton AC , Walling I , Smith A , O'Connor KA , Campbell JC , Calos M , Yu W , Pilitsis JG , Brotchie JM , Shin DS ((2015) ) Gap junction blockers attenuate beta oscillations and improve forelimb function in hemiparkinsonian rats. Exp Neurol 265: , 160–170. |
[15] | Johnson LA , Nebeck SD , Muralidharan A , Johnson MD , Baker KB , Vitek JL ((2016) ) Closed-loop deep brain stimulation effects on parkinsonian motor symptoms in a non-human primate - is beta enough? Brain Stimul 9: , 892–896. |
[16] | van Wijk BCM , Pogosyan A , Hariz MI , Akram H , Foltynie T , Limousin P , Horn A , Ewert S , Brown P , Litvak V ((2017) ) Localization of beta and high-frequency oscillations within the subthalamic nucleus region. NeuroImage Clin 16: , 175–183. |
[17] | Sharott A , Vinciati F , Nakamura KC , Magill PJ ((2017) ) A population of indirect pathway striatal projection neurons is selectively entrained to parkinsonian beta oscillations. J Neurosci 37: , 9977–9998. |
[18] | Deffains M , Iskhakova L , Katabi S , Israel Z , Bergman H ((2018) ) Longer p oscillatory episodes reliably identify pathological subthalamic activity in Parkinsonism. Mov Disord 33: , 1609–1618. |
[19] | Halje P , Tamte M , Richter U , Mohammed M , Cenci MA , Petersson P ((2012) ) Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. J Neurosci 32: , 16541–16551. |
[20] | Tamte M , Brys I , Richter U , Ivica N , Halje P , Petersson P ((2016) ) Systems level neurophysiological state characteristics for drug evaluation in an animal model of levodopa-induced dyskinesia. J Neurophysiol 115: , 1713–1729. |
[21] | Swann NC , de Hemptinne C , Miocinovic S , Qasim S , Wang SS , Ziman N , Ostrem JL , San Luciano M , Galifianakis NB , Starr PA ((2016) ) Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s disease. J Neurosci 36: , 6445–6458. |
[22] | Miller KJ , Leuthardt EC , Schalk G , Rao RPN , Anderson NR , Moran DW , Miller JW , Ojemann JG ((2007) ) Spectral changes in cortical surface potentials during motor movement. J Neurosci 27: , 2424–2432. |
[23] | Richter U , Halje P , Petersson P ((2013) ) Mechanisms underlying cortical resonant states: Implications for levodopa-induced dyskinesia. Rev Neurosci 24: , 415–429. |
[24] | Dupre KB , Cruz A V , McCoy AJ , Delaville C , Gerber CM , Eyring KW , Walters JR ((2015) ) Effects of L-dopa priming on cortical high beta and high gamma oscillatory activity in a rodent model of Parkinson’s disease. Neurobiol Dis 86: , 1–15. |
[25] | Miller KJ , Zanos S , Fetz EE , den Nijs M , Ojemann JG ((2009) ) Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci 29: , 3132–3137. |
[26] | Goda SA , Piasecka J , Olszewski M , Kasicki S , Hunt MJ ((2013) ) Serotonergic hallucinogens differentially modify gamma and high frequency oscillations in the rat nucleus accumbens. Psychopharmacology (Berl) 228: , 271–282. |
[27] | Hunt MJ , Raynaud B , Garcia R ((2006) ) Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol Psychiatry 60: , 1206–1214. |
[28] | Foffani G , Priori A , Egidi M , Rampini P , Tamma F , Caputo E , Moxon KA , Cerutti S , Barbieri S ((2003) ) 300-Hz subthalamic oscillations in Parkinson’s disease. Brain 126: , 2153–2163. |
[29] | Tsiokos C , Hu X , Pouratian N ((2013) ) 200-300Hz movement modulated oscillations in the internal globus pallidus of patients with Parkinson’s disease. Neurobiol Dis 54: , 464–474. |
[30] | Uhlhaas PJ , Singer W ((2006) ) Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 52: , 155–168. |
[31] | Baker SN , Pinches EM , Lemon RN ((2003) ) Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol 89: , 1941–1953. |
[32] | Courtemanche R , Fujii N , Graybiel AM ((2003) ) Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci 23: , 11741–11752. |
[33] | Fuentes R , Petersson P , Nicolelis MAL ((2010) ) Restoration of locomotive function in Parkinson’s disease by spinal cord stimulation: Mechanistic approach. Eur J Neurosci 32: , 1100–1108. |
[34] | Paul L. Nunez ; Ramesh Srinivasan ((2005) ) Electric Fields of the Brain - The Neurophysics of EEG, Oxford University Press. |
[35] | Dehghani N , Bedard C , Cash SS , Halgren E , Destexhe A ((2010) ) Comparative power spectral analysis of simultaneous elecroencephalographic and magne-toencephalographic recordings in humans suggests non-resistive extracellular media. J Comput Neurosci 29: , 405–421. |
[36] | Schalk G , Leuthardt EC ((2011) ) Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng 4: , 140–154. |
[37] | Nelson MJ , Pouget P , Nilsen EA , Patten CD , Schall JD ((2008) ) Review of signal distortion through metal micro-electrode recording circuits and filters. J Neurosci Methods 169: , 141–157. |
[38] | Linden H , Pettersen KH , Einevoll GT ((2010) ) Intrinsic dendritic filtering gives low-pass power spectra of local field potentials. J Comput Neurosci 29: , 423–444. |
[39] | Buzsaki G , Anastassiou CA , Koch C ((2012) ) The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: , 407–420. |
[40] | Herreras O ((2016) ) Local field potentials: Myths and misunderstandings. Front Neural Circuits 10: , 101. |
[41] | Mitzdorf U ((1985) ) Current source-density method and application in cat cerebral cortex: Investigation of evoked potentials and EEG phenomena. Physiol Rev 65: , 37–100. |
[42] | Scheffer-Teixeira R , Belchior H , Leao RN , Ribeiro S , Tort ABL ((2013) ) On high-frequency field oscillations (>100Hz) and the spectral leakage of spiking activity. J Neurosci 33: , 1535–1539. |
[43] | Kajikawa Y , Schroeder CE ((2011) ) How local is the local field potential? Neuron 72: , 847–858. |
[44] | Jasper EH , Andrews HL ((1938) ) Electro-encephalography. Arch Neurol Psychiatry 39: , 96. |
[45] | Wen H , Liu Z ((2016) ) Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr 29: , 13–26. |
[46] | Jenkinson N , Kühn AA , Brown P ((2013) ) Gamma oscillations in the human basal ganglia. Exp Neurol 245: , 72–76. |
[47] | Tsang EW , Hamani C , Moro E , Mazzella F , Lozano AM , Hodaie M , Yeh I-J , Chen R ((2012) ) Movement related potentials and oscillatory activities in the human internal globus pallidus during voluntary movements. J Neurol Neurosurg Psychiatry 83: , 91–97. |
[48] | Cassidy M , Mazzone P , Oliviero A , Insola A , Tonali P , Di Lazzaro V , Brown P ((2002) ) Movement-related changes in synchronization in the human basal ganglia. Brain 125: , 1235–1246. |
[49] | Williams D , Tijssen M , Van Bruggen G , Bosch A , Insola A , Di Lazzaro V , Mazzone P , Oliviero A , Quartarone A , Speelman H , Brown P ((2002) ) Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 125: , 1558–1569. |
[50] | Alonso-Frech F , Zamarbide I , Alegre M , Rodriguez-Oroz MC , Guridi J , Manrique M , Valencia M , Artieda J , Obeso JA ((2006) ) Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 129: , 1748–1757. |
[51] | Alegre M , Alonso-Frech F , Rodriguez-Oroz MC , Guridi J , Zamarbide I , Valencia M , Manrique M , Obeso JA , Artieda J ((2005) ) Movement-related changes in oscillatory activity in the human subthalamic nucleus: Ipsilateral vs. contralateral movements. Eur J Neurosci 22: , 2315–2324. |
[52] | Trottenberg T , Fogelson N , Kühn AA , Kivi A , Kupsch A , Schneider G-H , Brown P ((2006) ) Subthalamic gamma activity in patients with Parkinson’s disease. Exp Neurol 200: , 56–65. |
[53] | Tsang EW , Hamani C , Moro E , Mazzella F , Poon YY , Lozano AM , Chen R ((2010) ) Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology 75: , 950–959. |
[54] | Kempf F , Brücke C , Salih F , Trottenberg T , Kupsch A , Schneider G-H , Doyle Gaynor LMF , Hoffmann K-T , Vesper J , Wohrle J , Altenmüller D-M , Krauss JK , Mazzone P , Di Lazzaro V , Yelnik J , Kuhn AA , Brown P ((2009) ) Gamma activity and reactivity in human thalamic local field potentials. Eur J Neurosci 29: , 943–953. |
[55] | Brücke C , Bock A , Huebl J , Krauss JK , Schönecker T , Schneider G-H , Brown P , Kuhn AA ((2013) ) Thalamic gamma oscillations correlate with reaction time in a Go/noGo task in patients with essential tremor. Neuroimage 75: , 36–45. |
[56] | Fogelson N , Pogosyan A , Kühn AA , Kupsch A , van Brüggen G , Speelman H , Tijssen M , Quartarone A , Insola A , Mazzone P , Di Lazzaro V , Limousin P , Brown P ((2005) ) Reciprocal interactions between oscillatory activities of different frequencies in the subthalamic region of patients with Parkinson’s disease. Eur J Neurosci 22: , 257–266. |
[57] | Litvak V , Eusebio A , Jha A , Oostenveld R , Barnes G , Foltynie T , Limousin P , Zrinzo L , Hariz MI , Friston K , Brown P ((2012) ) Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci 32: , 10541–10553. |
[58] | Lalo E , Thobois S , Sharott A , Polo G , Mertens P , Pogosyan A , Brown P ((2008) ) Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci 28: , 3008–3016. |
[59] | Cheyne D , Ferrari P ((2013) ) MEG studies of motor cortex gamma oscillations: Evidence for a gamma "fingerprint" in the brain? Front Hum Neurosci 7: , 575. |
[60] | Huo X , Wang Y , Kotecha R , Kirtman EG , Fujiwara H , Hemasilpin N , Degrauw T , Rose DF , Xiang J ((2011) ) High gamma oscillations of sensorimotor cortex during unilateral movement in the developing brain: A MEG study. Brain Topogr 23: , 375–384. |
[61] | Muthukumaraswamy SD ((2010) ) Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol 104: , 2873–2885. |
[62] | Ball T , Demandt E , Mutschler I , Neitzel E , Mehring C , Vogt K , Aertsen A , Schulze-Bonhage A ((2008) ) Movement related activity in the high gamma range of the human EEG. Neuroimage 41: , 302–310. |
[63] | Crone NE , Miglioretti DL , Gordon B , Lesser RP ((1998) ) Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121: (Pt 12), 2301–2315. |
[64] | Pfurtscheller G , Graimann B , Huggins JE , Levine SP , Schuh LA ((2003) ) Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol 114: , 1226–1236. |
[65] | Groiss SJ , Wojtecki L , Südmeyer M , Schnitzler A ((2009) ) Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2: , 20–28. |
[66] | Miocinovic S , Swann NC , de Hemptinne C , Miller A , Ostrem JL , Starr PA ((2018) ) Cortical gamma oscillations in isolated dystonia. Parkinsonism Relat Disord 49: , 104–105. |
[67] | Delaville C , McCoy AJ , Gerber CM , Cruz AV , Walters JR ((2015) ) Subthalamic nucleus activity in the awake hemi-parkinsonian rat: Relationships with motor and cognitive networks. J Neurosci 35: , 6918–6930. |
[68] | Belic JJ , Halje P , Richter U , Petersson P , Hellgren Kotaleski J ((2016) ) Untangling cortico-striatal connectivity and cross-frequency coupling in L-DOPA-induced dyskinesia. Front Syst Neurosci 10: , 26. |
[69] | Tort ABL , Scheffer-Teixeira R , Souza BC , Draguhn A , Brankack J ((2013) ) Theta-associated high-frequency oscillations (110-160Hz) in the hippocampus and neocortex. Prog Neurobiol 100: , 1–14. |
[70] | Brys I , Halje P , Scheffer-Teixeira R , Varney M , Newman-Tancredi A , Petersson P ((2018) ) Neurophysiological effects in cortico-basal ganglia-thalamic circuits of antidyskinetic treatment with 5-HT 1A receptor biased agonists. Exp Neurol 302: , 155–168. |
[71] | Tort ABL , Kramer MA , Thorn C , Gibson DJ , Kubota Y , Graybiel AM , Kopell NJ ((2008) ) Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA 105: , 20517–20522. |
[72] | Wang J , Hirschmann J , Elben S , Hartmann CJ , Vesper J , Wojtecki L , Schnitzler A ((2014) ) High-frequency oscillations in Parkinson’s disease: Spatial distribution and clinical relevance. Mov Disord 29: , 1265–1272. |
[73] | Lopez-Azcarate J , Tainta M , Rodriguez-Oroz MC , Valencia M , Gonzalez R , Guridi J , Iriarte J , Obeso JA , Artieda J , Alegre M ((2010) ) Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci 30: , 6667–6677. |
[74] | Ozkurt TE , Butz M , Homburger M , Elben S , Vesper J , Wojtecki L , Schnitzler A ((2011) ) High frequency oscillations in the subthalamic nucleus: A neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol 229: , 324–331. |
[75] | Hirschmann J , Schoffelen JM , Schnitzler A , van Gerven MAJ ((2017) ) Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin Neurophysiol 128: , 2029–2036. |
[76] | Bevan MD , Hallworth NE , Baufreton J ((2007) ) GABAergic control of the subthalamic nucleus. Prog Brain Res 160: , 173–188. |
[77] | Fieblinger T , Graves SM , Sebel LE , Alcacer C , Plotkin JL , Gertler TS , Chan CS , Heiman M , Greengard P , Cenci MA , Surmeier DJ ((2014) ) Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun 5: , 5316. |
[78] | Chu H-Y , Atherton JF , Wokosin D , Surmeier DJ , Bevan MD ((2015) ) Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron 85: , 364–376. |
[79] | Schwab BC , Heida T , Zhao Y , van Gils SA , van Wezel RJA ((2014) ) Pallidal gap junctions-triggers of synchrony in Parkinson’s disease? Mov Disord 29: , 1486–1494. |
[80] | Hjorth J , Blackwell KT , Kotaleski JH ((2009) ) Gap junctions between striatal fast-spiking interneurons regulate spiking activity and synchronization as a function of cortical activity. J Neurosci 29: , 5276–5286. |
[81] | Lindenbach D , Conti MM , Ostock CY , George JA , Goldenberg AA , Melikhov-Sosin M , Nuss EE , Bishop C ((2016) ) The role of primary motor cortex (M1) glutamate and GABA signaling in l-DOPA-induced dyskinesia in parkin-sonian rats. J Neurosci 36: , 9873–9887. |
[82] | Gittis AH , Hang GB , LaDow ES , Shoenfeld LR , Atallah BV , Finkbeiner S , Kreitzer AC ((2011) ) Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron 71: , 858–868. |
[83] | Gittis AH , Kreitzer AC ((2012) ) Striatal microcircuitry and movement disorders. Trends Neurosci 35: , 557–564. |
[84] | Hunt MJ , Olszewski M , Piasecka J , Whittington MA , Kasicki S ((2015) ) Effects of NMDA receptor antagonists and antipsychotics on high frequency oscillations recorded in the nucleus accumbens of freely moving mice. Psychopharmacology (Berl) 232: , 4525–4535. |
[85] | Arvanov VL , Liang X , Russo A , Wang RY ((1999) ) LSD and DOB: Interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci 11: , 3064–3072. |
[86] | Lee J , Hudson MR , O'Brien TJ , Nithianantharajah J , Jones NC ((2017) ) Local NMDA receptor hypofunction evokes generalized effects on gamma and high-frequency oscillations and behavior. Neuroscience 358: , 124–136. |
[87] | Hunt MJ , Falinska M , Kasicki S ((2010) ) Local injection of MK801 modifies oscillatory activity in the nucleus accumbens in awake rats. J Psychopharmacol 24: , 931–941. |
[88] | Hunt MJ , Falinska M , Leski S , Wojcik DK , Kasicki S Differential effects produced by ketamine on oscillatory activity recorded in the rat hippocampus, dorsal striatum and nucleus accumbens. J. Psychopharmacol 25: , 808–821. |
[89] | Haufler D , Pare D ((2014) ) High-frequency oscillations are prominent in the extended amygdala. J Neurophysiol 112: , 110–119. |
[90] | Nicolas MJ , Lopez-Azcarate J , Valencia M , Alegre M , Perez-Alcazar M , Iriarte J , Artieda J ((2011) ) Ketamine-induced oscillations in the motor circuit of the rat basal ganglia. PLoS One 6: , e21814. |
[91] | Phillips KG , Cotel MC , McCarthy AP , Edgar DM , Tricklebank M , O'Neill MJ , Jones MW , Wafford KA ((2012) ) Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology 62: , 1359–1370. |
[92] | Hunt MJ , Kasicki S ((2013) ) A systematic review of the effects of NMDA receptor antagonists on oscillatory activity recorded in vivo. J Psychopharmacol 27: , 972–986. |
[93] | Santana MB , Halje P , Simplicio H , Richter U , Freire MAM , Petersson P , Fuentes R , Nicolelis MAL ((2014) ) Spinal cord stimulation alleviates motor deficits in a primate model of Parkinson disease. Neuron 84: , 716–722. |
[94] | Herz DM , Siebner HR , Hulme OJ , Florin E , Christensen MS , Timmermann L ((2014) ) Levodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson’s disease. Neuroimage 90: , 15–23. |
[95] | Shepherd GMG ((2013) ) Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14: , 278–291. |
[96] | Cumin D , Unsworth CP ((2006) ) Generalising the Kuramoto model for the study of neuronal synchronisation in the brain. Phys D Nonlinear Phenom 226: , 181–196. |
[97] | Restrepo JG , Ott E , Hunt BR ((2006) ) Synchronization in large directed networks of coupled phase oscillators. Chaos 16: , 015107. |
[98] | Saleem AB , Lien AD , Krumin M , Haider B , Roson MR , Ayaz A , Reinhold K , Busse L , Carandini M , Harris KD ((2017) ) Subcortical source and modulation of the narrowband gamma oscillation in mouse visual cortex. Neuron 93: , 315–322. |
[99] | Jones EG ((2009) ) Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci 1157: , 10–23. |
[100] | Brazhnik E , Novikov NI , Dupre KB , Wahba MI , McCoy AJ , Walters JR ((2013) ) Dissociation of high frequency (100Hz) oscillatory activity within the thalamocortical networkfromL-DOPA-induced dyskinesiain hemiparkinsonian rats. Soc Neurosci Abstr 648.03/RR1. |
[101] | Lindahl M , Hellgren-Kotaleski J ((2017) ) Untangling basal ganglia network dynamics and function - role of dopamine depletion and inhibition investigated in a spiking network model. eNeuro 3: , ENEURO.0156-16.2016. |
[102] | Kotaleski JH , Blackwell KT ((2010) ) Modelling the molecular mechanisms of synaptic plasticity using systems biology approaches. Nat Rev Neurosci 11: , 239–251. |
[103] | Lindahl M , Kamali Sarvestani I , Ekeberg O , Kotaleski JH ((2013) ) Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity in the direct, indirect, and hyperdirect pathways. Front Comput Neurosci 7: , 76. |
[104] | Berthet P , Lindahl M , Tully PJ , Hellgren-Kotaleski J , Lansner A ((2016) ) Functional relevance of different basal ganglia pathways investigated in a spiking model with reward dependent plasticity. Front Neural Circuits 10: , 53. |
[105] | Ellenbroek B , Youn J ((2016) ) Rodent models in neuroscience research: Is it a rat race? Dis Model Mech 9: , 1079–1087. |
[106] | Okano H , Kishi N ((2018) ) Investigation of brain science and neurological/psychiatric disorders using genetically modified non-human primates. Curr Opin Neurobiol 50: , 1–6. |
[107] | Waters S , Svensson P , Kullingsjo J , Ponten H , Andreasson T , Sunesson Y , Ljung E , Sonesson C , Waters N ((2017) ) In vivo systems response profiling and multivariate classification of cns active compounds: A structured tool for cns drug discovery. ACS Chem Neurosci 8: , 785–797. |
[108] | Buhmann C , Huckhagel T , Engel K , Gulberti A , Hidding U , Poetter-Nerger M , Goerendt I , Ludewig P , Braass H , Choe C-U , Krajewski K , Oehlwein C , Mittmann K , Engel AK , Gerloff C , Westphal M , Koppen JA , Moll CKE , Hamel W ((2017) ) Adverse events in deep brain stimulation: A retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS One 12: , e0178984. |
[109] | Hosain MK , Kouzani A , Tye S ((2014) ) Closed loop deep brain stimulation: An evolving technology. Australas Phys Eng Sci Med 37: , 619–634. |
[110] | Little S , Pogosyan A , Neal S , Zavala B , Zrinzo L , Hariz M , Foltynie T , Limousin P , Ashkan K , FitzGerald J , Green AL , Aziz TZ , Brown P ((2013) ) Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: , 449–457. |
[111] | Rosin B , Slovik M , Mitelman R , Rivlin-Etzion M , Haber SN , Israel Z , Vaadia E , Bergman H ((2011) ) Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 72: , 370–384. |
[112] | Herron JA , Thompson MC , Brown T , Chizeck HJ , Ojemann JG , Ko AL ((2017) ) Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg 127: , 580–587. |
[113] | Meidahl AC , Tinkhauser G , Herz DM , Cagnan H , Debarros J , Brown P ((2017) ) Adaptive deep brain stimulation for movement disorders: The long road to clinical therapy. Mov Disord 32: , 810–819. |
[114] | Swann NC , de Hemptinne C , Thompson MC , Miocinovic S , Miller AM , Gilron R , Ostrem JL , Chizeck HJ , Starr PA ((2018) ) Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng 15: , 046006. |




