A Systematic Review and Case Series of Ziprasidone for Psychosis in Parkinson’s Disease
Abstract
The atypical antipsychotic ziprasidone has been considered inappropriate for use in patients with Parkinson’s disease (PD), as most atypical antipsychotics worsen parkinsonism. However, the current evidence for safety and efficacy of ziprasidone in PDP has not been evaluated in a systematic fashion. We review published experience with ziprasidone for treating psychosis in PD via systematic search of MEDLINE, Embase, Cochrane CENTRAL, and Clinicaltrials.gov with terms related to “ziprasidone” and “Parkinson’s disease”, inclusive of case reports and prospective studies. We also add seven cases of ziprasidone exposure in patients in our center with idiopathic PD or Lewy body dementia (DLB), selected by retrospective query of all clinical data since 1996. In our review, two prospective trials and 11 case reports or series were found, with ziprasidone found to be generally effective for treatment of psychosis and with few adverse events reported. Our case series did not support efficacy of ziprasidone; it was generally safe in PD, but two patients with DLB had adverse motor events. We conclude that, although ziprasidone occasionally can produce substantial worsening of motor signs, it usually is well tolerated, and may provide in some cases a useful alternative to quetiapine, clozapine and pimavanserin, particularly in the acute care setting. Further randomized controlled studies are needed.
INTRODUCTION
Parkinson’s disease (PD) has classically been described as a motor syndrome; indeed James Parkinson, in his seminal report, described “powers of [the] mind, unimpaired” [1]. More recently, however, PD has better been characterized as a neurodegenerative disorder affecting widespread brain regions outside motor pathways, with cognitive, behavioral and other non-motor symptoms of the disease being some of its most disabling features [2]. Psychotic symptoms are common in PD, though criteria for Parkinson’s disease psychosis (PDP) have only recently been standardized. PDP requires psychotic symptoms or illusions, lasting at least 1 month, preexisting PD by standard clinical definitions, and exclusion of alternative diagnoses, while it may occur independently or in association with dopaminergic therapy [3]. PDP varies in severity but generally carries a poor prognosis; it limits treatment of motor symptoms, is associated with dementia, and increases mortality [4].
The treatment of psychotic symptoms in PD is limited by the antidopaminergic properties of most antipsychotic medications, and therapy has generally been restricted to quetiapine, clozapine, and pimavanserin, the last of which is the only pharmaceutical with an FDA indication for treatment of PDP [5–7]. However, significant limitations exist with these medications regarding efficacy, safety and cost, respectively [8–10]. Furthermore, none of these medications can be administered parenterally, limiting their utility in acute care settings [10]. Other atypical antipsychotics have been used in PDP with varying degrees of success, though generally limited by their tendency to exacerbate motor symptoms of PD due to their antidopaminergic effects [7]. Ziprasidone has been the subject of some curiosity for the treatment of psychotic symptoms in PD. In common with other atypical antipsychotics, ziprasidone is both a dopamine and serotonin antagonist, which preferentially binds to D2 over D1 receptors at a 100 to 1 ratio and 5–HT2A over D2 at a 6.9 to 1 ratio. While this affinity for D1 and D2 is within the range of many atypical antipsychotics, the higher relative affinity for 5–HT2A as compared with risperidone has been suggested as a mechanism for lower motor side effects [11]. Additionally, ziprasidone is one of the few atypical antipsychotics available in a parenteral form and has a relatively short half-life [10].
However, ziprasidone is generally now lumped in with other atypical antipsychotics and avoided in PDP due to concerns for exacerbating parkinsonism [4, 12]. Furthermore, the U.S. Food and Drug Administration issued a black box warning of “elderly patients with dementia-related psychosis treated with antipsychotic drugs are at increased risk of death” for all antipsychotics, although for pimavanserin this warning is specified as being for psychosis unrelated to PDP [10]. On the other hand, a recent review on treating PDP found little data on ziprasidone in PD, much of it surprisingly positive, prompting a closer examination [13]. Several previous reviews have been performed on antidopaminergic therapy in PDP [5, 14–16], though few have focused primarily on ziprasidone [17]. A comprehensive, systematic review of the available literature has not yet been performed and would be useful to help define what role ziprasidone may have in the treatment of PDP. Here we summarize published literature on the efficacy, safety and side effects of ziprasidone in PDP and add clinical data from our center for the use of ziprasidone in PDP.
METHODS
Question
The question addressed is the efficacy, safety and side effect profile of ziprasidone for the treatment of symptoms of psychosis in PD.
Survey methodology
The protocol for this systematic review was registered with PROSPERO (https://www.crd.york.ac.uk/PROSPERO), CRD42018099918. We performed a search of MEDLINE (1946 to January, 2018), Embase, Cochrane CENTRAL, and Clinicaltrials.gov with combinations and variations of the terms “Parkinson disease”, “Parkinson’s disease”, “psychosis”, “psychotic disorders”, “hallucinations”, “ziprasidone”, and “Geodon”. The search was limited to those reporting original clinical data in humans and was not limited by language or date. Complete search details are reported in a supplemental file. On reading of the citations, all articles that were relevant to the topic were identified for review. To be included in the review, articles must have reported data from clinical cases or trials in which ziprasidone was used to treat symptoms of psychosis in PD.
This search was performed in May 2018. We also asked the manufacturer of Geodon® for any information they had on ziprasidone in PD which did not reveal any additional information.
Inclusion criteria:
• Publications reporting original clinical data or experience in humans;
• Publications involving patients with a diagnosis of idiopathic PD and psychotic symptoms;
• Publications in which ziprasidone was administered for treatment of psychotic symptoms.
Exclusion criteria:
• Publications not reporting original clinical data, including reviews and book chapters;
• Publications in which ziprasidone may have been one of many medications administered but which do not report data specifically for this medication.
• Publications reporting data only on patients with other parkinsonian disorders, including drug-induced parkinsonism, DLB, multisystems atrophy, corticobasal degeneration, and progressive supranuclear palsy.
• Publications for which full text was unavailable online.
Data extraction and analysis
Selection of publications was performed by JRY, KJB, and AAD, all of whom have clinical expertise and research experience in movement disorders. JRY performed initial screening and selection of studies which were reviewed and verified by KJB. The initial round of selection was performed by screening title, abstract, and keywords for inclusion and exclusion criteria. If the publication potentially met inclusion and exclusion criteria, authors disagreed regarding eligibility, or if insufficient information was available, the full text was reviewed for eligibility (Fig. 1).
Fig.1
PRISMA flowsheet of literature search and selection.
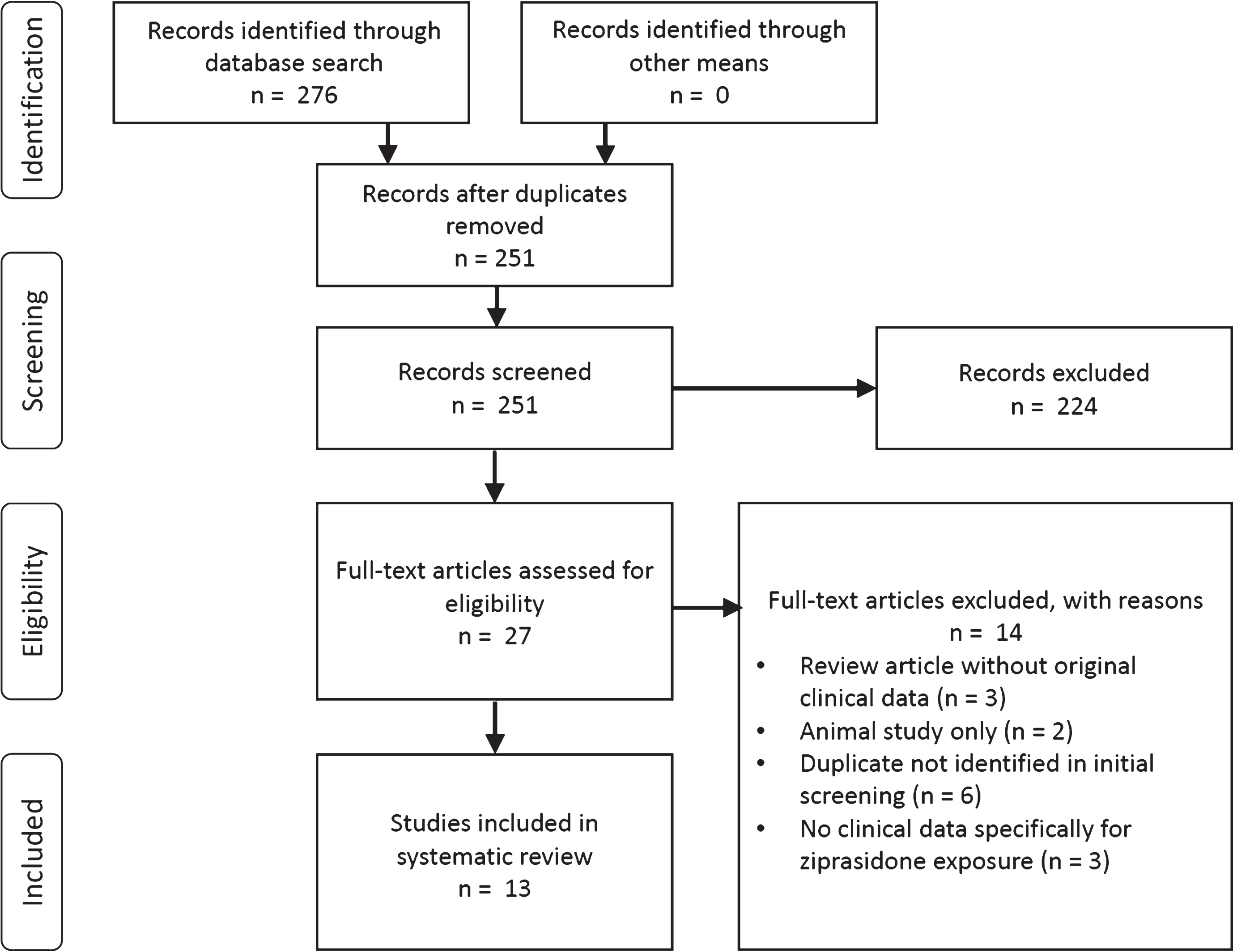
Data was independently extracted by JRY and KJB into a standardized form and inserted into the study database. Data was reviewed by all authors, and discrepancies were mediated by discussion between authors with AAD serving as referee. Data extracted included type of report or study, number of patients or subjects included, metrics used for evaluating efficacy and side effects, the presence of blinding in relevant study types, and clinical outcomes. Principal measures were defined as any original clinical data regarding the use of ziprasidone in PDP.
Given the wide range of data measures, high prevalence of subjective and case report data, and uncontrolled nature of the two small prospective studies resulting from the search, no metaanalysis could be meaningfully performed, and no attempt to mathematically combine the results of different studies was made.
Risk of bias
In order to assess the risk of bias for the two open-label prospective studies, the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) was used [18]. Although Pintor et al did have an element of randomization in their study design, the open label nature with lack of patient blinding and lack of placebo control made tools designed for evaluation of randomized controlled trials less appropriate here, while RoBANS criteria were generally applicable. JRY applied criteria for judging risk of bias, while KJB verified these results. This was used to inform the review authors regarding study quality in reaching conclusions.
Case series
We also reviewed our own clinical data for cases in which ziprasidone was used to treat psychotic symptoms in PD. All patients were evaluated in the Washington University Movement Disorders Center between 1996 and January 2018 by a movement disorders specialist. This center has used an electronic medical record for over 20 years, Medical Automated Records System (MARS), which permits automated retrospective queries of collected clinical data [19]. A search was performed for patients with a diagnosis of idiopathic Parkinson’s disease OR ICD–9–CM code of 332.0 and a history of ziprasidone in the medication list. This search included cases in which ziprasidone was initiated and cases in which ziprasidone was stopped. Authors JRY and KJB reviewed the charts of the individuals returned by this search to identify patients in whom ziprasidone had been used for symptoms of psychosis in the setting of idiopathic PD or, given the similar pathophysiology, patients with a diagnosis of dementia with Lewy bodies (DLB) and significant parkinsonism. Clinical details including diagnoses, minimum and maximum dose of ziprasidone, exposure time, other concurrent or subsequent antipsychotics used, UPDRS scores before, during and after ziprasidone exposure, and subjective notes regarding clinical course were extracted and are reported in Table 2. All data were collected in accordance with a study protocol established with the Washington University Human Research Protection Office (Institutional Review Board, protocol ID 201712126), and no identifiable information is reported here.
RESULTS
Systematic review
The review protocol produced 13 publications addressing the primary question (Table 1). Of these, 2 were prospective open label trials totaling 18 subjects, and 11 were case reports and case series totaling 67 subjects, though the largest totaling 43 subjects presented minimal clinical data. No randomized controlled trials or other blinded studies were found. Efficacy outcomes varied by study and included Neuropsychiatric Inventory (NPI), Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression (CGI), and subjective observations. Side effect and safety measures also varied by study and included Unified Parkinson’s Disease Rating Scale (UPDRS), Abnormal Involuntary Movement Scale (AIMS), Schwab and England Activities of Daily Living Scale (SaE), Hamilton Depression Rating Scale (HDRS), Mini Mental Status Exam (MMSE), corrected QT segment on electrocardiography (QTc), vital signs, and subjective observations on neurologic examination.
Table 1
Publications with primary data on the use of ziprasidone in Parkinson disease, sorted by date
| First author | Year | N | Study type | Blinding | Efficacy measures | Safety measures | Key findings |
| Gray | 2004 | 1 | Case report | none | Subjective | Vital signs, laboratory, subjective | Psychosis improved slightly, motor symptoms worsened. NMS diagnosed after 2 weeks. Sertraline started 1 week prior. |
| Lopez del Val | 2004 | 43 | Case series | none | Subjective | Subjective | All patients achieved good control of symptoms without reported side effects. |
| Connemann | 2004 | 1 | Case report | none | Subjective | UPDRS | Ziprasidone improved psychosis by 2 weeks, UPDRS minimally changed. Aripiprazole increased UPDRS from 43 to 101, reverted to 42 on ziprasidone. |
| Gomez-Esteban | 2005 | 12 | Open label prospective trial | none | NPI | UPDRS, AIMS | 2 withdrew due to adverse effects. For other 10, UPDRS 3 unchanged, significant decrease in NPI from 32.2 to 9.0 in 2 months. UPDRS 3 from 40.4 to 37.7. |
| Oeschner | 2005 | 5 | Case series | none | BPRS | QTc, BP, temperature, subjective | In emergency setting with 10–20 mg×1, moderate to good effects on BPRS (mean 72.2 to 47.6). Unchanged safety and motor measures. |
| Micheli | 2005 | 6 | Case series | none | BPRS, CGI, HDRS | UPDRS, QTc | 3 patients showed improved psychiatric symptoms, 3 did not. 1 patient with slight increase in UPDRS. In 3 non-responders, 2 responded to clozapine. |
| Berkowitz | 2006 | 4 | Case series | none | Subjective | Subjective | All patients had good sustained psychiatric response without increased motor symptoms. |
| Shiah | 2006 | 1 | Case report | none | Subjective | UPDRS | Psychosis exacerbated after deep brain stimulation. Quetiapine improved psychosis but increased UPDRS 3. Crosstitration to ziprasidone improved both motor and psychotic symptoms. |
| Schindehütte | 2007 | 4 | Case series | none | Subjective | Subjective | 1 of 4 patients with prolonged “off” periods compared to baseline. 3 of 4 patients with reduction in psychosis from baseline. |
| Duggal | 2008 | 1 | Case report | none | BPRS | UPDRS, MMSE | Quetiapine ineffective, switching to ziprasodone improved psychosis with similar UPDRS 3. Change in MMSE 24 to 30, BPRS 115 to 31, UPDRS 30 to 31. |
| Stefanis | 2010 | 1 | Case report | none | Subjective | MMSE, subjective | 1 patient with paranoid symptoms, did not respond to ziprasidone, haloperidol effective but caused motor exacerbation. |
| Pintor | 2012 | 6 | Open label prospective trial | none | BPRS, SAPS, CGIS | UPDRS, AIMS, MMSE, Schwab and England Scale | Ziprasodone with large size effect on BPRS (1.33), SAPS (1.79), no significant difference vs clozapine. MMSE up by 2.0 at 30 days. UPDRS 3 changes nonsignificant compared to clozapine. |
| El-Okdi | 2014 | 1 | Case report | None | Subjective | Vital signs, subjective | Admitted with hallucinations, developed serotonin syndrome after 20 mg ziprasidone in combination with tramadol and rasagiline which resolved quickly. |
Side effects and safety
In one case series, ziprasidone was reported to cause worsening of PD with prolonged off periods in 1 of 4 patients [20]. In another case, addition of ziprasidone was associated with marked worsening of parkinsonian signs and features of neuroleptic malignant syndrome [21]. However, two other reports described no side effects or worsened parkinsonism [22, 23]. In one case, switching from high dose quetiapine (up to 700 mg daily) to ziprasidone at 120 mg/day improved motor symptoms of PD which had been exacerbated on quetiapine [24]. The authors of one large study summarized their clinical experience with ziprasidone in PD in two sentences: 43 patients were given 20–40 mg/day, which controlled psychotic symptoms “adequately,” while “no side effects have been reported in any of our patients”, although no objective clinical data or specific details on individual patients were provided [25]. Interestingly, five patients given parenteral ziprasidone 10–20 mg in an emergency setting had “no increase in bradykinesia, muscle rigidity or tremor” on follow-up examinations during the 24 hours after injections [26]. One study noted that aripiprazole had worsened motor symptoms while a subsequent switch to ziprasidone essentially restored UPDRS to the patient’s baseline [27]. In one unusual case, the concomitant administration of ziprasidone, tramadol and rasagiline was associated with the development of serotonin syndrome [28]. The bulk of case data did not support worsening of parkinsonian motor symptoms with the administration of ziprasidone.
In one open label prospective study, 12 patients with PD were administered ziprasidone in an open-label fashion for symptoms of psychosis [29]. Two subjects withdrew due to adverse effects, one due to sedation and the other due to worsening of gait, while two other patients were reported to have a “slight deterioration in motor symptoms”, though UPDRS values for these patients were not specifically provided. Overall, however, UPDRS part 3 scores did not show a significant change after pairwise analysis, from a mean of 40.4 at baseline to 37.7 at the final visit. Another open label prospective study compared ziprasidone (6 subjects) to clozapine (8 subjects). Motor symptoms did not worsen with either medication, with UPDRS decreasing by 4.25 in the ziprasidone group and 1.12 in the clozapine group, and no significant differences noted in AIMS or SaE between groups. There were no significant differences between groups, suggesting that ziprasidone was not inferior to clozapine in its side effect profile in PDP [30].
Efficacy
Ziprasidone was generally effective in the treatment of psychotic symptoms throughout the cases reviewed, particularly when compared with other atypical antipsychotic agents. One case cited modest subjective improvement in psychosis [21], while others noted more substantial benefit, particularly when compared with other antipsychotics. In one case, aripiprazole was ineffective but the patient responded to ziprasidone [27], while in other cases ziprasidone was superior to quetiapine for control of PDP [23, 24]. In the emergency setting, 10–20 mg IM ziprasidone was effective for control of acute psychosis in PD, as patients experienced a mean Brief Psychiatric Rating Scale (BPRS) score reduction of 34% [26]. 2 other case series showed generally good responses to ziprasidone as well [20, 22], though this efficacy was not universal, with one case series citing 3 of 6 patients with good response to ziprasidone, and of non-responders clozapine was effective in 2, while in another 1 patient did not respond to ziprasidone but did respond to haloperidol [31, 32].
In one small open-label prospective trial in which ziprasidone was compared with clozapine over 4 weeks, psychotic symptoms improved in both groups as BPRS decreased by 7.25 in the ziprasidone group and by 4.37 in the clozapine group, without a significant difference between treatment groups [30]. Another open-label prospective study over 12 weeks reported dramatic improvement in psychotic symptoms after ziprasidone administration, with average NPI decreasing from 32.2 to 9.0 [29].
Case series
We found 7 patients at our center with a diagnosis of idiopathic PD or DLB who had received ziprasidone for treatment of psychotic symptoms, which are summarized below (Table 2). The small number of such cases found was unexpected given the thousands of patients with iPD/DLB that were treated at our center during this interval.
Table 2
Cases in which ziprasidone was used to treat symptoms of psychosis in PD or DLB at Washington University Movement Disorders Center
| Age | Sex | Diagnoses | Min dose (mg/d) | Max dose (mg/d) | Exposure time | Other drugs | UPDRS 3 before | UPDRS 3 during | UPDRS 3 after | Notes |
| 84 | Female | DLB, dystonia | 20 | 40 | 14 months | quetiapine | 51 | 57.5 | 66 | Ziprasidone started by outside MD. Did not improve hallucinations. Stopped by us at time of consultation, parkinsonism not improved by its cessation. |
| 83 | Male | DLB, AD | 60 | 60 | 1 month | quetiapine clozapine | 32 | 62.5 | 69.5 | Started ziprasidone elsewhere, subsequent subjective increase in rigidity and bradykinesia. Did not respond to quetiapine and parkinsonism did not improve on cessation. |
| 73 | Female | PD | 20 | 20 | 1.5 months | quetiapine | 31 | 29 | 37.5 | Ziprasidone and quetiapine started as inpatient for hallucinations. Quetiapine stopped and ziprasidone controlled hallucinations without increased parkinsonism. Ziprasidone then stopped on consultation without improvement in UPDRS, tolerated cessation. |
| 86 | Male | PD, DLB* | 20 | 20 | 8 months | pimavanserin clozapine | none | 59 | 61 | On ziprasidone at time of consultation, this was tapered in favor of clozapine without notable improvement in parkinsonism or improvement in psychosis. |
| 73 | Male | PD, drug-induced parkinsonism, DLB* | 40 (prn) | 40 (prn) | unknown (likely years) | quetiapine chlor-promazine | none | 67.5 | 39 | Asymmetric onset parkinsonism but also complicated by antidopaminergic drugs. Ziprasidone and chlorpromazine stopped on consultation, levodopa also increased. |
| 67 | Female | PD+psychosis, dementia NOS | 40 | 40 | 5 months | aripiprazole quetiapine | 38.5 | 36.5 | 28 | Ziprasidone started for psychosis, psychosis and mood improved. Mild dyskinesia but no noted increased parkinsonism. Subsequently removed, modest improvement in tremor. |
| 72 | Female | PD | 80 | 80 | 22 months | olanzapine | none | none | 42 | Gait predominant PD, worsened with ziprasidone and olanzapine but did not improve after cessation. |
*indicates postmortem diagnosis of diffuse cortical Lewy body disease without DLB diagnosis at time of ziprasidone exposure.
In our data, patients with PDP generally tolerated ziprasidone well, although its efficacy was less clear. In one case, a combination of quetiapine and ziprasidone was effective for treating hallucinations in the inpatient setting without notable motor side effects. Quetiapine was subsequently stopped, and ziprasidone continued to be effective as monotherapy without exacerbation of parkinsonism. In another case of PDP with especially severe psychosis, ziprasidone was effective in improving psychotic symptoms and mood, with only mild dyskinesia and no increase in parkinsonism noted. Cognitive function was also not worsened, and MMSE in fact increased from 9 to 22 with treatment. Despite no increase in motor symptoms being noted on initiation of ziprasidone, its eventual cessation was associated with a modest improvement in tremor. In 2 other cases, ziprasidone along with another antipsychotic did produce worsened motor symptoms, in one case with chlorpromazine and in another with olanzapine. In neither case was it possible retrospectively to differentiate which agent was primarily responsible for the exacerbation in symptoms. In all cases, ziprasidone was not continued long-term after evaluation in clinic.
Patients with DLB experienced a less favorable course after ziprasidone exposure. In one case, a patient with DLB was started on ziprasidone and quetiapine for hallucinations, which did not improve. Motor symptoms slightly worsened after administration, but after cessation of ziprasidone continued to worsen rather than improving; whether this was due to medication exposure or disease progression was unclear. In a second case ziprasidone started in the inpatient setting caused clear increase in rigidity both by history and subsequent examination in clinic, though again motor symptoms did not improve after cessation.
DISCUSSION
The available literature on ziprasidone in PDP is quite limited. Keeping that caveat in mind, we conclude that although some cases experienced drug-induced worsening, which in a few patients was severe, the majority of PDP patients treated with ziprasidone tolerated it quite well. Of 85 patients throughout both cases and prospective studies, adverse effects were reported in 6 of 85 (7 percent). Serious adverse events, including serotonin syndrome and neuroleptic malignant syndrome, were reported in 2 (2 percent), though in the case of serotonin syndrome ziprasidone was only one of several possible culprit medications. While QT prolongation has been previously reported with ziprasidone use, this was not observed in the reports found here [26, 31, 33]. Clozapine has long been considered the gold standard antipsychotic in PDP in terms of tolerability, and yet a head-to-head comparison did not reveal a significant difference in motor worsening between clozapine and ziprasidone [30]. A limiting factor in the interpretation of the two open label trials found here, however, is the brief duration of exposure (4 weeks and 12 weeks), which may not allow enough time for parkinsonism to fully develop. In the case of other atypical antipsychotics (i.e., risperidone), early reports were promising but later data revealed the development of worsening parkinsonism [5, 34]. Our case data supports the tolerability of ziprasidone in PDP, as in our cases where ziprasidone was used as monotherapy it did not produce motor worsening. However, this side effect profile may not be the case in DLB as both cases we reviewed experienced severe motor worsening after ziprasidone exposure.
Ziprasidone was also generally but not universally effective for the treatment of psychotic symptoms in PD, though when compared head to head in limited samples with other commonly prescribed antipsychotics it appeared to perform better than quetiapine and similarly to clozapine. Additionally, since none of the other drugs that are commonly prescribed in PDP are available in a parenteral option, the generally positive case series reported by Oechsner and Korchounov (2005) suggests that intramuscular ziprasidone may be an appropriate treatment option in the relatively unusual hospitalized patient with severe PDP. This choice may be especially useful while starting and titrating the dose of a treatment with demonstrated efficacy, or in the setting of acute exacerbation of psychosis where oral therapy is impractical. A trial of low-dose oral ziprasidone may also be appropriate in PDP patients who do not respond to pimavanserin or clozapine, or for whom the cost or safety profile of these drugs is not acceptable. Ziprasidone may also be a reasonable alternative to quetiapine, as limited case data do suggest that it may be effective in some patients who do not respond to quetiapine, while still offering a reasonably favorable safety profile. It should be noted, however, that no cases were found where quetiapine was used after ziprasidone, and additionally quetiapine has a more extensive track record for safety [5]. Although the limited evidence for ziprasidone in PDP appears promising, much higher quality evidence exists for clozapine and pimavanserin in this condition [5, 14].
CONCLUSIONS
The available literature contains a notable lack of randomized placebo-controlled trials for ziprasidone in PD, although the limited data available would suggest that ziprasidone has a similar profile of efficacy, safety and side effects to other antipsychotics used in PD. Ziprasidone may be especially useful in the setting of acute psychosis in PD where a parenteral option may be necessary. A publication bias cannot be excluded, however, particularly with the predominance of uncontrolled data here. Randomized controlled trials in PDP of ziprasidone vs. placebo or quetiapine would be especially welcome and possibly allow an additional evidence-based option for treatment of PDP.
CONFLICT OF INTEREST
KJB has received research support from Acadia Pharmaceuticals and Sunovion Pharmaceuticals for clinical trials in PDP, and consulting and speaking fees from Acadia. Neither company contributed in any way to this publication. JRY and AAD declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors would like to acknowledge Michelle Doering, MLIS, Bernard Becker Medical Library, Washington University School of Medicine, for creating systematic search strategies.
The authors are supported by The Michael J. Fox Foundation for Parkinson’s Research (KJB), the Mary E. Groff Charitable Trust (AAD), and NIH (K08 NS101118, AAD, and T32 EB021955, JRY). None of these organizations contributed to nor approved this publication.
REFERENCES
[1] | Parkinson J ((2002) ) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14: , 223–236; discussion 222. |
[2] | Kehagia AA ((2016) ) Neuropsychiatric symptoms in Parkinson’s disease: Beyond complications. Front Psychiatry 7: , 110. |
[3] | Ravina B , Marder K , Fernandez HH , Friedman JH , McDonald W , Murphy D , Aarsland D , Babcock D , Cummings J , Endicott J , Factor S , Galpern W , Lees A , Marsh L , Stacy M , Gwinn-Hardy K , Voon V , Goetz C ((2007) ) Diagnostic criteria for psychosis in Parkinson’s disease: Report of an NINDS, NIMH work group. Mov Disord 22: , 1061–1068. |
[4] | Friedman JH ((2013) ) Parkinson disease psychosis: Update. Behav Neurol 27: , 469–477. |
[5] | Wilby KJ , Johnson EG , Johnson HE , Ensom MHH ((2017) ) Evidence-based review of pharmacotherapy used for Parkinson’s disease psychosis. Ann Pharmacother 51: , 682–695. |
[6] | Cruz MP ((2017) ) Pimavanserin (Nuplazid): A treatment for hallucinations and delusions associated with Parkinson’s disease. P T 42: , 368–371. |
[7] | Fernandez HH , Trieschmann ME , Friedman JH ((2003) ) Treatment of psychosis in Parkinson’s disease: Safety considerations. Drug Saf 26: , 643–659. |
[8] | Shotbolt P , Samuel M , David A ((2010) ) Quetiapine in the treatment of psychosis in Parkinson’s disease. Ther Adv Neurol Disord 3: , 339–350. |
[9] | Alvir JM , Lieberman JA , Safferman AZ , Schwimmer JL , Schaaf JA ((1993) ) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 329: , 162–167. |
[10] | Lexicomp Online, Lexi-Drugs Online; Hudson, Ohio: Wolters Kluwer Clinical Drug Information, Inc; 2018, September 14, 2018. |
[11] | Seeger TF , Seymour PA , Schmidt AW , Zorn SH , Schulz DW , Lebel LA , McLean S , Guanowsky V , Howard HR , Lowe JA ((1995) ) Ziprasidone (CP-88,059): A new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther 275: , 101–113. |
[12] | Rummel-Kluge C , Komossa K , Schwarz S , Hunger H , Schmid F , Kissling W , Davis JM , Leucht S ((2012) ) Second-generation antipsychotic drugs and extrapyramidal side effects: A systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull 38: , 167–177. |
[13] | Black K ((2017) ) Treatment of Parkinson’s disease psychosis. Med Int Rev 27: , 266–271. |
[14] | Divac N , Stojanović R , Savić Vujović K , Medić B , Damjanović A , Prostran M ((2016) ) The efficacy and safety of antipsychotic medications in the treatment of psychosis in patients with Parkinson’s disease. Behav Neurol 2016: , 4938154. |
[15] | Lertxundi U , Peral J , Mora O , Domingo-Echaburu S , Martínez-Bengoechea MJ , García-Moncó JC ((2008) ) Antidopaminergic therapy for managing comorbidities in patients with Parkinson’s disease. Am J Health Syst Pharm 65: , 414–419. |
[16] | Frieling H , Hillemacher T , Ziegenbein M , Neundörfer B , Bleich S ((2007) ) Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur Neuropsychopharmacol 17: , 165–171. |
[17] | Durán-Ferreras E , Alvarez-López M , García-Moreno JM , Chacón J ((2008) ) [Ziprasidone in Parkinsonian dopamine psychosis]. Rev Neurol 46: , 476–480. |
[18] | Kim SY , Park JE , Lee YJ , Seo HJ , Sheen SS , Hahn S , Jang BH , Son HJ ((2013) ) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66: , 408–414. |
[19] | Martin WRW , Hartlein J , Racette BA , Cairns N , Perlmutter JS ((2017) ) Pathologic correlates of supranuclear gaze palsy with parkinsonism. Parkinsonism Relat Disord 38: , 68–71. |
[20] | Schindehütte J , Trenkwalder C ((2007) ) Treatment of drug-induced psychosis in Parkinson’s disease with ziprasidone can induce severe dose-dependent off-periods and pathological laughing. Clin Neurol Neurosurg 109: , 188–191. |
[21] | Gray NS ((2004) ) Ziprasidone-related neuroleptic malignant syndrome in a patient with Parkinson’s disease: A diagnostic challenge. Hum Psychopharmacol 19: , 205–207. |
[22] | Berkowitz AL ((2006) ) Ziprasidone therapy in elderly patients with psychotic mood disorders and Parkinson’s disease. Psychiatry (Edgmont) 3: , 59–63. |
[23] | Duggal HS , Singh I ((2008) ) Ziprasidone for drug-induced psychotic symptoms in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 32: , 583–584. |
[24] | Shiah IS , Lin CL , Mao WC , Luu SU ((2006) ) Ziprasidone in the treatment of Parkinson’s disease psychosis. Eur Psychiatry 21: , 578–579. |
[25] | López del Val LJ , Santos S ((2004) ) [Quetiapine and ziprasidone in the treatment of the psychotic disorders in Parkinson’s disease]. Rev Neurol 39: , 661–667. |
[26] | Oechsner M , Korchounov A ((2005) ) Parenteral ziprasidone: A new atypical neuroleptic for emergency treatment of psychosis in Parkinson’s disease? Hum Psychopharmacol 20: , 203–205. |
[27] | Connemann BJ , Schönfeldt-Lecuona C ((2004) ) Ziprasidone in Parkinson’s disease psychosis. Can J Psychiatry 49: , 73. |
[28] | El-Okdi NS , Lumbrezer D , Karanovic D , Ghose A , Assaly R ((2014) ) Serotonin syndrome after the use of tramadol and ziprasidone in a patient with a deep brain stimulator for Parkinson disease. Am J Ther 21: , e97–9. |
[29] | Gómez-Esteban JC , Zarranz JJ , Velasco F , Lezcano E , Lachen MC , Rouco I , Barcena J , Boyero S , Ciordia R , Allue I ((2005) ) Use of ziprasidone in parkinsonian patients with psychosis. Clin Neuropharmacol 28: , 111–114. |
[30] | Pintor L , Valldeoriola F , Baillés E , Martí MJ , Muñiz A , Tolosa E ((2012) ) Ziprasidone versus clozapine in the treatment of psychotic symptoms in Parkinson disease: A randomized open clinical trial. Clin Neuropharmacol 35: , 61–66. |
[31] | Micheli F , Taubenslag N , Gatto E , Scorticati MC ((2005) ) Ziprasidone and psychosis in Parkinson disease. Clin Neuropharmacol 28: , 254. |
[32] | Stefanis N , Bozi M , Christodoulou C , Douzenis A , Gasparinatos G , Stamboulis E , Stefanis C , Stefanis L ((2010) ) Isolated delusional syndrome in Parkinson’s disease. Parkinsonism Relat Disord 16: , 550–552. |
[33] | Camm AJ , Karayal ON , Meltzer H , Kolluri S , O’Gorman C , Miceli J , Tensfeldt T , Kane JM ((2012) ) Ziprasidone and the corrected QT interval: A comprehensive summary of clinical data. CNS Drugs 26: , 351–365. |
[34] | Workman RH , Orengo CA , Bakey AA , Molinari VA , Kunik ME ((1997) ) The use of risperidone for psychosis and agitation in demented patients with Parkinson’s disease. J Neuropsychiatry Clin Neurosci 9: , 594–597. |




