Safety and Durability of Effect with Long-Term, Open-Label Droxidopa Treatment in Patients with Symptomatic Neurogenic Orthostatic Hypotension (NOH303)
Abstract
Background: Neurogenic orthostatic hypotension (nOH) is associated with insufficient norepinephrine release in response to postural change.
Objective: The objective of this study was to evaluate the long-term safety and durability of efficacy of the norepinephrine precursor droxidopa in patients with symptomatic nOH.
Methods: This multinational study consisted of 3 sequential phases: a 3-month open-label droxidopa treatment phase followed by a 2-week double-blind, placebo-controlled withdrawal phase, and a 9-month open-label extension phase in which all patients received droxidopa. Patients were adults diagnosed with symptomatic nOH associated with Parkinson’s disease, multiple system atrophy, pure autonomic failure, dopamine β-hydroxylase deficiency, or nondiabetic autonomic neuropathy. Efficacy was evaluated using patient- and investigator-reported questionnaire responses and the orthostatic standing test. Safety was assessed through adverse event (AE) reports and vital signs.
Results: A total of 102 patients received treatment with droxidopa. Initial improvements from baseline in patient-reported nOH symptom severity and impact on daily activities, evaluated using the Orthostatic Hypotension Questionnaire, exceeded 50% and were maintained throughout the 12-month study. Decreased nOH severity was also reflected in clinician and patient ratings on the Clinical Global Impression questionnaire. Standing systolic and diastolic blood pressures were increased from baseline throughout the study with droxidopa treatment. The most frequently reported AEs were falls, urinary tract infection, and headache. There was a low incidence (≤2%) of cardiac AEs (eg, first-degree atrioventricular block, supraventricular extrasystoles).
Conclusions: Long-term, open-label treatment with droxidopa for up to 12 months was generally well tolerated and provided durable improvements in nOH signs and symptoms.
INTRODUCTION
Orthostatic hypotension (OH) is a fall in systolic blood pressure (SBP) of ≥20 mmHg, or in diastolic blood pressure (DBP) of ≥10 mmHg, within 3 minutes of standing [1, 2]. Neurogenic orthostatic hypotension (nOH) is caused by inadequate norepinephrine release from sympathetic neurons in response to postural change [1, 3]. nOH is a frequent feature of primary autonomic failure occurring in peripheral autonomic neuropathies and in some neurodegenerative disorders, particularly the α-synucleinopathies (Parkinson’s disease [PD], dementia with Lewy bodies, multiple system atrophy [MSA], pure autonomic failure [1, 3]. In hospital-based and community-based studies of patients with PD, orthostatic hypotension was present in 58% and 47% of patients, respectively [4, 5].
In 2014, the US Food and Drug Administration approved droxidopa for the treatment of symptomatic nOH [6]. The conversion of droxidopa, a synthetic amino acid analog, into norepinephrine by dopa-decarboxylase is thought to underlie its efficacy; norepinephrine increases blood pressure by inducing vasoconstriction [6]. Results of 2 short-term, randomized controlled clinical studies have demonstrated the tolerability of droxidopa and suggest that it is effective in providing relief of nOH symptoms [7–9].
The objective of this study was to evaluate the durability of efficacy and the long-term safety of droxidopa in patients with symptomatic nOH in an open-label (OL) study. The study design consisted of a 3-month OL droxidopa treatment phase (OL-3 months) followed by a 2-week randomized, double-blind, placebo-controlled withdrawal phase (DB-2 weeks) to enable assessment of durability of effect, and a subsequent 9-month OL extension phase (OL-9 months) in which all patients received droxidopa. The total duration of OL treatment was 12 months (OL-12 months).
MATERIALS AND METHODS
Participants in this multinational (6 countries) 69-center study (NCT00738062) were ≥18 years of age with a clinical diagnosis of symptomatic nOH associated with primary autonomic failure (PD, MSA, pure autonomic failure), dopamine β-hydroxylase (DBH) deficiency, or nondiabetic autonomic neuropathy (NDAN). All patients had previously reported an improvement of symptoms (with or without a blood pressure response) during the open-label dose-optimization period of 1 of 2 previous droxidopa studies (NOH301 [NCT00782340] [7] or NOH302 [NCT00633880] [10]). Key exclusion criteria were current use of vasoconstrictor agents (eg, midodrine), long-acting antihypertensive drugs, or norepinephrine reuptake inhibitors; preexisting sustained severe hypertension (seated blood pressure ≥180/110 mmHg); significant systemic, hepatic, cardiac, or renal disorders; known or suspected malignancy; a history of closed-angle glaucoma; or diabetes mellitus or diabetes insipidus.
Study design
The study included 3 sequential phases: OL-3 months, DB-2 weeks (washout), and OL-9 months (Fig. 1). Long-term safety and efficacy were assessed during the full 12 months of OL droxidopa treatment (OL-12 months), which comprised the OL-3 months and OL-9 months phases. After the OL-3 months phase, a 2-week DB washout phase occurred during which patients were randomized to either ongoing droxidopa or placebo during the DB-2 weeks phase, to compare efficacy and safety. After the DB-2 weeks phase, all patients were continued on droxidopa.
The study was approved by local independent ethics committees or institutional review boards. It conformed to guidelines that provided the greatest patient protection (either the Declaration of Helsinki or those specific to the study site’s country) and was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Treatment and concomitant medications
All patients received an individualized dose of droxidopa (100–600 mg) three times daily during the OL phases. Based on treatment assignment, patients received either their individualized dose of droxidopa or matched placebo during the DB phase. Treating physicians were allowed to adjust droxidopa doses (in 100-mg increments) to optimize benefits (eg, to improve symptoms or tolerability) at any time during the study, except within a week before the start of the DB period. Physicians could prescribe any other medications considered necessary that would not interfere with evaluation of droxidopa. All drugs for OH (except vasoconstricting agents) were permitted during the study.
Efficacy assessments
Efficacy was evaluated using the composite score of the patient-rated Orthostatic Hypotension Questionnaire (OHQ) [11], which consists of the Orthostatic Hypotension Symptom Assessment (OHSA) and the Orthostatic Hypotension Daily Activity Scale (OHDAS). The OHQ composite score was the average of composite scores for the OHSA (ratings of 6 items: dizziness, problems with vision, weakness, fatigue, trouble concentrating, and head/neck discomfort) and the OHDAS (ratings of 4 items pertaining to activities requiring standing or walking for a short or long time). Patients completed the OHQ based on recall of the previous week at each study visit (months 1, 2, and 3; at randomization and week 2 of the withdrawal period; and at months 6, 9, and 12 of the extension period). Each question was scored between 0 (no interference with the activity) and 10 (complete interference with the activity).
Secondary efficacy assessments included the orthostatic standing test (OST) and the Clinical Global Impression (CGI) ratings questionnaire for nOH. During the OST, SBP and DBP were measured in supine (ie, semirecumbent with 30° elevation of head and torso), standing, and sitting positions: 3 assessments were conducted over 10 minutes while supine, 1 assessment was made 3 minutes after standing from the supine position, and 1 assessment was taken 5 minutes after sitting from a standing position. CGI surveys were rated at each study visit by clinicians and patients for nOH improvement (CGI-I) and severity (CGI-S).
Safety assessments
Safety was evaluated throughout the study. Adverse events (AEs) and vital signs were recorded, a 12-lead electrocardiogram was obtained, and blood and urine samples were collected for laboratory testing at each study visit. AEs were recorded by the investigator and coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 10.1. Supine hypertension was defined as SBP >180 mmHg assessed in the supine (ie, semirecumbent) position (head and torso elevated approximately 30° from horizontal).
Statistical analyses
Efficacy was analyzed separately for the OL and DB treatment phases. Efficacy over the OL-12 months phase (which included both the OL-3 months and OL-9 months phases) was analyzed in all patients who received ≥1 dose of study drug (the safety population), and the OHQ, OHSA, and OHDAS scores were summarized for each study visit. Clinician- and patient-rated CGI-I and CGI-S scores and SBP and DBP were measured during the OST. CGI-S data were categorized as normal-borderline OH (score 1–2), mild-moderate OH (score 3–4), and marked OH (score 5–7). CGI-I data were categorized as “slightly improved” (score 1–3), “no change” (score 4), and “slightly worse” to “very much worse” (score 5–7). Tolerability data are presented as descriptive summaries without statistical comparisons.
Efficacy in the DB-2 weeks phase was analyzed in all patients who had completed the OL-3 months phase and were randomized to continued droxidopa or placebo (intention-to-treat population). The primary endpoint was the mean change from randomization to the end of the withdrawal phase in the OHQ composite score in patients receiving droxidopa versus placebo. Based on the results of a previous study (NOH302 [10]), the DB-2 weeks phase had approximately 50% power to detect a difference between the treatment arms with 75 patients randomized. Secondary efficacy endpoints included composite and individual item scores for the OHSA and OHDAS; patient- and clinician-rated CGI-I and CGI-S; and standing SBP and DBP. Endpoints based on the OHQ questionnaire were evaluated using analysis of covariance (ANCOVA), with the randomization value as covariant and treatment as the main effect, provided that assumptions of independence, constant variance, and normality were met. Nonparametric Mantel-Haenszel statistics were employed to compare treatment groups if the assumptions were not met. Between-group differences on the CGI-S and CGI-I were compared using the Fisher exact test.
RESULTS
Patients
Among 103 enrolled patients, 102 received droxidopa treatment. The mean patient age was 66 years; 60% of the patients were men, 97% were white, and 91% had a clinical diagnosis of alpha-synucleinopathies (ie, PD, MSA, or pure autonomic failure; Table 1). Of the 79 patients who completed the OL-3 months phase, 75 were randomized to the DB-2 weeks withdrawal phase; 69 completed DB-2 weeks and 54 patients (52.4%) completed the12-month study (Supplementary Figure 1). Reasons for discontinuation at any point during the 12-month study among all enrolled patients were AEs (n = 20), withdrawal of consent (n = 16), treatment failure or lack of efficacy (n = 4 and n = 3, respectively; terminology determined by the investigator), protocol violation (n = 2), loss to follow-up (n = 1), investigator decision (n = 1), and “other” (n = 2).
Open-label–12 months efficacy results
Initial improvements relative to baseline for nOH symptom severity and impact on daily activities were maintained throughout the 12-month OL observation phase (Fig. 2A and B). Specifically, the initial >50% reduction from baseline in the OHQ composite score achieved at month 1 of OL droxidopa treatment (–3.29 units) was sustained throughout the OL-12 months phase (Fig. 2A). This outcome was also observed in a post hoc analysis that used the last observation carried forward method of imputation for missing data (eg, because of patient dropout). Similar results were observed for specific OHSA item scores (Fig. 2B), as well as OHSA and OHDAS composite scores and OHDAS item scores (Supplementary Figure 2A and B). Improvements in OHQ and OHSA composite scores were observed regardless of age, sex, or primary diagnosis (data not shown).
Clinician and patient ratings on the CGI-S also reflected decreased nOH severity (Fig. 2C and D). More patients were rated by clinicians as “normal” at month 1 (24.4%) compared with baseline (0%), and fewer patients were rated as having “marked OH” (17.8% at month 1 vs 67.6% at baseline; Fig. 2C). The percentage of patients who rated their nOH as “normal” increased from 2.0% at baseline to 36.7% at month 1, and the percentage of patients who rated their nOH as “marked” decreased from 67.6% at baseline to 12.2% at month 1 (Fig. 2D). On the CGI-I at month 1, clinicians reported improvement for 90.2% of patients; 83.5% of patients reported improvement of their nOH (Supplementary Figure 2C and D). Similar results for patient- and clinician-reported nOH severity and improvement were observed throughout the 12-month study period (Supplementary Figure 2C and D). Throughout the OL-12 months phase, increases in standing SBP and DBP from baseline were maintained with open-label droxidopa(Supplementary Table 1). Mean (SD) increases in SBP at various follow-up visits during OL-12 months ranged from 6.9 (17.5) to 14.0 (22.5) mmHg over the mean (SD) standing SBP at baseline of 87.9 (17.5) mmHg. At month 12, mean (SD) SBP was 101.9 (26.2) mmHg, representing an increase from baseline of 12.3 (26.6) mmHg. Mean (SD) increases for standing DBP were 2.3 (11.1) to 6.9 (12.5) mmHg throughout OL-12 months, compared with the baseline measurement of 57.6 (11.2) mmHg. Mean (SD) DBP at month 12 was 63.0 (15.0) mmHg, representing an increase from baseline of 4.3 (13.7) mmHg.
Double-blind–2 weeks efficacy results
No statistically significant differences were demonstrated between the droxidopa and placebo treatment groups during the DB-2 weeks withdrawal phase. Regardless of treatment assignment, the improvement observed in the mean OHQ composite score at OL-3 months was maintained. Although the mean change in the OHQ composite score from randomization to the end of the 2-week withdrawal period (the primary endpoint) was numerically lower in the droxidopa group compared with the placebo group (0.57 units versus 0.90 units), this difference did not reach statistical significance (Supplementary Table 2). Similar trends were also seen in the mean changes from randomization in OHSA and OHDAS composite and individual item scores. No statistically significant differences between the droxidopa and placebo groups were seen on the CGI-S or CGI-I.
The increase from baseline in mean standing SBP observed during the OL-3 months phase continued during the DB-2 weeks phase. Patients randomized to either droxidopa or placebo continued to experience improvement in mean standing SBP relative to baseline, with no significant differences between droxidopa and placebo groups (Supplementary Table 2).
Tolerability
The most frequently reported AEs (subsequently MedDRA coded) during the 12-month study (102 patients) included falls (20.6%), urinary tract infection (17.6%), headache (13.7%), syncope (12.7%), back pain (10.8%), and dizziness (7.8%; Table 2). Most AEs were mild or moderate in severity, considered to be unrelated or unlikely related to study treatment by the site investigator, and were reported as resolved. Serious AEs occurred in 26 patients (25.5%); events reported by >1 patient included syncope (n = 4 [3.9%]); hip fracture (n = 3 [2.9%]); and angina pectoris, pneumonia, and urinary tract infection (n = 2 each [2.0%]). The events of angina pectoris were reported for 2 patients with pure autonomic failure. In one case, the event of angina pectoris occurred 91 days after the first dose of droxidopa, lasted 2 days, was moderate in severity, and was considered unlikely to be related to study drug; the patient subsequently died of a myocardial infarction approximately 3 years after study participation. In the second case, the event occurred 290 days after the first dose of droxidopa, lasted 4 days, was reported as severe, and was considered not related to study drug.
Cardiac AEs reported by >1 patient included the 2 SAEs of angina pectoris described above as well as first-degree atrioventricular block and supraventricular extrasystoles (n = 2 patients each). The following AEs led to study discontinuation: anxiety, loss of consciousness, headache, hypoxic encephalopathy, ventricular extrasystoles, acute renal failure, agitation, visual hallucination, rash, hallucination, hypertension, acute respiratory failure, cognitivedisorder, pneumonia, amnesia, pelvic fracture, and chest pain. No individual AE led to study discontinuation in >1 patient.
Five AEs leading to death occurred during the study. One of these, attributed to hypoxic encephalopathy, was considered by the investigator to be possibly related to treatment with droxidopa. This patient, who had a primary diagnosis of MSA and was receiving 400 mg droxidopa three times daily, died during the OL-3 months phase. The event precipitating death began 71 days after the first dose of study drug, following admission for an unspecified arrhythmia and seizure activity. The 4 deaths considered unrelated to treatment with droxidopa occurred in 3 patients with PD (due to acute respiratory failure, pneumonia, pelvic fracture; n = 1 each) and 1 patient with MSA (sudden cardiac death).
The incidence of supine hypertension (SBP > 180 mmHg) over the course of the 12-monthstudy and ranged from 3.8% at 3 months (3/79 patients) to 12.3% at 12 months (7/57 patients); the increase was not statistically significant. No consistent trend toward increasing rates of supine hypertension with increased duration of exposure to droxidopa was observed: month 1, 7.6% (n = 7/92); month 2, 9.2% (n = 8/87); month 3, 3.8% (n = 3/79); month 6, 7.5% (n = 5/67); month 9, 4.7% (n = 3/64); month 12, 12.3% (n = 7/57).
DISCUSSION
To date, randomized clinical studies demonstrating the efficacy of droxidopa for the treatment of nOH have been short term, with a maximum duration of exposure of 10 weeks [7–10]. This 12-month open-label extension study assessed the long-term safety and durability of efficacy of droxidopa in one of the largest long-term cohorts to date of patients with nOH. Improvements in symptoms and standing SBP observed during the first month of the OL phase were sustained throughout 12 months of treatment with droxidopa. Droxidopa consistently improved nOH symptoms across a range of measures and decreased the impact of symptoms on daily activities. A decrease in symptom severity of approximately 50% was observed after 1 month of treatment with droxidopa and was sustained through 12 months of patient follow-up (among patients available for analysis at each time point). These results are consistent with the findings of shorter-term studies and extend previous reports by demonstrating a durability of effect for droxidopa lasting through 1 year of treatment [7–10]. Additionally, although dose adjustments and concomitant medications for the treatment of nOH were allowed per the study protocol, there was no marked trend for increased dosing of droxidopa or increased use of fludrocortisone over time, further suggesting maintenance of droxidopaefficacy.
There was not a statistically significant difference between droxidopa and placebo during the 2-week double-blind withdrawal period, although patients receiving placebo had a numerically higher symptom score. These results are consistent with the findings of another 2-week randomized withdrawal study of droxidopa versus placebo, in which a nonsignificant trend favoring droxidopa over placebo was found for the primary endpoint (item 1 of the OHSA) [10]. In both withdrawal design trials, the lack of significance was primarily due to continued symptom improvement in patients randomized to placebo. The authors of the previous trial suggested that a carryover effect (ie, a pharmacodynamic timeframe that differs from the pharmacokinetic timeframe) from the dose-optimization phase immediately before randomization may have played a role in the observed results [10]. Because droxidopa has a relatively short serum half-life (2.5 hours) [6], such an effect was not expected. However, carryover effects have also been reported for levodopa, which, like droxidopa, has a short half-life (1 hour) [10]. One potential cause of a carryover effect is storage of norepinephrine by catecholaminergic neurons, which would prolong norepinephrine availability after drug clearance. Other possible contributory factors to a carryover effect include a sustained increase in norepinephrine production, unequal fluid intake between droxidopa and placebo groups, and potential resetting of the baroreflex.
Droxidopa was generally well tolerated over the course of the 12-month trial. The observed discontinuation rate was not unexpected given the relatively long study duration and the considerable burden of illness characteristic of some diagnoses included in the study population (eg, MSA). The most common AE was falls, which are common among patients with orthostatic hypotension. These 2 observations underscore some of the overall challenges associated with conducting clinical trials in patients with nOH. Patients with nOH are generally older, with complex medical histories and comorbid conditions and may experience falls for a multitude of reasons, all of which may affect long-term participation in clinical trials. For patients with nOH, the considerable burden of disease makes it difficult to discern the extent of morbidity associated with any underlying condition versus drug treatment, although data from the randomized droxidopa clinical trial with an extended duration of treatment (8–10 weeks) suggest that AE rates were similar in the droxidopa and placebo groups.[8, 9] Furthermore, relatively few patients discontinued the study for the reasons of treatment failure or lack of efficacy (n = 4 and n = 3, respectively), and the majority of patients who did not complete the study did not show evidence of loss of effect in spaghetti plots (data not shown). This open-label extension patient population reflects the common clinical scenario where patients who demonstrate initial symptomatic benefit typically remain on treatment. The pattern of AEs observed was qualitatively consistent with the safety and tolerability profile demonstrated for droxidopa in short-term clinical studies [7, 8, 10], and there were no new or unexpected safety signals. The low incidence of supine hypertension, defined as SBP >180 mmHg, (3.8% to 12.3%) observed through 12 months of follow-up in our study was comparable to the 7.9% and 10.9% incidences previously reported in similar study populations ≤2 weeks of treatment with droxidopa [8, 10]. The only serious cardiac AE reported by >1 patient was angina pectoris, which occurred in 2 patients receiving droxidopa and resolved in both cases. Of the 5 fatalities reported during the 12-month study period, 4 were considered unrelated to droxidopa.
Potential limitations of the current study include elements of the study design, such as a study population that was composed of patients who met criteria for response to droxidopa during the earlier short-term trials, and a mostly open-label study period. Additionally, there was a lack of separation between the treatment groups during the double-blind treatment period, although it should be emphasized that the study was not adequately powered to show a statistically significant effect during this second phase of the trial. As previously discussed, the effects of contributing factors on the findings during the double-blind period may not be fully understood. Finally, bias may have been introduced by the prespecified method of handling missing data, last observation carried forward.
Results of this open-label study suggest that long-term treatment with droxidopa for up to 1 year was generally well tolerated and provided durable improvements in nOH symptoms and in standing SBP in patients with symptomatic nOH.
Disclosure and role of funding source
The data reported in this article were derived from clinical trials funded by Lundbeck. The study sponsor participated in the design and conduct of these analyses, interpretation of data, and the decision to submit this article for publication. The authors received editorial assistance from the CHC Group (North Wales, PA), which was supported by Lundbeck LLC.
CONFLICTS OF INTEREST
S Isaacson has received compensation for consulting, speaking, and research activities for Lundbeck. HA Shill has received compensation for research activities for Lundbeck. S Vernino has received personal compensation as an advisory board and speaker bureau member for Lundbeck, as a consultant for Athena Diagnostics (Quest), and as associate editor of JAMA Neurology. A Ziemann and GJ Rowse were full-time employees of Lundbeck throughout the analysis of the study and preparation of the manuscript.
ACKNOWLEDGMENTS
Appendices
Supplemental material for this article includes changes in OST standing blood pressure during long-term open-label treatment with droxidopa (Supplementary Table 1); OHQ composite score and orthostatic standing test results during the DB 2-week withdrawal period (Supplementary Table 2); patient disposition details (Supplementary Figure 1); and OHSA and OHDAS composite scores, OHDAS individual item scores, and clinician-reported and patient-reported CGI-I scores throughout the study (Supplementary Figure 2).
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-160860.
REFERENCES
[1] | Freeman R , Wieling W , Axelrod FB , Benditt DG , Benarroch E , Biaggioni I , Cheshire WP , Chelimsky T , Cortelli P , Gibbons CH , Goldstein DS , Hainsworth R , Hilz MJ , Jacob G , Kaufmann H , Jordan J , Lipsitz LA , Levine BD , Low PA , Mathias C , Raj SR , Robertson D , Sandroni P , Schatz I , Schondorff R , Stewart JM , & van Dijk JG ((2011) ) Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21: , 69–72. |
[2] | Goldstein DS , & Sharabi Y ((2009) ) Neurogenic orthostatic hypotension: A pathophysiological approach. Circulation 119: , 139–146. |
[3] | Freeman R ((2008) ) Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med 358: , 615–624. |
[4] | Allcock LM , Ullyart K , Kenny RA , & Burn DJ ((2004) ) Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75: , 1470–1471. |
[5] | Senard JM , Rai S , Lapeyre-Mestre M , Brefel C , Rascol O , Rascol A , & Montastruc JL ((1997) ) Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry 63: , 584–589. |
[6] | Northera (droxidopa). Full Prescribing Information. Lundbeck NA Ltd, Deerfield, IL, (2014) . |
[7] | Kaufmann H , Freeman R , Biaggioni I , Low P , Pedder S , Hewitt LA , Mauney J , Feirtag M , Mathias CJ , & NOH301 Investigators ((2014) ) Droxidopa for neurogenic orthostatic hypotension: A randomized, placebo-controlled, phase 3 trial. Neurology 83: , 328–335. |
[8] | Hauser RA , Isaacson S , Lisk JP , Hewitt LA , & Rowse G ((2015) ) Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (NOH306B). Mov Disord 30: , 646–654. |
[9] | Hauser RA , Hewitt LA , & Isaacson S ((2014) ) Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J Parkinsons Dis 4: , 57–65. |
[10] | Biaggioni I , Freeman R , Mathias CJ , Low P , Hewitt LA , Kaufmann H , & Droxidopa 302 Investigators ((2015) ) Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension 65: , 101–107. |
[11] | Kaufmann H , Malamut R , Norcliffe-Kaufmann L , Rosa K , & Freeman R ((2012) ) The Orthostatic Hypotension Questionnaire (OHQ): Validation of a novel symptom assessment scale. Clin Auton Res 22: , 79–90. |
Figures and Tables
Fig.1
Study design.
Fig.2
OHQ composite score (A), OHSA individual item scores (B), clinician-reported CGI-S scores (C), and patient-reported CGI-S scores (D) during long-term open-label droxidopa treatment. CGI-S = Clinical Global Impression of nOH severity; OH = orthostatic hypotension; OHQ = Orthostatic Hypotension Questionnaire; OHSA = Orthostatic Hypotension Symptom Assessment.
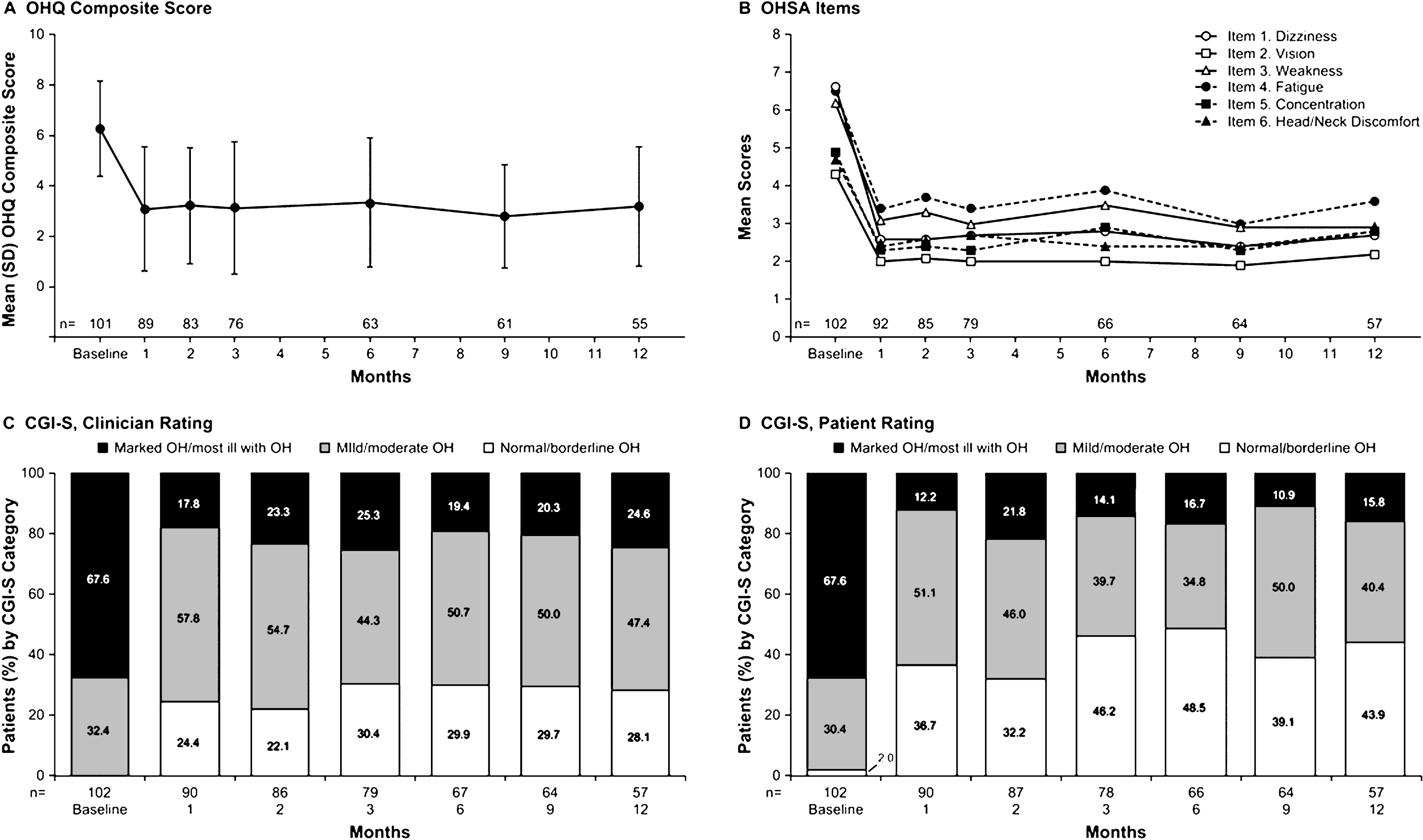
Table 1
Baseline demographic and disease characteristics
| Open-Label (12 months) | Double-Blind (2 weeks) | ||
| Variable | Droxidopa (n = 102) | Droxidopa (n = 38) | Placebo (n = 37) |
| Age, y | |||
| Mean (SD) | 65.8 (12.3) | 68.2 (13.0) | 66.2 (12.1) |
| Range | 30–88 | 30–86 | 30–88 |
| Sex, n (%) | |||
| Male | 61 (59.8) | 23 (60.5) | 24 (64.9) |
| Female | 41 (40.2) | 15 (39.5) | 13 (35.1) |
| Race/ethnicity, n (%) | |||
| White | 99 (97.1) | 37 (97.4) | 35 (94.6) |
| Other | 3 (2.9) | 1 (2.6) | 2 (5.4) |
| Primary clinical diagnosis, n (%) | |||
| PD | 48 (47.1) | 20 (52.6) | 18 (48.6) |
| MSA | 27 (26.5) | 8 (21.1) | 9 (24.3) |
| Pure autonomic failure | 18 (17.6) | 8 (21.1) | 7 (18.9) |
| DBH deficiency | 1 (1.0) | 1 (2.6) | 0 |
| NDAN | 5 (4.9) | 0 | 2 (5.4) |
| Other | 3 (2.9) | 1 (2.6) | 1 (2.7) |
| Mean (SD) OHQ composite score* | 6.27 (1.89) | 6.38 (1.85)† | 6.27 (1.95) |
| Mean (SD) OHSA composite score* | 6.01 (1.79) | 6.23 (1.63) | 5.80 (1.93) |
| Mean (SD) OHDAS composite score* | 6.56 (2.46) | 6.59 (2.53)† | 6.74 (2.37) |
| Mean (SD) change in SBP (pre-standing to standing), mmHg‡ | –43.7 (24.84) | –43.3 (21.6) | –41.8 (27.3) |
DBH = dopamine β-hydroxylase; MSA = multiple system atrophy; NDAN = nondiabetic autonomic neuropathy; OHDAS = Orthostatic Hypotension Daily Activity Scale; OHQ = Orthostatic Hypotension Questionnaire; OHSA = Orthostatic Hypotension Symptom Assessment; PD = Parkinson’s disease; SBP = systolic blood pressure. *Baseline values were measured before the first dose of study treatment in Study NOH301 or NOH302 unless the last visit in Study NOH301 or NOH 302 occurred >1 month before the start of the current study. †n = 37. ‡Change in standing SBP is the difference between SBP measured before standing and 3 minutes after standing from the supine (ie, 30° elevation of head and torso) position during the orthostatic standing test.
Table 2
Adverse events reported in >5% of patients in the total droxidopa group (safety population)
| DB-2 Weeks | |||||
| Adverse Event, n (%) | OL-3 Months (n = 102) | Droxidopa (n = 38) | Placebo (n = 37) | OL-9 Months (n = 74) | OL-12 Months (N = 102) |
| Fall | 7 (6.9) | 1 (2.6) | 1 (2.7) | 16 (21.6) | 21 (20.6) |
| Urinary tract infection | 9 (8.8) | 2 (5.3) | 0 | 12 (16.2) | 18 (17.6) |
| Headache | 6 (5.9) | 1 (2.6) | 2 (5.4) | 6 (8.1) | 14 (13.7) |
| Syncope | 5 (4.9) | 2 (5.3) | 0 | 8 (10.8) | 13 (12.7) |
| Back pain | 4 (3.9) | 2 (5.3) | 0 | 7 (9.5) | 11 (10.8) |
| Dizziness | 2 (2.0) | 1 (2.6) | 1 (2.7) | 5 (6.8) | 8 (7.8) |
| Muscle spasms | 2 (2.0) | 1 (2.6) | 0 | 4 (5.4) | 7 (6.9) |
| Orthostatic hypotension | 3 (2.9) | 0 | 0 | 4 (5.4) | 7 (6.9) |
| Confusional state | 5 (4.9) | 0 | 0 | 2 (2.7) | 6 (5.9) |
| Neck pain | 3 (2.9) | 0 | 0 | 3 (4.1) | 6 (5.9) |
| Somnolence | 5 (4.9) | 0 | 0 | 1 (1.4) | 6 (5.9) |
DB = double blind; OL = open label.




