Specific Attentional Disorders and Freezing of Gait in Parkinson’s Disease
Abstract
Background:
Due to its high prevalence in dual-task paradigms, freezing of gait in Parkinson’s disease is thought to be associated with dysexecutive syndrome and attentional disorders. However, the role of specific attentional disorders in patients with freezing of gait is still unclear.
Objective:
Here, we sought to specifically determine which basic attentional modalities are impaired in patients with freezing of gait.
Methods:
Seventy-eight parkinsonian patients performed a computer-controlled reaction-time paradigm designed to measure the different attentional subcomponents, controlled for visuospatial processing and motor participation.
Results:
The freezer (n = 42) and non-freezer (n = 36) groups were matched for age, educational level, MMSE and Mattis Dementia Rating Scale. There were no intergroup differences in simple reaction times, whereas choice reaction times were higher in the freezer group than in the non-freezer group for divided attention (p = 0.023).
Conclusions:
At equivalent levels of overall cognitive efficiency, freezer patients showed a greater slowdown than non-freezer patients with a specific impairment in divided attention.
INTRODUCTION
Freezing of gait (FoG) is defined as a sudden, brief and involuntary motor block, despite the intention to walk [1]. It has a major impact on quality of life [2, 3] and increases the risk of falls [4]. Several studies have sought to identify the determinants of FoG in Parkinson’s disease (PD) [1, 5] with motor and cognitive or interplaying (overload) hypotheses [6, 7]. Amboni et al.’s “cognitive hypothesis” [8] was based on the correlation between the severity of FoG on one hand and poor ability to resist interference (i.e. conflicting instructions) on the other. Hypotheses concerning the pathogenesis of FoG include impairments of executive functions (such as set-shifting, response inhibition) and attention [9]. These hypotheses are supported by the observation that (i) FoG occurs preferentially during attentional-costing tasks and (ii) patients with FoG are more impaired in executive function tasks. A pathophysiological model of FoG [6] suggested that cognitive or emotional inputs could temporary overload the motor pathways via the basal ganglia loops and thus account for the paroxysmal motor block observed during FoG. Rapid overload in patients with FoG is very prominent during dual-task paradigms [10, 11]. However, the mechanism of this overload has not yet been completely elucidated, since dual-task paradigms usually engage several attentional modalities that interfere with executive function [9]. Due to overlap between attention and executive function [12], attentional impairment in PD patients with FoG requires better characterization. In particular, it is necessary to precisely determine the role of cognition in this gait disorder, with a view to better understanding the neural substrates and improving rehabilitation [13]. In this regard, different aspects of attention have been explored: regulation of external factors [14], orienting [15], focusing [16], set-shifting [16–18], conflict resolution [19], error monitoring [20] and maintaining/disengaging attention [21]. Nevertheless, the specific nature of impairments in the various attentional components has not been systematically assessed in PD patients with FoG, previous studies investigating either a specific component either using more complex tasks involving executive functions.
The present study was based on Van Zomeren and Brouwer’s model [22], which is widely used in psychology to differentiate between different attentional subcomponents. As shown in Fig. 1, the model combines attentional processes in two dimensions: (i) intensity, with vigilance and sustained attention, and (ii) selectivity, with focused attention and divided attention. Selectivity allows the subject to ignore irrelevant stimuli on two levels; focused attention takes account of only one stimulus dimension (colour, size, shape, etc.), whereas divided attention considers at least two relevant stimulus dimensions. Lastly, an executive component called the supervisory attentional system [23] manages attention in complex, novel, non-automated or conflicting situations. A schematic representation of successive steps and levels of complexity of visuospatial attention is presented in Supplemental data.
Objectives and hypotheses
The main objective of the present study was to investigate the nature of attentional disorders in PD patients with FoG by using a paradigm based on Van Zomeren and Brouwer’s model. We sought to compare performance levels in patients with and without FoG under different attentional conditions. On the basis of previous studies in which gait disturbances in PD patients were exacerbated during dual-task paradigms that involved sustained and divided attention [24], we hypothesized that patients with FoG (referred to henceforth as “FoG patients”) would perform less well in specific attentional modalities (namely in divided attention or mental flexibility conditions) than patients without FoG (referred to henceforth as non-FoG patients).
METHODS
Experimental design and task
We assessed the patients’ performance in a computer-controlled reaction time (RT) paradigm designed to measure the different attentional subcomponents while controlling for visuospatial processing (via uniform central presentation) and motor participation (via a single-key response). The task has been described by Dujardin et al. [25]. The patients were assessed in an ecological “on” state after administration of their usual anti-parkinsonian medication(s).
Participants were seated in front of a 15-inch colour monitor. They were instructed to fix a grey square (2.5×2.5 cm) in the centre of the screen and to press the response key with their preferred hand as soon as the target stimulus appeared. The task comprised five levels:
- A simple RT task, intended to measure processing speed.
- A go/no-go choice RT task, intended to measure single-stimulus discrimination.
- A one-dimensional, focused-attention choice RT task, intended to assess attention on a one-dimensional stimulus. Subjects were instructed to ignore the distracters (green rectangles) and respond as in the go/no go RT task, i.e. by pressing the response key as quickly as possible when the central square turned blue, regardless of the number of distracters.
- A two-dimensional, divided-attention choice RT task, intended to assess attention between two stimulus dimensions. Subjects were instructed to respond as quickly as possible only when a blue square appeared surrounded by two green rectangles.
- An alternating choice RT task, intended to measure the flexibility of attention allocation. In the first phase of the task (30 trials), the instructions were the same as in the focused condition. Then, the instructions changed; the subjects were instructed to consider only the number of rectangles and to respond as quickly as possible when there were 2 rectangles (regardless of the color of the square).
The mean RT (in ms), the number of misses and the number of false alarms were recorded for each condition (except for the simple RT condition, in which false alarms were not possible). The test session lasted 45 minutes. The stimuli remained on the screen for 2 s at most or until a response was recorded during that time. Each level of the assessment was preceded by a practice block. The simple RT condition was always performed first. To limit order effects, the order of presentation of the four other conditions was counterbalanced.
All participants gave their informed consent to participation in the study. The study protocol was approved by the local independent ethics committee.
Population
Seventy-eight patients with PD (diagnosed according to accepted international criteria [26]) were recruited by the Department of Neurology and Movement Disorders. The FoG and non-FoG groups were constituted according to the patients’ responses to item 3 of Giladi’s FoG questionnaire [27]. All included patients suffered from ‘off-drug’ FoG [28], defined according the clinical response of FoG with levodopa administration. All patients performed a FoG trajectory [11] composed of gait initiation, turning, going through narrow passages and performing dual tasks. We determined a FoG trajectory score based on the duration of FoG episodes during the FoG trajectory: 1 point for a brief episode of FoG (<10 s), 2 points for a moderate episode (10–30 s) and 3 points for a long episode (>30 s).
Demographic and disease-related variables were collected during an ad hoc clinical interview. All patients were also assessed with the following instruments: part III of the Unified Parkinson’s Disease Rating Scale (UPDRS), the FoG questionnaire [27], the Mini Mental State Examination (MMSE) and the Mattis Dementia Rating Scale. The levodopa equivalent daily dose was calculated [29]. The main exclusion criteria were the presence of neurological or psychiatric disorders other than PD or an MMSE of 25 or less.
Statistical analyses
As distributions were not Gaussian, the demographic and clinical data from the FoG and non-FoG groups were compared by applying a Mann-Whitney test (for continuous variables) or a chi-squared test (for categorical variables). For RT data, the RTs’ coefficients of variance and accuracy (omissions and false alarms), that were normally distributed, analyses of covariance (ANCOVAs) were performed with group (FoG, non-FoG) as a between-group factor and with confounding factors as covariates (namely disease duration and deep brain stimulation (DBS), which are strongly associated with FoG [1]). Because disease duration and levodopa therapy were highly correlated, we used only one of these two parameters (the one that had the more robust association with RT data) as a covariable.
A Greenhouse-Geisser correction was applied when the assumption of sphericity did not hold. To explore putative associations between FoG characteristics and RTs, Spearman’s correlation coefficient was calculated.
The threshold for statistical significance was set to p < 0.05 in all cases. All analyses were performed with SPSS ® for Windows ® software (version 16.0, IBM Inc., Armonk, NY, USA).
RESULTS
Population
The demographic and clinical characteristics of the two PD groups are summarized in Table 1. Intergroup comparisons did not reveal any differences in terms of age, duration of formal education or global cognitive efficiency. The FoG group had a longer disease duration and a higher levodopa equivalent daily dose, relative to the non-FoG group (p < 0.05). The prevalence of DBS was also higher in the FoG group (p < 0.05). Thus, disease duration and the presence of DBS were included as covariates in subsequent statistical analyses. The levodopa equivalent daily dose was not included in the covariates because it correlated with disease duration (rho = 0.421, p < 0.05).
The two groups of patients did not differ in terms of the UPDRS motor score in the “on” state (p = 0.22). In contrast, the groups differed for variables that directly reflected the FoG phenomenon, such as the FoG questionnaire score (p < 0.05) and the FoG trajectory score (p < 0.05).
Attentional raw data
The mean (SD) performance levels of the two groups under the five conditions of the computer-controlled RT paradigm are shown in Table 1. In the absence of adjustment, RTs were higher in the FoG group than in the non-FoG group (p < 0.05) for all attentionalmodalities.
When disease duration and DBS were considered as covariates in ANCOVAs (Table 2), the only significant difference between the FoG and non-FoG groups concerned the RTs in the divided-attention condition (p = 0.02). There were no intergroup differences in RTs under the other conditions (p > 0.05). Furthermore, there were no intergroup differences in the accuracy of responses (p > 0.05 for all conditions).
Attentional RTs were correlated with FoG variables: FoG-questionnaire score and FOG-trajectory score (Table 3).
In Supplemental data, the specific effect of DBS in the subgroup of patient with FoG is also shown.
DISCUSSION
The present study sought to accurately define the attentional failure observed in PD patients with FoG (relative to those without FoG). By assessing performance in different attention modalities in the same individuals, we were able to show that patients with FoG displayed longer RTs than those without (whatever the modality of the attention task).
In view of the literature data [15–19], we could have expected to see extensive attentional failure in patients with FoG (caused by an increase in attentional cost as the task increased in complexity). In such a case, the RTs should have lengthened progressively from the simple RT to the go/no go choice RT, the focused-attention choice RT (with distractor inhibition), the divided-attention choice RT (with a need to take account of two attributes) and, lastly, the alternating choice RT. This was indeed the case when disease duration and DBS were not taken into account. When taking into account these covariates, thisslowing of information processing was only observed in the divided-attention condition. There were no intergroup differences in the accuracy of responses.
FoG: A failure of divided attention?
The presence of an impairment in divided attention distinguished between patients with FoG and those without. Importantly, our results clarified the role of attention in FoG and evidenced the specific role of divided-attention impairment in patients with FoG, which had not been possible with dual-task paradigms [9, 15, 21]. Indeed, dual-task paradigms involve the combined use of working memory and executive functions. In our present task, the patient had to simultaneously select two features of the stimulus before taking a decision (rather than having to cope with two different motor tasks). In fact, the motor involvement in our paradigm was low since the subjects had only to press a single response button. Moreover, the divided attention condition did not constitute a dual task. Hence, in a simple task with a single encoding modality (a visual modality, in the present study), PD patients with FoG processed concomitant information more slowly than patients without FoG –highlighting an early failure in simultaneous processing. These results increase knowledge of the mechanisms involved in the dual-task deficit in freezers. Usually, the explanation for dual-task difficulties in PD is that previously automated procedures (generally for motor activity, such as gait) are impaired by basal ganglia deficiency and are replaced by components controlled by the supervisory attentional system [23]. Here, the basic impairment in simultaneous two-stimulus integration (with no involvement of complex motor programs) suggests that patients with FoG have difficulty processing several simultaneous streams of data (rather than choosing relevant data - as in the flexibility task, where two orders had to be memorised simultaneously). Our results in patients with FoG suggest that their impairment in divided-attention tasking is located upstream of the high planning/regulating functions that are usually involved in dual-task impairments. The partial respect of their capacities in the flexibility task plead for a effective central executive [32] and a location of their impairment in divided-attention tasking even further upstream than the previously described limitation of attentional allocation.
However, this early impairment in processing several information concomitantly is probably greater when the patients with FoG have to deal with two different tasks at the same time (as is often the case in activities of daily living), with regard to capacity-sharing theories and a spectrum of prioritization [33].
FoG: An impairment of attention distribution?
Focused attention and mental flexibility were relatively unaffected in patients with FoG. This finding suggests that the necessary attentional resources are available but are poorly recruited. By considering attention simply as the sum of distinct subunits or modules (thus explaining limited attentional capacity) [34] in which several components run distinct processes and are functionally distinct [35], freezers are able to perform each correctly in an alternating choice RT condition (in which attention is alternately allocated to the number of squares and to their colour). That means that the sum of units is unaffected in freezers. However, when patients with FoG had to recruit all resources at the same time (in order to integrate information concerning the number and the colour of the squares), an impairment was observed. Although patients with FoG appear to have the same resources as patients without FoG and to be able to allocate attention to each single task, there does appear to be a difference in distribution of resources when required. Difficulty coping with several features at the same time might be a central feature of FoG and might be related to failure of the supervisory attentional system [23] or an overload in parallel processing of stimuli [31].
It is interesting to consider why this impairment could be particularly deleterious for freezers patients. Indeed, divided attention usually enables better exploration of the environment than focalized attention because the former is thought to involve “zoom lens” visuospatial attention [36]. When the subject fixes a single location in space, signal discrimination at that location is more difficult because all the signals have to be treated simultaneously by the same channel. Hence, the discrimination of incongruent sensory signals at this single, monitored location is more difficult because separation of the stimulus’ independent components is more complex. In divided attention tasks, the presentation of several modalities or locations enables the subject to independently select stimuli “in parallel”, which thus increases the overall amount of available resources. Processing two instructions in parallel is a means of recruiting separate pools of attentional resources [37]; this is especially true for the simultaneous processing of stimuli from different modalities [38]. In this respect, the specific impairment inincreasing attentional resources to process concomitant data (which appears to be crux of the divided-attention problem in patients with FoG) is particularly disabling; patients with FoG are capable of focusing their attentional resources but not distributing them between several parallel input streams.
Prioritization, interference resistance and accuracy
In both groups, the trade-off between speed and accuracy tended to reflect a lengthening of the RT and the maintenance of accuracy under all attentional conditions. Indeed, there were few false alarms and omissions in either group. Interestingly, a parallel can be drawn with the “posture second” strategy described by Bloem et al. [39], in which PD patients’ behaviour was marked by a preference for cognitive accuracy over motor performance. In divided-attention tasks with different inputs, the patient spontaneously considers cognitive aspects and does not prioritize the motor aspect, what was also noted in our results although the present study’s motor task was not very challenging. It reflects the poor central processing capacity [32] in patients with PD. Indeed, patients with PD are known to process tasks sequentially, in order to avoid system overload [30]. Here, patients with and without FoG displayed the same prioritization strategy and resisted interference to a similar extent, as evidenced by relatively unaffected performance in a focused attention task. The patients with FoG did not appear to be more impaired in information selection or filtering because the ability to inhibit a motor response (low number of false alarms and results in a “go–no go” task) or neglect a distractor (in a focused attention task) was unaffected; this may be due to the up-regulation of prefrontal dopaminergic receptors involved in resistance interference [40]. Previously, conflict resolution impairment was noted in freezers patients [19, 41] when they had to cope simultaneously with incongruent and relevant stimuli. Our results suggest that executive control is deficient in freezers, but also simply processing several features on a single stimulus at a same time.
What about neural substrates?
Our results agree with current hypotheses on FoG. Indeed, neuroimaging data revealed increased activity in the posterior parietal cortex when monitoring two modalities at different locations, while no specific region was recruited in the focused attention conditions [37]. In the same manner, in an object recognition divided attention paradigm the bilateral intraparietal sulcus was involved and correlated with attentional performance [42]. This region allows in-parallel processing when attention is spatially divided, referring to the cortical hypotheses of the origin of the FoG phenomenon [36] and explaining why the integration of external stimuli to drive movement is so deficient in freezers [43]. The frontoparietal regions of the cognitive control network [44], involved in the FoG phenomenon [45] also seems a good candidate for this low-level attentional discrimination processing (see Supplemental Figure).
Limitations
We performed a descriptive, cross-sectional study; further prospective follow-up studies are needed to establish whether early failure of divided attention in PD could predict the occurrence of FoG. Attentional tasks were performed in the “on drug” state and so the mean levodopa equivalent daily dose was higher in the FoG group. This may explain the comparable processing speed but may have modified attentional performance. We deliberately chose to test patients in the “on-drug” condition, in order to reflect ecological processing conditions.
Furthermore, it is important to keep in mind that the two groups were different: even with adjustment of analyses for disease duration and DBS, freezers patients had generally more advanced disease and could suffer from more diffuse lesions whose attentional results could also reflect. Concerning the others potential bias, we did not include levodopa therapy as a covariate because of the redundancy with disease duration. However, it could partly explain our results in ecological situation [46, 47]. Finally, the subgroup analysis highlighted the weight of DBS in the impairment in divided attention inside the FoG group. All these factors related to PD progression could impact attentional performance and act as a confounding factor in the evaluation of FoG [48].
In previous research [25], a specific impairment in mental flexibility in patients with PD was described. This impairment is critical for attention failure. However, mental flexibility does not appear to be factor that most strongly discriminates between PD patients with FoG and those without.
Usually, neuropsychological tests are performed in on drug condition as in this study, in order to reduce interference due to the akinesia or slowness. Because the off-FoG mainly occurs when the levodopa level decreased, the specific evaluation of attentional performance at this time would be very helpful, for example related to the levodopa pharmacokinetics. Thelimiting factor would be the necessity to take into account non-motor fluctuations (depression, anxiety or apathy) frequent in such circumstances and that could also interfere with attentional performance and trigger the FoG phenomenon [49]. Further studies are necessary to better apprehend the interplay between therapies, cognitive and affective aspects in the FoG occurence [7].
CONCLUSION AND PERSPECTIVES
Our study helped to determine the nature of attention failure in PD patients with FoG. These findings may explain why the influence of attention on gait is so ambivalent. For instance, focusing attention on walking enables the partial correction of disorders by modulating the voluntary step length. However, performing an attention-requiring double task worsens gait. The ability to focus attention appears to be unaffected in PD patients with FoG; this observation may explain the beneficial effect of single, external cues. In contrast, attention resources distribution was impaired in PD patients with FoG, which explains the worsening of walking and FoG during dual-task paradigms.
Current rehabilitation involves educative cognitive training [3, 50–53]. According to our results, cognitive training could specifically focus on treating simultaneous information or enlarge the bottleneck before more complex executive considerations, as soon as basic attentional features integration is deficient.
Conflict of Interest
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
We thank David Fraser (Biotech Communication, Damery, France) for editorial assistance and Julia Salleron for statistical analysis.
Appendices
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JPD-140498.
REFERENCES
1 | Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A (2011) Freezing of gait: Moving forward on a mysterious clinical phenomenon Lancet Neurol 10: 734 744 |
2 | Ellis T, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Dibble LE (2011) Which measures of physical function and motor impairment best predict quality of life in Parkinson’s disease? Parkinsonism Relat Disord 17: 693 697 |
3 | Walton CC, Shine JM, Mowszowski L, Naismith SL, Lewis SJG (2014) Freezing of gait in Parkinson’s disease: Current treatments and the potential role for cognitive training Restor Neurol Neurosci 32: 411 422 |
4 | Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena Mov Disord 19: 871 884 |
5 | Nieuwboer A, Giladi N (2013) Characterizing freezing of gait in Parkinson’s disease: Models of an episodic phenomenon Mov Disord 28: 1509 1519 |
6 | Lewis SJG, Barker RA (2009) A pathophysiological model of freezing of gait in Parkinson’s disease Parkinsonism Relat Disord 15: 333 338 |
7 | Shine JM, Naismith SL, Lewis SJG (2013) The differential yet concurrent contributions of motor, cognitive and affective disturbance to freezing of gait in Parkinson’s disease Clin Neurol Neurosurg 115: 542 545 |
8 | Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease Mov Disord 23: 395 400 |
9 | Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait Mov Disord 23: 329 342 |
10 | Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A (2010) Freezing of gait in Parkinson’s disease: The impact of dual-tasking and turning Mov Disord 25: 2563 2570 |
11 | Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR (2008) Clinimetrics of freezing of gait Mov Disord 23: Suppl 2 S468 S474 |
12 | LaBar KS, Gitelman DR, Parrish TB, Mesulam M (1999) Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects Neuroimage 10: 695 704 |
13 | Heremans E, Nieuwboer A, Spildooren J, Vandenbossche J, Deroost N, Soetens E, Kerckhofs E, Vercruysse S (2013) Cognitive aspects of freezing of gait in Parkinson’s disease: A challenge for rehabilitation J Neural Transm 120: 543 557 |
14 | Hallett M (2008) The intrinsic and extrinsic aspects of freezing of gait Mov Disord 23: Suppl 2 S439 S443 |
15 | Wright MJ, Burns RJ, Geffen GM, Geffen LB (1990) Covert orientation of visual attention in Parkinson’s disease: An impairment in the maintenance of attention Neuropsychologia 28: 151 159 |
16 | Moretti R, Torre P, Antonello RM, Esposito F, Bellini G (2011) The on-freezing phenomenon: Cognitive and behavioral aspects Parkinsons Dis 2011: 746303 |
17 | Naismith SL, Shine JM, Lewis SJG (2010) The specific contributions of set-shifting to freezing of gait in Parkinson’s disease Mov Disord 25: 1000 1004 |
18 | Shine JM, Naismith SL, Palavra NC, Lewis SJG, Moore ST, Dilda V, Morris TR (2013) Attentional set-shifting deficits correlate with the severity of freezing of gait in Parkinson’s disease Parkinsonism Relat Disord 19: 388 390 |
19 | Vandenbossche J, Deroost N, Soetens E, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E (2011) Freezing of gait in Parkinson disease is associated with impaired conflict resolution Neurorehabil Neural Repair 25: 765 773 |
20 | Walton CC, Shine JM, Mowszowski L, Gilat M, Hall JM, O’Callaghan C, Naismith SL, Lewis SJG (2014) Impaired cognitive control in Parkinson’s disease patients with freezing of gait in response to cognitive load J Neural Transm 10.1007/s00702-014-1271-6 |
21 | Partiot A (1996) Delayed response tasks in basal ganglia lesions in man: Further evidence for a striato-frontal cooperation in behavioural adaptation Neuropsychologia 34: 709 721 |
22 | Van Zomeren AH, Brouwer WH (1994) Clinical Neuropsychology of Attention Oxford University Press |
23 | Raz A, Buhle J (2006) Typologies of attentional networks Nat Rev Neurosci 7: 367 379 |
24 | Lord S, Rochester L, Hetherington V, Allcock LM, Burn D (2010) Executive dysfunction and attention contribute to gait interference in “off” state Parkinson’s disease Gait Posture 31: 169 174 |
25 | Dujardin K, Tard C, Duhamel A, Delval A, Moreau C, Devos D, Defebvre L (2013) The pattern of attentional deficits in Parkinson’s disease Parkinsonism Relat Disord 19: 300 305 |
26 | Gibb WR (1988) Accuracy in the clinical diagnosis of parkinsonian syndromes Postgrad Med J 64: 345 351 |
27 | Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD (2000) Construction of freezing of gait questionnaire for patients with Parkinsonism Parkinsonism Relat Disord 6: 165 170 |
28 | Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (2003) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease Eur J Neurol 10: 391 398 |
29 | Wenzelburger R, Zhang B-R, Pohle S, Klebe S, Lorenz D, Herzog J, Wilms H, Deuschl G, Krack P (2002) Force overflow and levodopa-induced dyskinesias in Parkinson’s disease Brain 125: 871 879 |
30 | Malapani C, Pillon B, Dubois B, Agid Y (1994) Impaired simultaneous cognitive task performance in Parkinson’s disease: A dopamine-related dysfunction Neurology 44: 319 326 |
31 | Klingberg T (2000) 95 102 Limitations in information processing in the human brain: Neuroimaging of dual task performance and working memory tasks. In Progress in Brain Research Uylings HBM, Van Eden CG, De Bruin JPC, Feenstra MGP, Pennartz CMA Elsevier 95 102 |
32 | Baddeley A (1996) Exploring the central executive Q J Exp Psychol A 49: 5 28 |
33 | Canning CG (2005) The effect of directing attention during walking under dual-task conditions in Parkinson’s disease Parkinsonism Relat Disord 11: 95 99 |
34 | Norman DA, Shallice T (1986) In Consciousness and Self-Regulation Attention to action: Willed and automatic control of behaviour Davidson RJ, Schwartz GE, Shapiro D New York Plenum |
35 | Sternberg S (2001) Separate modifiability, mental modules, and the use of pure and composite measures to reveal them Acta Psychol (Amst) 106: 147 246 |
36 | Eriksen CW, St James JD (1986) Visual attention within and around the field of focal attention: A zoom lens model Percept Psychophys 40: 225 240 |
37 | Santangelo V, Fagioli S, Macaluso E (2010) The costs of monitoring simultaneously two sensory modalities decrease when dividing attention in space Neuroimage 49: 2717 2727 |
38 | Talsma D, Doty TJ, Strowd R, Woldorff MG (2006) Attentional capacity for processing concurrent stimuli is larger across sensory modalities than within a modality Psychophysiology 43: 541 549 |
39 | Bloem BR, Grimbergen YAM, van Dijk JG, Munneke M (2006) The “posture second” strategy: A review of wrong priorities in Parkinson’s disease J Neurol Sci 248: 196 204 |
40 | Cools R, Miyakawa A, Sheridan M, D’Esposito M (2010) Enhanced frontal function in Parkinson’s disease Brain 133: 225 233 |
41 | Vandenbossche J, Deroost N, Soetens E, Zeischka P, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E (2012) Conflict and freezing of gait in Parkinson’s disease: Support for a response control deficit Neuroscience 206: 144 154 |
42 | Santangelo V, Macaluso E (2013) The contribution of working memory to divided attention Hum Brain Mapp 34: 158 175 |
43 | Wise SP, Boussaoud D, Johnson PB, Caminiti R (1997) Premotor and parietal cortex: Corticocortical connectivity and combinatorial computations Annu Rev Neurosci 20: 25 42 |
44 | Cole MW, Schneider W (2007) The cognitive control network: Integrated cortical regions with dissociable functions Neuroimage 37: 343 360 |
45 | Shine JM, Matar E, Ward PB, Bolitho SJ, Pearson M, Naismith SL, Lewis SJG (2013) Differential neural activation patterns in patients with Parkinson’s disease and freezing of gait in response to concurrent cognitive and motor load PLoS One 8: e52602 |
46 | Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM (2005) Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease Neuropsychologia 43: 823 832 |
47 | Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands Cereb Cortex 11: 1136 1143 |
48 | Dirnberger G, Jahanshahi M (2013) Executive dysfunction in Parkinson’s disease: A review J Neuropsychol 7: 193 224 |
49 | Ehgoetz Martens KA, Ellard CG, Almeida QJ (2014) Does anxiety cause freezing of gait in Parkinson’s disease? PLos One 9: e106561 |
50 | Milman U, Atias H, Weiss A, Mirelman A, Hausdorff JM (2014) Can cognitive remediation improve mobility in patients with Parkinson’s disease? Findings from a 12 week pilot study J Parkinsons Dis 4: 37 44 |
51 | Yogev-Seligmann G, Giladi N, Brozgol M, Hausdorff JM (2012) A training program to improve gait while dual tasking in patients with Parkinson’s disease: A pilot study Arch Phys Med Rehabil 93: 176 181 |
52 | Fok P, Farrell M, McMeeken J (2012) The effect of dividing attention between walking and auxiliary tasks in people with Parkinson’s disease Hum Mov Sci 31: 236 246 |
53 | Sturm W, Fuhr P, Zimmermann R, Gschwandtner U (2015) Cognitive training in Parkinson disease: Cognition-specific vs nonspecific computer training Neurology 84: 104 105 |
Figures and Tables
Fig.1
Attentional modalities schematised.
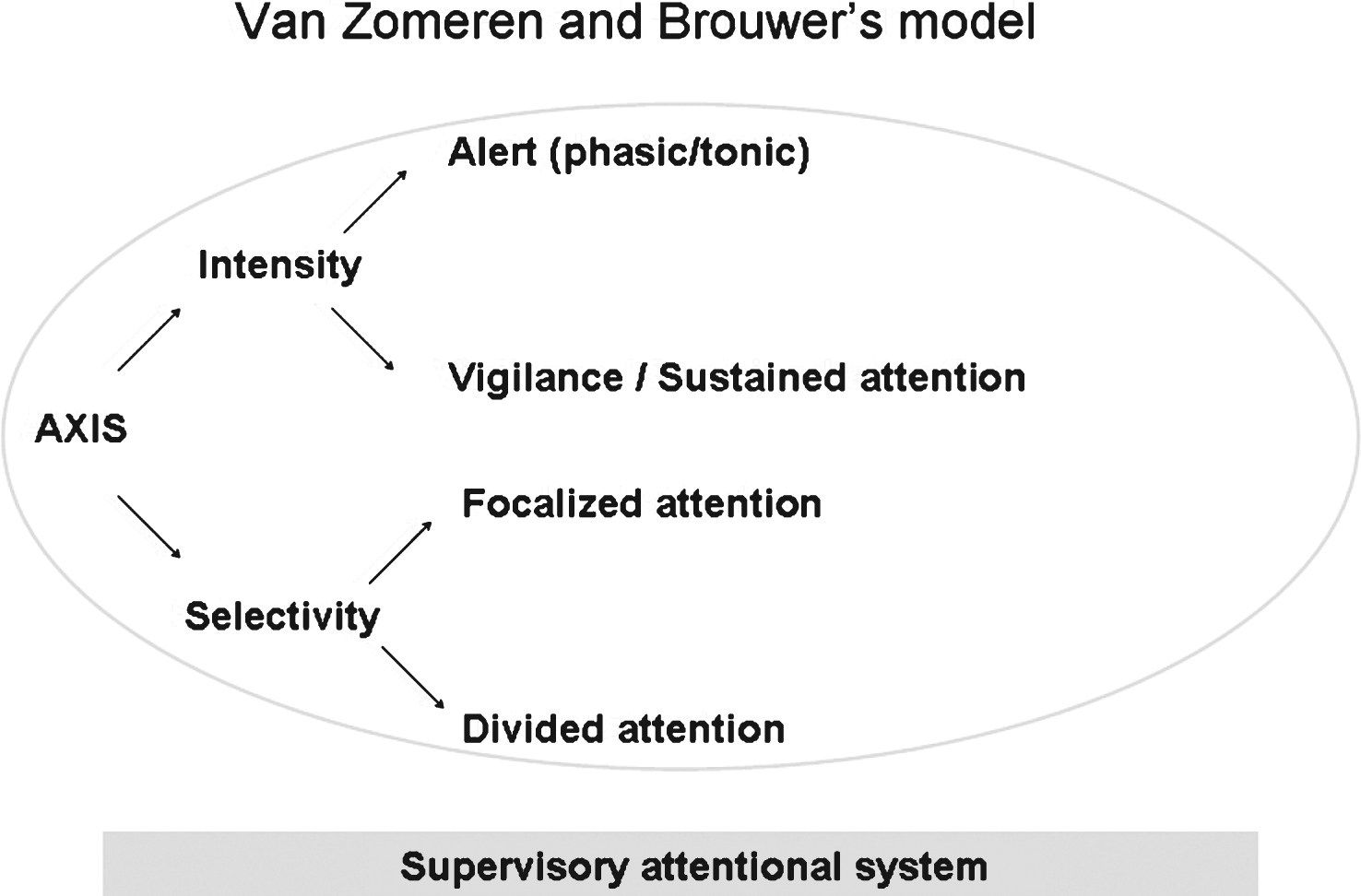
Table 1
Demographic, clinical and attentional characteristics of the two PD groups and statistical comparisons
| FoG group | Non-FoG group | Statistical results | p | |
| N | 42 | 36 | ||
| Demographical data | ||||
| Age (in years) | 61.9 (±8.7) | 59.3 (±9.7) | Z =−1.33 | 0.184 |
| Sex-ratio (male/female) | 17/25 | 23/13 | χ 2 =0.23 | 0.039 |
| Duration of formal education (in years) | 11.2 (±3.4) | 11.1 (±3.3) | Z =−0.17 | 0.863 |
| Mini Mental State Examination (/30) | 28 (±1.6) | 28.1 (±2.0) | Z =−0.55 | 0.586 |
| Mattis Dementia Scale (/144) | 137.1 (±5.1) | 137.5 (±5.7) | Z =−0.61 | 0.543 |
| Disease Characteristics | ||||
| Disease duration (in years) | 14.3 (±7.3) | 4.2 (±5.5) | Z =−6.07 | <0.001 |
| LEDD (mg daily) | 970 (±566) | 565 (±903) | Z =−4.47 | <0.001 |
| Deep brain stimulation (yes/no) | 26/16 | 3/33 | χ 2 =−0.55 | <0.001 |
| UPDRS 3 On drug (/108) | 25.5 (±11.1) | 22.8 (±9.2) | Z =−1.22 | 0.221 |
| FoG Characteristics | ||||
| FoG-Questionnaire (/24)a | 13.2 (±3.9) | 1.4 (±2.1) | Z =−7.54 | <0.001 |
| FoG trajectory On drugb | 3.8 (±4.8) | 0.0 (±0.0) | Z =−5.75 | <0.001 |
| Attentional Parameters | ||||
| Simple reaction time (in msec) | 363 (±89) | 303 (±58) | Z =−3.55 | <0.001 |
| Go-no go reaction time (in msec) | 530 (±116) | 460 (±64) | Z =−2.57 | 0.010 |
| Focused reaction time (in msec) | 555 (±123) | 479 (±70) | Z =−2.89 | 0.003 |
| Divided reaction time (in msec) | 637 (±122) | 532 (±93) | Z =−3.81 | <0.001 |
| Alternating reaction time (in msec) | 635 (±148) | 534 (±100) | Z =−3.35 | <0.001 |
Data are quoted as the mean (standard deviation). Significant differences between the two groups are marked in bold. afrom Giladi et al. (2000). bFoG trajectory score was based on the duration of FoG episodes during the FoG trajectory: 1 point for a brief episode of FoG (<10 s), 2 points for a moderate episode (10–30 s) and 3 points for a long episode (>30 s).
Table 2
Attentional results: means (standards deviations) and statistical results after adjustment on disease duration and deep brain stimulation
| FoG group | Non-FoG group | p | |
| Speed of Processing | |||
| Simple reaction times | 361.62 (±89.61) | 302.99 (±57.94) | 0.193 |
| Go-no go reaction times | 529.04 (±118.16) | 459.75 (±64.23) | 0.088 |
| Focused reaction times | 552.52 (±124.58) | 478.66 (±69.73) | 0.128 |
| Divided reaction times | 634.45 (±123.44) | 531.98 (±93.00) | 0.023 |
| Alternating reaction times | 631.55 (±150.27) | 534.30 (±99.74) | 0.099 |
| Accuracy of Responses | |||
| Go-no go omissions | 0.33 (±1.03) | 0.19 (±0.47) | 0.982 |
| Focused omissions | 0.55 (±1.56) | 0.08 (±0.28) | 0.557 |
| Divided omissions | 0.21 (±0.52) | 0.08 (±0.37) | 0.824 |
| Alternating omissions | 1.02 (±1.83) | 0.50 (±1.52) | 0.304 |
| Go-no go false alarms | 0.31 (±0.75) | 0.17 (±0.38) | 0.914 |
| Focused false alarms | 0.26 (±0.59) | 0.22 (±0.54) | 0.552 |
| Divided false alarms | 0.88 (±1.47) | 0.92 (±1.83) | 0.351 |
| Alternating false alarms | 0.88 (±1.74) | 0.61 (±0.87) | 0.713 |
Significant differences between the two groups are marked in bold. Reaction times are given in msec.
Table 3
Spearman correlation coefficients (on the first line) and significance (on the second line) between FoG parameters and reaction times. Significant correlations are marked in bold
| Simple RT | Go-no go RT | Focused RT | Divided RT | Alternating RT | Power of attention RT | |
| FoG-Questionnary (/24) | 0.3998 | 0.3157 | 0.3250 | 0.4019 | 0.3531 | 0.4033 |
| 0.0003 | 0.0049 | 0.0037 | 0.0003 | 0.0015 | 0.0003 | |
| FoG trajectory On drug | 0.4018 | 0.3402 | 0.3385 | 0.3705 | 0.3013 | 0.3727 |
| 0.0003 | 0.0025 | 0.0026 | 0.0009 | 0.0077 | 0.0008 |
RT: reaction times. Significant correlations are marked in bold.



