Six Years Follow-Up of an 11-Year-Old Girl with Anti-HMGCR Myopathy
Abstract
Anti-HMGCR myopathy is decribed as an immune-mediated necrotizing myopathy which is characterised by subacute, progressive proximal muscle weakness and elevated creatine kinase (CK) level. In pediatric population, anti-HMGCR myopathy has been reported solely as small case reports, albeit rare. Although treatment consensus has not yet been established, proper treatment with several immunomodulators to include IVIg can show remarkable improvement. We report an 11-year-old-girl diagnosed with anti-HMGCR myopathy with 6 years of follow-up.
INTRODUCTION
Anti-HMGCR myopathy was mostly defined in adults with statin exposure, presenting by muscle weakness. However, anti-HMGCR myopathy may have various forms, including presentation in children and young adults lacking statin exposure [1]. Clinical presentation mainly shows subacute, progressive proximal muscle weakness along with elevated CK level (1,000–20,000 IU/L) seen in immune mediated necrotising myopathy (IMNM) [1, 2]. Anti-HMGCR myopathy has been described in pediatric patients as case reports or small series. Although there is no consensus on biomarkers of disease progression and long-term treatment strategies, there are some treatment recommendations by experts [3]. We report a childhood case with long term follow up.
CASE REPORT
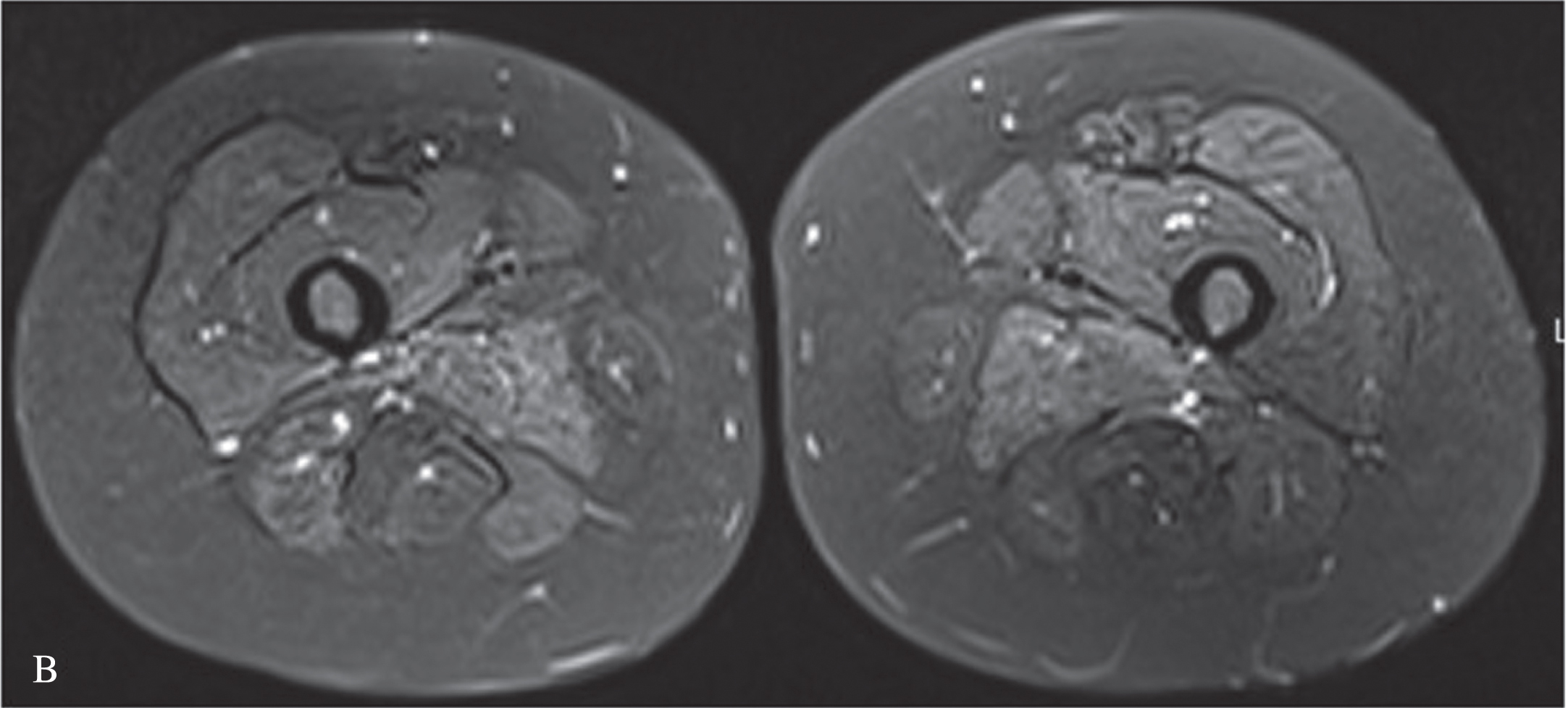
We present an 11-year-old-girl with anti-HMGCR myopathy with a 6 years of follow-up. Initial clinical findings were characterized by subacute, progressive proximal and truncal weakness for two months, at the age of 11 years-old. At the nadir of her symptoms she was weak to the extent that marked difficulty in climbing stairs and getting up from the ground. Her proximal muscle power was 4/5 Medical Research Council (MRC) with a positive Gowers sign of 12 seconds. CK was elevated at (10,488 IU/L), electromyography was myopathic. Muscle biopsy revealed variation in fiber size, scattered necrotic and regenerating fibers, mild endomysial fibrosis, few inflammatory cells and sarcolemmal HLA-ABC expression on some fibers; compatible with necrotising myopathy (Fig. 1). High anti-HMGCR autoantibody level (>200 U/ml, reference value < 20U/Ml) led to the diagnosis of anti-HMGCR myopathy. She was initially treated with oral corticosteroid (1 mg/kg once daily), followed by intravenous immunoglobulin (IVIg, 2 g/kg every 4 weeks), azathioprine, rituximab (Rtx) and methotrexate (Mtx), in combinations. We observed that combining IVIg and methotrexate contributed to better clinical improvement. At the beginning of the COVID19 pandemic, the patient could not get one dose of IVIG and that probably resulted increased CK level at 7049 IU/L without clinical deterioration (Figure 2). Later on, IVIg (2 gr/kg) was changed to sub-cutaneous Ig (SCIg) (1.6 gr/kg) for more feasible home delivery. She had been stable with this regimen (SCIg+Mtx) for more than one year with no overt muscle weakness, however her CK remained at 4000 IU/L range. In the first year of SCIg treatment, Mtx was discontinued due to her stable clinical course and also the dose of SCIg treatment was halved. Within the next six months, she first complained of muscle cramps and then developed persistent difficulty with walking, climbing stairs and arising from a chair. She could not rise from the floor during a Gowers maneuver. Upper and lower extremity, as well as both shoulder magnetic resonance imaging (MRI) examinations were performed. There was noticeable loss of volume and fatty atrophy, especially in the proximal part of the gastrocnemius muscle. Volume loss and fatty atrophy were observed in bilateral rectus femoris, adductor longus, brevis and magnus muscles, biceps femoris muscles, gluteal muscles sartorius muscles, iliopsoas muscles, semimembranosus and semitendinosus muscles, and the gracilis muscle. Short tau inversion recovery (STIR) signal increase was noted in adductor longus and brevis muscles (Fig. 3A, B). Volume loss and fatty atrophy were evident in bilateral brachialis and pronator teres muscles, bilateral subscapularis and serratus anterior and latissimus dorsi muscles, as well as in biceps brachii and brachialis muscles.Therefore, Mtx was added. Within the next four months, SCIg treatment was changed to IVIg and pulse IV corticosteroid treatment once was administered due to lack of significant clinical improvement. After one month, since the patient did not improve clinically despite all these treatment changes, the Mtx dose was doubled (20 mg/week). Within the next one month, she was able to rise from the floor with a positive Gowers sign of 10 seconds. Eventually, her Gowers sign lowered to six seconds. While using SCIg, CK levels were around 3000–4000 IU/L, they gradually decreased to below 1000 IU/L during the combination of IVIg and doubled dose Mtx (Figure 2). In addition, HLA-DR antigen was investigated and it was found the alleles HLA DRB1*08 and DRB1*15. Currently she is able to walk comfortably with a marked Gowers sign of six seconds. Her proximal muscle power is MRC 4 + /5.
Fig. 1
Muscle biopsy showing mild variation in fiber size and scattered necrotic fibers (black arrow).
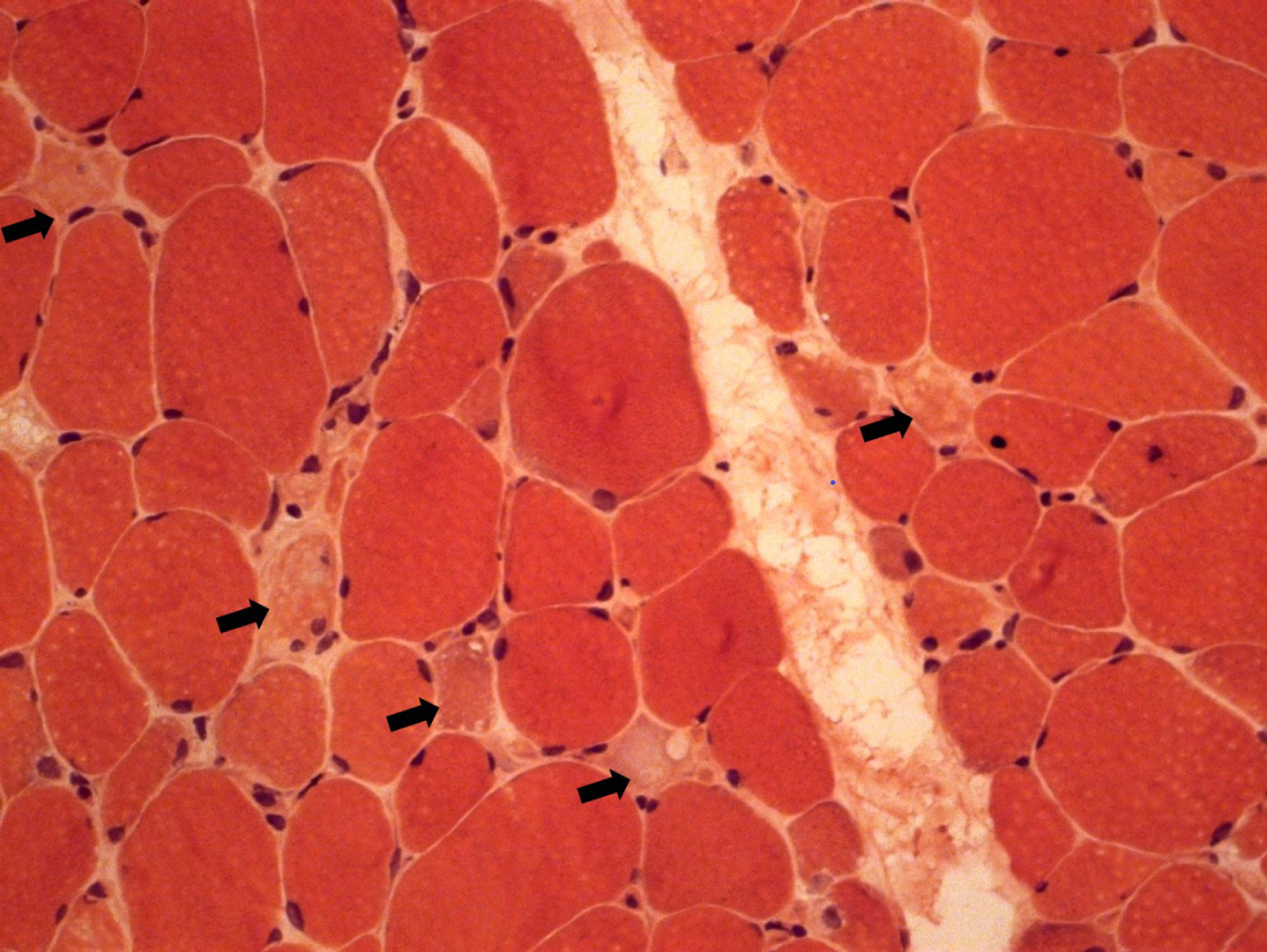
Fig. 2
Treatment regime and follow-up with CK level.
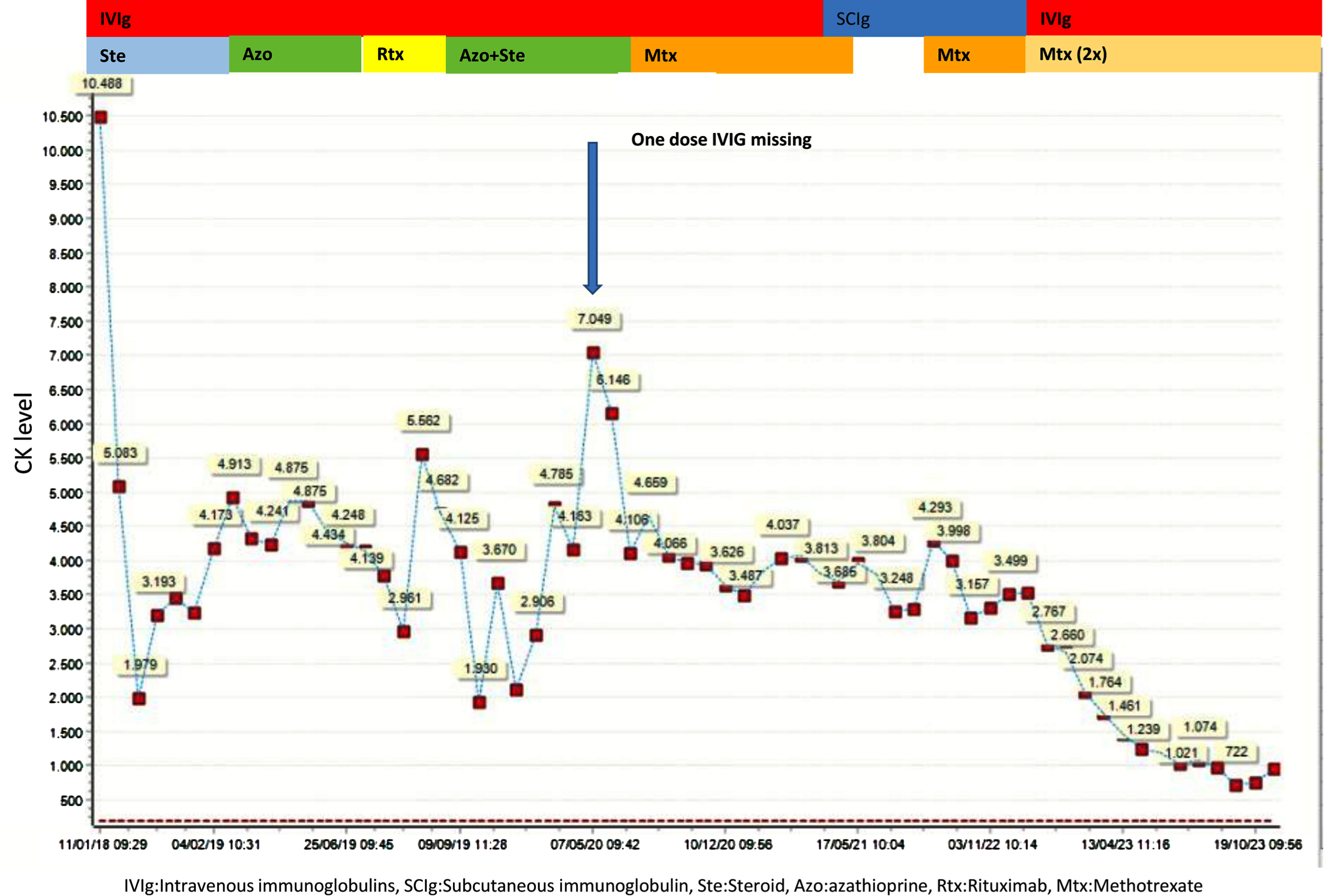
Fig. 3A
T1 weighted images shows fatty atrophy in bilateral bilateral rectus femoris, adductor longus, brevis and magnus muscles, biceps femoris muscles, gluteal muscles sartorius muscles, iliopsoas muscles.
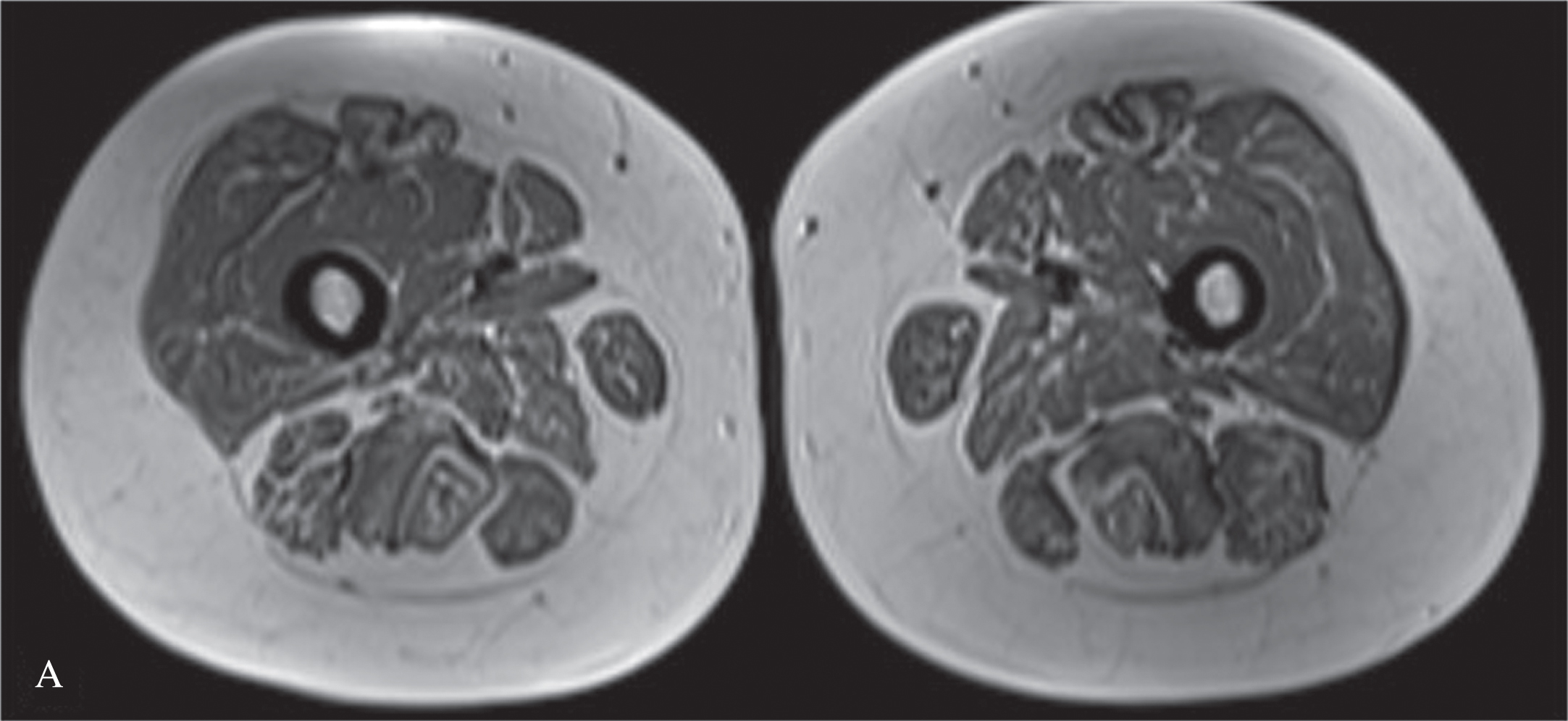
Fig. 3B
Short tau inversion recovery (STIR) signal is increased in adductor longus and brevis muscles.
DISCUSSION
Among idiopathic inflammatory myopathies (IIM), immune-mediated necrotizing myopathy (IMNM) has been defined as a disease with proximal weakness, high CK level and histopathological changes including muscle fiber necrosis and regeneration with unremarkable inflammation [4, 5]. Anti-HMGCR myopathy is a subgroup of IMNM and diagnosed high anti-HMGCR autoantibody level in addition to these clinical and muscle biopsy findings [1, 4].Anti-HMGCR myopathy was first recognized in patients with statin exposure, but later has been determined also in statin-naive patients, particularly young adults and pediatric patients. Clinical spectrum has been expanded to typical classic type with acute/subacute progressive, proximal weakness and chronic limb-girdle muscular dystrophy (LGMD)-like phenotype [1, 6]. Our patient’s clinical phenotype is also associated with the classic type (i.e sub-acute). This condition should be considered within the differential diagnosis of LGMD in general [5].
Although the pathogenesis of anti-HMGCR myopathy is still unknown, one of the most accepted hypotheses is immune activation and interactions between genetically predisposed individuals and particular environmental triggers [7, 8]. The human leucocyte antigen (HLA) domain of the major histocompatibility complex (MHC) is suggested the strongest genetic risk factor in IIM [7]. In the previous studies, it was determined the HLA-DRB1*07 : 01 allele in children and HLA-DRB1*11 : 01 allele in adult patients are associated with anti-HMGCR myopathy [7, 9]. However, our patient had alleles HLA DRB1*08 and DRB1*15.
Some reports suggest that the CK level can be utilized as a biomarker for disease activity in anti-HMGCR myopathy [1, 4]. Remarkably, there are sufficient data that CK is an excellent marker of disease activity in anti-HMGCR, determining the level of muscle necrosis and being strongly correlated with levels of anti-HMGCR autoantibodies. Patients with fatty replacement tend to experience a decrease in the maximum levels of CK for the same level of activity of the disease. Moreover, In anti-HMGCR, younger patients are more likely to experience more aggressive forms of the disease [10]. In the first admission of our patient, the CK level was 10,488 IU/L. After performing IVIg/ SCIg and azathioprine/Rtx/Mtx combinations, the CK level decreased to around 4,000 IU/L and it continued like that for a long time. At the clinical management of this patient, it was observed there was a decrease in the levels of CK from 5500U/L to 1800U/L after administering rituximab and IVIg. According to some experts, the combination of IVIg (saturates the neonatal Fc receptor and reduces the lifespan of endogenous antibodies) and Rtx (reduces the amount of endogenous immunoglobulins produced) appears to be the most effective treatment option available [11, 12]. In young patients with active disease, the combination of these two drugs is often not enough to control the disease. Therefore, it may be necessary to add other immunosuppressant agents such as Mtx, which happened in this individual to achieve long-lasting remission. Preventing the development of fatty replacement over time is crucial in young patients. In general, levels of CK below 500U/L do not usually lead to progression of fatty replacement, and treatment is usually focused on achieving these levels of CK. To achieve remission, rituximab can be added to IVIg and Mtx if this patient shows any disease activity.
In the COVID-19 pandemic, only one dose IVIg was missed and then the CK level increased to 7,049 IU/L. After regular IVIg/SCIg and azathioprine/Rtx/Mtx combinations, the CK level sustained around 3,000–4,000 IU/L. However, the CK level did not increase during the clinical deterioration after stopping Mtx and reducing SCIg. Morover, the CK level dropped gradually to 722 IU/L after starting IVIg and higher dose Mtx. Therefore, the neurological examination should be taken into consideration, first, as CK levels (increase or decrease) may sometimes precede clinical worsening or improvement by several months.
Treatment consists of corticosteroids, IVIg, and steroid-sparing immunosuppressants based on reports from pediatric patients due to a lack of consensus on biomarkers of disease progression and long-term treatment strategies [2, 8]. Some case reports suggest that IVIg monotherapy restores CK from elevated values to normal in mildly affected patients [13]. Among the limited treatment agents, IVIg and Mtx combination has been the most effective option for clinical improvement and lower CK level at the present case. Treatment selection, maintenance, escalation and reduction are modified individually. Moreover, there is not yet sufficient consensus on the treatment doses to be used [1]. It can be attributed to the enigma of the natural course of the disease. Suarez et al. reported an eight-year-old girl diagnosed with anti-HMGCR myopathy who had prolonged spontaneous recovery after four years of follow-up [5].
CONCLUSION
Although long-term observational studies and consensus guideline on management of anti HMGCR myopathy in children are still lacking, appropriate treatment strategies may provide significant improvement. In our experience, the best results were achieved by combination of IVIg and Mtx. In addition, we have emphasized that the neurological examination should be given priority, as clinical worsening or improvement can sometimes be preceded by an increase or decrease in CK levels for several months.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest regarding this publication.
DISCLOSURE
Financial support: None.
Conflict of interest: None.
Haluk Topaloglu is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
FUNDING
This study was supported by the Neuromuscular Research Association of Türkiye.
REFERENCES
[1] | Mohassel P , Mammen AL . Anti-HMGCR Myopathy. J Neuromuscul Dis. (2018) ;5: (1):11–20. |
[2] | Gupta L , Nune A , Naveen R , Verma R , Prasad P , Kharbanda R , et al.The prevalence and clinical characteristics of anti-HMGCR (anti-3-hydroxy-3-methyl-glutaryl-coenzyme A reductase) antibodies in idiopathic inflammatory myopathy: an analysis from the MyoCite registry. Rheumatol Int. (2022) ;42: (7):1143–54. |
[3] | Velardo D , Faravelli I , Cinnante C , Moggio M , Comi GP . Pediatric anti-HMGCR necrotizing myopathy: diagnostic challenges and literature review. Neurol Sci. (2020) ;41: (10):3009–13. |
[4] | Liang WC , Wang CH , Chen WZ , Kuo YT , Lin HF , Suzuki S , et al. Treatment experience of Taiwanese patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase myopathy. Kaohsiung J Med Sci. (2020) ;36: (8):649–55. |
[5] | Suárez B , Jofré J , Lozano-Arango A , Ortega X , Diaz J , Calcagno G , et al. Spontaneous symptomatic improvement in a pediatric patient with anti-3-hydroxy-3-methylglutraryl-coenzyme A reductase myopathy. Neuromuscul Disord. (2020) ;30: (6):503–9. |
[6] | Mohassel P , Landon-Cardinal O , Foley AR , Donkervoort S , Pak KS , Wahl C , et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol Neuroimmunol Neuroinflamm. (2019) ;6: (1):e523. |
[7] | Rothwell S , Chinoy H , Lamb JA , Miller FW , Rider LG , Wedderburn LR , et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann Rheum Dis. (2019) ;78: (7):996–1002. |
[8] | Khan N , Kazmi Z . A Rare Case of Anti-HMGCR and Anti-SRP-Positive Immune-Mediated Necrotizing Myopathy. Qatar Med J. (2022) ;2022: (1): 6. |
[9] | Mekmangkonthong A , Amornvit J , Numkarunarunrote N , Veeravigrom M , Khaosut P . Dengue infection triggered immune mediated necrotizing myopathy in children: a case report and literature review. Pediatr Rheumatol Online J. (2022) ;20: (1):40. |
[10] | Tiniakou E , Pinal-Fernandez I , Lloyd TE , Albayda J , Paik J , Werner JL , et al.More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford). (2017) ;56: (5):787–94. |
[11] | Landon-Cardinal O , Allenbach Y , Soulages A , Rigolet A , Hervier B , Champtiaux N , et al.Rituximab in the Treatment of Refractory Anti-HMGCR Immune-mediated Necrotizing Myopathy. J Rheumatol. (2019) ;46: (6):623–7. |
[12] | Gupta S , Rakhra A , Thallapally V , Nahas J . Rituximab use for refractory anti-HMGCR immune-mediated necrotizing myopathy: A case report. Intractable Rare Dis Res. (2021) ;10: (2):122–5. |
[13] | Della Marina A , Pawlitzki M , Ruck T , van Baalen A , Vogt N , Schweiger B , et al. Clinical Course, Myopathology and Challenge of Therapeutic Intervention in Pediatric Patients with Autoimmune-Mediated Necrotizing Myopathy. Children (Basel). (2021) )8: (9). |




