Relationship Between Hand Strength and Function in Duchenne Muscular Dystrophy and Spinal Muscular Atrophy: Implications for Clinical Trials
Abstract
Background:
Measurement of muscle strength and motor function is recommended in clinical trials of neuromuscular diseases, but the loss of hand strength at which motor function is impacted is not documented.
Objectives:
To establish the relationship between hand strength and function, and to determine the strength threshold that differentiates normal and abnormal hand function in individuals with Duchenne Muscular Dystrophy (DMD) or Spinal Muscular Atrophy (SMA).
Methods:
Maximal handgrip and key pinch strength were measured with the MyoGrip and MyoPinch dynamometers, respectively. Hand function was assessed using the MoviPlate, the Motor Function Measure items for distal upper limb (MFM-D3-UL) and the Cochin Hand Function Scale (CHFS).
Results:
Data from 168 participants (91 DMD and 77 SMA, age 6–31 years) were analyzed. Relationships between strength and function were significant (P < 0.001). Hand function was generally preserved when strength was above the strength threshold determined by Receiver-Operating Characteristic (ROC) analysis: For MFM-D3-UL, the calculated handgrip strength thresholds were 41 and 13% of the predicted strength for a healthy subject (% pred) and the key pinch strength thresholds were 42 and 26% pred for DMD and SMA, respectively. For the MoviPlate, handgrip strength thresholds were 11 and 8% pred and key pinch strength thresholds were 21 and 11% pred for DMD and SMA, respectively. For participants with sub-threshold strength, hand function scores decreased with decreasing strength. At equal % pred strength, individuals with SMA had better functional scores than those with DMD.
Conclusions:
Hand function is strength-dependent for most motor tasks. It declines only when strength falls below a disease-specific threshold. Therefore, therapies capable of maintaining strength above this threshold should preserve hand function.
INTRODUCTION
Upper limb function is fundamental in everyday life. Self-administrated questionnaires (SAQ) [1, 2], rater-controlled scales (RCS) [3–5] and the MoviPlate [6] have been developed to assess hand or upper limb function. The MyoGrip and MyoPinch dynamometers have been designed to accurately measure handgrip and key pinch strength [6–8] in individuals with neuromuscular disorders (NMD). Although significant correlations have been found between strength measured using the MyoGrip and MyoPinch dynamometers and function assessed with the MoviPlate, the Brooke or the Motor Function Measure (MFM) scales [6–8], to our knowledge, the impact of handgrip or key pinch strength changes on hand function has not been documented so far. However, understanding the decline of motor function and strength as well as the relationship between both is paramount in the design of clinical trials in these populations. The aims of this study were (i) to explore the relationships between hand function and handgrip or key pinch strength in individuals with Duchenne Muscular Dystrophy (DMD) or Spinal Muscular Atrophy (SMA), (ii) to find out whether the strength-function relationship is identical in different NMDs, namely DMD and SMA, (iii) to define the minimum handgrip and key pinch strength required for normal hand function (according to the functional scores) and to document the strength values which correspond to normal or impaired hand function in these two patient populations, (iv) to identify the tasks, which are particularly dependent on hand strength, and to examine whether this strength dependence is similar in the DMD and the SMA.
The MyoGrip and MyoPinch dynamometers were used because of their high sensitivity able to capture residual strength, even of the weakest individuals with neuromuscular diseases. In order to assess hand function in clinic and in daily activities, on acute and enduring tasks, we selected MFM D3 UL items as rater-controlled scale (RCS) [3–5], CHFS as self-administrated questionnaire (SAQ) [1, 2] and the MoviPlate [6], which aims to evaluate distal coordination and endurance of the upper limb.
To find out whether the strength-function relationship is identical in different NMDs, we chose to analyse two neuromuscular pathologies that are regularly the subject of clinical trials, namely DMD and SMA. We targeted SMA types 2 and 3 only, whose motor impairment of the hand is widely described in the literature and for which we had data. Indeed, in literature, a strength deficit measured with the MyoGrip and MyoPinch dynamometers was demonstrated in SMA types 2 and 3, with a significant decline in 12 or 24 months [9, 10]. In addition, grip strength and functional hand RULM score improved in SMA 3 and 4 after 22 months of treatment with nusinersen [11].
MATERIALS AND METHODS
Study design and participants
This study pools the results of three multicenter observational studies of the natural history of muscle strength and function in ambulant and/or non-ambulant patients with NMD: (i) Upper Limb Evaluation in Non-Ambulant Patients (ULENAP) With Neuromuscular Disorder (only the DMD and SMA data are treated here), (ii) Observational Study of Patients With Duchenne Muscular Dystrophy Theoretically Treatable With Exon 53 Skipping (preU7) and (iii) Prospective Study of the Natural History of Patients With Type 2 and 3 Spinal Muscular Atrophy (NatHis SMA). The data presented here were collected between January 2010 and January 2013 (ULENAP), between October 2011 and December 2016 (PreU7) and between May 2015 and May 2018 (NatHis SMA). All the centers were located in Western Europe (see Acknowledgements). In each site, the protocols were approved by the Local Ethics Review Board and guidelines on human experimentation were followed. All the patients, or their guardian(s) when patients were younger than 18 years old, gave written informed consent.
The protocols were registered on https://clinicaltrials.gov/ under the numbers NCT00993161 (ULENAP), NCT01385917 (PreU7) and NCT02391831 (NatHis SMA).
The patients in these three studies had similar age ranges (8–30, 6–20, 6–30 years old, respectively) and were able to comply with all protocol requirements. Patients with cognitive impairment limiting the understanding of the exercises were excluded. The participants were all naive to innovative therapies that were not yet available at the time. All MyoGrip, MyoPinch, MoviPlate and MFM assessments analysed in this study were performed following identical instructions in all three studies. The Cochin Manual Function Scale (CHFS) was only used in the ULENAP study. For the three studies, inclusion and exclusion criteria were previously defined, patients were assessed every six months, and results on the natural history of DMD and SMA have been published [6–10, 12–14]. In contrast, this present study focuses on the relationships between hand strength and function.
Strength measurements
Patients were evaluated for handgrip and key pinch strength with the MyoGrip and MyoPinch dynamometers, respectively (Ateliers Laumonier, Nesles-la-Vallée, France), as previously described [6, 10, 12].
Strength was expressed in absolute value (kg) or as a percentage of the predicted value for healthy subjects of the same age (% pred). The strength predicted for age was calculated similarly to a previous study [15] with models developed from normative data aggregated at the Institute of Myology (n > 500).
Function measurements
Functional hand abilities were assessed using the MoviPlate [6] (ValoTec, Villejuif, France) and specific items of the MFM, a 4-point based RCS validated for use in NMD. The items, detailed in the MFM manual [4] (https://mfm-nmd.org/?lang=en), reflect daily activities [16]. Possible scores for each item are 0 (greatest disability), 1, 2, 3 (no disability). Among the 32 items, only the six items (17 to 22) concerning distal upper limb function were analyzed in the present study. MFM-D3-UL score refers here to the total score of these six items. It ranges from 0 (greatest disability) to 18 (no disability) when expressed in absolute value and can be expressed in percentage of the maximum score (no disability). Participants younger than 7-years old at baseline, performed the MFM-20, which includes 20 of the 32 items of the MFM-32 scale, adapted to children between 2 and 7 years old. Items 18, 21 and 22 are the only distal upper limb items performed in the MFM-20.
The Cochin Hand Function Scale (CHFS), also called the Duruöz Hand Index (DHI) [3], is a 6-point based SAQ assessing hand abilities in everyday life (without the use of assistive devices). Score 0 is “without difficulty”, while score 5 corresponds to “impossible” [1, 3]. The total score for the 18 items ranges from 0 (no disability) to 90 (greatest disability).
A brief description of the items of the MFM-D3-UL and the CHFS is provided in Supplemental Table 1.
Statistical analyses
IBM SPSS Statistics version 22 software and R 4.3.1 software (the R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
• Baseline results
The comparison of baseline data between participants with DMD and SMA was performed using a Mann-Whitney test with Bonferroni correction.
• Strength-function relationships
Taking into account the repetition of visits within patients, strength-function relationships were analyzed using a mixed model. Mixed models are statistical models containing both fixed effects and random effects. They are particularly useful in contexts where repeated measurements are made on the same statistical units (longitudinal studies), or where measurements are made on groups of related statistical units, and are valued for their advantage in dealing with missing values.
So, instead of only baseline measurements, data of all visits were analysed while taking into account the repetition of visits per patient so that each participant had equal weighting in the analysis. Only strength data from the dominant hand were considered since the MFM-D3-UL and CHFS items were performed on this side.
– Strength versus MFM-D3-UL/CHFS/MoviPlate scores relationships
For continuous variables, a mixed linear model was performed to determine both (i) whether the MFM-D3-UL score, the total CHFS score, and the MoviPlate score were significantly dependent on strength and (ii) whether participants with DMD and SMA performed functional tests differently at equal strength. Continuous variables were log-transformed when it was necessary to get linearity of the relationship.
This analysis of the overall MFM-D3-UL and CHFS scores was then extended by an analysis of each item making them up (see next paragraph).
– Strength versus items scores relationships
In order to identify the items, and therefore the tasks, which are particularly dependent on hand strength, and to examine whether this strength dependence is similar in the DMD and the SMA, the following analysis was carried out.
For the items of the MFM-D3-UL and CHFS scales, distributions of the scores were unbalanced: The frequency of some modalities was too small. Therefore, it was necessary to merge modalities according to the item score distribution.
For binary outcome variables, a mixed effect logistic regression was used both (i) to identify the items whose score was significantly dependent on strength and (ii) to determine the items whose score was significantly different between DMD and SMA patients. The following equation was used:
Logit (Item in two class|handgrip or key pinch strength, diagnosis)=β0 +β1*strength (% pred) +β2*1|SMA
Strength was expressed in % pred, diagnosis was equal to 0 for DMD or to 1 for SMA and β0, β1 and β2 were the coefficients for the intercept, the strength and the diagnosis, respectively. From the coefficients of the model, odds ratios (OR) were computed to determine whether SMA patients performed better or worse than DMD patients. OR > 1 means that SMA patients are significantly more likely than DMD patients to have an item score higher than the median, while OR < 1 means the opposite. Note that since the 0 score means “full disability” for the MFM items but “no disability” for the CHFS items, OR > 1 means a better performance in MFM items and a worse performance in CHFS items for the SMA patients compared to DMD patients. One model was performed for each of the 27 covariates (i.e. MFM-D3-UL scale and its 6 items, CHFS and its 18 items, MoviPlate). Bonferroni threshold was set at p = 0.05/27 = 0.0018 for each of the 27 models.
• Strength threshold delimiting normal and abnormal hand function
The strength threshold delimiting normal and abnormal hand function was estimated using Receiver-Operating Characteristic (ROC) curves, which are used to show the connection between sensibility and specificity of a test, calculated for all possible thresholds [17]. The optimal threshold was selected by the closest to (0,1) criteria on the ROC curve [18]. The lowest functional scores of healthy subjects were estimated from the mean–1.96*SD of the MFM-D3-UL and MoviPlate scores and from the mean+1.96*SD of the CHFS scores in order to delimit normal and abnormal hand function. The function thresholds used here were 97.13% (= 99.89–1.96*1.41) for MFM-D3-UL [19], 52.96 (= 80.6–1.96*14.1) for MoviPlate (n = 66 healthy subjects, unpublished data from the ULENAP study) and 2.45 (= 0.33 + 1.96*1.08) for CHFS [20]. Pairwise deletion technique was used to handle missing data.
RESULTS
Participants
A total of 168 patients (91 with DMD and 77 with SMA (60% type 2 and 40% type 3) were included. Pooling all their visits, they totaled 650 visits from which 57 (9%) were missing visits (no strength and function data were available). The remaining 593 visits (337 for DMD and 256 for SMA patients) were analyzed. The number of missing measurements of handgrip strength, key pinch strength, MFM-D3-UL or MoviPlate in the analyzed visits and the follow-up duration for each study are indicated in Supplemental Table 2. Each participant took part in one of the 3 studies (ULENAP, PreU7 or NatHis-SMA) with the exception of a single patient involved into 2 studies (1 Ulenap visit in 2012 and 5 NatHis-SMA follow-up visits between 2015 and 2018). Clinical features at baseline were previously published for the ULENAP [7, 8], preU7 [14] and the NatHis-SMA studies [9]. Table 1 summarizes baseline data in the present study where patients data from the ULENAP, the preU7 and the NatHis-SMA studies where pooled. The DMD and SMA groups had similar age, weight and height and exhibited similar handgrip and key pinch strength. Nevertheless, participants with SMA performed better than those with DMD in the following functions: Brooke score, MFM D1 and D3 sub scores. CHFS scores were not significantly different between the two populations at baseline.
Table 1
Baseline data of participants with DMD, SMA and pooled data
| DMD | SMA | Total (DMD and SMA) | P DMD vs SMA | ||||||||||
| n | mean | SD | median [min–max] | n | mean | SD | median [min–max] | n | mean | SD | median [min–max] | ||
| Number | 91 | 77 | 168 | ||||||||||
| Male number (proportion) | 91 (100%) | 49 (64%) | 140 (83%) | ||||||||||
| Age (years) | 91 | 14.9 | 4.9 | 14.2 [6.0–28.1] | 77 | 15.2 | 7.0 | 14.9 [6.0–31.0] | 168 | 15.0 | 6.0 | 14.3 [6.0–31.0] | 0.920 |
| Weight (kg) | 91 | 46.4 | 17.1 | 46.0 [20.0–94.0] | 75 | 41.1 | 19.5 | 38.0 [15.0–117.0] | 166 | 44.0 | 18.4 | 42.3 [15.0–117.0] | 0.031 |
| Height (cm) | 85 | 150.0 | 16.8 | 154.0 [111.0–176.0] | 74 | 144.6 | 20.7 | 149.5 [107.0–183.0] | 159 | 147.5 | 18.9 | 151.0 [107.0–183.0] | 0.096 |
| Brooke score | 86 | 3.8 | 1.6 | 5 [1–6] | 74 | 2.7 | 1.3 | 3 [1–6] | 160 | 3.3 | 1.6 | 3 [1–6] | <0.001 |
| Maximal strength | |||||||||||||
| Handgrip (kg) | 89 | 4.08 | 3.31 | 3.38 [0.09–18.14] | 73 | 4.75 | 6.21 | 1.61 [0.29–28.43] | 162 | 4.38 | 4.83 | 2.95 [0.09–28.43] | 0.199 |
| Key pinch (kg) | 91 | 1.40 | 0.99 | 1.18 [0.20–5.03] | 73 | 1.63 | 1.81 | 0.66 [0.60–6.928] | 164 | 1.50 | 1.42 | 1.08 [0.06–6.928] | 0.116 |
| Handgrip (% pred) | 89 | 20.48 | 19.98 | 13.60 [0.20–79.60] | 73 | 20.53 | 25.02 | 9.60 [1.60–106.20] | 162 | 20.50 | 22.32 | 11.45 [0.20–106.20] | 0.630 |
| Key pinch (% pred) | 91 | 26.23 | 20.65 | 20.60 [2.60–81.90] | 73 | 28.53 | 29.61 | 15.80 [0.90–112.30] | 164 | 27.25 | 24.98 | 18.50 [0.90–112.30] | 0.365 |
| MFM | |||||||||||||
| MFM Total (%) | 91 | 40.3 | 22.0 | 37.5 [2.1–92.7] | 77 | 48.5 | 21.9 | 42.7 [4.2–94.8] | 168 | 44.1 | 22.3 | 39.6 [2.1–94.8] | 0.011 |
| MFM D1 (%) | 91 | 11.0 | 23.1 | 0 [0–84.6] | 77 | 15.8 | 25.5 | 2.6 [0–87.2] | 168 | 13.2 | 24.3 | 2.6 [0–87.2] | 0.001 |
| MFM D2 (%) | 91 | 52.4 | 30.5 | 52.8 [0–100] | 77 | 62.9 | 28.8 | 58.3 [2.6–100] | 168 | 57.2 | 30.1 | 54.2 [0–100] | 0.030 |
| MFM D3 (%) | 91 | 73.7 | 19.7 | 81.0 [9.5–100] | 77 | 84.1 | 17.0 | 90.5 [14.3–100] | 168 | 78.5 | 19.2 | 82.2 [9.5–100] | <0.001 |
| Item 17 – pick up coins | 89 | 2.2 | 0.9 | 3 [0–3] | 69 | 2.3 | 0.8 | 3 [0–3] | 158 | 2.3 | 0.9 | 3 [0–3] | 0.459 |
| Item 18 – finger turns around CD | 90 | 2.4 | 0.9 | 3 [0–3] | 77 | 2.8 | 0.6 | 3 [0–3] | 167 | 2.6 | 0.8 | 3 [0–3] | 0.010 |
| Item 19 – writing loops | 89 | 2.2 | 0.7 | 2 [0–3] | 69 | 2.6 | 0.7 | 3 [1–3] | 158 | 2.4 | 0.7 | 2 [0–3] | <0.001 |
| Item 20 – tearing paper | 89 | 1.7 | 0.9 | 2 [0–3] | 69 | 2.0 | 0.9 | 2 [0–3] | 158 | 1.8 | 0.9 | 2 [0–3] | 0.045 |
| Item 21 – supination | 91 | 2.0 | 1.0 | 2 [0–3] | 77 | 2.4 | 0.8 | 3 [0–3] | 168 | 2.2 | 0.9 | 2 [0–3] | 0.001 |
| Item 22 – finger pointing on diagram | 91 | 2.9 | 0.4 | 3 [1–3] | 77 | 2.9 | 0.4 | 3 [0–3] | 168 | 2.9 | 0.4 | 3 [0–3] | 0.695 |
| MoviPlate | 87 | 46.1 | 13.7 | 44.0 [19–82] | 71 | 53.6 | 18.0 | 51.0 [14–95] | 158 | 49.5 | 16.1 | 48.0 [14–95] | 0.004 |
| CHFS | 36 | 47.2 | 28.6 | 47 [1–90] | 18 | 42.7 | 25.7 | 47.00 [2–90] | 54 | 46.2 | 27.5 | 47.0 [1–90] | 0.497 |
| Kitchen | 45 | 24.8 | 12.8 | 27 [0–40] | 20 | 23.6 | 12.2 | 26 [1–40] | 65 | 24.5 | 12.5 | 27 [0–40] | 0.653 |
| Item 1 – holding a bowl | 47 | 2.4 | 2.3 | 2 [0–5] | 23 | 1.8 | 1.9 | 1 [0–5] | 70 | 2.2 | 2.1 | 2 [0–5] | 0.360 |
| Item 2 – grasp and raise a full bottle | 47 | 4.0 | 1.7 | 5 [0–5] | 23 | 3.1 | 1.9 | 3 [0–5] | 70 | 3.7 | 1.8 | 5 [0–5] | 0.030 |
| Item 3 – holding a plate full of food | 47 | 3.9 | 1.9 | 5 [0–5] | 22 | 3.6 | 2.0 | 5 [0–5] | 69 | 3.8 | 1.9 | 5 [0–5] | 0.465 |
| Item 4 – pouring a liquid from a bottle into a glass | 47 | 3.4 | 2.1 | 5 [0–5] | 23 | 2.7 | 1.9 | 3 [0–5] | 70 | 3.2 | 2.1 | 4 [0–5] | 0.067 |
| Item 5 – unscrew the lid from a jar opened before | 46 | 2.8 | 2.1 | 3.5 [0–5] | 23 | 3.0 | 2.0 | 4 [0–5] | 69 | 2.9 | 2.1 | 4 [0–5] | 0.905 |
| Item 6 – cut meat with a knife | 48 | 3.2 | 2.1 | 5 [0–5] | 23 | 3.3 | 2.0 | 4 [0–5] | 71 | 3.2 | 2.1 | 5 [0–5] | 0.937 |
| Item 7 – prick things with a fork | 48 | 1.3 | 2.0 | 0 [0–5] | 23 | 0.8 | 1.5 | 0 [0–5] | 71 | 1.2 | 1.9 | 0 [0–5] | 0.553 |
| Item 8 – peal fruit | 46 | 3.9 | 1.9 | 5 [0–5] | 21 | 4.0 | 1.5 | 5 [0–5] | 67 | 3.9 | 1.8 | 5 [0–5] | 0.649 |
| Dressing | 44 | 6.3 | 4.1 | 8 [0–10] | 22 | 4.6 | 3.6 | 5 [0–10] | 66 | 5.7 | 4.0 | 6.5 [0–10] | 0.101 |
| Item 9 – button shirt | 45 | 3.2 | 2.3 | 5 [0–5] | 23 | 2.1 | 1.9 | 2 [0–5] | 68 | 2.8 | 2.2 | 3 [0–5] | 0.047 |
| Item 10 – open and close zip | 47 | 3.1 | 2.1 | 4 [0–5] | 22 | 2.5 | 1.9 | 2.5 [0–5] | 69 | 2.9 | 2.0 | 3 [0–5] | 0.175 |
| Hygiene | 46 | 4.4 | 4.2 | 4.5 [0–10] | 23 | 3.7 | 3.0 | 3 [0–10] | 69 | 4.2 | 3.8 | 4 [0–10] | 0.691 |
| Item 11 – squeeze a new toothpaste tube | 46 | 2.1 | 2.2 | 1 [0–5] | 22 | 2.5 | 1.8 | 3 [0–5] | 68 | 2.2 | 2.1 | 2 [0–5] | 0.436 |
| Item 12 – hold a toothbrush | 47 | 2.3 | 2.3 | 1 [0–5] | 23 | 1.3 | 1.6 | 1 [0–5] | 70 | 2.0 | 2.2 | 1 [0–5] | 0.172 |
| At the office | 48 | 1.5 | 2.9 | 0 [0–10] | 23 | 1.0 | 2.3 | 0 [0–10] | 71 | 1.4 | 2.8 | 0 [0–10] | 0.476 |
| Item 13 – write a sentence with a pen | 48 | 1.0 | 1.7 | 0 [0–5] | 23 | 0.6 | 1.2 | 0 [0–5] | 71 | 0.9 | 1.6 | 0 [0–5] | 0.409 |
| Item 14 – write a letter with a pen | 48 | 0.5 | 1.4 | 0 [0–5] | 23 | 0.4 | 1.1 | 0 [0–5] | 71 | 0.5 | 1.3 | 0 [0–5] | 0.985 |
| Other | 39 | 9.7 | 6.3 | 10 [0–20] | 21 | 9.3 | 6.1 | 10 [0–20] | 60 | 9.6 | 6.2 | 10 [0–20] | 0.864 |
| Item 15 – turn a round door knob | 40 | 3.6 | 2.1 | 5 [0–5] | 21 | 3.5 | 2.0 | 5 [0–5] | 61 | 3.6 | 2.1 | 5 [0–5] | 0.488 |
| Item 16 – cut a piece of paper with scissors | 47 | 1.5 | 2.1 | 0 [0–5] | 23 | 1.4 | 1.5 | 1 [0–5] | 70 | 1.4 | 1.9 | 0 [0–5] | 0.664 |
| Item 17 – pick up coins | 48 | 1.0 | 1.5 | 0 [0–5] | 23 | 1.0 | 1.4 | 0 [0–5] | 71 | 1.0 | 1.5 | 0 [0–5] | 0.994 |
| Item 18 – turn a key in a lock | 45 | 3.5 | 2.0 | 5 [0–5] | 22 | 3.5 | 2.0 | 5 [0–5] | 67 | 3.5 | 2.0 | 5 [0–5] | 1.000 |
SD. standard deviation; P for Mann-Whitney test. Bold values indicate significant differences between DMD and SMA patients taking into account the Bonferroni correction: P < (0.05/43 = 0.001).
Strength thresholds for hand dysfunction
The relationships between the MFM-D3-UL, the MoviPlate score, the CHFS score and the handgrip or key pinch strength are presented in Fig. 1. They are not linear as there is a function ceiling effect at the highest strengths. Above a given handgrip or key pinch strength, hand function was (almost) preserved. Below these handgrip and key pinch strength thresholds, hand function generally decreased with strength loss.
Fig. 1
Relationships between strength and function assessed using the MFM-D3-UL, MoviPlate and CHFS scores, and illustration of strength thresholds. Relationship between the MFM-D3-UL (Fig. 1A), MoviPlate (Fig. 1B), CHFS (Fig. 1C) scores and the handgrip (left panels) or key pinch (right panels) strength expressed in percentage of predicted strength in DMD (black symbols), SMA type 3 (light blue symbols) and SMA type 2 (red symbols). Panels a and b are the superposition of panels c, e and d, f, respectively. Only the non-ambulant subjects of the ULENAP study performed the CHFS (Fig. 1C). Data from all visits of each patient are pooled. Strength is expressed in % pred to avoid the confounding factor of age (the younger and older participants have low absolute strength, but their predicted strength is low or high, respectively). Function thresholds are displayed with dark blue lines and were defined as described in the Methods. Strength thresholds were determined by ROC analysis and are displayed with vertical lines for DMD (black), SMA type 3 (light blue), SMA type 2 (red) and SMA type 2 and 3 together (purple).
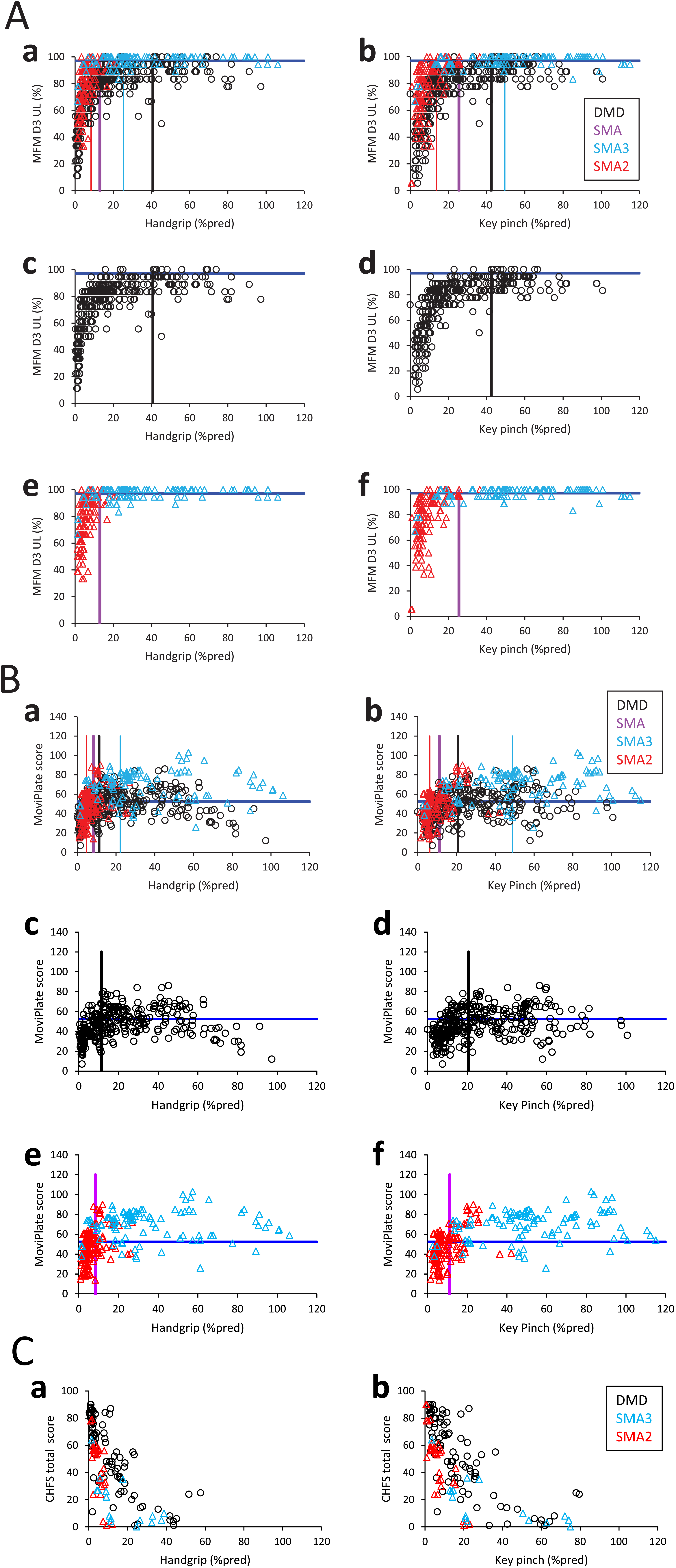
A ROC analysis estimated handgrip and key pinch strength thresholds delimiting normal and abnormal function, separately for DMD, SMA (types 2 and 3 together), SMA type 2 (SMA2) and SMA type 3 (SMA3). For MFM-D3-UL, the handgrip strength thresholds were determined to be 41 and 13 % pred and the key pinch strength thresholds were calculated to be 42 and 26 % pred for DMD and SMA, respectively (Fig. 1A). For the MoviPlate, the handgrip strength thresholds were 11 and 8 % pred and the key pinch strength thresholds 21 and 11 % pred for DMD and SMA, respectively (Fig. 1B).
Not enough CHFS data were available for the ROC analysis, in particular in the highest strength range, because this scale was only performed in non-ambulant patients from the ULENAP study (Fig. 1C).
Significance of the strength – function relationship
A highly significant relationship was found between handgrip or key pinch strength and the continuous variables, such as the MFM-D3-UL, the CHFS scores and the MoviPlate score (Table 2).
The relationship between the handgrip or key pinch strength and discrete variables such as the functional items scores was analyzed for each item of the MFM D3 UL and of the CHFS in Supplemental Table 3. The analysis revealed a significant relationship between handgrip or key pinch strength and the score of all the MFM D3 UL items, except item 19 (write) and 22 (place a finger on drawings), and all the CHFS items, except item 4 (pour liquid), 6 (cut meat), 7 (prick with a fork) and 14 (write). Picking up coins required handgrip strength in the MFM item 17 but not in the CHFS item 17, while key pinch strength was required for both items. Handgrip but not key pinch strength appeared necessary for CHFS items 15 (turn a door knob).
Difference in strength – function relationship between DMD and SMA patients
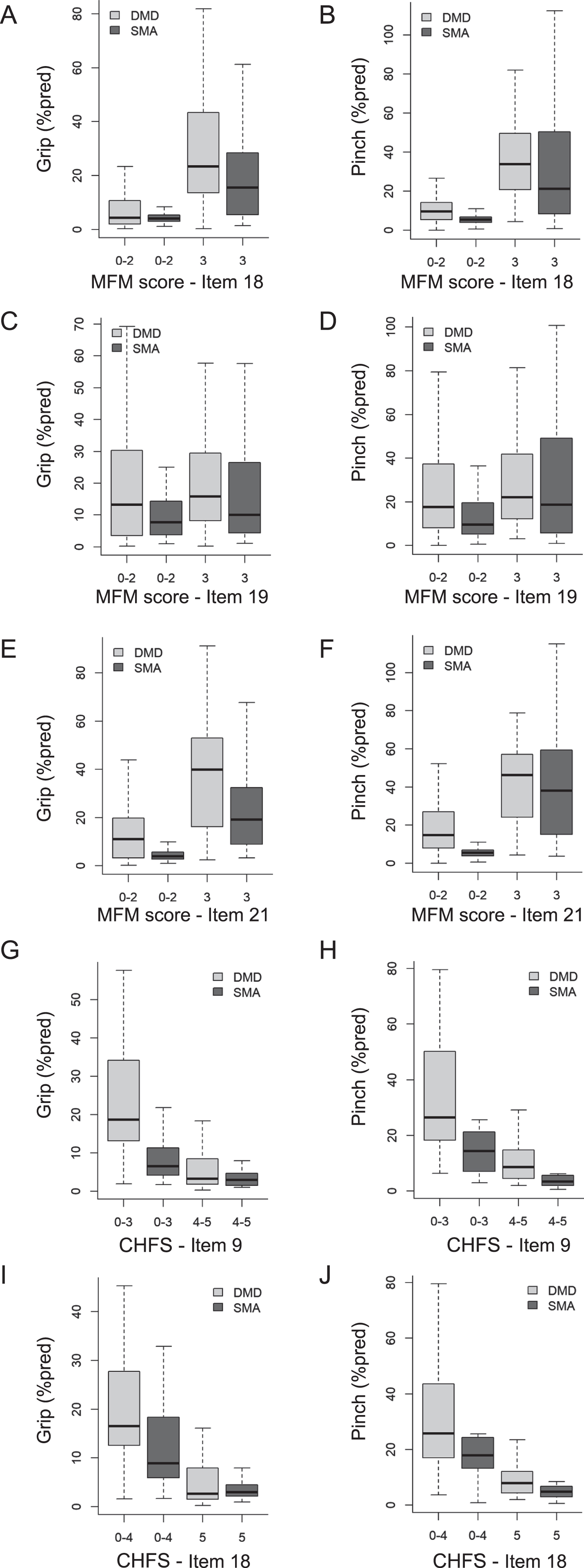
The mixed models also demonstrated that the log strength (% pred) – function relationships were different between the DMD and the SMA patients for the MFM-D3-UL, the CHFS and the MoviPlate scores (Table 2): Patients with SMA could obtain the same functional score as patients with DMD however with a lower maximum handgrip and key pinch strength. In other words, for the same strength (% pred), patients with SMA generally obtained better functional scores than patients with DMD on the MFM-D3-UL, the CHFS and the MoviPlate (Figs. 1 and 2). In particular, at equivalent strength, participants with SMA obtained better scores than individuals with DMD on MFM items 18 (sliding a finger around a CD), 19 (writing), 21 (picking up and turning over a tennis ball), as well as CHFS items 9 (buttoning a shirt) and 18 (turning a key, pinch strength only) (Suppl. Table 3).
Table 2
Mixed linear model for analysis of the strength – function relationships and of the difference in relationships between DMD and SMA patients
| Log handgrip strength (% pred) | Log Key pinch strength (% pred) | |||||||||||
| Log strength – Function relationship | SMA versus DMD | Log strength – Function relationship | SMA versus DMD | |||||||||
| Log strength | Log | Strength | SMA | SMA | SMA | Log strength | Log | Strength | SMA | SMA | SMA | |
| coefficient | strength SD | p-value | coefficient | SD | p-value | coefficient | strength SD | p-value | coefficient | SD | p-value | |
| MFM-D3-UL total score (%) | 10.42 | 1.42 | <0.001 | 13.58 | 1.82 | <0.001 | 12.76 | 0.89 | <0.001 | 14.28 | 2.07 | <0.001 |
| CHFS total score | –16.04 | 1.91 | <0.001 | –15.35 | 4.42 | <0.001 | –19.74 | 2.00 | <0.001 | –20.33 | 4.40 | <0.001 |
| MOVIPLATE score | 6.67 | 0.72 | <0.001 | 10.11 | 1.92 | <0.001 | 7.76 | 0.86 | <0.001 | 10.70 | 1.98 | <0.001 |
P-values < 0.0018 reflect statistical significance and are indicated in bold.
Fig. 2
Strength – item score relationships: Differences between DMD and SMA patients. Only the strength – item score relationships for which a significant difference was found between DMD and SMA are shown here, with grip strength on the left panels and key pinch strength on the right panels. Suppl Table 3 details the significance of the strength – item score relationships and the differences between the DMD and SMA groups. The items correspond to sliding a finger around a CD (A-B, MFM item 18), writing (C-D, MFM item 19), picking up and turning over a tennis ball (E-F, MFM item 21), buttoning a shirt (G-H, CHFS item 9) and turning a key (I-J, item CHFS 18). Data from all visits of each patient are pooled. Only the non-ambulant subjects of the ULENAP study performed the CHFS. Extremities of the lines extending from the boxes (whiskers) represent the minimum and maximum values. For all items in the MFM-D3-UL and CHFS scales, the modalities had to be merged (e.g. scores 0– 2, 0– 4, 3– 5) because the frequency of some modalities was too low for the analysis.
Strength loss associated with functional scores impairment
The relationship between handgrip or key pinch strength and functional scores was significant for most of the items of the MFM-D3-UL and the CHFS (significant β1 p-value in Supplemental Table 3). This means that the weaker the patients’ strength, the poorer the functional score. For the MFM-D3-UL and CHFS items, the distribution of strength by functional scores is presented in Fig. 2 (which shows only those items for which a significant difference was found between DMD and SMA) and in Fig. e-1 (full set of items). Some scores were merged when the number of participants per score was not sufficient. The force distribution shown for each functional score category can sometimes be wide, ranging for some items from ∼0 to ∼100 % pred. For most of the MFM-D3-UL items, the median strength was generally around 20 % pred for score 3 (no disability) and < 10 % pred for scores 2, 1 and 0 (greatest disability).
The strength distribution across the CHFS scores refers only to non-ambulant subjects (from the ULENAP study). As a result, some items did not have a sufficient number of patients with the score of 0 (no disability) and this modality had to be merged with the next score(s). For CHFS items with a sufficient number of patients with the score of 0 (no disability), the strength median was around 10 % pred for handgrip and 20 % pred for key pinch. For the scores 1 to 5 (greatest disability), the strength median was around 5 % pred for handgrip and key pinch.
DISCUSSION
This study demonstrates a significant relationship between handgrip or key pinch strength and hand function as assessed using the MFM-D3-UL, the CHFS and the MoviPlate, and documents the handgrip and key pinch strength distribution corresponding to the MFM-D3-UL and CHFS item scores. The strength threshold delineating normal and abnormal hand function is lower for SMA patients than for DMD patients.
To our knowledge, the relationship between hand strength and function has never been published before. Yet it is crucial for the choice of outcome measures in clinical practice and in trials. In fact, it shows that for participants with hand strength above the force threshold, only force measurements are worthwhile, because hand function remains preserved. When hand strength falls below the threshold, hand function gradually deteriorates and it becomes useful to assess this too, in particular in DMD and SMA type 2. The functional tasks significantly affected by a loss of strength are picking up and holding objects (MFM items 17, 21 and CHFS items 1, 2, 3, 12), using a touch screen or touchpad (MFM item 18), tearing a sheet of paper (MFM items 20), unscrewing (CHFS items 5), peeling (CHFS item 8), fine manipulation (CHFS items 9, 10), squeezing and pinching (CHFS item 11) as well as writing (CHFS item 13) (Suppl Table 1 and 3, [16]).
Surprisingly, based on the literature [9–11], it was unexpected that even the weakest participants with SMA type 3 had virtually no functional limitation of the hand, at least as assessed by MFM D3 UL, CHFS and MoviPlate. In clinical practice or trials involving participants with SMA type 3, it is therefore judicious to include measures of hand strength, but not necessarily of hand function.
In our analyses focusing on the hand, we did not apply a correction for the Brooke score, which was significantly different at baseline in DMD and SMA. This is because the Brooke score assesses the function of the entire upper limb, including the shoulder [21]. In addition, we compared hand function in the DMD and SMA groups at equal hand strength, expressed as a percentage of the predicted value for age. Finally, hand function was measured by the MFM D3 UL, CHFS and Moviplate tests, which generally assess distal motor skills without involving the shoulder (Suppl Table 1).
Patients with impaired hand function present with lower handgrip and key pinch strength than patients with normal hand function. However, those with normal or almost normal hand function demonstrate a wide range of handgrip and key pinch strengths, from low to high. Therefore, strength is far to be the sole determinant of hand function, and the different functional scores cannot be associated with specific levels of strength, probably because of the heterogeneity of individual compensation strategies.
Using ROC analysis we estimated strength thresholds delimiting normal and impaired hand function. Determining a strength threshold common to both SMA subtypes (SMA2 and SMA3 together) seems more relevant than a threshold specific to each subtype. As shown in Fig. 1A and B, almost no individual with SMA3 has an impaired MFM D3 UL or MoviPlate score, and none of the participants with SMA2 had high strength values. For each of the SMA subtypes, it is therefore not appropriate to determine a strength threshold when the function is fairly homogeneous in the group. On the other hand, there is a phenotypic continuum between SM3 and SM2. The strength threshold common to both SMA subtypes (SMA2 and SMA3 together) also roughly marks the distinction between SMA type 2 and type 3 (Fig. 1A and B).
Therapies for DMD or SMA that could maintain handgrip and key pinch strength above the threshold values would preserve hand function, at least as assessed using the MFM-D3-UL and the MoviPlate.
The European Medicines Agency (EMA) guideline on the clinical investigation of medicinal products for the treatment of Duchenne and Becker muscular dystrophy recommends the use of two endpoints selected from the domains of muscle strength and motor function. We have previously demonstrated that handgrip strength, as measured as % pred, decreases even in the early stages of disease [22], including in ambulant patients. This decrease does not yet translate into significant loss of function, as there is a clear ceiling effect on the upper limb scales in these patients [9, 23, 24]. The benefit of delaying strength loss at early stage translates into delaying the onset of hand loss of function. In advanced stages of the disease, when patients present with weakness lower than the strength threshold, a small reduction in handgrip and key pinch strength can be associated with large functional loss. Therapies capable of preventing even a slight loss of strength may therefore prove crucial in maintaining a functionalhand.
Despite significant relationships between hand strength and function, large variability in hand function is observed at each given strength, particularly below the strength threshold. It is also questionable whether each functional score of a given item has a corresponding range of strength and whether, as strength decreases, specific functional scores are achieved. Despite the significant relationship between strength and functional scores for some MFM D3 UL or CHFS items (Supplementary Table 3, Fig. 2), the distribution of strength corresponding to each MFM D3 UL or CHFS functional score can be very broad and often overlaps with that of the other functional scores (Fig. 2). For example, for an MFM item score equal to 3 (no disability) or a CHFS item score equal to 0 (no disability), there is a wide distribution of strengths, ranging for certain items from ∼0 to ∼100 % pred. This suggests that even some of the weakest patients are able to achieve optimal functional goals with their hand and that factors other than strength also contribute to hand function. Therefore, even below the strength threshold, hand performance cannot be predicted from only handgrip or key pinchstrength.
As suggested in a previous study of the lower limbs in SMA type 2 and 3, contractures may be involved in motor function [25]. The lower strength thresholds for SMA patients and the better hand functional performance at equal strength compared to DMD patients might be explained by a lower contracture index (% of subjects with contracture x Mean maximal loss of range in degrees/1000) at the elbow and wrist in SMA compared to DMD [26]. Patients with type 3 SMA are generally free of elbow and wrist contractures [27] and can often have an almost fully functional hand until very significant loss of strength (Fig. 1). Although we do not have the data to verify this, we nevertheless assume that with fewer contractures, SMA patients probably have a wider range of compensatory strategies for their muscle weakness. Based on questionnaires, no upper limb pain or stiffness difference was noticed between DMD and SMA patients [28]. Other factors such as dexterity, position sense, skin sensation, bimanual coordination, arm stability [29] and trunk control [30] contribute to upper limb and hand performance but remain to be investigated in these populations.
Despite the large number of patients included, this study suffers from certain limitations. (i) The three studies (ULENAP, PreU7, NatHis SMA) took place at different times and in most cases at different sites. The same trainers in dynamometric and functional assessments in the three studies may have limited this source of bias to some extent. (ii) The CHFS is not validated for NMD. Nevertheless, we did not only use the total score of the scale, but we also considered each item in order to define precise hand functions. (iii) Upper limb scales, such as the PUL [5] and the PROMs [2] for DMD or the RULM [31] for SMA, which were validated after the beginning of the three studies could not be included. However, only the PUL and RULM distal items would have been analyzed here, the majority of which resemble all of the MFM-D3-UL items except item 18. (iv) We did not quantify contractures through a standardized protocol in the different studies and were therefore unable to study their influence on the strength – function relationship. But referring to the literature, it is reasonable to assume that the poorer functional performance of the hand in DMD and SMA type 2 compared to SMA type 3 is related to the development of contractures that do not usually occur in SMA type 3 [26, 27]. (v) As the preU7 study only included DMD participants amenable to exon 53 skipping, the proportion of these severely affected patients [13, 32] is higher in our study compared to that in the DMD population. (vi) Our study establishes a link between hand strength and hand function, but it should be kept in mind that hand function may require stabilisation of the elbow and shoulder, and therefore a more proximal muscle contribution that is more or less important depending on the task. Weakness in the proximal muscles may therefore also have an impact on certain hand functions.
In conclusion, our study demonstrates a significant relationship between hand strength and function, with function decreasing below disease-specific strength threshold. By understanding the relationship between hand strength and function, our results highlights the importance of delaying the loss of hand strength. Whether handgrip and key pinch strength is above or below threshold, maintaining it through prophylactic and therapeutic interventions is crucial to keeping the hand as functional as possible, particularly in DMD and type 2 SMA.
For the clinician, it is therefore essential to monitor the evolution of maximum hand strength in order to prevent, where possible, and anticipate the functional repercussions of a loss of strength.
For clinical trials, knowledge of the relationship between hand strength and function and of the strength threshold should improve the homogenisation of participants and the selection of appropriate outcome measures: (i) If participants with hand strength above threshold and therefore little or no functional impairment were included, a therapeutic intervention would only have an impact on strength. (ii) Including participants with sub-threshold strength and therefore a progressive functional deficit, a therapeutic intervention should influence both hand strength and function. Thus, while hand strength measurement will be informative in all cases, functional measures will be relevant primarily in participants with sub-threshold hand strength. Our study makes it possible to consider functional tests of the hand only when the force becomes equal to or less than the threshold, alleviating evaluations of the hand in individuals stronger than the force threshold. Disease-specific handgrip and key pinch strength thresholds should be used since we have shown that they differ depending on the pathology.
ACKNOWLEDGMENTS
The authors are grateful to the healthy volunteers for normative data collection, as well as the patients and their families, and everyone involved in the ULENAP, PreU7 and NatHis SMA studies, particularly at the different sites in France (Institute of Myology in Paris for the three studies, Trousseau Hospital and Necker Hospital in Paris and Raymond Poincaré Hospital in Garches for the ULENAP study, University Hospital in Lille for both ULENAP and NatHis-SMA studies, University Hospitals in Nantes, Toulouse, Lyon and Strasbourg for NatHis-SMA study), in Belgium (University Hospital in Gent for the ULENAP study, CHR La Citadelle in Liège for both ULENAP and NatHis-SMA studies, and Gasthuisberg Hospital in Leuven for NatHis-SMA study), in the United Kingdom (Great Ormond Street Hospital in London for the preU7 study) and in Germany (University Hospital in Essen for NatHis-SMA study). They also acknowledge their colleagues at the Institut de Myologie: Charlotte Lilien for data entry and Simone Birnbaum for proof-reading of the manuscript.
The ULENAP and PreU7 projects were supported by the Association Française contre les Myopathies (AFM) and by the Advanced Diagnostics for New Therapeutic Approaches (ADNA), a program dedicated to personalized medicine, coordinated by Institut Mérieux and supported by research and innovation aid from the French public agency, OSEO. The NatHis-SMA study was co-funded by Institut Roche and Association Institut de Myologie.
CONFLICT OF INTEREST
V. Decostre reports no disclosure. M. De Antonio reports no disclosure. L. Servais is co-inventor of the MoviPlate. He has received consulting fees from Roche, Biogen, Novartis, Biohaven, Scholar Rock Pfizer, Sarepta, RegenexBio, Affinia, Dyne and Sysnav. He chairs the DSMB of Fibrogen and Lupin Therapeutics. J-Y. Hogrel is co-inventor of the MyoGrip, MyoPinch, and MoviPlate. He received consulting fees from Roche.
DATA AVAILABILITY STATEMENT
Anonymized data will be shared on request from any qualified investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with the European Union legislation on general data protection regulation.
SUPPLEMENTARY MATERIALS
The supplementary materials are available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230182.
Supplementary Figure: Strength – items score relationships. Strength – items score relationships are shown for handgrip (left panels) and key pinch (right panels) strength. Data from all visits for each patient are pooled. Only the non-ambulant subjects of the ULENAP study performed the CHFS. Extremities of the lines extending from the boxes (whiskers) represent the minimum and maximum values.
REFERENCES
[1] | Duruoz MT , Poiraudeau S , Fermanian J , Menkes CJ , Amor B , Dougados M , et al. Development and validation of a rheumatoid hand functional disability scale that assesses functional handicap. J Rheumatol. (1996) ;23: (7):1167–72. |
[2] | Klingels K , Mayhew AG , Mazzone ES , Duong T , Decostre V , Werlauff U , et al. Development of a patient-reported outcome measure for upper limb function in Duchenne muscular dystrophy: DMD Upper Limb PROM. Dev Med Child Neurol. (2017) ;59: (2):224–31. |
[3] | Poole JL . Measures of hand function: Arthritis Hand Function Test (AHFT), Australian Canadian Osteoarthritis Hand Index (AUSCAN), Cochin Hand Function Scale, Functional Index for Hand Osteoarthritis (FIHOA), Grip Ability Test (GAT), Jebsen Hand Function Test (JHFT), and Michigan Hand Outcomes Questionnaire (MHQ). Arthritis Care Res (Hoboken). (2011) ;63: (Suppl 11):S189–99. |
[4] | Berard C , Payan C , Hodgkinson I , Fermanian J . A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. (2005) ;15: (7):463–70. |
[5] | Mayhew A , Mazzone ES , Eagle M , Duong T , Ash M , Decostre V , et al. Development of the Performance of the Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. (2013) ;55: (11):1038–45. |
[6] | Servais L , Deconinck N , Moraux A , Benali M , Canal A , Van Parys F , et al. Innovative methods to assess upper limb strength and function in non-ambulant Duchenne patients. Neuromuscul Disord. (2013) ;23: (2):139–48. |
[7] | Seferian AM , Moraux A , Annoussamy M , Canal A , Decostre V , Diebate O , et al. Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne Muscular Dystrophy: An observational multicenter trial. PLoS One. (2015) ;10: (2):e0113999. |
[8] | Seferian AM , Moraux A , Canal A , Decostre V , Diebate O , Le Moing AG , et al. Upper limb evaluation and one-year follow up of non-ambulant patients with spinal muscular atrophy: An observational multicenter trial. PLoS One. (2015) ;10: (4):e0121799. |
[9] | Chabanon A , Seferian AM , Daron A , Pereon Y , Cances C , Vuillerot C , et al. Prospective and longitudinal natural history study of patients with Type 2 and 3 spinal muscular atrophy: Baseline data NatHis-SMA study. PLoS One. (2018) ;13: (7):e0201004. |
[10] | Annoussamy M , Seferian AM , Daron A , Pereon Y , Cances C , Vuillerot C , et al. Natural history of Type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann Clin Transl Neurol. (2020) . |
[11] | De Wel B , De Schaepdryver M , Poesen K , Claeys KG . Biochemical and clinical biomarkers in adult SMA 3-4 patients treated with nusinersen for 22 months. Ann Clin Transl Neurol. (2022) ;9: (8):1241–51. |
[12] | Hogrel JY , Wary C , Moraux A , Azzabou N , Decostre V , Ollivier G , et al. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology. (2016) ;86: (11):1022–30. |
[13] | Servais L , Montus M , Guiner CL , Ben Yaou R , Annoussamy M , Moraux A , et al. Non-Ambulant Duchenne Patients Theoretically Treatable by Exon 53 Skipping have Severe Phenotype. J Neuromuscul Dis. (2015) ;2: (3):269–79. |
[14] | Lilien C , Reyngoudt H , Seferian AM , Gidaro T , Annoussamy M , Che V , et al. Upper limb disease evolution in exon 53 skipping eligible patients with Duchenne muscular dystrophy. Ann Clin Transl Neurol. (2021) . |
[15] | Annoussamy M , Lilien C , Gidaro T , Gargaun E , Che V , Schara U , et al. X-linked myotubular myopathy: A prospective international natural history study. Neurology. (2019) ;92: (16):e1852–e67. |
[16] | Duong T , Braid J , Staunton H , Barriere A , Petridis F , Reithinger J , et al. Understanding the relationship between the 32-item motor function measure and daily activities from an individual with spinal muscular atrophy and their caregivers’ perspective: A two-part study. BMC Neurol. (2021) ;21: (1):143. |
[17] | Florkowski CM . Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin Biochem Rev. (2008) ;29: (Suppl 1):S83–7. |
[18] | Pepe MS . The statistical evaluation of medical tests for classification and prediction. Oxford: Oxford University Press; 2003. |
[19] | de Lattre C , Payan C , Vuillerot C , Rippert P , de Castro D , Berard C , et al. Motor function measure: Validation of a short form for young children with neuromuscular diseases. Arch Phys Med Rehabil. (2013) ;94: (11):2218–26. |
[20] | Yildirim MH , Yildirim EA , Carpar E , Coskun T , Ipekcioglu D , Canturk G . Hand functions in patients with schizophrenia: A clinical comparison with bipolar disorder and healthy subjects. Compr Psychiatry. (2018) ;87: , 53–8. |
[21] | Brooke MH , Griggs RC , Mendell JR , Fenichel GM , Shumate JB , Pellegrino RJ . Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. (1981) ;4: (3):186–97. |
[22] | Hogrel JY , Decostre V , Ledoux I , de Antonio M , Niks EH , de Groot I , et al. Normalized grip strength is a sensitive outcome measure through all stages of Duchenne muscular dystrophy. J Neurol. (2020) ;267: (7):2022–8. |
[23] | Pane M , Mazzone ES , Sivo S , Fanelli L , De Sanctis R , D’Amico A , et al. The 6 minute walk test and performance of upper limb in ambulant duchenne muscular dystrophy boys. PLoS Curr. (2014) ;6. |
[24] | Pera MC , Coratti G , Mazzone ES , Montes J , Scoto M , De Sanctis R , et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve. (2019) ;59: (4):426–30. |
[25] | Salazar R , Montes J , Dunaway Young S , McDermott MP , Martens W , Pasternak A , et al. Quantitative Evaluation of Lower Extremity Joint Contractures in Spinal Muscular Atrophy: Implications for Motor Function. Pediatr Phys Ther. (2018) ;30: (3):209–15. |
[26] | Johnson ER , Fowler WM Jr , Lieberman JS. Contractures in neuromuscular disease. Arch Phys Med Rehabil. (1992) ;73: (9):807–10. |
[27] | Wang HY , Ju YH , Chen SM , Lo SK , Jong YJ . Joint range of motion limitations in children and young adults with spinal muscular atrophy. Arch Phys Med Rehabil. (2004) ;85: (10):1689–93. |
[28] | Bergsma A , Janssen M , Geurts ACH , Cup EHC , de Groot IJM . Different profiles of upper limb function in four types of neuromuscular disorders. Neuromuscul Disord. (2017) ;27: (12):1115–22. |
[29] | Ingram LA , Butler AA , Walsh LD , Brodie MA , Lord SR , Gandevia SC . The upper limb Physiological Profile Assessment: Description, reliability, normative values and criterion validity. PLoS One. (2019) ;14: (6):e0218553. |
[30] | Bulut N , Alemdaroglu-Gurbuz I , Topaloglu H , Yilmaz O , Karaduman A . The association between trunk control and upper limb functions of children with Duchenne muscular dystrophy. Physiother Theory Pract. (2020) :1–9. |
[31] | Davoli GBQ , Cardoso J , Silva GC , Moreira RFC , Mattiello-Sverzut AC . Instruments to assess upper-limb function in children and adolescents with neuromuscular diseases: A systematic review. Dev Med Child Neurol. (2021) . |
[32] | Brogna C , Pane M , Coratti G , D’Amico A , Pegoraro E , Bello L , et al. Upper limb changes in DMD patients amenable to skipping exons 44, 45, 51 and 53: A 24-month study. Children (Basel). (2023) ;10(4). |




