DySMA – an Instrument to Monitor Swallowing Function in Children with Spinal Muscular Atrophy ages 0 to 24 Months: Development, Consensus, and Pilot Testing
Abstract
Background:
The manifestation of bulbar symptoms, especially swallowing, is important for evaluating disease-modifying therapies for spinal muscular atrophy (SMA). Due to the lack of instruments, the topic is still underrepresented in research.
Objective:
This study aimed to develop a tool to monitor swallowing development in children aged 0 to 24 months with SMA.
Methods:
The method was guided by the COSMIN guidelines and followed a multi-stage Delphi process. The first step was a rapid review of swallowing outcomes in children with SMA younger than 24 months. In the second step, online group interviews with experts (n = 7) on dysphagia in infants were conducted, followed by an anonymous online survey among experts in infants with SMA (n = 19). A predefined consensus threshold for nominal scaled voting was set at≥75 % and for 5-point Likert scale voting at 1.25 of the interquartile range. The third step was the pilot test of the instrument, performed with three groups (healthy controls n = 8; pre-symptomatic n = 6, symptomatic n = 6).
Results:
Based on the multi-level interprofessional consensus, the DySMA comprises two parts (history and examination), ten categories, with 36 items. Implementation and scoring are clearly articulated and easy to implement. The pilot test showed that swallowing development could be recorded in all groups.
Conclusion:
The DySMA is well suited for monitoring swallowing development in pre-symptomatic and symptomatic treated infants with SMA. It can be performed in a time-efficient and interprofessional manner. The resulting score is comparable to results from other instruments measuring other domains, e.g., motor function.
INTRODUCTION
Spinal muscular atrophy (SMA) is a rare neurodegenerative disease affecting the lower motor neuron [1]. The most severe form (formally clinically classified as type 1) manifests its symptoms in the first months of life and, if untreated, leads to severe muscle wasting, dysphagia, respiratory failure, and ultimately death [2–4]. Close monitoring of swallowing ability is required due to the rapid death of motor neurons, which can lead to a loss of swallowing function within a few weeks [5, 6]. SMA is caused by a deletion of the survival motor neuron (SMN1) gene and a resulting decreased SMN protein production. The time of onset and severity are influenced by the number of copies [1–6] of the SMN2 gene [7, 8].
The introduction of newborn screening on SMA and the approval of three gene-modifying or gene-replacement therapies (nusinersen, onasemnogene, and risdiplam) have necessitated a rethinking of research and clinical care as new phenotypes emerge [3].
Manifestation of bulbar symptoms, particularly swallowing, will be important for future therapy evaluation. Increasing research interest can be observed. Since swallowing is a complex process and there is a general lack of valid instruments to assess swallowing function in children [9, 10], selecting appropriate measuring instruments is challenging.
The first studies systematically investigating swallowing in treated children under 24 months of age were published. Van der Heul et al. [5] studied a prospective cohort of five nusinersen-treated children with type 1 SMA using Clinical Swallowing Examination (CSE) and videofluoroscopic swallowing study (VFSS) up to ten months after the start of therapy. Two of the children were clinically pre-symptomatic, and three were already symptomatic. Initially, an improvement or maintenance of swallowing function was observed. However, swallowing deteriorated in all children between the ages of eight and 12 months.
Weststrate et al. [8] retrospectively examined 24 symptomatic children with type 1 SMA up to 24 months after initiation of nusinersen treatment using the Pediatric Functional Intake Scale (p-FOIS) [6]. Despite improvement in motor function, oral intake status could not be maintained.
In a pilot study of ten SMA patients with 2 SMN2 copies treated with nusinersen, onasemnogene, or risdiplam after symptom onset, Zang et al. [11] could not demonstrate significant improvement in swallowing function by flexible-endoscopic evaluation of swallowing (FEES) approximately six months after the start of therapy.
Berti and colleagues [12] followed a cohort of 20 symptomatic nusinersen-treated children for up to 62 months using the Oral and Swallowing Abilities Tool (OrSAT) [13]. They demonstrated a modest improvement in oral and swallowing abilities, particularly in children who did not show marked swallowing dysfunction before therapy initiation.
A larger retrospective study by McGrattan et al. [14], which retrospectively evaluated children treated early with onasemnogene from three study cohorts, showed that in the first 18 months after the start of therapy, 60 of 65 children were fully orally fed and had no significant swallowing disorders.
Concerning the measuring methods used in these studies, it should be emphasized that instrumental swallowing examinations and oral intake scales cannot capture and record the physiological development of swallowing. They cannot represent a normal development course and are not sensitive to small changes. For example, an exclusively breastfed newborn will score the same on an oral intake scale as a 24-month-old toddler who eats solid food but has undergone complex development to do so. The same applies to the instrumental swallowing examination; here, the healthy newborn will obtain the same result as the two-year-old child, although this child has developed tremendously from infancy.
A recently published international consensus on bulbar function in SMA [15] was an essential step toward interprofessional applicable assessment development and led to an item bank for further development. It has yet to be published which items are appropriate, especially for children younger than two years. Still, the classification of an instrument for children 0 to 24 months of age was deemed worthwhile. It is necessary to closely monitor the course of swallowing in children with SMA, even in children treated very early and pre-symptomatically [3, 5, 6, 11]. For this, the development of a change-sensitive instrument is crucial.
This study aimed to develop the monitoring tool DySMA (Dysphagia in Children with Spinal Muscular Atrophy). The construct to be measured by the tool was defined as the monitoring of swallowing development (physiological and pathological). The target population were infants and toddlers with SMA aged 0 to 24 months. Interprofessional feasibility by speech-language pathologists (SLP), physical therapists (PT), and neuropediatricians (NP) was intended. As an outcome, swallowing development should be expressed in a point value comparable to motor function tests such as the Children’s Hospital of Philadelphia Infant Test (CHOP-INTEND [16]). Further requirements for the tool were an objective, short, and frequently repeatable implementation with a low burden on the children and their families.
The tool did not intend to replace a detailed CSE or instrumental swallowing examination. Neither the severity of dysphagia nor therapy goals should be derived from the tool. Furthermore, it was not intended to develop a screening to indicate an aspiration risk or the need for differentiated swallowing diagnostics.
METHODS
The development of the DySMA instrument is part of the identically named study and consists of a multi-stage Delphi process (Fig. 1). The local ethics committee approved the study (2022-100827_2-BO-ff). The Study is registered in the German Clinical Trials Register (DRKS00029541, German WHO primary registry).
Fig. 1
Modified multi-stage Delphi process of the DySMA development; FS = formulation session; CD = cognitive debriefing; sym. = symptomatic.
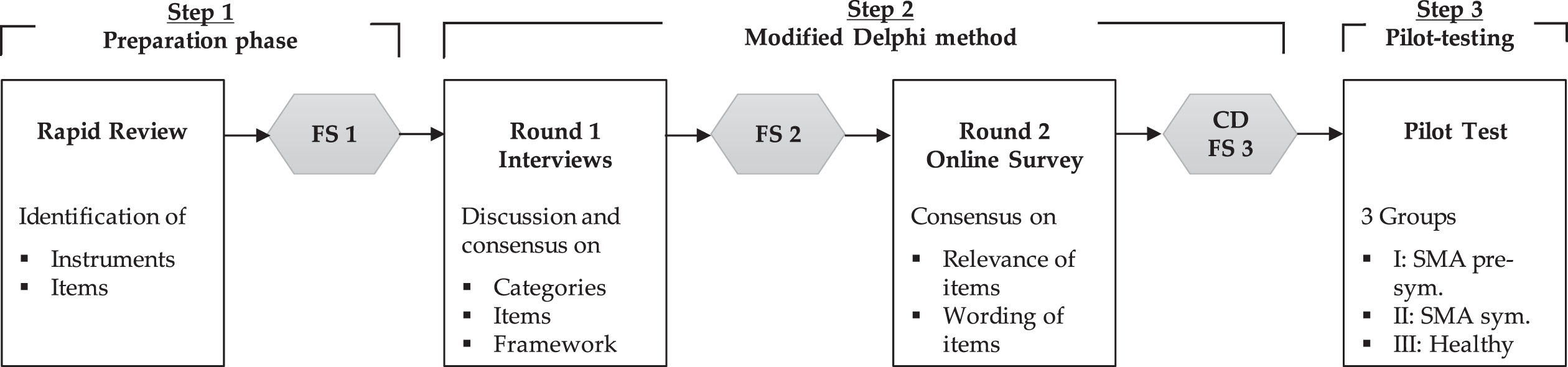
The method was guided by the “COSMIN criteria and rating system for evaluating the content validity of PROMs” [17]. All voting on relevance and wording refer to the German version of the DySMA. The categories and items illustrated here have been translated into English for publication.
Step 1: Rapid Review and preparation
As a first step, we searched for original articles reporting clinical items for swallowing in SMA type 1 children (or with predominantly 2 SMN2 copies) younger than 24 months. These could be either clinical instruments, parts of clinical instruments, or single items reported as outcomes. The exact inclusion and exclusion criteria are listed in Appendix 1.
The Medline and CINAHL databases were searched from inception to August 2022 for original research published in English using medical subject headings (MeSH) and key terms from “infant”, “spinal muscular atrophy”, and “swallowing disorder”. This search resulted in 229 publications. Additionally, six records were identified through other sources. After removing duplicates, 223 records were screened, and finally, six full texts were included in the qualitative synthesis. In the case of multiple studies by the same authors using the same instrument, only one publication was included in the analysis (see Appendix 1 for the PRISMA flow diagram of the reviewing process [18]).
We developed an initial item pool based on the items identified in the rapid review and preliminary studies of the working group [11, 19]. The exact wording of the items and categories for bundling items were determined in formulation session 1 (FS1). Two SLPs, one physician (MD), and two psychologists from the working group attended the meeting, and the initial item pool was generated.
Step 2: Modified Delphi method
A multi-stage modified Delphi process consisted of several formulation sessions, online group interviews, and an anonymous online survey (see Fig. 1).
Interviews
The experts for the interviews were recruited in Germany via mailing lists of pediatric dysphagia networks and by e-mail using the snowball system. The goal was to recruit ten participants. Due to the serious lack of experience with this patient group, the search was adapted and expanded to include experts who had no explicit experience with SMA but with infant dysphagia and extended to Switzerland.
Eligibility was also conditional on being an SLP (or equivalent) and having self-reported expertise in dysphagia in children younger than two years.
We conducted semi-standardized interviews via the Zoom video platform using an interview guide. All participants provided written informed consent. The interviews were audiotaped.
Two SLPs with expertise in both the construct (swallowing development in children) and the target population (young children with SMA) conducted the interviews. Neither was eligible to vote.
The interviews aimed to discuss and reach a consensus on the relevance of the preformulated categories and items for the construct and the target group. In addition, the participants could add items that had not been previously recorded. Approval or rejection was nominally scaled (yes/no/not sure).
Qualitative data, such as discussions or additional suggested items, were transcribed with simple coding and included in the following formulation session (FS2). Categories and items were excluded, added, or reformulated, resulting in a new pool.
Online Survey
In the following round, an online survey was created using Lime Survey 5.x, hosted at Hamburg University (https://www.rrz.uni-hamburg.de/services/software/alphabetisch/limesurvey\!.\!html). The questionnaire aimed to generate consensus on the categories and items of the DySMA in terms of their relevance to the planned instrument using a 5-point Likert scale (1 = disagree; 5 = fully agree). In addition, the wording was assessed on a nominal scale (yes/no), including an open text box. Demographic data on occupation, work location, and years of experience were collected. An SLP and an MD pretested the function and completeness of the online survey.
SLPs, PTs, and NPs with experience diagnosing children with SMA (<24 months) were invited to participate in the survey. Only members of these professional groups with relevant professional experience could participate. Participants were recruited via e-mails to neuromuscular centers treating SMA patients and professional association mailing lists. Participation was anonymous. It was not known in advance how many potential participants with appropriate qualifications there were. According to COSMIN criteria [17], a minimum of 30 participants would have been desirable. The participants from the previous interviews were all invited to participate again, so the questionnaire also served as a summary and feedback on the results of the interviews.
After the final analysis of the online questionnaire, another formulation session (FS3) was conducted, and the framework of the tool, including the scoring, was determined. Practicability was discussed with two PTs and two NPs who had tested the DySMA in their clinical practice with the target group (cognitive debriefing). They provided feedback on the feasibility of individual items and the practicality of the instrument, resulting in another adjustment to the questionnaire.
Consensus definition and data analysis
All type of data was tabulated in Excel (2016, Microsoft) and exported for further analysis to SPSS (Version 27, IBM). Demographic data, frequencies, and percentages were analyzed descriptively.
The consensus threshold for nominal scaled voting was set at≥75% [20]. Concerning the 5-point Likert scale, a predefined threshold of 1.25 of the interquartile range (IQR) was set for consensus, corresponding to 75% [21].
Step 3: Pilot testing of the DySMA
The pilot trial was conducted in a single neuropediatric center treating SMA by two SLPs with three groups of children. Group I included eight healthy controls from the ongoing DySMA norm study. The children were recruited between April and June 2023 through playgroups, maternity clinics, and daycare centers in Hamburg, Germany. Inclusion criteria were birth after 37 weeks of gestation, unremarkable development, no known chronic illnesses according to routine pediatric check-ups, and an apparent absence of infection on the examination day. Group II is part of the ongoing DySMA study and includes six infants with SMA who received early and clinically pre-symptomatic therapy. Group III consists of six infants and toddlers with symptomatic SMA who received treatment. These are four children from the DySMA study and two from the CHILDYS-RD study, for which data have been previously published [11]. Inclusion criteria were genetically proven 5q-associated spinal muscular atrophy with two SMN2 copies and therapy with one of the three currently approved treatments. All parents provided written informed consent.
Due to different study designs, the measurement time points varied: children from the DySMA study had up to five data collection points, children from the CHILDYS-RD study had two, and healthy controls had one.
The implementation of the DySMA consists of a patient history section and an examination section (see Appendix 3). An SLP assessed the DySMA for all children, and the OrSAT [13] was used as a comparison tool. The instrument was chosen because it is most similar to the DySMA in terms of the aim, the construct measured, and the scoring. The Neuromuscular Disease Swallowing Status Scale NdSSS [22] and the p-FOIS [6] were also captured. Although a CSE was also performed in symptomatic children, it is not reported as a comparative outcome due to the lack of standardization [9].
RESULTS
Step 1: Rapid Review and preparation of the item pool
After evaluating the six included studies, two oral intake scales (NdSSS [22, 23] and p-FOIS [6]), and one oral and swallowing abilities instrument (OrSAT) [13] were identified. One instrument, the HINE [24], includes an item on sucking/swallowing, as well as the Floppy Infant Module [25]. A total of nine outcome parameters could be identified in the included publications and were reported in the results as part of the instruments used: oral intake/ type of nutrition [6, 13, 23], tongue fasciculation [23, 26], difficulty managing secretions [23], weak sucking [5, 23, 26, 27], weak voice [5, 23], ineffective cough [5], wet voice/breathing [5], coughing while feeding [5, 13], fatigue while feeding [5, 13].
Based on the clinical experience of the research group and two preliminary studies [11, 19], additional items were added on gastrointestinal problems like vomiting and reflux, compensation while feeding, and eating behavior. These studies were published after the rapid review search period and, therefore, could not be included in the review.
From a clinical perspective, the questionnaire needed to be short and also usable by non-specialists in dysphagia. This resulted in a rather small item pool. After the first formulation session (FS1), 36 unique items classified into the following ten categories were included in the Delphi process: 1) type of nutrition, 2) compensation, 3) secretion management, 4) choking, 5) eating behavior, 6) fatigue, 7) gastrointestinal problems, 8) strength (voice and cough), 9) non-nutritive sucking and, 10) atrophy (see Appendix 2 for detailed item list/detailed process). A nominal rating system (yes/no) was adopted for the items of the tool.
Step 2: Modified Delphi method
Interviews
Nine SLPs were invited for the interviews; two could not participate due to time constraints, so seven experts participated. Five were from Germany, two from Switzerland, and all participants were female. Four SLPs had prior experience with SMA (Mdn 3,5 years; range 2–5). The average experience with swallowing disorders in infants and toddlers of all participants was 20 years (range 15–24 years).
In a discussion of the tool’s framework, there was agreement that the proposed instrument does not require observation of food intake for its intended purpose since it does not replace a CSE and must be able to be used quickly by non-SLPs with no experience in dysphagia (100%).
Voting on the planned categories of the instrument went as follows: the non-nutritive sucking category (NNS), including the three items it contained, was excluded. Broad consensus existed among the experts that no conclusions about nutritive sucking could be drawn from the NNS (0%). For example, non-nutritive sucking also depends on the current state (hungry, sleepy, etc.), and some children generally refuse a pacifier. A brand new jaw strength and stability category was added, which includes four items biting, chewing, drinking from a cup, and drinking from a straw. One item paradoxical breathing was added to the strength category, and the category was renamed. No clear consensus was found for the eating behavior (75%) and atrophy (50%) categories. It was consented that they retained their relevance to the instrument by excluding and adding individual items and renaming the categories. As a result, two items were excluded from the eating behavior category (eats too little and skips meals), and one was added (parental concern about food intake). Two unique items were added to the atrophy category (maximum jaw opening and open mouth breathing), and the category was renamed (see Appendix 2 for detailed information about construct and target group consensus).
A total of 39 items in ten categories emerged from FS2 after evaluating the interviews. They were included in the following step of the Delphi process. It should be noted that the category type of nutrition contains an oral intake scale consisting of eight items that cannot be scored individually. The following vote, therefore, refers to 31 individual items (see Appendix 2).
Online survey
Due to the acquisition via large mailing lists, it is unclear how many potential participants were initially reached. A total of 28 participants attempted to complete the questionnaire. Of these, 23 met the inclusion criteria. Finally, 19 questionnaires were filled in completely and used for analysis. Six participants had also taken part in the interviews in the first round.
Of the 19 participants, eight were SLPs with a median experience of three years (range 0–19 years) for the target group. Seven participants were PTs with a median experience of 28 years (range 6–32 years). The remaining four were NPs with a median experience in children with SMA younger than 24 months of 11 years (range 7–25 years).
Most participants worked in centers or hospitals with a neuropediatric focus (n = 13), three were employed in a clinic with a different focus, and three worked in an outpatient practice.
Consensus on relevance
Nine of the ten categories were deemed relevant and did not exceed the IQR threshold value of 1.25. The category gastrointestinal problems crossed the threshold with an IQR of 2 and were discussed in the following FS3 (see Appendix 2).
Of 31 items, a clear consensus on relevance was found for 26 items (IQR < 1.25) (see Appendix 2). Four items were finally excluded after discussion in the last FS and after a cognitive debriefing (CD) with users. Appendix 2 shows that the items bites of food, noticeably high/narrow palate, vomiting, and extremely picky eating habits were retained even though the threshold was exceeded. The application-related CD showed that parents frequently reported these parameters and that they represented a typical symptom for the target group. In addition, after discussion, the items requires many small meals, eats very slowly/takes a long time for a meal, falls asleep while eating, and suspected reflux were excluded because they overlapped with other items and could not be clearly scored in the application.
Consensus on wording
Consensus was reached on the wording for six categories; there was also minimal rephrasing for two items. Four categories were reworded because the 75% consensus threshold was not reached (see Appendix 2). Comments on the rewording in the open text field concerned the changing, omitting, or adding of individual words, which are not listed here in detail.
Framework
Regarding the framework, it was decided in the last FS that categories 2-6 are automatically scored as 0 if oral nutrition is no longer possible (type of nutrition≤1). This way, ambiguities in scoring during cognitive debriefing are eliminated (see Appendix 3 DySMA). Ambiguities in assessing children who were not fed orally also occurred in earlier prototypes and the implementation of the OrSAT, so this point was important to users.
The final pilot version of the DySMA consists of two parts (patient history and examination), ten categories (see Appendix 2), and 36 items. In the first Delphi round, there was consensus that the tool does not require direct observation of food intake (see Appendix 2). For this reason, all items related directly to food intake and the secretion management category were finally assigned to the patient history. All items that are considered non-nutritive were assigned to the examination section. These also require evaluation by the examiner for accurate assessment (e.g., whether the jaw opening is limited or if there is significant paradoxical breathing). A score between 0 and 35 points can be achieved; the higher the score, the more advanced the swallowing development (see Appendix 3).
Step 3: Pilot-testing of the DySMA
The characteristics of the three pilot groups are shown in Table 1. In groups I and II, there were more girls than boys overall. Further detailed information, such as the CHOP-INTEND [16] and long-term trajectories, has been published elsewhere [11, 19] or will be published after the completion of the DySMA study proper.
Table 1
Sample characteristics (N = 20)
| Group I (n = 8) | Group II (n = 6) | Group III (n = 6) | |
| healthy controls | SMA pre-symptomatic | SMA symptomatic | |
| Sex F:M | 5 : 3 | 5 : 1 | 3 : 3 |
| SMN2 copy number | – | 2 (100%) | 2 (100%) |
| Median age at start SMN modifying medication | – | 32.5 (26–38) | 81 (22–267) |
| Age range */Median age at first examination | 39–715 * | 31 (22–37) | 78,5 (12–234) |
| Type of medication | |||
| onasemnogene | – | 5 | 4 |
| nusinersen | – | 0 | 1 |
| onasemnogene + nusinersen | – | 1 | 0 |
| risdiplam + onasemnogene | – | 0 | 1 |
| Nutrition at inclusion | |||
| Oral | 6 | 6 | 4 |
| Tube feeding | 0 | 0 | 2 |
| Median DySMA (0–35) score at inclusion | 31 (23–35) * | 26.5 (25–27) | 17 (13–20) |
| Median OrSAT (0–12) score at inclusion | 10,5 (5–12) * | 7 (4–7) | 3.5 (1–5) |
| Median NdSSS (1–8) score at inclusion | 8 (8–8) * | 8 (8–8) | 8 (3–8) |
| Median p-FOIS (1–6) score at inclusion | 6 (6–6) * | 6 (5–6) | 5 (2–6) |
Note. All ages in days; median with range (in brackets); *These scores represent a range of all healthy controls aged 0–24 months, which were only examined once in contrast to the SMA sample.
Figure 2 shows the DySMA total scores of the three samples in the top row, with the OrSAT measurements below. It should be noted that, strictly speaking, no progress is shown for healthy controls, but only separate measurements of individual infants and toddlers of different ages are shown.
Fig. 2
Graphic representation of the development of swallowing measured with the DySMA (a-c) and the OrSAT (d-f); a; d) in a healthy control sample; each point represents one case, b; e) in an SMA sample, the children were clinically pre-symptomatic before the start of therapy, c; f) in an SMA sample, the children were symptomatic before the start of treatment, the lines represent the course of a case.
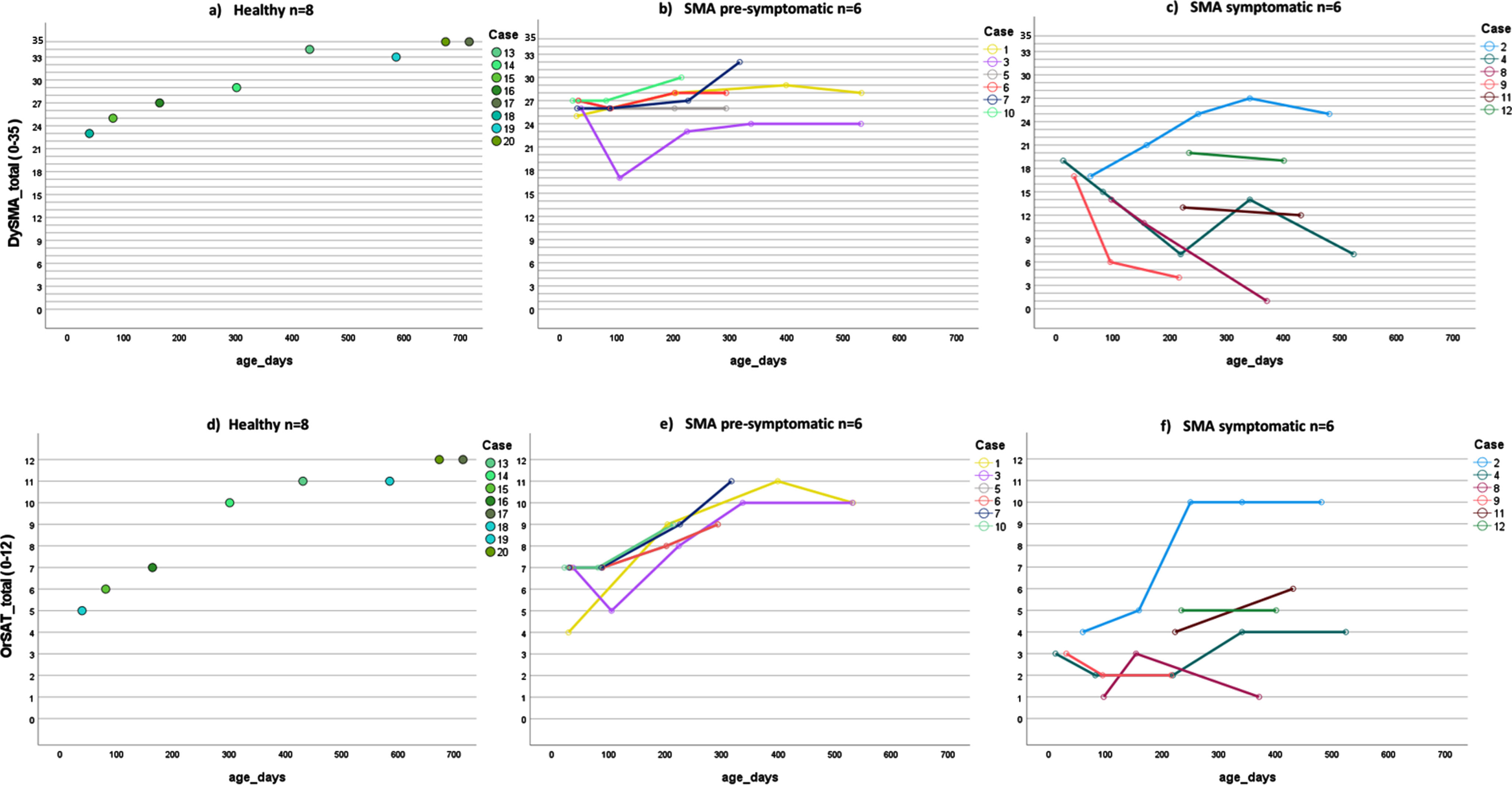
The DySMA score of healthy controls ranges from 23 to 35 (35 is the maximum score), and the OrSAT value ranges from 5 to 12 (12 is the maximum score); for both tools, the maximum value is reached from 22 months. For all children, the oral intake scales remained at the maximum value.
In pre-symptomatic children, the DySMA ranges from 17 to 32 and the OrSAT from 4 to 11. None of these children reached the maximum value. However, it should be noted that the maximum age in this group is 17 months, not 22 months like in the healthy control group. The NdSSS ranged from 7 to 8, and the p-FOIS from 4 to 6.
The symptomatic sample had a DySMA range from 1 to 27 and an OrSAT range of 1 to 10. The NdSSS ranged from 1 to 8, and the p-FOIS from 1 to 6. As in the pre-symptomatic sample, the maximum age was 17 months.
It is evident that the development of swallowing is more pathological in the symptomatically treated group than in the other groups. The trajectories in the healthy controls and the pre-symptomatically treated children could be similarly represented with the DySMA and the OrSAT. However, the OrSAT trajectory was somewhat steeper due to the distribution of scores. The symptomatic group started with a lower OrSAT score and stagnated or improved over time, whereas the DySMA tended to worsen (Fig. 2).
DISCUSSION
This study aimed to develop, reach consensus on, and pilot the DySMA instrument for monitoring swallowing development in infants and toddlers with SMA younger than 24 months. A multi-level interprofessional consensus indicated that the DySMA items are relevant to the construct of swallowing development and the target population of children with SMA and that the items are worded comprehensibly. The pilot test showed that the tool works properly and can be applied easily. Swallowing development and differences between the groups were established.
Compared to the OrSAT [13], conducted simultaneously, the instruments show that the trajectories were similar with some exceptions. Comparable to the studies by van der Heul and colleagues [5] and Weststrate et al. [6], the swallowing development of the children treated only symptomatically was unsatisfactory. Analogous to the results recently published by McGrattan and colleagues [14] in children treated very early, swallowing development in the presymptomatically treated group was predominantly good during the first 17 months of life.
It is undisputed that the treatment success of disease-modifying therapies in SMA patients will eventually have to be measured by swallowing outcome, in addition to other parameters. Due to the changing phenotype [1, 3] and infants treated very early, a change-sensitive instrument that can also reflect positive development is urgently needed.
The OrSAT [13] is a promising tool for this target group (children with SMA < 24 months). However, in addition to the construct of swallowing, this instrument also measures the production of syllables and words, which is explicitly not the goal of the DySMA. When applying the OrSAT in everyday clinical practice, the research group encountered uncertainties in the assessment. These are: the scoring for a non-orally fed child remains unclear, items concerning tiring, completing a meal, and duration of a meal overlap, and children may score higher simply by moving up an age category. It is, therefore, possible for the score to change from one day to the next, even with otherwise identical results.
In principle, in our opinion, it is not useful to assess several aspects of bulbar symptomatology with one instrument. Language and phonetic disorders probably occur with a frequency similar to typically developing children in the target group. Speech and articulation should, therefore, be surveyed separately because, like swallowing, they are complex phenomena. For this reason, speech-language pathology will play an essential role in interprofessional research teams in the future. The work of Dunaway Young and colleagues [15], with a large pool of items, provides a good basis to assess bulbar symptoms in SMA as broadly as possible in different age groups. This could lead to different instruments in the future that capture these different constructs individually.
In this study, we demonstrated that oral intake scales (NdSSS [22]; p-FOIS [6]) are not sufficient to monitor swallowing development in a new phenotype (pre-symptomatic treatment) of SMA. Although the oral intake scales can illustrate the steps between oral and parenteral feeding and show differences between groups, they cannot monitor physiological development. For example, in the pilot sample, all healthy controls received the highest score on the oral intake scales, and this no longer changes with physiological development. However, to monitor developmental steps in the future, as is the case with motor function, tools such as the DySMA are helpful. For example, it can be used to record when children begin to eat or chew solid food.
The DySMA is well-suited as a basis for developing further tools for various age groups. For example, as suggested by Dunaway Young and colleagues for the age group 2 to 12 years [15].
The DySMA will be a useful adjunct to motor function tests in registration trials, for patient registers (e.g., SMArtCARE [28]), and outpatient follow-up. Especially when SLP expertise is unavailable, it is a useful tool to systematically record the progression of the swallowing outcomes in the target population.
Limitations and considerations for future studies
As far as the Delphi method is concerned, the small number of participants was a clear weakness. According to the COSMIN recommendations [17], a sample size of less than 30 persons is insufficient. In German-speaking countries, however, there has been little but increasing expertise in the field of swallowing in infants and toddlers. At the same time, there is little experience with children with SMA under 24 months of age. In addition, newborn screening in Germany only began in autumn 2021, so the number of cases is still low. For this reason, 19 individuals can be considered adequate. The same applies to the sample size of the pilot sample.
Regarding the classification of the groups, it should be mentioned that the pre-symptomatic sample was clinically classified and not based on device measurements, such as compound motor action potential (CMAP).
It is important to note here that ideally, children with SMA should receive a detailed swallowing examination by an SLP, which cannot be replaced by performing the DySMA. Due to a general shortage of SLPs in Germany in this area and the underrepresentation of SLPs in SMA centers and research, an interprofessional approach will be necessary. In our online survey sample, we saw that PT (Mdn 28y) and NP (Mdn 11y) currently have significantly longer experience with the target group than SLP (Mdn 3y); there is still some catching up to do here. Regarding the pilot testing of the DySMA, it must be taken into account that SLPs used the tool in our study. Although two PTs and two NPs tested the applicability of the tool and contributed to the final version in a cognitive debriefing, in the future, the reliability between PT, NP, and SLP must also be checked in order to make a final statement about the interprofessional applicability.
Concerning the DySMA framework, it can undoubtedly be critically discussed that it has to do without direct observation of food intake and that it is a combined tool of patient history and examination. In the formulation sessions, it was discussed that parents of children with SMA already have to fill in several Parent-Reported Outcome Measures (PROMS) at the examination appointments (at least, this is the case in our center). In the experience of the examiners, it was easier to ask questions and give examples in an oral patient history. However, it also became apparent that some items require specific assessment by the examiners (examination section, e.g., reduced maximum jaw opening) as they cannot be judged effortlessly by parents.
DySMA tool’s next steps are to check the reliability and validation using larger samples (DySMA study) and norming using healthy controls (DySMA norm study). In addition, the English translation needs to be reviewed by an international expert consensus and made available internationally. Due to the condition’s rarity and the so far poorly-researched target group under 24 months of age, collaboration with expert groups worldwide is valuable here.
CONCLUSION
The DySMA is well suited for mapping swallowing development in presymptomatically and symptomatically treated infants and toddlers with SMA. It can be administered time-efficient and interprofessional by SLPs, PTs, or NPs. The resulting score can be correlated with results from other instruments measuring different constructs, e.g., motor function.
AUTHOR CONTRIBUTIONS
JZ, SW, JHQ, CP and TF contributed to the study conception and design. Material preparation and data collection were performed by JZ, JJ, DW, JD and CD. Data analysis and interpretation were performed by JZ, SW, JHQ, AN, and TF. The first draft of the manuscript was written by JZ and critically revised by all authors. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank those who participated in the interviews and the online survey. They also thank the children and their families who participated in the study.
FUNDING INFORMATION
We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf and DFG –German Research Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests regarding the manuscript.
DATA AVAILABILITY STATEMENT
Data are tabulated in the manuscript. Further data are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230177.
REFERENCES
[1] | Mercuri E , Sumner CJ , Muntoni F , Darras BT , Finkel RS Spinal muscular atrophy. Nat Rev Dis Primers. (2022) ;8: (1):52. |
[2] | Finkel RS , McDermott MP , Kaufmann P , Darras BT , Chung WK , Sproule DM , et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. (2014) ;83: (9):810–7. |
[3] | McGrattan KE , Graham RJ , DiDonato CJ , Darras BT Dysphagia Phenotypes in Spinal Muscular Atrophy: The Past, Present, and Promise for the Future. Am J Speech Lang Pathol. (2021) ;30: (3):1008–22. |
[4] | Lunn MR , Wang CH Spinal muscular atrophy. Lancet. (2008) ;371: (9630):2120–33. |
[5] | van der Heul AMB , Cuppen I , Wadman RI , Asselman F , Schoenmakers M , van de Woude DR , et al. Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type an Observational Study. J Neuromuscul Dis. (2020) ;7: (3):323–30. |
[6] | Weststrate H , Stimpson G , Thomas L , Scoto M , Johnson E , Stewart A , et al.Evolution of bulbar function in spinal muscular atrophy type 1 treated with nusinersen. Dev Med Child Neurol. 2022. |
[7] | Bernal S , Alias L , Barcelo MJ , Also-Rallo E , Martinez-Hernandez R , Gamez J , et al. The c. 859G>C variant in the SMN2 gene is associated with types II and III SMA and originates from a common ancestor. J Med Genet. (2010) ;47: (9):640–2. |
[8] | Ruhno C , McGovern VL , Avenarius MR , Snyder PJ , Prior TW , Nery FC , et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum Genet. (2019) ;138: (3):241–56. |
[9] | Garand KLF , McCullough G , Crary M , Arvedson JC , Dodrill P Assessment Across the Life Span: The Clinical Swallow Evaluation. Am J Speech Lang Pathol. (2020) ;29: (2S):919–33. |
[10] | Heckathorn DE , Speyer R , Taylor J , Cordier R Systematic Review: Non-Instrumental Swallowing and Feeding Assessments in Pediatrics. Dysphagia. (2016) ;31: (1):1–23. |
[11] | Zang J , Johannsen J , Denecke J , Weiss D , Koseki JC , Niessen A , et al. Flexible endoscopic evaluation of swallowing in children with type 1 spinal muscular atrophy. Eur Arch Otorhinolaryngol. (2023) ;280: (3):1329–38. |
[12] | Berti B , Fanelli L , Stanca G , Onesimo R , Palermo C , Leone D , et al.Oral and Swallowing Abilities Tool (OrSAT) in nusinersen treated patients. Arch Dis Child. 2022. |
[13] | Berti B , Fanelli L , de Sanctis R , Onesimo R , Palermo C , Leone D , et al. Oral and Swallowing Abilities Tool (OrSAT) for Type 1 SMA Patients: Development of a New Module. J Neuromuscul Dis. (2021) ;8: (4):589–601. |
[14] | McGrattan KE , Shell RD , Hurst-Davis R , Young SD , O’Brien E , Lavrov A , et alPatients with Spinal Muscular Atrophy Type 1 Achieve and Maintain Bulbar Function Following Onasemnogene Abeparvovec Treatment. J Neuromuscul Dis. 2023. |
[15] | Dunaway Young S , McGrattan K , Johnson E , van der Heul M , Duong T , Bakke M , et al. Development of an International SMA Bulbar Assessment for Inter-professional Administration. J Neuromuscul Dis. (2023) ;10: (4):639–52. |
[16] | Glanzman AM , Mazzone E , Main M , Pelliccioni M , Wood J , Swoboda KJ , et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. (2010) ;20: (3):155–61. |
[17] | Terwee CB , Prinsen CAC , Chiarotto A , Westerman MJ , Patrick DL , Alonso J , et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. (2018) ;27: (5):1159–70. |
[18] | Moher D , Liberati A , Tetzlaff J , Altman DG , Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. (2009) ;3: (3):e123–30. |
[19] | Zang J , Flügel T , Nießen, A , Koseki, J-C., , Nienstedt, JC. , Pflug, C. Clinical Parameter for the Assessment of Swallowing Disorders in Infants with Spinal Muscular Atrophy type 1. European Society for Swallowing Disorders; 12-16 September 2022; Leuven, Belgium2022. |
[20] | Diamond IR , Grant RC , Feldman BM , Pencharz PB , Ling SC , Moore AM , et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. (2014) ;67: (4):401–9. |
[21] | Beiderbeck D , Frevel N , von der Gracht HA , Schmidt SL , Schweitzer VM Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX. (2021) ;8: , 101401. |
[22] | Wada A , Kawakami M , Liu M , Otaka E , Nishimura A , Liu F , et al. Development of a new scale for dysphagia in patients with progressive neuromuscular diseases: the Neuromuscular Disease Swallowing Status Scale (NdSSS). J Neurol. (2015) ;262: (10):2225–31. |
[23] | Choi YA , Suh DI , Chae JH , Shin HI Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol. (2020) ;130: , 109818. |
[24] | Haataja L , Mercuri E , Regev R , Cowan F , Rutherford M , Dubowitz V , et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. (1999) ;135: (2 Pt 1):153–61. |
[25] | Cutrona C , Pede E , De Sanctis R , Coratti G , Tiberi E , Luciano R , et al. Assessing floppy infants: a new module. Eur J Pediatr. (2022) ;181: (7):2771–8. |
[26] | Pane M , Donati MA , Cutrona C , De Sanctis R , Pirinu M , Coratti G , et al. Neurological assessment of newborns with spinal muscular atrophy identified through neonatal screening. Eur J Pediatr. (2022) ;181: (7):2821–9. |
[27] | De Vivo DC , Bertini E , Swoboda KJ , Hwu WL , Crawford TO , Finkel RS , et al. Nusinersen initiated in infants during the pre-symptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. (2019) ;29: (11):842–56. |
[28] | Pechmann A , Konig K , Bernert G , Schachtrup K , Schara U , Schorling D , et al. SMArtCARE - A platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis. (2019) ;14: (1):18. |




