Facioscapulohumeral Muscular Dystrophy European Patient Survey: Assessing Patient Reported Disease Burden and Preferences in Clinical Trial Participation
Abstract
Background:
Facioscapulohumeral muscular dystrophy (FSHD) is a genetic disorder characterized by progressive muscle weakness leading to permanent disability. There are no curative treatments, however, there are several upcoming clinical trials testing new therapies in FSHD.
Objective:
This study aimed to explore the disease burden and patient preferences of people with FSHD to ensure that clinical trials can be designed to include outcome measures that are relevant and important to patients.
Methods:
A survey was developed with a steering committee clinicians and physiotherapists with relevant experience in the disease, patient representatives, a registry expert and industry consultants. Themes of the survey included; participant demographics, disease progression and impact on function, factors encouraging or discouraging clinical trial participation, and positive outcomes of a clinical trial.
Results:
1147 participants responded to the online survey, representing 26 countries across Europe and a range of disease severities. The study highlighted the key symptoms causing concern for FSHD patients - muscle weakness and mobility issues - reflecting what participants want targeted for future therapies. The need for clear information and communication throughout clinical trials was emphasised. Factors most encouraging trial participation included access to new investigational therapies, access to trial results and benefits for the FSHD community. Factors most discouraging trial participation included travel related issues and fear of side effects.
Conclusions:
The results from this study identify the patient reported burden of FSHD and should provide researchers and industry with areas of therapeutic research that would be meaningful to patients, as well as supporting the development of patient centric outcome measures in clinical trials.
INTRODUCTION
Facioscapulohumeral muscular dystrophy (FSHD) is a progressive muscular dystrophy caused by the aberrant expression of the DUX4 gene in skeletal muscle [1]. The reported incidence of FSHD varies across Europe, affecting between 5 and 12 people in 100,000 [2–5].
FSHD is diagnosed by both clinical and genetic assessments and is categorized as either Type 1 or Type 2 FSHD. Approximately 95% of patients are Type 1 [6]. Both FSHD types 1 and 2 are clinically similar. Onset of symptoms typically occurs in the first or second decade of life, however, can begin at any age from infancy to late adulthood. FSHD can present with considerable variation in the onset and severity of symptoms, even within the same family.
The pattern of muscle involvement in FSHD often affects the facial, shoulder and upper arm muscles initially, followed by weakness of the abdominal, lower limb and pelvic girdle muscles later in the progression of the disease [7], however, presentation can be highly variable. There may be significant asymmetry with the affected muscles, with muscles on one side of the body being weaker than the other. FSHD does not typically affect the life span of the patient, however can cause permanent and irreversible disability, with approximately 20% of patients over 50 requiring a wheelchair [8].
Unfortunately, there are no curative treatments for FSHD, with current standards of care focusing on the management of symptoms. However, several clinical trials have been run in FSHD aiming to increase muscle mass or strength or to reduce the rate of disease progression [9–13].
Developing new therapies in rare disease can present with several challenges [14]. There are small numbers of patients, and they can present with considerable phenotypic and genotypic heterogeneity, so finding a significant number of individuals that would fit within inclusion criteria of a clinical trial can be challenging. Stakeholders such as industry and regulators are therefore increasingly seeking input from patients in order to advance understanding the disease and the patient community’s needs [15].
Patient preferences are defined as ‘qualitative or quantitative statements of the relative desirability or acceptability of attributes that differ among alternative health interventions’ [16]. The use of patient preference studies in informing clinical decision making can lead to relevant, well-informed, patient centric decisions [16, 17]. Furthermore, consideration of patient preferences in clinical trial design can provide insights into the relative importance of clinical trial outcomes for patients, and result in improved recruitment, retention and compliance of patients [18]. For example, long travel with limited mobility to a clinical trial could limit patient participation, but including remote assessments could potentially ease that burden [19]. Patient preference studies can also provide valuable insights into identifying unmet healthcare needs [20, 21].
There is a growing interest from several pharma companies to run clinical trials in FSHD across Europe [22]. This study aimed to understand the FSHD community’s perspective, so that when clinical trials are developed, they are designed and organized in a way to maximize patient involvement and participation, by targeting key symptoms and including outcome measures that are relevant and important to patients.
METHODS
Approval for this study was obtained from Newcastle University Faculty of Medical Sciences Ethics Committee (Ref: 2251/16858). Whilst demographic information was collected in the survey, it was not possible to trace the participant and therefore all answers were anonymous. The full consent agreement can be found in Appendix 1, pages 1-2.
SURVEY DESIGN
Themes of the survey were developed with a steering committee which included clinicians and physiotherapists with relevant experience in the disease, patient representatives, a registry expert and industry consultants. The themes included:
1. Demographic information to understand the representation and diversity of participants across Europe.
2. Disease progression and impact on function: Questions were included to understand the patient’s current function and mobility. The effectiveness of their current treatment regimen was explored. The symptoms causing most concern and difficulty in daily life were also highlighted.
3. Factors encouraging or discouraging clinical trial participation: Questions were designed to understand what barriers are in place for clinical trial participation and how trial design might be able to mitigate these factors.
4. What would patients see as a positive outcome of a clinical trial: Understanding the preferences of participants when designing new therapies, regarding both symptomatic and social benefits.
There were a range of qualitative question types in the survey, including multiple choice, free text options and Likert scales. Likert scales were used to understand participant’s opinions of clinical trials, as well as the severity of their condition (e.g., not at all affected, mildly affected, moderately affected, and severely affected). Multiple choice questions were used where symptoms or clinical trial preferences were known. Free text options were used in order to capture any additional opinions or symptoms not included in the survey.
The survey was developed in English and translated into Dutch, French, German, Italian and Spanish (Eldon Bureau Limited, Newcastle upon Tyne). This was distributed by FSHD Europe through their network of patient registries, patient organisations and social media across several European countries. No incentives were offered for completion of the survey. (A copy of the survey can be found in Appendix 1).
The survey was designed and made available online via Jisc Online Surveys and responses were collected for a period of three weeks in April-May 2022. FSHD Europe coordinated the dissemination of the survey through their member networks. It was shared across national patient organisations, patient registries and social media.
DATA ANALYSIS
Responses from Jisc Online surveys were exported via excel in each language and merged to allow comparisons of all results in English. Results were prepared as tabulated descriptive statistics and presented as numbers (n) and percentage (%) of total respondents per question. Statistical analyses were completed in Graphpad Prism v9.0. Unpaired t-tests were used to compare age, gender and ambulation status with clinical trial decision making factors.
RESULTS
A total of 1209 responses were collected over a period of three weeks. Participants who did not have a diagnosis of FSHD by a healthcare professional (n = 26) or who did not live in Europe (n = 36) were screened out of the results, leaving a total of 1147 responses.
DEMOGRAPHIC INFORMATION
The majority of respondents were patients (92%), with caregivers representing 5% of participants. The final 3% identified as both a patient and a caregiver. Patients had to have a diagnosis of FSHD by a healthcare professional to complete the suvey. 68% of participants reported to be FSHD type 1, with 7% of participants reporting to be FSHD type 2. The remaining participants either did not know the genetic diagnosis (17%) or did not have their FSHD diagnosis genetically confirmed (8%) (Supplemental Figure 1).
Responses were obtained from patients and caregivers across 26 European countries. The largest number of responses were from countries in which the native language was available; United Kingdom (30%), Germany (21%), France (12%), Italy (12%), Spain (10%) and the Netherlands (7%) (Supplemental Figure 1).
50% of respondents were female, 49% were male, with 1% selecting ‘prefer not to say’. The current age of individuals with FSHD ranged from 2 to 86 years old, with an mean age of 50.5 years.
DISEASE PROGRESSION AND IMPACT ON FUNCTIONING
40% of patients first experienced symptoms of FSHD between the ages of 11–20 (Fig. 1A, B). The average time from onset of symptoms to diagnosis, calculated by the difference in age reported at each of these milestones, was 7.9 + /- 0.3 years (Fig. 1C).
Over the past six months, the majority of respondents (55%) reported that their condition had got minimally worse, and 18% of respondents reported no change in their condition. Over the past three years, 46% of respondents reported that their condition had got much worse, and 13% reported their condition had got very much worse (Fig. 1D, E).
Fig. 1
Onset, diagnosis and severity of FSHD. [A] Age at onset of symptoms. Bar chart showing percentage of participants presenting with symptoms at different age ranges. Colours indicate the first symptom experienced; Facial weakness (green), pain (light blue), scapular winging (dark blue), weakness in arms/hands (dark purple), weakness in legs/feet (light purple), other (pink). [B] Age at diagnosis of FSHD. Bar chart showing the percentage of participants diagnosed with FSHD by age. [C] Average time from onset of symptoms to FSHD diagnosis. Bar chart indicating the time (years) between initial symptoms and diagnosis with FSHD by country. (United Kingdom n = 357; Germany n = 248; France n = 139; Italy n = 139; Spain n = 114; Netherlands n = 80) European average (red) shows the average time from across all European participants (n = 1147). Error showing+/- SEM [D] Current age and severity of condition. Bar chart showing the current age of participants and the current severity of their condition. Green –not at all affected; yellow –mildly affected; orange –moderately affected; red –severely affected. [E] Change in severity over time. Participants reported the changes in their condition over the past 6 months (top bar) and 3 years (bottom bar). Colours indicate severity: blue –very much improved; dark green –much improved; light green –minimally improved; grey –no change; yellow –minimally worse; orange –much worse; red –very much worse.
![Onset, diagnosis and severity of FSHD. [A] Age at onset of symptoms. Bar chart showing percentage of participants presenting with symptoms at different age ranges. Colours indicate the first symptom experienced; Facial weakness (green), pain (light blue), scapular winging (dark blue), weakness in arms/hands (dark purple), weakness in legs/feet (light purple), other (pink). [B] Age at diagnosis of FSHD. Bar chart showing the percentage of participants diagnosed with FSHD by age. [C] Average time from onset of symptoms to FSHD diagnosis. Bar chart indicating the time (years) between initial symptoms and diagnosis with FSHD by country. (United Kingdom n = 357; Germany n = 248; France n = 139; Italy n = 139; Spain n = 114; Netherlands n = 80) European average (red) shows the average time from across all European participants (n = 1147). Error showing+/- SEM [D] Current age and severity of condition. Bar chart showing the current age of participants and the current severity of their condition. Green –not at all affected; yellow –mildly affected; orange –moderately affected; red –severely affected. [E] Change in severity over time. Participants reported the changes in their condition over the past 6 months (top bar) and 3 years (bottom bar). Colours indicate severity: blue –very much improved; dark green –much improved; light green –minimally improved; grey –no change; yellow –minimally worse; orange –much worse; red –very much worse.](https://content.iospress.com:443/media/jnd/2024/11-2/jnd-11-2-jnd230171/jnd-11-jnd230171-g001.jpg)
The walking ability of participants is shown in Supplemental Figure 2. Those who responded that their walking ability was moderately affected or severely affected (n = 625) were asked a further question about what walking aids they currently used. 63% of participants have experienced falls due to their FSHD. 64% of this subset of participants had experienced minor injuries (e.g. bruising) and 17% had experienced major injuries (e.g. broken bones) (Supplemental Figure 2).
Participant’s upper extremity function was measured using the 20-item Upper Extremity Functional Index (UEFI-20) [23, 24]. Each item (e.g. brushing your hair, driving, opening a jar etc.), uses a 5-point response scale to rate difficulty in performing from 0 to 4. Summing the items yields a total score from 0 (worst) to 80 (best) points. Supplemental Figure 2E shows the upper extremity function of participants by age group. There was one participant under 5 years old, aged 2 years old.
Weakness in orofacial muscles can cause swallowing and communication difficulties in people with FSHD [25, 26]. 64% of participants reported that their swallowing ability had not been affected by their FSHD, while 28% reported that they were mildly affected (occasional feeling of solids sticking); 7% were moderately affected (frequent feeling of solids ‘sticking’ requiring some adaptations to diet although coughing or choking was infrequent) and 1% were severely affected (required adapted diet, regular coughing or choking episodes). 61% of participants reported that their speech has not been affected by their FSHD while 33% of participants reported to be mildly affected (usually understood and rarely asked to repeat things) and 6% were moderately affected (poorly understood by strangers, frequently asked to repeat things).
Nine percent of participants reported their respiratory health was either moderately or severely affected by their FSHD and required some level of ventilation. A further 41% reported that their respiratory health has been mildly affected by their condition.
The majority (89%) of participants were using some form of therapy to help with their condition, with 11% reporting that they were not currently using anything. The largest proportion of respondents reported that they were using physical or occupational therapy (49%), exercise (47%), and use of mobility aids (42%) to manage their condition. (Supplemental Figure 3).
Participants reported to be taking several medications to manage a wide range of symptoms, such as pain relief, anti-inflammatories, anti-anxiety or anti-depression medications, medications for gastro-intestinal symptoms or hypertension medications. Painkillers were most frequently mentioned, with patients often taking several types of painkillers with varying strengths depending on their pain level. These included medications such as paracetamol, ibuprofen, amitriptyline, tramadol, codeine and morphine. 14% of respondents reported that they are taking dietary or herbal supplements to help their condition. Several participants mentioned the study by Passerieux et al (2015) and reported to be taking a combination of vitamin C, vitamin E, zinc gluconate and selenomethionine supplements [27]. Several respondents also reported that cannabis was part of their treatment regimen.
Participants indicated that the biggest limitations of their current treatment regimen were that it was not very effective (24.7%), it requires a lot of effort (18%), is time consuming (15.6%) and they have limited availability of accessibility e.g., do not live close to a specialist centre (15.8%) (Supplemental Figure 3).
Not being able to walk, or impaired mobility was the most reported symptom to cause difficulty in daily life, followed by general muscle weakness, difficulty using arms and hands, and fatigue, lack of energy and endurance (Fig. 2A). Participants reported that losing independence or the ability to walk concerned them the most, as well as not having the energy to live or work as they want to (Fig. 2B).
Fig. 2
Participants experience of FSHD. [A] Symptoms causing difficulty in daily life. Participants (n = 1147) were asked to rank how particular symptoms caused difficulty in daily life from 0 (dark green; no difficulty) to 5 (red; causes great difficulty). Black box highlights symptoms which caused the largest majority of participants difficulty. [B] Future concerns for FSHD. Participants (n = 1147) were asked to rank what concerned them most about the future from 0 (dark green; not at all concerned) to 5 (red; causes great concern). Black box indicates the issue that cause the largest majority of participants concerns for the future.
![Participants experience of FSHD. [A] Symptoms causing difficulty in daily life. Participants (n = 1147) were asked to rank how particular symptoms caused difficulty in daily life from 0 (dark green; no difficulty) to 5 (red; causes great difficulty). Black box highlights symptoms which caused the largest majority of participants difficulty. [B] Future concerns for FSHD. Participants (n = 1147) were asked to rank what concerned them most about the future from 0 (dark green; not at all concerned) to 5 (red; causes great concern). Black box indicates the issue that cause the largest majority of participants concerns for the future.](https://content.iospress.com:443/media/jnd/2024/11-2/jnd-11-2-jnd230171/jnd-11-jnd230171-g002.jpg)
DECISION FACTORS FOR CLINICAL TRIAL PARTICIPATION
The most encouraging factor for patients to take part in a clinical trial was access to the investigational product or therapy. This was followed by access to trial results when published and benefits for the FSHD community. Compensation for travel and time ranked lowest amongst participants for being an encouraging factor for clinical trial participation. (Fig. 3). Participants’ gender and ambulation did not have a significant effect on what factors they found most encouraging when deciding to take part in a clinical trial. Unpaired t-test showed that age had a significant effect, with younger participants selecting compensation for travel as an encouraging factor (P = 0.0140). (See supplemental Figures 4–6).
Fig. 3
Positive factors for clinical trial participation. Participants (n = 1147) were asked to rank what factors would most encourage them to participate in a clinical trial from 0 (red; not encouraging) to 5 (dark green; most encouraging). Black boxes indicate the top three factors that would be the most encouraging for the largest proportion of participants.
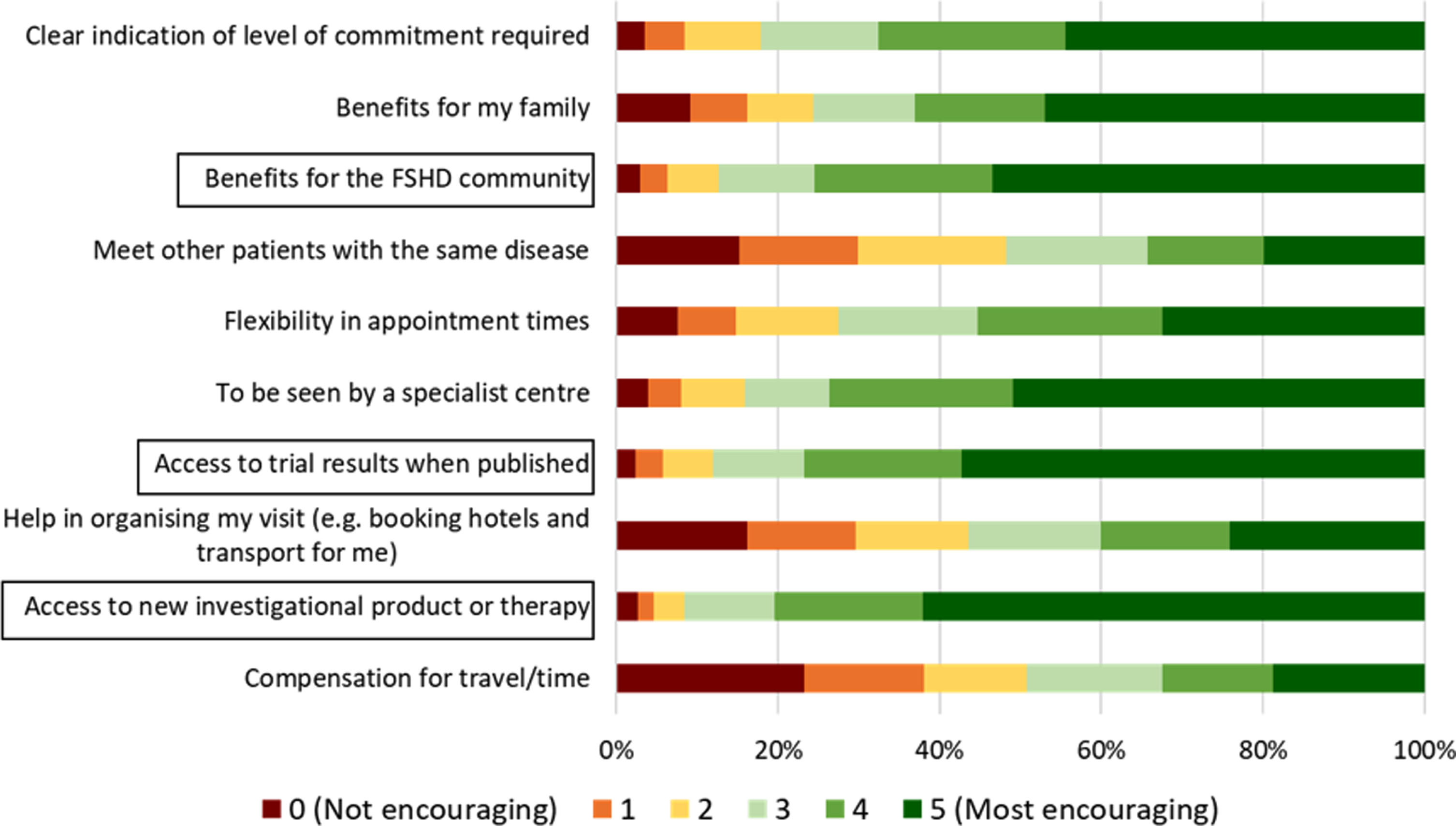
Participants commented in the free text responses that being seen locally or remotely (i.e. over video call) would be encouraging, which would remove the need for long travel. It was also important to patients that there be a thorough explanation of the trial and the risks that would be involved, as well as a transparent selection process. Participants also cited altruistic reasons, such as helping scientific research or helping family members and future generations with FSHD. Participants commented that flexibility around work would be encouraging. Psychological support throughout the trial would be encouraging, as well as allowing for a companion to attend the trial site visits (e.g., partner or friend).
During a clinical trial, participants would be willing to take part in blood samples (95%), magnetic resonance imaging (MRI) (85%), functional assessments such as grip strength or timed up and go (92%), lung function assessments (87%), use a wearable device (75%), open muscle biopsy (44%) and needle biopsy (48%). From those who stated they would be willing to undertake a muscle biopsy, 82% indicated that they would be willing to undergo multiple biopsies. For participants that indicated they would be willing to undergo an MRI scan, 30% would be willing to sit in an MRI machine for 2 hours or more, 17% for up to 90 minutes, 36% for up to an hour and 17% for up to 30 minutes.
The most discouraging factor for patients was if the facility conducting the trial was far away, followed by fear of side effects of treatment (Fig. 4). Participants’ gender and ambulation did not have a significant effect on what factors they found most encouraging when deciding to take part in a clinical trial. Unpaired t-test showed that age had a significant effect on participants selecting fear of side effects from treatment (P = 0.0387), Facility of the clinical trial is far away (P = 0.0024), Would have to miss work/school (P < 0.0001) and Lack of financial compensation for time spent on the trial (P = 0.0004) (See supplemental Figures 7–9).
Fig. 4
Negative factors for clinical trial participation. [A] Participants (n = 1147) were asked to rank what factors would most discourage from participating in a clinical trial from 0 (dark green; not discouraging) to 5 (red; most discouraging). Black box indicates the factor that would be the most discouraging factors for the largest proportion of participants. [B] Participants were asked if there was a particular procedure that would discourage them from participating in a clinical trial. [C] Bar chart showing proportion of participants that would be concerned about experiencing a particular side effect from clinical trial participation.
![Negative factors for clinical trial participation. [A] Participants (n = 1147) were asked to rank what factors would most discourage from participating in a clinical trial from 0 (dark green; not discouraging) to 5 (red; most discouraging). Black box indicates the factor that would be the most discouraging factors for the largest proportion of participants. [B] Participants were asked if there was a particular procedure that would discourage them from participating in a clinical trial. [C] Bar chart showing proportion of participants that would be concerned about experiencing a particular side effect from clinical trial participation.](https://content.iospress.com:443/media/jnd/2024/11-2/jnd-11-2-jnd230171/jnd-11-jnd230171-g004.jpg)
Fig. 5
Preferences of new therapies. [A] Symptom participants would like to be targeted first by a new therapy. Pie chart showing participants preferences about which symptom they would like to be improved first. Participants could select up to 3 options from the list on the right. [B] Most important outcome of a new therapy. Participants were asked what they considered to be the most important outcome of a new therapy (n = 1147).
![Preferences of new therapies. [A] Symptom participants would like to be targeted first by a new therapy. Pie chart showing participants preferences about which symptom they would like to be improved first. Participants could select up to 3 options from the list on the right. [B] Most important outcome of a new therapy. Participants were asked what they considered to be the most important outcome of a new therapy (n = 1147).](https://content.iospress.com:443/media/jnd/2024/11-2/jnd-11-2-jnd230171/jnd-11-jnd230171-g005.jpg)
In the participant’s comments through the free text option, how the trial was organised was an important factor in deciding to take part in a clinical trial. Participants expressed their wish of being well informed of what the aims of the trial are and what is expected from them as participants. Trials should offer support to participants, especially if adverse events are experienced or if the trial is paused or ended unexpectedly. Participants would also like to be well informed on the progress or the results of the trial. Flexibility around work schedules or compensation for missed work could encourage better participation.
Several uncertainties of participants were also noted, such as fear of painful or invasive tests, use of placebo or additional deterioration of their condition. Participants were asked about which specific tests or side effects they may be concerned about experiencing. Muscle biopsies were again shown to be the procedure that participants found to be most discouraging from taking part in a clinical trial. 24% of participants said that no procedure would discourage them from participation in a clinical trial. Participants noted that they may be more willing to participate with adequate and thorough explanation of the procedures and trial processes.
Despite the distance of a trial facility being a discouraging factor for trial participation, the majority of participants would be willing to travel to take part in a clinical trial and stay overnight in a hotel that was suitably equipped to meet their needs (Supplemental Figure 10). Overnight stays should be clearly indicated when advertising for a clinical trial –travel with limited mobility can be difficult and participants may need to arrange childcare for example.
DEFINING OUTCOMES FROM A CLINICAL TRIAL
Impaired mobility and general muscle weakness were the symptoms that participants would like to be improved first through a new therapy. Figure 5 shows what participants consider to be the most important when trialling a new therapy. Stopping the progression of the disease was ranked most highly by participants (31%), followed by regaining strength (18%) and improved mobility (14%).
Free text responses indicated that increased ability to take part in social activities and events, such as going for a walk with friends or family, looking after children and grandchildren as well as generally being more active were considered important social benefits of a therapy. Improved mood, confidence, and energy, as well as easing pain were also noted. Some symptoms were directly mentioned, such as improvements in swallowing/eating, cardiac problems and weight. Overall, increased independence showed the highest score for participants.
DISCUSSION
This study explored the unmet healthcare needs, patient reported burden of FSHD and treatment expectations of caregivers and patients with FSHD in an international sample of individuals across Europe. This study aimed to inform potential stakeholders with patient preferences at the early stages of therapeutic development and the design of clinical trials, to benefit the FSHD community without considering a particular therapeutic option. The symptomatic disease burden of FSHD patients and caregivers and their current therapeutic options were also reviewed, to better understand the gaps in healthcare for people with FSHD.
Patient groups have a nuanced understanding of the preferences and limitations of their patient communities. Previous studies have shown that including patient preferences in clinical trial design and recruitment can lead to better retention [28, 29]. Furthermore, patient involvement has shown to improve the outcomes of interventions, as well as their safety, efficacy, and relevance to the patients’ needs [30–32]. It therefore brings significant value to include the voice of the patient throughout the medicinal product life cycle. Rare disease patients play an active role in the in the European Medicines Agency (EMA) therapeutic development and licencing processes, in the development of European Reference Networks, and in the European Joint Programme for Rare Disease Research [32]. The European Neuromuscular Centre (ENMC) also emphasises the importance of shared decision making, highlighting the need for the patient voice to be included in the design of clinical trials from the start, and identifies methods to help encourage this [33, 34].
The survey was available online in six European languages,. The inclusion of caregivers in this study allowed for the representation of paediatric patients or more severely affected patients whose disease burden may have precluded them from participation otherwise. The availability and accessibility of the survey undoubtedly contributed to the large number of participants that took part, reflecting the willingness of the FSHD community to participate in research activities.
Diagnostic delays in rare diseases are common. This can lead to inappropriate or missed treatments and is associated with increased morbidity [35–37]. The variability of onset of FSHD was demonstrated in Fig. 1A, with facial weakness being more common in infants, scapular winging in children and young adults, and weakness in the feet being the prevalent symptom in adults. This variability can lead to further clinical investigations delaying diagnosis [38]. In general, the average time for accurate diagnosis of a rare disease is 4-5 years, but can be as long as a decade [39]. The average time from onset of symptoms to diagnosis across all participants in this study was 7.9 years but varied between countries, demonstrating the inequalities in diagnosis of FSHD across European countries.
The patient experience can vary greatly due to the complexities of the disease, as well as legal, organisational, cultural, economic and social factors across diverse national health care systems in different countries within Europe [40]. The FSHD European Trial Network created in 2021 aims to address some of these issues, through education and standardisation of FSHD diagnosis and treatment across Europe [41]. This survey provided an opportunity to understand patient preferences from a broad range across the FSHD community, for instance from differing disabilities (mild, moderate and severely affected patients), ages (from aged 2 to 86) and diagnoses (FSHD type I and II).
Despite most participants in this study reporting to be moderately or severely affected by their FSHD, half were not taking any medications to help their condition. Approximately 30% of participants felt that their treatment regimen did not control their overall condition and symptoms or controlled them ‘very little’. Several participants reported the use of supplements, referencing the study by Passerieux et al (2015). This study reported that taking a combination of vitamin C, vitamin E, zinc gluconate and selenomethionine supplements improved the maximum voluntary contraction and endurance of the quadriceps, however it did not improve the functionality of the patients in the two-minute walk test [27]. Despite these results, patients are still willing to try this combination of supplements in order to help their condition.
The symptoms causing the most difficulty in daily life were primarily due to the progressive muscle weakness that is experienced by people with FSHD, and these symptoms were reflected in what patients would like to be improved first when designing clinical trials, as well as what concerned them for the future. This is similar to patient preference studies in other neuromuscular diseases, which found ‘muscle strength’ and ‘energy and endurance’ as the top two unmet health needs in mitochondrial disease and myotonic dystrophy patients [15]. Another symptom highlighted in this study was fatigue, lack of energy and endurance. This has been shown to have a severe impact on patient’s lives [42] and is an important outcome measure in previous clinical trials in FSHD [43, 44]. Mobility, muscle strength, and fatigue are clearly important to FSHD patients and should be considered when designing therapies as well as meaningful endpoints for clinical trials in FSHD.
It is crucial that the expectations of participants are managed carefully and that adequate time is given for informed consent. Several participants also noted that they had previously taken part in trials and had not received results, reflecting. Verhaart et al (2019) found that communication of results was considered important, but only received by 26% of participants [45]. The desire for better access to information is not unique to FSHD, and was noted in a recent study regarding patient preferences in spinal muscular atrophy [46]. Whilst educational sources are available, patients are not sufficiently informed of where to access this. An open and clear communications plan has the potential to increase trial recruitment and improve participant experiences, with a harmonised message from clinicians, clinical research organisations, and sponsors. Further funding in patient education initiatives as well as communication for healthcare professionals could also help to bridge the gap revealed in this study.
Travel with neuromuscular disease can be challenging and should be accounted for when planning clinical trials. These results show that adequate help in organising travel, reimbursement of travel costs or the possibility to be seen by a local clinic or remotely could encourage greater participation in clinical trials. Alternatively, it could be beneficial to develop outcome measures that can be performed from home. Furthermore, if overnight stays in a hotel are necessary for trial participants, it is critical that hotels offering accessible room options are made available to make the process easier for participants.
Muscle biopsies were highlighted as a procedure that could also discourage participation in clinical trials. Verhaart et al (2019) highlighted the concerns and risks of muscle biopsies in clinical trials in Duchenne muscular dystrophy. It was suggested that while a muscle biopsy may be unavoidable in early phase trials, in later phase placebo-controlled trials biopsies are considered unethical by caregivers, as functional evidence is adequate for approval by regulators [45]. Recent studies have shown that MRI can adequately measure disease heterogeneity in FSHD [47, 48]. A recent European Neuromuscular Centre (ENMC) workshop discussed muscle imaging in FSHD and its relevance for clinical trials, and found that MRI is widely tolerated by patients [49], reflecting what was found in this study.
LIMITATIONS
This study aimed to get a broad representation of patient preferences in FSHD across Europe. The majority of participants were from Western Europe, likely due to the limitations in languages that the survey was translated in to. 92% of participants were from the six countries whose native language was available (Netherlands, France, Germany, Italy, Spain and United Kingdom) with the remaining 8% being from 20 other countries in Europe. Links with patient organisations in more Eastern European countries could help mitigate this in future studies.
Whilst several routes of dissemination were used to advertise the survey, it could be said that patients who are highly engaged with patient organisations and research may have differing preferences compared to the average patient. People who are less computer literate or do not use social media may be less exposed compared to these patients, as they receive less information. While some extra information of what a clinical trial can involve, including the use of placebo, as well as explanations of some procedures such as muscle biopsy were included throughout the survey, some participants may not have fully understood some areas of the survey.
Finally, it is important to note that all the data is self-reported and not confirmed by clinicians. Patient opinions could be swayed by optimism or pessimism around their condition. For instance, the majority of patients reported to be moderately or severely affected by their FSHD, which was not necessarily reflected in the mobility of patients. Some variables such as gender, age and ambulation were explored as factors that could have had an effect on what would encourage or discourage a participant from taking part in a clinical trial, but future work could include further analysis of variables such as level of education or healthcare systems (e.g., public vs private).
CONCLUSIONS
The results from this study should provide researchers and industry with areas of therapeutic research that would be meaningful to patients, as well as supporting the development of clinical trial outcome measures. It could also provide an insight into symptoms that are most important to patients, and gaps in the therapies that are available for FSHD patients to their healthcare providers. Patient preference studies are lacking in general for neuromuscular diseases highlighting the importance of this study in FSHD. Furthermore, this study could provide as a template for other diseases such as limb girdle muscular dystrophy.
FUNDING
This work was supported with a grant provided by FSHD Europe under the FSHD European Patient Pharma Project to the John Walton Muscular dystrophy research centre, Newcastle University (Grant reference number 008893).
ACKNOWLEDGMENTS
We are grateful for the willingness of all participants to complete the survey. Thanks to Facio Therapies who provided advice on this study.
CONFLICT OF INTEREST
Several authors of this publication are members of the European Reference Network for rare neuromuscular diseases (EURO-NMD).
SUPPLEMENTARY MATERIAL
[1] The supplementary materials and the Appendix part is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230171.
REFERENCES
[1] | van der Maarel SM , Tawil R , Tapscott SJ . Facioscapulohumeral muscular dystrophy and DUX breaking the silence. Trends in Molecular Medicine. (2011) ;17: :252–258. |
[2] | Deenen JC , Arnts H , van der Maarel SM , Padberg GW , Verschuuren JJ , Bakker E et al. Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology. (2014) ;83: :1056–1059. |
[3] | Guien C , Blandin G , Lahaut P , Sanson B , Nehal K , Rabarimeriarijaona S et al. The French National Registry of patients with Facioscapulohumeral muscular dystrophy. Orphanet Journal of Rare Diseases. (2018) ;13: :1–10. |
[4] | Camacho A , Esteban J , Paradas C Report by the Spanish Foundation for the Brain on the social impact of amyotrophic lateral sclerosis and other neuromuscular disorders. Neurología (English Edition). (2018) ;33: :35–46. |
[5] | Bettio C , Salsi V , Orsini M , Calanchi E , Magnotta L , Gagliardelli L et al. The Italian National Registry for FSHD: an enhanced data integration and an analytics framework towards Smart Health Care and Precision Medicine for a rare disease. Orphanet Journal of Rare Diseases. (2021) ;16: :1–13. |
[6] | Jeffrey Statland RT Facioscapulohumeral Muscular Dystrophy. Neurologic Clinics. 2014;32. |
[7] | Wang LH , Tawil R Facioscapulohumeral dystrophy. Current Neurology and Neuroscience Reports. (2016) ;16: :1–8. |
[8] | Statland JM , Tawil R . Facioscapulohumeral muscular dystrophy. CONTINUUM: Lifelong Learning in Neurology. (2016) ;22: :1916. |
[9] | Wang LH , Tawil R . Current therapeutic approaches in FSHD. Journal of Neuromuscular Diseases. (2021) ;8: :441–451. |
[10] | Tawil R , McDermott M , Pandya S , King W , Kissel J , Mendell J et al. A pilot trial of prednisone in facioscapulohumeral muscular dystrophy. Neurology. (1997) ;48: :46–49. |
[11] | Kissel J , McDermott M , Mendell J , King W , Pandya S , Griggs R et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. (2001) ;57: :1434–1440. |
[12] | Wagner KR , Fleckenstein JL , Amato AA , Barohn RJ , Bushby K , Escolar DM et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Annals of Neurology. (2008) ;63: :561–571. |
[13] | Payan C , Hogrel J , Hammouda E , Lacomblez L , Ollivier G , Doppler V et al. Periodic salbutamol in facioscapulohumeral muscular dystrophy: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation. (2009) ;90: :1094–1101. |
[14] | Berry SA , Coughlin CR , McCandless S , McCarter R , Seminara J , Yudkoff M et al. Developing interactions with industry in rare diseases: Lessons learned and continuing challenges. Genetics in Medicine. (2020) ;22: :219–226. |
[15] | Jimenez-Moreno AC , van Overbeeke E , Pinto CA , Smith I , Sharpe J , Ormrod J et al. Patient preferences in rare diseases: a qualitative study in neuromuscular disorders to inform a quantitative preference study. The Patient-Patient-Centered Outcomes Research. (2021) ;14: :601–612. |
[16] | Medical Device Innovation Consortium, Medical Device Innovation Consortium (MDIC) Patient Centred Benefit-Risk Project Report, in A framework for incorporating information on patient preferences regarding benefit and risk into regulatory assessments of newmedical technology. 2015. |
[17] | Egbrink MO , IJzerman M . The value of quantitative patient preferences in regulatory benefit-risk assessment. Journal of Market Access & Health Policy. (2014) ;2: :22761. |
[18] | Chhatre S , Jefferson A , Cook R , Meeker CR , Kim JH , Hartz KM et al. Patient-centered recruitment and retention for a randomized controlled study. Trials. (2018) ;19: :1–10. |
[19] | Govindarajan R , Berry JD , Paganoni S , Pulley MT , Simmons Z . Optimizing telemedicine to facilitate amyotrophic lateral sclerosis clinical trials. Muscle & Nerve. (2020) ;62: :321–326. |
[20] | Cook NS , Cave J , Holtorf A-P Patient preference studies during early drug development: aligning stakeholders to ensure development plans meet patient needs. Frontiers in Medicine. 2019:82. |
[21] | van Overbeeke E , Whichello C , Janssens R , Veldwijk J , Cleemput I , Simoens S et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: A literature review. Drug Discovery Today. (2019) ;24: :57–68. |
[22] | Voermans N , Bravo MV-M , Padberg G , Laforêt P , van Alfen N , Attarian S et al. 1st FSHD European Trial Network workshop: Workingtowards trial readiness across Europe. Neuromuscular Disorders. (2021) ;31: :907–918. |
[23] | Stratford PW Development and initial validation of the Upper Ectremity Functional Index. Physiother Can. (2001) ;52: :259–267. |
[24] | Chesworth BM , Hamilton CB , Walton DM , Benoit M , Blake TA , Bredy H et al. Reliability and validity of two versions of the upper extremity functional index. Physiotherapy Canada. (2014) ;66: :243–253. |
[25] | Mul K , Berggren KN , Sills MY , McCalley A , van Engelen BG , Johnson NE et al. Effects of weakness of orofacial muscles on swallowing and communication in FSHD. Neurology.. (2019) ;92: :e957–e963. |
[26] | Mul K , Wijayanto F , Loonen TG , Groot P , Vincenten SC , Knuijt S et al. Development and validation of the patientreported “Facial Function Scale” for facioscapulohumeral muscular dystrophy. Disability and Rehabilitation. 2022:1-6. |
[27] | Passerieux E , Hayot M , Jaussent A , Carnac G , Gouzi F , Pillard F et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: A double-blind randomized controlled clinical trial. Free Radical Biology and Medicine. (2015) ;81: :158–169. |
[28] | Bloom D , Beetsch J , Harker M , Hesterlee S , Moreira P , Patrick-Lake B et al. The rules of engagement: CTTI recommendations for successful collaborations between sponsors and patient groups around clinical trials. Therapeutic Innovation & Regulatory Science. (2018) ;52: :206–213. |
[29] | Morel T , Cano SJ Measuring what matters to rare disease patients–reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet Journal of Rare Diseases. (2017) ;12: :1–13. |
[30] | Sharma AE , Knox M , Mleczko VL , Olayiwola JN . The impact of patient advisors on healthcare outcomes: A systematic review. BMC Health Services Research. (2017) ;17: :1–14. |
[31] | Brett J , Staniszewska S , Mockford C , Herron-Marx S , Hughes J , Tysall C et al. Mapping the impact of patient and public involvement on health and social care research: A systematic review. Health Expectations.. (2014) ;17: :637–650. |
[32] | Bolz-Johnson M , Kenny T , Le Cam Y , Hernando I Our greatest untapped resource: our patients. Journal of Community Genetics. (2021) ;12: :241–246. |
[33] | Lochmüller H , Ambrosini A , van Engelen B , Hansson M , Tibben A , Breukel A et al. The position of neuromuscular patients in shared decision making. Report from the 235th ENMC workshop: Milan, Italy, January 19-20, Journal of Neuromuscular Diseases. (2019) ;6: :161–172. |
[34] | Ambrosini A , Quinlivan R , Sansone VA , Meijer I , Schrijvers G , Tibben A et al. “Be an ambassador for change that you would like to see”: A call to action to all stakeholders for co-creation in healthcare and medical research to improve quality of life of people with a neuromuscular disease. Orphanet Journal of Rare Diseases.. (2019) ;14: :1–12. |
[35] | Blöß S , Klemann C , Rother A-K , Mehmecke S , Schumacher U , Mücke U et al. Diagnostic needs for rare diseases and shared prediagnostic phenomena: Results of a German-wide expert Delphi survey. PLoS One. (2017) ;12: :e0172532. |
[36] | Wang RT , Fadlon CAS , Ulm JW , Jankovic I , Eskin A , Lu A et al. Online self-report data for duchenne muscular dystrophy confirms natural history and can be used to assess for therapeutic benefits. PLoS Currents. 2014;6. |
[37] | Kishnani PS , Amartino HM , Lindberg C , Miller TM , Wilson A , Keutzer J et al. Timing of diagnosis of patients with Pompe disease: data from the Pompe registry. American Journal of Medical Genetics Part A. (2013) ;161: :2431–2443. |
[38] | Pastorello E , Cao M , Trevisan CP . Atypical onset in a series of 122 cases with FacioScapuloHumeral Muscular Dystrophy. Clinical Neurology and Neurosurgery. (2012) ;114: :230–234. |
[39] | Marwaha S , Knowles JW , Ashley EA . A guide for the diagnosis of rare and undiagnosed disease: beyond the exome. Genome Medicine. (2022) ;14: :1–22. |
[40] | Tumiene B , Graessner H . Rare disease care pathways in the EU: from odysseys and labyrinths towards highways. Journal of Community Genetics. (2021) ;12: :231–239. |
[41] | EURO-NMD. FSHD European Trial Network 2021 [cited 30/09/2022];Available from: https://ern-euro-nmd.eu/fshdeuropean-trial-network/. |
[42] | Schipper K , Bakker M , Abma T . Fatigue in facioscapulohumeral muscular dystrophy: a qualitative study of people’s experiences. Disability and Rehabilitation. (2017) ;39: :1840–1846. |
[43] | Voet N , Bleijenberg G , Hendriks J , de Groot I , Padberg G , van Engelen B et al. Both aerobic exercise and cognitive-behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology. (2014) ;83: :1914–1922. |
[44] | Veenhuizen Y , Cup EH , Jonker MA , Voet NB , van Keulen BJ , Maas DM et al. Self-management program improves participation in patients with neuromuscular disease: A randomized controlled trial. Neurology. (2019) ;93: :e1720–e1731. |
[45] | Verhaart IE , Johnson A , Thakrar S , Vroom E , De Angelis F , Muntoni F et al. Muscle biopsies in clinical trials for Duchenne muscular dystrophy–Patients’ and caregivers’ perspective. Neuromuscular Disorders. (2019) ;29: :576–584. |
[46] | Peterson IS , Mazzella AJ , Belter LT , Curry MA , Cruz RE , Jarecki J The Cure SMA Clinical Trial Experience Survey: A Study of Trial Participant Perspectives on Clinical Trial Management and Patient-Centric Management Practices. Neurology and Therapy. 2022:1-15. |
[47] | Wang LH , Friedman SD , Shaw D , Snider L , Wong C-J , Budech CB et al. MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD. Human Molecular Genetics. (2019) ;28: :476–486. |
[48] | Mellion ML , Widholm P , Karlsson M , Ahlgren A , Tawil R , Wagner KR et al. Quantitative muscle analysis in FSHD using whole-body fat-referenced MRI: composite scores for longitudinal and cross-sectional analysis. Neurology. (2022) ;99: :e877–e889. |
[49] | Monforte M , Attarian S , Vissing J , Diaz-Manera J , Tasca G 265th ENMC international workshop: Muscle imaging in Facioscapulohumeral Muscular Dystrophy (FSHD): Relevance for clinical trials. 22–24 April 2022, Hoofddorp, The Netherlands. Neuromuscular Disorders. 2022. |




