Age Related Burden of Swallowing in Adult Patients Affected by Duchenne Muscular Dystrophy
Abstract
Background:
In Duchenne muscular dystrophy (DMD), dysphagia is a common but often overlooked symptom, which may affect quality of life (QoL). Its possible causes are progressive deterioration of muscle groups involved in swallowing function (oropharyngeal, inspiratory muscles) or impairment of autonomic function.
Objectives:
In adult patients with DMD, we aimed to identify predictors of swallowing-related QoL and to compare swallowing-related QoL at different ages.
Methods:
Forty-eight patients aged 30.0±6.6 years were enrolled. Questionnaires were administered: the Swallowing Quality of Life questionnaire (SWAL-QOL) for swallowing-related QoL assessment, and the Compass 31 for autonomic symptoms assessment. The Brooke Upper Extremity Scale was used for upper limbs muscular function assessment. Respiratory and muscle function tests were performed, including spirometry, arterial blood gases, polysomnography, maximal inspiratory pressure (MIP), maximal expiratory pressure and sniff nasal inspiratory pressure.
Results:
An abnormal composite SWAL-QOL score (≤86) was found in 33 patients. Autonomic symptoms were mild, while a severe impairment was shown by the Brooke Upper Extremity Scale. Spirometry and muscle strength tests demonstrated severe alterations, while diurnal and nocturnal blood gases were normal, due to effective use of noninvasive ventilation. Independent predictors of the composite SWAL-QOL score were age, MIP and Compass 31. A MIP < 22 had an accuracy of 92% in predicting altered swallowing-related QoL. The composite SWAL-QOL score was worse in subjects > 30 years old than in younger patients (64.5±19.2 vs 76.6±16.3, p < 0.02), due to worse scores in items pertinent to mental and social functioning; scores in domains pertinent to the physical function were similar in both groups.
Conclusions:
In adult DMD, swallowing-related QoL, which is altered in most patients, can be predicted by age, inspiratory muscles strength and autonomic dysfunction symptoms. While swallowing function is already altered in young patients, swallowing-related QoL can progressively worsen with advancing age due to psychological and social factors.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a progressive disease due to absence of dystrophin, which involves striated and smooth muscles. Muscle dysfunction leads to several problems, including motor impairment, respiratory failure and loss of independence. In patients with DMD, quality of life (QoL) scales have shown variable degrees of satisfaction. Indeed, while motor impairment negatively affects well-being, the psycho-social sphere is often preserved [1–4]. However, other poorly considered factors may contribute to reduce QoL in this disease.
Dysphagia can be an important component of the symptomatology of patients with DMD, although it is often overlooked [5, 6]. In addition to swallowing disturbances, patients with DMD often complain symptoms of gastrointestinal dysfunction, such as gastric dilatation and intestinal pseudo-obstruction, [7–9].
In DMD, dysphagia is considered mainly a consequence of the impairment of oropharyngeal striated muscles function, which leads to slower and effortful bolus transportation [5]. Absence of dystrophin in gastro-intestinal smooth muscles and dysfunction of the enteric autonomic nervous system may also contribute, as suggested by studies in mice models of DMD [10, 11]. Furthermore, altered autonomic control in the gastroenteric duct, which was demonstrated by the Compass 21 questionnaire [4], could also play a role. Some studies observed a relationship between swallowing performance and respiratory function, as assessed by common respiratory tests [12, 13], or by the severity of ventilator-dependence [14]. Since the alterations responsible for dysphagia in DMD worsen over time, it is conceivable that older DMD patients complain worse dysphagia symptoms, which may be associated to a deterioration of QoL.
To our knowledge, only one study evaluated how dysphagia impairs QoL in adult DMD patients [15]. In that study, the Sydney Swallow Questionnaire (SSQ) was used, which demonstrated to satisfactorily detect dysphagia in subjects with DMD. However, the study did not include patients older than 26. Today, thanks to current management methods, most patients affected by DMD can survive long beyond that age, so that there is a gap in our knowledge about QoL in older DMD patients. Besides, data from a recent review suggest that for the assessment of the impact of dysphagia on QoL the Swallowing Quality of Life questionnaire (SWAL-QOL) should be preferred over other tools. This indication was based on psychometric evaluation and clinical utility, including adherence to the WHO ICF framework [16]. The SWAL-QOL probes for the body function and structure across multiple stages of swallowing, such as oral, pharyngeal and esophageal [17, 18].
In this study, we administered the SWAL-QOL to adult patients with DMD. The aims were: 1) to describe swallowing-related QoL in this category of patients; 2) to identify potential independent predictors of its impairment; 3) to evaluate the functional impact of swallowing disorders on QoL at different ages.
PATIENTS AND METHODS
This was a prospective, cross-sectional study. We recruited consecutive adult patients with diagnosis of DMD, defined by clinical and genetic criteria, who came to the regional center for respiratory complications of neuromuscular diseases of Villa Sofia-Cervello hospital between 2017 and 2020 for a periodic follow-up evaluation. Criteria of inclusion were age≥18 years and ability to perform a complete battery of tests of respiratory muscle strength. In all patients, dysphagia related QoL, as well as autonomic function impairment, were assessed by questionnaires. Questionnaires were self-administered or were answered with the help of a trained interviewer. Upper limb muscle function was assessed according to standard criteria. Finally, both diurnal and nocturnal respiratory function were evaluated. A clinician especially skilled in neuromuscular disorders was in charge of the functional assessment of the patients (GC). The study was approved by the local ethical committee (Palermo 2 verb 14, prot. amm.vo 325 AOR 05.10.2016) and all patients gave informed consent.
Dysphagia related quality of life assessment
The impact of dysphagia on QoL was evaluated by the SWAL-QOL questionnaire [19]. The first thirty items of the questionnaire are used to assess ten quality of life concepts, seven of which are dysphagia-related (food selection, burden, mental health, social functioning, fear, eating duration, eating desire) and three pertain to general QoL (communication, sleep and fatigue). The questions are intended to reflect experience within the preceding month and are scored on a 5-point Likert scale, which can be transformed to achieve scores ranging from 0 (least favorable state) to 100 (most favorable state). A composite SWAL-QOL score can be derived by averaging the ten scale scores. As a clinical cut-off score to identify individuals with significantly altered QoL, previous research suggested a decrease≥14 points from the maximum SWAL-QOL composite score (100 points) [20]. The SWAL-QOL also includes a symptom frequency battery of 14 questions (DSB, dysphagia symptoms battery), which has been used as an of index dysphagia status. Possible responses range from 0 to 100, where 100 is the best score. Responses to all items are averaged to obtain a mean score.
The SWAL-QOL has been used in patients affected by neuromuscular disease but no one of these patients was affected by DMD [21]. Therefore, in the patients of this study we tested its reliability with the evaluation of internal consistency and test-retest reproducibility when administered two weeks apart. This period is considered adequately long to prevent recall, but short enough to expect no clinical change to occur [19]. No participant underwent a therapeutic intervention for dysphagia either before or between the questionnaire administrations.
Autonomic symptoms assessment
Autonomic symptoms were evaluated by the Compass 31 questionnaire [22]. This is a self-assessment instrument exploring six domains of autonomic function: orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder and pupillomotor function. Altogether, the questionnaire consists of 31 items, 12 of which pertain to the gastrointestinal domain. Score in the latter domain can range between 0 and 24. The final Compass 31 score derives from the sum of the scores of the six domains, and can range between 0 and 100. Higher scores indicate worse autonomic symptoms.
Upper limbs muscular function assessment
Muscular function of the upper limbs was evaluated by the Brooke Upper Extremity Scale. This is a 6-point scale that allows classification of upper extremity muscular function. One is the best score, indicating that the patient is able to start with arms at the sides and can abduct the arms in a full circle until they touch above the head, while 6 corresponds to the worst score, indicating inability to raise hands to the mouth and absence of any useful function of the hands [23].
Respiratory function assessment
Respiratory muscle strength was assessed by maximal static inspiratory pressure (MIP), maximal expiratory pressure (MEP) and sniff nasal inspiratory pressure (SNIP). Prior to each test, participants were given detailed instructions and a demonstration of the procedure by the examiner. MIP was measured following maximal inspiration from residual volume. MEP was obtained through maximal expiratory effort from total lung capacity. For both measurements, the highest value obtained with three acceptable manoeuvres from at least five attempts was selected [24]. SNIP was evaluated according to standardized methodology [25]. Spirometry was performed in a sitting position with a flow meter attached to a flanged rubber mouthpiece with the nose occluded in accordance with American Thoracic Society/ European Respiratory Society recommendations [26]. Arterial blood gases were measured early in the morning in the supine position during administration of noninvasive ventilation (NIV).
To complete the follow-up evaluation, patients were submitted to in-hospital nocturnal polysomnography (PSG) (SomnoLab 2 AASM, Weinmann, Hamburg, Germany) with simultaneous transcutaneous CO2 (PtcCO2) monitoring during administration of NIV as previously prescribed [4]. Sleep was scored according to AASM rules [27].
Statistics
Data are presented as mean±standard deviation, or median (interquartile range). The Kolmogorov-Smirnov test was used to test the data for normal distribution.
Internal consistency of the SWAL-QOL was determined by Cronbach’s α coefficient. A coefficient > 0.70 was taken as acceptable. Test-retest reproducibility was assessed by intraclass correlation coefficient (ICC) using a 2-way random effect model with 95% confidence intervals (C.I.). An ICC > 0.70 indicated sufficient test-retest reproducibility.
Univariate correlations were analyzed using Pearson’s correlation coefficient. To identify potential independent predictors of the composite SWAL-QOL score, a stepwise regression model was used. In this regression, all variables that were correlated with the composite score with a p-value<0.10 were entered. The optimal cut-off value to predict an altered dysphagia– related QoL (composite SWAL-QOL≤86) and its diagnostic accuracy were evaluated by receiver operating characteristics (ROC) curve analysis.
The sample was divided according to age < or≥30 years. Unpaired t-test and U-Mann-Whitney test were used for comparisons between normally and non-normally distributed data, respectively.
A p < 0.05 was considered significant. Statistical analysis was performed using a commercial software package (IBM SPSS v. 22 and MedCal v. 20.115).
RESULTS
Forty-eight subjects met the inclusion criteria. In these patients, all items of the questionnaires were completed without missing data. Mean age of the patients was 30.0±6.6 years. Body mass index was 19.7±5.8 kg/m2.
The average composite SWAL-QOL score in the sample was 71.1±18.5. It was below the threshold of 86 in 33/48 patients. The mean DSB in the sample was 72.3±19.6. The Compass 31 score was 12.5±10.5, while gastrointestinal symptoms score was 5.8±3.9. The Brooke UES score was 5.4±0.5.
Characteristics of muscular function, diurnal and nocturnal respiratory function and sleep structure of the patients are shown in Table 1. Twelve patients used NIV only at night (≤12 hours/day), while the remaining 36 patients used it also during the day (>12 hours/day). All patients made use of a wheelchair and of a cough assist device.
Table 1
Respiratory function and sleep structure*
| FVC (% predicted) | 20.4±14.3 |
| MIP (cmH2O) | 17.9±12.6 |
| MEP (cmH2O) | 15.1±10.1 |
| SNIP (cmH2O) | 21.0±11.1 |
| PCF (L/min) | 134.8±61.6 |
| pH | 7.40±0.04 |
| PaO2 (mmHg) | 93.4±18.7 |
| PaCO2 (mmHg) | 42.6±7.5 |
| HCO3-(mmol/L) | 26.5±3.7 |
| BE (mmol/L) | 2.1 [0.2-4.3] |
| Mean SpO2 (%) | 97.2±1.0 |
| ODI (n/h) | 0.2 [0.0-1.0] |
| Nadir (%) | 88.8±3.2 |
| T 90 (min) | 0.0 [0.0-0.7] |
| mean nocturnal PtcCO2 (mmHg) | 38.0±6.2 |
| peak nocturnal PtcCO2 (mmHg) | 44.4±5.3 |
| TST (min) | 349.4±68.4 |
| SE (% time in bed) | 73.6±14.5 |
| SL (min) | 36.1±24.6 |
| WASO (min) | 48.7±31.7 |
| N1 (% TST) | 15.5±8.4 |
| N2 (% TST) | 50.3±9.0 |
| N3 (% TST) | 17.6±7.8 |
| R (% TST) | 16.6±7.3 |
| Arousals (n/h) | 17.0±6.2 |
FVC, forced vital capacity; MIP, maximal inspiratory pressure; SNIP, sniff nasal pressure; MEP, maximal expiratory pressure; PCF, peak cough flow; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of CO2; HCO3–,bicarbonates; BE, base excess; SpO2, pulse oxygen saturation; ODI, number of oxygen desaturation ≥ 3% per hour of sleep; Nadir, lowest oxygen saturation; T90, time spent with oxygen saturation below 90%; PtcCO2, transcutaneous PCO2; TST, total sleep time; SE%, sleep efficiency; SL, sleep latency; WASO, wake after sleep onset; N1, NREM stage 1; N2, NREM stage 2; N3, NREM stage 3; R, REM stage. *Arterial blood gases and polysomnography were performed in 45 subjects.
All participants completed the test-retest assessment of the composite SWAL-QOL. The Cronbach’s α coefficient was 0.87 (95% lower CI 0.82) and ICC was 0.99 (95% lower CI: 0.98), indicating good internal consistency and reproducibility.
Table 2 shows univariate correlations between the composite score of the SWAL-QOL and other variables. Age, spirometry and respiratory muscle function indices, as well as the Compass 31 score, were significantly correlated to the composite SWAL-QOL score, whereas arterial blood gases and PSG indices were not.
Table 2
Univariate correlations between composite SWAL-QOL score and other variables
| Composite score | p | |
| Age | -0.50 | 0.0003 |
| FVC (% predicted) | 0.60 | <0.0001 |
| MIP (cmH2O) | 0.71 | <0.0001 |
| MEP (cmH2O) | 0.65 | <0.0001 |
| SNIP (cmH2O) | 0.44 | 0.006 |
| PCF (L/min) | 0.31 | 0.03 |
| PaO2 (mmHg) | 0.14 | 0.35 |
| PCO2 mmHg | -0.20 | 0.17 |
| HCO3– (mmol/L) | -0.15 | 0.32 |
| BE (mmol/L) | -0.15 | 0.31 |
| Mean SpO2 (%) | 0.20 | 0.18 |
| ODI | 0.14 | 0.34 |
| Nadir (%) | -0.18 | 0.21 |
| T90 (min) | 0.23 | 0.12 |
| mean nocturnal PtcCO2 mmHg | -0.31 | 0.03 |
| peak nocturnal PtcCO2 mmHg | -0.16 | 0.26 |
| TST (min)* | -0.11 | 0.46 |
| SE (%) | -0.15 | 0.30 |
| SL (min) | 0.19 | 0.19 |
| WASO (min) | -0.10 | 0.50 |
| N1 (%) | 0.11 | 0.46 |
| N2 (%) | -0.15 | 0.29 |
| N3 (%) | 0.08 | 0.59 |
| R (%) | -0.14 | 0.34 |
| Arousals (n/h) | -0.17 | 0.25 |
| Compass 31 | -0.51 | 0.0002 |
| Gastrointestinal domain | -0.36 | 0.01 |
FCV, forced vital capacity; MIP, maximal inspiratory pressure; SNIP, sniff nasal pressure; MEP, maximal expiratory pressure; PCF, peak cough flow; PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of CO2; HCO3–, bicarbonates; BE, base excess; SpO2, pulse oxygen saturation; ODI, number of oxygen desaturation≥3% per hour of sleep; Nadir, lowest oxygen saturation; T90, time spent with oxygen saturation below 90%; PtcCO2, transcutaneous PCO2; PtCO2, transcutaneous PCO2; TST, total sleep time; SE%, sleep efficiency; SL, sleep latency; WASO, wake after sleep onset; N1, NREM stage 1; N2 NREM stage2; N3, NREM stage 3; R, stage REM.
Table 3 shows results of multiple regression with the composite SWAL-QOL as dependent variable. We identified MIP, age and Compass 31 as independent predictors.
Table 3
Multiple regression for predictors of composite SWAL-QOL score
| Independent variable | Coefficient | Std Error | t | p |
| (Constant) | 89.1 | |||
| MIP | 0.8006 | 0.1347 | 5.945 | <0.0001 |
| Age | -0.8712 | 0.2422 | -3.597 | 0.0008 |
| Compass 31 | -0.5003 | 0.1569 | -3.189 | 0.002 |
MIP, maximal inspiratory pressure.
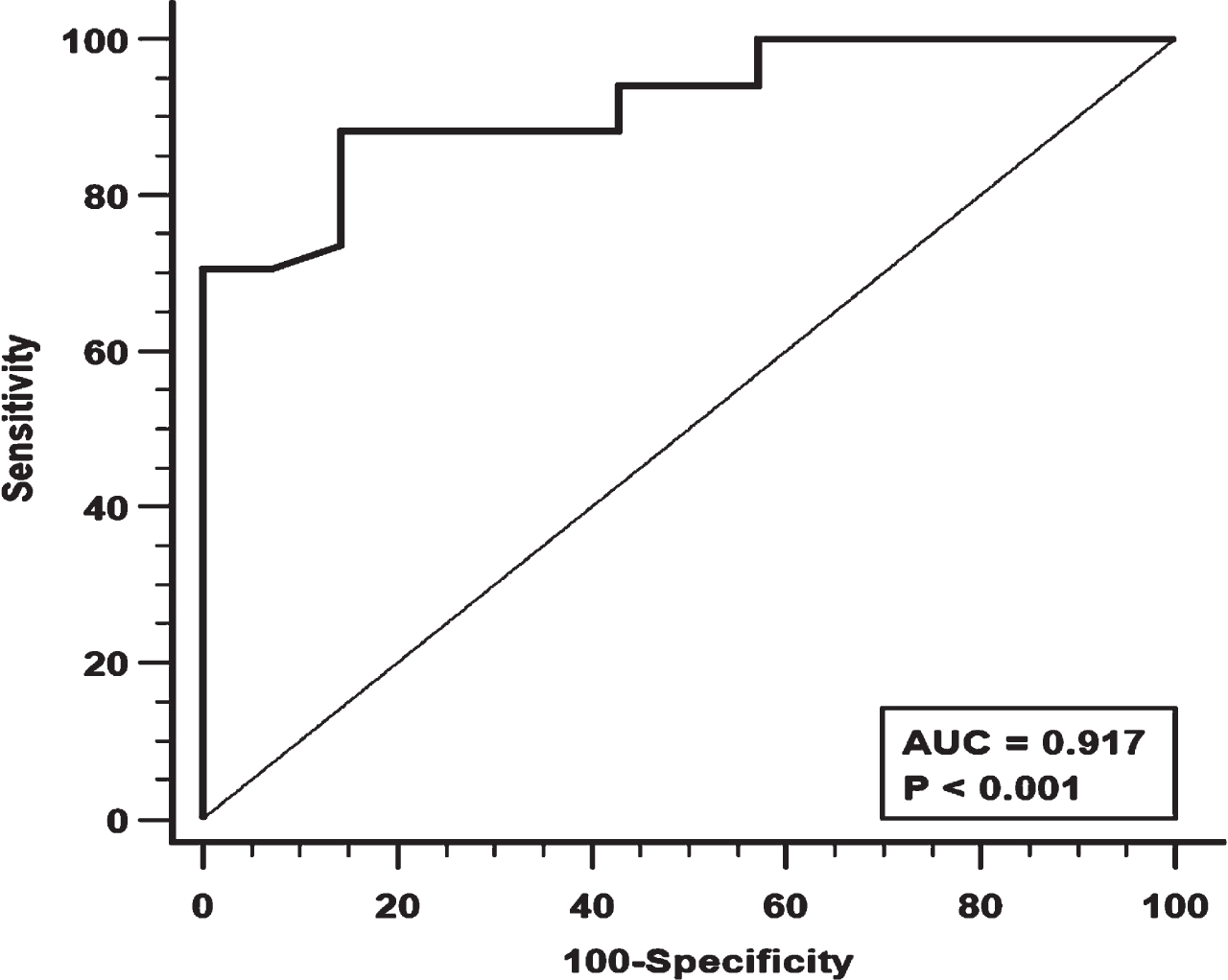
Figure 1 shows the ROC curve for MIP. A MIP value < 22 cmH2O was associated with the largest AUC (0.917) with good sensitivity and specificity (88.2% and 85.7% respectively).
Fig. 1
Receiver Operating Characteristics (ROC) analysis for Maximal Inspiratory Pressure (MIP) and its diagnostic performance. AUC = area under the curve.
Twenty-six patients were < 30 and 22 were≥30 years old. Results of respiratory and muscular function tests were worse in the older subjects, but no significant difference between groups was found in arterial blood gases, PSG indices and autonomic symptoms (Table S1). The composite SWAL-QOL was worse in the older subjects. However, not all items differed significantly between groups: eating duration and food selection, that were already heavily compromised in the younger subjects, marginally differed between younger and older subjects, while other items, especially burden and mental health, were significantly worse in the older patients (Table 4).
Table 4
SWAL-QOL scores in patients < 30 and≥30 years old
| Age<30 (n = 26) | Age≥30 (n = 22) | p | |
| Burden | 82.2±26.9 | 55.6±35.7 | 0.005 |
| Eating duration | 41.3±40.5 | 47.7±46.2 | 0.61 |
| Eating desire | 74.9±15.7 | 74.7±23.1 | 0.96 |
| Food selection | 66.8±34.4 | 54.5±31.9 | 0.20 |
| Fear of eating | 79.3±20.4 | 61.8±25.8 | 0.01 |
| Communication | 82.6±28.9 | 72.7±31.2 | 0.25 |
| Mental health | 89.8±19.6 | 67.9±27.3 | 0.002 |
| Social functioning | 88.6±16.9 | 73.4±28.5 | 0.02 |
| Sleep | 85.0±20.9 | 75.5±18.2 | 0.10 |
| Fatigue | 78.5±23.4 | 62.8±22.8 | 0.02 |
| Composite score | 76.6±16.3 | 64.5±19.2 | 0.02 |
| DBS* | 76.8±17.0 | 67.0±21.6 | 0.08 |
DBS, dysphagia battery score.
Twenty-six of the 36 patients using NIV > 12 hours applied NIV also during their meals. We re-evaluated the composite SWAL-QOL and its predictors in the 22 patients who did not use NIV during mealtime to avoid the possible confounding effect of this therapy. As compared to patients using NIV during their meals, these patients were younger and had better respiratory and muscular function (Table S2). In this group, age no longer predicted the composite SWAL-QOL score, while MIP remained the only independent predictor (Table S3).
DISCUSSION
This study was performed on a relatively large number of adult patients with DMD, including a high proportion of subjects over the age of 30. The main findings were as follows: 1) An altered swallowing-related QoL was present in most patients; 2) Age, inspiratory muscle strength and autonomic symptoms independently predicted degree of impairment of swallowing-related QoL; 3); QoL aspects related to the mental and social sphere were significantly worse in subjects≥30 years old, whereas those more closely related to physical impairment were heavily compromised already at young ages and barely differed between younger and older subjects.
Although dysphagia is a common symptom in DMD, to our knowledge the SWAL-QOL questionnaire has been used in other neuromuscular diseases [28], but not specifically in DMD. The analysis in our patients showed a good reliability of this questionnaire, supporting its use to identify perturbations in QoL related to the swallowing function even in DMD. We found that swallowing-related QoL is often altered in DMD, confirming the results obtained in a previous study where another questionnaire was used [15]. We identified age, MIP and Compass 31 score as independently related to the outcome of the SWAL-QOL questionnaire.
Our study demonstrated a significant role of age as a predictor of swallowing-related QoL. This is not in agreement with a recent study that found that age was not a determinant of gastrointestinal symptoms in adult patients with DMD [12]. However, that study included patients with a narrow range of age. By contrast, we studied patients in a broad age range and, as a consequence, with highly variable functional impairment, which could allow us to demonstrate a relationship between age and SWAL-QOL scores. Actually, symptoms of dysphagia, which hampered quality of life, were previously reported in a sample of DMD patients much younger than in our study [15]. Hence, dysphagia may already appear in young DMD patients, but its impact on patients’ well-being may progressively worsen with advancing age.
The role of MIP as a predictor of the composite score may be explained considering that the degeneration of inspiratory and oropharyngeal muscle groups proceeds in parallel in the course of the disease, so that inspiratory muscle strength may closely reflect ability in swallowing. Moreover, oropharyngeal muscles, which are the most directly involved in the swallowing process, cooperate with inspiratory muscles when a subject swallows, so that if inspiratory muscles function is compromised, swallowing is affected, too. A previous study found a relationship between swallowing impairment, as assessed by an 8-stage scale, and respiratory function, as assessed by FVC, but in this study respiratory muscle strength was not evaluated [14]. In our study, MIP predicted swallowing impairment better than FVC. In fact, FVC reflects functional properties of the respiratory system, not all of which are related to swallowing. Instead, MIP is only relevant to the strength of inspiratory muscles. In agreement with our finding, in a previous study MIP was found to be associated with swallowing ability and specifically with the number of swallows per bolus and time of swallowing [29]. In common clinical practice, the SWAL-QOL questionnaire is rarely administered because it is considered burdensome. This may lead to neglect the impact of swallowing-related disorders on QoL. The assessment of MIP in DMD may be clinically relevant not only to evaluate the degree of impairment of inspiratory muscles function, but also to estimate the possible impact of swallowing problems on QoL. According to our results, a cut off of MIP < 22 cmH2O is associated with an increased risk of an impact of dysphagia on QoL.
Autonomic impairment, as resulting from the Compass 31 questionnaire, was the last independent predictor of composite SWAL-QOL. We and other authors have already described an altered autonomic function in DMD [4, 30, 31]. Gastrointestinal and secretomotor subdomains were the most heavily affected [4]. Furthermore, esophageal dysphagia and early satiety may be prominent symptoms in some DMD patients [32, 33], possibly as a consequence of gastroparesis due to autonomic dysfunction. Through these effects, autonomic impairment may considerably contribute to harm QoL.
When we divided our sample into a group < 30 and a group≥30 years old, we found that various aspects gave a different contribution to the worse QoL in the older patients. The most prominent differences were found in items reflecting mental and psychological aspects of QoL, while items more closely related to physical dysfunction, like eating duration and food selection, were already severely affected in younger patients and did not differ between groups. A longer duration of eating may be linked to an ineffective bolus propulsion, which generates frequent swallows or ‘piecemeal deglutition’. A long duration of meals was also observed in a previous study [15] where the age of the patients was lower than in our study. These findings suggest that muscular and, possibly, autonomic dysfunction are already enough to cause significant dysphagia in young adult patients with DMD. However, in these subjects they mildly affect the psychological and social spheres. The latter make a much greater contribution to worsening QoL in older subjects. It remains to be established to what extent the worse psychological attitudes of the older patients may depend on the persistence of swallowing disorders throughout their life, and how much other factors may contribute.
It has been suggested that NIV during meals may reduce dysphagia [34], as it can improve breathing and swallowing coordination. Then, we re-evaluated predictors of the composite SWAL-QOL after excluding patients using NIV during meals from our sample. The remaining patients had a narrow age range, so that the effect of age was no longer evident. However, the independent effect of MIP was confirmed.
This study has several strengths. We used a questionnaire that is considered the gold standard to evaluate the impact of swallowing dysfunction on QoL. Besides, the sample of DMD patients we studied can be considered large proportionally to the low prevalence of the disease, and included subjects in a wide age range. Therefore, data could be generalized to DMD patients with similar characteristics that receive the same level of healthcare. The large proportion of relatively old patients allowed us to assess effects of advancing age and to recognize what might make QoL worse in older DMD patients. However, a limitation of the study was that only cross-sectional data were collected so that the role of age in our findings was not deduced from longitudinal observations, but from comparisons between groups of patients of different ages.
In conclusion, swallowing-related QoL is impaired in most adult subjects with DMD. In these patients, age, degree of weakness of inspiratory muscles and symptoms of autonomic dysfunction predict swallowing-related QoL. Since MIP closely reflects discomfort associated with swallowing dysfunction, its alteration may lead to suspect deglutition problems. Swallowing-related QoL is worse in older patients, mainly due to psychological and social factors. Management and specifically designed programs of rehabilitation should address with greater care dysphagia to mitigate deterioration of QoL in DMD. The use of the SWAL-QOL, in addition to more commonly used tests, may lead to a more comprehensive evaluation of QoL in patients with this disease.
ACKNOWLEDGMENTS
We thank R. Di Pietro, president of the Sicilian Section of Italian Association of Glycogenosis and A.M. Franzoni, president of the Sicilian Section of Autoimmune Rare Disease, for their unrelenting support. We also thank the Italian Union Against Muscular Dystrophy, Section of Palermo, for providing funds to support this work.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230055.
REFERENCES
[1] | Kohler M , Clarenbach CF , Böni L , Brack T , Russi EW , Bloch KE Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med. (2005) ;172: (8):1032–6. |
[2] | Travlos V , Patman S , Wilson A , Simcock G , Downs J Quality of Life and Psychosocial Well-Being in Youth With Neuromuscular Disorders Who Are Wheelchair Users: A Systematic Review. Arch Phys Med Rehabil. (2017) ;98: (5):1004–1017.e1. doi: 10.1016/j.apmr.2016.10.011. |
[3] | Pangalila RF , van den Bos GA , Bartels B , Bergen MP , Kampelmacher MJ , Stam HJ , Roebroeck ME Quality of life of adult men with Duchenne muscular dystrophy in the Netherlands: implications for care. J Rehabil Med. (2015) ;47: (2):161–6. doi: 10.2340/16501977-1898. |
[4] | Crescimanno G , Greco F , D’Alia R , Messina L , Marrone O Quality of life in long term ventilated adult patients with Duchenne muscular dystrophy. Neuromuscul Disord. (2019) ;29: (8):569–575. doi: 10.1016/j.nmd.2019.06.599. |
[5] | Toussaint M , Davidson Z , Bouvoie V , Evenepoel N , Haan J , Soudon P Dysphagia in Duchenne muscular dystrophy: practical recommendations to guide management. Disabil Rehabil. (2016) ;38: (20):2052–62. doi: 10.3109/09638288.2015.1111434. |
[6] | Audag N , Toussaint M , Liistro G , Vandervelde L , Cugy E , Reychler G European Survey: Dysphagia Management in Patients with Neuromuscular Diseases. Dysphagia. (2022) ;37: (5):1279–1287. doi: 10.1007/s00455-021-10392-3. |
[7] | Lo Cascio CM , Goetze O , Latshang TD , Bluemel S , Frauenfelder T , Bloch KE Gastrointestinal Dysfunction in Patients with Duchenne Muscular Dystrophy. PLoS One. (2016) 11: (10):e0163779. doi: 10.1371/journal.pone.0163779. |
[8] | Leon SH , Schuffler MD , Kettler M , Rohrmann CA Chronic intestinal pseudoobstruction as a complication of Duchenne’s muscular dystrophy. Gastroenterology. (1986) ;90: (2):455–9. doi: 10.1016/0016-5085(86)90948-0. |
[9] | Vianello A , Arcaro G , Ferrarese S , Molena B , Giraudo C Acutecolonic pseudo-obstruction causing Acute Respiratory Failure inDuchenne Muscular Dystrophy. Pulmonology. (2021) ;27: (3):273–276. doi: 10.1016/j.pulmoe.2020.04.018. |
[10] | Lazzarin MC , de Oliveira F Consequences of Duchenne Muscular Dystrophy in gastrointestinal tract. Adv Res Gastroenterol Hepatol. (2018) ;10: (2):555782. |
[11] | Vannucchi MG , Corsani L , Giovannini MG , Faussone-Pellegrini MS Expression of dystrophin in the mouse myentericneurones. Neurosci Lett. (2001) ;300: (2):120–4. doi: 10.1016/s0304-3940(01)01555-5. |
[12] | Lee JW , Oh HJ , Choi WA , Kim DJ , Kang SW Relationship between Eating and Digestive Symptoms and Respiratory Function in Advanced Duchenne Muscular Dystrophy Patients. J Neuromuscul Dis. (2020) ;7: (2):101–107. doi: 10.3233/JND-190435. |
[13] | Fayssoil A , Chaffaut C , Prigent H , Laforet P , Clair B , Orlikowski D , et al Nutritional status, swallowing disorders, and respiratory prognosis in adult Duchenne muscular dystrophy patients. Pediatr Pulmonol. (2021) ;56: (7):2146–2154. doi: 10.1002/ppul.25430. |
[14] | Yamada Y , Kawakami M , Wada A , Fukui S , Haruyama K , Otsuka T , Liu M Long-term follow-up of dysphagia in adult patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. ((2018) ) 22: (5):786–790. doi: 10.1016/j.ejpn.2018.06.004. |
[15] | Archer SK , Garrod R , Hart N , Miller S Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. Int J Lang Commun Disord. (2013) ;48: (2):240–6. doi: 10.1111/j.1460-6984.2012.00197.x. |
[16] | Keage M , Delatycki M , Corben L , Vogel A A systematic review of self-reported swallowing assessments in progressive neurological disorders. Dysphagia. (2015) ;30: (1):27–46. doi: 10.1007/s00455-014-9579-9. |
[17] | McHorney CA , Bricker DE , Kramer AE , Rosenbek JC , Robbins J , Chignell KA , et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. (2000) ;15: (3):115–21. doi: 10.1007/s004550010012. |
[18] | McHorney CA , Robbins J , Lomax K , Rosenbek JC , Chignell K , Kramer AE , et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. (2002) ;17: (2):97–114. doi: 10.1007/s00455-001-0109-1. |
[19] | Rinkel RN , Verdonck-de Leeuw IM , Langendijk JA , van Reij EJ , Aaronson NK , Leemans CR The psychometric and clinical validity of the SWAL-QOL questionnaire in evaluating swallowing problems experienced by patients with oral and oropharyngeal cancer. Oral Oncol. (2009) ;45: (8):e67–71. doi: 10.1016/j.oraloncology.2009.03.003. |
[20] | Plowman-Prine EK , Sapienza CM , Okun MS , Pollock SL , Jacobson C , Wu SS , et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. (2009) ;24: (9):1352–8. doi: 10.1002/mds.22617. |
[21] | Youssof S , Romero-Clark C , Warner T , Plowman E Dysphagia-related quality of life in oculopharyngeal muscular dystrophy: Psychometric properties of the SWAL-QOL instrument. Muscle Nerve. (2017) ;56: (1):28–35. doi: 10.1002/mus.25441. |
[22] | Sletten DM , Suarez GA , Low PA , Mandrekar J , Singer W COMPASS arefined and abbreviated Composite Autonomic Symptom Score. Mayo ClinProc. (2012) ;87: (12):1196–201. doi: 10.1016/j.mayoc2012.10.013. |
[23] | Hiller LB , Wade CK Upper extremity functional assessment scales in children with Duchenne muscular dystrophy: a comparison. Arch Phys Med Rehabil. (1992) ;73: (6):527–34. |
[24] | Evans JA , Whitelaw WA The assessment of maximal respiratory mouth pressures in adults. Respir Care. (2009) ;54: (10):1348–59. |
[25] | Lofaso F , Nicot F , Lejaille M , Falaize L , Louis A , Clement A , et al. Sniff nasal inspiratory pressure: what is the optimal number of sniffs? Eur Respir J. (2006) ;27: (5):980–2. doi: 10.1183/09031936.06.00121305. |
[26] | Graham BL , Steenbruggen I , Miller MR , Barjaktarevic IZ , Cooper BG , Hall GL , et al. Standardization of Spirometry Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. (2019) ;200: (8):e70–e88. doi: 10.1164/rccm.201908-1590ST. |
[27] | Berry RB , Quan SF , Abreu AR , et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; (2020) . |
[28] | Argov Z , de Visser M Dysphagia in adult myopathies. Neuromuscul Disord; (2021) Jan;31: (1):5–20. doi: 10.1016/j.nmd.2020.11.001. |
[29] | Terzi N , Orlikowski D , Aegerter P , Lejaille M , Ruquet M , Zalcman G , et al. Breathing-swallowing interaction in neuromuscular patients: a physiological evaluation. Am J Respir Crit Care Med. (2007) ;175: (3):269–76. doi: 10.1164/rccm.200608-1067OC. |
[30] | Inoue M , Mori K , Hayabuchi Y , Tatara K , Kagami S Autonomic function in patients with Duchenne muscular dystrophy. Pediatr Int. (2009) ;51: (1):33–40. doi: 10.1111/j.1442-200X.2008.02656.x. |
[31] | Lanza GA , Dello Russo A , Giglio V , De Luca L , Messano L , Santini C , et al. Impairment of cardiac autonomic function in patients with Duchenne muscular dystrophy: relationship to myocardial and respiratory function. Am Heart J. (2001) ;141: (5):808–12. doi: 10.1067/mhj.2001.114804. |
[32] | Chung BC , Park HJ , Yoon SB , Lee HW , Kim KW , Lee SI , et al. Acute gastroparesis in Duchenne’s muscular dystrophy. Yonsei Med J. (1998) Apr;39: (2):175–9. doi: 10.3349/ymj.1998.39.2.175. |
[33] | Dhaliwal A , Madiraju S , Dhindsa BS , Hassen GW , Rochling FA Gigantic Stomach: A Rare Manifestation of Duchenne Muscular Dystrophy. Cureus. (2019) May 7;11: (5):e4609. doi: 10.7759/cureus.4609. |
[34] | Garguilo M , Lejaille M , Vaugier I , Orlikowski D , Terzi N , Lofaso F , et al. Noninvasive Mechanical Ventilation Improves Breathing-Swallowing Interaction of Ventilator Dependent Neuromuscular Patients: A Prospective Crossover Study. PLoS One. (2016) ;11: (3):e0148673. doi: 10.1371/journal.pone.0148673. |




