Living with Dysphagia: A Survey Exploring the Experiences of Adults Living with Neuromuscular Disease and their Caregivers in the United Kingdom
Abstract
Background:
Dysphagia is common in adults living with neuromuscular disease (NMD). Increased life expectancy, secondary to improvements in standards of care, requires the recognition and treatment of dysphagia with an increased priority. Evidence to support the establishment of healthcare pathways is, however, lacking. The experiences of people living with NMD (pplwNMD) and their caregivers are valuable to guide targeted, value-based healthcare.
Objective:
To generate preliminary considerations for neuromuscular dysphagia care and future research in the United Kingdom, based on the experiences of those living with, or caring for, people with NMD.
Methods:
Two surveys (one for adults living with NMD and dysphagia, and a second for caregivers) were co-designed with an advisory group of people living with NMD. Surveys were electronically distributed to adults living with NMD and their caregivers between 18th May and 26th July 2020. Distribution was through UK disease registries, charity websites, newsletters, and social media.
Results:
Adults living with NMD receive little information or education that they are likely to develop swallowing difficulties. Most respondents report wanting this information prior to developing these difficulties. Difficulties with swallowing food and medication are common in this group, and instrumental assessment is considered a helpful assessment tool. Both adults living with NMD and caregivers want earlier access to neuromuscular swallowing specialists and training in how best to manage their difficulties.
Conclusions:
Improvement is needed in the dysphagia healthcare pathway for adults living with NMD to help mitigate any profound physical and psychological consequences that may be caused by dysphagia. Education about swallowing difficulties and early referral to a neuromuscular swallowing specialist are important to pplwNMD and their caregivers. Further research is required to better understand the experiences of pplwNMD and their caregivers to inform the development of dysphagia healthcare pathways.
INTRODUCTION
Dysphagia is common in adults living with neuromuscular disease (NMD) [1–3]. It can present early in the disease and lead to complications such as dehydration, malnutrition, aspiration pneumonia and difficulty in managing oral medication and secretions [2, 4, 5]. It may also have social and psychological consequences, leading to a negative impact on a person’s quality of life (QoL) [6]. The prevalence of dysphagia in NMD ranges between 30–84% [1, 3, 7, 8]. This wide range of prevalence is due to differences in the type and severity of the NMD, and the method of dysphagia diagnosis. Diseases such as myotonic dystrophy type 1 (DM1) [9], Duchenne muscular dystrophy (DMD) [7, 8], spinal muscular atrophy (SMA) [10, 11], inclusion body myositis (IBM) [12], oculopharyngeal muscular dystrophy (OPMD) [13] and motor neuron disease (MND) [14, 15] are those most commonly reported to cause dysphagia. Improvements in standards of care and life expectancy [16, 17], due to medical and therapeutic advancement, have dictated the need to recognise and treat dysphagia with increased priority, with an emphasis on a proactive approach [18]. This is challenging in an already pressured healthcare system where appointment delays and cancellations exist [19].
The absence of high-quality studies exploring symptoms and management of dysphagia in NMD makes it difficult for healthcare providers to develop care pathways that prioritise the needs of this patient population [20, 21]. As such, the experiences of people living with the disease, and their caregivers [22], are a valuable resource to advocate for targeted, value-based healthcare [23]. Co-development of research resources with patient advisory groups ensures that the aims, methods, and outcomes of research meet the needs of those for whom it is intended to serve [24]. The aim of this study was to understand how people living, and caring for people, with NMD experience dysphagia and how it impacts their lives. It also aimed to understand the priority with which people living with NMD place on the assessment and management of dysphagia. Using the experiences of both adults living with dysphagia due to NMD, and their caregivers, we generate preliminary considerations for neuromuscular dysphagia healthcare and research in the United Kingdom.
MATERIALS AND METHODS
Study design
This cross-sectional study utilised a survey concept, design and dissemination that were co-developed by the lead author and an advisory group of seven people: four living with DM1, one living with IBM and two caregivers for people living with DM1 (pplwDM1). Three advisors had dysphagia diagnosed by a healthcare professional and one experienced symptoms of dysphagia. One advisor with DM1 denied any symptoms of dysphagia. Both caregivers had experience of living with a person with dysphagia.
The advisory group met five times with the lead author between September 2019 and August 2020 (supplementary material 1). The first two meetings were held in person and the latter three were held remotely via Zoom, due to Covid-19. In the initial meeting, members shared their experiences of living, or caring for a person, with NMD and dysphagia. Administrative support from a second person (CM) was provided to ensure all contributions from the advisory group were captured. Experiences were recorded by the lead author using pen and paper, with the support of CM. Thematic analysis [25] was completed by the lead author to identify data-driven themes describing the experiences of the advisory group. At a second meeting, advisors were invited to review the data-driven themes to ensure they accurately reflected what they intended to say. They were also given opportunity to contribute further experience, and offer suggestions on how they felt these themes could be best used to meet the aims of the project. The advisory group felt it paramount to explore the extent to which their experiences reflected those living with NMD across the UK. An online exploratory survey was deemed an appropriate methodology to reach large numbers of people across the UK [26]. Ethical approvals were provided by HRA and Heath and Care Research Wales (HCRW), REC Reference 20/WA/0107.
Survey development
Two surveys were developed: one for people living with NMD (pplwNMD) and dysphagia, and one for caregivers. Co-development of the survey is described in supplementary material 1 (meetings 3-5) and includes question development and design. Each survey was grouped into five sections based on the themes generated by the advisory group: i) neuromuscular and dysphagia symptoms, ii) education and knowledge, iii) assessment and diagnosis, iv) management, and v) impact. The survey for pplwNMD contained 40 questions with 3-15 questions per section. All questions were multiple choice except one, where participants were asked to rank their neuromuscular symptoms.
The survey for caregivers contained 43 questions with 4-16 questions per section. All but three questions were multiple choice. One was free-text and two required participants to rank the neuromuscular symptoms of the person they cared for according to how they affected both the person, and themselves. At the end of each survey, participants were asked to indicate how they had become aware of the survey and given opportunity to comment on any further aspects of swallowing and neuromuscular difficulties they felt were not sufficiently covered. Both surveys are available to view in the supplementary material 2.
Eligibility
At the start of the survey, participants completed a series of screening questions to self-assess their eligibility. Participants confirmed they were i)≥18 years of age, ii) living with a diagnosed neuromuscular condition, and iii) experiencing swallowing difficulties at the time of survey completion. Caregivers confirmed they were i)≥18 years of age, and ii) a current or previous caregiver for a person living with NMD and swallowing difficulties within the last 18 months. Previous caregivers were asked to respond to questions based on the last 6-12 months of being in their caregiver role.
Surveys were developed electronically using Opinio software (https://objectplanet.com/opinio/). Hard copies were available on request. Respondents who did not confirm eligibility criteria were automatically prevented from continuing with the survey. The survey was available in English. Swallowing difficulties included those with a formal diagnosis of dysphagia, plus those experiencing symptoms yet to be confirmed by a healthcare professional. A neuromuscular condition was defined as any disease affecting the peripheral nervous system, the neuromuscular junction, or skeletal muscle. Participants were required to have had their condition confirmed by neurological assessment and asked to disclose any medical diagnosis unrelated to their condition. Participants were requested not to complete the survey more than once and were unable to do so if they logged in from the same device. Survey software enabled participants to complete the survey in instalments by allowing them to save and return to their responses prior to the survey deadline.
Dissemination
Electronic surveys were open for ten weeks from 18th May to 26th July 2020, and distributed via NMD registries for DM1, SMA and FSHD. Neuromuscular charities (Muscular Dystrophy UK, CureDM1, SMAUK, TreatSMA, Pathfinders Neuromuscular Alliance, Lily Foundation, Action Duchenne, Rare Disease UK, Motor Neurone Disease Association, Myositis UK, Independent Living UK, MNDA and the Myotonic Dystrophy Support Group) also disseminated via their websites, newsletters, and social media. Survey links were distributed to clinical-academic professional groups in neuromuscular centres across London, Newcastle, and Oxford for dissemination to colleagues and patients. The project advisory group shared the survey with friends, family, and peers.
Data analysis
Surveys less than 50% complete were excluded from analysis. Descriptive statistics were used to summarise participant demographics. Categorical variables were described with frequencies and percentages. Scores for ranked responses were aggregated to provide an overall rank (1-10) for each sub-domain (1 = highest rank or impact, 10 = lowest rank or impact). Non-responses were defined per question from the analyses. Results were stratified by disease type (DM1, FSHD, SMA and other NMD), based upon the diagnosis provided by participants. Due to the unique and aggressive clinical profile of MND, for which bespoke clinical care recommendations exist [27], responses from participants and caregivers living with MND were analysed and reported separately. For ‘please specify’ and ‘other’ response categories, thematic analysis [25] was used. This involved three authors (JA, AGS, GQ) manually and independently developing data-derived themes, before reaching a consensus on over-arching themes. Where only a small number (<3) of participant responses were provided, quotes are provided verbatim.
RESULTS
Six hundred and fifty-five responses were received (484 from participants living with NMD and 171 from caregivers). Two-hundred and forty-eight surveys were less than 50% complete and excluded from analysis. Forty-three responses were from participants living with MND, or their caregivers, and an additional 55 surveys were excluded for other reasons (Fig. 1). A total of 310 surveys were available for analysis: n = 272 from participants living with NMD (plwNMD) and = 38 from caregivers, as shown in Fig. 1.
Fig. 1
Flow chart of survey exclusion methods.
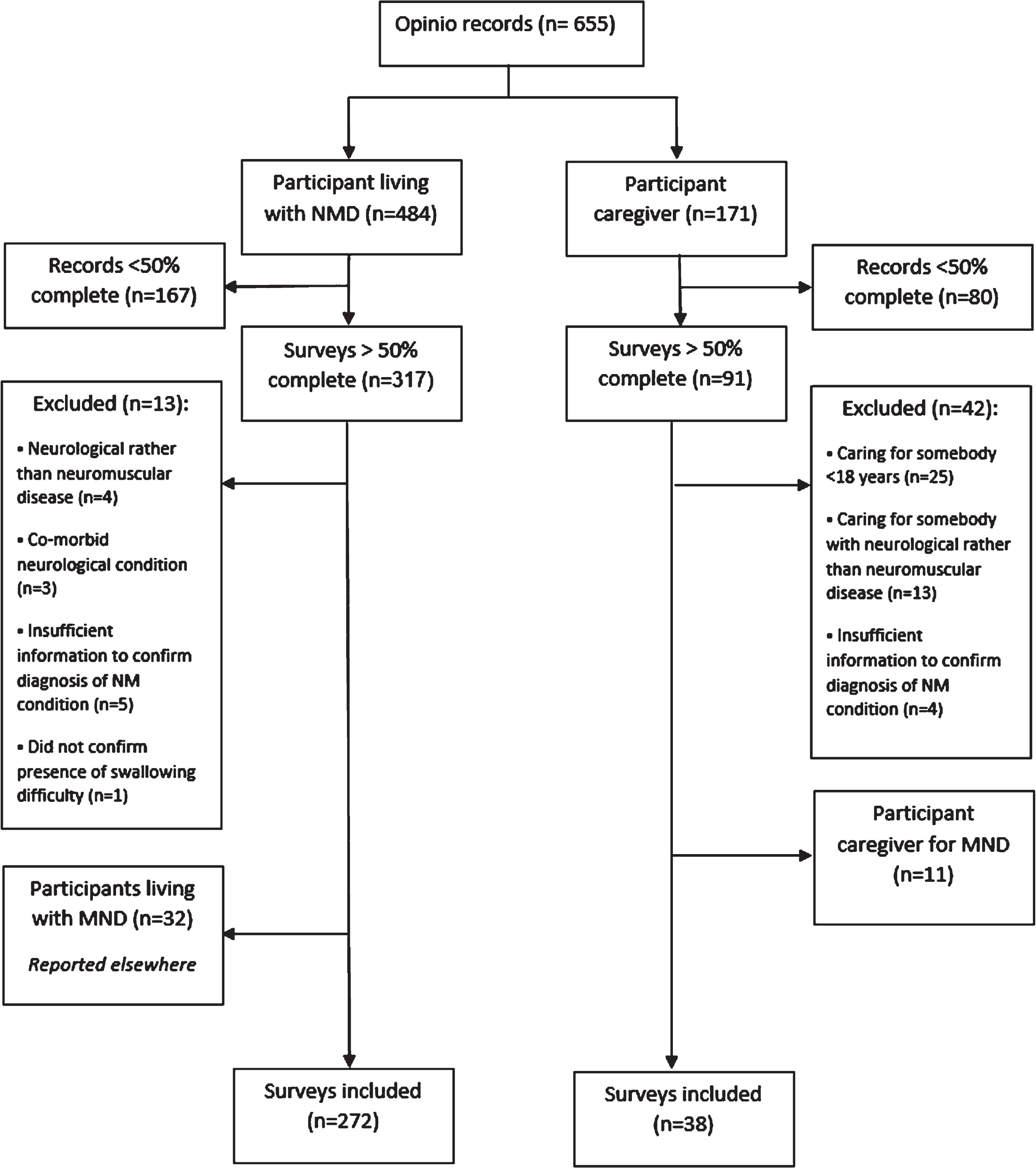
PlwNMD (n = 272) accessed the survey via disease registry (n = 176), charity website or newsletter (n = 42), social media (n = 32), usual healthcare setting or professional (n = 16) or via another method (n = 6). Participant caregivers (pCGs) (n = 38) accessed the survey via charity website or newsletter (n = 14), disease registry (n = 10), social media (n = 6), healthcare setting or professional (n = 4) or a friend or family member (n = 4).
Results are presented separately for plwNMD and pCGs. Each group is sub-divided according to the specific NMD affecting the participant. Whilst this has enabled within-group comparison of survey domains, the reader should exercise caution where comparisons are made between groups due to uneven representation of participant population, small sample size and absence of statistical comparison.
PlwNMD
Population characteristics
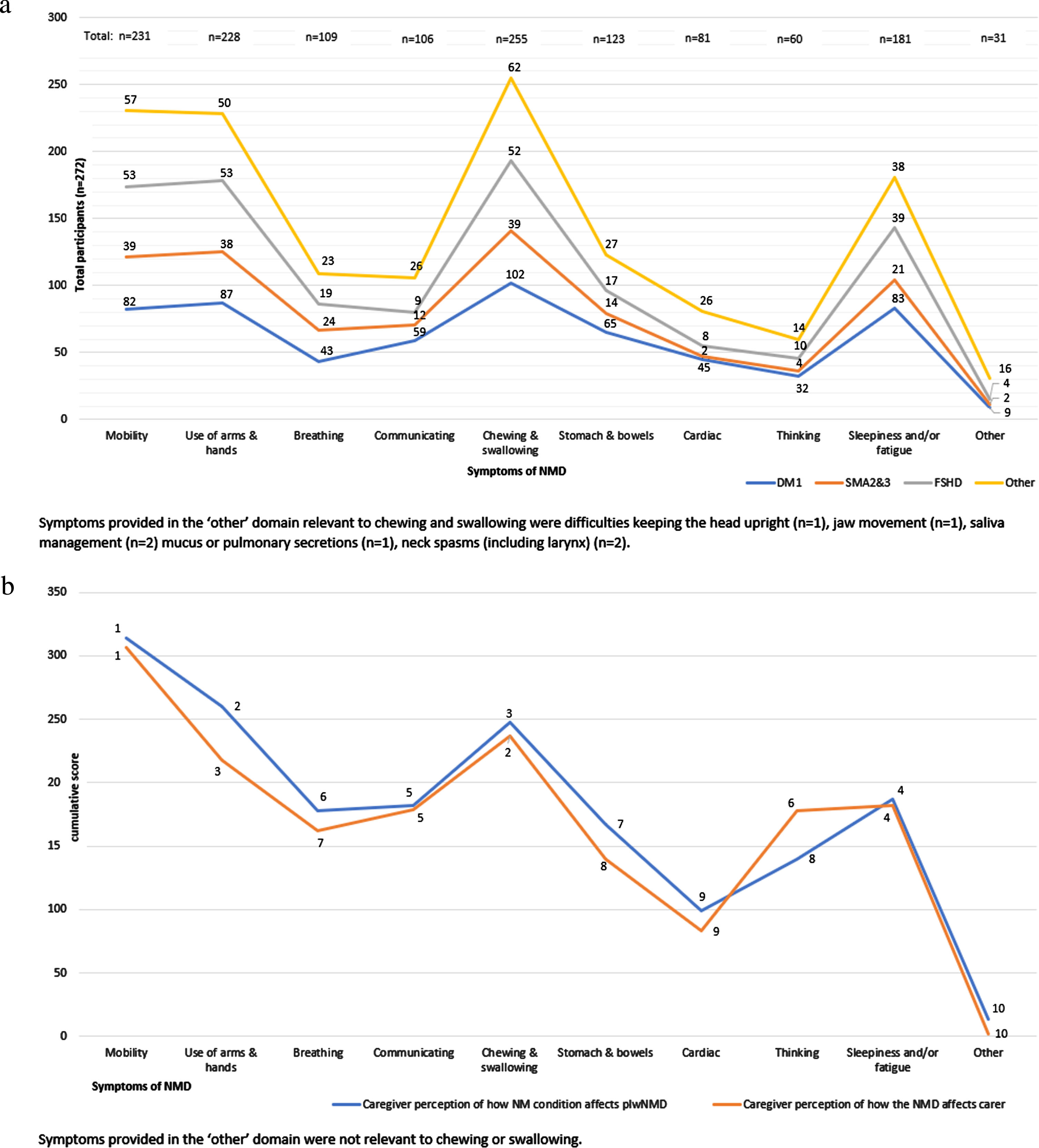
Seventy-six percent (n = 206) of plwNMD either had DM1 (n = 111), FSHD (n = 56) or SMA type 2 or 3 (n = 39). The remaining 24% (n = 66) lived with a variety of neuromuscular conditions detailed in Table 1 (part A). Sixty-one percent (n = 167) of all plwNMD had dysphagia confirmed by a healthcare professional. Participant demographics, including age, method of diagnosis, length of time living with i) NMD and ii) dysphagia, plus additional medical diagnosis are provided in Table 1 (part A) and separated into participants living with DM1 (plwDM1), participants living with FSHD (plwFSHD), participants living with SMA (plwSMA) and participants living with other NMD (plwotherNMD). The neuromuscular profile of participants relative to their dysphagia symptoms is shown in Fig. 2(a). After chewing and swallowing difficulties, most participants were frequently affected by difficulties with mobility (n = 231), use of hands and arms (n = 228), and daytime fatigue or tiredness (n = 181).
Table 1
Demographics of participants living with NMD
| Part A: Demographic information for participants living with neuromuscular disease (plwNMD) | |||||
| DM1 | FSHD | SMA | Other NMD1 | All NMD | |
| (n = 111, 40.8%) | (n = 56, 20.6%) | (n = 39, 14.3%) | (n = 66, 24.3%) | (n = 272) | |
| Participant age | |||||
| 18–30 years | 5 (4.5%) | 3 (5.4%) | 16 (41.0%) | 6 (9.1%) | 30 (11.0%) |
| 31–45 years | 26 (23.4%) | 7 (12.5%) | 9 (23.1%) | 13 (19.7%) | 55 (20.2%) |
| 46–60 years | 53 (47.7%) | 20 (35.7%) | 11 (28.2%) | 21 (31.8%) | 105 (38.6%) |
| 61–75 years | 23 (20.7%) | 25 (44.6%) | 3 (7.7%) | 23 (34.8%) | 74 (27.2%) |
| 76 + years | 4 (3.6%) | 1 (1.8%) | 0 (0%) | 3 (4.5%) | 8 (2.9%) |
| Method of NMD diagnosis | |||||
| Disease standard2 | 89 (80.2%) | 45 (80.4%) | 22 (56.4%) | 49 (74.2%) | 205 (75.4%) |
| Other methods3 | 11 (9.9%) | 9 (16.1%) | 11 (28.2%) | 13 (19.7%) | 44 (16.2%) |
| Unable to recall/unclassified | 11 (9.9%) | 2 (3.6%) | 6 (15.4%) | 4 (6.1%) | 23 (8.5%) |
| Length of time living with symptoms of NMD | |||||
| <12 months | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1–5 years | 7 (6.3%) | 4 (7.1%) | 0 (0.0%) | 6 (9.1%) | 17 (6.3%) |
| 6–15 years | 34 (30.6%) | 7 (12.5%) | 0 (0.0%) | 17 (25.8%) | 58 (21.3%) |
| >15 years | 58 (52.3%) | 28 (50%) | 10 (25.6%) | 29 (43.9%) | 125 (46.0%) |
| Since birth | 11 (9.9%) | 17 (30.4%) | 29 (74.4%) | 14 (21.2%) | 71 (26.1%) |
| Can’t remember | 1 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Healthcare professional responsible for diagnosis of dysphagia | |||||
| GP | 1 (0.9%) | 2 (3.6%) | 0 (0%) | 3 (4.5%) | 6 (2.2%) |
| Neurologist | 8 (7.2%) | 6 (10.7%) | 3 (7.7%) | 10 (15.2%) | 27 (9.9%) |
| Speech &Language Therapist | 56 (50.5%) | 16 (28.6%) | 23 (59.0%) | 37 (56.1%) | 132 (48.5%) |
| Other | 1 (0.9%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) |
| Self-reported | 45 (40.5%) | 31 (55.4%) | 13 (33.3%) | 16 (24.2%) | 105 (38.6%) |
| Length of time participant had experienced symptoms of dysphagia | |||||
| <12 months | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1–5 years | 7 (6.3%) | 4 (7.1%) | 0 (0.0%) | 7 (10.6%) | 18 (6.6%) |
| 6–15 years | 34 (30.6%) | 7 (12.5%) | 0 (0.0%) | 17 (25.8%) | 58 (21.3%) |
| >15 years | 58 (52.3%) | 28 (50%) | 10 (25.6%) | 28 (42.4%) | 124 (45.6%) |
| Since birth | 11 (9.9%) | 17 (30.4%) | 29 (74.4%) | 14 (21.2%) | 71 (26.1%) |
| Not sure/Can’t remember | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) |
| Other relevant medical diagnosis | |||||
| COPD/asthma | 6 (5.4%) | 1 (1.8%) | 1 (2.6%) | 4 (6.1%)4 | 12 (4.4%) |
| Reflux | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | 1 (0.4%) |
| Sleep apnea | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | 1 (0.4%) |
| Depression/anxiety | 4 (3.6%) | 2 (3.6%) | 4 (10.3%) | 2 (3.0%)4 | 12 (4.4%) |
| Other/unrelated | 24 (21.6%) | 36 (64.3%) | 3 (7.8%) | 1 (1.5%) | 64 (23.5%) |
| None | 77 (69.4%) | 17 (30.4%) | 29 (74.4%) | 60 (90.9%)4 | 183 (67.3%) |
| Part B: Demographic information for participant caregivers (pCGs) | |||||
| DM1 | SMA | IBM | Other | All NMD | |
| (n = 24) | (n = 6) | (n = 3) | (n = 6) | (n = 39) | |
| Caregiver age | |||||
| 18-30 years | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| 31-45 years | 2 (8.3%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 3 (7.7%) |
| 46-60 years | 9 (37.5%) | 5 (83.3%) | 0 (0.0%) | 4 (66.7%) | 18 (46.2%) |
| 61-75 years | 12 (50.0%) | 0 (0%) | 2 (66.7%) | 2 (33.3%) | 16 (41.0%) |
| >75 years | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 1 (2.6%) |
| Length of time in caregiving role | |||||
| <12 months | 1 (4.2%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 2 (5.1%) |
| 1-5 years | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| 6-10 years | 4 (16.7%) | 0 (0.0%) | 2 (66.7%) | 0 (0.0%) | 6 (15.4%) |
| Over 10 years | 11 (45.8%) | 1 (16.7%) | 1 (33.3%) | 3 (50%) | 16 (41.0%) |
| Since birth | 6 (25.0%) | 4 (66.7%) | 0 (0.0%) | 3 (50%) | 13 (33.3%) |
| Other | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| Age of person living with NMD | |||||
| 18-30 years | 6 (25.0%) | 5 (83.3%) | 0 (0.0%) | 2 (33.3%) | 13 (33.3%) |
| 31-45 years | 5 (20.8%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 6 (15.4%) |
| 46-60 years | 6 (25.0%) | 1 (16.7%) | 0 (0%) | 2 (33.3%) | 9 (23.1%) |
| 61-75 years | 7 (29.2%) | 0 (0.0%) | 2 (66.7%) | 0 (0.0%) | 9 (23.1%) |
| 76+ | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) | 1 (16.7%) | 2 (5.1%) |
| Method of NMD diagnosis | |||||
| Disease standard* | 19 (79.2%) | 3 (50%) | 3 (100%) | 2 (33.3%) | 27 (69.2%) |
| Other | 5 (20.8%) | 3 (50%) | 0 (0.0%) | 4 (66.7%) | 12 (20.8%) |
| Length of time person had been living with symptoms of NMD | |||||
| <12 months | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1-5 years | 1 (4.2%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 2 (5.1%) |
| 6-15 years | 6 (25.0%) | 0 (0.0%) | 2 (66.7%) | 2 (33.3%) | 10 (25.6%) |
| >15 years | 8 (33.3%) | 3 (50%) | 0 (0.0%) | 1 (16.7%) | 12 (30.8%) |
| Since birth | 9 (37.5%) | 3 (50%) | 0 (0.0%) | 3 (50%) | 15 (38.5%) |
| Can’t remember | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Relationship to person living with NMD | |||||
| Spouse/romantic partner | 13 (54.2%) | 1 (16.7%) | 3 (100%) | 2 (33.3%) | 19 (48.7%) |
| Parent | 4 (16.7%) | 1 (16.7%) | 0 (0.0%) | 2 (33.3%) | 7 (17.9%) |
| Son or daughter | 5 (20.8%) | 4 (66.7%) | 0 (0.0%) | 2 (33.3%) | 11 (28.2%) |
| Sibling | 2 (8.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (5.1%) |
| Friend | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Paid caregiver | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unpaid caregiver | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Healthcare professional responsible for diagnosis of dysphagia | |||||
| GP | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Neurologist | 4 (16.7%) | 2 (33.3%) | 2 (66.7%) | 2 (33.3%) | 10 (25.6%) |
| Speech &Language Therapist | 12 (50%) | 1 (16.7%) | 1 (33.3%) | 2 (33.3%) | 16 (41.0%) |
| Other | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| Self-reported | 7 (29.2%) | 3 (50%) | 0 (0%) | 2 (33.3%) | 12 (30.8%) |
| Other relevant medical diagnosis of person living with NMD | |||||
| COPD/asthma | 1 (4.2% | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 2 (5.1%) |
| Reflux | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Barrett’s oesophagus | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| Sleep apnea | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Depression/anxiety | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other/unrelated | 6 (25.0%) | 0 (0.0%) | 2 (66.7%) | 3 (50.0%) | 11 (28.2%) |
| None | 16 (6.7%) | 6 (100%) | 1 (33.3%) | 2 (33.3%) | 25 (64.1%) |
DM1: Myotonic Dystrophy, FSHD: Facioscapulohumeral Muscular Dystrophy, SMA: Spinal muscular Atrophy, IBM: Inclusion Body Myositis NMD: Neuromuscular disease. 1) Responses included participants living with mitochondrial disease (n = 9), inclusion body myositis (n = 8), Duchenne muscular dystrophy (n = 6), Charcot Marie Tooth (n = 6), those with unconfirmed/unclassified neuromuscular disease (n = 3), myasthenia gravis (n = 5), myotonic dystrophy type 2 (n = 4) and≤2 responses each from participants with oculopharyngeal muscular dystrophy, primary lateral sclerosis, Kennedy’s disease, myopathy, and neuropathy. 2) Genetic testing considered gold standard except for diagnosis of myasthenia gravis, peripheral neuropathy, primary lateral sclerosis, and IBM. 3) Included nerve conduction, muscle biopsy and family history. 4) One participant had both asthma and depression.
Fig. 2
(a): Neuromuscular symptom profile of plwNMD relative to difficulties in chewing and swallowing. (b): Range and impact of neuromuscular impairments ranked 1–10 as perceived by pCGs on i) the person living with NMD and ii) themselves.
Physical consequences of dysphagia
Dysphagia
The most frequent consequences of dysphagia reported by plwNMD were food sticking in the throat (n = 207, 76.1%), taking extra care when eating and drinking (n = 187, 68.8%), coughing or choking while eating (n = 177, 65.1%), and adapting what and how they eat (n = 168, 61.8%). Thickening drinks, signifying difficulties with swallowing liquids, was the least frequent consequence (n = 6, 2.2%).
Earliest consequences of dysphagia
The earliest consequences of dysphagia reported most often by plwNMD were food sticking in the throat (n = 120, 44.1%) and coughing or choking whilst eating (n = 109, 40.1%). This was observed in all NMD groups, though plwSMA additionally reported difficulty swallowing tablet medications (n = 18, 46.2%).
Dysphagia consequences affecting QoL
The most troublesome consequences of dysphagia reported most often by plwNMD were coughing and choking whilst eating (n = 106, 39.0%) and feeling food sticking in the throat (n = 105, 38.6%). This was observed in all NMD groups, though plwSMA additionally reported longer mealtimes (n = 14, 35.9%). Dysphagia consequences, including earliest and most troublesome symptoms, are provided in supplementary material 3.
Life-threatening consequences of dysphagia
Sixteen percent (n = 44) of plwNMD reported being underweight. The group with the highest percentage of underweight participants were plwDM1 (18.9%) and the lowest was plwFSHD (12.5%). Eleven percent (n = 29) of plwNMD reported≥1 choking event requiring either emergency attention or hospital admission in their lifetime. The group with the highest percentage of choking events was plwSMA (n = 9, 23%) and the lowest was plwFSHD (n = 3, 5.4%). Fourteen percent (n = 39) of plwNMD reported≥1 chest infection potentially related to aspiration of food and/or drink in≤3 years. Of these, 53.8% (n = 21) required domiciliary antibiotic treatment, 28.2% (n = 11) required hospital admission (n = 11) and 7.7% (n = 3) required a combination of both. The group with the highest percentage of chest infections thought to be related to food and/or drink was plwotherNMD (n = 12, 18.2%) and the lowest was plwFSHD (n = 6, 10.7%). Frequency of choking, chest infections and low weight per disease group are provided in supplementary material 3. An additional seven plwNMD reported hospital admission(s) relating to dysphagia > 3 years ago. Two participants described their experiences:
“[. . .] in 2014, when I choked on a piece of roast beef, I very quickly developed food aspirated pneumonia resulting in three months on intensive care” (plwotherNMD)
“I had one [hospital admission] due to choking a long time ago which turned into pneumonia and collapsed lung” (plwSMA).
Progression of dysphagia
Sixty-six percent (n = 180) of plwNMD felt their swallowing difficulties had progressed since onset. The group with the highest reported progression were plwSMA (n = 34, 87.2%) and those with the lowest were plwFSHD (n = 32, 57.1%). Supplementary material 3 details the stability of caregiver-perceived symptoms according to length of time since symptom onset and type of NMD.
Dysphagia Assessment and Diagnosis
Time taken for swallowing investigations
Twenty percent (n = 56) of participants who had received a healthcare assessment of their swallowing had undergone this assessment prior to experiencing any symptoms. Table 2 shows the timeline of dysphagia assessment according to disease group.
Table 2
Details of dysphagia assessment and participant perception
| Participants living with NMD (plwNMD) | Participant caregivers (pCGs) | |||||||||
| DM1 | FSHD | SMA | Other NMD | All NMD | DM1 | SMA | IBM | Other | All NMD | |
| (n = 111) | (n = 56) | (n = 39) | (n = 66) | (n = 272) | (n = 24) | (n = 6) | (n = 3) | (n = 5) | (n = 38) | |
| Time to healthcare assessment | ||||||||||
| Prior to onset of difficulties | 32 (28.8%) | 8 (14.3%) | 3 (7.7%) | 13 (19.7%) | 56 (20.6%) | 8 (33.3%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 9 (23.7%) |
| Soon after onset of symptoms | 3 (2.7%) | 1 (1.8%) | 3 (7.7%) | 7 (10.6%) | 14 (5.1%) | 4 (16.7%) | 1 (16.6%) | 0 (0.0%) | 1 (20.0%) | 6 (15.8%) |
| <6 months | 5 (4.5%) | 3 (5.4%) | 6 (15.4%) | 5 (7.6%) | 19 (7.0%) | 4 (16.7%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 5 (13.2%) |
| <12 months | 10 (9.0%) | 5 (8.9%) | 2 (5.1%) | 6 (9.1%) | 23 (8.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| >12 months | 19 (17.1%) | 8 (14.3%) | 5 (12.8%) | 11 (16.7%) | 43 (15.8%) | 1 (4.2%) | 0 (0.0%) | 1 (33.3%) | 0 (0.0%) | 2 (5.3%) |
| After hospital admission | 2 (1.8%) | 3 (5.4%) | 5 (12.8%) | 5 (7.6%) | 15 (5.5%) | 2 (8.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (5.3%) |
| Never | 26 (23.4%) | 21 (37.5%) | 8 (20.5%) | 8 (12.1%) | 63 (23.2%) | 1 (4.2%) | 3 (50%) | 0 (0.0%) | 2 (40.0%) | 6 (15.8%) |
| Can’t remember/unclear | 12 (10.8%) | 3 (5.4%) | 6 (15.4%) | 7 (10.6%) | 28 (10.3%) | 1 (4.2%) | 0 (0.0%) | 1 (33.3%) | 1 (20.0%) | 3 (7.9%) |
| Other1 | 2 (1.8%) | 4 (7.1%) | 1 (2.6%) | 4 (6.1%) | 11 (4.0%) | 3 (12.5%) | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | 5 (13.2%) |
| Assessment modality | ||||||||||
| Instrumental (FEES and/or VFSS) | 43 (38.7%) | 12 (21.4%) | 19 (48.7%) | 32 (48.5%) | 106 (39.0%) | 11 (45.8%) | 2 (33.3%) | 2 (66.7%) | 2 (40%) | 17 (44.7%) |
| Clinical | 24 (21.6%) | 10 (17.9%) | 7 (17.9%) | 15 (22.7%) | 56 (20.6%) | 9 (37.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 9 (23.7%) |
| Not assessed | 42 (37.8%) | 33 (58.9%) | 13 (33.3%) | 17 (25.7%) | 105 (38.6%) | 4 (16.7%) | 3 (50%) | 1 (33.3%) | 3 (60%) | 11 (21.1%) |
| Other2 | 2 (1.8%) | 1 (1.8%) | 0 (0.0%) | 2 (3.0%) | 5 (1.8%) | 0 (0.0%) | 1 (16.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| DM1 | FSHD | SMA | Other NMD | All NMD | DM1 | SMA | IBM | Other | All NMD | |
| (n = 43) | (n = 12) | (n = 19) | (n = 32) | (n = 106) | (n = 11) | (n = 2) | (n = 2) | (n = 2) | (n = 17) | |
| Type of instrumental assessment | ||||||||||
| VFSS | 24 (55.8%) | 4 (33.3%) | 15 (78.9%) | 15 (46.9%) | 58 (54.7%) | 11 (100%) | 2 (100%) | 2 (100%) | 1 (50.0%) | 16 (94.1%) |
| FEES | 7 (16.3%) | 4 (33.3%) | 0 (0.0%) | 6 (18.8%) | 17 (16.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 1 (5.9%) |
| Both | 12 (27.9%) | 4 (33.3%) | 4 (21.1%) | 11 (34.4%) | 31 (29.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Participant perception (instrumental) | ||||||||||
| Helpful | 30 (69.8%) | 8 (66.6%) | 14 (73.7%) | 27 (84.4%) | 79 (74.5%) | 9 (81.8%) | 1 (50.0%) | 2 (100%) | 2 (100%) | 14 (82.4%) |
| Not helpful | 9 (20.9%) | 2 (16.7%) | 4 (21.1%) | 2 (6.3%) | 17 (16.0%) | 1 (9.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.9%) |
| Unsure | 4 (9.3%) | 2 (16.7%) | 1 (5.2%) | 3 (9.4%) | 10 (9.4%) | 1 (9.1%) | 1 (50.0%) | 0 (0.0%) | 0 (0.0%) | 2 (11.8%) |
| DM1 | FSHD | SMA | Other NMD | All NMD | DM1 | SMA | IBM | Other | All NMD | |
| (n = 24) | (n = 10) | (n = 7) | (n = 15) | (n = 56) | (n = 9) | (n = 0) | (n = 0) | (n = 0) | (n = 12) | |
| Participant perception (clinical) | ||||||||||
| Helpful | 12 (50.0%) | 5 (50%) | 4 (57.1%) | 12 (80.0%) | 33 (58.9%) | 5 (55.6%) | N/A | N/A | N/A | 5 (%) |
| Not helpful | 6 (25.0%) | 3 (30.0%) | 3 (42.9%) | 1 (6.7%) | 13 (23.2%) | 2 (22.2%) | N/A | N/A | N/A | 2 (%) |
| Unsure | 6 (25.0%) | 2 (20.0%) | 0 (0.0%) | 2 (13.3%) | 10 (17.9%) | 2 (22.2%) | N/A | N/A | N/A | 2 %) |
DM1: Myotonic Dystrophy, FSHD: Facioscapulohumeral Muscular Dystrophy, SMA: Spinal muscular Atrophy, IBM: Inclusion Body Myositis NMD: Neuromuscular disease. 1) Participants living with DM1: Symptoms have been discussed but not investigated (n = 1), I chose not to be assessed (n = 1). Participants living with FSHD: Symptoms have been discussed but not investigated (n = 4). Participants living with SMA: Symptoms have been discussed but not investigated (n = 1). Participants living with other NMD: Symptoms have been discussed but not investigated (n = 3), I chose not to be assessed (n = 1). 2) Participants living with DM1: Discussed problems with a speech and language therapist (n = 2). Participant living with FSHD: No detail given. Participant living with other NMD: Discussed problems with a speech and language therapist (n = 2). Caregivers of participants living with SMA: assessment of weight (n = 1).
Method and perception of assessment
Sixty-three percent (n = 106) of plwNMD who had received a healthcare assessment, also underwent an instrumental evaluation of their swallowing. This equated to 39.0% of all plwNMD who completed the survey. Of these, 54.7% (n = 58) had a videofluoroscopic swallowing study (VFSS), 16.1% (n = 17) had fibreoptic endoscopic evaluation of swallowing (FEES) and 29.2% (n = 31) had both. Table 2 provides figures on which participant groups found instrumental evaluation helpful versus unhelpful, compared with those who underwent clinical assessment only.
Themes of why plwNMD found instrumental evaluation helpful were:
i) for identification or ‘pinpointing’ of the problem; for example, “I thought my issue was swallowing foods, however it turned out after the x-ray that sometimes liquids pass into my lungs [. . .]” (plwDM1).
ii) confirmation; for example, “investigation allowed [a] healthcare professional to confirm my symptoms [. . .]” (plwotherNMD).
iii) differential diagnosis or ‘ruling out’ a problem; for example, “I was worried about cancer [. . .] and was relieved it was clear” (plwotherNMD)
iv) validation; for example, “[...] most of my life I had struggled with swallowing but was never believed” (plwotherNMD).
v) understanding and awareness; for example, “having the test helped me understand more about my swallowing which led to getting further help [. . .]” (plwSMA)
vi) advice and management; for example, “we could see how the food went down my throat and that it was useful to bend my head forward and to the left” (plwSMA).
Themes generated by participants who did not consider instrumental assessment helpful, or were unsure of its value, were:
i) not revealing the issue; for example, “The test result didn’t show any signs of swallowing difficulties. It only happens sometimes and can’t be provoked” (plwDM1).
ii) not evaluating relevant aspects of the swallow; for example, “[. . .] they gave me very soft food that was easy to swallow [during the assessment] unlike meat, bread [. . .] etc” (plwDM1).
iii) providing information of what was already known; for example, “I was just told I have weak muscles, which I knew anyway” (plwDM1).
iv) not providing any solutions to identified problems; for example, “They [. . .] could offer no remedy” (plwotherNMD).
v) results were never received
Those who considered clinical assessment helpful gave responses under the themes of i) improved understanding of their dysphagia diagnosis and its relationship to NMD, ii) receipt of advice to manage the problem, iii) identification and iv) confirmation of the problem, plus v) better awareness of how to manage their difficulties. Those who did not find clinical assessment helpful, or were unsure of its value, described:
i) lack of belief, or disappointment in the assessment (or assessor); for example, “It did not take a trained medical professional with a banana to identify the issue [. . .]” (plwSMA).
ii) dissatisfaction with the recommended actions; for example, “I couldn’t eat the suggested way; I could only eat and swallow the way I had taught myself” (plwSMA).
iii) confirmation of what was already known,
iv) lack of action after results were provided, and
v) lack of available treatment after diagnosis.
Impact of dysphagia
Eighty-six percent (n = 234) of plwNMD reported dysphagia-related anxiety and 74.3% (n = 202) reported embarrassment associated with their dysphagia. Most participants were ‘a little anxious’ (48.0%, n = 130) rather than ‘quite anxious’ (30.5%, n = 83) or ‘very anxious’ (7.7%, n = 21). Fifty-nine percent (n = 159) reported greater concern for their dysphagia than their caregivers. Table 3 (part A) details the psychological impact of dysphagia by diagnosis.
Table 3
Physical and psychological impact of dysphagia on participants living NMD and their caregivers
| Part A: Concern, anxiety and embarrassment | ||||||||||
| Participants living with NMD (plwNMD) | Participants caregivers (pCGs) | |||||||||
| DM1 | FSHD1 | SMA | Other NMD | All NMD1 | DM1 | SMA | IBM | Other | All NMD | |
| (n = 111) | (n = 55) | (n = 39) | (n = 66) | (n = 271) | (n = 23) | (n = 6) | (n = 3) | (n = 6) | (n = 38) | |
| i) Who is most concerned? | ||||||||||
| plwNMD | 62 (55.9%) | 40 (72.7%) | 20 (51.3%) | 37 (56.1%) | 159 (58.7%) | 4 (17.4%) | 2 (33.3%) | 2 (66.7%) | 2 (33.3%) | 10 (26.3%) |
| pCG | 43 (38.7%) | 8 (14.5%) | 15 (38.5%) | 19 (28.8%) | 85 (31.4%) | 17 (73.9%) | 4 (66.7%) | 1 (33.3%) | 2 (33.3%) | 24 (63.1%) |
| Healthcare team | 0 (0.0%) | 1 (1.8%) | 1 (2.6%) | 4 (6.1%) | 6 (2.2%) | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 2 (5.3%) |
| Nobody | 5 (4.5%) | 6 (10.9%) | 3 (7.7%) | 6 (9.1%) | 20 (7.4%) | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 2 (5.3%) |
| Other2 | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| ii) How anxious are you? | ||||||||||
| Very | 7 (6.3%) | 2 (3.6%) | 6 (15.4%) | 6 (9.1%) | 21 (7.7%) | 10 (43.5%) | 1 (16.7%) | 1 (33.3%) | 1 (16.7%) | 13 (34.2%) |
| Quite | 39 (35.1%) | 17 (30.9%) | 15 (38.5%) | 12 (18.2%) | 83 (30.6%) | 7(30.4%) | 4 (66.7%) | 1 (33.3%) | 2 (33.3%) | 14 (36.8%) |
| A little | 49 (44.1%) | 25 (45.5%) | 14 (35.9%) | 42 (63.6%) | 130 (48.0%) | 6 (26.1%) | 1 (16.7%) | 1 (33.3%) | 2 (33.3%) | 10 (26.3%) |
| Not at all | 16 (14.4%) | 11 (20.0%) | 4 (10.3%) | 6 (9.1%) | 37 (13.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 1 (2.6%) |
| iii) How embarrassed are you? | ||||||||||
| Very | 23 (20.7%) | 7 (12.7%) | 9 (23.1%) | 10 (15.2%) | 49 (18.1%) | N/A | N/A | N/A | N/A | N/A |
| Quite | 40 (36.0%) | 11 (20%) | 10 (25.6%) | 12(18.2%) | 73 (26.9%) | N/A | N/A | N/A | N/A | N/A |
| A little | 26 (23.4%) | 19 (34.5%) | 9 (23.1%) | 26 (39.4%) | 80 (29.5%) | N/A | N/A | N/A | N/A | N/A |
| Not at all | 22 (19.8%) | 18 (32.7%) | 11 (28.2%) | 18 (27.3%) | 69 (25.5%) | N/A | N/A | N/A | N/A | N/A |
| Part B: Daily impact of dysphagia | ||||||||||
| Participant caregivers (pCGs) | ||||||||||
| All NMD | DM1 | SMA | IBM | Other NMD | ||||||
| (n = 38) | (n = 23) | (n = 6) | (n = 3) | (n = 6) | ||||||
| I prepare special food or meals for the person I care for | Daily | 21 55.3%) | 13 (56.5%) | 2 (33.3%) | 2 (66.7%) | 4 (66.7%) | ||||
| Challenging | 5 (13.6%) | 3 (13.0%) | 0 (0.0%) | 2 (66.7%) | 0 0.0%) | |||||
| We can’t talk or socialise during mealtimes any more | Daily | 13 (34.2%) | 9 (39.1%) | 1 (33.3%) | 2 (66.7%) | 1 (16.7%) | ||||
| Challenging | 2 (5.3%) | 1 (4.3%) | 0 (0.00%) | 1 (33.3%) | 0 0.0%) | |||||
| I cut food up small for the person I care for | Daily | 26 (68.4%) | 16 (69.6%) | 4 (66.7%) | 2 (66.7%) | 4 (66.7%) | ||||
| Challenging | 2 (5.3%) | 2 (8.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
| I am present at mealtimes to watch them | Daily | 23 (60.5%) | 12 (52.2%) | 3 (50.0%) | 2 66.7 %) | 6 (100.0%) | ||||
| Challenging | 11 (28.9%) | 5 (21.7%) | 1 (16.7%) | 0 (0.0%) | 5 (83.3%) | |||||
| I remind them to take extra care when eating and drinking | Daily | 19 (50%) | 16 (69.6%) | 0 (0.0%) | 1 (33.3%) | 2 (33.3%) | ||||
| Challenging | 2 (5.3%) | 2 (8.7%) | 0 (0.0%) | 0 (0.0%) | 0 (16.7%) | |||||
| I crush or disperse medications | Daily | 10 (26.3%) | 4 (17.4%) | 3 (50.0%) | 1 (33.3%) | 2 33.3%) | ||||
| Challenging | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
| I give medications for saliva | Daily | 3 (7.9%) | 1 (4.3%) | 1 (16.7%) | 0 0.0%) | 1 (16.7%) | ||||
| Challenging | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 0.0%) | |||||
| I provide food and fluids via a gastrostomy | Daily | 9 (23.7%) | 5 (21.7%) | 2 (33.3%) | 0 (0.0%) | 2 (33.3%) | ||||
| Challenging | 3 (7.9%) | 1 4.3%) | 1 (16.7%) | 0 (0.0%) | 1 (16.7%) | |||||
| I wipe or clear food debris from the mouth or throat | Daily | 5 (13.2%) | 3 (13.0%) | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | ||||
| Challenging | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
| I get anxious and stressed when watching at mealtimes | Daily | 18 (47.4%) | 12 (52.2%) | 2 (33.3%) | 2 (66.7%) | 2 (33.3%) | ||||
| Challenging | 10 (26.3%) | 8 (34.8%) | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | |||||
| Other | Daily | 4 (10.5%) | 1 (4.3%) | 2 (33.3%) | 0 (0.0%) | 1 (16.7%) | ||||
| Challenging | 3 (7.9%) | 1 (4.3%) | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | |||||
DM1: Myotonic Dystrophy, FSHD: Facioscapulohumeral Muscular Dystrophy, SMA: Spinal uscular Atrophy, IBM: Inclusion Body Myositis NMD: Neuromuscular disease. 1) One plwFSHD did not respond to this section of the survey. Numbers have been adjusted accordingly 2) Described as ‘casual acquaintances’
Knowledge and understanding of dysphagia
Dysphagia preparedness
Sixty-seven percent (n = 183) of plwNMD were not aware their NMD could cause dysphagia at the time of NMD diagnosis. The most informed group were plwSMA (n = 26, 66.7%) and the least informed were plwFSHD (n = 11, 19.6%). Most participants who were prepared for NMD-related dysphagia (n = 87) had self-educated (n = 34, 39.1%).
Timing, importance, and approach to dysphagia education
Ninety-four percent (n = 255) of plwNMD felt it was important for swallowing difficulties to be identified early. Of this group, 60.4% (n = 154) considered it very important and 39.6% (n = 101) quite important. The following themes were identified in the responses from those who considered it very important to have swallowing problems diagnosed early:
i) physical risk and its associated management; for example, “choking on your food could kill you” “chances of inhaling food or liquid into lungs could be reduced” (plwDM1)
ii) differential diagnosis; for example, “you need to know if it’s a symptom of NMD or not, so you know whether to consider other options” (plwotherNMD)
iii) treatment & management; for example, “the sooner you find a problem, the sooner you can fix it or learn to deal with it” (plwDM1)
iv) education and awareness; for example, “so you know why food gets stuck, not knowing what is happening is the worst part” (plwDM1)
v) understanding; for example, “it’s good to know your limitations” (plwDM1)
vi) psychological well-being; for example, “early diagnosis can prevent stress” (plwotherNMD)
vii) social wellbeing; for example, “eating in public places need not be avoided and self-confidence can be improved” (plwotherNMD)
viii) future research; for example, “the more patients that are diagnosed, increases [the] priority for medication [treatment] to be developed” (plwDM1).
i) acceptance; for example, “it is what it is” (plwotherNMD)
ii) the low impact of their dysphagia on their safety and QoL, relative to other aspects of their disease
iii) lack of available treatment; and
iv) not wanting to cause unnecessary panic.
Seventy-one percent (n = 192) of plwNMD felt the best time to raise dysphagia awareness was at the same time (n = 110) or shortly after (n = 82) NM diagnosis. Fifty-three percent (n = 145) of plwNMD felt that they should be responsible for identifying the early signs of dysphagia. Three participants highlighted the importance of education to support early identification and management:
“I should [be responsible] but only once I’ve been advised as to what the early signs and symptoms are . . . ” (plwotherNMD)
“ . . . although I might be the first person to notice, I should know what the next steps are to assess and address the problem and which professional is responsible for that” (plwSMA)
“Early discussion and being made aware is important, but referral to an individual who is solely qualified and regularly refreshed in methods and treatment of this condition is the shortest, safest and effective way to treat” (plwotherNMD).
Table 4 outlines how prepared plwNMD are for the possible onset of dysphagia as well as when, how, and by whom plwNMD believe dysphagia education should be provided.
Perspectives on dysphagia services for plwNMD
Participants selected nine different ways to improve services for plwNMD and dysphagia. The three most popular were: access to a neuromuscular swallowing specialist (n = 93, 34.2%), swallowing training for patients (n = 90, 33.1%), and earlier discussion and assessment of management swallowing problems (n = 78, 28.7%). Table 4 provides details of service improvement suggestions according to caregiver disease group.
Table 4
Knowledge and understanding of dysphagia reported by participants living with NMD and their caregivers
| Participants living with NMD (plwNMD) | Participant caregivers (pCGs) | |||||||||
| DM1 | FSHD | SMA | Other NMD | All NMD | DM1 | SMA | IBM | Other | All NMD | |
| (n = 111) | (n = 56) | (n = 39) | (n = 66) | (n = 272) | (n = 24) | (n = 6) | (n = 3) | (n = 5) | (n = 38) | |
| Did you know NMD could cause dysphagia? | ||||||||||
| Yes | 32 (28.8%) | 11 (19.6%) | 26 (66.7%) | 19 (28.8%) | 87 (32.0%) | 13 (54.2%) | 3 (50.0%) | 1 (33.3%) | 1 (20.0%) | 18 (47.4%) |
| No | 77 (69.4%) | 45 (80.4%) | 13 (33.3%) | 47 (71.2%) | 183 (67.3%) | 11 (45.8%) | 3 (50.0%) | 2 (66.6%) | 4 (80.0%) | 20 (52.6%) |
| Unsure | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Who should identify dysphagia? | ||||||||||
| plwNMD | 51 (45.9%) | 37 (66.1%) | 20 (51.3%) | 37 (56.1%) | 145 (53.3%) | 1 (4.2%) | 2 (33.3%) | 1 (33.3%) | 0 (0.0%) | 4 (10.5%) |
| Neurologist | 22 (19.8%) | 7 (12.5%) | 9 (23.1%) | 14 (21.2%) | 52 (19.1%) | 4 (16.7%) | 1 (16.7%) | 1 (33.3%) | 1 (20.0%) | 7 (18.4%) |
| GP | 6 (5.4%) | 4 (7.2%) | 1 (2.6%) | 6 (9.1%) | 17 (6.3%) | 3 (12.5%) | 0 (0.0%) | 0 (0.0%) | 1 (20.0%) | 4 (10.5%) |
| Swallowing specialist | 14 (12.6%) | 2 (3.6) | 2 (5.1%) | 5 (7.6%) | 23 (8.5%) | 3 (12.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (7.9%) |
| Family/caregiver | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.7%) | 5 (20.8%) | 1 (16.7%) | 0 (0.0%) | 2 (40.0%) | 8 (21.1%) |
| Unsure | 16 (14.4%) | 6 (10.7%) | 6 (15.4%) | 4 (6.1%) | 32 (11.8%) | 7 (29.2%) | 2 (33.3%) | 1 (33.3%) | 1 (20.0%) | 11 (28.9%) |
| Other1 | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | 1 (0.4%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
| How important is early diagnosis? | ||||||||||
| Very | 56 (50.5%) | 29 (51.8%) | 26(66.7%) | 43 (65.2%) | 154 (56.6%) | 18 (75.0%) | 5 (83.3) | 2 (66.6%) | 4 (80.0%) | 29 (76.3%) |
| Quite | 47 (42.3%) | 22 (39.3%) | 11 (28.2%) | 21 (31.8%) | 101 (37.1%) | 6 (25.0%) | 1 (16.7%) | 1 (33.3%) | 1 (20.0%) | 9 (23.7%) |
| Not very | 8 (7.2%) | 4 (7.1%) | 2 (5.1%) | 2 (3.0%) | 16 (5.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Not at all | 0 (0.0%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| When is the best time to raise awareness? | ||||||||||
| At NM diagnosis | 53 (47.7%) | 19 (33.9%) | 10 (25.6%) | 28 (42.4%) | 110 (40.4%) | 10 (41.7%) | 3 (50.0%) | 1 33.3%) | 3 (60.0%) | 17 (44.7%) |
| After NM diagnosis | 25 (22.5%) | 14 (25.0%) | 21 (53.8%) | 22 (33.3%) | 82 (30.1%) | 7 (29.2%) | 0 (0.0%) | 1 (33.3%) | 1 20.0%) | 9 (23.7%) |
| At first symptom | 15 (13.5%) | 13 23.2%) | 6 (15.4%) | 12 (18.2%) | 46 (16.9%) | 3 (12.5%) | 1 (16.7%) | 1 (33.3%) | 1 (20.0%) | 6 (15.8%) |
| After prolonged symptoms | 9 (8.1%) | 2 (3.6%) | 0 (0.0%) | 2 (3.0%) | 13 (4.8%) | 1 (4.2%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 2 (5.3%) |
| Only if significant issues | 9 (8.1%) | 8 (14.3%) | 2 (5.1%) | 1 (1.5%) | 20 (7.3%) | 2 (8.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (5.3%) |
| Other2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 1 (0.4%) | 1 (4.2%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 2 (5.3%) |
| How would you improve services? | ||||||||||
| Better communication | 19 (17.1%) | 14 (25.0%) | 10 (25.6%) | 18 (27.3%) | 61 (22.4%) | 2 (8.3%) | 1 (16.7%) | 1 (33.3%) | 1 (20.0%) | 5 (5.3%) |
| Earlier access to local SLT | 29 (26.1%) | 7 (12.5%) | 5 (12.8%) | 7 (10.6%) | 48 (17.6%) | 6 (25.0%) | 0 (0.0%) | 0 (0.0%) | 3 (60.0%) | 9 (23.7%) |
| Access to NM SLT | 36 (32.4%) | 11 (19.6%) | 15 (38.5%) | 31 (47.0%) | 93 (34.2%) | 7 (29.2%) | 0 (0.0%) | 2 (66.7%) | 2 (40.0%) | 11 (28.9%) |
| Patient training | 38 (34.2%) | 17 (30.4%) | 15 (38.5%) | 20 (30.3%) | 90 (33.1%) | 10 (41.7%) | 0 (0.0%) | 1 (33.3%) | 0 (10.0%) | 11 (28.9%) |
| Healthcare training | 9 (8.1%) | 5 (8.9%) | 5 (12.8%) | 9 (13.6%) | 28 (10.3%) | 4 (16.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (10.5%) |
| Earlier discussion and assessment | 26 (23.4%) | 19 (33.9%) | 7 (17.9%) | 26 (39.4%) | 78 (28.7%) | 6 (25.0%) | 1 (16.7%) | 1 (33.3%) | 0 (0.0%) | 8 (21.1%) |
| Better treatment | 19 (17.1%) | 4 (7.1%) | 12 (30.8%) | 6 (9.1%) | 41 (15.1%) | 4 (16.7%) | 3 (50.0%) | 1 (33.3%) | 0 (0.0%) | 8 (21.1%) |
| Access to online forums or support groups | 1 (0.9%) | 2 (3.6%) | 3 (7.7%) | 4 (6.1%) | 10 (3.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unsure | 5 (4.5%) | 13 (23.2%) | 1 (2.6%) | 4 (6.1%) | 23 (8.4%) | 3 (12.5%) | 3 (50.0%) | 0 (0.0%) | 2 (40.0%) | 8 (21.1%) |
| Other3 | 2 (1.8%) | 0 (0.0%) | 1 (2.6%) | 0 (0.0%) | 3 (1.1%) | 0 (0.0%) | 1 (16.7%) | 0 (0.0%) | 0 (0.0%) | 1 (2.6%) |
DM1: Myotonic Dystrophy, FSHD: Facioscapulohumeral Muscular Dystrophy, SMA: Spinal muscular Atrophy, IBM: Inclusion Body Myositis NMD: Neuromuscular disease. 1) Participant living with SMA (plwSMA); Either the person themselves or the doctor (n = 1) participant caregiver (pCG) for person living with DM1: It should be a combined responsibility (n = 1). 2) Participant living with Charcot Marie Tooth: When raised by the patient (n = 1). 3) Participant living with DM1: Emergency swallowing/choking training for patients, families and caregivers (n = 2) plwSMA: better training for family and caregivers (n = 1), pCG for pplwSMA: better care when patients move to adult services (n = 1).
pCGs
Population characteristics
Thirty-eight participant caregiver (pCG) responses were included, of which 63.1% (n = 24) provided care for pplwDM1, 15.8% (n = 6) for people living with SMA (pplwSMA), 7.9% (n = 3) for people living with IBM (pplwIBM) and 5.3% (n = 2) for people living with FSHD (pplwFSHD). The remaining pCGs provided care for people living with limb girdle muscular dystrophy (LGMD) (n = 1), Kearns-Sayre sydrome (KSS) (n = 1), and mitochondrial myopathy (n = 1). Table 1 (Part B) provides an overview of pCG demographics, including age of the pCG, age and diagnosis of the person they cared for, length of time in a caregiver role and relationship to the person living with NMD. Caregivers have been grouped according to the diagnosis of the person they care for.
Perception of neuromuscular impairments and dysphagia indications
Neuromuscular impairments
All pCGs cared for pplwNMD who had signs and symptoms of NMD additional to dysphagia. Difficulties with chewing and swallowing were ranked by pCGs as the third most impactful on the lives of pplwNMD, and second most impactful on the lives of pCGs. Figure 2(b) shows the range of neuromuscular impairments and their perceived impact on pplwNMD compared with caregivers.
Indications of dysphagia
The indications of dysphagia most frequently observed by pCGs were coughing or choking whilst eating (n = 31, 81.6%), taking extra care when eating and drinking (n = 29, 76.3%) and longer mealtimes (n = 29, 76.3%). Thickening drinks, signifying difficulties with swallowing liquids, was the least frequent indication reported by pCGs (n = 2, 5.3%).
Earliest indications of dysphagia observed by pCGs
The earliest indication of dysphagia observed by most pCGs was coughing or choking whilst eating (n = 26, 68.4%). This was witnessed by those caring for pplwDM1 (n = 18, 78.2%), IBM (n = 3, 100%) and other NMD (n = 5, 100.0%). Caregivers for pplwSMA more often observed extra care when eating and drinking (n = 3, 50%).
Indications of dysphagia affecting caregiver QoL
The dysphagia indication considered to be ‘worst’ by most pCGs was coughing or choking whilst eating (n = 22, 57.9%). This was consistent in those caring for pplwDM1 (n = 15, 65.2%), IBM (n = 3, 100%) and other NMD (n = 3, 60.0%). Most caregivers for pplwSMA reported that extra care when eating and drinking was the worst indication to live with (n = 3, 50%). Dysphagia indications, including earliest onset and worst to live with, are provided in supplementary material 3.
Life-threatening indications of dysphagia
Thirty-four percent (n = 13) of pCGs considered the person living with NMD to be underweight. Most was reported by pCGs of pplwSMA (n = 4, 66.7%). Eight percent (n = 3) of pCGs had witnessed more than one choking incident requiring emergency or hospital attention. All were pCGs of pplwDM1. Fifty three percent (n = 20) of pCGs had observed indications of chest infections; 40.0% (n = 8) of which were thought to be related to eating and drinking.
Signs of progression
Almost ninety-five percent (n = 36) of pCGs felt that swallowing difficulties had progressed whilst caring for the person living with NMD. Supplementary material 3 details symptom progression according to symptom onset and pCG group.
Dysphagia assessment and diagnosis
Time taken for swallowing investigations
Of those who had undergone healthcare assessment of their swallowing (n = 24), 37.5% (n = 9) of pCGs reported that the initial assessment took place before the onset of any swallowing difficulty. Table 2 shows the timeline of dysphagia assessment according to caregiver disease group.
Method & perception of assessment
Of those who had undergone healthcare assessment of their swallowing, 65% (n = 17) of pGCs cared for pplwNMD who underwent an instrumental evaluation as part of this assessment. Table 2 details the type of instrumental assessment each person underwent alongside the pCG perspective on its helpfulness compared with clinical assessment.
Five pCGs described why they found instrumental assessment helpful. Reasons for this fell into four themes: identification, confirmation, explanation, and advice and/or management of the problem. Those who did not find instrumental assessment helpful did not provide rationale. One pCG who was uncertain of its value, described a conflict (or disconnect) between VFSS and real-world experience:
“[the VFSS] showed he was slightly aspirating but [he] has only ever [had] one chest infection” (pCG of plwSMA).
Impact of dysphagia
Table 3 (part B) shows the daily impact of dysphagia on pCGs across disease groups. Most frequently caregivers were required to cut food up into small pieces for pplwNMD (68.4%, n = 26) and ensure they were present during mealtimes (60.5%, n = 23). Reminders to take extra care at mealtimes were common for pCGs of pplwDM1 (n = 16, 69.6%) compared with pCGs of other groups. Mealtime anxiety and/or stress was ranked the most challenging aspect of caring for pplwDM1 (n = 8, 34.8%) and pplwSMA (n = 2, 33.3%), whereas the need to prepare special foods was most challenging for pCGs of pplwIBM (n = 2, 66.7%) and the need to be present at mealtimes most challenging for pCGs of pplwotherNMD (n = 5, 83.3%). Two pCGs provided free-text examples of how dysphagia impacted their mealtimes:
“I find it hard [at mealtimes] as he doesn’t like to be reminded and says I’m nagging” (pCG of plwDM1).
“We thought the loss of ability [to swallow] was bad but losing the ability to socialise with a meal or drink has isolated him even more” (pCG of plwSMA).
Psychological Impact
Ninety-seven percent (n = 37) of caregivers reported anxiety related to swallowing. The highest levels of anxiety were reported by pCGs of pplwDM1 (43.5%, n = 10), and lowest in pCGs of pplwSMA (16.7%, n = 1) and pplwotherNMD (16.7%, n = 1). Sixty-three percent (n = 24) of pCGs felt their concern about dysphagia was higher than the person they cared for. Participant caregivers of pplwDM1 expressed greater concern (n = 17, 73.9%) than those who cared for other pplwNMD (n = 7, 46.7%). One pCG stated:
“As a [caregiver], I think I am much more anxious than [relative] about his swallowing problems. He seems to accept them and find his own ways of coping. But for me it is heart-breaking to see him struggling” (pCG of plwDM1).
Knowledge and understanding of dysphagia
Dysphagia preparedness
Fifty-three percent (n = 20) of pCGs were unaware that NMD could cause swallowing difficulties. Of the pCGs who were prepared for NMD-related dysphagia (n = 18), most were self-educated (n = 10, 55.6%).
Timing, importance, and approach to dysphagia education
One hundred percent (n = 38) of pCGs felt it was important for swallowing difficulties to be identified early: 76.3% (n = 29) felt it was very important and 23.7% (n = 9) quite important. Reasons for this included avoidance of dysphagia-associated risks, education of self and others, access to treatment, and management of psychosocial well-being. Sixty-eight percent of pCGs (n = 26) felt the best time to raise awareness of dysphagia was at the same time (n = 17) or shortly after (n = 9) NM diagnosis. Two pCGs provided contrasting perspectives on the timing of dysphagia assessment and education:
“It should be routinely discussed from early on to remove stigma and fear [. . .]” (pCG of plwDM1).
“[It’s a] lot to take in of first diagnosis [. . .] I think in the first year of diagnosis we only took in half of what we were told” (pCG of plwSMA).
Most pCGs felt that healthcare professionals should be responsible for identifying dysphagia in pplwNMD (n = 14, 36.8%). The fewest pCGs felt it should be the responsibility of pplwNMD (n = 4, 10.5%). Table 4 outlines how prepared pCGs were for the possible onset of dysphagia as well as when, how and by whom pCGs believed dysphagia education should be provided.
Perspectives on dysphagia services for plwNMD
Caregivers chose eight from nine different suggestions to improve services for pplwNMD and swallowing difficulties. The two most popular were: access to a neuromuscular swallowing specialist (n = 11, 28.9%), and swallowing training for patients (n = 11, 28.9%). Table 4 provides details of service improvement suggestions according to disease group.
DISCUSSION
The purpose of this study was to understand how people living, and caring for people, with NMD experience dysphagia, and the impact it has on their lives. From the survey, we have generated preliminary considerations for the current and future care of adults living with dysphagia caused by NMD, as well as suggestions for future research. Key considerations include:
‘One size does not fit all’ with respect to dysphagia symptom profiles
Our survey findings show uniformity across neuromuscular disease groups with respect to the most and least frequently reported dysphagia symptoms. These findings (i.e. swallowing food is more difficult than swallowing liquid) are consistent with previous studies describing the clinical presentation [9, 11] and mechanisms [28, 29] of dysphagia in NMD. Symptom profiles are however distinct from other neurological disease groups where tools to detect dysphagia are predominantly focused on difficulties swallowing liquids [30–32]. Despite similarities within the NMD group, findings also indicate that some dysphagia symptoms occur more frequently in some groups, compared to others. This aligns with previous research showing within-group variations and symptom heterogeneity associated with individual disease patterns, severity and progression [1, 2, 20].
PlwSMA frequently reported longer mealtimes compared to other groups. This supports the findings of Audag et al., [33] who identified that mealtime length was one of twelve questionnaire domains that scored more highly amongst plwSMA when compared with other NMD groups. Longer length of mealtime may represent a more severely impaired participant group who have accommodated for their dysphagia by pacing mealtimes; and/or who experience more substantial whole-body neuromuscular impairment, including impairment of hand and arm function, which makes self-delivery of food and drink to the mouth more difficult [34]. Co-occurring impairments of respiratory muscle function in pplwSMA may contribute to a higher prevalence of reported choking episodes in this group, given the positive association between respiratory weakness and cough strength [35]. Central nervous system involvement, including cognitive deficits, associated with some NMD groups such as pplwDM1, may implicate how well a person might perceive and accommodate for their progressive dysphagia symptoms [36, 37], further contributing to between-group differences. Whilst the differences identified in this study require further substantiation, the importance of a dysphagia assessment that is tailored to meet the individual requirements of each neuromuscular group is highlighted and supports the proposal for eventual categorisation of dysphagia according to the underlying NMD [33].
• Dysphagia evaluation should include physical and psychosocial impact
Whilst the prevalence of aspiration pneumonia in people living with dysphagia varies widely [38], reports of eating and drinking-related chest infection (9.1%) was lower than anticipated in a population where respiratory failure is a common cause of death [39]. The reason for this is likely multifactorial, not least the requirement for participants to have had a previous formal diagnosis of chest infection and discussion about its cause. The consequence of aspiration caused by dysphagia in pplwNMD is however likely to be greater than in those living with dysphagia caused by a disease that implicates mobility, self-feeding and respiratory function; known risk factors in the development of pneumonia [40, 41]. This supports a multidisciplinary approach to the physical evaluation of dysphagia in people living with NMD [2, 42].
Dysphagia-related anxiety and embarrassment is not confined to those living with the condition [43, 44]. In the caregiver group, anxiety was almost three-times higher than those living with dysphagia. PlwNMD judged caregiver concern to be greatest in those who cared for adults with DM1 and SMA. This level of concern may reflect dysphagia severity and/or the effectiveness of symptom management programmes in these groups. It may also reflect the ability and/or willingness of the person living with dysphagia to implement management approaches, and the ability of the healthcare team to overcome any factors that affect adherence [5, 45]. The mental health burden associated with caring for people living with chronic disease may also affect a caregivers perceived need for vigilance and adherence to dysphagia recommendations [46, 47]. It may additionally explain why high numbers of caregivers report a need to be physically present at mealtimes. Considering this, the potential for both participant and caregiver physical and psychological burden should be acknowledged as part of the dysphagia assessment process. Use of a caregiver-specific dysphagia questionnaire [48] may be helpful to understand the physical and psychological impact of dysphagia on the caregiver and target management holistically.
• Inclusion of caregiver experience may be helpful to support identification and monitoring of dysphagia.
Neuromuscular diseases are degenerative, with increasing symptoms over time [49]. There are few longitudinal studies of dysphagia in this patient group, but cross-sectional studies describe a progressive worsening of symptoms, associated with more advanced disease [9, 28, 29]. Caregiver experience closely reflected those who experienced the symptoms first-hand; however, in relation to symptom progression and weight loss, these were reported considerably more by caregivers than those living with NMD. Whilst the reason for this is not evident, inclusion of caregiver experience may be helpful to support with the identification and monitoring of dysphagia and could be examined in more detail through future participant interviews or focus groups.
• Proactive education about dysphagia in NMD
Forty percent of participants living with symptoms of neuromuscular dysphagia had not received input from a healthcare professional. This gap between pplwNMD and healthcare services may exist for a number of reasons: a greater focus on the underlying cause of dysphagia and/or other disease symptoms [50], patient decision to manage dysphagia independently before seeking professional help [50], and/or lack of awareness (and therefore, lack of enquiry) about dysphagia and its health implications. Care guidelines [18, 51, 52] recommend assessment of dysphagia to safeguard against avoidable health complications and improve QoL. Of those who had received healthcare input, one third received it prior to experiencing symptoms. There was variation between disease groups: almost two-thirds plwDM1 (57.1%) versus one twentieth of plwSMA (5.3%) experienced proactive assessment. Variations in clinical care recommendations and healthcare pathways [42] may account for differences in assessment timing, as might differences in awareness and reporting of dysphagia symptoms, patient priority and consent for assessment.
The ‘benefit versus burden’ of proactive dysphagia assessment requires further investigation as to whether this changes the clinical course of dysphagia and/or its associated complications, particularly as robust treatment programmes are still emerging [21]. Despite this, the majority (approximately 70%) of pCGs and plwNMD placed high importance on dysphagia education prior to symptom-onset. A priority area for improvement of neuromuscular services is patient education and training. This training should empower people living with dysphagia to better advocate for themselves. If offered jointly with the caregiver, training may facilitate a shared understanding of dysphagia and the responsibilities each party has with respect to dysphagia management. The responsibility with whom detection of early dysphagia symptoms exists needs elucidating to ensure clinical care pathways are transparent. A collaborative approach to symptom monitoring between patients, caregivers and healthcare staff may be most desirable [53]. Agreement on this will influence the nature, level and recipients of future education and training initiatives.
• Specialist assessment is helpful
• More research is needed to understand dysphagia, particularly in plwFSHD
The high response rates from plwFSHD is likely to reflect survey dissemination methods. Swallowing difficulties are usually considered rare and mild in FSHD [54] and swallowing assessment is not part of the current standard of care for pplwFSHD [55]. There is, however, an increased awareness of swallowing difficulties in FSHD and its relationship with cheek compression strength with potential for treatment [54]. This survey has highlighted the importance that pplwFSHD place on early discussion and assessment of swallowing problems. There is, therefore, a need for further research to understand the specific requirements of this patient group.
Limitations
Recall bias and miscomprehension of survey questions are recognised limitations of survey methodology. Whilst use of an advisory group to develop and pilot survey questions aimed to minimise such limitations, the possibility of response bias from those more troubled by their dysphagia symptoms may have led to an over-estimation of the physical and psychological impact of dysphagia in pplwNMD. To the same effect, the life-threatening consequences of dysphagia may have been under-estimated due to lack of representation from those who were critically unwell or did not survive. The voices of those not engaged with disease registries or neuromuscular charities, as well as those unable to access sufficient levels of written English are also likely to be under-represented.
Approximately one-third of participants did not complete≥50% of the survey. Whilst this ‘drop-out’ rate may reflect the length and/or complexity of the survey, further exploration of this data showed that over half did not complete the survey much beyond the eligibility screening questions. This is therefore the likely outcome of disseminating a web link to an unspecified group of people who explored the link with no intention to complete. The method of survey dissemination also prohibited a calculation of response rate. Whilst a range of NMD groups and ages were represented in the survey responses, data on disease and dysphagia severity was not directly captured. Absence of disease sub-types for SMA and phenotypic classification of DM1 could also influence the replicability and reproducibility of results. For the reasons described above, the perspectives of those living with, or caring for, people with the most prevalent NMD (MND) are also not represented in this paper.
There was comparatively low uptake from people living with DMD given the relative prevalence of this disease compared with those with higher response rates. Uptake was also low from people living with OPMD, where dysphagia is a major symptom [56]. Dissemination from disease registries is likely to explain high engagement rates for participants living with DM1, FSHD and SMA. A more targeted approach to dissemination within other neuromuscular groups, and caregivers is likely to have improved representation. Whilst low in comparison to response rates from plwNMD, caregiver responses were higher than any previous studies in this area [57]. However, these findings should be interpreted with caution –the qualitative findings in particular are unlikely to offer the same depth and insight compared with data collected via focus group methodology [58]. It was not possible to directly compare responses from plwNMD and their respective caregivers due to anonymity. Matching participant with caregiver responses may be of benefit in future studies.
Conclusion
Dysphagia can have profound physical and psychological consequences for pplwNMD and their caregivers. Despite this, high numbers of pplwNMD experiencing symptoms of dysphagia have not been assessed by a healthcare professional. Many pplwNMD would like better education and information about swallowing difficulties; and most would prefer this to be provided before they develop these difficulties. Whilst pplwNMD share similar symptoms of dysphagia, some are more unique to certain groups. Further work is therefore required to better understand the profile of dysphagia in individual disease groups. Instrumental swallowing assessment is considered helpful by the majority of pplwNMD and should therefore be considered an important part of the specialist assessment. Clarity is needed as to whose role it is to identify the early signs of dysphagia, but inclusion of caregivers - where permitted by the plwNMD - seems likely to add valuable information to the assessment and clinical decision-making process. There is a future need to provide access to well-informed staff, with sufficient knowledge and skill, to meet the above key patient priorities. These findings provide a starting point for future research amongst patients, families, and health care professionals, which may expand to the use of qualitative methodology and co-production of guidelines or recommendations for NMD dysphagia practice.
ACKNOWLEDGMENTS
With thanks to Charlotte Massey (CM) who provided administration support during the first advisory group meeting. Thanks also to Professor Maggie-Lee Huckabee, Professor Ros Quinlivan, Dr Enrico Bugiardini and Dr Chris Turner who provided a pre-submission review of this manuscript. Additional thanks to the journal reviewers for their detailed feedback and suggestions which have helped to improve the quality of the final manuscript.
FUNDING
The research costs for this study were supported by the National Institute for Health Research (NIHR) as part of a Pre-Clinical Academic Fellowship (PCAF) and Small Acorns Charitable Funding from The National Brain Appeal, University College London NHS Foundation Trust.
CONFLICT OF INTEREST
The authors have no conflict of interest to report
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-230002.
REFERENCES
[1] | Willig TN , Paulus J , Lacau Saint∧Guily J , Béon C , Navarro J . Swallowing problems in neuromuscular disorders. Archives of Physical Medicine and Rehabilitation (1994) ;75: :1175–81. https://doi.org/10.1016/0003-9993(94)90001-9 |
[2] | Britton D , Karam C , Schindler JS . Swallowing and Secretion Management in Neuromuscular Disease. Clinics in Chest Medicine. (2018) ;39: :449–57. https://doi.org/10.1016/j.ccm.2018.01.007. |
[3] | Knuijt S , Kalf JG , de Swart BJM , Drost G , Hendricks HT , Geurts ACH et al. Dysarthria and dysphagia are highly prevalent among various types of neuromuscular diseases. Disability and Rehabilitation (2014) ;36: :1285–9. https://doi.org/10.3109/09638288.2013.845255. |
[4] | Verin E , Clavé P , Bonsignore MR , Marie JP , Bertolus C , Similowski T et al. Oropharyngeal dysphagia: when swallowingdisorders meet respiratory diseases. Eur Respir J (2017) ;49: :1602530. https://doi.org/10.1183/13993003.02530-2016 |
[5] | Fitzgerald BP , Conn KM , Smith J , Walker A , Parkhill AL , Hilbert JE et al. Medication adherence in patients with myotonic dystrophy and facioscapulohumeral muscular dystrophy. J Neurol (2016) ;263: :2528–37. https://doi.org/10.1007/s00415-016-8300-3 |
[6] | Salera S , Menni F , Moggio M , Guez S , Sciacco M , Esposito S . Nutritional Challenges in Duchenne Muscular Dystrophy. Nutrients. (2017) ;9: :594. https://doi.org/10.3390/nu9060594 |
[7] | Aloysius A , Born P , Kinali M , Davis T , Pane M , Mercuri E . Swallowing difficulties in Duchenne muscular dystrophy: Indications for feeding assessment and outcome of videofluroscopic swallow studies. European Journal of Paediatric Neurology. (2008) ;12: :239–45. https://doi.org/10.1016/j.ejpn.2007.08.009 |
[8] | Pane M , Vasta I , Messina S , Sorleti D , Aloysius A , Sciarra F et al. Feeding problems and weight gain in Duchenne muscular dystrophy. European Journal of Paediatric Neurology. (2006) ;10: :231–6. https://doi.org/10.1016/j.jsurg.2016.07.008 10.1016/j.ejpn.2006.08.008. |
[9] | Pilz W , Baijens LWJ , Passos VL , Verdonschot R , Wesseling F , Roodenburg N et al. Swallowing assessment in myotonic dystrophy type 1 using fiberoptic endoscopic evaluation of swallowing (FEES). Neuromuscular Disorders (2014) ;24: :1054–62. https://doi.org/10.1016/j.nmd.2014.06.002. |
[10] | Choi Y-A , Suh DI , Chae J-H , Shin H-I Trajectory of change in the swallowing status in spinal muscular atrophy type I. International Journal of Pediatric Otorhinolaryngology (2020) ;130: :109818. https://doi.org/10.1016/j.ijporl.2019.109818. |
[11] | van den Engel-Hoek L , Erasmus CE , van Bruggen HW , de Swart BJM , Sie LTL , Steenks MH et al. Dysphagia in spinal muscular atrophy type II: More than a bulbar problem? Neurology (2009) ;73: :1787–91. https://doi.org/10.1212/WNL.0b013e3181c34aa6 |
[12] | Houser SM , Calabrese LH , Strome M . Dysphagia in patients with inclusion body myositis. Laryngoscope. (1998) ;108: :1001–5. https://doi.org/10.1097/00005537-199807000-00009 |
[13] | Kroon RHMJM , Horlings CGC , de Swart BJM , van Engelen BGM , Kalf JG Swallowing, Chewing and Speaking: Frequently Impaired in Oculopharyngeal Muscular Dystrophy. JND. 2020:1-12. https://doi.org/10.3233/JAD-200511. |
[14] | Kawai S , Tsukuda M , Mochimatsu I , Enomoto H , Kagesato Y , Hirose H et al. A Study of the Early Stage of Dysphagia in Amyotrophic Lateral Sclerosis. Dysphagia. (2003) ;18: :1–8. https://doi.org/10.1007/s00455-002-0074-3 |
[15] | Chiò A , Logroscino G , Traynor BJ , Collins J , Simeone JC , Goldstein LA et al. Global Epidemiology of Amyotrophic Lateral Sclerosis: A Systematic Review of the Published Literature. Neuroepidemiology. (2013) ;41: :118–30. https://doi.org/10.1159/000351153 |
[16] | Landfeldt E , Thompson R , Sejersen T , McMillan HJ , Kirschner J , Lochmüller H Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta-analysis. Eur J Epidemiol. (2020) ;35: :643–53. https://doi.org/10.1007/s10654-020-00613-8 |
[17] | Carey IM , Banchoff E , Nirmalananthan N , Harris T , DeWilde S , Chaudhry UAR et al. Prevalence and incidence of neuromuscular conditions in the UK between 2000 and 2019: A retrospective study using primary care data. PLoS ONE. (2001) ;16: :e0261983. https://doi.org/10.1371/journal.pone.0261983 |
[18] | on behalf of the ANSN Narayan S , Pietrusz A , Allen J , DiMarco M , Docherty K et al. Adult North Star Network (ANSN): Consensus Document for Therapists Working with Adults with Duchenne Muscular Dystrophy (DMD) - Therapy Guidelines. JND. (2022) ;9: :365–81. https://doi.org/10.3233/JND-210707 |
[19] | Papanicolas I , Mossialos E , Gundersen A , Woskie L , Jha AK Performance of UK National Health Service compared with other high income countries: observational study. BMJ. 2019:l6326. https://doi.org/10.1136/bmj.l6326. |
[20] | Audag N , Goubau C , Toussaint M , Reychler G Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: a systematic review. Therapeutic Advances in Chronic Disease (2019) ;10: :204062231882162. https://doi.org/10.1177/2040622318821622 |
[21] | Jones K , Pitceathly RD , Rose MR , McGowan S , Hill M , Badrising UA et al. Interventions for dysphagia in long-term, progressive muscle disease. Cochrane Database of Systematic Reviews. 2016;2016. https://doi.org/10.1002/14651858.CD004303.pub4. |
[22] | Shune SE , Namasivayam-MacDonald A Dysphagia-Related Caregiver Burden: Moving Beyond the Physiological Impairment. Perspect ASHA SIGs (2020) ;5: :1282–9. https://doi.org/10.1044/2020_PERSP-20-00067 |
[23] | Kumah E , Osei-Kesse F , Anaba C . Understanding and Using Patient Experience Feedback to Improve Health Care Quality: Systematic Review and Framework Development. Journal of Patient-Centered Research and Reviews. (2017) ;4: :24–31. https://doi.org/10.17294/2330-0698.1416 |
[24] | Using Principles of Co-Production to Improve Patient Care and Enhance Value. AMA Journal of Ethics, (2017) ;19: :1125–31. https://doi.org/10.1001/journalofethics.2017.19.11.pfor1-1711. |
[25] | Braun V , Clarke V . Using thematic analysis in psychology. Qualitative Research in Psychology. (2006) ;3: :77–101. https://doi.org/10.1191/1478088706qp063oa |
[26] | Jamsen J , Corley K E-Survey Methodology: In: Reynolds RA, Woods R, Baker JD, editors. Handbook of Research on Electronic Surveys and Measurements, IGI Global; 2007, p. 1-8. https://doi.org/10.4018/978-1-59140-792-8.ch001. |
[27] | National Clinical Guideline Centre (UK). Motor Neurone Disease: Assessment and Management. London: National Institute for Health and Care Excellence (UK); 2016. |
[28] | Engel-Hoek L , Erasmus CE , Hendriks JCM , Geurts ACH , Klein WM , Pillen S et al. Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment. J Neurol (2013) ;260: :1295–303. https://doi.org/10.1007/s00415-012-6793-y. |
[29] | Wohlgemuth M , de Swart BJM , Kalf JG , Joosten FBM , Van der Vliet AM , Padberg GW Dysphagia in facioscapulohumeral muscular dystrophy. Neurology (2006) ;66: :1926–8. https://doi.org/10.1212/01.wnl.0000219760.76441.f8. |
[30] | Wang T-G , Chang Y-C , Wu M-C , Lin L-C . Evaluating Swallowing Dysfunction Using a 100-ml Water Swallowing Test. Dysphagia. (2004) ;19: :43–7. https://doi.org/10.1007/s00455-003-0030-x |
[31] | Bhanu K , Kanna Sv A simple bedside test to assess the swallowing dysfunction in Parkinson′s disease. Ann Indian Acad Neurol. (2014) ;17: :62. https://doi.org/10.4103/0972-2327.128556 |
[32] | Martino R , Silver F , Teasell R , Bayley M , Nicholson G , Streiner DL et al. The Toronto Bedside Swallowing Screening Test (TOR-BSST): Development and Validation of a Dysphagia Screening Tool for Patients With Stroke. Stroke. (2009) ;40: :555–61. https://doi.org/10.1161/STROKEAHA.107.510370 |
[33] | Audag N , Liistro G , Goubau C , Vandervelde L , Poncin W , Toussaint M et al. Screening for oropharyngeal dysphagia in adult patients with neuromuscular diseases using the Sydney Swallow Questionnaire. Muscle and Nerve. (2021) ;64: :277–84. https://doi.org/10.1002/mus.27254 |
[34] | Werlauff U , Steffensen BF , Bertelsen S , Fløytrup I , Kristensen B , Werge B Physical characteristics and applicability of standard assessment methods in a total population of spinal muscular atrophy type II patients. Neuromuscular Disorders. (2010) ;20: :34–43. https://doi.org/10.1016/j.nmd.2009.11.008 |
[35] | Schroth MK Special Considerations in the Respiratory Management of Spinal Muscular Atrophy. Pediatrics. (2009) ;123: :S245–9. https://doi.org/10.1542/peds.2008-2952K |
[36] | Miller JN , Kruger A , Moser DJ , Gutmann L , van der Plas E , Koscik TR et al. Cognitive Deficits, Apathy, and Hypersomnolence Represent the Core Brain Symptoms of Adult-Onset Myotonic Dystrophy Type 1. Front Neurol (2021) ;12: :700796. https://doi.org/10.3389/fneur.2021.700796 |
[37] | Okkersen K , Buskes M , Groenewoud J , Kessels RPC , Knoop H , Van Engelen B et al. The cognitive profile of myotonic dystrophy type 1: A systematic review and meta-analysis. Cortex. (2017) ;95: :143–55. https://doi.org/10.1016/j.cortex.2017.08.008 |
[38] | DeLegge MH . Aspiration Pneumonia: Incidence, Mortality, and At-Risk Populations. JPEN J Parenter Enteral Nutr. (2002) ;26: :S19–25. https://doi.org/10.1177/014860710202600604 |
[39] | Bourke SC Respiratory involvement in neuromuscular disease. Clin Med. (2014) ;14: :72–5. https://doi.org/10.7861/clinmedicine.14-1-72. |
[40] | Langmore SE , Terpenning MS , Schork A , Chen Y , Murray JT , Lopatin D et al. Predictors of Aspiration Pneumonia: How Important Is Dysphagia? . Dysphagia (1998) ;13: :69–81. https://doi.org/10.1007/PL00009559 |
[41] | Langmore SE , Skarupski KA , Park PS , Fries BE . Predictors of Aspiration Pneumonia in Nursing Home Residents. Dysphagia. (2002) ;17: :298–307. https://doi.org/10.1007/s00455-002-0072-5 |
[42] | Audag N , Toussaint M , Liistro G , Vandervelde L , Cugy E , Reychler G . European Survey: Dysphagia Management in Patients with Neuromuscular Diseases. Dysphagia (2022) ;37: :1279–87. https://doi.org/10.1007/s00455-021-10392-3 |
[43] | Nund RL , Ward EC , Scarinci NA , Cartmill B , Kuipers P , Porceddu SV . The lived experience of dysphagia following non-surgical treatment for head and neck cancer. International Journal of Speech-Language Pathology. (2014) ;16: :282–9. https://doi.org/10.3109/17549507.2013.861869 |
[44] | Jacobsson C , Axelsson K , Osterlind PO , Norberg A How people with stroke and healthy older people experience the eating process. J Clin Nurs. (2000) ;9: :255–64. https://doi.org/10.1046/j.1365-2702.2000.00355.x |
[45] | Boussaïd G , Lofaso F , Santos DB , Vaugier I , Pottier S , Prigent H et al. Factors influencing compliance with non-invasiveventilation at long-term in patients with myotonic dystrophy type 1: A prospective cohort. Neuromuscular Disorders. (2016) ;26: :666–74. https://doi.org/10.1016/j.nmd.2016.07.014 |
[46] | Landfeldt E , Lindgren P , Bell CF , Guglieri M , Straub V , Lochmüller H et al. Quantifying the burden of caregiving in Duchenne muscular dystrophy. J Neurol. (2016) ;263: :906–15. https://doi.org/10.1007/s00415-016-8080-9 |
[47] | Kurauchi G , Endo M , Odaira K , Ono R , Koseki A , Goto M et al. Caregiver Burden and Related Factors Among Caregivers of Patients with Myotonic Dystrophy Type 1. JND. (2019) ;6: :527–36. https://doi.org/10.3233/JND-190386 |
[48] | Shune SE , Resnick B , Zarit SH , Namasivayam-MacDonald AM . Creation and Initial Validation of the Caregiver Analysis of Reported Experiences with Swallowing Disorders (CARES) Screening Tool. Am J Speech Lang Pathol (2020) ;29: :2131–44. https://doi.org/10.1044/2020_AJSLP-20-00148 |
[49] | Gellman MD editor. Encyclopedia of Behavioral Medicine. Cham: Springer International Publishing. 2020. https://doi.org/10.1007/978-3-030-39903-0. |
[50] | Lisiecka D , Kelly H , Jackson J How do people with Motor Neurone Disease experience dysphagia? A qualitative investigation of personal experiences. Disability and Rehabilitation. (2021) ;43: :479–88. https://doi.org/10.1080/09638288.2019.1630487 |
[51] | Ashizawa T , Gagnon C , Groh WJ , Gutmann L , Johnson NE , Meola G et al. Consensus-based care recommendations for adults with myotonic dystrophy type 1. Neurol Clin Pract. (2018) ;8: :507–20. https://doi.org/10.1212/CPJ.0000000000000531 |
[52] | Mercuri E , Finkel RS , Muntoni F , Wirth B , Montes J , Main M et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscular Disorders. (2018) ;28: :103–15. https://doi.org/10.1016/j.nmd.2017.11.005 |
[53] | Logemann JA Multidisciplinary management of dysphagia. Acta Otorhinolaryngol Belg (1994) ;48: :235–8. |
[54] | Mul K , Berggren KN , Sills MY , McCalley A , van Engelen BGM , Johnson NE , et al. Effects of weakness of orofacial muscles on swallowing and communication in FSHD. Neurology (2019) ;92: :e957–63. https://doi.org/10.1212/WNL.0000000000007013 |
[55] | Tawil R , van der Maarel S , Padberg GW , van Engelen BGM . 171st ENMC International Workshop: Standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscular Disorders. (2010) ;20: :471–5. https://doi.org/10.1016/j.nmd.2010.04.007 |
[56] | Brisson J , Gagnon C , Brais B , Côté I , Mathieu J A study ofimpairments in oculopharyngeal muscular dystrophy. Muscle Nerve. (2020) ;62: :201–7. https://doi.org/10.1002/mus.26888 |
[57] | LaDonna KA , Koopman WJ , Ray SL , Venance SL Hard to Swallow: A Phenomenological Exploration of the Experience of Caring for Individuals With Myotonic Dystrophy and Dysphagia. Journal of Neuroscience Nursing. (2016) ;48: :42–51. https://doi.org/10.1097/JNN.0000000000000178 |
[58] | Wilkinson S . Focus group methodology: a review. International Journal of Social Research Methodology. (1998) ;1: :181–203. https://doi.org/10.1080/13645579.1998.10846874 |




