Inspiratory Muscle Training in Nemaline Myopathy
Abstract
Background:
Respiratory muscle weakness is a common feature in nemaline myopathy. Inspiratory muscle training (IMT) is an intervention that aims to improve inspiratory muscle strength.
Objective:
The aim of this controlled before-and-after pilot study was to investigate if IMT improves respiratory muscle strength in patients with nemaline myopathy.
Methods:
Nine patients (7 females; 2 males, age 36.6±20.5 years) with respiratory muscle weakness and different clinical phenotypes and genotypes were included. Patients performed eight weeks of sham IMT followed by eight weeks of active threshold IMT. The patients trained twice a day five days a week for 15 minutes at home. The intensity was constant during the training after a gradual increase to 30% of maximal inspiratory pressure (MIP).
Results:
Active IMT significantly improved MIP from 43±15.9 to 47±16.6 cmH2O (p = 0.019). The effect size was 1.22. There was no significant effect of sham IMT. Sniff nasal inspiratory pressure, maximal expiratory pressure, spirometry, and diaphragm thickness and thickening showed no significant improvements.
Conclusions:
This pilot study shows that threshold IMT is feasible in patients with nemaline myopathy and improves inspiratory muscle strength. Our findings provide valuable preliminary data for the design of a larger, more comprehensive trial.
INTRODUCTION
Nemaline myopathy is a genetically and clinically heterogeneous congenital myopathy with characteristic nemaline rods in the muscle biopsy [1, 2]. The prevalence estimate of the all-age population is 0.20 per 100,000 [3]. Inspiratory and expiratory muscle weakness are common features in patients with nemaline myopathy which in some patients leads to respiratory failure [4]. The primary aims of mechanical ventilation and cough augmentation techniques are to unload the weakened respiratory muscles and improve coughing efficacy by increasing lung volume, respectively [5]. In contrast, there is no evidence on therapies which improve the respiratory muscle strength in patients with nemaline myopathy.
Inspiratory muscle training (IMT) is an intervention that aims to improve strength or endurance of the diaphragm and accessory muscles of inspiration by training with a specific training device [6]. The devices used apply normocapnic hyperpnea, flow resistive loading, or pressure threshold loading [7]. There is evidence that IMT improves respiratory muscle strength in several conditions, such as chronic obstructive pulmonary disease, heart failure and in various neurological conditions, including stroke and cervical spinal cord injury [8–11]. In the past, skeletal muscle training in neuromuscular disorders was controversial due to concerns of inducing muscle injury. However, in the last decade it was demonstrated that aerobic exercise is safe and only high intensity training is discouraged due to the risk of muscle injury [12]. In line with this, multiple IMT studies in neuromuscular disorders, i.e. late-onset Pompe disease and myasthenia gravis, were performed and show a positive effect on respiratory muscle strength [13–17]. In contrast, studies in patients with amyotrophic lateral sclerosis and Duchenne muscular dystrophy failed to show benefit of IMT on respiratory muscle strength [18–21].
As the effect of IMT in various neuromuscular disorders is heterogeneous, we cannot extrapolate these findings to other neuromuscular disorders. Moreover, congenital myopathies are underrepresented in IMT studies. With the exception of a case report [22], no studies on the effects of IMT on inspiratory muscle strength in patients with nemaline myopathy have been performed. Therefore, we need to assess the effect of IMT in nemaline myopathy in a separate trial. A recent study showed an inspiratory muscle strength below normal reference range in 96% of a cohort of patients with nemaline myopathy [23], hence IMT could be beneficial for these patients.
Therefore, the aim of our pilot study was to investigate whether an eight week inspiratory IMT programme is able to improve inspiratory muscle strength in nemaline myopathy patients with respiratory muscle weakness. In addition, we measured expiratory muscle strength, spirometry, and diaphragm thickness and thickening during inspiration (assessed by ultrasound).
PATIENTS AND METHODS
Study design
This controlled before-and-after pilot study was performed from November 2018 until March 2021 at the Radboud University Medical Centre, Nijmegen, the Netherlands. The patients were recruited from a cohort of 42 patients with nemaline myopathy. Ethical approval (NL65214.091.18) was obtained from the local ethics committee and written informed consent was obtained from all patients or legal representatives. The study was registered in the ClinicalTrials.gov database (NCT03728803).
Study population
The inclusion criteria were genetically confirmed nemaline myopathy or the combination of a nemaline myopathy clinical phenotype, a biopsy confirming nemaline myopathy, and a first degree family member with genetically confirmed nemaline myopathy. The age range included was six to 80 years. By excluding younger and older patients we aimed to increase the likelihood for the patients to be cognitively and physically able to carry out the tests and training. Only patients with respiratory muscle weakness were included, defined as a maximal inspiratory pressure (MIP) of less than 70% of predicted values [24, 25]. These levels of reduced MIP correspond to chronic respiratory failure and could imply the need for respiratory support, thus these patients might benefit the most from IMT. Patients with a history of other conditions that affected pulmonary or respiratory muscle function, such as chronic obstructive pulmonary disease, were excluded. Patient demographics (age, sex, BMI), genotype, form of nemaline myopathy, motor function, requirement of home mechanical ventilation, and the presence of scoliosis (Adam’s forward bend test [26]) were systematically collected. Patients were classified according to the recent classification, which distinguishes the different forms of nemaline myopathy based on the age of onset and severity [1, 2]: 1) severe nemaline myopathy (contractures, fractures, no respiratory effort or no movements at birth); 2) typical nemaline myopathy (perinatal onset and motor milestones delayed but reached); 3) mild nemaline myopathy (childhood or juvenile onset); 4) distal nemaline myopathy (distal weakness); 5) childhood-onset nemaline myopathy with slowness (slowness of movements and core-rod histology, caused by mutations in the KBTBD13 gene [27–29]); and 6) recessive TNNT1 (former Amish) nemaline myopathy.
The 32-item Motor Function Measure (MFM) was used to examine motor function. This quantitative scale consists of three different domains; standing position and transfers (domain 1), axial and proximal motor function (domain 2); and distal motor function (domain 3). The total score is expressed as a percentage of the maximum possible score (a lower score indicating the patient is clinically more affected). Scoring was conducted in line with the MFM third edition user manual (2009).
Inspiratory muscle training
The patients trained with a Threshold IMT device (Threshold IMT, Philips Respironics, Murrysville, PA, USA). The spring-loaded valve was removed to create the device for sham IMT. The patients performed eight weeks of sham IMT followed by active IMT for another eight weeks separated by one week of rest. Patients were explained that a low intensity training was compared to a high intensity training. They were instructed to perform deep and fast inspirations at functional residual capacity. The intensity for active IMT started at 15% of MIP and was increased each week by 5% until 30% in week 4. If 15% of the patient’s MIP was below the lowest intensity of the device, i.e. 9 cm H2O, the patient started at a higher intensity and reached 30% earlier or started at 30%. Patients were also included in the training if 9 cm H2O did not exceed 40% of MIP and the training was not too strenuous. The intensities were maintained constant during the training to allow comparison between patients. The training sessions were performed twice a day five days a week for 15 minutes at home. 10 repetitions were alternated with a recovery time with a maximum of 90 seconds. The patients filled in a diary to record their sessions and indicate the reason why they did not (fully) perform a session. They were called once a week by the researchers to assess adherence and adverse effects. The measurements of spirometry, respiratory muscle strength testing, and diaphragm ultrasound were performed at three separate visits: at baseline, post sham IMT and post active IMT. The patients and researchers were blinded to the results of the previous visits.
Outcome measures
The primary outcome measure was MIP. Secondary outcome measures were maximal expiratory pressure (MEP), sniff nasal inspiratory pressure (SNIP), forced vital capacity (FVC), peak cough flow (PCF), and diaphragm thickness and thickening ratio.
Spirometry was carried out with a handheld electronic spirometer (SpiroUSB, Vyaire Medical, Mettawa, IL, USA connected to PC Spirometry software, Spida CareFusion 2.3.0.10 for Windows 7) and FVC was compared with reference values [30]. Respiratory muscle strength testing was performed with a handheld electronic manometer (Micro RPM, Micro Medical, Rhymney, UK connected to Puma software version 1.4.2) and compared with reference values [31–33]. Both the spirometry and respiratory muscle strength tests were performed in accordance with the standards and recommendations of the statement of the American Thoracic Society and the European Respiratory Society [34–36]. In situations where a patient was not able or willing to visit the hospital, respiratory muscle strength testing was performed during a home visit.
Diaphragm thickness and thickening were measured in supine position using an Esaote MyLab Twice ultrasound machine (Esaote SpA, Genoa, Italy) equipped with a 3–13 MHz LA533 linear transducer (Esaote SpA, Genoa, Italy). We used a previously published and most commonly applied methodology [37, 38]. The ultrasound probe was placed at the zone of apposition of the diaphragm, typically in the 8th or 9th intercostal space, on the antero-axillary line. Thickness was measured at resting end-expiration (TEE), i.e. at expiratory reserve volume, and at maximal end-inspiration (TEI), i.e. at total lung capacity, from the inner part of the peritoneal layer to the inner part of the pleural layer. An average value was calculated from three separate measurements. Thickening ratio (TR) was calculated as TEI/ TEE [37] and absolute change in thickness was calculated as TEI - TEE.
Sample size calculation
We used MIP for our sample size calculation. Based on previous studies [13–16, 39], we expected an increase of at least 10 cmH2O in MIP as a result of an eight week training programme (δ= 10 cmH2O). Based on previous published work on measurement of MIP it was estimated that the within-subject SD (σ) of the change in MIP is approximately 15 cmH2O [40]. Type I error (α) is 0.05 (2-sided) and the type II error (power, 1-β) is 0.80.
The formula for calculation of sample sizes for a before-and-after trial is:
Considering the risk of drop-out we intended to recruit 5 additional patients.
Data analysis
The data was stored in Castor EDC (Castor clinical data management platform, Amsterdam, The Netherlands) and the statistical analysis was performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corporation, Armonk, NY, USA). GraphPad Prism software version 5.03 (GraphPad Software, San Diego, CA, USA) was used for visualisations. Descriptive statistics were presented as mean±SD. A one-way repeated measures ANOVA was performed to assess the effect of IMT. Mauchly’s Test of Sphericity was used to test whether or not the assumption of sphericity was met. If this assumption was violated the Greenhouse-Geisser correction was applied. A post-hoc Bonferroni test was performed when significant main effects were observed. The magnitude of significant post-hoc effects were determined using Cohen’s measure of effect size for within-subjects designs (dz) [41]. Cohen’s dz of 0.2 is considered a small effect, 0.5 a medium effect, and≥0.8 a large effect. The common language effect size was calculated to express the probability that a patient has a higher value on one measurement than the other [42]. A p-value < 0.05 was considered to be significant.
RESULTS
Patients
Respiratory muscle weakness was detected in 15 patients of the cohort of 42 patients with nemaline myopathy. The MIP of two patients was too low to perform IMT (40% of MIP≤9 cmH2O). One patient declined participation. Three patients stopped during the sham IMT period because of various reasons (inability to combine the training with school; mental stress because of the COVID-19 pandemic; inability to return for the follow-up visits due to COVID-19 restrictions). In total, nine patients completed sham IMT and active IMT, two of whom were visited at home. The patient characteristics are shown in Table 1.
Table 1
Patient characteristics
| Age (years) | Sex | BMI (kg/m2) | Baseline MIP (cmH2O) | Baseline MEP (cmH2O) | MFM (%) | Clinical form of nemaline myopathy | Genotype | Mechanical ventilation | Ambulation | Scoliosis | |
| Patient 1 | 15 | F | 18 | 52 | 27 | 64 | Typical | ACTA1 | Nocturnal NIV | Wheelchair-dependent | Yes, severe |
| Patient 2* | 16 | F | 23 | 35 | 80 | 98 | Mild | NEB | None | Unaided | Yes, mild |
| Patient 3 | 18 | F | 16 | 55 | 42 | 94 | Typical | TMP3 | Nocturnal NIV | Intermittent wheelchair use | No |
| Patient 4 | 28 | F | 21 | 35 | 32 | 50 | Typical | CFL2 | Nocturnal NIV | Wheelchair-dependent | Yes, severe |
| Patient 5 | 31 | M | 22 | 46 | 63 | 93 | Mild | MYPN | None | Unaided | No |
| Patient 6 | 34 | F | 22 | 38 | 35 | 81 | Childhood-onset | KBTBD13 | None | Unaided | No |
| with slowness | |||||||||||
| Patient 7 | 59 | F | 23 | 34 | 59 | 78 | Childhood-onset | KBTBD13 | None | Walking aid | Yes, mild |
| with slowness | |||||||||||
| Patient 8‡ | 62 | M | 23 | 25 | 43 | 56 | Typical | NEB | Nocturnal NIV | Intermittent wheelchair use | Yes, mild |
| Patient 9*§ | 66 | F | 17 | 23 | 27 | 72 | Mild | NEB | Nocturnal and daytime NIV | Intermittent wheelchair use | Yes, severe |
| Total (n = 9) | 36.6±20.5 | 7 F, 2 M | 20.6±2.8 | 38±11.1 | 45±18.3 | 76.2±17.2 |
*Home visit. ‡Trained at 36% of MIP during active IMT. §Trained at 39% of MIP during active IMT. Results are expressed as mean±SD. BMI, body mass index. MIP, maximal inspiratory pressure. MEP, maximal expiratory pressure. MFM, Motor Function Measure. NIV, non-invasive mechanical ventilation. IMT, inspiratory muscle training.
Adherence and adverse effects
Self-reported adherence was 96.9±3.8% for sham IMT and 96.6±4.4% for active IMT. There were several mild adverse effects. During sham IMT patients reported a dry mouth (n = 2), light-headedness (n = 2), gagging (n = 1), and chest pain (n = 1). Active IMT was experienced as strenuous (n = 3) and patients complained of palpitations (n = 1), dry mouth (n = 1), gagging (n = 1), and fatigue (n = 1). No serious adverse events occurred.
Effect on respiratory muscle function
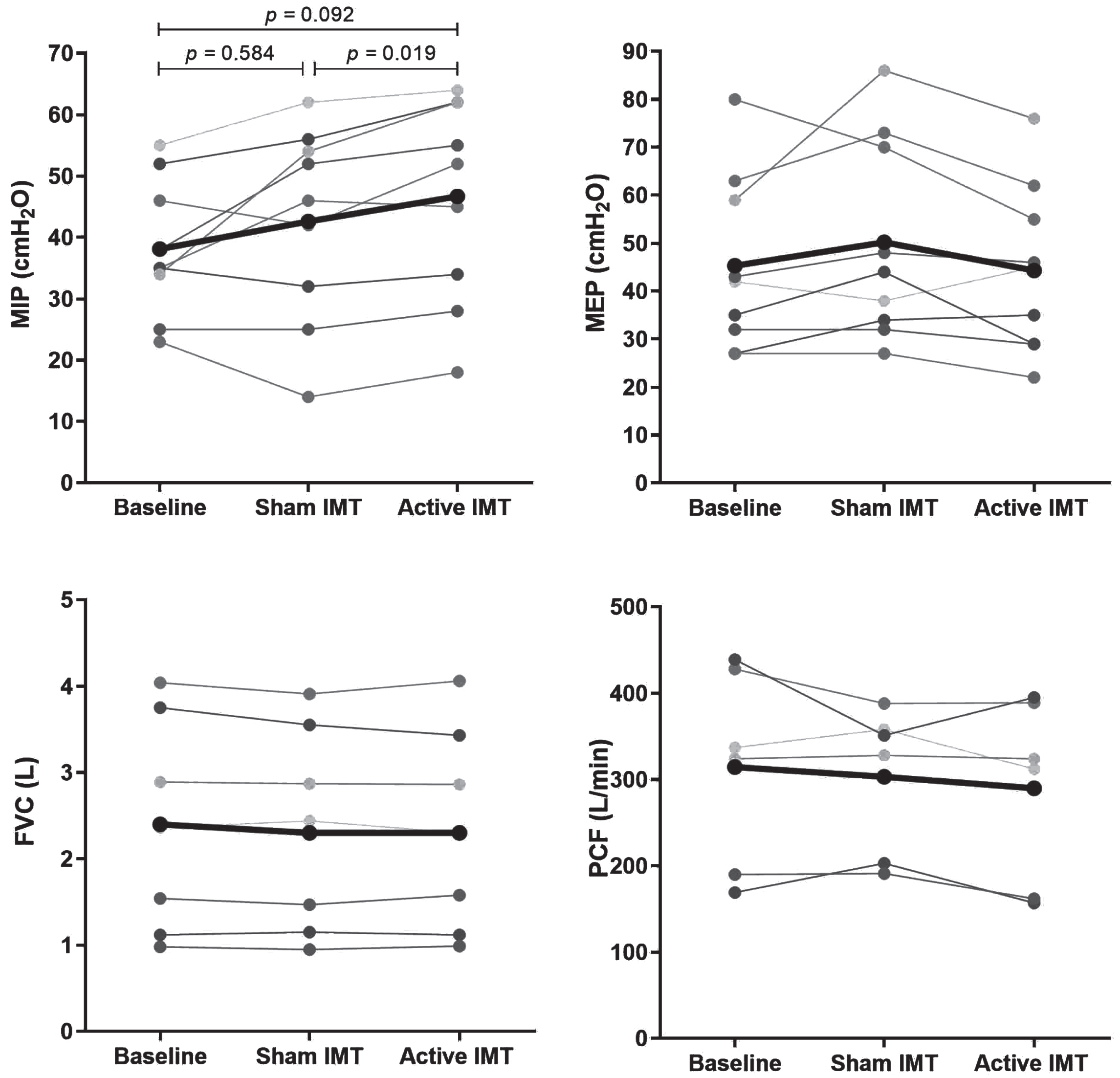
There was a significant effect of IMT on MIP, F(1.186, 9.489) = 5.046, p = 0.045, ηp2 = 0.387. Post-hoc analysis indicated a significant increase in MIP following active IMT (p = 0.019) from 43±15.9 to 47±16.6 cmH2O with an absolute difference of 4±3.4 cmH2O and a relative difference of 11.4±9.8%. The Cohen’s dz effect size was 1.22. The common language effect size indicates that after controlling for individual differences, the likelihood that a patient’s MIP increases after active IMT is 89%. MIP did not significantly improve after sham IMT due to a large variation in individual responses. MEP, SNIP, FVC, PCF, and diaphragm thickness showed no improvement or decline post sham IMT and post active IMT. An exception was a decrease of the right TEI at post active IMT from 2.0±0.8 to 1.7±0.8 mm (p = 0.038). The results are displayed in Table 2 and Fig. 1.
Table 2
Results of post sham IMT and post active IMT
| Baseline | Post sham IMT | Post active IMT | ANOVA (p-value) | |
| Respiratory muscle strength (n = 9) | ||||
| MIP (cmH2O) | 38±11.1 | 43±15.9 | 47±16.6* | 0.045 |
| MIP (% predicted) | 48.1±15.2 | 53.8±21.6 | 58.6±21.8 | 0.054 |
| MEP (cmH2O) | 45±18.3 | 50±21.0 | 44±17.6 | 0.206 |
| MEP (% predicted) | 45.9±20.7 | 50.2±20.9 | 44.7±18.0 | 0.291 |
| SNIP (cmH2O) | 40±17.1 | 40±19.2 | 40±18.4 | 0.940 |
| SNIP (% predicted) | 43.2±18.6 | 43.4±21.6 | 43.9±20.8 | 0.924 |
| Spirometry (n = 7) | ||||
| FVC (L) | 2.4±1.2 | 2.3±1.2 | 2.3±1.2 | 0.422 |
| FVC (% predicted) | 54.0±22.7 | 53.1±22.4 | 53.1±21.6 | 0.511 |
| PCF (L/min) | 314.5±114.6 | 303.2±84.5 | 289.8±106.3 | 0.266 |
| Diaphragm ultrasound | ||||
| Right (n = 6) | ||||
| TEE (mm) | 0.9±0.3 | 1.1±0.3 | 1.0±0.3 | 0.317 |
| TEI (mm) | 1.7±0.7 | 2.0±0.8 | 1.7±0.8* | 0.043 |
| TEI - TEE (mm) | 0.8±0.4 | 0.9±0.6 | 0.7±0.5 | 0.148 |
| TR | 1.8±0.4 | 1.8±0.6 | 1.6±0.4 | 0.363 |
| Left (n = 7) | ||||
| TEE (mm) | 1.3±0.7 | 1.3±0.4 | 1.2±0.4 | 0.861 |
| TEI (mm) | 1.9±1.1 | 2.3±1.2 | 1.8±0.8 | 0.294 |
| TEI - TEE (mm) | 0.7±0.4 | 1.0±1.0 | 0.5±0.5 | 0.242 |
| TR | 1.5±0.3 | 1.8±0.8 | 1.4±0.3 | 0.205 |
Spirometry and diaphragm ultrasound were performed in seven patients visiting the hospital and PCF in six patients as the data of one patient is missing. The right hemidiaphragm of one patient could not be visualised. *Significant post-hoc test: post active IMT vs post sham IMT. Results are expressed as mean±SD. MIP, maximal inspiratory pressure. MEP, maximal expiratory pressure. SNIP, sniff nasal inspiratory pressure. FVC, forced vital capacity. PCF, peak cough flow. TEE, end-expiration diaphragm thickness. TEI, end-inspiration diaphragm thickness. TEI - TEE, absolute change in thickness. TR, thickening ratio. IMT, inspiratory muscle training.
Fig. 1
Result of MIP (A), MEP (B), FVC (C), and PCF (D) at baseline, post sham IMT and post active IMT. The one-way repeated measures ANOVA was only significant for MIP (p = 0.045) and not for MEP (p = 0.206), FVC (p = 0.422) and PCF (p = 0.266). Therefore, the p-values of the post-hoc tests are only shown for MIP. MIP, maximal inspiratory pressure. MEP, maximal expiratory pressure. FVC, forced vital capacity. PCF, peak cough flow. IMT, inspiratory muscle training.
DISCUSSION
The main finding of this pilot study is that an eight week inspiratory muscle training programme in a cohort of patients with nemaline myopathy with respiratory muscle weakness is feasible and improves MIP with 11%. No consistent effect of IMT was found on MEP, SNIP, FVC, PCF, or diaphragm thickness. IMT was well tolerated and there were no serious adverse events.
In accordance with previous studies in patients with slowly progressive neuromuscular disorders [13–16, 39], IMT in patients with nemaline myopathy was associated with an increase of MIP. This is the first study to show this effect in patients with nemaline myopathy in a relatively short training period, and the findings add to the evidence for the use of IMT in neuromuscular disorders to improve inspiratory muscle strength. The absolute increase of MIP in our study (4 cmH2O) is relatively small in comparison with previous studies in other neuromuscular disorders (increases ranging from 9 to 38 cmH2O) [13–16, 39]. These studies had similar training protocols as our study, except for sham IMT.
The lack of effect in the sham IMT period in our study does suggest that the reported increase in inspiratory muscle strength is real. Note that even though the values of MIP from baseline (38 cmH2O) to post-sham IMT (43 cmH2O) suggest an increase of 5 cmH2O, this is not statistically significant and related to a large variation in individual responses. To highlight this, only five out of nine patients showed an improvement after sham IMT, in contrast to eight out of nine patients after active IMT. It might be that the selection procedure of nemaline myopathy patients with low values of MIP from the cohort was prone to regression to the mean from baseline to post-sham measurement or that there is a learning effect in the performance of the MIP manoeuvre [43]. However, we cannot rule out that some patients did have a real increase in MIP after sham IMT. In previous studies in patients with neuromuscular disorders [18, 40, 44], heart failure [45] and chronic obstructive pulmonary disease [46], an increase in MIP was shown as well in the sham group. Of note, some of these sham trainings used a low resistance instead of none, thus are more likely to have a training effect. Furthermore, a study in healthy subjects performing a training consisting of inhalations to total lung capacity has shown that this leads to an improvement of the vital capacity attributed to the increase of maximal shortening of the inspiratory muscles [47].
Both MIP and SNIP assess inspiratory muscle strength, but in the current study IMT only showed an improvement of MIP. This is in contrast with previous studies reporting an effect of respiratory muscle training on SNIP [40, 48, 49]. SNIP evaluates diaphragm strength more specifically than MIP, as demonstrated in previous studies where SNIP generated higher transdiaphragmatic pressures than MIP[50, 51]. IMT is a nonspecific training method in which all respiratory muscles can be recruited to overcome the imposed load. Thus, it might be that patients in our study recruited the accessory respiratory muscles more than the diaphragm during training. Note that no improvements in diaphragm thickening were found as well. Whether this holds true or SNIP is less sensitive to detect changes in inspiratory muscle strength, should be addressed in future studies that use outcomes measures of transdiaphragmatic pressure. We found that end-inspiratory thickness of the diaphragm at the right side decreased after IMT, but not on the left side. Therefore, it is unlikely that this truly reflects a decrease in diaphragm thickness. As expected, MEP and PCF did not improve with active IMT, as IMT specifically targets the inspiratory muscles and not the expiratory muscles. This is in line with other threshold IMT studies in patients with a neuromuscular disorder [13–15, 39].
The minimal clinically important difference for MIP is unknown in IMT studies in neuromuscular disorders, therefore it is unclear whether the effect on MIP in our study is clinically relevant. Furthermore, there is no effect on pulmonary function measured by spirometry, which is in line with other studies on threshold IMT in neuromuscular disorders[16, 18, 19, 39, 52, 53]. A possible explanation is that larger increases of respiratory muscle strength are needed to increase pulmonary function. Therefore, in this pilot study the difference in MIP with the intervention does not seem to change the severity of the chronic respiratory failure for a given individual or the cohort.
The motivation of the patients to perform IMT was very high with a self-reported adherence of ∼97%, and only mild adverse effects were reported. The mild adverse effects, the absence of serious adverse events, and the short training programme probably contributed to a high adherence. The high adherence and mild adverse effects are in accordance with previous IMT studies in neuromuscular disorders [13–16, 18, 19, 39, 53]. In our study, there was a drop-out of three patients, two of which related to the COVID-19 pandemic. This exceptional situation possibly explains why the drop-out rate is higher than in previous IMT studies (maximum of two patients) [13–16, 18, 39, 53].
Currently, several mechanical supportive therapies are available for patients with nemaline myopathy to prevent a decline in respiratory status, like (non-)invasive mechanical ventilation and cough augmentation techniques [54]. In addition to these mechanical supportive therapies, respiratory muscle training specifically targets the weakened respiratory muscles. To date, IMT has not been widely incorporated into the clinical management of patients with nemaline myopathy in contrast to mechanical supportive therapies. The positive results of respiratory muscle training in the studies in neuromuscular disorders should be interpreted with caution as a systemic review found heterogeneity, small sample sizes, and a large risk of bias in the published studies [55]. It is unknown to what extent the results could be interpreted for nemaline myopathy, as congenital myopathies are underrepresented in these studies. Moreover, the training protocols in the studies differ greatly making a comparison challenging. Whether IMT could prevent respiratory insufficiency or can improve respiratory muscle strength in patients with respiratory failure is unknown. Due to this lack of conclusive evidence, IMT is sparsely applied in patients with a neuromuscular disorder in clinical practice and not part of the treatment guidelines. Nevertheless, it is important to note that exercise in neuromuscular disorders, with the exception of high intensity, is generally considered to be safe [12]. Strength training or aerobic exercise programmes might maximise muscle and cardiorespiratory function and prevent additional disuse atrophy [56].
Several limitations of this study should be addressed. First, our sample size was relatively small and heterogeneous. Patients with different ages, genotypes and level of ventilatory support were included. Moreover, the phenotype differed much between the patients. This makes it difficult to extrapolate our results to the entire group of patients with nemaline myopathy. The intended 18 patients could not be included because of a relatively low occurrence of respiratory muscle weakness in the cohort of 42 patients. This can be explained by the fact that in 24 patients nemaline myopathy was caused by variants in the KBTBD13 gene, the most common genotype in the Netherlands [27–29, 57]. This genotype is known to have relatively spared respiratory muscles [29, 58, 59]. Moreover, the low prevalence of nemaline myopathy makes recruitment challenging. Second, we were unable to include patients with severe respiratory muscle weakness since their MIP was too low to overcome the lowest resistance of the IMT device. Thus, the effect of IMT in severely affected nemaline myopathy patients remains unknown. As yet, only a few studies have investigated the effects of IMT in patients with a neuromuscular disorder with severe respiratory muscle weakness. Two studies in Duchenne muscular dystrophy did not find an improvement of respiratory muscle endurance in patients with severe respiratory muscle weakness [60, 61]. The studies in patients requiring non-invasive mechanical ventilation show contradicting results ranging from improvement of MIP to no effect [15, 52, 62, 63]. Third, due to the inclusion criterium of a MIP of less than 70% of predicted values, we did not study the effect of IMT in patient with nemaline myopathy with a normal MIP. It is plausible that the initiation of respiratory muscle training prior to the manifestation of respiratory muscle weakness mitigates subsequent decline in respiratory muscle function. The use of a MIP of less than 70% of predicted values to indicate respiratory muscle weakness, is not a strict cut-off as there is sparse literature in neuromuscular disorders to support this. Fourth, we did not perform a randomised controlled trial as this was not feasible with the expected number of inclusions. A cross-over design was not suitable, as a carry-over effect of active IMT to sham IMT could not be excluded. However, all patients performed a sham training period to minimise a possible placebo effect or an effect of improved technique of test performance.
Future multicentre studies in a larger cohort of nemaline myopathy patients could assess/ confirm whether threshold IMT is an intervention that stabilises or improves respiratory muscle strength. International collaboration is essential to design a well-powered randomised controlled trial, as discussed in the ENMC workshop in 2019 [64]. A future trial should preferably consist of a prolonged training programme to assess the long term effect and the feasibility for patients to train for a longer period. A larger number of participants would also allow for better understanding of variability in genotype/phenotype and age. Moreover, functional and patient reported outcome measures should be included. Using electronic devices in these studies would make it possible to provide the patients with feedback, monitor the adherence and perform assessments remotely [65].
In conclusion, in our cohort of nemaline myopathy patients with respiratory muscle weakness, threshold IMT in an eight week training programme is feasible and improves inspiratory muscle strength. Our findings provide valuable preliminary data for the design of a larger, more comprehensive trial on IMT in patients with nemaline myopathy.
ACKNOWLEDGMENTS
The patients with nemaline myopathy included in this study are acknowledged for their participation. Several authors are members of the European Reference Network for rare neuromuscular diseases (EURO-NMD).
FUNDING
This work was financially supported by A Foundation Building Strength and the Princess Beatrix Fund (Grant number W.OR17-08).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Laitila J , Wallgren-Pettersson C . Recent advances in nemaline myopathy. Neuromuscul Disord. (2021) ;31: (10):955–67. |
[2] | Sewry CA , Laitila JM , Wallgren-Pettersson C . Nemaline myopathies: A current view. J Muscle Res Cell Motil. (2019) ;40: (2):111–26. |
[3] | Huang K , Bi FF , Yang H . A Systematic Review and Meta-Analysis of the Prevalence of Congenital Myopathy. Front Neurol. (2021) ;12: :761636. |
[4] | Wallgren-Pettersson C , Sewry CA , Nowak KJ , Laing NG . Nemaline myopathies. Semin Pediatr Neurol. (2011) ;18: (4):230–8. |
[5] | Morrow B , Argent A , Zampoli M , Human A , Corten L , Toussaint M . Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database Syst Rev. (2021) ;4: (4):Cd013170. |
[6] | Silva IS , Fregonezi GA , Dias FA , Ribeiro CT , Guerra RO , Ferreira GM . Inspiratory muscle training for asthma. Cochrane Database Syst Rev. (2013) ;2013: (9):Cd003792. |
[7] | Sapienza CM . Respiratory muscle strength training applications. Curr Opin Otolaryngol Head Neck Surg. (2008) ;16: (3):216–20. |
[8] | Cahalin LP , Arena RA . Breathing exercises and inspiratory muscle training in heart failure. Heart Fail Clin. (2015) ;11: (1):149–72. |
[9] | Gosselink R , De Vos J , van den Heuvel SP , Segers J , Decramer M , Kwakkel G . Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur Respir J. (2011) ;37: (2):416–25. |
[10] | Berlowitz DJ , Tamplin J . Respiratory muscle training for cervical spinal cord injury. Cochrane Database Syst Rev. 2013: (7):Cd008507. |
[11] | Menezes KK , Nascimento LR , Ada L , Polese JC , Avelino PR , Teixeira-Salmela LF . Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: A systematic review. J Physiother. (2016) ;62: (3):138–44. |
[12] | Preisler N , Orngreen MC . Exercise in muscle disorders: What is our current state? Curr Opin Neurol. (2018) ;31: (5):610–7. |
[13] | Aslan GK , Huseyinsinoglu BE , Oflazer P , Gurses N , Kiyan E . Inspiratory Muscle Training in Late-Onset Pompe Disease: The Effects on Pulmonary Function Tests, Quality of Life, and Sleep Quality. Lung. (2016) ;194: (4):555–61. |
[14] | Jevnikar M , Kodric M , Cantarutti F , Cifaldi R , Longo C , Della Porta R , et al. Respiratory muscle training with enzyme replacement therapy improves muscle strength in late - onset Pompe disease. Molecular Genetics and Metabolism Reports. (2015) ;5: :67–71. |
[15] | Wenninger S , Greckl E , Babacic H , Stahl K , Schoser B . Safety and efficacy of short- and long-term inspiratory muscle training in late-onset Pompe disease (LOPD): A pilot study. Journal of Neurology. (2019) ;266: (1):133–47. |
[16] | Fregonezi GA , Resqueti VR , Guell R , Pradas J , Casan P . Effects of 8-week, interval-based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest. (2005) ;128: (3):1524–30. |
[17] | Weiner P , Gross D , Meiner Z , Ganem R , Weiner M , Zamir D , et al. Respiratory muscle training in patients with moderate to severe myasthenia gravis. The Canadian Journal of Neurological Sciences Le Journal Canadien Des Sciences Neurologiques. (1998) ;25: (3):236–41. |
[18] | Cheah BC , Boland RA , Brodaty NE , Zoing MC , Jeffery SE , McKenzie DK , et al. INSPIRATIonAL–INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis: Official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. (2009) ;10: (5-6):384–92. |
[19] | Pinto S , Swash M , de Carvalho M . Respiratory exercise in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis: Official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. (2012) ;13: (1):33–43. |
[20] | Rodillo E , Noble-Jamieson CM , Aber V , Heckmatt JZ , Muntoni F , Dubowitz V . Respiratory muscle training in Duchenne muscular dystrophy. Archives of Disease in Childhood. (1989) ;64: (5):736–8. |
[21] | Stern LM , Martin AJ , Jones N , Garrett R , Yeates J . Training inspiratory resistance in Duchenne dystrophy using adapted computer games. Developmental Medicine and Child Neurology. (1989) ;31: (4):494–500. |
[22] | Smith BK , Bleiweis MS , Zauhar J , Martin AD . Inspiratory muscle training in a child with nemaline myopathy and organ transplantation. Pediatr Crit Care Med. (2011) ;12: (2):e94–8. |
[23] | Amburgey K , Acker M , Saeed S , Amin R , Beggs AH , Bönnemann CG , et al. A Cross-Sectional Study of Nemaline Myopathy. Neurology. (2021) ;96: (10):e1425–e36. |
[24] | Dall’Ago P , Chiappa GR , Guths H , Stein R , Ribeiro JP . Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: A randomized trial. J Am Coll Cardiol. (2006) ;47: (4):757–63. |
[25] | Chiappa GR , Roseguini BT , Vieira PJ , Alves CN , Tavares A , Winkelmann ER , et al. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. (2008) ;51: (17):1663–71. |
[26] | Côté P , Kreitz BG , Cassidy JD , Dzus AK , Martel J . A study of the diagnostic accuracy and reliability ofthe Scoliometer and Adam’s forward bend test. Spine (Phila Pa 1976). (1998) ;23: (7):796–802; discussion 3. |
[27] | Pauw-Gommans IM , Gerrits KH , de Haan A , van Engelen BG . Muscle slowness in a family with nemaline myopathy. Neuromuscul Disord. (2006) ;16: (8):477–80. |
[28] | Gommans IM , Davis M , Saar K , Lammens M , Mastaglia F , Lamont P , et al. A locus on chromosome 15q for a dominantly inherited nemaline myopathy with core-like lesions. Brain. (2003) ;126: (Pt 7):1545–51. |
[29] | Gommans IM , van Engelen BG , ter Laak HJ , Brunner HG , Kremer H , Lammens M , et al. A new phenotype of autosomal dominant nemaline myopathy. Neuromuscul Disord. (2002) ;12: (1):13–8. |
[30] | Quanjer PH , Stanojevic S , Cole TJ , Baur X , Hall GL , Culver BH , et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function equations. Eur Respir J. (2012) ;40: (6):1324–43. |
[31] | Uldry C , Fitting JW . Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax. (1995) ;50: (4):371–5. |
[32] | Wilson SH , Cooke NT , Edwards RH , Spiro SG . Predicted normal values for maximal respiratory pressures in caucasianadults and children. Thorax. (1984) ;39: (7):535–8. |
[33] | Verma R , Chiang J , Qian H , Amin R . Maximal Static Respiratory and Sniff Pressures in Healthy Children. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. (2019) ;16: (4):478–87. |
[34] | American Thoracic Society/European Respiratory S. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. (2002) ;166: (4):518–624. |
[35] | Graham BL , Steenbruggen I , Miller MR , Barjaktarevic IZ , Cooper BG , Hall GL , et al. Standardization of Spirometry Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. (2019) ;200: (8):e70–e88. |
[36] | Laveneziana P , Albuquerque A , Aliverti A , Babb T , Barreiro E , Dres M , et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. (2019) ;53: (6). |
[37] | Boon AJ , Harper CJ , Ghahfarokhi LS , Strommen JA , Watson JC , Sorenson EJ . Two-dimensional ultrasound imaging of the diaphragm: Quantitative values in normal subjects. Muscle & Nerve. (2013) ;47: (6):884–9. |
[38] | van Doorn JLM , Pennati F , Hansen HHG , van Engelen BGM , Aliverti A , Doorduin J . Respiratory muscle imaging by ultrasound and MRI in neuromuscular disorders. Eur Respir J. 2021. |
[39] | Yeldan I , Gurses HN , Yuksel H . Comparison study of chest physiotherapy home training programmes on respiratory functions in patients with muscular dystrophy. Clinical Rehabilitation. (2008) ;22: (8):741–8. |
[40] | Aslan GK , Gurses HN , Issever H , Kiyan E . Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: A randomized controlled trial. Clinical Rehabilitation. (2014) ;28: (6):573–81. |
[41] | Lakens D . Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. (2013) ;4: :863. |
[42] | McGraw KO , Wong SP . A common language effect size statistic. Psychological Bulletin. (1992) ;111: (2):361–5. |
[43] | Terzi N , Corne F , Mouadil A , Lofaso F , Normand H . Mouth and nasal inspiratory pressure: Learning effect and reproducibility in healthy adults. Respiration. (2010) ;80: (5):379–86. |
[44] | Jones HN , Kuchibhatla M , Crisp KD , Hobson-Webb LD , Case L , Batten MT , et al. Respiratory muscle training in late-onset Pompe disease: Results of a sham-controlled clinical trial. Neuromuscul Disord. (2020) ;30: (11):904–14. |
[45] | Marco E , Ramírez-Sarmiento AL , Coloma A , Sartor M , Comin-Colet J , Vila J , et al. High-intensity vs. sham inspiratory muscle training in patients with chronic heart failure: A prospective randomized trial. Eur J Heart Fail. (2013) ;15: (8):892–901. |
[46] | Langer D , Ciavaglia C , Faisal A , Webb KA , Neder JA , Gosselink R , et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J Appl Physiol. (2018) ;125: (2):381–92. |
[47] | Fanta CH , Leith DE , Brown R . Maximal shortening of inspiratory muscles: Effect of training. J Appl Physiol Respir Environ Exerc Physiol. (1983) ;54: (6):1618–23. |
[48] | Allen J , Astin R , Smith C , Banks D , Turner C . Expiratory muscle strength training improves measures of pressure generation and cough strength in a patient with myotonic dystrophy type 1. Neuromuscul Disord. (2020) ;30: (9):750–5. |
[49] | Araujo TL , Resqueti VR , Lima INDF , Dourado Junior ME , Fregonezi G . Effects of respiratory muscle training on respiratory muscle strength and heart rate variability in myotonic dystrophy patients type 1. Journal of Respiratory and CardioVascular Physical Therapy. (2012) ;1: (1):3–8. |
[50] | Nava S , Ambrosino N , Crotti P , Fracchia C , Rampulla C . Recruitment of some respiratory muscles during three maximal inspiratory manoeuvres. Thorax. (1993) ;48: (7):702–7. |
[51] | Prigent H , Orlikowski D , Fermanian C , Lejaille M , Falaize L , Louis A , et al. Sniff and Muller manoeuvres to measure diaphragmatic muscle strength. Respir Med. (2008) ;102: (12):1737–43. |
[52] | Klefbeck B , Lagerstrand L , Mattsson E . Inspiratory muscle training in patients with prior polio who use part-time assisted ventilation. Archives of Physical Medicine and Rehabilitation. (2000) ;81: (8):1065–71. |
[53] | Topin N , Matecki S , Le Bris S , Rivier F , Echenne B , Prefaut C , et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul Disord. (2002) ;12: (6):576–83. |
[54] | Toussaint M , Chatwin M , Gonzales J , Berlowitz DJ . 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders Naarden, The Netherlands, 3-5 March, 2017. Neuromuscul Disord. (2018) ;28: (3):289–98. |
[55] | Silva IS , Pedrosa R , Azevedo IG , Forbes AM , Fregonezi GA , Dourado Junior ME , et al. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database Syst Rev. (2019) ;9: :Cd011711. |
[56] | Voet NB , van der Kooi EL , van Engelen BG , Geurts AC . Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. (2019) ;12: (12):Cd003907. |
[57] | de Winter JM , Molenaar JP , Yuen M , van der Pijl R , Shen S , Conijn S , et al. KBTBD13 is an actin-binding protein that modulates muscle kinetics. J Clin Invest. (2020) ;130: (2):754–67. |
[58] | Kang ZX , Wei XJ , Miao J , Gao YL , Wang ZY , Yu XF . A family with nemaline myopathy type 6 caused by hseterozygous mutation (c. C>T) in the KBTBD13 gene in China: A case report. Neuropathology. (2020) ;40: (1):104–8. |
[59] | Olivé M , Goldfarb LG , Lee HS , Odgerel Z , Blokhin A , Gonzalez-Mera L , et al. Nemaline myopathy typeClinical and myopathological features. Muscle & nerve. (2010) ;42: (6):901–7. |
[60] | Vilozni D , Bar-Yishay E , Gur I , Shapira Y , Meyer S , Godfrey S . Computerized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul Disord. (1994) ;4: (3):249–55. |
[61] | Wanke T , Toifl K , Merkle M , Formanek D , Lahrmann H , Zwick H . Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. (1994) ;105: (2):475–82. |
[62] | Jones HN , Crisp KD , Robey RR , Case LE , Kravitz RM , Kishnani PS . Respiratory muscle training (RMT) in late-onset Pompe disease (LOPD): Effects of training and detraining. Molecular genetics and metabolism. (2016) ;117: (2):120–8. |
[63] | Smith BK , Martin AD , Lawson LA , Vernot V , Marcus J , Islam S , et al. Inspiratory muscle conditioning exercise and diaphragm gene therapy in Pompe disease: Clinical evidence of respiratory plasticity. Experimental Neurology. (2017) ;287: (Pt 2):216–24. |
[64] | Neuhaus SB , Wallgren-Pettersson C , Bönnemann CG , Schara U , Servais L . 250th ENMC International Workshop: Clinical trial readiness in nemaline myopathy 6-8 September 2019, Hoofdorp, the Netherlands. Neuromuscul Disord. (2020) ;30: (10):866–75. |
[65] | McNarry MA , Berg RMG , Shelley J , Hudson J , Saynor ZL , Duckers J , et al. Inspiratory Muscle Training Enhances Recovery Post COVID- A Randomised Controlled Trial. Eur Respir J 19: :2022. |




