A Targeted Approach for Evaluating DUX4-Regulated Proteins as Potential Serum Biomarkers for Facioscapulohumeral Muscular Dystrophy Using Immunoassay Proteomics
Abstract
Background:
Facioscapulohumeral muscular dystrophy (FSHD) is a progressive myopathy caused by misexpression of the double homeobox 4 (DUX4) embryonic transcription factor in skeletal muscle. Identifying quantitative and minimally invasive FSHD biomarkers to report on DUX4 activity will significantly accelerate therapeutic development.
Objective:
The goal of this study was to analyze secreted proteins known to be induced by DUX4 using the commercially available Olink Proteomics platform in order to identify potential blood-based molecular FSHD biomarkers.
Methods:
We used high-throughput, multiplex immunoassays from Olink Proteomics to measure the levels of several known DUX4-induced genes in a cellular myoblast model of FSHD, in FSHD patient-derived myotube cell cultures, and in serum from individuals with FSHD. Levels of other proteins on the Olink Proteomics panels containing these DUX4 targets were also examined in secondary exploratory analysis.
Results:
Placental alkaline phosphatase (ALPP) levels correlated with DUX4 expression in both cell-based FSHD systems but did not distinguish FSHD patient serum from unaffected controls.
Conclusions:
ALPP, as measured with the Olink Proteomics platform, is not a promising FSHD serum biomarker candidate but could be utilized to evaluate DUX4 activity in discovery research efforts.
INTRODUCTION
Facioscapulohumeral muscular dystrophy (FSHD) is an inherited myopathy with no disease-modifying or curative treatment options [1]. FSHD is caused by misexpression of the double homeobox 4 (DUX4) gene in skeletal muscle following epigenetic de-repression of the D4Z4 macrosatellite repeat array on chromosome 4q35 due to D4Z4 array contraction (type 1) or mutations in genes encoding D4Z4 chromatin modifiers (type 2) [2–5]. Sustained misexpression of DUX4, a transcription factor important for early embryonic development, is toxic [6–9]. Toxicity is thought to be caused by the dysregulation of genes and proteins involved in germline and stem cell development, myogenesis, innate immunity, and several other important cellular functions, though exactly how downstream molecular changes lead to pathology is not fully understood [10, 11]. In individuals with FSHD, DUX4-induced cell death leads to skeletal muscle fiber degeneration and replacement with fatty, fibrous tissue resulting in progressive muscle weakness and disabling physical limitations. Skeletal muscle atrophy typically first affects the face, shoulders, and arms, and then descends into the trunk and lower extremities.
As our understanding of FSHD pathophysiology has deepened, therapeutic efforts to prevent or delay FSHD disease progression have evolved from attempts to generally improve muscle function to approaches that specifically modulate DUX4 itself and the pathways that underlie DUX4 toxicity [12, 13]. With targeted therapies beginning to enter clinical trials [14, 15] it is essential to have robust tools with which to assess their effectiveness. Biomarkers are one such tool. Because many of the proposed disease-modifying therapies for FSHD selectively target DUX4, biomarkers designed to report on DUX4 level and/or activity are of critical importance in clinical development. Indeed, biomarker identification and validation has been a major goal of recent FSHD clinical trial workshops [16, 17]. To date, several studies have examined DUX4 target gene expression as a proxy for DUX4 activity from invasive muscle biopsies guided by MRI [18–20]. However, circulating blood-based molecular biomarkers are of particular interest as they have the potential to provide rapid, objective, minimally invasive, and quantitative measurements that can be assayed repeatedly over time using typically inexpensive methods, in contrast to tissue biomarkers that require repeated muscle biopsies. A circulating biomarker may also allow physiological changes to be detected at a time when functional differences may not yet be measurable. Additionally, since all skeletal muscles are exposed to the circulation, muscle-derived serum or plasma proteins have the potential to reflect average disease burden throughout the body [21], “smoothing out” the local spatial variability in DUX4 expression observed with needle biopsies. However, since only a small fraction of muscles may present with active DUX4 expression at any one time in FSHD, a useful circulating biomarker may require a highly sensitive assay and little to no background contribution from unaffected muscle and non-muscle tissue.
There are limited data exploring peripheral blood biomarkers in FSHD and no independently validated circulating markers that can be used as predictive or prognostic tools. A study using whole transcriptome analysis to interrogate blood RNA expression profiles did not find any gene expression differences between individuals with FSHD and unaffected controls that were significant after multiple hypothesis correction [22]. Studies using serum or plasma from FSHD patients and controls have identified proteins related to non-specific muscle damage [21, 23], immunity mediators [24–28], and miRNAs [26, 29, 30] as enriched in the disease context, but none were FSHD-specific and therefore might not reflect active DUX4-mediated disease processes. An expedient way to accelerate FSHD biomarker discovery is to utilize established, commercially available, clinical-grade proteomics platforms to measure levels of known DUX4 targets. Although the proteomic panels used in prior studies [21, 23, 25, 27, 28] in some cases incidentally included DUX4 targets, no studies to date have by design measured serum protein levels of DUX4 targets –despite the fact that quantifying DUX4 target expression in muscle is the only validated way to detect and track DUX4 activity [18, 19, 31]. While prior shotgun proteomics studies of FSHD serum [24, 26] could in principle have detected alterations in levels of DUX4 targets, more sensitive targeted assays may offer improved power to do so.
Here, we performed a focused and targeted study using the commercially available Proximity Extension Assay proteomics technology from Olink Proteomics to determine whether any already well-established DUX4-regulated proteins present on the Olink Proteomics panels would show differential levels in FSHD patient versus unaffected control serum with the ultimate goal of identifying markers warranting further investigation. Most established DUX4 targets are not present on any of the Olink Proteomics biomarker panels, but because we are using DUX4 target expression levels as a proxy for DUX4 activity –rather than making suppositions that any particular target plays a direct role in FSHD pathophysiology –this need not be an impediment. The results we present include our findings from using Olink Proteomics panels in comparisons of serum from FSHD patients versus unaffected controls, as well as comparisons of FSHD patient-derived myotubes versus control cells, and studies using an inducible DUX4 myoblast model of FSHD.
MATERIALS AND METHODS
Cell culture
MB135 (Control-A), 54-1 (Control-B), MB073 (FSHD-A, FSHD type 1), 54-2 (FSHD-B, FSHD type 1), MB200 (FSHD-C, FSHD type 2), and MB135-iDUX4 immortalized human myoblasts were a gift from Dr. Stephen Tapscott and originated from the FSHD Research Center at the University of Rochester Medical Center. MB135-iDUX4 cells have been described previously [32]. All cell lines were authenticated by karyotype analysis and determined to be free of mycoplasma by PCR screening. Cell line characteristics are provided in Supplementary Table 1. Myoblasts were maintained in Ham’s F-10 Nutrient Mix (Gibco) supplemented with 20% Fetal Bovine Serum (Gibco), 10 ng/mL recombinant human basic fibroblast growth factor (Promega), and 1μM dexamethasone (Sigma-Aldrich). MB135-iDUX4 myoblasts were additionally maintained in 2μg/mL puromycin dihydrochloride (VWR). Induction of the DUX4 transgene was achieved by culturing cells in 1μg/mL doxycycline hyclate (Sigma-Aldrich). Differentiation of myoblasts into myotubes was achieved by switching the fully confluent myoblast monolayer into Dulbecco’s Modified Eagle Medium (Gibco) containing 1% horse serum (Gibco) and Insulin-Transferrin-Selenium (Gibco). All cells were incubated at 37°C with 5% CO2.
Human serum
All serum samples (n = 20 unaffected controls and n = 20 FSHD patients) were obtained following informed, written consent through the FSHD Research Center at the University of Rochester Medical Center under a local IRB-approved protocol and were deidentified. Donor characteristics are described in Supplementary Table 2.
Preparation of cell lysates and supernatants for Olink Proteomics analysis
Myoblasts were seeded at a density of 2.5×105 cells/well (MB135, 54-1, MB073, 54-2, MB200) or 1.5×105 cells/well (MB135-iDUX4) on 12-well plates. Twenty-four hours prior to harvest, cells were washed three times with PBS and serum-free media was added. After 24 hours in serum-free media, supernatant and cell lysate were harvested as follows. The supernatant was removed, centrifuged for 5 minutes at 300 rcf to pellet any cell debris, transferred to a microcentrifuge tube, snap frozen with liquid nitrogen, and stored at -80°C. Fifty microliters of ice-cold 1X RIPA Lysis Buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate) supplemented with cOmplete Mini EDTA-free Protease Inhibitor Tablets (Roche) was added to each well, lysate was collected with a cell scraper, transferred to a microcentrifuge tube, incubated on ice for 15 minutes, sonicated with a Bioruptor (Diagenode) for 5 minutes on low (30 seconds on, 30 seconds off) to aid lysis, centrifuged for 5 minutes at 16,000 rcf at 4°C, transferred to a new microcentrifuge tube, quantified using the BCA Protein Assay Kit (Pierce), snap frozen with liquid nitrogen, and stored at -80°C. Samples were shipped on dry ice to Olink Proteomics.
Olink Proteomics assay
Olink Proteomics conducted targeted, high-throughput, multiplex immunoassays of protein levels using the provided cell lysates, supernatants, and serum. Samples were run on the Target 96 Development (v.3511) and/or Target 96 Oncology III (v.4001) panels –these panels were selected because they were the only to include DUX4 targets. The assay readout of normalized protein expression (NPX) values [33] –log2-scaled scores with additional Inter-Plate Control normalization –was obtained from Olink Proteomics for downstream analysis. Because NPX scores are on a log2 scale, fold changes are computed as 2(difference in NPX). The data from Olink Proteomics also included a limit of detection (LOD) score for each protein, defined to be the average score for negative control wells (buffer only) plus three standard deviations. Data below the LOD were used “as-is”, and although the response beneath the LOD can be non-linear, the effect of this on estimated fold changes is typically conservative (https://www.olink.com/faq/how-is-the-limit-of-detection-lod-estimated-and-handled).
siRNA transfections
Silencer Select siRNAs were obtained from Thermo Fisher Scientific and transfected into MB135-iDUX4 myoblasts 36 hours prior to doxycycline induction using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. The siRNAs used in this study are listed below:
siCTRL: Silencer Select Negative Control No. 1 siRNA
siDUX4: CCUACACCUUCAGACUCUATT (sense), UAGAGUCUGAAGGUGUAGGCA (anti-sense)
RNA isolation and RT-qPCR
Total RNA was extracted from whole cells using TRIzol Reagent (Invitrogen) following the manufacturer’s instructions. Isolated RNA was treated with DNase I (Invitrogen) and reverse transcribed into cDNA using SuperScript III Reverse Transcriptase (Invitrogen) and random hexamers (Invitrogen) according to the manufacturer’s protocol. Quantitative PCR was carried out on a CFX384 Touch Real-Time PCR Detection System (Bio-Rad) using primers specific to each gene of interest and iTaq Universal SYBR Green Supermix (Bio-Rad). The expression levels of target genes were normalized to that of the reference gene RPL27 using the delta-Ct method [34]. The primers used in this study are listed below:
CKM F: CACCCCAAGTTCGAGGAGAT
CKM R: AGCGTTGGACACGTCAAATA
DUX4 transgene F: TAGGGGAAGAGGTAGACGGC
DUX4 transgene R: CGGTTCCGGGATTCCGATAG
KHDC1L F: CACCAATGGCAAAGCAGTGG
KHDC1L R: TCAGTCTCCGGTGTACGGTG
MYOG F: GCCAGACTATCCCCTTCCTC
MYOG R: GAGGCCGCGTTATGATAAAA
RPL27 F: GCAAGAAGAAGATCGCCAAG
RPL27 R: TCCAAGGGGATATCCACAGA
ZSCAN4 F: TGGAAATCAAGTGGCAAAAA
ZSCAN4 R: CTGCATGTGGACGTGGAC
Statistical analysis
Data analysis and statistical tests were performed using GraphPad Prism software (version 9.0) or in the R programming environment using LIMMA [35] with plots generated using Prism or ggplot2 [36]. LIMMA computes empirical Bayes moderated t-statistics and F-statistics, with variances that are shrunken toward a global mean-variance trend (option trend = T); this helps stabilize results, particularly for studies in which the number of replicates is small. The focus of this study was on the small number of pre-defined DUX4 target proteins (from Table S1 of [31]) that were included on Olink Proteomics biomarker panels (one or two DUX4 target proteins on each of two 92-protein panels). In exploratory analyses of the full panels, false discovery rate (FDR) was used to control for multiple hypothesis testing.
RESULTS
Identification of DUX4 target genes on Olink Proteomics biomarker panels
We sought to identify candidate DUX4-induced genes for which commercial, clinical-grade proteomics assays were readily available, with a particular interest in those genes with low background expression in somatic tissues (based on Genotype-Tissue Expression (GTEx) Project data, www.gtexportal.org) and whose protein products were predicted to be secreted (based on Human Protein Atlas (HPA) secretome data [37], www.proteinatlas.org). We compared a set of 213 robust DUX4-upregulated target genes (Table S1 of [31]) to the 1,160 unique human protein targets found on 14 different Olink Proteomics Target 96 biomarker panels (Fig. 1A). Three targets were present on both lists: placental alkaline phosphatase (ALPP), carbonic anhydrase 2 (CA2), and corticotropin releasing hormone binding protein (CRHBP). Of these, ALPP and CRHBP were included in the HPA secretome database and are predicted to encode signal peptides (as determined by UniProt annotation), making them excellent candidates to be secreted and have potential utility as FSHD blood-based molecular biomarkers.
Fig. 1
DUX4-induced ALPP protein is present in muscle cell supernatant. (A) Venn diagram showing the overlap between unique protein targets on the Olink Proteomics human Target 96 panels and DUX4-induced genes as classified in [31]. (B) The protein level of ALPP (left), CA2 (middle), and CRHBP (right) as measured by Olink Proteomics assay in cell lysate (top) and supernatant (bottom) harvested from MB135-iDUX4 myoblasts left untreated (-DOX) or treated with doxycycline (+DOX) to induce DUX4 transgene expression for 12 or 24 hours. (C) ALPP protein levels as measured by Olink Proteomics assay in cell lysate (top) and supernatant (bottom) from MB135-iDUX4 myoblasts treated with or without doxycycline for 24 hours following transfection with no (-), non-targeting control (siCTRL), or DUX4 (siDUX4) siRNA. Protein levels in (B) and (C) are presented as normalized log2 expression values. LOD, limit of detection. Each data point in the plots represents a single replicate; replicates from comparable conditions in (B) and (C) are grouped together in Supplementary Figure 1C.
![DUX4-induced ALPP protein is present in muscle cell supernatant. (A) Venn diagram showing the overlap between unique protein targets on the Olink Proteomics human Target 96 panels and DUX4-induced genes as classified in [31]. (B) The protein level of ALPP (left), CA2 (middle), and CRHBP (right) as measured by Olink Proteomics assay in cell lysate (top) and supernatant (bottom) harvested from MB135-iDUX4 myoblasts left untreated (-DOX) or treated with doxycycline (+DOX) to induce DUX4 transgene expression for 12 or 24 hours. (C) ALPP protein levels as measured by Olink Proteomics assay in cell lysate (top) and supernatant (bottom) from MB135-iDUX4 myoblasts treated with or without doxycycline for 24 hours following transfection with no (-), non-targeting control (siCTRL), or DUX4 (siDUX4) siRNA. Protein levels in (B) and (C) are presented as normalized log2 expression values. LOD, limit of detection. Each data point in the plots represents a single replicate; replicates from comparable conditions in (B) and (C) are grouped together in Supplementary Figure 1C.](https://content.iospress.com:443/media/jnd/2023/10-6/jnd-10-6-jnd221636/jnd-10-jnd221636-g001.jpg)
ALPP distinguishes DUX4-expressing samples from controls in inducible DUX4 myoblasts
To measure the level of ALPP, CA2, and CRHBP in DUX4-expressing cells by Olink Proteomics assay we used the MB135-iDUX4 myoblast cell line, which has been engineered to inducibly express a DUX4 transgene upon addition of doxycycline [32]. MB135-iDUX4 myoblasts were left untreated or cultured with doxycycline for 12 or 24 hours. Doxycycline treatment led to robust, time-dependent DUX4 transgene induction as well as activation of DUX4 target genes [31] such as KHDC1L (Supplementary Figure 1A, Supplementary Table 3). Olink Proteomics analysis was performed on cell lysate and cell supernatant samples at both time points and normalized protein expression (NPX) values were reported on a log2 scale (Fig. 1B, Supplementary Figure 2A, Supplementary Table 4). Samples with no or low levels of DUX4 also have low levels of DUX4 targets and therefore might be expected to display only nominal target protein expression via Olink Proteomics assay. ALPP protein levels were above the limit of detection (LOD) in all cell lysate samples, with NPX values of ∼5.6 in both untreated and doxycycline-treated cells at 12 hours and also in untreated cells at 24 hours, but increasing to an NPX value of 8.7 in doxycycline-treated cells at 24 hours. Note that because NPX scores are on a log2 scale, the increase from ∼5.6 to 8.7 corresponds to a 23.1 = 8.6-fold increase in protein level; other references to fold changes are based on similar calculations of 2(delta NPX). In contrast, the level of CA2 and CRHPB protein in MB135-iDUX4 cell lysate hovered around the LOD, with CRHBP showing no correlation to DUX4 expression and CA2 displaying only a 1.4-fold increase after treatment with doxycycline for 24 hours. The inducible promoter in this cell system does exhibit some “leakiness” (Supplementary Figure 1B) [38], which may have contributed to the measurable cell lysate ALPP levels in the absence of doxycycline. In cell supernatants, CA2 and CRHBP protein levels were at or below the LOD, as were ALPP levels for samples left untreated or treated with doxycycline for 12 hours. However, after 24 hours of doxycycline treatment ALPP protein in cell supernatant was above the LOD and was 3.5-fold higher than the paired untreated sample. Because the NPX score for the untreated sample was below the LOD it may include a sizable contribution from non-specific background signal, so this fold change may be underestimated (see Methods) and the p-values should be regarded with caution; a similar caveat applies to other comparisons involving scores below the LOD. These results demonstrate that while CRHBP protein levels do not track with DUX4 expression, and CA2 levels only distinguish the highest DUX4-expressing condition from the rest in cell lysate, ALPP protein levels robustly correlate with DUX4 expression in both cell lysates and cell supernatants from MB135-iDUX4 myoblasts. Therefore, ALPP appeared to be a molecule worth pursuing further as a circulating FSHD biomarker.
To confirm that ALPP protein levels in MB135-iDUX4 cells are dependent on DUX4 expression, we knocked down the DUX4 transgene using siRNA-mediated depletion and measured ALPP protein in cell lysate and supernatant in untreated cells or after 24 hours of doxycycline via Olink Proteomics assay (Fig. 1C, Supplementary Figure 2B, Supplementary Table 4). Recapitulating our previous results, myoblasts treated with doxycycline to induce DUX4 for 24 hours showed elevated ALPP levels compared to untreated cells (∼20-fold in lysate, ∼3-fold in supernatant) when left untransfected or transfected with a non-targeting control siRNA. Also, as before, only in supernatant were ALPP levels in untreated cells below the LOD. This independent replication of prior ALPP measurements reveals low variability across replicates (Supplementary Figure 1C). Importantly, in doxycycline-treated cells transfected with siRNAs targeting the DUX4 transgene, ALPP levels decreased (∼4-fold in lysate, ∼2.5-fold in supernatant) and in supernatant fell below the LOD. DUX4 transgene and DUX4 target gene mRNA levels decreased >65% upon treatment with DUX4 siRNAs, showing the efficacy of the knockdown (Supplementary Figure 1D, Supplementary Table 3). As a control to confirm that doxycycline treatment alone does not impact ALPP expression, we treated parental MB135 myoblasts with doxycycline and saw no effect on the level of ALPP, which hovered at or below the LOD, in cell lysate or supernatant (Supplementary Figure 1E, Supplementary Table 4). Together, these results demonstrate that ALPP protein detected in the supernatant of MB135-iDUX4 myoblasts via Olink Proteomics distinguishes DUX4-expressing samples from controls in an inducible DUX4 myoblast system.
ALPP distinguishes FSHD from control myotubes
To determine if ALPP protein could distinguish FSHD cells expressing endogenous levels of DUX4 from controls, we employed two unaffected control and three FSHD patient-derived myoblast cell lines that were differentiated into myotubes for 2, 4, or 6 days. DUX4 levels are known to increase over this differentiation time course [39]. ALPP levels in cell lysate and supernatant were below the LOD in both control cell lines at most time points, whereas by day 2 (in lysate) or day 6 (in supernatant) of differentiation ALPP was above the LOD in all three FSHD cell lines (Fig. 2A, Supplementary Figure 2C, Supplementary Table 5). In supernatant, this increase occurred gradually, with one of the FSHD cell lines showing measurable ALPP levels at day 2 of differentiation, two at day 4, and all three at day 6. Elevation of ALPP in FSHD versus control myotubes was significant (p < 0.05 by LIMMA moderated t-test) at all three time points for lysate (day 2: p = 0.038; day 4: p = 0.016; day 6: p = 0.0012) but just at day 6 for supernatant (day 2: p = 0.26; day 4: p = 0.084; day 6: p = 0.018). The myogenic genes MYOG and CKM were induced over this course of differentiation in all cell lines, as expected (Supplementary Figure 3, Supplementary Table 6), as was the DUX4 target gene ZSCAN4 in the FSHD lines (Fig. 2B, Supplementary Table 6). Note that ZSCAN4 levels typically had the same rank order among FSHD cell lines (FSHD-C≥FSHD-B≥FSHD-A) as ALPP levels, consistent with both being induced by DUX4, but with just three samples such a concordance in ranks could also plausibly occur by chance. In conclusion, ALPP expression robustly distinguishes DUX4-expressing samples from controls in supernatants and lysates from differentiating muscle cell lines.
Fig. 2
ALPP protein levels increase upon FSHD myoblast differentiation. (A) The normalized log2 expression level of ALPP protein as measured by Olink Proteomics assay in the cell lysate (left) and supernatant (right) of two independent control and three independent FSHD myoblast cell lines differentiated into myotubes for 2, 4, or 6 days. (B) DUX4 target gene ZSCAN4 mRNA levels as measured by RT-qPCR in control and FSHD myoblast cell lines differentiated into myotubes for 0, 2, 4, or 6 days. LOD, limit of detection. n/a, not available.
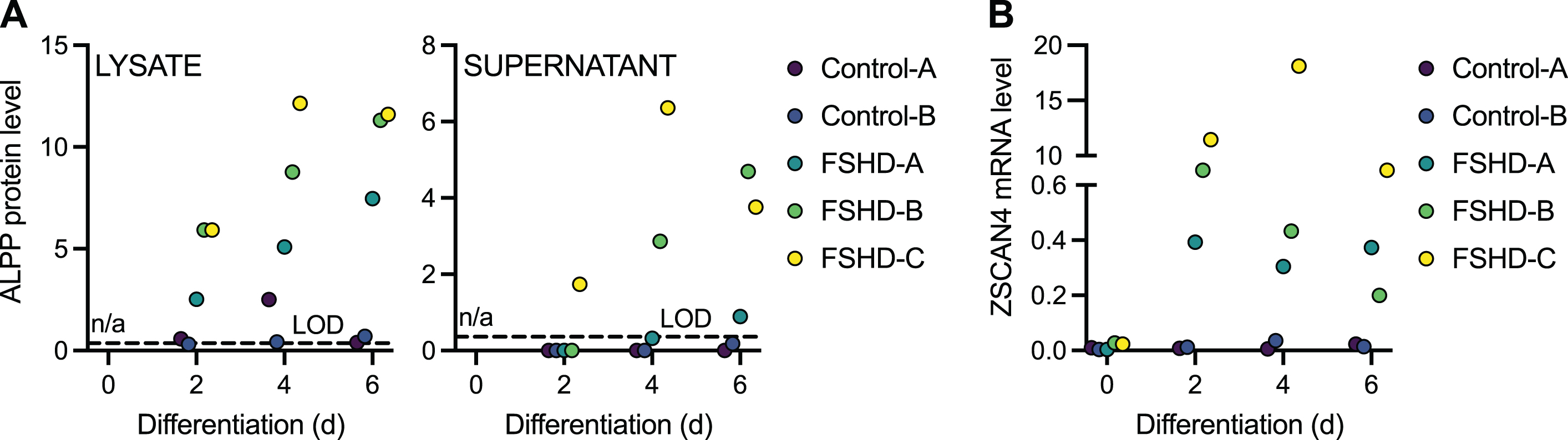
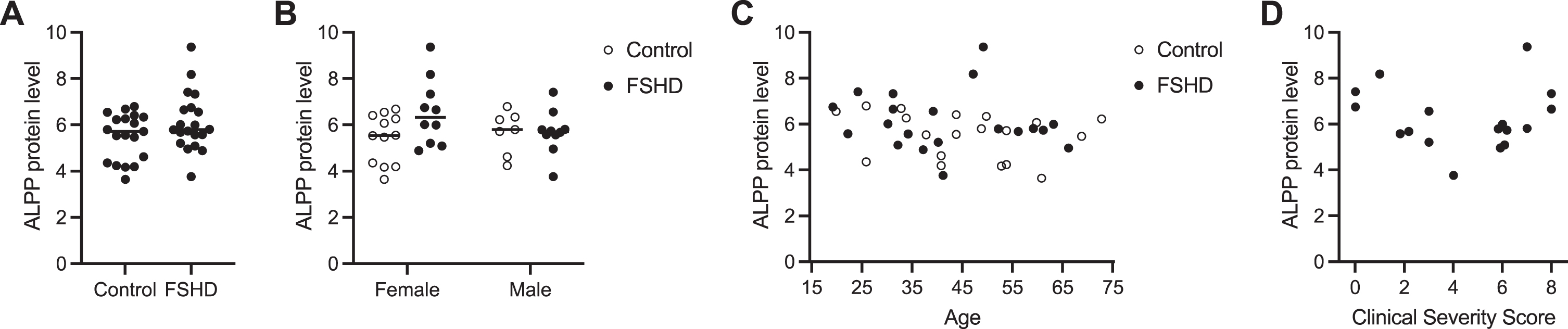
Fig. 3
Serum ALPP levels do not predict FSHD disease state. (A) ALPP protein levels presented as normalized log2 expression values measured by Olink Proteomics assay in serum from 20 individuals with FSHD and 20 unaffected controls. (B–D) ALPP protein levels shown in (A) stratified by sex (B), age (C), and FSHD Clinical Severity Score (D).
ALPP levels do not distinguish FSHD from control serum
To measure the level of ALPP protein in blood from individuals with FSHD and unaffected controls, we obtained 40 serum samples from the FSHD Research Center at the University of Rochester Medical Center and performed Olink Proteomics assays (Supplementary Figure 4). We fit a LIMMA linear model with factors for sex and disease status and their interaction (moderated two-way ANOVA) for the 92 proteins on the Target 96 Oncology III panel that includes ALPP. Here we summarize the results for ALPP, and results for all proteins are discussed in the Supplementary Material. There was not overall a significant association of ALPP protein levels in serum with FSHD status and/or sex (p-value = 0.104 for F-test) (Fig. 3A-B, Supplementary Table 7), a result in contrast to our findings from the MB135-iDUX4 cellular myoblast model of FSHD and FSHD patient-derived myotube cell cultures. ALPP levels were on average higher in FSHD than control samples among the female subjects (increase of 1.14 NPX or ∼2.2-fold; nominal p-value = 0.018 for this contrast), but as the overall F-test was non-significant this should be regarded with caution (Fig. 3B). There was also not a significant correlation between serum ALPP levels and age (rho = 0.29 and p-value = 0.07 by Spearman rank correlation test) (Fig. 3C), or with disease severity among those with FSHD (rho = 0.01 and p-value = 0.97 by Spearman rank correlation test) (Fig. 3D). The plot of FSHD clinical severity score (CSS) versus ALPP protein levels may have some hint of a V-shape but this could be a chance occurrence and is non-significant (p-value = 0.22 using a Hoeffding D-test for a not-necessarily monotonic association). Notably, serum ALPP levels were high at baseline even in unaffected controls, which may confound the circulating contribution from diseased muscle (see Discussion). Together, these results suggest that ALPP, as measured by the Olink Proteomics platform, is not a sensitive biomarker candidate for FSHD, though it could have utility as a tool in discovery research given its sensitivity and specificity in reporting out DUX4 activity in cell-based FSHD model systems.
As our goal was to identify DUX4-induced serum biomarkers, we used only the two Olink Proteomics panels that included protein products of known DUX4-induced genes. The two panels used in the cell culture studies contained 181 other protein targets, and although not of primary interest we also examined their levels. Several increased in expression following doxycycline induction of DUX4 in the MB135-iDUX4 myoblasts (Supplementary Figure 2, Supplementary Table 8). Of these, VMO1, PSPN, PTP4A1, and NOV are upregulated at the mRNA level in DUX4-expressing cells [32], but were not present on our original list of 213 robust DUX4-upregulated target genes. Only PTP4A1 protein levels correlated with DUX4 expression in both MB135-iDUX4 cell lysates and cell supernatants; however, PTP4A1 did not distinguish FSHD from control myotubes, illustrating the importance of validating any findings derived from inducible DUX4 cell models with FSHD patient-derived muscle cells expressing endogenous levels of DUX4.
DISCUSSION
As potential FSHD therapies enter clinical trials, having quantitatively sensitive and rapidly responsive molecular biomarkers is critical for assessment of therapeutic approaches. Circulating biomarkers in particular provide the promise of reflecting overall disease burden in ways not possible using physical examination, tissue biopsy, or imaging methods. In this study, we used a commercial platform provided by Olink Proteomics to measure protein levels of three known DUX4-induced targets –ALPP, CA2, and CRHBP –in lysate and supernatant from a cellular myoblast model of FSHD and in FSHD patient-derived myotube cell cultures. The two cell-based systems revealed ALPP as a promising secreted FSHD biomarker candidate, so we performed Olink Proteomics validation assays for ALPP in serum from individuals with FSHD and unaffected controls, but these did not show a correlation between serum ALPP levels and disease state or severity and revealed high background levels of ALPP in human serum. Therefore, we conclude that ALPP, as detected by the Olink Proteomics platform, is not a good clinical FSHD biomarker despite its promising performance in cellular systems.
Our initial examination of background tissue expression was primarily based on RNA expression levels from GTEx, which may not reflect serum protein levels. Moreover, the GTEx database does not include placenta, an organ where ALPP is expressed based on data from the HPA. Other confounding systemic non-muscle sources of ALPP may arise from the lung, gastrointestinal cells, and/or the cervix [40]. However, the Human Plasma Proteome Project (HPPP) database (www.hupo.org/plasma-proteome-project) showed little evidence for plasma ALPP expression at the time of this study, and the HPA page for ALPP says that it is not detected in plasma by mass spectrometry based on the PeptideAtlas database (build id = 465). We note however that HPA now also includes Proximity Extension Assay data from plasma based on a recent study using Olink Proteomics [41], and this does show fairly high levels of ALPP among unaffected controls, consistent with our observations. It may be possible that ALPP homologs could be contributing to the observed background serum ALPP levels, as we were unable to assess the specificity of the Olink Proteomics assay for ALPP (versus, for example, ALPG) and therefore cannot dismiss the possibility that the assay may be cross-reacting with other proteins. Another limitation of this study is that for comparisons between groups that involve values beneath the LOD, both the reported fold changes and p-values should be regarded with caution.
The prolonged, non-linear muscle degradation and highly variable clinical presentation typical of FSHD requires precise measurement of biomarker concentrations so that subtle changes in disease can be detected. The Olink Proteomics technology used in this study claims high specificity and sensitivity and could provide a powerful tool for quantifying low-abundance DUX4-induced proteins that might have been otherwise thought too variable to be useful biomarkers for disease assessment. In this study we were limited to a small number of DUX4-induced proteins that were present on existing Olink Proteomics panels. There is the possibility of designing custom Olink Proteomics panels that consolidate DUX4 target proteins and other FSHD-relevant proteins from existing panels onto smaller focused panels, but targeted mass spectrometry approaches using Parallel or Multiple Reaction Monitoring (PRM/MRM) offer an interesting alternative for profiling a larger collection of DUX4-induced proteins in serum, as has been done for microdialysates from FSHD muscle [24].
In the serum studies we used only one Olink Proteomics biomarker panel –the Target 96 Oncology III panel containing ALPP –since the two DUX4 targets on the Target 96 Development panel did not show clear DUX4-dependent induction in cell culture studies, which may be due in part to their levels being near or below the Olink Proteomics assay limit of detection. Incidental exploratory findings for the other proteins on the Target 96 Oncology III panel in serum are discussed in the legend to Supplementary Figure 4.
The decision to focus our circulating biomarker search on DUX4-induced genes was intended to ensure that any hits were clearly relevant to FSHD, but also required that the DUX4 target be secreted from muscle cells. Our time course differentiation data suggest that ALPP secretion requires high, sustained DUX4 (and DUX4 target) expression, a prerequisite that may eliminate other candidate molecules not detectable under the conditions used here. However, our study also clearly demonstrated the utility of ALPP as a discovery tool. Measuring ALPP levels in the supernatant of DUX4-expressing cells would allow for time course experiments not possible with currently used RNA and protein analysis methods.
Overall, it is possible that multiple assessment approaches will be necessary to evaluate changes in FSHD disease burden over the course of a clinical trial. Combining information from serum biomarkers, muscle biopsy, magnetic resonance imaging, and clinical strength and activity measurements may increase our ability to assess disease progression and evaluate FSHD therapeutics.
ACKNOWLEDGMENTS
We thank the FSHD families whose participation is critical for progress. This study was supported by the FSHD Society (S.J. and A.E.C.), the Geraldi Norton Foundation (S.J. and A.E.C.), NIH 5P50HD060848-15 Wellstone Center for FSHD (O.D.K.), and the RNA Bioscience Initiative at the University of Colorado Anschutz Medical Campus (S.J.). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
CONFLICT OF INTEREST
J.A. is the Chief Science Officer of the FSHD Society.
AUTHOR CONTRIBUTIONS
A.E.C., J.A., O.D.K., and S.J. conceived and designed the study. A.E.C. performed experiments. R.T. provided critical reagents. A.E.C., J.A., O.D.K., and S.J. analyzed data. A.E.C. wrote the manuscript with input from all authors.
REFERENCES
[1] | Hamel J , Tawil R . Facioscapulohumeral muscular dystrophy: Update on pathogenesis and future treatments, Neurotherapeutics (2018) ;15: (4):863–71. https://doi.org/10.1007/s13311-018-00675-3 |
[2] | Hamanaka K , Sikrova D , Mitsuhashi S , Masuda H , Sekiguchi Y , Sugiyama A , et al. Homozygous nonsense variant in LRIF1 associated with facioscapulohumeral muscular dystrophy, Neurology (2020) ;94: (23):e2441–e7. https://doi.org/10.1212/WNL.0000000000009617 |
[3] | Lemmers RJ , Tawil R , Petek LM , Balog J , Block GJ , Santen GW , et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2, Nat Genet (2012) ;44: (12):1370–4. https://doi.org/10.1038/ng.2454 |
[4] | Lemmers RJ , van der Vliet PJ , Klooster R , Sacconi S , Camano P , Dauwerse JG , et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. (2010) ;329: (5999):1650–3. https://www.science.org/doi/10.1126/science.1189044 |
[5] | van den Boogaard ML , Lemmers R , Balog J , Wohlgemuth M , Auranen M , Mitsuhashi S , et al. Mutations in DNMT3B modify epigenetic repression of the D4Z4 repeat and the penetrance of facioscapulohumeral dystrophy, Am J Hum Genet (2016) ;98: (5):1020–9. https://doi.org/10.1016/j.ajhg.2016.03.013 |
[6] | De Iaco A , Planet E , Coluccio A , Verp S , Duc J , Trono D DUX-family transcription factors regulate zygotic genome activation in placental mammals, Nat Genet (2017) ;49: (6):941–5. https://doi.org/10.1038/ng.3858 |
[7] | Hendrickson PG , Dorais JA , Grow EJ , Whiddon JL , Lim JW , Wike CL , et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons, Nat Genet (2017) ;49: (6):925–34. https://doi.org/10.1038/ng.3844. |
[8] | Rickard AM , Petek LM , Miller DG Endogenous DUX4 expression in FSHD myotubes is sufficient to cause cell death and disrupts RNA splicing and cell migration pathways, Hum Mol Genet (2015) ;24: (20):5901–14. https://doi.org/10.1093/hmg/ddv315 |
[9] | Kowaljow V , Marcowycz A , Ansseau E , Conde CB , Sauvage S , Matteotti C , et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein, Neuromuscul Disord (2007) ;17: (8):611–23. https://doi.org/10.1016/j.nmd.2007.04.002 |
[10] | Campbell AE , Belleville AE , Resnick R , Shadle SC , Tapscott SJ Facioscapulohumeral dystrophy: Activating an early embryonic transcriptional program in human skeletal muscle, Hum Mol Genet (2018) ;27: (R2):R153–62. https://doi.org/10.1093/hmg/ddy162 |
[11] | Lim KRQ , Nguyen Q , Yokota T DUX4 signalling in the pathogenesis of facioscapulohumeral muscular dystrophy, Int J Mol Sci (2020) ;21: (3). https://doi.org/10.3390/ijms21030729 |
[12] | Wang LH , Tawil R Current therapeutic approaches in FSHD, J Neuromuscul Dis (2021) ;8: (3):441–51. doi: 10.3233/JND-200554 |
[13] | Cohen J , DeSimone A , Lek M , Lek A Therapeutic approaches in facioscapulohumeral muscular dystrophy, Trends Mol Med (2021) ;27: (2):123–37. https://doi.org/10.1016/j.molmed.2020.09.008 |
[14] | Mellion ML , Ronco L , Berends CL , Pagan L , Brooks S , van Esdonk MJ , et al. Phase 1 clinical trial of losmapimod in facioscapulohumeral dystrophy: Safety, tolerability, pharmacokinetics, and target engagement, Br J Clin Pharmacol (2021) ;87: (12):4658–69. https://doi.org/10.1111/bc14884 |
[15] | Efficacy and safety of losmapimod in subjects with facioscapulohumeral muscular dystrophy (FSHD) [Internet]. 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04003974. |
[16] | Tawil R , Padberg GW , Shaw DW , van der Maarel SM , Tapscott SJ , Participants FW Clinical trial preparedness in facioscapulohumeral muscular dystrophy: Clinical, tissue, and imaging outcome measures 29-30 May Rochester, New York, Neuromuscul Disord (2016) ;26: (2):181–6. https://doi.org/10.1016/j.jsurg.2016.07.008 10.1016/j.nmd.2015.10.005}. |
[17] | Tawil R , Shaw DW , van der Maarel SM , Tapscott SJ Clinical trial preparedness in facioscapulohumeral dystrophy: Outcome measures and patient access: 8-9 April Leiden, The Netherlands, Neuromuscul Disord (2014) ;24: (1):79–85. https://doi.org10.1016/j.nmd.2013.07.009 |
[18] | Wang LH , Friedman SD , Shaw D , Snider L , Wong CJ , Budech CB , et al. MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD, Hum Mol Genet (2019) ;28: (3):476–86. https://doi.org/10.1093/hmg/ddy364 |
[19] | Wong CJ , Wang LH , Friedman SD , Shaw D , Campbell AE , Budech CB , et al. Longitudinal measures of RNA expression and disease activity in FSHD muscle biopsies, Hum Mol Genet (2020) ;29: (6):1030–43. https://doi.org/10.1093/hmg/ddaa031. |
[20] | van den Heuvel A , Lassche S , Mul K , Greco A , San Leon Granado D , Heerschap A , et al. Facioscapulohumeral dystrophy transcriptome signatures correlate with different stages of disease and are marked by different MRI biomarkers, Sci Rep (2022) ;12: (1):1426. https://doi.org/10.1038/s41598-022-04817-8 |
[21] | Petek LM , Rickard AM , Budech C , Poliachik SL , Shaw D , Ferguson MR , et al. A cross sectional study of two independent cohorts identifies serum biomarkers for facioscapulohumeral muscular dystrophy (FSHD), Neuromuscul Disord (2016) ;26: (7):405–13. https://doi.org/10.1016/j.nmd.2016.04.012 |
[22] | Signorelli M , Mason AG , Mul K , Evangelista T , Mei H , Voermans N , et al. Evaluation of blood gene expression levels in facioscapulohumeral muscular dystrophy patients, Sci Rep (2020) ;10: (1):17547. https://doi.org/10.1038/s41598-020-74687-5 |
[23] | Statland J , Donlin-Smith CM , Tapscott SJ , van der Maarel S , Tawil R Multiplex screen of serum biomarkers in facioscapulohumeral muscular dystrophy, J Neuromuscul Dis (2014) ;1: (2):181–90. https://doi.org/10.3233/JND-140034 |
[24] | Corasolla Carregari V , Monforte M , Di Maio G , Pieroni L , Urbani A , Ricci E , et al. Proteomics of muscle microdialysates identifies potential circulating biomarkers in facioscapulohumeral muscular dystrophy, Int J Mol Sci (2020) ;22: (1). https://doi.org/10.3390/ijms22010290 |
[25] | Gros M , Nunes AM , Daoudlarian D , Pini J , Martinuzzi E , Barbosa S , et al. Identification of serum interleukin 6 levels as a disease severity biomarker in facioscapulohumeral muscular dystrophy, J Neuromuscul Dis (2022) ;9: (1):83–93. https://doi.org/10.3233/JND-210711 |
[26] | Heier CR , Zhang A , Nguyen NY , Tully CB , Panigrahi A , Gordish-Dressman H , et al. Multi-omics identifies circulating miRNA and protein biomarkers for facioscapulohumeral dystrophy, J Pers Med (2020) ;10: (4). https://doi.org/10.3390/jpm10040236 |
[27] | Wong CJ , Wang L , Holers VM , Frazer-Abel A , van der Maarel SM , Tawil R , et al. Elevated plasma complement components in facioscapulohumeral dystrophy, Hum Mol Genet (2022) ;31: (11):1821–9. https://doi.org/10.1093/hmg/ddab364 |
[28] | Tasca G , Monforte M , Corbi M , Granata G , Lucchetti D , Sgambato A , et al. Muscle microdialysis to investigate inflammatory biomarkers in facioscapulohumeral muscular dystrophy, Mol Neurobiol (2018) ;55: (4):2959–66. https://doi.org/10.1007/s12035-017-0563-x |
[29] | Matsuzaka Y , Kishi S , Aoki Y , Komaki H , Oya Y , Takeda S , et al. Three novel serum biomarkers, miR-1, miR-133a, and miR-206 for limb-girdle muscular dystrophy, facioscapulohumeral muscular dystrophy, and Becker muscular dystrophy, Environ Health Prev Med (2014) ;19: (6):452–8. https://doi.org/10.1007/s12199-014-0405-7 |
[30] | Nunes AM , Ramirez M , Jones TI , Jones PL Identification of candidate miRNA biomarkers for facioscapulohumeral muscular dystrophy using DUX4-based mouse models. Dis Model Mech. (2021) ;14: (8). https://doi.org/10.1242/dmm.049016 |
[31] | Yao Z , Snider L , Balog J , Lemmers RJ , Van Der Maarel SM , Tawil R , et al. DUX4-induced gene expression is the major molecular signature in FSHD skeletal muscle, Hum Mol Genet (2014) ;23: (20):5342–52. https://doi.org/10.1093/hmg/ddu251 |
[32] | Jagannathan S , Shadle SC , Resnick R , Snider L , Tawil RN , van der Maarel SM , et al. Model systems of DUX4 expression recapitulate the transcriptional profile of FSHD cells, Hum Mol Genet (2016) ;25: (20):4419–31. https://doi.org/10.1093/hmg/ddw271 |
[33] | Assarsson E , Lundberg M , Holmquist G , Bjorkesten J , Thorsen SB , Ekman D , et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability, PLoS One. (2014) ;9: (4):e95192. https://doi.org/10.1371/journal.pone.0095192 |
[34] | Livak KJ , Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. (2001) ;2: (4):402–8. https://doi.org/10.1006/meth.2001.1262 |
[35] | Ritchie ME , Phipson B , Wu D , Hu Y , Law CW , Shi W , et al. limma powers differential expression analyses for RNA-sequencing and microarray studies, Nucleic Acids Res (2015) ;43: (7):e47. https://doi.org/10.1093/nar/gkv007 |
[36] | Wickham H ggplot2: Elegant graphics for data analysis. New York, NY: Springer-Verlag; 2016. |
[37] | Uhlen M , Karlsson MJ , Hober A , Svensson AS , Scheffel J , Kotol D , et al. The human secretome. Sci Signal. (2019) ;12: (609). https://www.science.org/doi/10.1126/scisignal.aaz0274. |
[38] | Bosnakovski D , Chan SSK , Recht OO , Hartweck LM , Gustafson CJ , Athman LL , et al. Muscle pathology from stochastic low level DUX4 expression in an FSHD mouse model, Nat Commun. (2017) ;8: (1):550. https://doi.org/10.1038/s41467-017-00730-1 |
[39] | Balog J , Thijssen PE , Shadle S , Straasheijm KR , van der Vliet PJ , Krom YD , et al. Increased DUX4 expression during muscle differentiation correlates with decreased SMCHD1 protein levels at D4Z4, Epigenetics (2015) ;10: (12):1133–42. https://doi.org/10.1080/15592294.2015.1113798 |
[40] | Uhlen M , Oksvold P , Fagerberg L , Lundberg E , Jonasson K , Forsberg M , et al. Towards a knowledge-based Human Protein Atlas, Nat Biotechnol (2010) ;28: (12):1248–50. https://doi.org/10.1038/nbt1210-1248 |
[41] | Zhong W , Edfors F , Gummesson A , Bergstrom G , Fagerberg L , Uhlen M Next generation plasma proteome profiling to monitor health and disease, Nat Commun (2021) ;12: (1):2493. https://doi.org/10.1038/s41467-021-22767-z |
[42] | Wang X , Middleton FA , Tawil R , Chen XJ Cytosolic adaptation to mitochondria-induced proteostatic stress causes progressive muscle wasting, iScience (2022) ;25: (1):103715. https://doi.org/10.1016/j.isci.2021.103715 |
[43] | Gabellini D , Green MR , Tupler R Inappropriate gene activation in FSHD: A repressor complex binds a chromosomal repeat deleted in dystrophic muscle, Cell (2002) ;110: (3):339–48. https://doi.org/10.1016/S0092-8674(02)00826-7 |




