Improvements in Walking Distance during Nusinersen Treatment – A Prospective 3-year SMArtCARE Registry Study
Abstract
Background and objectives:
Disease progression in patients with spinal muscular atrophy (SMA) has changed dramatically within the past years due to the approval of three different disease-modifying treatments. Nusinersen was the first drug to be approved for the treatment of SMA patients. Clinical trials provided data from infants with SMA type 1 and children with SMA type 2, but there is still insufficient evidence and only scarcely reported long-term experience for nusinersen treatment in ambulant patients. Here, we report data from the SMArtCARE registry of ambulant patients under nusinersen treatment with a follow-up period of up to 38 months.
Methods:
SMArtCARE is a disease-specific registry in Germany, Austria and Switzerland. Data are collected as real-world data during routine patient visits. Our analysis included all patients under treatment with nusinersen able to walk independently before start of treatment with focus on changes in motor function.
Results:
Data from 231 ambulant patients were included in the analysis. During the observation period, 31 pediatric walkers (27.2%) and 31 adult walkers (26.5%) experienced a clinically meaningful improvement of≥30 m in the 6-Minute-Walk-Test. In contrast, only five adult walkers (7.7%) showed a decline in walking distance≥30 m, and two pediatric walkers (1.8%) lost the ability to walk unassisted under treatment with nusinersen. HFMSE and RULM scores improved in pediatric and remained stable in adult patients.
Conclusion:
Our data demonstrate a positive effect of nusinersen treatment in most ambulant pediatric and adult SMA patients. We not only observed a stabilization of disease progression or lack of deterioration, but clinically meaningful improvements in walking distance.
Trial registration DRKS00012699
INTRODUCTION
The development and approval of three different disease-specific drugs for the treatment of patients with spinal muscular atrophy (SMA) has dramatically changed the course of the disease. With the leading symptom of a progressive muscle weakness and atrophy, SMA is a rare neuromuscular disorder with a broad clinical spectrum affecting patients of all ages. Disease severity is determined by different biomarkers such as, SMN2 copy number, and correlates inversely with the age at symptom onset. Patients developing the first symptoms of muscle weakness at an age older than 18 months are expected to have a milder phenotype of SMA type 3 [1, 2]. These patients gain the ability to walk independently but often lose ambulation as the disease progresses [3, 4].
Three different disease-specific and approved drugs (nusinersen, onasemnogene abeparvovec and risdiplam) are now available for the treatment of SMA patients. Nusinersen (Spinraza®, Biogen, Cambridge, USA), an antisense oligonucleotide, acts as a splicing modifier targeting the intronic splicing silencer N1 in SMN2 [5]. Sham-controlled clinical trial data showed significant improvements in motor function in a small cohort of pediatric patients with either early-onset SMA or non-ambulant later-onset SMA under treatment with nusinersen with a follow-up period of 15 months, leading to the approval of nusinersen in Europe in 2017 [6, 7]. A small cohort of 13 ambulant children participated in the open-label phase I/II clinical trials [8], but ambulant and adult patients were not enrolled in the sham-controlled phase III clinical trials. Real-world data from different countries and disease registries indicate that nusinersen has the potential to positively influence disease progression in ambulant patients in the short-term follow-up [9–11]. However, data on the long-term effect of nusinersen in ambulant patients is still scarce. Motor function of SMA type 3 patients might be stable or slowly progressive for several years over the natural course of the disease, but still an average decline in 6MWT of -7.8 m per year has been observed [4]. Accordingly, in patients aged 30 years, the probability of remaining ambulant is 33% with symptom onset before 3 years (SMA type 3a) and to 69% with symptom onset after 3 years (SMA type 3b) [3]. Moreover, a persistent improvement of walking distance in patients with SMA is highly unlikely without any intervention.
Here, we report data on ambulant patients treated with nusinersen derived from the SMArtCARE registry with a follow-up time of up to 38 months focusing on long-term effects on motor function.
MATERIALS AND METHODS
SMArtCARE registry
SMArtCARE is a disease-specific registry with currently 58 participating centers in Germany, Austria and Switzerland [12]. All neuromuscular centers in these three countries are invited to participate in the SMArtCARE data collection. The registry encompasses data of 1190 patients of any age, SMA type, and treatment regime, as of November 2021. Inclusion criteria are genetically confirmed 5q-SMA and written consent of patients or caregivers. Within the SMArtCARE registry, data are collected during routine patient visits as real-life-outcome data. After the initial dosing phase within the first two months, visits are scheduled every four months during nusinersen treatment. To ensure completeness and data quality, we use standardized case report forms that are aligned with the international consensus for SMA registries [13]. The main focus of the data collection is the evaluation of motor function of patients using standardized physiotherapeutic assessments. Participating physiotherapists and raters were regularly trained to ensure interrater reliability. In contrast to clinical trials, these physiotherapeutic assessments are not mandatory within the SMArtCARE data collection und thus not available for all patients at all time-points.
Patient cohort
We identified all patients under nusinersen treatment in our database (data analysis 15th of November 2021). Inclusion criterion for this data analysis was that patients were able to walk independently at start of treatment –equivalent to a phenotype of SMA type 3, but excluding type 3 patients who had previously lost ambulation. Patients with a documentation of baseline characteristics and motor function before start of treatment were included. Patients were stratified to subgroups according to age at start of treatment: cohort “pediatric walkers” included all patients younger than 18 years and cohort “adult walkers” all patients older than 18 years of age.
Outcomes
Primary outcome of this analysis were changes in walking distance assessed with the 6-Minute-Walk-Test (6MWT) [14]. A minimum 10 minute rest was recommended before performing the 6MWT for all patients. A difference≥30 m in walking distance was considered clinically meaningful for SMA, Duchenne muscular dystrophy and other chronic diseases [14–16]. Further, changes in Hammersmith Functional Motor Scale Expanded (HFMSE) and Revised Upper Limb Module (RULM) scores were evaluated. The HFMSE consists of 33 items with a total score of 66 points with higher scores indicating better motor function. A change of≥3 points in the HFMSE score was considered clinically meaningful [17, 18]. The RULM comprises 20 items that focus on changes in function of upper limb. Total score is 37, with a change of≥2 points considered clinically meaningful [19]. To evaluate a possible ceiling effect of these motor function tests, patients with≥500 m in 6MWT [20], ≥60 points in HFMSE score and≥35 points in RULM score were depicted at baseline and at m38. The ability to walk independently was defined according to WHO criteria: taking five steps in upright position with the back straight without any contact with persons or objects [21]. In addition, longitudinal data on the need for ventilator support, the need for tube feeding, fatigue, and mortality were evaluated. The symptom of fatigue was captured by the clinician by taking the interval history and examination. At each visit, patients were asked whether they experience fatigue with acitivities of daily living. We further performed post-hoc analysis of 6MWT in all patients with a walking distance > 100 m at baseline and last observation to compare the time needed for the first 50 m with the time needed for the last 50 m. Adverse events (AE) were recorded as AE with or without hospitalization and specified using the Medical Dictionary for Regulatory Activities (MedDRA) code [22]. AEs were captured by the treating physicians at least during each planned patient visit. We asked the treating physician to assess whether the AE was related or possibly related to nusinersen treatment.
Statistical analysis
Before data export, primary and secondary outcomes and cohorts for subgroup analysis were defined in a statistical analysis plan. Descriptive analysis was performed by calculating absolute frequencies and percentages. Continuous data were analyzed as mean and 99% confidence interval. Analysis was based on comparisons from baseline to month 14 (m14), month 26 (m26), and month 38 (m38). The first visit of each patient corresponded to start of treatment, with a follow-up time of a maximum of 38 months. The last available visit was set as the individual endpoint for all patients and was considered for the analysis of clinically meaningful changes in 6MWT, HFMSE and RULM. Data for m38 were not available for all patients due to different timing of treatment initiation. For patients who stopped treatment within 38 months of follow-up, data up to a maximum of six months after treatment discontinuation was included into the analysis. If patients changed drug treatment, no further data were evaluated after discontinuation of nusinersen treatment. Inferential analysis was applied to evaluate the effect of age at symptom onset, age at start of treatment, SMN2 copy number, sex, fatigue and past time from baseline on changes in 6MWT. A p-value of≤0.01 was considered statistically significant.
Standard protocol approvals, registrations, and patient consents
Central ethics approval was obtained by the Ethics committee of the University of Freiburg (EK-Freiburg 56/18), and local ethics approvals were obtained from all participating centers. All patients or caregivers gave written informed consent before data were entered in the SMArtCARE registry.
Data availability
All data included in this analysis are recorded in the SMArtCARE registry. Anonymized and aggregated data will be provided by the corresponding author upon request and approval of the SMArtCARE steering committee.
RESULTS
Data from 231 patients from 38 neuromuscular centers were included in the data analysis. Table 1 summarizes baseline characteristics of all patients. Age at symptom onset was considerably higher in adult walkers than in pediatric walkers. This was probably due to the fact that patients with earlier onset lost ambulation and were thus not part of the adult population. Beyond that, cohorts were comparable regarding baseline motor function as assessed by 6MWT, HFMSE and RULM, and SMN2 copy number. As this study focused on ambulant patients, it did not include more severely affected patients with SMA type 3 (e.g. type 3a) as they typically loose ambulation early in the course of the disease.
Table 1
Baseline characteristics of all patients
| Pediatric walker | Adult walker | |
| (n = 114) | (n = 117) | |
| Age at symptom onset (months) | 40.8 [30.4;51.5] | 138.6 [113.6;163.7] |
| Age at start of treatment (months) | 103.3 [89;117.6] | 443.5 [405.1;481.9] |
| SMN2 copy number | ||
| 1 SMN2 | 0 (0%) | 2 (1.7%) |
| 2 SMN2 | 10 (8.8%) | 4 (3.4%) |
| 3 SMN2 | 35 (30.7%) | 24 (20.5%) |
| ≥4 SMN2 | 57 (50%) | 67 (57.3%) |
| unknown | 12 (10.5%) | 20 (17.1%) |
| FVC% (n) | 89.6 [85.4;93.8] (29) | 93.0 [89.6;96.4] (85) |
| HMFSE score (n) | 51.1 [48.6;53.7] (88) | 46.2 [43.2;49.5] (105) |
| RULM score (n) | 32.4 [30.8;34.2] (70) | 34.7 [33.7;35.9] (101) |
| 6MWT (n) | 329.2 [264.9;393.6] (50) | 353.8 [312.5;403.7] (84) |
| Fatigue | 27 (23.7%) | 42 (35.9%) |
Baseline characteristics of all patients allocated to pediatric and adult walkers. Data are listed as mean and 99% confidence interval or n (%).
During the observation period of 38 months, three patients (1.3%) stopped treatment with nusinersen. Of these, two patients changed treatment to risdiplam. None of the patients received a combination therapy with nusinersen and risdiplam or onasemnogene abeparvovec. Fourteen patients (6.1%) were lost to follow-up with no data entered over more than 12 months. Despite the Covid-19 pandemic, only 17 patients included in the analysis (7.4%) had intervals greater than six months between nusinersen treatments. Further, of all documented 2132 nusinersen treatments, only 1.3% were given at intervals greater than 6 months. Varying follow-up times were mainly caused by different timing of treatment initiation and not due to missing data. Table 2 lists the number of patients per cohort and time-points.
Table 2
Number of patients per cohort and time-point
| Baseline | m14 | m26 | m38 | |
| Pediatric walkers | 114 | 103 | 84 | 55 |
| Adult walkers | 117 | 109 | 81 | 55 |
6MWT
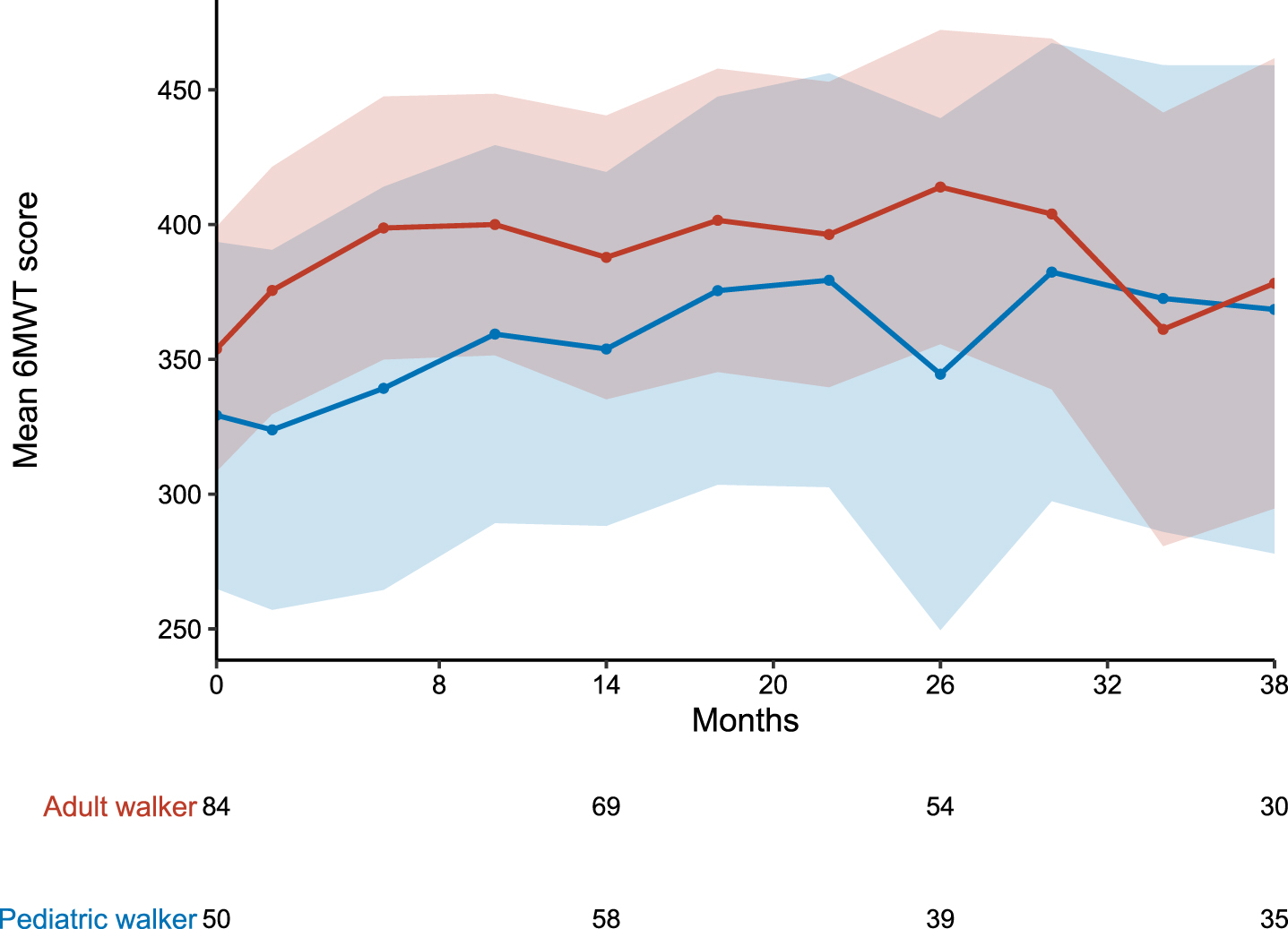
Both pediatric and adult patients improved their walking distance in the 6MWT during the observation period. In the overall cohorts, mean walking distance changed from baseline to m38 by 39.3 m in pediatric walkers and 24.4 m in adult walkers. Figure 1 displays the longitudinal progression of all patients in the different cohorts. Only considering patients who performed 6MWT at baseline and m38, mean walking distance changed by 83.0 m (n = 18) in pediatric walkers and 46.0 m (n = 27) in adult walkers. During the observation period, 31 pediatric walkers (27.2%) and 31 adult walkers (26.5%) experienced a clinically meaningful improvement of≥30 m. In contrast, five adult walkers (4.3%) showed a decline in walking distance≥30 m (none of these stopped treatment with nusinersen), and two pediatric walkers (1.8%) lost the ability to walk unassisted under treatment with nusinersen (loss of ambulation at the age of 10 and 7.5 years). One of the latter patients changed treatment to risdiplam. Of note, both patients who lost ambulation had three SMN2 copies. Loss of ambulation occurred 13.5 and 25.8 months after start of treatment. Baseline results of the 6MWT of these two patients were not available. A walking distance in the 6MWT≥500 m was observed in nine pediatric (7.9%) and 18 adult walkers (15.4%) at baseline, and 10 pediatric (18.2%) and nine adult walkers (16.4%) at m38, respectively (see Fig. 4).
Fig. 1
Longitudinal progression of the 6MWT. 6MWT for pediatric (blue) and adult (red) walkers. Data are listed as mean and 99% confidence interval. Available patients at baseline, m14, m26 and m38 are added. For a group size fewer than 10 patients no data are shown. As the performance of the 6MWT is not mandatory within the SMArtCARE data collection, data are not available for all patients at all time-points.
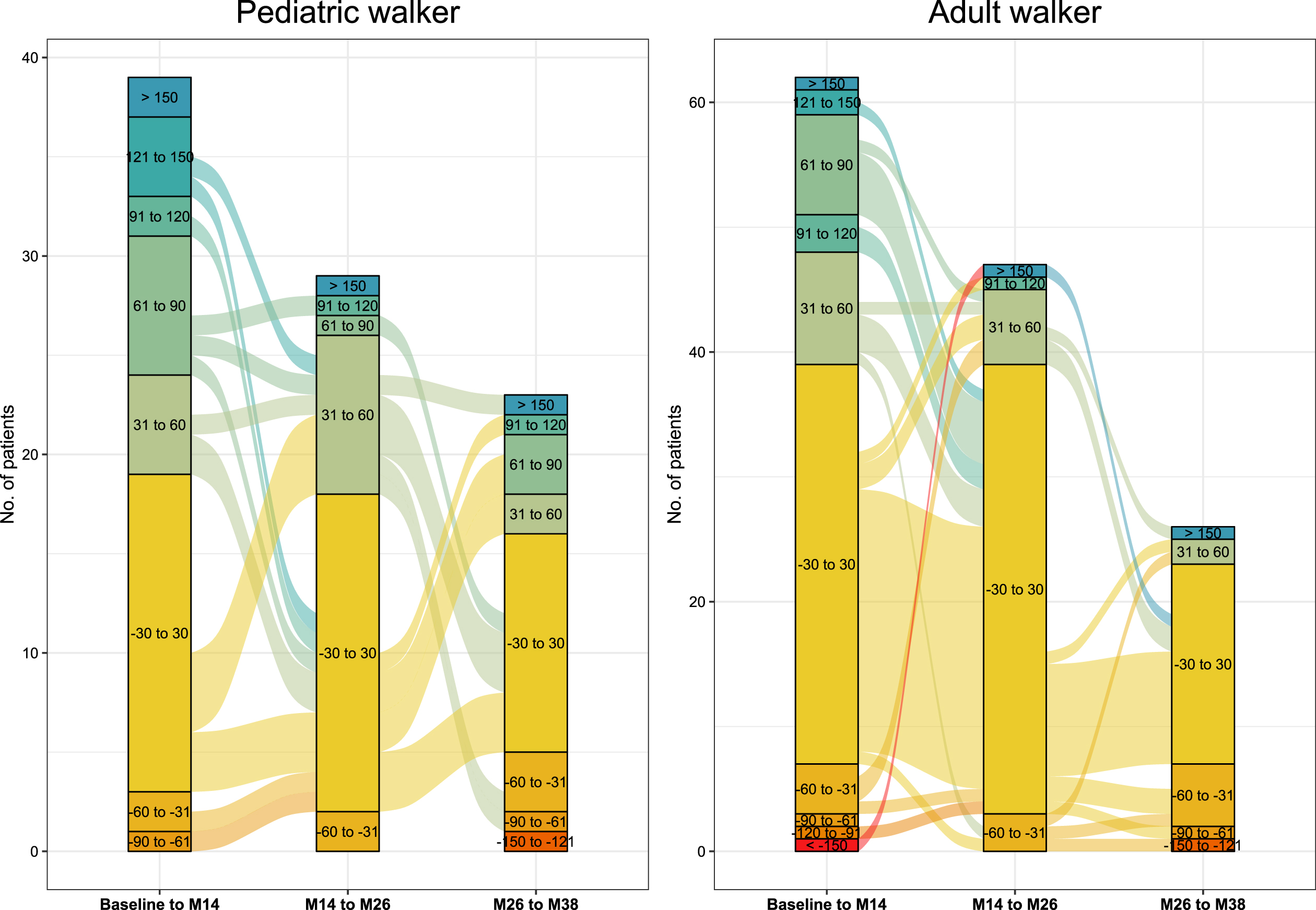
During the first 14 months of treatment, 20 pediatric (19.4%) and 23 adult walkers (21.1%) experienced improvements in the 6MWT≥30 m. In contrast, only 5 pediatric (4.9%) and 8 adult patients (7.3%) showed a decline≥30 m. In adult walkers, improvements≥30 m in walking distance occurred less frequently between m14 and m26 (11 pediatric (13.1%) and 8 adult walkers (9.9%)), and between m26 and m38 (7 pediatric (12.7%) and 3 adult walkers (5.5%)) (see Fig. 2).
Fig. 2
Responder analysis 6MWT. Responder analysis for pediatric walkers and adult walkers. Colors indicate response groups according to changes in 6MWT per time-period (baseline-m14, m14-m26, m26-m38). Lines between columns indicate the progression of patients over time with improvements or worsening in walking distance.
Inferential analysis revealed a higher SMN2 copy number as only covariate to have a significant influence on improvements in the 6MWT. The absence of fatigue and a lower walking distance at baseline were associated with greater improvements in the 6MWT (see Table 3).
Table 3
Inferential analysis 6MWT
| Value | SE | DF | t-value | p-value | |
| Baseline_m14 vs. m14_m26 | 9.810 | 4.216 | 700 | 2.327 | 0.02 |
| Baseline_m14 vs. m26_m38 | 3.155 | 6.598 | 700 | 0.478 | 0.63 |
| Age at symptom onset | 0.034 | 0.034 | 146 | 0.982 | 0.33 |
| Walking distance at baseline | –0.044 | 0.017 | 146 | –2.660 | 0.01 |
| Age at start of treatment | 0.011 | 0.015 | 146 | 0.704 | 0.48 |
| SMN2 copy number | –17.901 | 5.875 | 146 | –3.047 | 0.003 |
| Sex M | –2.683 | 5.656 | 146 | –0.474 | 0.64 |
| Fatigue | –6.894 | 3.574 | 700 | –1.929 | 0.05 |
Inferential analysis evaluates the effect of age at diagnosis, age at start of treatment, SMN2 copy number (≤3 SMN2 copies vs.≥4 SMN2 copies), gender, baseline 6MWT, and past time from baseline on changes in 6MWT. *Standard error = SE; degree of freedom = DF.
HFMSE and RULM
During the observation period of 38 months, mean HFMSE scores improved in pediatric walkers (+5.3 points) and remained stable in adult walkers (–1.4 points). Clinically meaningful improvements in HFMSE score were experienced by 38 pediatric (33.3%) and 42 adult walkers (35.9%). Eleven adult walkers (9.4%) showed a worsening in HFMSE≥3 points. A HFMSE score≥60 points was observed in 22 pediatric (19.3%) and 14 adult walkers (12.0%) at baseline, and 17 pediatric (31.0%) and four adult walkers (7.3%) at m38, respectively (see Fig. 4). Mean RULM scores improved in pediatric patients (+2.8 points) and remained stable in adult patients (–0.5 points) (see Fig. 3). Twenty-seven pediatric (23.7%) and 17 adult walkers (14.5%) showed clinically meaningful improvements in RULM score. Only three adult walkers (2.6%) experienced a worsening in RULM score≥2 points. A RULM score≥35 points was observed in 34 pediatric (29.8%) and 79 adult walkers (67.5%) at baseline, and 24 pediatric (43.6%) and 31 adult walkers (56.4%) at m38, respectively (see Fig. 4). Inferential analysis revealed a lower baseline score as only covariate to have a significant influence on improvements in RULM and HFMSE score (see supplementary Table 1).
Fig. 3
Longitudinal progression of HFMSE and RULM score. A HMFSE and B RULM score for pediatric (blue) and adult walkers (red). Data are listed as mean and 99% confidence interval. Available patients at baseline, m14, m26 and m38 are added. For a group size fewer than 10 patients no data are depicted.
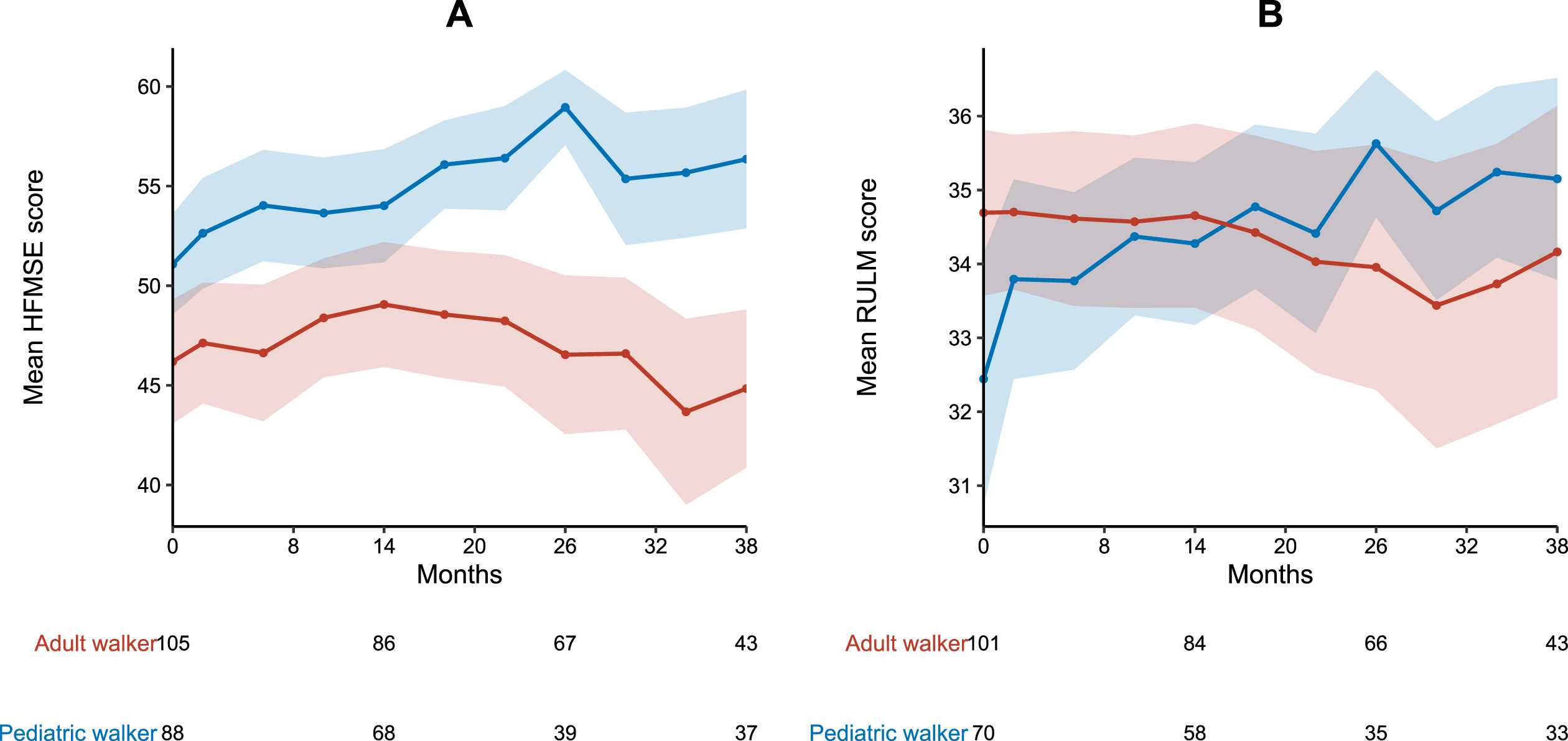
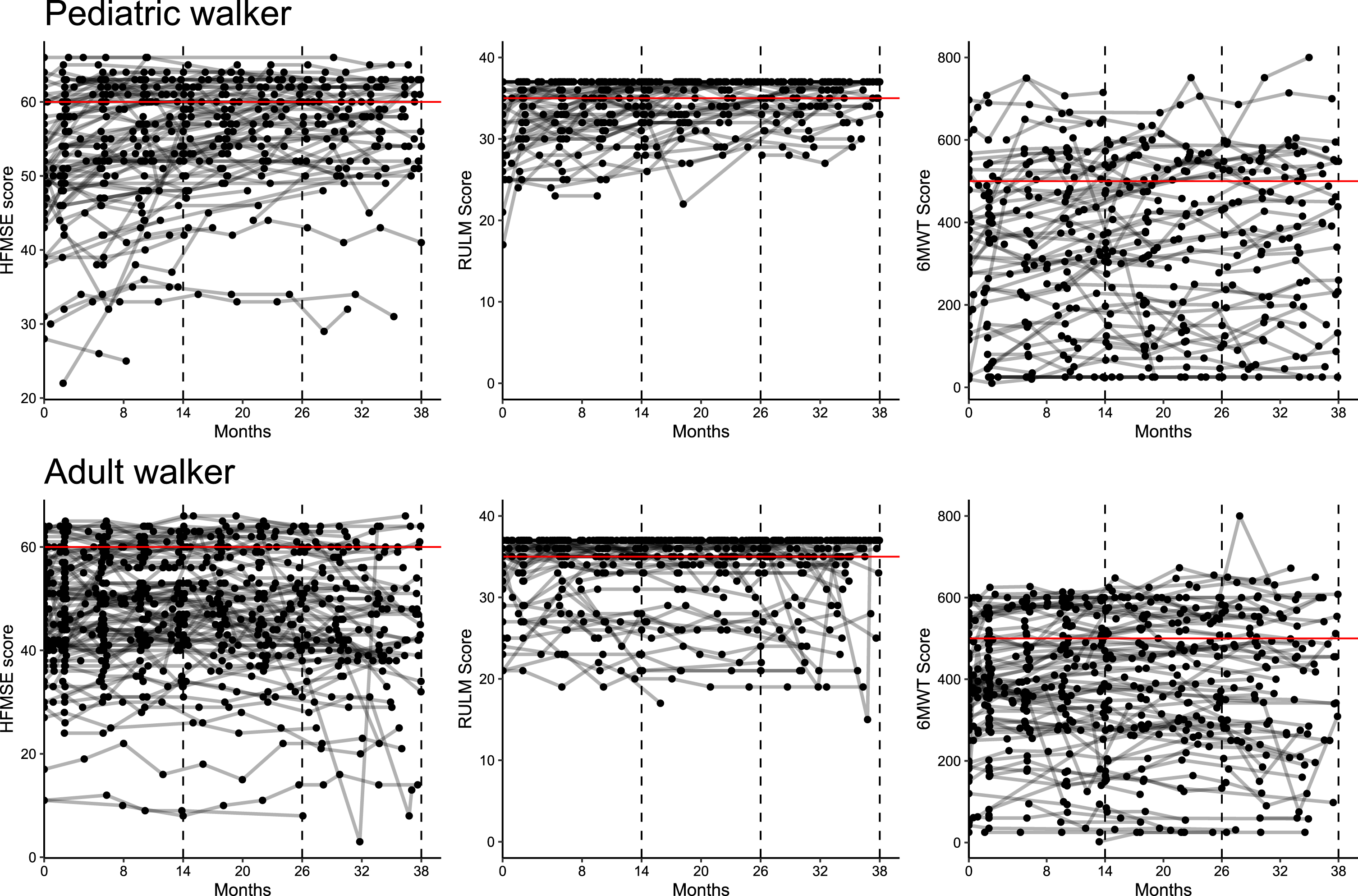
Fig. 4
Ceiling effect of outcome measures. Longitudinal progression for HFMSE, RULM and 6WMT in pediatric (A) and adult walkers (B). Lines represent individual patients. Red horizontal lines reflect a ceiling effect with≥60 points in HFMSE,≥35 points in RULM and≥500 m in 6MWT.
Respiratory and bulbar function
At baseline, none of the patients required ventilator support or tube feeding. Mean FVC(%) was normal at baseline (s. Table 1) and remained stable in both cohorts: FVC(%) at m38 was on average 93.3 [86.1;100.5] (n = 17) in pediatric walkers and 96.3 [93.5;99.1] (n = 39) in adult walkers. During the observation period, three adult patients (2.6%) started to use non-invasive, part-time ventilator support. One pediatric patient (0.9%) required tube feeding for a period of 6 months. None of the patients became dependent on invasive or permanent ventilation.
Fatigue
The symptom of fatigue decreased in both cohorts during the 38 months follow-up period: of all patients at m38, only 10 patients (9.1%) indicated to experience fatigue in contrast to 69 patients (29.9%) at baseline –two children (3.6%) and 8 adult patients (14.5%). Post-hoc analysis of the 6MWT did not reveal signs of fatigability neither at baseline nor after treatment (see supplementary Table 2).
Adverse events
In total, 50 adverse events (AE) among 40 patients were reported during the observation period. Of all AEs, 32 (64.0%) were AEs with hospitalization and 18 (36.0%) without hospitalization. The most common types of adverse events were post-lumbar puncture syndrome (26.0%), fractures and accidents (36.0%), infectious diseases (16.0%), cardiac symptoms (6%), pain (2%), and others (14.0%). No patient died during the observation period. 16 AEs (32%) were considered as possibly related to drug treatment by the treating physician.
DISCUSSION
The approval of nusinersen was mainly based on data of pediatric patients with SMA type 1 and type 2. Nevertheless, in Europe and other regions, the drug was approved for all patients with SMA irrespective of age and motor function. Especially for ambulant patients, data on response to treatment and long-term follow-up are very scarce. Due to reimbursement policies, this led to limited access to treatment for adult or ambulant patients in different countries.
Consistent with results from previous real-world data collections [9–11], our results demonstrate a stabilization of disease progression and, more importantly, clinically meaningful improvements in walking distance in both pediatric and adult SMA patients. In the natural course of the disease, a decline in walking distance is expected with a mean age at loss of ambulation of 14 years. Further, patients with SMA type 3a have been reported to have a poorer prognosis of remaining ambulatory than patients with SMA type 3b [3]. In our analysis, we mainly included patients categorized as SMA type 3b. These patients might have a greater probability of achieving improvements in the 6MWT due to the slower disease progression and a greater functional reserve capable of partially reversing the symptoms of the disease. On the one hand, most pediatric walkers were treated early before the age of 10 and on the other hand, adult walkers showed a good functional level at baseline with remaining ambulation at start of treatment at a mean age of 36 years. As patients who lost ambulation prior to treatment were not included in this analysis, our cohort does not represent the whole spectrum of SMA type 3 patients but rather SMA type 3b patients with slower disease progression. Nevertheless, stabilization or rather slowed disease progression or improvement in walking distance in patients with SMA without any intervention is highly unlikely and thus reflects a positive response to nusinersen treatment.
In RULM score, 49% of patients already achieved≥35 points at baseline, preventing changes in motor function to be sufficiently captured due to a ceiling effect (see Fig. 4). For the 6MWT, reference data from a large cohort study with healthy 11 to 14 years-old children showed a mean (±standard deviation) distance of 576±93 m in boys and 545±92 m in girls[20]. We therefore considered a walking distance of more than 500 m as a possible ceiling effect in the 6MWT. In our cohort, only 17.2% of patients showed a walking distance≥500 m at m38, so that changes in motor function could be depicted in most of the patients. Although solely data analysis was limited to ambulant patients, only 19.1% of patients were scored with more than 60 points in HFMSE with a mean score of 56.4 [52.9;59.8] in pediatric and 44.8 [40.9;48.8] in adult walkers at m38. Further research is needed to assess which motor skills improve upon disease-specific drug treatments, and which motor skills are nevertheless difficult to achieve. Comparing these three physiotherapeutic assessments, changes in the 6MWT seem to be a better indicator of treatment response than HFMSE and RULM in ambulant SMA patients.
In adult or pediatric ambulant patients, a significant reduction of fatigue under nusinersen treatment has already been described in previous studies [23, 24]. Within the SMArtCARE registry, both pediatric and adult patients reported a remarkable reduction of perceived fatigue after 38 months of treatment. But, comparing the time needed for the first with last 50 m in 6MWT, we did not detect changes in performance fatigability under treatment with nusinersen. It is thus important to further investigate how the subjective improvement of fatigue relates to activities of daily living, walking ability or other outcomes.
The main strength, but also limitation of this study is the real-world data approach. In contrast to clinical trials, data items and physiotherapeutic assessments are not mandatory and thus not available for all patients at all time points. However, to ensure the best possible completeness and quality of data, we established detailed recommendations for the follow-up visits and provided standardized case report forms and outcome measures for data collection. Physiotherapists are regularly trained in face-to-face workshops and webinars to ensure interrater reliability. Before analysis, data was reviewed for completeness, consistency, and plausibility.
In conclusion, our data demonstrate a positive effect of nusinersen treatment on motor function in ambulant pediatric and adult SMA patients. We not only observed a stabilization of disease progression or lack of deterioration, but clinically meaningful improvements in walking distance in a subgroup of patients. The 6MWT seems to be the most suitable outcome measure to evaluate response to disease-specific treatments in ambulant SMA patients.
ACKNOWLEDGMENTS
We would like to thank all patients and families that agreed to share their data within the SMArtCARE registry. We further thank all participating centers in Germany, Austria and Switzerland.
SOURCES OF SUPPORT
Biogen and Novartis Gene Therapies provide financial support for the SMArtCARE registry. AP was supported by the Berta Ottenstein clinician scientist program of the University of Freiburg.
CONFLICTS OF INTEREST AND DISCLOSURES
A. Pechmann received compensation for advisory boards, training activities and research grants from Novartis and Biogen. M. Schimmel received compensation for advisory boards from Roche; A. Blaschek received personal fees for advisory board services / speakers honoraria from Novartis, Roche, Ad medicum, Sanofi Genzyme and Avexis; JC Koch received compensation for advisory boards and talks from Biogen and Roche; D. Zeller received compensation for scientific consultancy from Novartis, for advisory boards from Biogen, and for training activities from Desitin; M. Baumann received compensation for advisory boards and speakers honoraria from Novartis, Biogen and Roche; R. Trollmann reports personal fees from Desitin Pharma, Novartis, PTC Therapeutics; M. Weiler received compensation for advisory boards, consultant and speaker honoraria and/or financial support for conference attendances from Akcea Therapeutics, Alnylam Pharmaceuticals, Biogen, Pfizer, Roche, and Sobi; C. Neuwirth received compensation for advisory boards and training activities by Biogen, Roche, Genzyme and Mitsubishi Tanabe; J. Friese received compensation for an advisory board from Novartis Gene Therapies; B. Schrank received compensation for advisory board from Biogen; M. Flotats-Bastardas received compensation for advisory boards from Biogen, Novartis and Roche; M.C. Walter received compensation for advisory boards, funding for travel or speaker honoraria, and research support from Biogen, Novartis, and Roche; R. Günther received compensation for advisory boards and research support from Biogen and compensation for advisory boards from Hofmann La-Roche; M. Türk received compensation for a presentation and an advisory board from Sanofi Genzyme; T. Hagenacker received compensation for advisory board, lectures and research grants from Biogen, Roche and Novartis; J. Johansen received honoraria for advisory board participation and/or lectures and/or manuscript writing from Avexis/Novartis, Biogen, Roche, PTC, Pfizer and Sarepta Th., respectively, and financial support from Biogen for the SMArtCARE registry (paid to institution); S. Petri received compensation for advisory boards from Biogen, Roche and Zambon, speaker honoraria from Biogen, Roche, Italfarmaco and Novartis, grants from the German Israeli Foundation (GIF), German Neuromuscular Society (DGM) and Neurodegenerative Research (NDR); C. Stadler received fees for speeches & participation in discussions from Merz Pharma; B. Becker received financial support for a publication from Biogen; C. Kamm received honoraria as a speaker and advisory board member from Biogen, Roche and Ipsen; C. Köhler received honoraria from Roche, PTC, Biogen and Genzyme; M. Smitka received funding for educational activities and participation in advisory boards concering SMA from Novartis Gene Therapies, Biogen and Roche; H. Kölbel received compensation for presentations and consultancy from Avexis, Biogen, Sanofi, Pfizer, Novartis, and Roche; A. Ziegler receives honoraria for advisory boards and speaker honoraria from Novartis Gene Therapies, Roche Pharma and Biogen. His institution receives research grants from Biogen; G. Bernert receives research suppert from PTC and honoraria from Novartis Gene Therapies, PTC, Biogen, Roche, Pfizer, Santhera; E. Wilichowski received compensation for advisory boards from Biogen, Novartis and Roche; H. Lochmüller received consultancy and financial support for research projects and clinical trials from Amplo Biotechnology, AMO Pharma, argenx, Biogen, Desitin, Fulcrum Therapeutics, Harmony Biosciences, KYE Pharmaceuticals, Milo Biotechnology, Novartis, Pfizer, PTC Therapeutics, Hoffman-La Roche Limited, Sanofi-Genzyme, Santhera, Sarepta, Satellos, Spark Therapeutics and Ultragenyx. HL is the Editor-in-chief for the Journal of Neuromuscular Diseases (IOS Press); J. Kirschner received support from Biogen, Novartis, Roche and ScholarRock for consultancy, educational activities and/or clinical research.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-221600.
REFERENCES
[1] | Farrar MA , Vucic S , Johnston HM , Du Sart D , Kiernan MC , Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy, The Journal of Pediatrics (2013) ;162: :155–9. doi: 10.1016/j.jpeds.2012.05.067. |
[2] | Zerres K , Rudnik-Schöneborn S , Natural history in proximal spinal muscular atrophy, Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol (1995) ;52: :518–23. doi: 10.1001/archneur.1995.00540290108025. |
[3] | Lusakowska A , Jedrzejowska M , Kaminska A , Janiszewska K , Grochowski P , Zimowski J , et al., Observation of the natural course of type 3 spinal muscular atrophy: Data from the polish registry of spinal muscular atrophy, Orphanet J Rare Dis. (2021) ;16: :150. doi: 10.1186/s13023-021-01771-y. |
[4] | Montes J , McDermott MP , Mirek E , Mazzone ES , Main M , Glanzman AM , et al. Ambulatory function in spinal muscular atrophy: Age-related patterns of progression, PLoS One. (2018) ;13: :e0199657. doi: 10.1371/journal.pone.0199657. |
[5] | Singh NK , Singh NN , Androphy EJ , Singh RN , Splicing of a critical exon of human Survival Motor Neuron is regulated by a unique silencer element located in the last intron, Mol Cell Biol (2006) ;26: :1333–46. doi: 10.1128/MCB.26.4.1333-1346.2006. |
[6] | Mercuri E , Darras BT , Chiriboga CA , Day JW , Campbell C , Connolly AM , et al., Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy, N Engl J Med (2018) ;378: :625–35. doi: 10.1056/NEJMoa1710504. |
[7] | Finkel RS , Mercuri E , Darras BT , Connolly AM , Kuntz NL , Kirschner J , et al., Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy, N Engl J Med (2017) ;377: :1723–32. doi: 10.1056/NEJMoa1702752. |
[8] | Darras BT , Chiriboga CA , Iannaccone ST , Swoboda KJ , Montes J , Mignon L , et al., Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies, Neurology (2019) ;92: :e2492–e2506. doi: 10.1212/WNL.0000000000007527. |
[9] | Hagenacker T , Wurster CD , Günther R , Schreiber-Katz O , Osmanovic A , Petri S , et al., Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study, The Lancet Neurology (2020) ;19: :317–25. doi: 10.1016/S1474-4422(20)30037-5. |
[10] | Walter MC , Wenninger S , Thiele S , Stauber J , Hiebeler M , Greckl E , et al., Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 - A Prospective Observational Study, JND (2019) ;6: :453–65. doi: 10.3233/JND-190416. |
[11] | Pera MC , Coratti G , Bovis F , Pane M , Pasternak A , Montes J , et al., Nusinersen in pediatric and adult patients with type III spinal muscular atrophy, Ann. Clin. Transl. Neurol (2021) ;8: :1622–34. doi: 10.1002/acn3.51411. |
[12] | Pechmann A , König K , Bernert G , Schachtrup K , Schara U , Schorling D , et al., SMArtCARE - A platform to collect real-life outcome data of patients with spinal muscular atrophy, Orphanet J Rare Dis. (2019) ;14: :18. doi: 10.1186/s13023-019-0998-4. |
[13] | Mercuri E , Finkel R , Scoto M , Hall S , Eaton S , Rashid A , et al., Development of an academic disease registryfor spinal muscular atrophy, Neuromuscul Disord (2019) ;29: :794–9. doi: 10.1016/j.nmd.2019.08.014. |
[14] | Dunaway Young S , Montes J , Kramer SS , Marra J , Salazar R , Cruz R , et al., Six-minute walk test is reliable and valid in spinal muscular atrophy, Muscle Nerve (2016) ;54: :836–42. doi: 10.1002/mus.25120. |
[15] | McDonald CM , Henricson EK , Abresch RT , Florence J , Eagle M , Gappmaier E , et al., The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study, Muscle Nerve (2013) ;48: :357–68. |
[16] | Lachmann R , Schoser B , The clinical relevance of outcomes used in late-onset Pompe disease: Can we do better? Orphanet J Rare Dis (2013) ;8: :160. |
[17] | Glanzman AM , O’Hagen JM , McDermott MP , Martens WB , Flickinger J , Riley S , et al., Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III, J Child Neurol (2011) ;26: :1499–507. doi: 10.1177/0883073811420294. |
[18] | Pera MC , Coratti G , Forcina N , Mazzone ES , Scoto M , Montes J , et al., Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy, BMC Neurol. (2017) ;17: :39. doi: 10.1186/s12883-017-0790-9. |
[19] | Mazzone ES , Mayhew A , Montes J , Ramsey D , Fanelli L , Young SD , et al., Revised upper limb module for spinal muscular atrophy: Development of a new module, Muscle Nerve (2017) ;55: :869–74. doi: 10.1002/mus.25430. |
[20] | Kasović M , Stefan L , Petrić V , Normative data for the 6-min walk test in 11-14 year-olds: A population-based study, BMC Pulm Med. (2021) ;21: :297. doi: 10.1186/s12890-021-01666-5. |
[21] | Wijnhoven TM , de Onis M , Onyango AW , Wang T , Bjoerneboe G-EA , Bhandari N , et al., Assessment of gross motor development in the WHO Multicentre Growth Reference Study, Food Nutr Bull (2004) ;25: :S37–45. doi: 10.1177/15648265040251S105. |
[22] | Brown EG , Wood L , Wood S , The medical dictionary for regulatory activities (MedDRA), Drug Saf (1999) ;20: :109–17. doi: 10.2165/00002018-199920020-00002. |
[23] | Kizina K , Stolte B , Totzeck A , Bolz S , Schlag M , Ose C , et al., Fatigue in adults with spinal muscular atrophy under treatment with nusinersen, Sci Rep (2020) ;10: :11069. doi: 10.1038/s41598-020-68051-w. |
[24] | Montes J , Dunaway Young S , Mazzone ES , Pasternak A , Glanzman AM , Finkel RS , et al., Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy, Muscle Nerve (2019) ;60: :409–14. doi: 10.1002/mus.26633. |




