Functional and Clinical Outcomes Associated with Steroid Treatment among Non-ambulatory Patients with Duchenne Muscular Dystrophy1
Abstract
Background:
Evidence on the long-term efficacy of steroids in Duchenne muscular dystrophy (DMD) after loss of ambulation is limited.
Objective:
Characterize and compare disease progression by steroid treatment (prednisone, deflazacort, or no steroids) among non-ambulatory boys with DMD.
Methods:
Disease progression was measured by functional status (Performance of Upper Limb Module for DMD 1.2 [PUL] and Egen Klassifikation Scale Version 2 [EK] scale) and by cardiac and pulmonary function (left ventricular ejection fraction [LVEF], forced vital capacity [FVC] % -predicted, cough peak flow [CPF]). Longitudinal changes in outcomes, progression to key disease milestones, and dosing and body composition metrics were analyzed descriptively and in multivariate models.
Results:
This longitudinal cohort study included 86 non-ambulatory patients with DMD (mean age 13.4 years; n = 40 [deflazacort], n = 29 [prednisone], n = 17 [no steroids]). Deflazacort use resulted in slower average declines in FVC % -predicted vs. no steroids (+3.73 percentage points/year, p < 0.05). Both steroids were associated with significantly slower average declines in LVEF, improvement in CPF, and slower declines in total PUL score and EK total score vs. no steroids; deflazacort was associated with slower declines in total PUL score vs. prednisone (all p < 0.05). Both steroids also preserved functional abilities considered especially important to quality of life, including the abilities to perform hand-to-mouth function and to turn in bed at night unaided (all p < 0.05 vs. no steroids).
Conclusions:
Steroid use after loss of ambulation in DMD was associated with delayed progression of important pulmonary, cardiac, and upper extremity functional deficits, suggesting some benefits of deflazacort over prednisone.
LIST OF ABBREVIATIONS
AAN: | American Academy of Neurology |
BMI: | body mass index |
CPF: | cough peak flow |
DMD: | Duchenne muscular dystrophy |
EK: | Egen Klassifikation |
FDA: | Food and Drug Administration |
FVC: | forced vital capacity |
HR: | hazard ratio |
LVEF: | left ventricular ejection fraction |
PUL: | Performance of Upper Limb Module for DMD 1.2 |
SD: | standard deviation |
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a rare genetic disorder characterized by progressive deterioration of skeletal, cardiac, and pulmonary muscle fibers [1]. DMD affects only males and has an estimated incidence of approximately 1 in 3,800 to 6,200 live male births [2–4]. A hallmark of DMD is the eventual loss of independent ambulation, with progressive involvement of upper limb function, typically between age 9 and 14 years [5–8]. Subsequent declines in pulmonary and cardiac function contribute to a median survival of approximately 25 years [9, 10].
Pharmacologic treatment with the corticosteroids prednisone/prednisolone (hereafter “prednisone”) or deflazacort is the standard of care to delay disease progression in DMD [11, 12]. Although prednisone does not have a Food and Drug Administration (FDA)-approved indication specifically for DMD, it has been widely used for treating it for decades. Deflazacort is an oxazoline derivative of prednisone initially approved by the FDA in February 2017 for boys with DMD aged ≥5 years and later approved for use in ages ≥2 years [13, 14]. The American Academy of Neurology (AAN) guidelines, last updated in 2016, classify both corticosteroids as being “probably” or “possibly” effective and potentially showing benefit on markers of disease progression [11]. Indeed, corticosteroid use has been associated with better functional outcomes in DMD, including delaying loss of ambulation and improving strength, timed motor function, pulmonary function, and cardiac function, as well as possibly lengthening survival [11, 12, 15, 16]. However, differences in the side effect profiles of prednisone and deflazacort could impact whether patients need to reduce the dose to a potentially less efficacious level due to tolerability concerns. For example, prednisone carries a higher risk of weight gain while deflazacort carries a higher risk of cataracts [15, 17]. Additionally, differences in lipid solubility between the two steroids could influence their bioavailability to muscle fibers as DMD progresses [18, 19].
The AAN guidelines classify a greater share of prednisone’s efficacy evidence as Level B (“probably” effective) than Level C (“possibly” effective) [11] compared to deflazacort, but a 2016 Cochrane Review considered the quality of comparative evidence to be low [20]. More recent studies have suggested that relative to patients treated with prednisone, patients treated with deflazacort experience similar or slower rates of functional decline and different side effects [21]. A meta-analysis of placebo arm data from phase III trials found that functional decline over 48 weeks was significantly slower for deflazacort versus prednisone for five measures of ambulatory function and comparable for a sixth measure [18]. A single-center study found that, relative to prednisone, deflazacort was associated with longer time to loss of ambulation and scoliosis, but similar bone health as assessed by whole body bone mineral density [22]. A multicenter, international study found that, compared to prednisone, deflazacort was associated with 2-3 year delays in loss of ability to stand from supine, loss of ambulation, and loss of hand-to-mouth function [16]. Finally, a recent randomized trial among 196 corticosteroid-naïve boys with DMD aged 4 to 7 years found comparable efficacy between the two steroids, significantly greater weight gain with prednisone, and more cataracts with deflazacort [23].
There is relatively little research on the non-ambulatory period of DMD, and steroid use is highly variable despite evidence showing a benefit of steroid continuation [6, 16]. Non-use or discontinuation of steroids may be attributable to tolerability concerns for some patients, [24] but may reflect the perception that steroids provide less benefit in the non-ambulatory period. A 2019 analysis of 569 non-ambulatory patients with DMD found that only 49% used corticosteroids (equally split between prednisone and deflazacort); among those not on corticosteroids, 23% had never used them and 28% had formerly used them [24]. This is consistent with tolerability only partially explaining non-use of steroids. Further research is needed to identify the optimal therapy for patients at different, including later, disease stages [25].
To address the need for more evidence on the long-term efficacy of prednisone and deflazacort in DMD after loss of ambulation, this study characterized and compared disease progression by steroid treatment (prednisone, deflazacort, or no steroids) among a geographically diverse sample of boys with DMD who had lost ambulation. Disease progression was measured by functional status and cardiac and pulmonary function, and analyzed both longitudinally and relative to key disease milestones. Additionally, this study explored trends in steroid dosing and body composition metrics.
METHODS
Study design
Data source
This study used data from the PRO-DMD-01 national history study (Clinicaltrials.gov identifier: NCT01753804), a prospective observational study of disease progression in males with confirmed DMD sponsored by BioMarin Pharmaceutical Inc. Participants were aged 3 to 18 years at study entry and underwent functional and clinical assessments every 6 months for up to 3 years. Both ambulatory and non-ambulatory participants were eligible for enrollment.
Data from PRO-DMD-01 were provided by CureDuchenne, a 501(3)c DMD patient foundation. The dataset contains longitudinal, patient-level data for 269 boys with DMD from 16 centers in the United States (US), Argentina, Belgium, Brazil, France, Germany, Italy, Netherlands, Sweden, and Turkey. Data were available on demographics, steroid use, and several measures of ambulatory, pulmonary, and cardiac function.
Patient consent and institutional board review was conducted during the original PRO-DMD-01 study. As this is a post-hoc analysis of previously collected data and does not involve any new experimentations with human participants performed by any of the authors, no ethical review was required.
Study population
The study population included the participants in PRO-DMD-01 who entered the study non-ambulatory or became non-ambulatory during the study. Each patient’s index visit was defined as the first observed visit while non-ambulatory. Patients were additionally required to have at least one follow-up visit after the index visit and non-missing data for the following variables at baseline: age, age at DMD diagnosis, steroid type (including none), and duration of steroid use (if applicable). Sample sizes for analyses of each outcome varied based on data availability over the follow up period.
Study measures
Patient characteristics
Patient characteristics collected at the index visit included information on demographics and vitals (age, height [standing or calculated based on ulnar length], weight, body mass index [BMI]) and steroid use (type [deflazacort, prednisone, or none], regimen, duration of use, and dose). Vital characteristics were measured in absolute terms and as z-scores based on distributions for healthy boys from the US Centers for Disease Control and Prevention [26]. Dose was measured both by weight (mg/kg) and as a share of the recommended dose.
Patients were classified and analyzed according to three treatment groups based on the type of steroid (or lack thereof) observed at the index visit: deflazacort, prednisone, or none. No restriction was imposed with respect to switching or discontinuation of therapy over the follow-up period.
Longitudinal outcomes
Pulmonary and cardiac outcomes included forced vital capacity (FVC) % -predicted, cough peak flow (CPF), and left ventricular ejection fraction (LVEF). For FVC % -predicted, selected outlier observations were excluded based on expert opinion on clinically possible levels and changes usually due to obvious one-time errors in anthropometric measurements producing large fluctuations in % -predicted values.
Functional status was recorded according to the Performance of Upper Limb Module for DMD 1.2 (PUL) [27, 28] and the Egen Klassifikation Scale Version 2 (EK) scale [29]. The PUL total score ranges from 0–74, where lower scores represent greater functional impairment, and is calculated by summing the scores from three sub-dimensions corresponding to high level (shoulder), mid-level (elbow), and distal (wrist and hand) function. Sub-dimensions contain items scored from 0 to between 2 and 5. The entry item (not included in the total score) defines starting functional level on an integer scale of 0–6 and is based on a revised version of the Brooke Scale of Upper Extremity Function (Supplemental Methods) [27, 30].
The EK scale total score ranges from 0–51, with lower scores representing greater functional impairment. It is calculated by adding scores for 17 sub-items, which each range from 0–3. Subitems pertain to specific tasks or movements, with a score of 0 representing inability.
Milestone outcomes
Milestone outcomes related to cardiac and pulmonary function included LVEF <55%, clinically defined tachycardia in DMD, [31] and FVC % -predicted <60%, <50%, and <30%. FVC % -predicted<60% often leads to initiation of mechanical cough assistant devices, 50% is when guidelines recommend evaluation for nocturnal non-invasive ventilation, and 30% is associated with the need for diurnal ventilation day and night and an increased mortality risk [32, 33].
The following functional milestones were also assessed: (1) loss of ability to perform hand-to-mouth function (part of PUL); (2) loss of ability to turn in bed at night unaided (EK subitem); (3) loss of ability to use a manual wheelchair (EK subitem); (4) loss of ability to eat independently and without elbow support (EK subitem); and (5) loss of ability to transfer independently from wheelchair (EK subitem).
Statistical analysis
Baseline characteristics
Patient characteristics at the index visit, including outcome measures, were summarized overall and stratified by steroid type at the index visit using means and standard deviations (SD) for continuous measures and counts and percentages for categorical/binary measures. Total duration of follow-up in months was also assessed.
Longitudinal analysis
Mixed-effects models with random intercepts were used to evaluate changes in outcomes over time. Model covariates included age, age squared, steroid type and duration at index, age at DMD diagnosis, an interaction of age and index steroid type, and a binary indicator for whether the patient entered the PRO-DMD-01 study non-ambulatory or lost ambulation during the study. As these models assume normality of the underlying residuals, quantile-quantile plots of the residuals for each model were inspected to rule out non-normality. Predicted values from the models were then used to construct figures displaying average outcome trajectories over time stratified by index steroid type.
Analysis of DMD milestones
Time from birth to DMD disease milestones was assessed using Kaplan-Meier analyses and Cox proportional hazards models. Cox model covariates included age, steroid type, and duration at index, and a binary indicator for entry into the PRO-DMD-01 study non-ambulatory as noted above. For patients who had already reached a given milestone at their index visit, age at index was used as a conservative measure of the age at first reaching the milestone.
Additional analyses
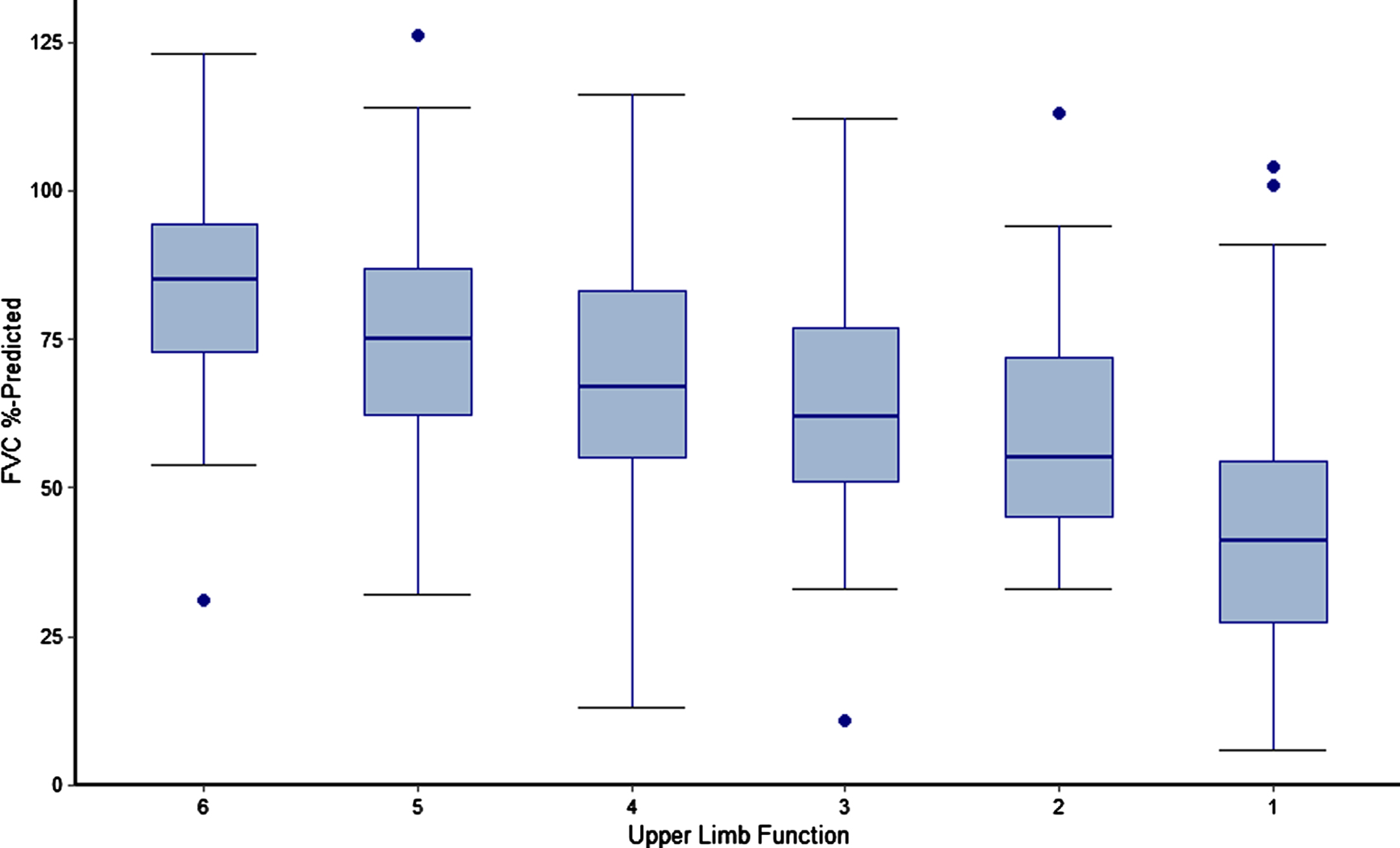
Analyses of steroid dose trajectories (mg/kg) over time by index steroid type and of the relationship between upper limb function and pulmonary function (measured by FVC % -predicted) were performed. In the latter analysis, upper limb function was defined using the entry item to the PUL and boxplots of FVC % -predicted were plotted for each observed level of the PUL entry score.
RESULTS
Baseline characteristics
Of 86 non-ambulatory patients with DMD who met the inclusion criteria, 40 (46.5%) were receiving deflazacort, 29 (33.7%) were receiving prednisone, and 17 (19.8%) were not using steroids at the index visit (Table 1). The mean age at the index visit was 13.4 (SD: 2.9) years overall and was similar across groups (prednisone: 12.6 [3.0], deflazacort: 13.8 [3.0], no steroids: 13.89 [2.2] years). On average, patients receiving deflazacort were older, shorter, and weighed less at baseline. A greater share of deflazacort- than prednisone-treated patients were on daily regimens (95.0% vs. 41.4%) and received a higher mean dose (0.61 [SD: 0.22] vs. 0.39 [0.20] mg/kg). Among the subgroup of patients on daily steroids (n = 50), the average daily dose remained higher for deflazacort (0.61 [SD: 0.22] mg/kg/day, equivalent to 68.2% [24.8% ] of the recommended daily dose; n = 38) relative to prednisone (0.27 [0.16] mg/kg/day, equivalent to 36.4% [21.0% ] of the recommended daily dose; n = 12) (data not shown). Patients were followed for an average of 21.4 months after index, with deflazacort patients followed for a slightly shorter time relative to others (17.4 vs. 25.9 [prednisone] and 23.1 months [no steroids]) (data not shown). Deflazacort patients also experienced a lower number of visits over the follow-up period (4.3 visits per person vs. 4.7 [prednisone] and 4.8 [no steroids]) (data not shown). Most patients remained on their index steroid throughout follow-up, with only 11 switching or discontinuing therapy (2/40 [from deflazacort]; 6/29 [from prednisone]; 3/17 [from no steroids]) (data not shown).
Table 1
Baseline characteristics of non-ambulatory patients with DMD, by steroid use
| Total | Prednisone | Deflazacort | No steroid | ||
| N = 86 | N = 29 | N = 40 | N = 17 | p-valuea | |
| Demographics (mean±SD) | |||||
| Age, years | 13.39±2.86 | 12.60±2.98 | 13.75±2.98 | 13.89±2.15 | 0.19 |
| Age at DMD diagnosis, years | 5.39±3.02 | 5.55±2.83 | 5.71±3.28 | 4.37±2.63 | <0.01* |
| Height, cm | 145.95±15.71 | 149.50±17.11 | 139.04±11.48 | 154.01±15.66 | <0.01* |
| Weight, kg | 49.99±17.33 | 56.51±21.65 | 45.82±12.28 | 48.68±16.89 | <0.05* |
| BMI, kg/m2 | 23.09±6.27 | 24.50±5.88 | 22.90±5.44 | 21.01±8.08 | 0.2 |
| Weight Z-score | –0.15±1.98 | 0.93±1.29 | –0.75±2.10 | –0.60±1.97 | <0.001* |
| BMI Z-score | 0.67±2.13 | 1.31±1.06 | 0.83±1.26 | –0.79±3.80 | <0.01* |
| Steroid regimen, n (%) | <0.01* | ||||
| Every other day | 1 (1.16%) | 1 (3.45%) | 0 (0.00%) | 0 (0.00%) | <0.001* |
| Once daily | 50 (58.14%) | 12 (41.38%) | 38 (95.00%) | 0 (0.00%) | <0.05* |
| Otherb | 18 (20.93%) | 16 (55.17%) | 2 (5.00%) | 0 (0.00%) | 0.52 |
| No steroids | 17 (19.77%) | 0 (0.00%) | 0 (0.00%) | 17 (100.00%) | 0.43 |
| Steroid duration, years | 5.04±3.80 | 5.57±3.39 | 5.95±3.53 | 1.97±3.72 | 0.36 |
| Steroid dose, mg | 23.67±8.07 | 20.24±9.65 | 26.22±5.52 | – | 0.3 |
| Steroid dose by weight, mg/kg | 0.52±0.24 | 0.39±0.20 | 0.61±0.22 | – | 0.19 |
| % of recommended starting dosec | 61.41±26.24 | 52.33±26.25 | 68.17±24.43 | – | <0.01* |
| Current ACE inhibitor, n (%) | 37 (43.02%) | 10 (34.48%) | 19 (47.50%) | 8 (47.06%) | <0.05* |
| Current beta blocker, n (%) | 15 (17.44%) | 3 (10.34%) | 8 (20.00%) | 4 (23.53%) | 0.2 |
| Current diuretic, n (%) | 10 (11.63%) | 2 (6.90%) | 7 (17.50%) | 1 (5.88%) | <0.001* |
| Functional measures (mean±SD) | |||||
| Functional ability | |||||
| PUL total score | 58.58±12.13 | 59.87±11.97 | 60.17±8.71 | 47.88±19.94 | <0.05* |
| Total Egen Klassifikation score | 42.22±8.40 | 42.08±7.03 | 46.76±4.24 | 34.42±9.82 | <0.001* |
| Ability to use a wheelchair | 1.99±1.03 | 2.00±1.02 | 2.18±1.03 | 1.50±0.94 | 0.12 |
| Ability to move arms | 2.54±0.71 | 2.61±0.66 | 2.71±0.68 | 2.07±0.70 | <0.05* |
| Ability to cough | 2.71±0.59 | 2.78±0.42 | 2.91±0.29 | 2.13±0.92 | <0.001* |
| Ability to eat | 2.43±0.90 | 2.52±0.85 | 2.71±0.58 | 1.67±1.18 | <0.001* |
| Pulmonary & cardiac function | |||||
| FVC % -predicted (%) | 70.32±22.44 | 68.15±15.64 | 78.20±22.61 | 54.64±24.82 | 0.9 |
| CPF (L/min) | 197.03±84.08 | 202.60±117.86 | 199.35±82.38 | 187.02±59.86 | <0.01* |
| LVEF (%) | 57.68±8.95 | 55.94±7.82 | 61.13±4.13 | 51.53±14.30 | <0.001* |
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; CPF, cough peak flow; DMD, Duchenne muscular dystrophy; FVC, forced vital capacity; LVEF, left ventricular ejection fraction; PUL, Performance of Upper Limb; SD, standard deviation. *p < 0.05. Note: aReported p-values correspond to a comparison across all three steroid categories; bOther regimens may include weekend dosing or other intermittent dosing (e.g., 10 days on/off); cThe recommended doses are 0.9 mg/kg for deflazacort and 0.75 mg/kg for prednisone.
Baseline values of outcome measures are summarized in Table 1. The PUL total score was higher among patients treated with steroids vs. no steroids (59.9 [prednisone] and 60.2 [deflazacort] vs. 47.9 [no steroids]). EK total score and scores on ability to move arms, cough, and eat were all higher among patients treated with steroids vs. no steroids, while the ability to use a wheelchair was similar across groups. In terms of pulmonary and cardiac function, FVC % -predicted was similar between groups, but CPF and LVEF were better among patients receiving steroids. Additional baseline measures are summarized in Supplemental Table 1.
Pulmonary and cardiac outcomes
Annualized rates of progression
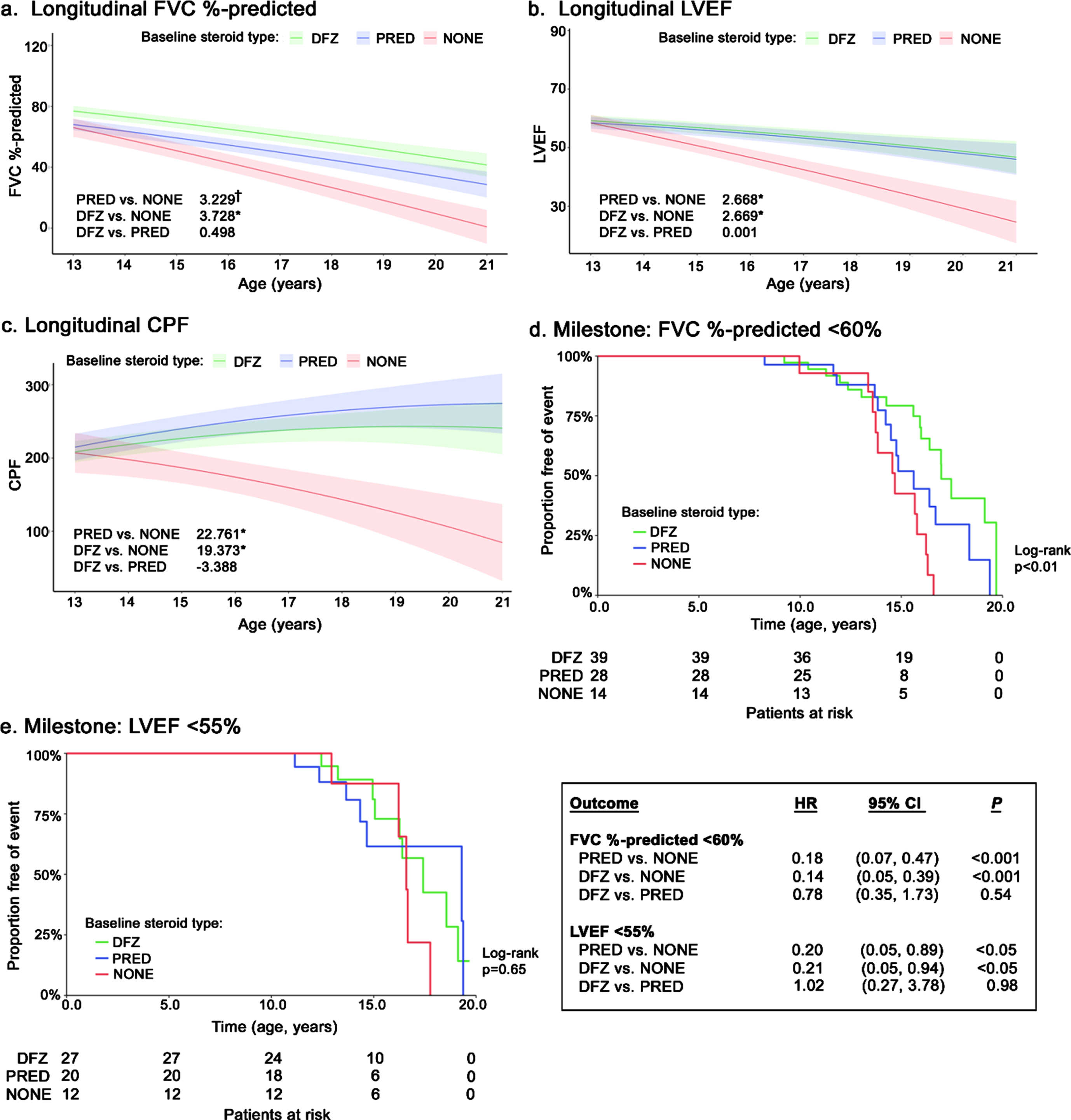
Compared with patients not receiving steroids, those receiving prednisone or deflazacort experienced slower average declines in change in FVC % -predicted (+3.23 percentage points [ppts] and +3.73 ppts/year, respectively), although the difference was only significant for deflazacort (p < 0.05) (Fig. 1a). Both steroid groups experienced significantly slower average declines in LVEF compared to patients not receiving steroids (both prednisone and deflazacort: +2.67 units/year; p < 0.05) (Fig. 1b). Longitudinal changes in FVC % -predicted and LVEF were not significantly different between patients treated with prednisone or deflazacort. Similar results were observed among patients with a FVC % -predicted within the 30–80% range at index (Supplemental Figure 1a). While both prednisone- and deflazacort-treated patients experienced rising levels of CPF over time, CPF declined significantly for patients not receiving steroids (–22.76 [prednisone] and –19.37 [deflazacort] L/min/year; both p < 0.05) (Fig. 1c). There were no significant differences between prednisone and deflazacort on CPF.
Fig. 1
Longitudinal pulmonary and cardiac outcomes among non-ambulatory patients with DMD, by steroid use. Caption: Shaded regions on trajectory plots are bounded by±1 standard error. Estimates overlaid on trajectory plots are slope differences between steroid groups. HRs and slope differences are obtained from two model specifications, which differ only by the baseline steroid reference group (none or prednisone). Four follow-up measurements of FVC % -predicted among 3 patients were deemed to be outliers and excluded from analysis. **p < 0.01, *p < 0.05, †p < 0.10. Abbreviations: CI, confidence interval; CPF, cough peak flow; DFZ, deflazacort; DMD, Duchenne muscular dystrophy; FVC, forced vital capacity; HR, hazard ratio; LVEF, left ventricular ejection fraction; PRED, prednisone.
Disease progression milestones
During follow-up, 42 patients reached FVC % -predicted<60% (prednisone: 50.0% [n = 14/28]; deflazacort: 41.0% [n = 16/39]; no steroids: 85.7% [n = 12/14]) (Fig. 1d). Compared to no steroids, both steroids were associated with significant delays in the median age upon reaching FVC % -predicted<60% (prednisone: +0.9 years; deflazacort: +2.3 years; log-rank p < 0.01). Slower progression to this milestone persisted for both steroid groups in the adjusted Cox model, where the hazard ratios (HRs) for prednisone and deflazacort vs. no steroids were 0.18 and 0.14, respectively (both p < 0.001); the HR for deflazacort vs. prednisone was not significantly different (box in Fig. 1). Similar results were observed for FVC % -predicted<50% and <30% (Supplemental Figures 1b and 1c).
During follow-up, 21 patients reached LVEF <55% (prednisone: 33.3% [n = 9/27]; deflazacort: 35.0% [n = 7/20]; no steroids: 41.7% [n = 5/12]) (Fig. 1e). Compared with patients not receiving steroids, those who received steroids had numerically but not significantly higher median ages at reaching LVEF <55% (prednisone: +2.7 years; deflazacort +0.8 years; log-rank p = 0.65) (Fig. 1f). After adjustment in a Cox model, use of either steroid was associated with a significantly lower hazard of reaching LVEF <55% vs. no steroids (HR: 0.20 [prednisone] and 0.21 [deflazacort]; both p < 0.05); the HR was not significantly different for deflazacort vs. prednisone (box in Fig. 1).
Functional outcomes
Annualized rates of progression
Prednisone-treated patients experienced a significantly slower decline in functional ability, as measured by the total PUL score, compared with patients not on steroids (+2.5 points/year; p < 0.05) (Fig. 2a). Deflazacort-treated patients experienced even slower declines in total PUL scores compared with those not on steroids (+4.0 points/year) as well as prednisone-treated patients (+1.5 points/year; both p < 0.05). Relative to patients not on steroids, EK total scores declined significantly slower for patients receiving prednisone (+2.0 points/year; p < 0.01) and deflazacort (+2.4 points/year; p < 0.001); declines in EK total scores did not differ significantly by steroid type (Fig. 2b).
Fig. 2
Longitudinal functional outcomes among non-ambulatory patients with DMD, by steroid use. Caption: Shaded regions on trajectory plots are bounded by±1 standard error. Estimates overlaid on trajectory plots are slope differences between steroid groups. HRs and slope differences are obtained from two model specifications, which differ only by the baseline steroid reference group (none or prednisone). **p < 0.01, *p < 0.05. Abbreviations: CI, confidence interval; DFZ, deflazacort; DMD, Duchenne muscular dystrophy; HR, hazard ratio; PRED, prednisone.
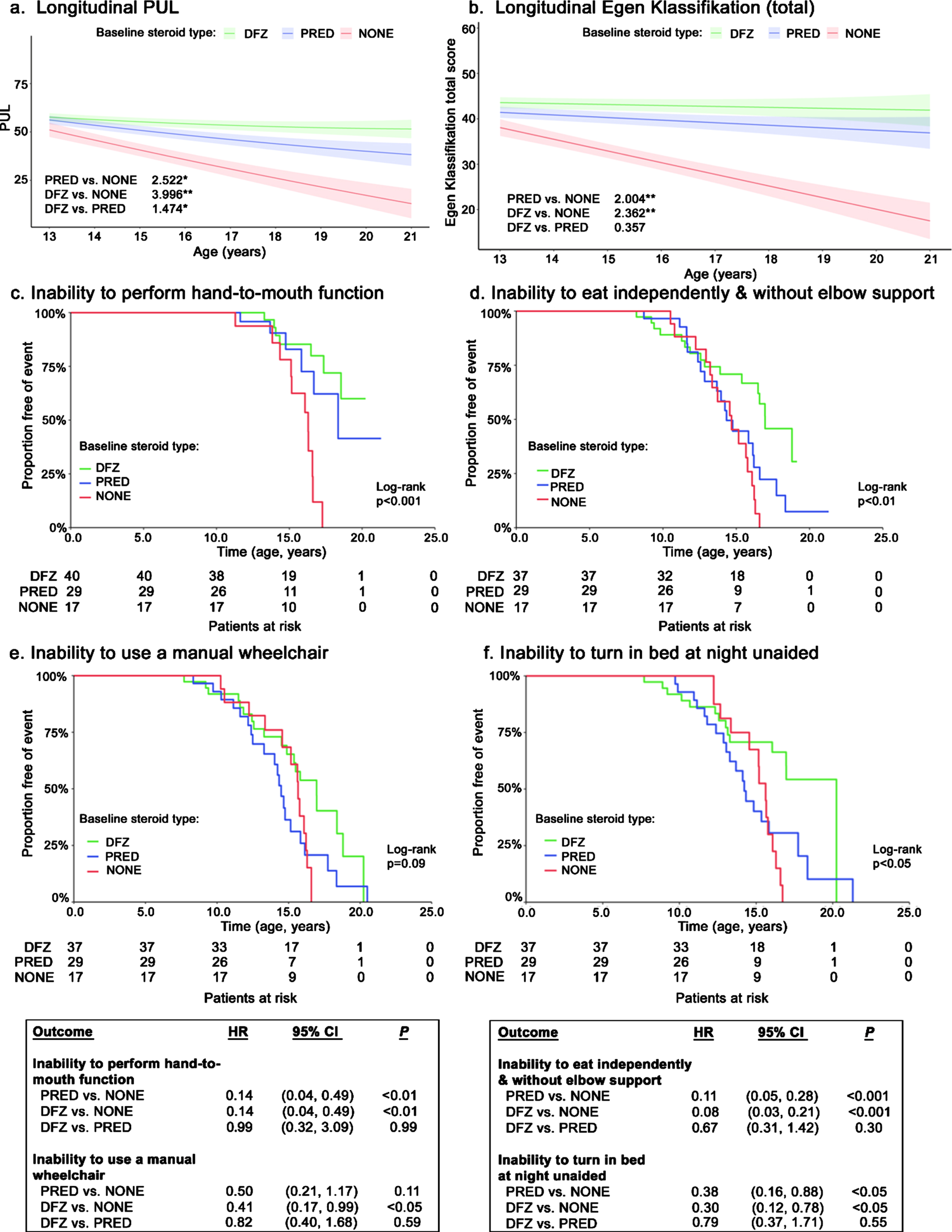
Disease progression milestones
The median age at loss of hand-to-mouth function was not consistently reached; however, significantly higher proportions of steroid-treated patients maintained function at age 15 years (deflazacort: 85%; prednisone: 83%) vs. patients not receiving steroids (78%; p < 0.001) (Fig. 2c). The median age at loss of the ability to use a manual wheelchair was numerically but not significantly higher for patients on deflazacort compared to the other groups (+2.5 years vs. prednisone; +1.3 years vs. no steroids; log-rank p < 0.10) (Fig. 2d). The median age at loss of ability to eat independently and without elbow support occurred significantly later among deflazacort-treated patients compared to the other treatment groups (+2.6 years vs. prednisone; +2.3 years vs. no steroids; log-rank p < 0.01) (Fig. 2e). Additionally, the median age at loss of ability to turn in bed at night unaided was significantly prolonged for deflazacort-treated patients compared to the other groups, and by a considerable amount (+6.0 years vs. prednisone; +4.6 years vs. no steroids; log-rank p < 0.05) (Fig. 2f).
In adjusted Cox models, patients receiving either steroid had a significantly lower hazards of losing hand-to-mouth function (both HR: 0.14, p < 0.01), the ability to eat independently and without elbow support (0.11 [prednisone] and 0.08 [deflazacort], both p < 0.001), and the ability to turn in bed at night unaided (0.38 [prednisone] and 0.30 [deflazacort], both p < 0.05) relative to patients taking no steroids (boxes in Fig. 2). In adjusted Cox models of loss of ability to use a manual wheelchair, only deflazacort-treated patients experienced a significantly lower hazard vs. patients receiving no steroids (HR: 0.41; p < 0.05); there were no significant differences between deflazacort- and prednisone-treated patients. The ability to transfer independently from a wheelchair was quickly lost, with no patients maintaining this ability by age 20 years. Prednisone-treated patients experienced a significantly lower hazard of inability to transfer independently from a wheelchair relative to patients receiving no steroids (HR: 0.36; p < 0.01) or deflazacort (1.96; p < 0.05) (Supplemental Figure 2).
Annualized changes in body weight and height
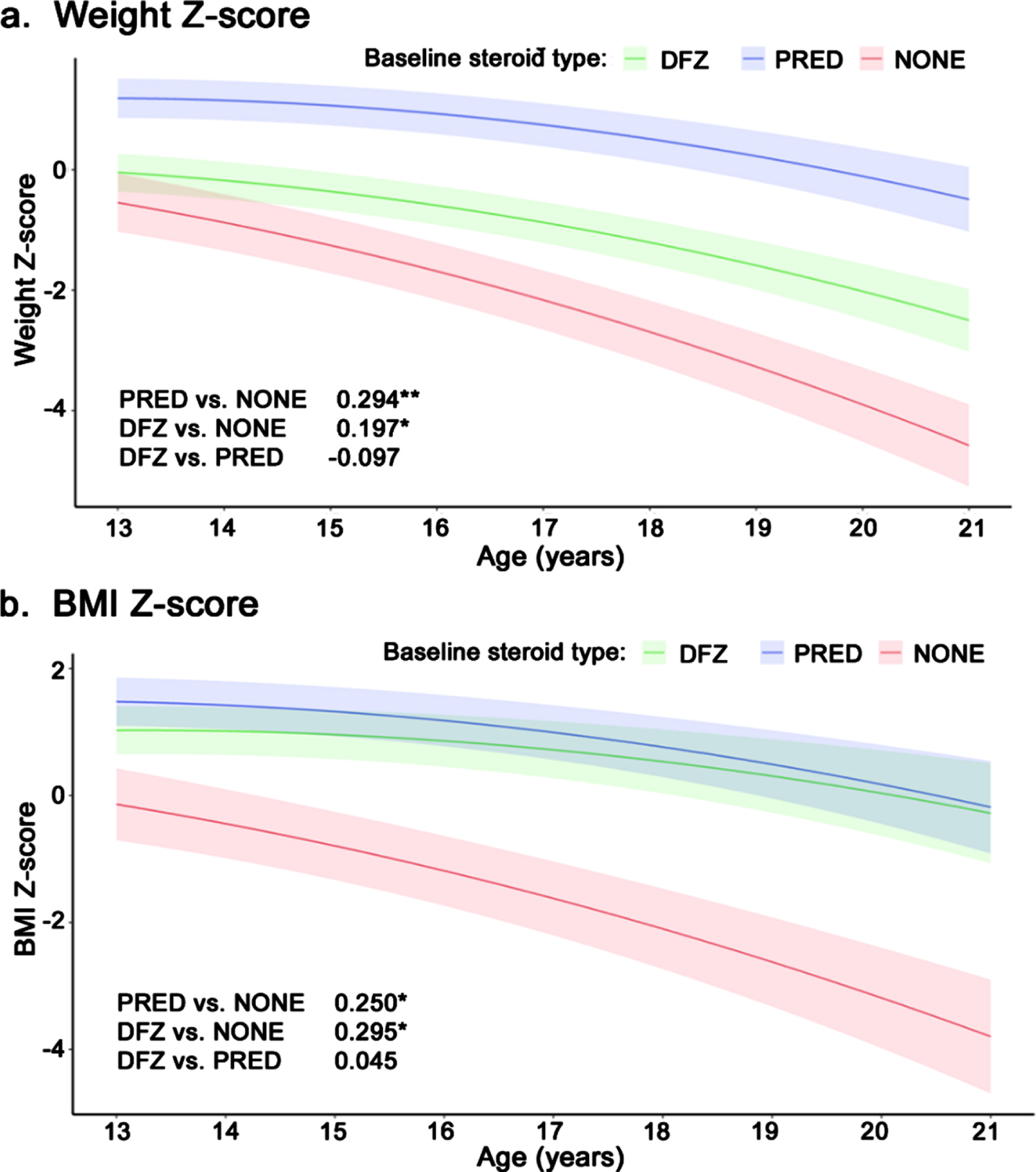
Weight z-score declined significantly faster among patients who received no steroids compared to patients treated with prednisone (–0.29 units/year; p < 0.001) or deflazacort (–0.20 units/year; p < 0.05) (Fig. 3a). Deflazacort-treated patients experienced a slightly faster decline in weight vs. prednisone-treated patients, but the difference was not significant (–0.10 units/year, p = 0.14). Similarly, BMI z-score declined significantly faster among patients who received no steroids vs. prednisone- or deflazacort-treated patients (–0.25 and –0.3 units/year, respectively; both p < 0.01); there was no significant difference in BMI z-score between the steroid types (Fig. 3b). Height z-score declined significantly faster among deflazacort-treated patients vs. those who received no steroids (–0.24 units/year; p < 0.05) or prednisone (–0.37 units/year; p < 0.01); there was no significant difference in height z-score change between prednisone patients and patients not on steroids (Supplemental Figure 3).
Fig. 3
Longitudinal z-score for (a) weight and (b) BMI among non-ambulatory patients with DMD, by steroid use. Caption: Shaded regions are bounded by±1 standard error. Estimates overlaid on plot are slope differences between steroid groups. Slope differences are obtained from two model specifications, which differ only by the baseline steroid reference group (none or prednisone). **p < 0.01, *p < 0.05. Abbreviations: BMI, body mass index; DFZ, deflazacort; DMD, Duchenne muscular dystrophy; PRED, prednisone.
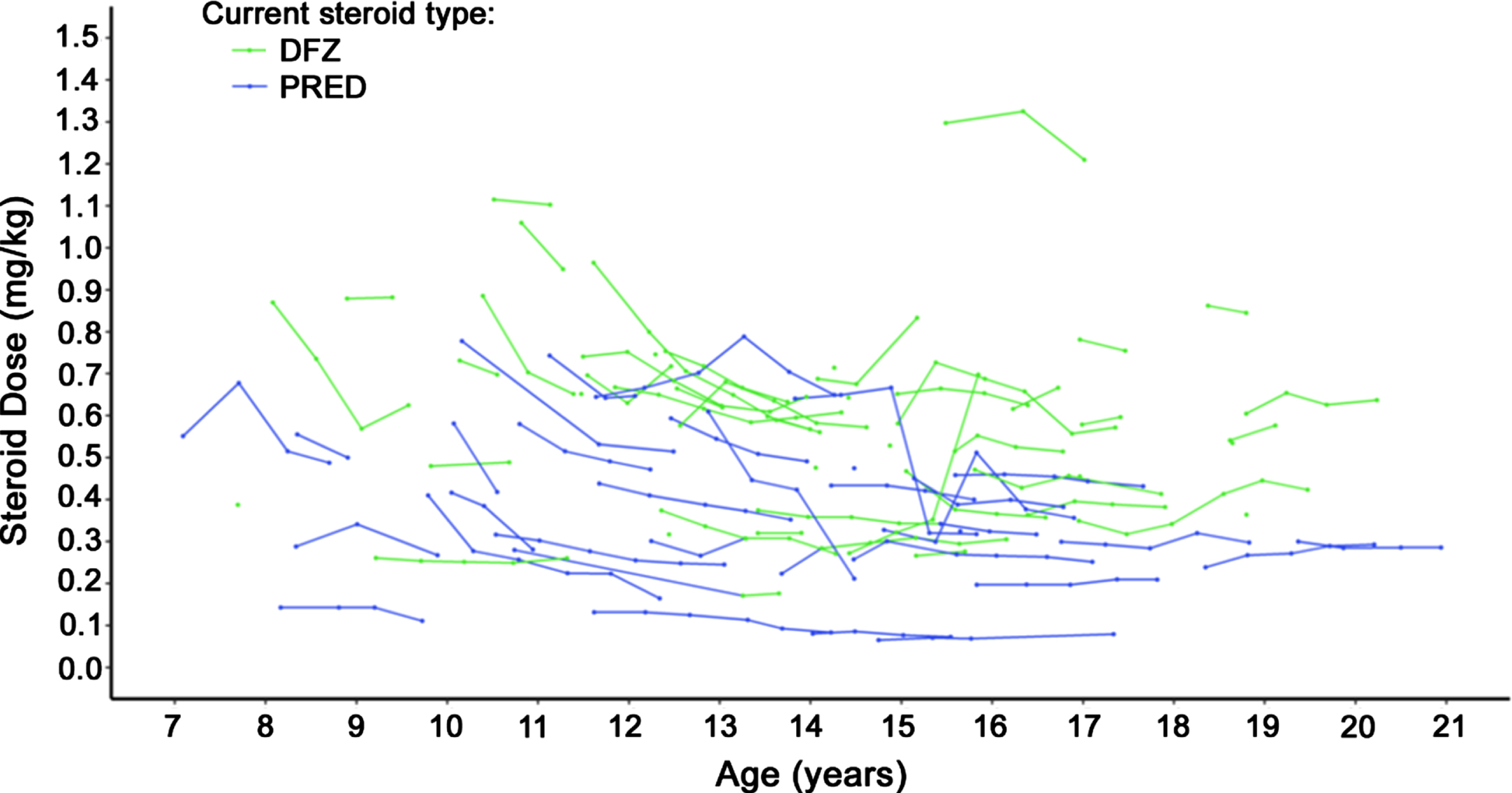
Steroid dosing
Steroid dosage over time is displayed in Fig. 4. Deflazacort-treated patients had slightly higher steroid dose by weight trajectories over time compared to prednisone-treated patients. During follow-up, 7 deflazacort patients and 3 prednisone patients experienced dose increases, while 1 and 4, respectively, experienced dose reductions.
Fig. 4
Steroid dosage over time among non-ambulatory patients with DMD. Caption: Dose reported for patients with non-missing weight whose dose was available in milligrams. Each continuous line represents one patient. Abbreviation: DFZ, deflazacort; DMD, Duchenne muscular dystrophy; PRED, prednisone.
Associations between pulmonary and upper-limb function
The relationship between FVC % -predicted and upper limb function among all patients is presented in Fig. 5. There was a direct correlation between loss of upper limb function and loss of pulmonary function, with lower levels of FVC % -predicted observed among patients with lower levels of upper limb function. Median FVC % -predicted was over twice as high among patients with the highest vs. lowest observed levels of upper limb function (FVC % -predicted of 85% [PUL entry item = 6] vs. only 41% [PUL entry item = 1]).
Fig. 5
FVC % -predicted by Upper Limb Function among non-ambulatory patients with DMD. Caption: Upper limb function was defined using the entry item to the Performance of Upper Limb Module for DMD 2.0 (lowest value of 0 not observed), which is based on a revised version of the Brooke Scale of Upper Extremity Function. Higher entry item values represent greater functional ability rather than greater functional impairment. Figure includes measurements of FVC % -predicted and PUL from all visits for sample patients, including visits while ambulatory for those patients who lost ambulation over the course of the PRO-DMD-01 study. Four follow-up measurements of FVC % -predicted among 3 patients were deemed to be extreme outliers and excluded from analysis. Dots represent outliers. Abbreviations: DMD, Duchenne muscular dystrophy; FVC, forced vital capacity.
DISCUSSION
This study found consistent and significant associations between steroid use and slower disease progression across all outcome domains (pulmonary, cardiac, functional ability) among non-ambulatory boys with DMD. In particular, steroid use was associated with the preservation of two tasks considered by patients and their caregivers to especially impact quality of life: the abilities to independently bring hands to mouth and to reposition at night unaided [34].
Deflazacort-treated patients experienced similar or greater preservation of function relative to prednisone-treated patients. Notably, deflazacort patients received a higher dose –both as a proportion of the recommended dose and as a result of more frequent regimens - and experienced less weight gain than prednisone-treated patients. Higher doses and more patients on daily regimens may reflect greater tolerability (either expected or experienced), which in turn, could influence outcomes. However, higher dosages when measured in mg per kg may simply reflect lower or declining body weight, independent of tolerability. Indeed, prior studies among ambulatory patients with DMD have found mixed evidence regarding the tolerability of deflazacort relative to prednisone, [15, 18, 35] and further assessment of their comparative tolerability profiles after loss of ambulation is warranted.
Relative to prior studies that quantified steroid use during the non-ambulatory period in DMD, we observed more patients on steroids (80% vs. 48–53%), greater deflazacort use among steroid-users (58% vs. 48%), and more steroid-naïve patients among those not on steroids (76% vs. 42–45%) [6, 24]. These results may reflect differences in population demographics, including age and geography, treatment approaches at participating sites in PRO-DMD-01, differing time periods of study, and variability attributable to small samples inherent to studying a rare disease. Our findings indicate that the well-described benefits of steroid use for slowing disease progression among ambulatory patients extend into the non-ambulatory period, and suggest that deflazacort may provide greater benefits in slowing upper limb skeletal muscle disease progression measures than prednisone [6, 15, 16, 18, 22]. Our observation that steroid use was associated with greater preservation of upper limb function while non-ambulatory is consistent with Pane et al., which reported that the mean changes in PUL scores at 12 months was worse among those who never received steroids (–4.44) or discontinued steroids at loss of ambulation (–5.52) compared with steroid-treated patients (–3.79) [6]. Similarly, McDonald et al. reported that steroid treatment was associated with significantly higher median age at loss of mobility and upper limb milestones, and deflazacort was superior to prednisone in the length of the delay [16]. However, our results, showing differences in the rates of decline in FVC % -predicted and EK total score, differ from those of another study of non-ambulatory patients with DMD reporting that steroids did not impact either measure [36]. That study had a higher average age at baseline (17 vs. 13 years) and required boys to be non-ambulatory for more than a year, which could have impacted the differences in results. Finally, the results of the present study are consistent with prior research linking prednisone with more weight gain and deflazacort with shorter stature [15, 17, 35]. Lack of steroid treatment was associated with a faster progression to low BMI z-scores, and impaired pulmonary and cardiac function may play a role in accelerating this decline.
An important finding of this study was the correlation between pulmonary outcomes and upper limb function during the non-ambulatory period, which was remarkably consistent with an existing analysis of the relationship between FVC % -predicted and the Brooke Scale [37]. That study included both ambulatory and non-ambulatory patients with DMD from another natural history database and compared outcomes among steroid-naïve patients and those who received steroids. In the prior study and ours, the loss of hand-to-mouth function was associated with a FVC % -predicted crossing the 50% threshold. Prior results and the present findings confirm that, for patients with developmental disabilities or autistic spectrum features who cannot reliably participate in pulmonary function testing, the loss of hand-to-mouth function could potentially be used as a proxy for an FVC % -predicted<50%, at which point evaluation for nocturnal hypoventilation and the need for non-invasive ventilation should be considered [37].
The results of this study are subject to several limitations. First, due to the rarity of DMD, the sample sizes were generally small, limiting statistical precision when comparing treatment subgroups. Second, our treatment of milestones that were already reached as of the index visit may increase the apparent ages at which milestones are reached; however, as this affects all treatment groups, differences in median ages at milestones across groups will be less affected. Third, this study did not include all possible adverse events/side effects associated with long-term steroid use (e.g., fracture risk, Cushingoid syndrome, cataracts, insulin resistance) because they were not systematically assessed in PRO-DMD-01. Thus, the benefits of long-term steroid use during the non-ambulatory period cannot be comprehensively weighed against the risks. Finally, we cannot rule out confounding due to the limited post-index steroid switching or unobserved differences between patients receiving prednisone, deflazacort, and no steroids (e.g., differences in care received before PRO-DMD-01 enrollment; socioeconomic status, which may impact therapy choice/adherence). Finally, there may be a payer bias implicit in results for loss of ability to use a manual wheelchair, particularly for US patients, as some insurance will only reimburse one wheelchair in a given 5-year period, leading physicians to prescribe more expensive power wheelchairs over manual wheelchairs [38].
In conclusion, steroid use after loss of ambulation was associated with delayed progression of important pulmonary, cardiac, and functional deficits in DMD, with suggestive evidence that deflazacort may be more effective than prednisone as judged by certain measures of function. Clinicians should consider continuing steroid use for patients with DMD after ambulation is lost to slow disease progression and delay further loss of functional abilities considered clinically meaningful.
ACKNOWLEDGMENTS
Medical writing was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc., and supported by PTC Therapeutics, Inc. The authors also wish to thank the PRO-DMD-01 consortium investigators for their work in the design and conduct of the study. All members of the PRO-DMD-01 consortium are listed in the Supplemental Materials. This manuscript is based on research using data from data contributor Cure Duchenne that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Financial support of this study was provided by PTC Therapeutics, Inc.
CONFLICT OF INTEREST
PT and KK are employees of PTC Therapeutics, Inc. and hold stock/options. JRM, JF, HL, AZ, MF, and JS are employees of Analysis Group, Inc., which has received consulting fees from PTC Therapeutics, Inc. for the conduct of this study. CMM has acted as a consultant on clinical trials of DMD for Astellas, Avidity Biosciences, Capricor Therapeutics, Catabasis, Edgewise Therapeutics, Entrada therapeutics, Epirium Bio (formerly Cardero Therapeutics), FibroGen, Italfarmaco, Pfizer, PTC Therapeutics, Roche, Santhera Pharmaceuticals and Sarepta Therapeutics. He reports honoraria for presentations from PTC Therapeutics, Sarepta Therapeutics, Solid Biosciences, Santhera Pharmaceuticals, Capricor Therapeutics, and Catabasis. He has received compensation for participation in advisory boards from PTC Therapeutics, Sarepta Therapeutics, Avidity Biosciences, Edgewise Therapeutics, and Santhera Pharmaceuticals. He has received research support for clinical trials from Capricor Therapeutics, Catabasis, Italfarmaco, Pfizer, PTC Therapeutics, Santhera Pharmaceuticals, and Sarepta Therapeutics, and reports grants from the U.S. Department of Defense, U.S. National Institutes of Health (NINDS), Parent Project Muscular Dystrophy, and NIDILRR. OHM has served on an advisory board, as a consultant or speaker for Santhera Pharmaceuticals, Sarepta Therapeutics, Capricor Therapeutics, Entrada Therapeutics, Edgwise Therapeutics and FibroGen. KNH has acted as a consultant, advisory board or data safety monitor board member related to DMD for Bristol-Myers Squibb, Capricor Therapeutics, Catabasis Pharmaceuticals, Daiichi Sankyo, FibroGen, Revidia Therapeutics, PTC Therapeutics, Sarepta Therapeutics, Stealth Biotherapeutics, Vertex Pharmaceuticals, and Wave Life Science. He has received research support related to DMD provided by the US Food and Drug Administration (FDA), Parent Project Muscular Dystrophy, and Sarepta Therapeutics. NG has served in advisory boards or data monitoring committees related to DMD for Sarepta Therapeutics, BioMarin, PTC Therapeutics, Italfarmaco, Daiichi Sankyo, Dyne, Wave Life Science, Pfizer, Genethon, and Santhera Pharmaceuticals. EKH reports consulting and advisory committee work for PTC Therapeutics, Glaxo-Smith Kline, Pfizer, Santhera Pharmaceuticals, Sarepta Therapeutics, Cardero Therapeutics, and Capricor Therapeutics, as well as grant support and travel assistance from the Muscular Dystrophy Association and Parent Project Muscular Dystrophy.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-221575.
REFERENCES
[1] | Chung J , Smith AL , Hughes SC , Niizawa G , Abdel-Hamid HZ , Naylor EW , et al. Twenty-year follow-up of newborn screening for patients with muscular dystrophy. Muscle Nerve (2015) ;53: (4):570–8. |
[2] | Romitti PA , Zhu Y , Puzhankara S , James KA , Nabukera SK , Zamba GK , et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics (2015) ;135: (3):513–21. |
[3] | Mendell JR , Shilling C , Leslie ND , Flanigan KM , al-Dahhak R , Gastier-Foster J , et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology (2012) ;71: (3):304–13. |
[4] | Crisafulli S , Sultana J , Fontana A , Salvo F , Messina S , Trifirò G Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet Journal of Rare Diseases (2020) ;15: (1):141. |
[5] | Ricotti V , Ridout DA , Scott E , Quinlivan R , Robb SA , Manzur AY ,et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. Journal of Neurology, Neurosurgery, and Psychiatry (2013) ;84: (6):698–705. |
[6] | Pane M , Fanelli L , Mazzone ES , Olivieri G , D’Amico A , Messina S , et al. Benefits of glucocorticoids in non-ambulant boys/men with Duchenne muscular dystrophy: A multicentric longitudinal study using the Performance of Upper Limb test. Neuromuscul Disord (2015) ;25: (10):749–53. |
[7] | Mazzone E , Bianco F , Main M , van den Hauwe M , Ash M , de Vries R ,et al. Six minute walk test in type III spinal muscular atrophy: A 12-month longitudinal study. Neuromuscul Disord (2013) ;23: (8):624–8. |
[8] | Seferian AM , Moraux A , Annoussamy M , Canal A , Decostre V , Diebate O , et al. Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne muscular dystrophy: An observational multicenter trial. PLoS One (2015) ;10: (2):e0113999. |
[9] | Ishikawa Y , Miura T , Ishikawa Y , Aoyagi T , Ogata H , Hamada S , et al. Duchenne muscular dystrophy: Survival by cardio-respiratory interventions. Neuromuscul Disord (2011) ;21: (1):47–51. |
[10] | LoMauro A , D’Angelo MG , Aliverti A Assessment and management of respiratory function in patients with Duchenne muscular dystrophy: Current and emerging options. Ther Clin Risk Manag (2015) ;11: :1475–88. |
[11] | Gloss D , Moxley RT , 3rd, Ashwal S , Oskoui M Practice guideline update summary: Corticosteroid treatment of Duchenne muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology (2016) ;86: (5):465–72. |
[12] | Birnkrant DJ , Bushby K , Bann CM , Apkon SD , Blackwell A , Brumbaugh D , et al. Diagnosis and management of Duchenne muscular dystrophy, part Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol (2018) ;17: (3):251–67. |
[13] | US Food and Drug Administration Highlights of prescribing information: EMFLAZA (deflazacort) [2017] 2017. Available from: https://www.accessdata.fda.gov/drugsatfda docs/label/2017/208684s000,208685s000lbl.pdf. Accessed: January 25, 2022. |
[14] | United States Food and Drug Administration Administration. Highlights of prescribing information: EMFLAZA (deflazacort) [2019 update] 2019. Available from: https://www.accessdata.fda.gov/drugsatfdadocs/label/2019/208684s003,208685s003lbl.pdf. Accessed: February 24, 2022. |
[15] | Griggs RC , Miller JP , Greenberg CR , Fehlings DL , Pestronk A , Mendell JR , et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology (2016) ;87: (20):2123–31. |
[16] | McDonald CM , Henricson EK , Abresch RT , Duong T , Joyce NC , Hu F , et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study. Lancet (2018) ;391: (10119):451–61. |
[17] | Rice ML , Wong B , Horn PS , Yang MB Cataract development associated with long-term glucocorticoid therapy in Duchenne muscular dystrophy patients. Journal of AAPOS: The Official Publication of the American Association for Pediatric Ophthalmology and Strabismus (2018) ;22: (3):192–6. |
[18] | McDonald CM , Sajeev G , Yao Z , McDonnell E , Elfring G , Souza M , et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: A meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve (2020) ;61: (1):26–35. |
[19] | Assandri A , Buniva G , Martinelli E , Perazzi A , Zerilli L Pharmacokinetics and metabolism of deflazacort in the rat, dog, monkey and man. Adv Exp Med Biol (1984) ;171: , 9–23. |
[20] | Matthews E , Brassington R , Kuntzer T , Jichi F , Manzur AY Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev (2016) (5):CD003725. |
[21] | Biggar WD , Skalsky A , McDonald CM Comparing deflazacort and prednisone in Duchenne muscular dystrophy. J Neuromuscul Dis (2022) ;9: (4):463–76. |
[22] | Marden JR , Freimark J , Yao Z , Signorovitch J , Tian C , Wong BL Real-world outcomes of long-term prednisone and deflazacort use in patients with Duchenne muscular dystrophy: Experience at a single, large care center. J Comp Eff Res (2020) ;9: (3):177–89. |
[23] | Guglieri M , Bushby K , McDermott MP , Hart KA , Tawil R , Martens WB , et al. Effect of different corticosteroid dosing regimens on clinical outcomes in boys with duchenne muscular dystrophy: A randomized clinical trial. JAMA (2022) ;327: (15):1456–68. |
[24] | Cowen L , Mancini M , Martin A , Lucas A , Donovan JM Variability and trends in corticosteroid use by male United States participants with Duchenne muscular dystrophy in the Duchenne Registry. BMC Neurol (2019) ;19: (1):84. |
[25] | Zhang T , Kong X Recent advances of glucocorticoids in the treatment of Duchenne muscular dystrophy (Review). Exp Ther Med (2021) ;21: (5):447. |
[26] | Kuczmarski RJ , Ogden CL , Guo SS , Grummer-Strawn LM , Flegal KM , Mei Z , et al. CDC growth charts for the United States: Methods and development. Vital Health Stat 11 (2002) (246):1–190. |
[27] | Mayhew A , Mazzone ES , Eagle M , Duong T , Ash M , Decostre V , et al. Development of the Performance of the Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol (2013) ;55: (11):1038–45. |
[28] | Mercuri E , McDonald C , Mayhew A , Florence J , Mazzone E , Bianco F , et al. International workshop on assessment of upper limb function in Duchenne Muscular Dystrophy: Rome, 15-16 February Neuromuscul Disord (2012) ;22: (11):1025–8. |
[29] | Emery AEH , Muntoni F , Quinlivan R Egen Klassifikation Scale Version 2 (EK2). Duchenne muscular dystrophy. 4th ed. Oxford: Oxford University Press; (2015) . p. ix, p. 308. |
[30] | Brooke MH , Griggs RC , Mendell JR , Fenichel GM , Shumate JB , Pellegrino RJ Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve (1981) ;4: (3):186–97. |
[31] | Fayssoil A , Abasse S , Silverston K Cardiac involvement classification and therapeutic management in patients with Duchenne muscular dystrophy. J Neuromusc Dis (2017) ;4: (1):17–23. |
[32] | Birnkrant DJ , Bushby K , Bann CM , Alman BA , Apkon SD , Blackwell A , et al. Diagnosis and management of Duchenne muscular dystrophy, part Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurology (2018) ;17: (4):347–61. |
[33] | Birnkrant DJ , Panitch HB , Benditt JO , Boitano LJ , Carter ER , Cwik VA , et al. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest (2007) ;132: (6):1977–86. |
[34] | Peay H , Kennedy A , Fischer R , Bronson A , Furlong P Promoting meaningful clinical trial outcome measures for Duchenne muscular dystrophy. Neuromuscul Dis (2016) ;26: :S187. |
[35] | Bello L , Gordish-Dressman H , Morgenroth LP , Henricson EK , Duong T , Hoffman EP , et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology (2015) ;85: (12):1048–55. |
[36] | Connolly AM , Florence JM , Zaidman CM , Golumbek PT , Mendell JR , Flanigan KM , et al. Clinical trial readiness in non-ambulatory boys and men with duchenne muscular dystrophy: MDA-DMD network follow-uMuscle Nerve (2016) ;54: (4):681–9. |
[37] | McDonald CM , Gordish-Dressman H , Henricson EK , Duong T , Joyce NC , Jhawar S , et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: Long-term natural history with and without glucocorticoids. Neuromuscul Disord (2018) ;28: (11):897–909. |
[38] | Centers for Medicare & Medicaid Services. Durable medical equipment. Available from: https://www.cms.gov/Center/Provider-Type/Durable-Medical-Equipment-DME-Center. Accessed: February 14, 2022. |




