Assessing Bulbar Function in Spinal Muscular Atrophy Using Patient-Reported Outcomes
Abstract
Background:
Novel Spinal Muscular Atrophy (SMA) treatments have demonstrated improvements on motor measures that are clearly distinct from the natural history of progressive decline. Comparable measures are needed to monitor bulbar function, which is affected in severe SMA.
Objective:
To assess bulbar function with patient-reported outcome measures (PROs) and determine their relationships with clinical characteristics.
Methods:
We recruited 47 non-ambulatory participants (mean (SD) age = 29.8 (13.7) years, range = 10.3–73.2) with SMA. PROs including Voice Handicap Index (VHI) and Eating Assessment Tool-10 (EAT-10) were collected alongside clinical characteristics and standardized motor assessments. Associations were assessed using Spearman correlation coefficients and group comparisons were performed using Wilcoxon rank sum tests.
Results:
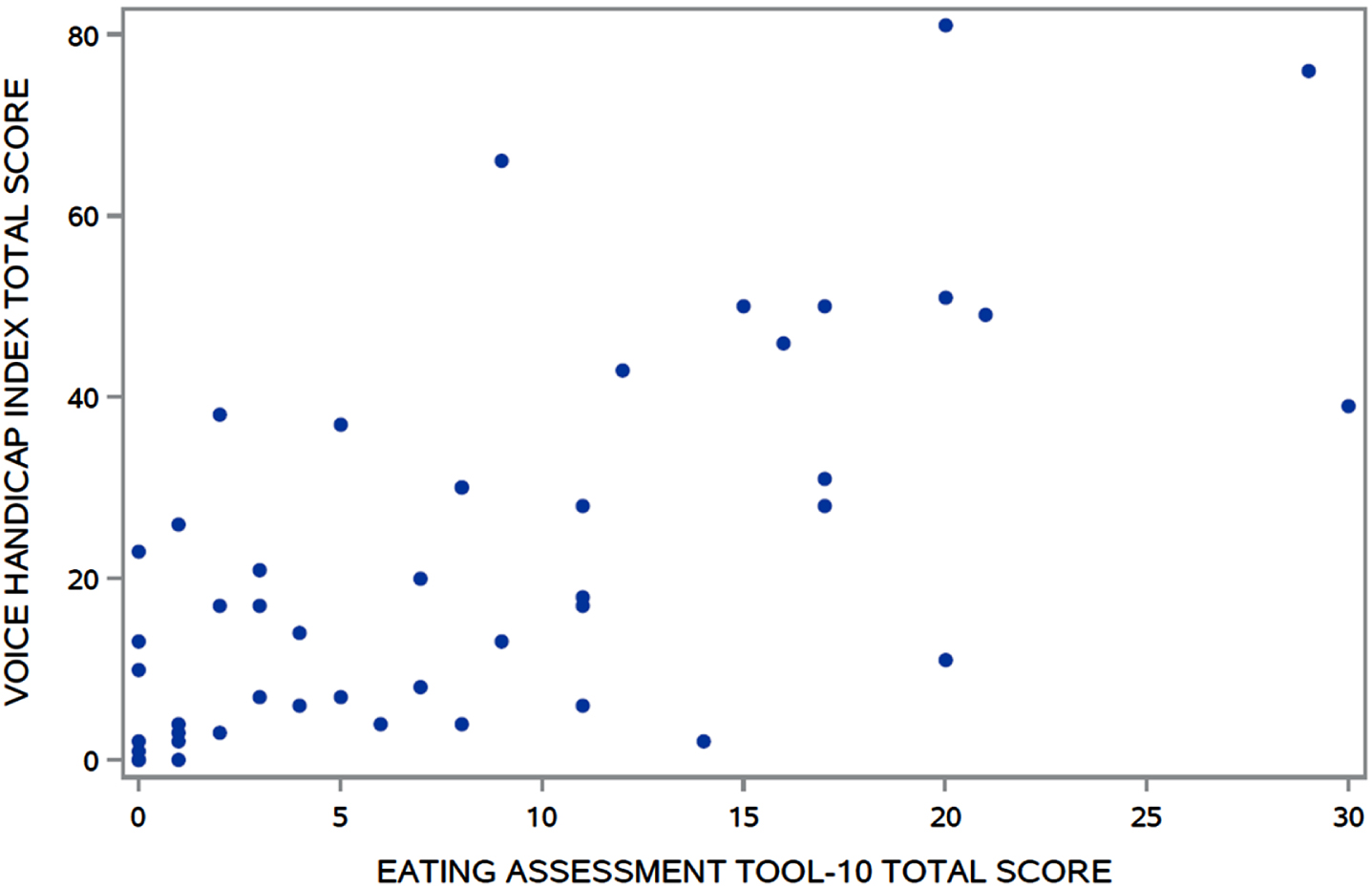
A majority of the 47 participants were SMA type 2 (70.2%), non-sitters (78.7%), 3 copies of SMN2 (77.5%), and using respiratory support (66.0%). A majority (94%) reported voice issues primarily in 8/30 VHI questions. Problems included: difficulty understanding me in a noisy room (87.2%); difficult for people to hear me (74.5%); and people ask me to repeat when speaking face-to-face (72.3%). A majority (85.1%) reported swallowing issues primarily in 3/10 EAT-10 questions: swallowing pills (68.1%); food sticks to my throat (66.0%); and swallowing solids (61.7%). The two PROs were moderately associated (rs = 0.66).
Conclusions:
Weaker individuals with SMA experience bulbar problems including difficulties with voice and swallowing. Further refinement and assessment of functional bulbar scales will help determine their relevance and responsiveness to changes in SMA. Additional study is needed to quantify bulbar changes caused by SMA and their response to disease-modifying treatments.
INTRODUCTION
Spinal muscular atrophy (SMA) is an autosomal recessive motor neuron disease characterized by progressive muscle weakness caused by degeneration of lower motor neurons in the spinal cord and brainstem. The disease affects an estimated 10–16 per 100,000 infants [1]. SMA has a broad phenotypic spectrum of disease severity with a clinical classification based on age of onset and maximal function achieved. Patients with the intermediate form, type 2, are moderately affected as they achieve the ability to sit but never walk. Patients with the mildest form, type 3, are less affected as they achieve the ability to walk independently but may lose this ability over time with varying degrees of disability [2].
Individuals with more severe forms of SMA have historically experienced bulbar dysfunction that includes problems in communication and deglutition resulting from weakness in the oropharyngeal musculature controlled by the cranial nerves in the pons and medulla [3–8]. Abnormal bulbar function is a well-known complication of SMA with common physical complaints and prevalence previously reported [6, 9–13] with a negative impact on activities of daily living, health, well-being and social participation [3–5, 7, 8].
Previous questionnaire-based studies examining specifications of these deficits using patient-reported outcome (PRO) measures reported the three most common deficits in deglutition in individuals with SMA type 2 and 3 (age 3 to 45 years) to be choking (31%), difficulty conveying food to the mouth (20%), and difficulty chewing (20%), with patients with lower levels of motor function having 7.6 greater odds of reporting dysphagia [10]. Another commonly reported impairment using a PRO in those age 1 to 75 years is the perception of fatigue during mealtimes that prohibits an individual’s ability to safely consume full oral nutrition [6, 14]. Though fewer investigations have reported on specifications of communication deficits, those that have examined this domain, using questionnaires and structured interviews in individuals age 31 to 67 years, have indicated high reports of voice and speech problems that, similar to swallowing, were more common in patients with type 1 and 2 (45%) than in those with type 3 (17%), although no significant correlation was found between these symptoms and disease duration [13].
The approval of disease-modifying therapies for SMA was based on clinical trials of infants, children, and young adults [15–23] with no formal studies conducted in the chronic adult SMA population. Real-world evidence from adults with SMA has suggested improvement on treatment in several motor function and respiratory measures distinct from the progressive decline typically seen in untreated chronic SMA [24–30]. However, there is limited objective data regarding the impacts of disease-modifying therapies on communication and deglutition due, in part, to the scarcity of tools specific to assessing bulbar function in individuals with SMA [4, 31].
The use of PROs has been strongly supported by regulatory authorities both in US and in Europe to provide a better understanding of disease burden and impact of treatment on patients [4]. A Canadian adult SMA consensus-derived clinical outcome measure toolkit recognized gaps and poor availability of assessments to detect changes in multiple aspects of life including bulbar function (communication and swallowing), particularly in non-sitter and sitter populations [32], as this was previously identified as a priority for stabilization for persons with SMA type 2 and 3 [33]. Monitoring perceived changes in communication and deglutition is important in severely affected adult patients, in whom motor function testing of limb musculature is typically less sensitive or applicable due to the advanced disease stage and floor effects of available measures [34].
The primary objective of this study is to systematically assess perceived bulbar function (voice and deglutition) among treated and untreated non-ambulatory patients with SMA using two currently available PROs, the Voice Handicap Index (VHI) and Eating Assessment Tool-10 (EAT-10), and determine their relationships with clinical characteristics. Understanding perceived bulbar function was deemed clinically feasible as these PROs do not require the use of instrumental assessments or need for specialized equipment or training and could be administered during a routine clinic visit. We also sought to examine if perceived voice and deglutition deficits are associated with one another. We hypothesize that more severe, lower functioning individuals with SMA will experience greater perceived voice and deglutition deficits, and that these deficits will be weakly related to age.
MATERIALS AND METHODS
For this cross-sectional study, data were acquired prospectively as part of an IRB-approved multicenter natural history study of SMA at two of the Pediatric Neuromuscular Clinical Research (PNCR) Network sites (Stanford University School of Medicine and Boston Children’s Hospital / Harvard Medical School). All participants spoke English, and informed consent was obtained prior to enrollment. This study is in accord with the Helsinki Declaration of 1975.
Subjects
Forty-seven non-ambulatory participants with SMA were included between December 2018 and February 2021 while nusinersen and risdiplam treatment were available. Exclusion criteria were unstable medical conditions that would preclude participation or other conditions precluding safe participation [35].
Procedures
Participants in the natural history study had age, sex, SMA type, SMN2 copy number, current functional status, symptom duration, treatment status, respiratory and nutritional support collected at the first routine clinical care visit at which both PROs of bulbar function were obtained. Voice was evaluated using the Voice Handicap Index (VHI) and deglutition using the Eating Assessment Tool-10 (EAT-10), both completed via paper and pen/pencil with specifications of each metric outlined below. The PROs were provided to the participant by a rehabilitation clinician for self-report; however, a caregiver or clinician was present to help circle the participants responses if the participant was unable to do so themselves. Motor function was evaluated using the Hammersmith Functional Motor Scale Expanded (HFMSE), the Revised Upper Limb Module (RULM), the Children’s Hospital of Philadelphia Adult Test of Neuromuscular Disorders (CHOP-ATEND), as well as the Egen Klassifikation Scale 2 (EK2), a PRO.
VHI. The VHI was designed to quantify the psychosocial handicapping effects of voice disorders and is used to assess the patient’s judgement about the relative impact of his or her voice disorder on daily activities. The 30-item scale encompasses 3 subscales: Functional (10 items), Physical (10 items), and Emotional (10 items). The items are statements that many people have used to describe their voices and the effects of their voices on their lives. Participants are asked to circle the response that indicates how frequently they have the same experience using a five-point ordinal scale (0 = never; 1 = almost never; 2 = sometimes; 3 = almost always; 4 = always) [36, 37]. Severity levels are identified based on total score (0–120) in which 0 to 30 is mild, 31 to 60 is moderate, and 61 to 120 is severe, representing greater psychosocial consequences of a patient’s voice disorder. The VHI has demonstrated strong internal consistency reliability and test-retest reliability, and has been applied in other neurological disease groups including Parkinson’s disease and neurofibromatosis type 1 [36, 37].
EAT-10. The EAT-10 was designed as a symptom-specific dysphagia outcome tool. The 10-item scale asks to what extent are the following scenarios problematic using a five-point ordinal scale ranging from 0 = no problem to 4 = severe problem. A total score (0–40) that is 3 or greater is indicative of dysphagia and reflective of a problem with swallowing efficiency and safety [38]. The EAT-10 has shown excellent internal consistency reliability, test-retest reliability, and criterion-based validity. It is validated in multiple languages and has been applied in other neurological disease groups including differentiated safe versus unsafe swallowing in amyotrophic lateral sclerosis, childhood onset muscular dystrophy, Parkinson’s disease, myasthenia gravis, multiple sclerosis, and myopathy [38–42].
HFMSE. The HFMSE is a disease-specific scale consisting of 33 items measuring gross motor function in SMA types 2 and 3. It has demonstrated good reliability and validity in patients with SMA [43, 44], requires standard equipment and has minimal participant burden [43] for the intended population of sitters and walkers.
RULM. The RULM is a disease-specific scale consisting of 20 items evaluating upper limb function and performance on ADLs in SMA types 2 and 3. It has been validated and demonstrated good test-retest reliability [45–47].
CHOP ATEND. The CHOP-ATEND is a scale modified from the original Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) excluding items that cannot be performed with adults (items 11, 15, and 16) [48]. As the CHOP-INTEND was originally developed to assess infants with SMA type 1 [49], criteria for start positions, contracture considerations, and grading methodology were clarified for administration in non-ambulatory adults while keeping the construct of the items intact [24].
EK2. The EK2 assesses functional abilities for people who are non-ambulatory with neuromuscular disease by evaluating activities and abilities such as transfers, trunk mobility, wheelchair use, bed mobility, cough, feeding, bulbar issues, distal hand function, and well-being. A patient is interviewed to determine how certain items are normally performed and to demonstrate certain skills based on initial responses to a question. The test includes 17 items, each of which is scored on an ordinal scale (0 to 3). The EK2 scale demonstrates reliability and validity in SMA [50, 51].
Data analysis
Demographic and clinical characteristics were summarized using means, standard deviations, range, and medians for continuous variables or using frequencies and percentages for categorical variables. Reported perceived voice and deglutition deficits were defined as non-zero total scores on the respective PRO. Associations between PROs of bulbar function and continuous clinical characteristics were assessed using Spearman correlation coefficients. Wilcoxon rank sum tests were used to compare subgroups defined by clinical characteristics with respect to bulbar function PROs. Upper extremity function was classified into two groups: unable to lift hands against gravity (RULM entry item A score 0 or 1) and able to lift hand(s)/arms against gravity (RULM entry item A score 2–6). A significance level of 0.05 was used for all analyses.
RESULTS
We recruited 47 non-ambulatory participants (mean (SD) age 29.8 (13.7) years, range 10.3 –73.2) with a genetically confirmed diagnosis of SMA who completed both bulbar function PROs at their clinic visit. Twenty-five participants (53.2%) were recruited from Stanford University School of Medicine and 22 participants (46.8%) from Boston Children’s Hospital / Harvard Medical School. Demographic and clinical characteristics are described in Table 1. A majority of the 47 participants were SMA type 2 (70.2%), non-sitters (78.7%), with 3 SMN2 copies (77.5%), using any respiratory support (66.0%) and receiving disease-modifying therapy at the time of PRO completion (66.0%). In all cases the disease-modifying therapy that had been received was nusinersen.
Table 1
Demographic and clinical characteristics
| Variable | N | Mean (SD) | Median | Min | Max |
| Male, n (%) | 23 (48.9) | ||||
| SMA Type, n (%) | |||||
| 1 | 3 (6.4) | ||||
| 2 | 33 (70.2) | ||||
| 3 | 11 (23.4) | ||||
| SMN2 copy number, n (%) † | |||||
| 2 copies | 5 (12.5) | ||||
| 3 copies | 31 (77.5) | ||||
| 4 copies | 4 (10.0) | ||||
| Non-Sitter, n (%) | 37 (78.7) | ||||
| Gastrostomy tube, n (%) | 16 (34.0) | ||||
| Tracheostomy, n (%) | 4 (8.5) | ||||
| Respiratory Support with NIV, n (%) ‡ | 27 (57.4) | ||||
| Night time NIV, n (%) | 18 (81.8) | ||||
| Night and Day time NIV, n (%) | 1 (3.7) | ||||
| Other, n (%) | 1 (3.7) | ||||
| Age, y | 47 | 29.8 (13.7) | 26.8 | 10 | 73 |
| 10 to < 20 y, n (%) | 12 (25.5) | ||||
| 20 to < 40 y, n (%) | 27 (57.4) | ||||
| 40 to < 60 y, n (%) | 6 (12.8) | ||||
| >60 y, n (%) | 2 (4.3) | ||||
| Symptom Onset, y | 47 | 1.0 (0.6) | 1 | 0.2 | 2.9 |
| Symptom Duration, y | 47 | 28.8 (13.7) | 25.6 | 9.7 | 71.2 |
| Nusinersen use, n (%) | 31 (65.9) | ||||
| Duration of Nusinersen use, y | 31 | 1.4 (0.9) | 1.5 | 0.3 | 3.5 |
SD, Standard Deviation; NIV, Non-Invasive Ventilation; y, year. † SMN2 copy number was unknown for n = 7 participants. ‡ Type of NIV was missing for n = 7 participants.
Motor function testing (Table 2) revealed weaker phenotypes with all participants scoring≤22 out of 66 on the HFMSE and nearly half (42.9%) scoring zero, exhibiting a floor effect. Mean RULM score was 9.6 out of 37 with only 19.1% scoring zero, exhibiting a floor effect. Of the 40 participants assessed with RULM entry item A, 16 (40.0%) were unable to lift hands against gravity (entry item A score 0 or 1). No participants could raise their arms above their head with or without compensations (entry item A score 5 or 6). The CHOP-ATEND was able to capture subtler distal upper and lower extremity movements with a mean score of 24.0 out of 64 and no participants demonstrating a floor effect. Of the 22 participants assessed with the CHOP-ATEND, 11 exhibited full head control with trunk supported as described by item 12 [49]. Not all motor function testing outcomes were appropriate for administration based on SMA severity level and therefore limited data are available for each motor outcome (Table 2).
Table 2
Motor function and patient-reported outcomes
| Motor function outcome | N | Mean (SD) | Median | Min | Max |
| HFMSE (total score 66) † | 21 | 3.6 (5.9) | 1 | 0 | 22 |
| RULM (total score 37) | 42 | 9.6 (7.6) | 9 | 0 | 29 |
| CHOP-ATEND (total score 64) † | 22 | 24.0 (12.7) | 25 | 1 | 44 |
| Patient-reported outcomes | N | Mean (SD) | Median | Min | Max |
| EK2 (total score 51) ‡ | 25 | 24.6 (10.3) | 23 | 9 | 41 |
| EAT-10 (total score 40) | 47 | 8.3 (7.9) | 7 | 0 | 30 |
| VHI Total (total score 120) | 47 | 22.4 (20.9) | 17 | 0 | 81 |
| Functional domain | 47 | 9.5 (6.9) | 8 | 0 | 28 |
| Physical domain | 47 | 7.5 (8.1) | 5 | 0 | 34 |
| Emotional domain | 47 | 5.4 (7.6) | 2 | 0 | 30 |
| VHI-10 (total score 40) | 47 | 8.6 (7.4) | 7 | 0 | 28 |
| Mild Severity Level (total score 0-30), n (%) | 34 (72.3) | ||||
| Moderate Severity Level (total score 31-60), n (%) | 10 (21.3) | ||||
| Severe Severity Level (total score 61-120), n (%) | 3 (6.4) |
SD, Standard Deviation; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; CHOP-ATEND, Children’s Hospital of Philadelphia Adult Test of Neuromuscular Disorders; EK2, Egen Klassifikation Scale 2; EAT-10, Eating Assessment Tool-10; VHI, Voice Handicap Index. † Limited data are available due to the appropriateness of outcome administration based on SMA severity level. ‡ Administered at Stanford University School of Medicine only.
Patient-reported issues of bulbar function
Ninety-four percent of SMA participants reported perceived voice deficits, noted primarily in 8 of the 30 VHI items (Fig. 1). The problems most frequently reported include items related to sound production and articulation: ‘people have difficulty understanding me in a noisy room’ (87.2%); ‘my voice makes it difficult for people to hear me’ (74.5%); and ‘people ask me to repeat myself when speaking face-to-face’ (72.3%). Percentages of item scoring responses for all individual items of the VHI are provided (Supplementary Fig. 1 and Supplementary Table 1). Seventy-two percent of participant VHI total scores fell in the mild severity level, 21% in the moderate severity level, and 6% in the severe severity level (Table 2).
Fig. 1
Items in which greater than 50% of participants reported any problems VHI, Voice Handicap Index; F, Functional Domain; P, Physical Domain; EAT-10, Eating Assessment Tool-10.
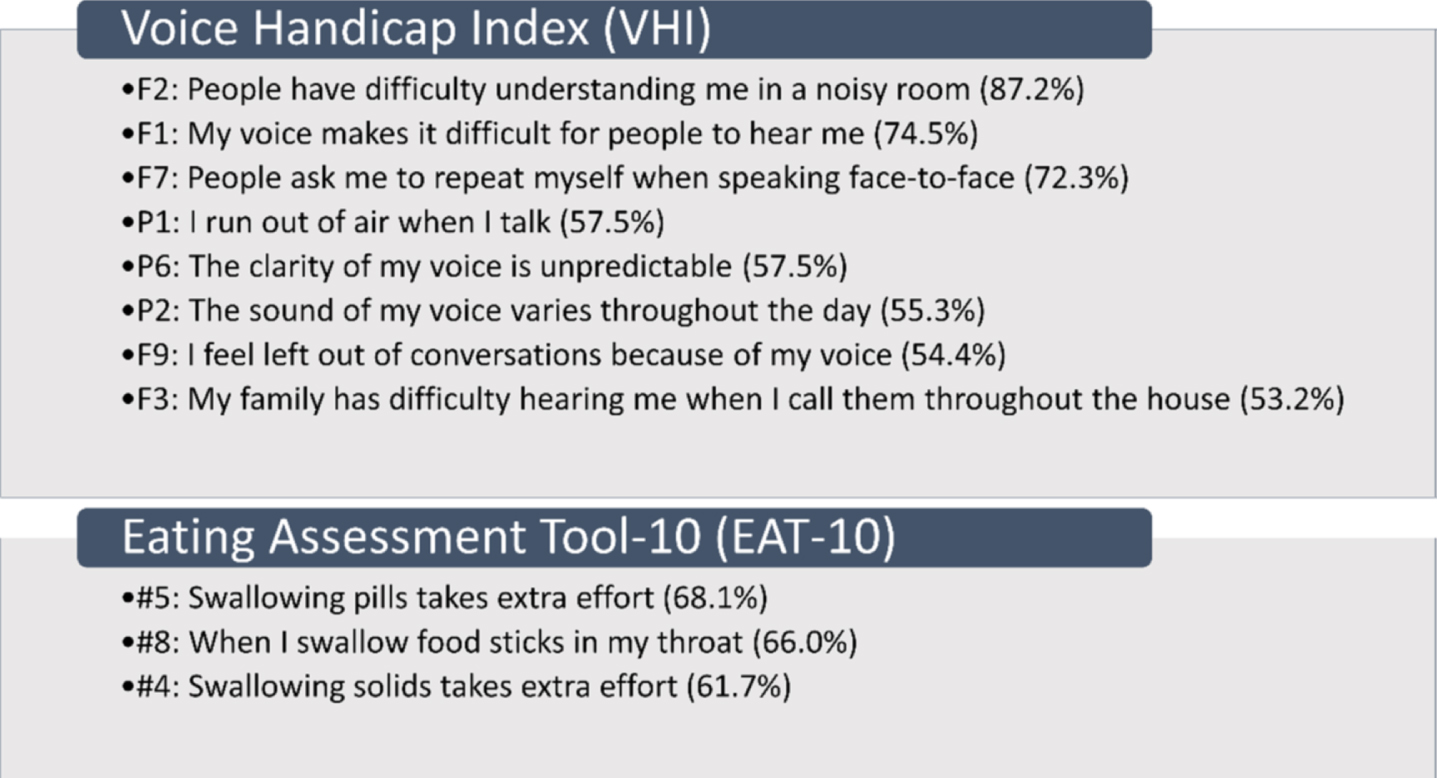
Eighty-five percent of SMA participants reported perceived swallowing deficits, noted primarily in 3 of the 10 EAT-10 items (Fig. 1). The problems most frequently reported include: ‘swallowing pills takes extra effort’ (68.1%); ‘when I swallow food sticks in my throat’ (66.0%); and ‘swallowing solids takes extra effort’ (61.7%). Percentages of item scoring responses for all individual items of the EAT-10 are provided (Supplementary Fig. 2 and Supplementary Table 2). Sixty-eight percent of participants scored greater than or equal to 3 out of 40 indicating a problem with swallowingefficiency/safety.
Fig. 2
Association between VHI and EAT-10 total scores (r = 0.66, p < 0.0001). VHI, Voice Handicap Index; EAT-10, Eating Assessment Tool-10.
Although the EK2 is not a PRO of bulbar function, there are a few items assessed that are applicable to voice/speech and swallowing problems. Sixty-four percent of participants reported they are unable to cough effectively (item 8), 40.0% do not exhibit powerful speech as they were unable to sing or speak loudly (item 9), 64.0% require head support when driving or sitting still in wheelchair (item 12), 56.0% modify their foods in any way in order to eat them (item 14), 72.0% are unable to consume a whole meal in the same time as others (item 15), and 64.0% experience problems with swallowing, including choking (item 16).
Bulbar function associations
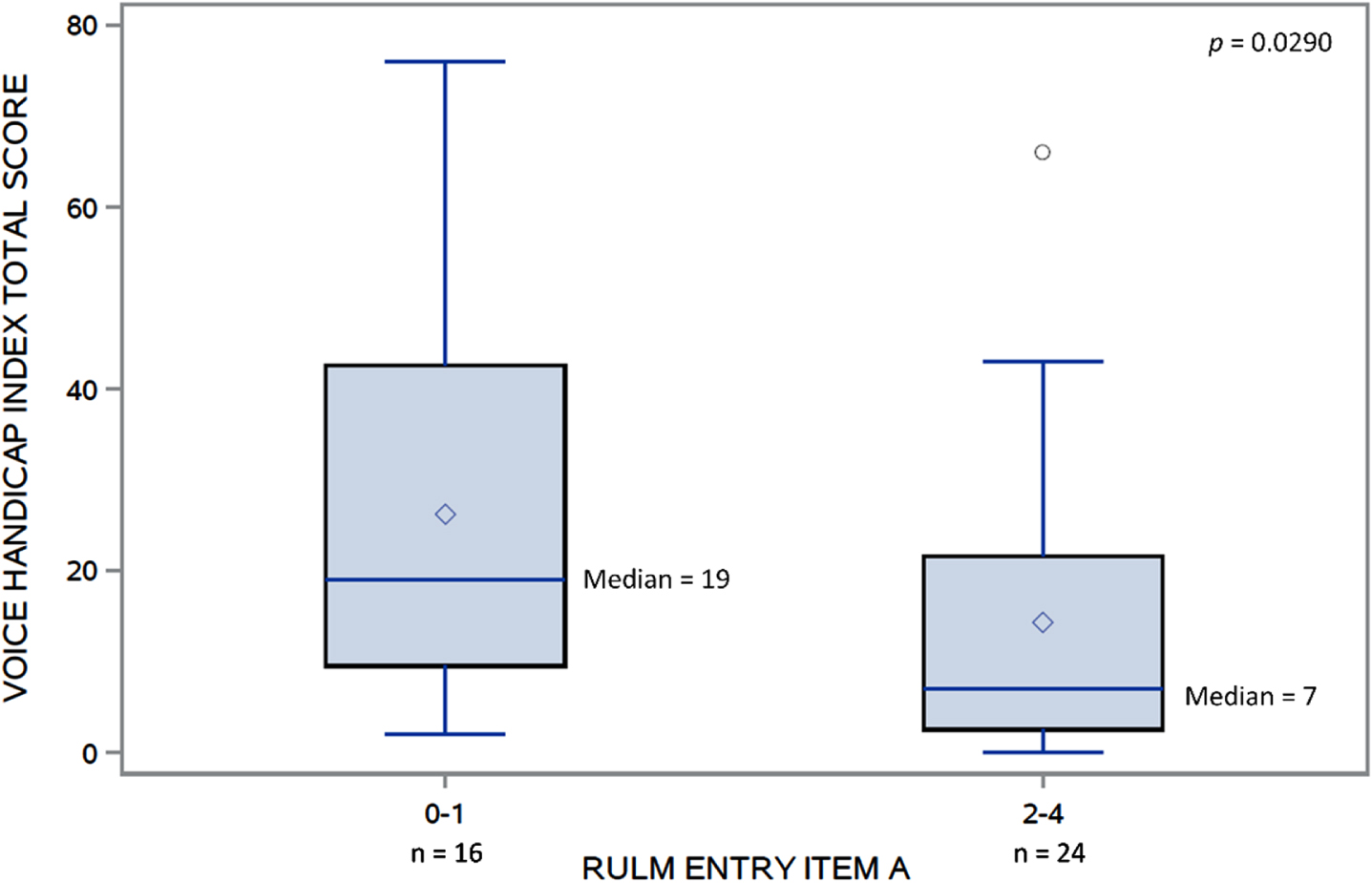
The VHI and EAT-10 total scores were moderately associated (rs = 0.66, p < 0.0001) (Fig. 2). Significant differences in VHI total scores were found (Fig. 3) between those who scored 0–1 (median = 19) and those who scored 2–4 (median = 7) on RULM entry item A (W = 407, p = 0.03). No other clinical characteristics were associated with the perceived voice issues.
Fig. 3
Distributions of VHI total score in those with RULM entry item A 0-1 and 2-4.VHI, Voice Handicap Index; RULM, Revised Upper Limb Module.
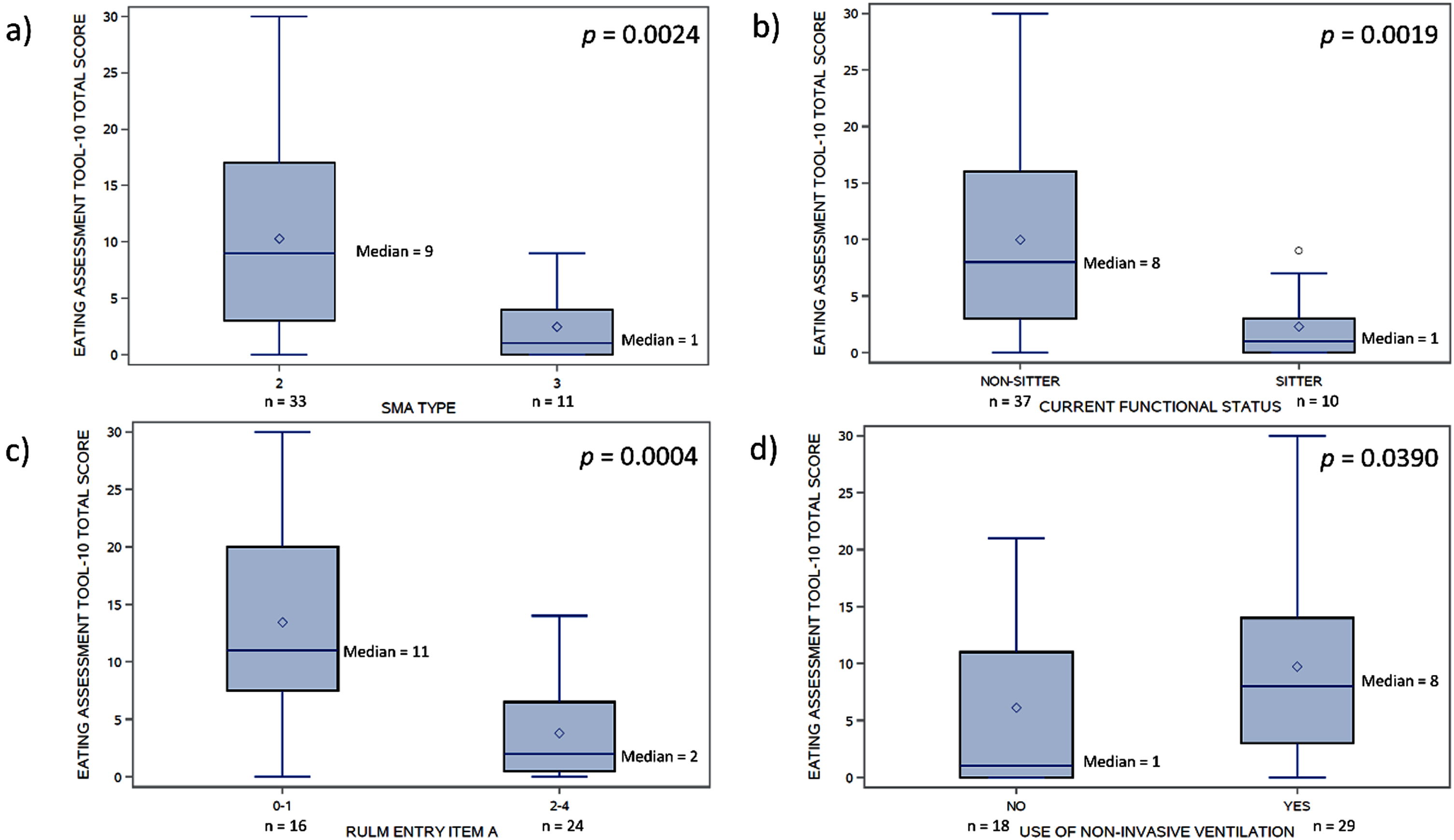
The EAT-10 total scores were weakly associated with age (rs = 0.32, p = 0.03) and symptom duration (rs = 0.35, p = 0.02). Significant differences in EAT-10 total scores (Fig. 4) were found between those with SMA type 2 (median = 9) and type 3 (median = 1) (W = 136, p = 0.002), those with current functional status of non-sitter (median = 8) and sitter (median = 1) (W = 121, p = 0.002), those with RULM entry item A score 0–1 (median = 11) and score 2–4 (median = 2) (W = 456, p = 0.0004), and those using non-invasive ventilation (median = 8) and not on any respiratory support (median = 1) (W = 338, p = 0.04).
Fig. 4
Distributions of EAT-10 total scores by: a) SMA type, b) current functional status, c) RULM entry item A, and d) use of non-invasive ventilation. EAT-10, Eating Assessment Tool-10; RULM, Revised Upper Limb Module.
DISCUSSION
In this investigation we evaluated voice and deglutition using two PROs of bulbar function among non-ambulatory patients with SMA. Our results demonstrate: 1) weaker individuals with SMA experience high rates of perceived deficits in specific components of communication and deglutition and their relationship to one another; and 2) perceived deglutition is associated with other metrics of disease characteristics such as SMA type, current upper extremity function, and use of respiratory support.
These results are consistent with those of previous investigations that have reported high rates of bulbar impairment among patients with SMA that are correlated with disease severity [6, 10]. Specifically, work by Chen et al. (2012) reported 77% of individuals with SMA type 2 reported dysphagia, while only 23% of those with SMA type 3 reported such deficits. Investigators in this study found the best predictors of bulbar integrity, however, to not be in the broad SMA classifications but in the more refined measures of motor function [10]. It is notable that the primary perceived dysphagia symptoms identified within these previous investigations as well as our current findings were not in the individual’s ability to ingest liquids, but in their ability to ingest solids. This is in contrast to deficits in swallowing liquids that are frequently reported in other patient populations (e.g., stroke), and may reflect differences in underlying impairments. For example, it is plausible that in contrast to impairments in airway closure, patients with SMA more frequently suffer from deficits in bolus propulsion and mandibular movement required for effective mastication. Future investigations examining the correlation between these PROs including the EAT-10 and instrumental assessments are required to better understand how perceived deficits relate to true physiology.
We found age to be weakly correlated with perceived swallowing ability, suggesting that swallowing and mastication complaints become more common with increasing age for individuals with SMA [6, 9, 10, 12]. This is consistent with the known progression of muscle weakness, atrophy, and fatty infiltration of SMA muscles that mediate mouth opening (lateral pterygoid) and mastication [9, 52]. Postmortem examination in SMA has shown degeneration of motor neurons in the brainstem as the trigeminal motor nucleus is affected in patients with severe SMA, but less than lower cranial nerve nuclei [53], perhaps due to differences in motor unit size [52]. Alternatively, specific bulbar muscle groups (or specific motor neuron pools) may differ in vulnerability to SMN deficiency [52]. Progressive weakness of neck and bulbar muscles can impact temporomandibular joint morphology, head balance, and function leading to contracture and reduced mouth opening. These limitations have been shown to occur at a very young age (7 years); therefore, it is important to investigate clinically whether earlier therapeutic intervention might be more efficacious in maintaining function [54].
Interestingly, our participants reported low mean perceived swallowing deficits despite the severity of our SMA cohort as 34.0% had a gastrostomy tube. Clinically, older individuals with SMA often report only supplemental use of their gastrostomy tubes for their meals and primarily eat by mouth. The frequency and volume of gastrostomy intake would be helpful information when interpreting these results, but this information was not collected in our cohort. Also, of clinical significance are the reported deficits in communication that impeded the ability to be understood. Despite the fact that the majority (57.4%) of our participants required respiratory support, the reports of mild voice difficulties in the older, chronic SMA cohort queried in this investigation are consistent with the limited previous investigations examining this area of function; in one such investigation, 27% of individuals with SMA reported ‘a weak voice’ [6]. The ability to effectively communicate, i.e., able to be understood by an unknown listener in a variety of environments, requires integrity within the respiratory system to enable sufficient breath support for phonation, as well as the neuromuscular and skeletal system to enable rapid and precise movement of the articulators including the lips, tongue, and jaw. While the evaluation of all of these separate components of communication was beyond the scope of this investigation, it is likely that the aforementioned cranial nerve degradation in advanced SMA influences all of these sub-systems. Determining the sub-systems involved in these functional communication deficits is certainly a critical next step in establishing future optimal rehabilitation approaches.
Utilization of more refined communication assessments is also important in fully understanding the true physiologic impairment. It is notable that unlike PROs examining motor control, an individual’s perceived communication effectiveness is largely based on their ability to effectively transmit their intended message to their listener. Research in other populations who have profound communication deficits indicates it is common for these individuals to limit communication to close family members and friends most familiar with the communicator’s unique communication limitations, or to use these communication partners to translate their message to those communication partners less familiar with their deficits. In both cases, it is common for the communicator to not perceive a profound communication impairment as their communicative intent gets transmitted effectively [55], further reiterating the need to combine these functional outcomes with refined clinical assessments.
The evaluation of bulbar function in SMA is becoming an increasingly greater area of interest especially given the need to detect clinically meaningful response to changes in treatment with the growing availability of FDA approved therapies [31, 56]. Previous studies have included PROs or semi-structured interviews to describe bulbar dysfunction, although many focused-on infants and children with more severe SMA type 1 [4, 57–59]. A recent German study examined patient-reported treatment expectations for adult SMA individuals receiving nusinersen [34]. Although adults had high expectation of treatment effectiveness regarding increases in muscle strength and disease stabilization, only 4% expected an improvement in bulbar function in the type 2 population, although perhaps it was not a prominent impairment in their cohort. Encouragingly, 13% of individuals reported experiencing improvement in ventilatory and bulbar function, both mainly in SMA type 2, and no individuals reported deterioration of bulbar or respiratory function over a follow-up period of ten months [34]. This highlights the need to identify dedicated bulbar assessments, both physiologic and PRO, in SMA [4, 31] as the use of PROs is tremendously beneficial in elucidating the functional impacts of the disease burden on individuals [4]; however, the reliance on perception likely limits their sensitivity to some extent [6, 9, 13].
Our study was limited given the novelty and application of using these standardized PROs (often used for clinical dysarthria and dysphagia in other disease groups) to assess bulbar function in SMA. Neither PRO used in this study was shown to clearly assess all aspects and sub-systems involved in the perception of voice and deglutition deficits, and our results need to be considered with caution as limited data were available for pediatric SMA, and for those participants under age 18 years (n = 9), the Pediatric Eating Assessment Tool (PEDI-EAT-10) and Pediatric Voice Handicap Index (pVHI) for caregivers would have been more appropriate and informative. There are currently no longitudinal data available on either the VHI or EAT-10 in SMA, nor any investigations of validity, reliability, or minimal detectable changes, which will be necessary to help assess normal patient variability and determine clinically meaningful changes with treatment. The perceived impaired vocal communication issues our patients reported might reflect a complex combination of dysphonia, dysarthria, impaired stamina, and ventilatory insufficiency making it challenging to evaluate these impairments with a patient-reported assessment of voice. Further research is needed to identify relevant items to develop a SMA-specific PRO that yields a reliable, valid, responsive, and clinically relevant measure of bulbar function.
Perceived voice and swallowing issues in SMA are common yet may be underreported in the chronic SMA cohort. Our findings highlight the need for objective and quantitative measures of these aspects of this neurodegenerative disorder. Future studies of a larger sample may provide more insight on the ability of these PROs to measure a range of bulbar function, and a supplementary review of other currently available PROs may enable the development of a better disease-specific measure of bulbar function, which may be more sensitive to the effects of disease-modifying treatments for SMA. Multidisciplinary efforts are currently underway including collaboration of speech language pathologists, physical/occupational therapists, neurologists, and dentists with expertise in SMA to identify and refine assessment tools for relevance and evaluation of bulbar function in SMA [60]. The ideal tool should be standardized and aim to determine the presence, severity, and progression of dysarthria and dysphagia, and help to identify rehabilitation and management interventions, as well as quantify treatment effects[24, 61].
ACKNOWLEDGMENTS
We gratefully thank the PNCR/iSMAC study group of investigators, coordinators, and therapists as well as the patients and families who participated. The authors wish to thank the Cure SMA Industry Collaboration for the funding that supported this project. The members of the Cure SMA Industry Collaboration during the time of program development were, Astellas, Biogen, Cytokinetics Inc., Genentech/ Roche Pharmaceuticals, Novartis Gene Therapies, Novartis, and Scholar Rock, Inc. This work was supported by Cure SMA, which had no involvement in the preparation of the article, study design, collection, analysis and interpretation of data, or in the writing of the report.
CONFLICTS OF INTEREST
S. D. Y. has been a member of advisory boards for Biogen, Roche/Genentech, and Scholar Rock; received personal compensation for activities with Biogen, Cure SMA, and Scholar Rock as a consultant; and received research support from Cure SMA and the SMA Foundation.
A. P. has received research support from the SMA Foundation; served as a consultant for Biogen; and has served on advisory boards for AveXis, Biogen, Roche, and Scholar Rock.
T. D. has been an advisory board member of Cure SMA, Biogen, Cytokinetics, Roche/Genentech, Scholar Rock, Sarepta, Bristol Myers Squibb, Audentes, and Novartis and served as a consultant for Roche, Audentes, and Biohaven; receives research support and grants from Biogen and Ionis.
K. M. has received grant funding and consulting income from Biogen and AveXis but has no financial interest in these companies. She is founder and scientific director of nuBorn Medical and Science Stand. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
S. S. has received consulting fees from Roche, Genetech, Inc., doc.ai.
E. M. has no disclosures to report.
C. D. has no disclosures to report.
W. T. has no disclosures to report.
D. P. has no disclosures to report.
A. L. has no disclosures to report.
A. R. has no disclosures to report.
C. W. has served on advisory boards and speaker bureaus for Biogen.
W. M. has no disclosures to report.
M. M. has received research support from the NIH, FDA, Cure SMA, and PTC Therapeutics; served as a consultant for Fulcrum Therapeutics, Inc. and NeuroDerm, Ltd.; and served on Data and Safety Monitoring Boards for NIH, Eli Lilly and Company, Catabasis Pharmaceuticals, Inc., Vaccinex, Inc., Neurocrine Biosciences, Inc., Voyager Therapeutics, Prilenia Therapeutics Development, Ltd., ReveraGen BioPharma, Inc., and NS Pharma, Inc
B. T. D. has been a member of advisory boards for AveXis, Biogen, Cytokinetics, Dynacure, PTC, Roche, and Sarepta; received research support from Cure SMA, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Slaney Family Fund for SMA, the SMA Foundation, and the Working on Walking Fund; and received grants from Biogen and Ionis Pharmaceuticals, Inc. during the ENDEAR, CHERISH, CS2, CS12, and CS11 studies, and from Cytokinetics, Fibrogen, PTC, Roche, Santhera, Sarepta, and Summit, and reports no personal financial interests in these companies.
J. W. D. has received consulting fees from Audentes, Biogen, Ionis Pharmaceuticals, Roche/Genentech Pharmaceuticals, Cytokinetics, Pfizer, AveXis, AMO Pharmaceuticals, Sarepta Therapeutics, Santhera Pharmaceuticals, and Scholar Rock. He has received grant support from Biogen; Ionis Pharmaceuticals, Cytokinetics, Roche Pharmaceuticals, AveXis, Sanofi-Genzyme, Sarepta Therapeutics, and Scholar Rock.
REFERENCES
[1] | Darras BT , Markowitz JA , Monani UR , et al. Chapter 8 –Spinal Muscular Atrophies. In: Basil T. Darras Monique M. Ryan, Darryl C. De Vivo HRJ (ed) Neuromuscular Disorders of Infancy, Childhood, and Adolescence (Second Edition). San Diego: Academic Press, 117–45. |
[2] | Dubowitz V . Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. (1995) ;5: :3–5. |
[3] | Kooi-van Es M , Erasmus CE , de Swart BJM , et al. Dysphagia and Dysarthria in Children with Neuromuscular Diseases, a Prevalence Study. J Neuromuscul Dis. (2020) ;7: :287–95. |
[4] | Berti B , Fanelli L , de Sanctis R , et al. Oral and Swallowing Abilities Tool (OrSAT) for Type 1 SMA Patients: Development of a New Module. J Neuromuscul Dis. (2021) ;8: :589–601. |
[5] | Mercuri E , Finkel RS , Muntoni F , et al. Diagnosis and management of spinal muscular atrophy: Part Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. (2018) ;28: :103–15. |
[6] | van der Heul AMB , Wijngaarde CA , Wadman RI , et al. Bulbar Problems Self-Reported by Children and Adults with Spinal Muscular Atrophy. J Neuromuscul Dis. (2019) ;6: :361–8. |
[7] | Lloyd Morris EH , Estilow T , Glanzman AM , et al. Improving Temporomandibular Range of Motion in People With Duchenne Muscular Dystrophy and Spinal Muscular Atrophy. Am J Occup Ther Off Publ Am Occup Ther Assoc. (2050) ;74: :7402205080p1-10. |
[8] | Kooi-van Es M , Erasmus CE , Houwen S , et al. Early detection of dysphagia and dysarthria in children with neuromuscular disorders: Diagnostic accuracy of a Screeninglist for Physicians. J Pediatr Rehabil Med. (2020) ;13: :17–23. |
[9] | van Bruggen HW , Wadman RI , Bronkhorst EM , et al. Mandibular dysfunction as a reflection of bulbar involvement in SMA type 2 and 3. Neurology. (2016) ;86: :552–9. |
[10] | Chen Y-S , Shih H-H , Chen T-H , et al. Prevalence and risk factors for feeding and swallowing difficulties in spinal muscular atrophy types II and III. J Pediatr. (2012) ;160: :447–451.e1. |
[11] | Willig TN , Paulus J , Lacau Saint Guily J , et al. Swallowing problems in neuromuscular disorders. Arch Phys Med Rehabil. (1994) ;75: :1175–81. |
[12] | Messina S , Pane M , De Rose P , et al.Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. (2008) ;18: :389–93. |
[13] | de Groot IJ , de Witte LP . Physical complaints in ageing persons with spinal muscular atrophy. J Rehabil Med. (2005) ;37: :258–62. |
[14] | Bartels B , Habets LE , Stam M , et al. Assessment of fatigability in patients with spinal muscular atrophy: development and content validity of a set of endurance tests. BMC Neurol. (2019) ;19: :21. |
[15] | Chiriboga CA , Swoboda KJ , Darras BT , et al. Results from a phase 1 study of nusinersen (ISIS-SMN Rx) in children with spinal muscular atrophy. Neurology. (2016) ;86: :890–7. |
[16] | Finkel RS , Chiriboga CA , Vajsar J , et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. (2016) ;388: :3017–26. |
[17] | Finkel RS , Mercuri E , Darras BT , et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. (2017) ;377: :1723–32. |
[18] | Mercuri E , Darras BT , Chiriboga CA , et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. (2018) ;378: :625–35. |
[19] | Darras BT , Chiriboga CA , Iannaccone ST , et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology. (2019) ;92: :e2492–e2506. |
[20] | Mendell JR , Al-Zaidy S , Shell R , et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med. (2017) ;377: :1713–22. |
[21] | Baranello G , Darras BT , Day JW , et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N Engl J Med. (2021) ;384: :915–23. |
[22] | Darras BT , Masson R , Mazurkiewicz-Bełdzińska M , et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N Engl J Med. (2021) ;385: :427–35. |
[23] | Day JW , Finkel RS , Chiriboga CA , et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. (2021) ;20: :284–93. |
[24] | Duong T , Wolford C , McDermott MP , et al. Nusinersen Treatment in Adults With Spinal Muscular Atrophy. Neurol Clin Pract. (2021) ;11: :e317–27. |
[25] | Hagenacker T , Wurster CD , Günther R , et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. (2020) ;19: :317–25. |
[26] | Jochmann E , Steinbach R , Jochmann T , et al. Experiences from treating seven adult 5q spinal muscular atrophy patients with Nusinersen. Ther Adv Neurol Disord. (2020) ;13: :1756286420907803. |
[27] | Moshe-Lilie O , Visser A , Chahin N , et al. Nusinersen in adult patients with spinal muscular atrophy: Observations from a single center. Neurology. (2020) ;95: :e413–6. |
[28] | Yeo CJJ , Simeone SD , Townsend EL , et al. Prospective Cohort Study of Nusinersen Treatment in Adults with Spinal Muscular Atrophy. J Neuromuscul Dis. (2020) ;7: :257–68. |
[29] | Walter MC , Wenninger S , Thiele S , et al. Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3 – A Prospective Observational Study. J Neuromuscul Dis. (2019) ;6: :453–65. |
[30] | Veerapandiyan A , Eichinger K , Guntrum D , et al. Nusinersen for older patients with spinal muscular atrophy: A real-world clinical setting experience. Muscle and Nerve. (2020) ;61: :222–6. |
[31] | McGrattan KE , Graham RJ , DiDonato CJ , et al. Dysphagia Phenotypes in Spinal Muscular Atrophy: The Past, Present, and Promise for the Future. Am J Speech-Language Pathol. (2021) ;30: :1008–22. |
[32] | Slayter J , Hodgkinson V , Lounsberry J , et al. A Canadian Adult Spinal Muscular Atrophy Outcome Measures Toolkit: Results of a National Consensus using a Modified Delphi Method. J Neuromuscul Dis. (2021) ;8: :579–88. |
[33] | Rouault F , Christie-Brown V , Broekgaarden R , et al. Disease impact on general well-being and therapeutic expectations of European Type II and Type III spinal muscular atrophy patients. Neuromuscul Disord. (2017) ;27: :428–38. |
[34] | Osmanovic A , Ranxha G , Kumpe M , et al. Treatment expectations and patient-reported outcomes of nusinersen therapy in adult spinal muscular atrophy. J Neurol. (2020) ;267: :2398–407. |
[35] | Mercuri E , Finkel R , Scoto M , et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscul Disord. (2019) ;29: :794–9. |
[36] | Jacobson BH , Johnson A , Grywalski C , et al. The Voice Handicap Index (VHI). Am J Speech-Language Pathol. (1997) ;6: :66–70. |
[37] | Cosyns M , Mortier G , Janssens S , et al. Voice-related quality of life in adults with neurofibromatosis type 1. J Voice. (2012) ;26: :e57–62. |
[38] | Belafsky PC , Mouadeb DA , Rees CJ , et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. (2008) ;117: :919–24. |
[39] | Plowman EK , Tabor LC , Robison R , et al. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. (2016) ;28: :85–90. |
[40] | Printza A , Goutsikas C , Triaridis S , et al. Dysphagia diagnosis withquestionnaire, tongue strength measurement, and FEES in patientswith childhood-onset muscular dystrophy. Int J PediatrOtorhinolaryngol. (2019) ;117: :198–203. |
[41] | Cheney DM , Siddiqui MT , Litts JK , et al. The Ability of the 10-Item Eating Assessment Tool (EAT-10) to Predict Aspiration Risk in Persons With Dysphagia. Ann Otol Rhinol Laryngol. (2015) ;124: :351–4. |
[42] | Arslan SS , Demir N , Kilinç HE , et al. The Ability of the Eating Assessment Tool-10 to Detect Aspiration in Patients With Neurological Disorders. J Neurogastroenterol Motil. (2017) ;23: :550–4. |
[43] | Glanzman AM , O’Hagen JM , McDermott MP , et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. (2011) ;26: :1499–507. |
[44] | O’Hagen JM , Glanzman AM , McDermott MP , et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord. (2007) ;17: :693–7. |
[45] | Mazzone E , Bianco F , Martinelli D , et al. Assessing upper limb function in nonambulant SMA patients: development of a new module. Neuromuscul Disord. (2011) ;21: :406–12. |
[46] | Mazzone ES , Mayhew A , Montes J , et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle and Nerve. (2017) ;55: :869–74. |
[47] | Sivo S , Mazzone E , Antonaci L , et al. Upper limb module in non-ambulant patients with spinal muscular atrophy: 12 month changes. Neuromuscul Disord. (2015) ;25: :212–5. |
[48] | Kichula E , Duong T , Glanzman A , et al. Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) Feasibility for Individuals with Severe Spinal Muscular Atrophy II (S46.004). |
[49] | Glanzman AM , McDermott MP , Montes J , et al. Validation of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. (2011) ;23: :322–6. |
[50] | Steffensen B , Hyde S , Lyager S , et al. Validity of the EK scale: a functional assessment of non-ambulatory individuals with Duchenne muscular dystrophy or spinal muscular atrophy. Physiother Res Int J Res Clin Phys Ther. (2001) ;6: :119–34. |
[51] | Steffensen BF , Lyager S , Werge B , et al. Physical capacity in non-ambulatory people with Duchenne muscular dystrophy or spinal muscular atrophy: a longitudinal study. Dev Med Child Neurol. (2002) ;44: :623–32. |
[52] | Wadman RI , van Bruggen HW , Witkamp TD , et al. Bulbar muscle MRI changes in patients with SMA with reduced mouth opening and dysphagia. Neurology. (2014) ;83: :1060–6. |
[53] | Byers RK , Banker BQ . Infantile muscular atrophy. Arch Neurol. (1961) ;5: :140–64. |
[54] | van Bruggen HW , van den Engel-Hoek L , van der Pol WL , et al. Impaired mandibular function in spinal muscular atrophy type II: need for early recognition. J Child Neurol. (2011) ;26: :1392–6. |
[55] | van den Engel-Hoek L , de Groot IJM , de Swart BJM , et al. Feeding and Swallowing Disorders in Pediatric Neuromuscular Diseases: An Overview. J Neuromuscul Dis. (2015) ;2: :357–69. |
[56] | Brakemeier S , Stolte B , Thimm A , et al. Assessment of Bulbar Function in Adult Patients with 5q-SMA Type 2 and 3 under Treatment with Nusinersen. Brain Sci. (2021) ;11: :1244. |
[57] | Zappa G , LoMauro A , Baranello G , et al. Intellectual abilities, language comprehension, speech, and motor function in children with spinal muscular atrophy type 1. J Neurodev Disord. (2021) ;13: :9. |
[58] | Choi Y-A , Suh DI , Chae J-H , et al. Trajectory of change in the swallowing status in spinal muscular atrophy type I. Int J Pediatr Otorhinolaryngol. (2020) ;130: :109818. |
[59] | van der Heul AMB , Cuppen I , Wadman RI , et al. Feeding and Swallowing Problems in Infants with Spinal Muscular Atrophy Type an Observational Study. J Neuromuscul Dis. (2020) ;7: :323–30. |
[60] | Dunaway Young S , Muni Lofra R , Coratti G , et al. Development of an International SMA Bulbar Function Assessment for Inter-professional Administration. Cure SMA Virtual PT Poster Walkthrough Session June. 2021. |
[61] | Audag N , Goubau C , Toussaint M , et al. Screening and evaluation tools of dysphagia in children with neuromuscular diseases: a systematic review. Dev Med Child Neurol. (2017) ;59: :591–6. |




