Characterizing the Occurrence of Key Clinical Milestones in Duchenne Muscular Dystrophy in the United States Using Real-World Data
Abstract
Background:
Data on the clinical course of Duchenne muscular dystrophy (DMD) exist from well-characterized clinical cohorts but estimates from real-world populations are fewer.
Objective:
The objective was to estimate the prevalence of key clinical milestones by age, among real-world commercially-insured DMD patients in the United States.
Methods:
MarketScan claims (2013–2018) were used to identify males with DMD. The percentages with wheelchair use or experiencing scoliosis, neurologic/neuropsychiatric involvement, cardiomyopathy, and respiratory involvement were tabulated; as were the median (interquartile range [IQR]) ages at first observed occurrence within the claims data.
Results:
Among DMD patients (n = 1,964), the median (IQR) baseline age was 15 (9–21) years, and median follow-up was 1.7 years. Wheelchair use was observed in 55% of those aged 8 to 13 years at cohort entry; scoliosis, among 38% of those 8 to 10 and 52% of those 11 to 13 years; neurologic/neuropsychiatric involvement, among 41–43% of those 8 to 13 years; respiratory involvement, among 45% of those 14 to 19 years; and cardiomyopathy, among 68% of those 14 to 16 and 58% of those 17 to 19 years.
Conclusions:
The prevalence of key clinical milestones across ages was broadly consistent with published findings. Variability in estimates reflect clinical heterogeneity; these contemporary estimates from real-world data help characterize clinical outcomes in DMD.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a severe X-linked progressive neuromuscular degeneration caused by mutations in the gene for dystrophin, a protein required for the structural integrity of muscle cells [1–4]. Affected patients typically present in early childhood with gait abnormalities, muscle weakness, and delayed motor and cognitive function [1, 5–8]. Several well-described clinical cohorts in the United States (US) have documented the inexorable progression of DMD, including from the Cooperative International Neuromuscular Research Group (CINRG), [9] Duchenne Registry, [10] and Centers for Disease Control and Prevention’s Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) [11]. Although there is heterogeneity in the exact timing of events, patients inevitably experience key clinical milestones in their progression. Muscular weakness leads to loss of ambulation (LOA), wheelchair use, and scoliosis in late childhood [12–14]. Loss of strength in active breathing muscles contributes to respiratory insufficiency and the need for ventilation in the teenage years [15]. Cardiomyopathy also develops in late adolescence and the progression of DMD culminates with early mortality in the third or fourth decade of life [13, 16–19].
While estimates of the frequency and approximate timing of key clinical milestones are available from these well-defined US clinical cohorts, [9–11] estimates from generalizable population-based real-world cohorts to characterize the progression experienced by those with DMD in routine clinical practice are limited. Administrative claims studies are based on healthcare usage data that document patient diagnoses, procedures performed, medications dispensed, physicians visited, and inpatient stays. Such data are often used to characterize patient populations, understand treatment patterns, and assess aspects of the clinical or economic burden of a health condition from a large population perspective [20]. Claims database assessments for DMD however are limited. At the time of this study, the only published claims-based study providing estimates of the clinical burden of DMD was prior to the widespread use of corticosteroids, and included a small number of patients from one commercial plan only [21]. The objective of this study was to estimate the prevalence of key clinical milestones by age among commercially-insured patients with DMD in the US using real-world data.
MATERIALS AND METHODS
This study was a US-based, real-world, retrospective cohort study to describe the clinical course of patients with DMD, treated under commercial health insurance plans. The study cohort and entry criteria were described previously in a study examining the characteristics of and economic burden among those with DMD in the US [22].
Data source
Data were derived from the IBM MarketScan commercial databases, [23] a set of large, nationally representative healthcare databases. The commercial database contains data for employer-sponsored, privately insured employees and their families, [23] and a total of 78,371,462 unique individuals were covered within the 5-year period from 2013 to 2018. These data have been widely validated for clinical, pharmacoepidemiological and pharmacoeconomic research [24–32].
Study sample
MarketScan claims (2013–2018) were used to identify the eligible DMD population, which included all males≤30 years of age, with a muscular dystrophy (MD) International Classification of Diseases (ICD)-9 code 359.1, MD-related ICD-10 code G71.0, or the Becker/Duchenne MD-specific ICD-10 code G71.01, in any position on≥2 outpatient DMD medical claims (with > 30 days between claims) or as the primary or secondary diagnosis of≥1 inpatient claim; or, a dispensation for eteplirsen. To remove those likely to have other congenital dystrophies, individuals with the following were excluded (see Table A1 and A.2 for applicable codes): [21]≥2 medical claims for ventilator use separated by at least 180 days before age 6 years;≥1 medical claim with a Current Procedural Terminology (CPT) code for an orthopedic procedure on the foot before age 3 years;≥1 medical claim for a power, power-assist, and/or manual wheelchair before age 5 years;≥1 medication fill (NDC 64406005801) or an injection code (HCPCS J2326) for nusinersen at any point during the study period.
To understand the impact of both follow-up time and age on the observation of relevant outcomes, patients were stratified by age at cohort entry (8 to 10 years, 11 to 13 years, 14 to 16 years, and 17 to 19 years). Within these stratified analyses, to ensure some amount of follow-up was available on all of the patients contributing to these analyses, a one-year minimum follow-up requirement was imposed.
Study design
The identification period for study enrollment was April 1, 2013 to March 31, 2018. All DMD cohort members were enrolled at their index visit, the first eligible inpatient or outpatient visit with a relevant diagnostic code or dispensation for the DMD-specific medication, eteplirsen. All cohort members were followed until death (if known), deregistration, or the end of the follow-up period unless otherwise specified in analyses.
Identifying key clinical milestones
Key clinical milestones of interest included LOA, scoliosis, cardiomyopathy, respiratory involvement, and neurologic or neuropsychiatric involvement. As there is no widely-used or well-validated diagnosis code for LOA, it was identified via the proxy outcome of procedural codes for wheelchair use. Scoliosis was identified by either diagnosis codes for scoliosis or procedural codes for spinal surgery. Cardiomyopathy was identified by diagnosis codes for cardiomyopathy or heart failure, or any dispensations for angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and/or diuretics (spironolactone or eplerenone). For respiratory involvement, diagnostic codes for respiratory failure as well as procedural codes for tracheostomy, assisted ventilation, and selected codes for pulmonary management were used; more severe respiratory conditions were identified by the subset of diagnostic codes for respiratory failure and the procedural code for tracheostomy only. Finally, identification of neurologic and/or neuropsychiatric involvement was based on diagnosis codes for attention deficit disorders, learning disabilities, pervasive developmental disorders, or behavioral disorders; and procedural codes for neuropsychological testing. See Appendix Table A.2 for details of how the clinical outcomes were identified.
Analysis
To describe the study sample, consistent with the previous analyses, [22] the demographic characteristics were summarized, and median duration of follow-up estimated. Health status over the follow-up period was summarized by the percentage observed with at least one prescription for corticosteroids; comorbidity burden (using the median with interquartile range [IQR] Elixhauser Index score, [33] and the frequency of individual Elixhauser comorbidities); and the prevalence of other key comorbidities of interest. These key comorbidities were identified based on literature review, and including respiratory infectious diseases, anxiety, asthma, depression, fractures/osteoporosis, epilepsy, diabetes mellitus and cystic fibrosis. See Appendix Table A.2 for codes.
Primary analyses
In the primary (non-age-restricted) analyses, the percentages of patients who experience key clinical milestones were tallied and the median (IQR) ages at the first observation during the study timeframe of each outcome were calculated within the available follow-up. Due to the limited follow-up available, incidence of events could not be ascertained as the window of observation per individual will impact whether or not an initial event is observed (Appendix Figure A.1).
Age-restricted analyses
To understand the impact of both follow-up time and age on the likelihood of capturing prevalence of patients experiencing key clinical milestones in each cohort, age-restricted analyses were performed. From the cohorts identified by those restrictions, the percentage of patients with wheelchair use or who experienced scoliosis, and neurologic and/or neuropsychiatric involvement was estimated among those aged 8 to 10 and 11 to 13 at cohort entry; and the percentage experiencing cardiomyopathy, respiratory involvement, or severe respiratory outcomes among those 14 to 16 and 17 to 19 years at cohort entry. These age categories were selected for clinical relevance, based on evidence from published studies on the mean age at occurrence of LOA, [34, 35] respiratory involvement, [36, 37] and cardiomyopathy in the US. [13, 36, 37] Estimates of the prevalence of key clinical milestones was compared between the age-restricted cohorts and the overall cohort. Age-restricted Kaplan-Meier (KM) analyses were also conducted to account for censoring to better understand the prevalence of events captured by follow-up time available.
Sensitivity analyses
The impact of key design assumptions on the results were tested in sensitivity analyses. Two age-restricted sensitivity analyses were performed. Firstly, the minimum continuous follow-up requirement of one year in the primary age-restricted analysis was removed, to minimize the impact of any time-related biases (i.e. requiring a minimum of one-year of follow-up could help contribute to higher observed rates of clinical outcomes). Secondly, the observation window was reduced to just one year, without a minimum continuous follow-up requirement. This was to allow for an estimation of the lower-end of the potential range of estimates of the prevalence of key clinical milestones among age-restricted patients, to better understand the impact of follow-up time.
To increase the likelihood of eliminating uncertain DMD cases, the criteria for identifying DMD was tested by applying three definitions: 1) restricting the cohort to those < 18 years old at index, to eliminate individuals with other potential MDs 2) adding an inclusion criterion of having≥1 diagnosis from a specialist, and 3) adding an exclusion criterion of having≥2 claims for any of the following: myoneural disorder (358.x or G70.x), Guillain-Barre syndrome (357.0 or G61.0), or hereditary motor and sensory neuropathy (356.2 or G60.0). For all of these sensitivity analyses around DMD definitions, the median age at and proportion experiencing key clinical milestones was tabulated and compared to those of the primary analyses.
Data in the MarketScan commercial databases are de-identified and are compliant with the Health Insurance Portability and Accountability Act (HIPAA) regulations, thus, Institutional Review Board approval was not required to conduct this study.
RESULTS
Patient characteristics and health status
Table 1 summarizes patient characteristics and health status over the study period. In total, 1,964 patients with DMD were identified in the primary analysis. Median (IQR) age at cohort entry was 15 (9–21) years, with a median of 1.7 years of follow-up. At least one corticosteroid dispensation was observed among 38.8% over available follow-up; additional breakdown of corticosteroid use by age group at cohort entry is included in the appendix (Appendix Figure A.2). Over the study period, the median (IQR) unweighted Elixhauser score was 1 (0–3), and cardiac and pulmonary comorbidities were the most frequent individual Elixhauser comorbidities observed. Of the other key comorbidities, respiratory infectious disease was the most common (observed in 48.8% of the cohort) followed by anxiety disorders (15.4%) and asthma (14.5%).
Table 1
Patient demographics and characteristics of the DMD cohort
| DMD cohort | |
| (n = 1,964) | |
| Follow-up time, median (IQR), years | 1.7 (0.9–3.4) |
| Age at cohort entry, median (IQR), years | 15 (9–21) |
| Age at cohort entry categories, n(%) | |
| 0 to 3 | 88 (4.5) |
| 4 to 7 | 260 (13.2) |
| 8 to 13 | 497 (25.3) |
| 14 to 17 | 379 (19.3) |
| 18 to 25 | 527 (26.8) |
| 26+ | 213 (10.8) |
| Region, n(%) | |
| Northeast | 357 (18.2) |
| North central | 511 (26.0) |
| South | 738 (37.6) |
| West | 354 (18.0) |
| Unknown | 4 (0.2) |
| Corticosteroid* use during follow-up, n(%) | |
| Any corticosteroid use | 762 (38.8) |
| None | 1,202 (61.2) |
| Unweighted Elixhauser score over follow-up, median (IQR) | 1 (0–3) |
| Elixhauser comorbidities observed during follow-up, n(%) | |
| Congestive heart failure | 585 (29.8) |
| Cardiac arrhythmias | 525 (26.7) |
| Chronic pulmonary disease | 428 (21.8) |
| Other neurological disorders | 399 (20.3) |
| Depression | 299 (15.2) |
| Fluid and electrolyte disorders | 255 (13.0) |
| Obesity | 254 (12.9) |
| Valvular disease | 212 (10.8) |
| Hypertension, uncomplicated | 198 (10.1) |
| Paralysis | 181 (9.2) |
| Weight loss | 176 (9.0) |
| Other key comorbidities observed during follow-up, n(%) | |
| Respiratory infectious disease | 958 (48.8) |
| Anxiety, dissociative, somatoform disorders | 302 (15.4) |
| Asthma | 285 (14.5) |
| Depressive disorder | 185 (9.4) |
| Fracture and/or osteoporosis | 148 (7.5) |
| Epilepsy | 89 (4.5) |
| Diabetes mellitus | 56 (2.9) |
| Cystic fibrosis | 39 (2.0) |
Note: Only Elixhauser comorbidity categories with a prevalence≥5% are presented*including prednisone, prednisolone, and deflazacort; Abbreviations: IQR = interquartile range; DMD = Duchenne muscular dystrophy.
The prevalence of of key clinical milestones
Among the DMD cohort from the primary analysis, neurologic and/or neuropsychiatric involvement was observed among 27%, wheelchair use among 45%, scoliosis among 30%, cardiomyopathy among 46%, and respiratory involvement among 34%. The ages at first observed claim for each key clinical milestone in this cohort are presented in Fig. 1. The median (IQR) age at neurologic and/or neuropsychiatric involvement was first recorded at 11 (8–16) years old; wheelchair use was 16 (12–22) years old; for scoliosis, 16 (12–20) years old; for cardiomyopathy, 17 (13–22) years old; and respiratory involvement, 19 (15–24) years old. Age-restricted analyses were also performed to estimate the occurrence of those key outcomes stratified by age at cohort entry. Compared to the overall cohort, the prevalence of key clinical milestones was higher among the age- and time-restricted subset (Table 2), as expected given that the age and time restrictions aimed to focus the observation window on the time period where these outcomes were more likely to have first occurred. As the higher observed prevalence could also be due, in part, to the minimum follow-up requirement, a variation of this analysis was explored where the minimum follow-up requirement was removed (sensitivity analysis #1), and varied to explore the impact of a shorter observation window (sensitivity analysis #2). Due to the nature of age-restriction, the median age at outcome was largely correlated with the respective age restriction across all scenarios.
Fig. 1
Age at first record of key clinical milestones, and the number and percentage observed with each, among patients with DMD. *N/N involvement: neurologic/neuropsychiatric involvement; Box represents the interquartile range (IQR), from the first quartile (Q1:25%) to the third quartile (Q3:75%), with the line in the middle depicting the median; dots represent outliers, defined as greater than the range of Q1 - 1.5×the interquartile range to Q3 + 1.5×the IQR, as bounded by the extended lines.
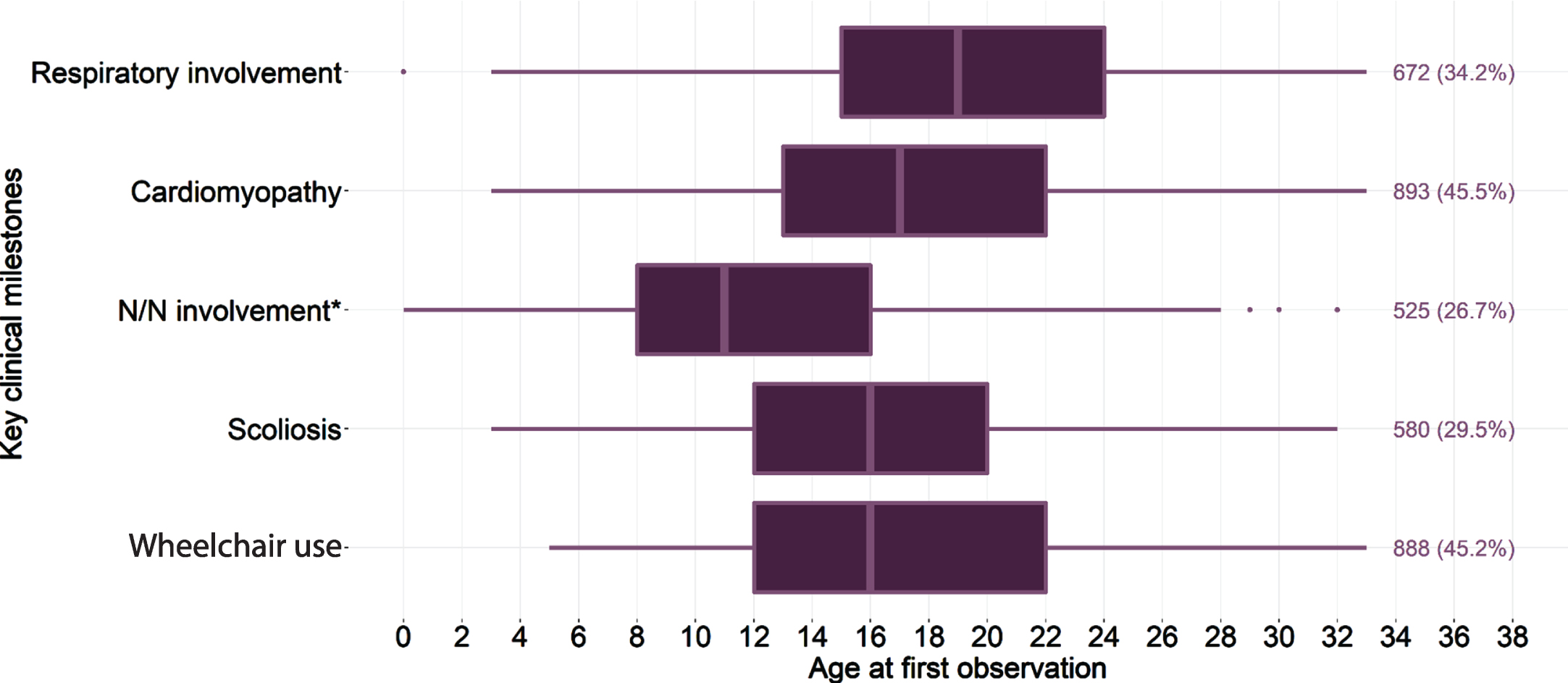
Table 2
The percentage with a record of a key clinical milestone, and median age per milestone, among patients with DMD stratified by age, follow-up, and observation window
| Age at cohort entry | Median (IQR) follow-up | Key clinical milestone | n(%)* | Median (IQR) age at outcome |
| Two-year observation window, among those with a minimum follow-up of one year (main age-restricted analysis) | ||||
| 8 to 10 years (n = 202, 10.3% of total cohort) | 2.0 (1.7 to 2.0) | Wheelchair use | 112 (55.4) | 10 (10 to 11) |
| Scoliosis | 76 (37.6) | 10 (10 to 11) | ||
| Neurologic/neuropsychiatric involvement | 86 (42.6) | 10 (9 to 10.75) | ||
| 11 to 13 years (n = 188, 9.6% of total cohort) | 2.0 (1.7 to 2.0) | Wheelchair use | 104 (55.3) | 13 (12 to 14) |
| Scoliosis | 97 (51.6) | 13 (12 to 14) | ||
| Neurologic/neuropsychiatric involvement | 77 (41.0) | 13 (12 to 14) | ||
| 14 to 16 years (n = 198, 10.1% of total cohort) | 2.0 (1.8 to 2.0) | Respiratory involvement | 89 (44.9) | 16 (15 to 16) |
| Severe respiratory outcomes | 47 (23.7) | 16 (15 to 17) | ||
| Cardiomyopathy | 135 (68.2) | 16 (15 to 16) | ||
| 17 to 19 years (n = 129, 6.7% of total cohort) | 2.0 (1.6 to 2.0) | Respiratory involvement | 59 (45.7) | 18 (18 to 19) |
| Severe respiratory outcomes | 29 (22.5) | 18 (18 to 20) | ||
| Cardiomyopathy | 75 (58.1) | 18 (17 to 18) | ||
| Sensitivity analysis variation #1: Two-year observation window; no minimum follow-up imposed | ||||
| 8 to 10 years (n = 258, 13.2% of the total cohort) | 2.0 (1.1 to 2.0) | Wheelchair use | 109 (42.1) | 10 (9 to 11) |
| Scoliosis | 65 (25.1) | 10 (9 to 11) | ||
| Neurologic/neuropsychiatric involvement | 95 (36.7) | 9 (9 to 10) | ||
| 11 to 13 years (n = 238, 12.1% of the total cohort) | 2.0 (1.2 to 2.0) | Wheelchair use | 114 (47.9) | 13 (12 to 13) |
| Scoliosis | 97 (40.8) | 13 (12 to 13) | ||
| Neurologic/neuropsychiatric involvement | 79 (33.2) | 13 (12 to 13) | ||
| 14 to 16 years (n = 281, 14.3% of the total cohort) | 2.0 (0.9 to 2.0) | Respiratory involvement | 108 (38.4) | 16 (15 to 16) |
| Severe respiratory outcomes | 52 (18.5) | 16 (15 to 16) | ||
| Cardiomyopathy | 156 (55.5) | 15.5 (15 to 16) | ||
| 17 to 19 years (n = 174, 8.8% of the total cohort) | 1.7 (1.0 to 2.0) | Respiratory involvement | 61 (35.1) | 18 (17 to 18) |
| Severe respiratory outcomes | 27 (15.5) | 18 (17 to 19) | ||
| Cardiomyopathy | 95 (54.6) | 18 (17 to 18) | ||
| Sensitivity analysis variation #2: One-year observation window; no minimum follow-up imposed | ||||
| 8 to 10 years (n = 258, 13.2% of the total cohort) | 1.0 (1.0 to 1.0) | Wheelchair use | 86 (33.2) | 10 (9 to 10) |
| Scoliosis | 53 (20.5) | 10 (9 to 10) | ||
| Neurologic/neuropsychiatric involvement | 81 (31.3) | 9 (9 to 10) | ||
| 11 to 13 years (n = 238, 12.1% of the total cohort) | 1.0 (1.0 to 1.0) | Wheelchair use | 97 (40.8) | 13 (12 to 13) |
| Scoliosis | 81 (34.0) | 13 (12 to 13) | ||
| Neurologic/neuropsychiatric involvement | 74 (31.1) | 12 (12 to 13) | ||
| 14 to 16 years (n = 281, 14.3% of the total cohort) | 0.9 (1.0 to 1.0) | Respiratory involvement | 99 (35.2) | 15 (15 to 16) |
| Severe respiratory outcomes | 45 (16.0) | 15 (15 to 16) | ||
| Cardiomyopathy | 143 (50.9) | 15 (15 to 16) | ||
| 17 to 19 years (n = 174, 8.8% of the total cohort) | 1.0 (1.0 to 1.0) | Respiratory involvement | 57 (32.8) | 18 (17 to 18) |
| Severe respiratory outcomes | 24 (13.8) | 18 (17 to 18) | ||
| Cardiomyopathy | 94 (54.0) | 18 (17 to 18) | ||
*Denominator: all patients within the age-range at cohort entry. Abbreviations: IQR = interquartile range; DMD = Duchenne muscular dystrophy.
The age-restricted KM analyses showed that as follow-up (time from cohort entry) increases, the prevalence of events captured increases (Table 3). By year one, approximately 25% of patients aged 8 to 10 years at cohort entry had an event of wheelchair use captured, compared to 40% by year two, and over 50% by year three. The corresponding values were higher among those aged 11 to 13 years, ranging from approximately 40% by year one to 60% by year three. Similar trends are observed for other key clinical milestones, with a few showing trends of plateau between year two and three, such as respiratory involvement among those aged 17 to 19 years at index which started to plateau at approximately 42% by the end of year two.
Table 3
Estimates from Kaplan-Meier analysis of age-specific prevalence by follow-up\\ time among patients with DMD
| Cumulative age-specific prevalence at the end of each follow-up year | |||
| Year 1 | Year 2 | Year 3 | |
| Wheelchair use | |||
| Age 8 to 10; n = 259 | 24.9% | 40.2% | 52.9% |
| Age 11 to 13; n = 237 | 37.8% | 50.8% | 59.0% |
| Scoliosis | |||
| Age 8 to 10; n = 259 | 15.0% | 24.1% | 30.4% |
| Age 11 to 13; n = 237 | 29.5% | 43.1% | 47.4% |
| Neurologic/neuropsychiatric involvement | |||
| Age 8 to 10; n = 259 | 32.8% | 44.4% | 46.1% |
| Age 11 to 13; n = 237 | 26.3% | 32.4% | 38.0% |
| Respiratory involvement | |||
| Age 14 to 16; n = 264 | 27.9% | 39.5% | 44.4% |
| Age 17 to 19; n = 267 | 35.8% | 42.1% | 43.5% |
| Cardiomyopathy | |||
| Age 14 to 16; n = 264 | 44.9% | 52.7% | 61.3% |
| Age 17 to 19; n = 267 | 54.4% | 58.1% | 64.1% |
DMD = Duchenne muscular dystrophy.
Additional sensitivity analyses
In the sensitivity analysis where the criterion for a minimum of one-year of continuous follow-up was removed, the observed percentage experiencing key clinical milestones was less than in the age-restricted analyses. However, estimates generally remained higher than in the primary analyses (Table 2). In addition, in the sensitivity analysis where the observation window was shortened to one year, the observed percentage experiencing key clinical milestones was expectedly even lower than other scenarios explored.
The sensitivity analyses exploring different DMD cohort definitions (i.e. restricting to patients < 18 years old at index, requiring patients to have at least one diagnosis from a specialist, or excluding patients with myoneural disorder, Guillain-Barre syndrome, or hereditary motor and sensory neuropathy) showed that patients identified by these definitions had similar durations of follow-up, and frequency of corticosteroid use compared to the primary analyses cohort. Age at index, by definition, was lower among the cohort < 18 years old at index, however remained comparable across the other sensitivity analysis cohorts. The frequency and age at key clinical milestones were also comparable between the sensitivity and primary analyses, with the exception of the cohort who was < 18 years old at index, which by definition would have lower estimated median ages at the occurrence of these comorbidities due to the elimination of older DMD patients. Among the cohort < 18 years old at index, the overall frequency of patients with a record of LOA was comparable to that of the base case definition; median ages were higher for outcomes occurring earlier in patients’ lives, and lower for outcomes that tend to occur in the later stages (Appendix Table A.3).
DISCUSSION
Large well-conducted clinical studies and registries have documented the characteristics of the clinical progression of DMD; [12, 13, 18, 19, 39] but how these align with estimates based on real-world data has not previously been reported. This health insurance claims study used the large representative MarketScan commercial database to estimate the prevalence of notable clinical milestones that characterize the natural history of DMD, by age. Approximately 2,000 patients with commercial insurance coverage in the US were included.
The results of the primary (non-age-restricted) analyses showed a slightly older median age at key clinical milestones compared with published estimates from clinical studies, [12, 13, 18, 19, 39] which suggested that at least for some patients, the events captured were potentially not the initial diagnosis related to that milestone. However, as is expected given the relatively short follow-up available in the dataset, the observed frequency of patients experiencing each milestone was less than anticipated overall. As a result, age-restricted analyses were performed. These analyses generally showed higher frequencies of key clinical milestones compared to the primary analysis, as expected given that the age and time restrictions aimed to focus the observation window on the time period where these outcomes were more likely to have occurred. The frequency of wheelchair use, as a proxy for LOA, among children and young teenagers in the present study – observed in 55% – was consistent with published estimates of the age at wheelchair use range from 30% by 10 years [12] through 95% by 15 years of age [13]. The frequency of scoliosis, documented in up to 50% of young teenagers over their first two years of follow up, was also consistent with published estimates suggesting 60% of DMD patients have scoliosis by 15 years of age [13]. Neurologic and/or neuropsychiatric involvement was observed in less than half of children and young teenagers in the current study, which is also broadly consistent with published estimates of the age at diagnosis of common neuropsychiatric complications in DMD; [40] unlike LOA, for example, it is not necessarily to be expected that these complications would be identified in everyone with DMD [41]. Respiratory involvement was observed in almost half of the cohort of older teenagers, and published estimates suggest 40% to 50% of patients require ventilation over 20 years of age [39, 42]. Finally, estimates of cardiomyopathy and heart failure were on the lower end of published estimates of 68% to 93% diagnosed with cardiomyopathy by 20 years of age [13, 18, 19].
The potential challenges in ascertaining clinical outcomes when using administrative data warrant further discussion. The MarketScan databases are based on claims submitted for reimbursement and not research purposes, and billing practices vary between providers and by insurance type. In the current study, identification of scoliosis, neurologic involvement, cardiomyopathy, respiratory failure, and heart failure primarily relied on diagnosis codes. However, the presence of a diagnostic code alone cannot indicate the severity of the underlying condition; nor are the underlying reasons for a physician selecting a particular diagnostic code available. For example, whether the use of a specific code indicates a clinical suspicion of early signs of a complication versus a severe manifestation, or whether a medication is used prophylactically or for acute treatment, is not clear. Some outcomes may not be easily identified in administrative data, particularly capture of initial symptoms or behaviors. For example, some of the diagnoses contributing to the neuropsychiatric/neurologic involvement outcome are challenging to identify using claims datasets [43]. Of necessity, selected outcomes in the current study were ascertained based on proxy measures within the claims datasets and the reliability of these is unclear [44]. For LOA, as a specific diagnostic code is not available, assessment was based on wheelchair use. However, identification of a wheelchair code does not indicate if it is a first purchase or a replacement; or give information of the frequency of wheelchair use. Equally, wheelchairs may be acquired via other sources. In a similar vein, for identifying respiratory involvement, ventilation use may indicate the start of respiratory decline or a management strategy for more severe disease. Finally, across all the outcomes considered, it is possible that a given encounter was coded as a visit for ‘DMD’, rather than for the specific manifestation of interest. Despite these challenges, these data provide indicators of how patients progress when managed in a standard clinical practice setting and have implications for using real-world data to monitor the clinical burden of patients with DMD over time.
There are some additional limitations to the analyses that should be noted. A clinically validated case definition for use in administrative claims databases is not available and there may have been some misclassification on exposure (e.g. patients with other MDs including limb-girdle or Becker muscular dystrophy may have been inadvertently included in the study cohort and patients with DMD over age 30 may have been inadvertently excluded). Other methods to help eliminate other MD cases, for example restricting the timing of the first observed MD diagnosis to early childhood, were not implemented because of the relatively short follow-up per patient available to allow true ascertainment of the first diagnosis, and also to avoid 1) a prohibitively small sample size for analysis, and 2) disregarding true cases of DMD who entered the cohort at older ages (due to the limited follow-up available within MarketScan). Nevertheless, additional sensitivity analyses performed to explore the impact of case definition assumptions showed little variability in estimates of study outcomes. In addition, while the sensitivity analysis restricting to those < 18 years at index led to lower median age estimates, the direction of variation in the frequency of key clinical outcomes observed supported the base case findings on the prevalence of these events. Wheelchair use was consistently documented across sensitivity analysis cohorts; and events occurring earlier in life were observed at a higher frequency (while events occurring later in life were observed at a lower frequency) among the cohort restricted to those < 18 years at index. One additional limitation to note with regard to the definition of DMD, is that individuals with DMD who appear in the MarketScan databases over age 30 years for the first time would be missed; however, this would likely occur quite rarely.
Given the limited follow-up available per patient and that the age at cohort entry of the overall population was in the teenage years, the timing of key clinical milestones occurring prior to cohort entry cannot be accurately ascertained. It therefore cannot be determined whether the first event observed was the first occurrence of the key milestone in a patient’s life (rather than an acknowledgement of the patient having already passed that milestone or ongoing documentation of what has become a chronic condition). As exemplified through the age-restricted sensitivity analyses varying follow-up and observation window requirements, follow-up duration plays a large role in the capture of key milestones in a patient’s life. As a result, this study presents the prevalence of key milestones by age at cohort entry and fixed observation windows to allow better interpretation of findings. To supplement this, the KM analyses showed that the prevalence of events captured increases as follow-up increases.
Additionally, a challenge in comparing the occurrence of outcomes with earlier studies is introduced by developments in research and diagnostics such that certain complications (e.g. early signs of cardiomyopathy) may be identified at a younger age through the increased sensitivity of tools such as cardiac MRI. This may therefore lead to apparent higher rates and earlier identification compared to earlier studies with outcomes based on older technology. While understanding mortality is of interest, very limited data were available (i.e. only from inpatient records, and only from the beginning of the study period through 2016); this outcome was not investigated further. Assessing corticosteroid treatment patterns was not a primary objective of the study and as such it was not designed to comprehensively assess these. As with the analyses of key clinical milestones, it is important to consider the window of follow-up data availability when interpreting available data on corticosteroid use [45]. Given that the majority of this cohort was outside of the age range with the highest anticipated corticosteroid use (6 to 12 years of age), and a large proportion of patients had lost ambulation during the study, the lower rate of overall corticosteroid use in this study cohort is expected and should be interpreted in this context. Finally, the results of this study are specific to individuals covered under a subset of commercial plans and may not be generalizable to all commercial or to government payer segments in the US.
The use of well-validated datasets that provided a large sample size of DMD patients, including children and young adults, from varied commercial insurance plans, was a key strength of this study. Performing age- and time-restricted analyses helped to address potential biases associated with estimating time-dependent outcomes from individuals of differing ages over the course of a non-standardized follow-up window. However, it is important to remember that there is between-patient heterogeneity in the timing of the occurrence of these outcomes, [14, 16] which complicates accurate ascertainment when considered in the context of relatively short follow-up windows per patient available in claims datasets. The results of the sensitivity analyses for the cohort definition were consistent with those of the primary analyses, providing support that the study findings were robust. With respect to the cohort definition, identifying patients with DMD-genotype specific treatments (such as eteplirsen) or DMD/BMD-specific ICD-10 codes (G71.01) was also explored, but did not identify any additional patients beyond those already captured by the study inclusion criteria.
CONCLUSIONS
This is the first study to document the occurrence of key clinical milestones among real-world patients with DMD with a wide range of commercial insurance plans. The prevalence of key clinical milestones observed by age was broadly consistent with published findings from large registries. Variability in estimates reflect the clinical heterogeneity experienced by those with DMD but also the impact of observation windows on the ability to ascertain key clinical outcomes in DMD. As such, the findings of this study also help delineate the types of outcomes that one can well characterize using existing claims data for cohort studies, and considerations on the methodology to do so. These data summarizing the occurrence of relevant clinical outcomes among patients with DMD with commercial insurance coverage add to the growing body of evidence describing the clinical course of DMD patients using real-world data.
DISCLOSURES
FUNDING
This work was supported by Sarepta Therapeutics, Inc.
CONFLICT OF INTEREST
SMS, CQ and EP are employees of Broadstreet HEOR, which received funds from Sarepta Therapeutics Inc for this work.
ACK and KLG are employees of Sarepta Therapeutics, Inc.
SI has received research funding or consulting fees from Avexis, Biogen, Fibrogen, Mallinkrodt, Regeneron, Sarepta, Scholar Rock, PTC Therapeutics, Pfizer, MDA, CureSMA, NIH, Genentech-Roche, and BCBS.
SUPPLEMENTARY MATERIAL
[1] The Appendix is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-220816.
REFERENCES
[1] | Wein N , Alfano L , Flanigan KM Genetics and emerging treatments for Duchenne and Becker muscular dystrophy, Pediatric Clinics of North America. (2015) ;62: (3):723–42. |
[2] | Crisafulli S , Sultana J , Fontana A , Salvo F , Messina S , Trifiro G Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis, Orphanet J Rare Dis. (2020) ;15: (1):141. |
[3] | McDonald CM , Henricson EK , Abresch RT , Han JJ , Escolar DM , Florence JM , et al. The cooperative international neuromuscular research group Duchenne natural history study–a longitudinal investigation in the era of glucocorticoid therapy: Design of protocol and the methods used, Muscle & Nerve. (2013) ;48: (1):32–54. |
[4] | Hoffman EP , Brown RH Jr , Kunkel LM , Dystrophin: The protein product of the Duchenne muscular dystrophy locus, Cell. (1987) ;51: (6):919–28. |
[5] | Yiu EM , Kornberg AJ Duchenne muscular dystrophy, Journal of Paediatrics and Child Health. (2015) ;51: (8):759–64. |
[6] | Mirski KT , Crawford TO Motor and cognitive delay in Duchenne muscular dystrophy: Implication for early diagnosis, The Journal of Pediatrics. (2014) ;165: (5):1008–10. |
[7] | Connolly AM , Florence JM , Cradock MM , Eagle M , Flanigan KM , McDonald CM , et al. One year outcome of boys with Duchenne muscular dystrophy using the Bayley-III scales of infant and toddler development, Pediatr Neurol. (2014) ;50: (6):557–63. |
[8] | Connolly AM , Florence JM , Cradock MM , Malkus EC , Schierbecker JR , Siener CA , et al. Motor and cognitive assessment of infantsand young boys with Duchenne MuscularDystrophy: Results from the Muscular Dystrophy Association DMDClinical Research Network, Neuromuscular Disorders: NMD. (2013) ;23: (7):529–39. |
[9] | McDonald CM , Henricson EK , Abresch RT , Han JJ , Escolar DM , Florence JM ,et al. The cooperative international neuromuscular research group Duchenne natural history study—a longitudinal investigation in the era of glucocorticoid therapy: Design of protocol and the methods used, Muscle & Nerve. (2013) ;48: (1):32–54. |
[10] | Parent Project Muscular Dystrophy The Duchenne Registry [Available from: 2019 https://www.duchenneregistry.org/. |
[11] | Centers for Disease Control and Prevention Muscular Dystrophy Research and Tracking, 2019 [Available from: https://www.cdc.gov/ncbddd/musculardystrophy/researchhtml. |
[12] | Bello L , Gordish-Dressman H , Morgenroth LP , Henricson EK , Duong T , Hoffman EP , et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study, Neurology. (2015) ;85: (12):1048–55. |
[13] | Kim S , Zhu Y , Romitti PA , Fox DJ , Sheehan DW , Valdez R , et al. Associations between timing of corticosteroid treatment initiation and clinical outcomes in Duchenne muscular dystrophy, Neuromuscular Disorders: NMD. (2017) ;27: (8):730–7. |
[14] | Bello L , Morgenroth LP , Gordish-Dressman H , Hoffman EP , McDonald CM , Cirak S , et al. DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study, Neurology. (2016) ;87: (4):401–9. |
[15] | McDonald CM , Gordish-Dressman H , Henricson EK , Duong T , Joyce NC , Jhawar S , et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: Long-term natural history with and without glucocorticoids, Neuromuscular Disorders: NMD. (2018) ;28: (11):897–909. |
[16] | Szabo SM , Salhany RM , Deighton A , Harwood M , Mah J , Gooch KL The clinical course of Duchenne muscular dystrophy in the corticosteroid treatment era: A systematic literature review, Orphanet J Rare Dis. (2021) ;16: (1):237. |
[17] | Landfeldt E , Thompson R , Sejersen T , McMillan HJ , Kirschner J , Lochmuller H Life expectancy at birth in Duchenne muscular dystrophy: A systematic review and meta-analysis, Eur J Epidemiol. (2020) ;35: (7):643–53. |
[18] | McKane M , Soslow JH , Xu M , Saville BR , Slaughter JC , Burnette WB , et al. Does Body Mass Index Predict Premature Cardiomyopathy Onset for Duchenne Muscular Dystrophy? Journal of Child Neurology. (2017) ;32: (5):499–504. |
[19] | Barber BJ , Andrews JG , Lu Z , West NA , Meaney FJ , Price ET , et al. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy, The Journal of Pediatrics. (2013) ;163: (4):1080–4 e1. |
[20] | Pharmacoepidemiology Strom BL , Kimmel SE , Hennessy S , editors New York, NY: Wiley-Blackwell; 2019. |
[21] | Thayer S , Bell C , McDonald CM The Direct Cost of Managing a Rare Disease: Assessing Medical and Pharmacy Costs Associated with Duchenne Muscular Dystrophy in the United States, Journal of Managed Care & Specialty Pharmacy. (2017) ;23: (6):633–41. |
[22] | Klimchak AC , Szabo SM , Qian C , Popoff E , Iannacccone S , Gooch K Characterizing demographics, comorbidities and costs of care among populations with Duchenne muscular dystrophy with Medicaid and commercial coverage, Manuscript in preparation. 2021. |
[23] | IBM Watson Health COMMERCIAL CLAIMS AND ENCOUNTERS DATABASE AND MEDICARE SUPPLEMENTAL AND COORDINATION OF BENEFITS DATABASE, IBM Watson Health; 2017. |
[24] | Gauthier G , Guérin A , Zhdanava M , Jacobson W , Nomikos G , Merikle E , et al.Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: A retrospective US claims database analysis, BMC Psychiatry. 2017;17:222. |
[25] | Marsico M , Mehta V , Chastek B , Liaw K-L , Derkay C Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States, Sexually Transmitted Diseases. (2014) ;41: (5):300–5. |
[26] | Szabo SM , Gooch K , Schermer C , Walker D , Lozano-Ortega G , Rogula B ,et al. Association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder: US-based retrospective cohort study, BMJ Open. (2019) ;9: (5):e026391. |
[27] | Wu JM , Matthews CA , Conover MM , Pate V , Funk MJ Lifetime Risk of Stress Incontinence or Pelvic Organ Prolapse Surgery, Obstetrics and Gynecology. (2014) ;123: (6):1201–6. |
[28] | Zhao Y , Johnston SS , Smith DM , McMorrow D , Krege J , Krohn K Association between teriparatide adherence and healthcare utilization and costs among hip fracture patients in the United States, Bone. (2014) ;60: :221–6. |
[29] | Broulette J , Yu H , Pyenson B , Iwasaki K , Sato R The incidence rate and economic burden of community-acquired pneumonia in a working-age population, American Health & Drug Benefits. (2013) ;6: (8):494. |
[30] | Lykins J , Wang K , Wheeler K , Clouser F , Dixon A , El Bissati K , et al. Understanding Toxoplasmosis in the United States Through “Large Data” Analyses, Clinical Infectious Diseases. (2016) ;ciw356. |
[31] | Song X , Shi N , Badamgarav E , Kallich J , Varker H , Lenhart G , et al. Cost burden of second fracture in the US Health System, Bone. (2011) ;48: (4):828–36. |
[32] | Durden E , Walker D , Gray SL , Fowler R , Juneau P , Gooch KG , editors. The direct and indirect costs due to work loss associated with overactive bladder in the United States, American Urological Association;. 2017. |
[33] | Hude Q , Vijaya S , Patricia H , Andrew F , Bernard B , Jean-Christophe L , et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data, Medical Care. (2005) ;43: (11):1130–9. |
[34] | Kim S , Campbell KA , Fox DJ , Matthews DJ , Valdez R STARnet MD. Corticosteroid Treatments in Males With Duchenne Muscular Dystrophy: Treatment Duration and Time to Loss of Ambulation, Journal of Child Neurology. (2015) ;30: (10):1275–80. |
[35] | King W , Ruttencutter R , Nagaraja HN , Matkovic V , Landoll J , Hoyle C , et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy, Neurology. (2007) ;68: (19):1607–13. |
[36] | Gambetta K , Wittlieb-Weber C , Bock M , Villa C , Johnson J , Lal A , et al. Impact of Genotype on Boys with Duchenne Muscular Dystrophy, The Journal of Heart and Lung Transplantation. (2018) ;37: (4):S122. |
[37] | Pandya S , James KA , Westfield C , Thomas S , Fox DJ , Ciafaloni E , et al. Health profile of a cohort of adults with Duchenne muscular dystrophy, Muscle & Nerve. (2018) ;58: (2):219–23. |
[38] | 38 !!! INVALID CITATION !!! 16. |
[39] | McDonald CM , Henricson EK , Abresch RT , Duong T , Joyce NC , Hu F , et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study, The Lancet. (2018) ;391: (10119):451–61. |
[40] | Hendriksen RGF , Vles JSH , Aalbers MW , Chin RFM , Hendriksen JGM Brain-related comorbidities in boys and men with Duchenne Muscular Dystrophy: A descriptive study, European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society. (2018) ;22: (3):488–97. |
[41] | Ricotti V , Mandy WP , Scoto M , Pane M , Deconinck N , Messina S , et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations, Developmental Medicine and Child Neurology. (2016) ;58: (1):77–84. |
[42] | Koeks Z , Bladen CL , Salgado D , Van Zwet E , Pogoryelova O , McMacken G ,et al. Clinical outcomes in Duchenne muscular dystrophy: A study of patients from the TREAT-NMD DMD global database, Journal of Neuromuscular Diseases. (2017) ;4: (4):293–306. |
[43] | Shi Y , Schulte PJ , Hanson AC , Zaccariello MJ , Hu D , Crow S , et al. Utility of medical record diagnostic codes to ascertain attention-deficit/hyperactivity disorder and learning disabilities in populations of children, BMC Pediatr. (2020) ;20: (1):510. |
[44] | Gooch KL , Szabo SM , Audhya I , Salhany R , Qian C , Klimchak AC , editors. The feasibility of using US claims data to assess outcomes in Duchenne muscular dystrophy. AMCP; 2020. |
[45] | Cowen L , Mancini M , Martin A , Lucas A , Donovan JM Variability and trends in corticosteroid use by male United States participants with Duchenne muscular dystrophy in the Duchenne Registry, BMC Neurol. (2019) ;19: (1):84. |




