Timing of Clinical Interventions in Patients With Duchenne Muscular Dystrophy: A Systematic Review and Grading of Evidence
Abstract
Background:
Clinical medical management guidelines of Duchenne muscular dystrophy (DMD) emphasize prevention and early identification and treatment.
Objective:
The objective of our study was to review, synthesize, and grade published evidence of the impact of the timing of clinical interventions in DMD.
Methods:
We searched PubMed, Embase, and the Cochrane Library for records published from inception up until November 19, 2021, reporting evidence of the impact of the timing of clinical interventions in DMD. We assessed the quality of evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.
Results:
We included 12 publications encompassing 1,623 patients with DMD from seven countries (Australia, France, Germany, Italy, Japan, the United Kingdom, and the United States of America). Six (50%) studies reported evidence of an impact of the timing of initiation of glucocorticoids on loss of ambulation, cardiomyopathy, fractures, forced vital capacity, and height and BMI; four (33%) of cardiac medication (i.e., angiotensin-converting enzyme inhibitors, β-blockers, and eplerenone) on left ventricular size and function and survival; one (8%) of lower limb surgery on motor quotient and loss of ambulation; and one (8%) of ataluren on lower extremity and motor function. The overall quality of the body of evidence was low.
Conclusion:
While there is a clinical rationale for anticipatory diagnostic and therapeutic strategies, evidence of the impact of the timing of initiation of treatments in patients with DMD is still emerging. Further research of this topic is warranted to inform treatment guidelines in this indication.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is a rare, genetic disease caused by mutations in the gene that produces dystrophin, a cell membrane protein required to maintain muscle integrity [1]. Deficiency or complete absence of dystrophin causes plasma membrane leakage and muscle fibre degeneration leading to progressive muscle weakening, loss of independent ambulation, and a wide array of serious multisystem complications, including cardiomyopathy and respiratory muscle dysfunction [2]. In addition to the detrimental impact of the disease on the health status and quality of life of affected patients [3], DMD has been shown to be associated with a considerable burden on family caregivers [4], as well as large costs to society [5].
Advancements in DMD standards of care have resulted in significant improvements to life expectancy, and many patients now live into their fourth decade of life [6]. In line with this development, the medical management of DMD has shifted to more anticipatory diagnostic and therapeutic strategies, to achieve prevention, early identification, and treatment of disease complications [2]. Indeed, as a chronic, monotonically progressive disease with onset in early childhood, there is a naturally strong case for preventive treatment to help halt or delay muscle wasting. For example, at the time of diagnosis (around the age of 5 years [2]), many boys with DMD are already subject to significant lower extremity impairment, and currently used drugs, such as glucocorticoids, are not able to restore lost functional ability. Moreover, new treatment strategies, including nonsense readthrough therapies for boys with premature stop codon mutations and exon-skipping by means of antisense oligonucleotides, can only rescue and protect existing muscle fibres. Therefore, these treatments will likely yield more benefits if they are started as early as possible [7].
There is currently no up-to-date scientific summary of the impact of early treatment in DMD. To bridge this evidence gap, the objective of this study was to review, synthesize, and grade published evidence of the impact of the timing of clinical interventions in patients with DMD.
MATERIALS AND METHODS
This literature review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [8].
Search strategy
We searched PubMed, Embase, and the Cochrane Library for records of studies published from inception up until November 19, 2021, reporting evidence of the impact of the timing of clinical interventions in patients with DMD. The search string contained a combination of the following Medical Subject Heading terms, title/abstract, and topic/all-field tags: “Duchenne muscular dystrophy”, “prophylactic”, “early”, “late”, “delayed”, “timely”, “timing”, “treatment”, “therapy”, and “intervention” (full search strings are provided in the Appendix).
Selection criteria
Eligibility criteria based on the Population, Intervention, Comparison, Outcomes and Study design (PICOS) framework for study inclusion are presented in Table 1. For inclusion, we also required all studies to report results from comparative/relative assessments of the timing of clinical interventions, for example treatment initiation at < X vs. ≥X years of age, as opposed to estimates for treatment initiation “earlier than usual”.
Table 1
PICOS eligibility criteria for study inclusion
| Inclusion | Exclusion | |
| Population | Patients diagnosed with DMD | Patients without a diagnosis of DMD |
| Intervention | Any | None |
| Comparators | Any | None |
| Outcome | Any clinical outcome | None |
| Study design | Any | None |
Note: Population, Intervention, Comparison, Outcomes and Study design (PICOS). Duchenne muscular dystrophy (DMD).
Screening and data extraction
One investigator (EL) initially screened article titles and abstracts for eligibility, and subsequently reviewed full-text versions of selected records. The reason for exclusion was recorded and confirmed by a second investigator (NF). For all articles that met the inclusion criteria upon full-text review, the following information was extracted into a pre-designed data extraction form: Author; title; study year; geographical setting; study design; pharmacological interventions (incl. dose and duration of exposure); sample population characteristics; and efficacy and/or effectiveness results relating to the impact of the timing of clinical interventions in DMD. We synthesised extracted evidence of the impact of the timing of clinical interventions in DMD into nine outcome categories: cardiac health and function; gastrointestinal health; lower extremity and motor function; mental health; respiratory health and function; scoliosis; survival; upper extremity function; and weight, height, and BMI.
Level of evidence
We assessed the quality of the identified evidence of the impact of the timing of clinical interventions in patients with DMD using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [9]. GRADE rates the overall quality of evidence based on design limitations, risk of bias, consistency of the results across available studies, the precision of the results, directness, and likelihood of publication, and has four levels of evidence –also known as certainty in evidence or quality of evidence: (1) very low (i.e., the true effect is probably markedly different from the estimated effect), (2) low (i.e., the true effect might be markedly different from the estimated effect), (3) moderate (i.e., the authors believe that the true effect is probably close to the estimated effect), and (4) high (i.e., the authors have a lot of confidence that the true effect is similar to the estimated effect). Per the GRADE manual, two investigators (EL and NF) independently provided an initial rating of all included records. Next, the quality of evidence (at the outcome level) was rated down for issues or limitations pertaining to study limitations, inconsistency of results, imprecision, indirectness of evidence, and publication bias, and/or rated up in case of a large magnitude of effect, a dose response, or if confounders are likely to minimize the effect. Finally, each investigator independently provided an overall GRADE quality rating of each outcome and study [9].
RESULTS
The database searches resulted in the identification of 1,549 publications (Fig. 1). Of these, 404 were duplicates, 1,120 excluded following title and abstract screening, and 25 selected for full-text review. Finally, 12 full-text articles [10–21] were included for evidence extraction, synthesis, and grading. Summary details of the included publications are presented in Table 2.
Fig. 1
PRISMA diagram of the selection process of the included publications. Note: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
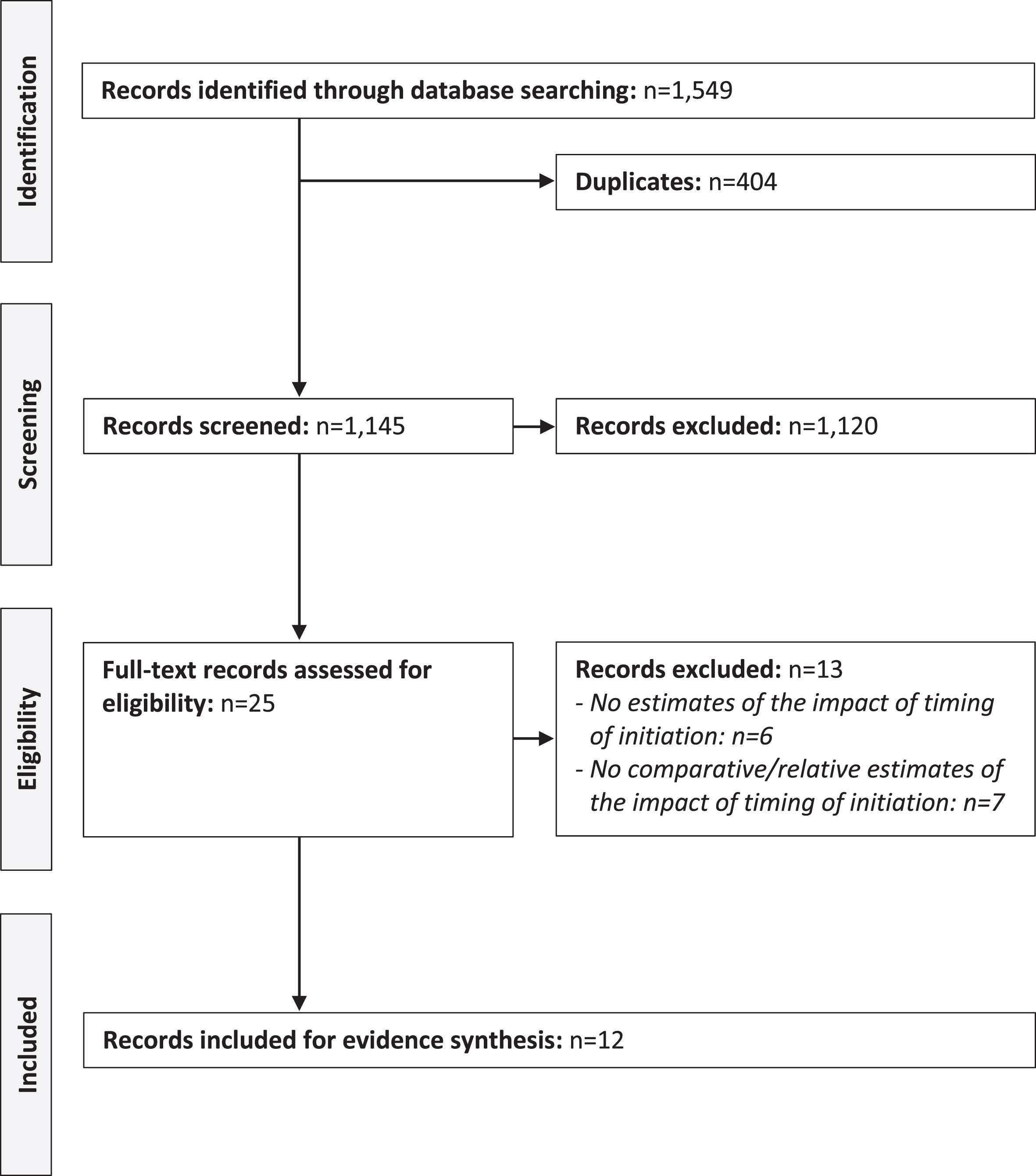
Table 2
Characteristics of included studies
| Clinical intervention(s) | |||||||
| Author (year) [country] | Study design | Sample, n (age) | Intervention(s) | Dose, mean | Duration of exposure | Timing of initiation | Study follow-up |
| Aikawa et al. (2019) [JP] [10] | Retrospective cohort study | 21 patients with DMD (median age: 10 years, IQR: 6–14 years) and 13 with BMD (median age: 6 years, IQR: 8–18 years) | ACE inhibitors (cilazapril and enalapril), or ACE inhibitor and β-blockers (bisoprolol) | Cilazapril: 1.5 mg/day | 3.0 yearsb | At LVEF≥55% vs. < 55% | 3.0 yearsb |
| Enalapril: 10 mg/daya | |||||||
| Bisoprolol: 2.5 mg/daya | |||||||
| Bonifati et al. (2006) [IT] [11] | Retrospective cohort study | 48 patients with DMD (mean age: 8 years; range: 4–12 years) | Glucocorticoids (prednisone or deflazacort) | Predisone: 0.75 mg/kg/day (year 1) and 1.5 mg/kg (until LoA). | ≥1 year | Based on age at treatment initiation | NR |
| Deflazacort: 0.9 mg/kg/day (prednisone dose equivalent) (year 1) and 1.8 mg/kg (until LoA). | |||||||
| Davidson et al. (2012) [AUS] [12] | Retrospective cohort study | 144 patients with DMD (mean age: 12 years; range: NR) | Glucocorticoids (drugs NR) | NR | NR | Based on age at treatment initiation | NR |
| Duboc et al. (2005) [FR] [13] | RCT | Group 1 (early treatment) 28 patients with DMD (mean age: 11 years; range: NR) | Year 1–3 | Perindopril: 2–4 mg/dayc | Group 1 5 years | Group 1 (early treatment) At study initiation | 5 years |
| ACE inhibitor (perindopril); or placebo Year 4–5 | β-blockers: NR | Group 2 2 years | Group 2 (late treatment) 3 years after study initiation | ||||
| Duboc et al. (2007) [FR] [14] | RCT | Group 2 (late treatment) 29 patients with DMD (mean age: 11 years; range: NR) | ACE inhibitor (perindopril) or ACE inhibitor (perindopril) and β-blockers (drugs NR) | 10 years | |||
| Forst et al. (1995) [DE] [15] | Prospective cohort study | Group 1 (early surgery) 57 patients with DMD (mean age: 6 years; range: NR) | Lower limb surgery | NA | NA | Group 1 (early surgery) At early ambulatory disease stage (retractions of the lower limb joints were just emerging) | 4 yearsd |
| Group 2 (late surgery) 66 patients with DMD (mean age: 9 years; range: NR) | Group 2 (late surgery) At end of ambulatory disease stage (mild contractures of the joints) | ||||||
| Kim et al. (2015) [USA] [16] | Retrospective cohort study | 220 patients with DMD (mean age: 7 years; range: NR) | Glucocorticoids (prednisone and/or deflazacort) | NR | Group 1 (short treatment) 1.4 years Group 1 (long treatment) 5.4 years | Group 1 (short treatment) Age at treatment initiation: 7.3 years Group 2 (long treatment) Age at treatment initiation: 6.8 years | NR |
| Kim et al. (2017) [USA] [17] | Retrospective cohort study | Group 1 (early treatment) 59 patients with DMD (distribution of age NR) | Glucocorticoids (deflazacort, prednisone, or prednisolone) | NR | 5.9–6.4 years (depending on outcome) | Group 1 (early treatment) Age at treatment initiation: 4.2 years | ≥1 year |
| Group 2 (late treatment) 259 patients with DMD (distribution of age NR) | Group 2 (late treatment) Age at treatment initiation: 7.6 years | ||||||
| Lamb et al. (2016) [USA] [18] | Retrospective cohort study | 324 patients with DMD (mean age: 7 years, range: 2–12 years) | Glucocorticoids (deflazacort and/or prednisone) | NR | 3.2 yearse | Based on age at treatment initiation | NR |
| Raman et al. (2017) [USA] [19] | Prospective cohort study | 11 patients with DMD (median age: 18 years, range: 8–26 years) | Eplerenone (on top of ACE inhibitors, angiotensin receptor blockers, and/or β-blockers) | 25 mg once daily | 2 years | Median age at treatment initiation: young patients (11 years) vs. old patients (18 years) | 2 years |
| Ricotti et al. (2013) [UK] [20] | Prospective cohort study | 360 patients with DMD (mean age: 6 years; range: 3–10 years) | Glucocorticoids (prednisolone and deflazacort) | By regimenf | 4 yearsd | Based on age at treatment initiation (< 5 years vs. ≥5 years) | 4 yearsd |
| Ruggiero et al. (2018) [IT] [21] | Case series | 3 patients with DMD (case 1: 11 years; case 2: 8 years; and case 3: 6 years) | Ataluren | 40 mg/kg/day | 12 months | Case 1: 10 years of age | 12 months |
| Case 2: 8 years of age | |||||||
| Case 3: 5 years of age | |||||||
Note: Angiotensin-converting enzyme (ACE). Left ventricular ejection fraction (LVEF). Loss of ambulation (LoA). Not reported (NR). Randomized controlled trial (RCT). The North Star Ambulatory Assessment (NSAA). Australia (AUS). Germany (DE). France (FR). Italy (IT). Japan (JP). United Kingdom (UK). United States of America (USA). aMedian dose at end of study. bMedian. cThe placebo group received perindopril (2–4 mg/day) year 4–5. dMean. eDuration of exposure until loss of ambulation, or an age of 12 years. fSee article for details of doses.
Summary of the evidence
Identified studies encompassed a total of 1,623 male patients with DMD from seven countries (Australia, France, Germany, Italy, Japan, the United Kingdom [UK], and the United States of America [USA]). Six (50%) studies reported evidence of an impact of the timing of initiation of glucocorticoids [11, 12, 16–18, 20]; four (33%) of cardiac medication (i.e., angiotensin-converting enzyme [ACE] inhibitors, β-blockers, and eplerenone) [10, 13, 14, 19]; one (8%) of lower limb surgery [15]; and one (8%) of ataluren [21].
Cardiac health and function
We found four studies reporting evidence of an impact of the timing of clinical interventions on cardiac health and function in patients with DMD. Specifically, Aikawa et al. [10] investigated the progression of left ventricular dysfunction and myocardial fibrosis in 34 Japanese patients with DMD or Becker muscular dystrophy (BMD) treated with ACE inhibitors, or ACE inhibitors and β-blockers in combination. Compared with no treatment, the authors found that only those who started treatment at LVEF < 55% (n = 13) had significantly higher left ventricular ejection fraction (LVEF) after a median study follow-up of 3 years. The corresponding estimate for myocardial fibrosis (detected by late gadolinium enhancement) was not significant. Similar findings were reported by Duboc et al. [13], who studied the effect of ACE inhibitors, or ACE inhibitors and β-blockers in combination, initiated in 57 French patients with DMD with LVEF > 55%. The authors found that early versus delayed administration of ACE inhibitors (i.e., at study initiation versus three years after study initiation) was not associated with a significant increase in LVEF after a total follow-up of five years. Moreover, Kim et al. [17] investigated the long-term risk of cardiomyopathy in 660 US patients with DMD treated with glucocorticoids. The authors found that patients initiating glucocorticoid treatment in early childhood (≤5 years of age; n = 59) had a significantly higher risk of cardiomyopathy compared to those who initiated treatment in late childhood (> 5 years of age; n = 259). Lastly, Raman et al. [19] investigated the efficacy of eplerenone in 11 US patients with DMD across a follow-up of two years, and estimated the median change in left ventricular systolic strain at -4.4% and 0.2% for those who started treatment at < 5 years of age (n = 6) and≥5 years (n = 5), respectively.
Gastrointestinal health
We found no evidence of an impact of the timing of clinical interventions on gastrointestinal health in patients with DMD.
Lower extremity and motor function
We found seven studies reporting evidence of an impact of the timing of clinical interventions on lower extremity and motor function in patients with DMD. Specifically, Bonifati et al. [11] investigated glucocorticoid receptor gene polymorphisms in 48 Italian patients with DMD treated with glucocorticoids. The authors found that those who initiated glucocorticoid treatment earlier in life had a significantly lower risk of losing ambulation across follow-up. Davidson et al. [12] reached a similar result in a study of 144 Australian patients with DMD, where a longer duration of glucocorticoid therapy was found to be associated with a reduction in the risk of losing ambulation before 10 years of age. Contrary to these findings, Kim et al. [17] found that the risk of loss of ambulation was very similar in a sample comprising of 312 US patients initiating glucocorticoid treatment in early and late childhood (≤5 vs. > 5 years of age). Kim et al. [16] investigated the association between glucocorticoid treatment and time to loss of ambulation in 220 patients with DMD. The authors compared two groups of patients specified in terms of duration of glucocorticoid exposure (1.4 vs. 5.4 years prior to loss of ambulation) and found that longer treatment exposure was associated with prolonged ambulation. However, interestingly, mean age at treatment initiation was similar between the two groups (7.3 vs. 6.8 years). Ricotti et al. [20] investigated long-term benefits of glucocorticoids in 360 UK patients with DMD. Patients who initiated glucocorticoid therapy before 5 years of age had better functional ability (as measured using the North Star Ambulatory Assessment total score) across a mean follow-up of four years compared to those starting later in life, although the result was not statistically significant (p = 0.06). Forst et al. [15] investigated the effect of lower limb surgery in 123 German patients with DMD. The authors found that patients who had the procedure prophylactically, as retractions of the lower limb joints were just emerging (at a mean age of 6 years; n = 57), had favourable motor quotient (p < 0.001) and prolonged ambulation (p = NR) compared to those who had the survey when exhibiting mild contractures of the joints at the end of the ambulatory disease stage (at a mean age of 9 years: n = 66). Finally, Ruggiero et al. [21] investigated the impact of different timings of treatment initiation of ataluren in a case series comprising of three Italian children with nmDMD, and found that earlier therapy initiation was associated with better timed function test results after 12 months.
Mental health
We found no evidence of an impact of the timing of clinical interventions on mental health in patients with DMD.
Respiratory health and function
We found one study reporting evidence of the impact of the timing of clinical interventions on respiratory health and function in patients with DMD. Specifically, Kim et al. [17] found that those initiating glucocorticoid treatment in early childhood (≤5 years of age) had significantly lower expected FVC values than those who started treatment in late childhood (> 5 years of age) when adjusting for age at onset of first sign or symptoms and age at each test in a linear regression model.
Scoliosis
We found one study reporting evidence of the impact of the timing of clinical interventions on scoliosis in patients with DMD. Specifically, Kim et al. [17] found that age at scoliosis diagnosis did not differ statistically among those initiating glucocorticoid treatment in early versus late childhood (≤5 versus > 5 years of age).
Survival
We found one study reporting evidence of the impact of the timing of clinical interventions on survival in patients with DMD. Specifically, Duboc et al. [14] investigated the long-term impact of preventive treatment with ACE inhibitors on mortality in 57 French patients with DMD with normal LVEF. The authors found that early versus delayed administration of ACE inhibitors (at study initiation versus three years after study initiation) was associated with a significant reduction in all-cause mortality.
Upper extremity function
We found no evidence of an impact of the timing of clinical interventions on upper extremity function in patients with DMD.
Weight, height, and BMI
We found one study reporting evidence of the impact of the timing of clinical interventions on weight, height, and/or BMI in patients with DMD. Lamb et al. [18] investigated growth patterns in 324 ambulatory males with DMD treated with glucocorticoids, and found age at treatment initiation to be significantly and positively associated with height Z-score and negatively with BMI Z-score.
Rating of the quality of the evidence
Per the manual of GRADE, we initially attributed included RCTs a high rating, observational studies a low rating, and case reports a very low rating. Next, we downgraded the rating for Aikawa et al. [10] for indirectness (as results were reported for a sample comprising of patients with DMD and BMD), Davidson et al. [12] due to serious limitations (risk of bias) pertaining to lack of information on exposure, Duboc et al. [13] due to inconsistency of results, Duboc et al. [14] due to risk of bias because of confounding, and Raman et al. [19] due to a small sample size. Finally, we provided an overall rating of the quality of the evidence of each publication (Table 3).
Table 3
GRADE assessment of included studies
| Author (year) [country] | Intervention(s) | Outcome measure(s) | Method of analysis | Outcome results | Initial GRADE | Outcome GRADE modification | Overall GRADE |
| Aikawa et al. (2019) [JP] [10] | ACE inhibitors (cilazapril and enalapril), or ACE inhibitors and β-blockers (bisoprolol) | LVEF | Mixed-effects regression model | •ACE inhibitor initiation at LVEF < 55% (vs. no treatment): β= 3.7; p = 0.010. | Low | Very low (indirectness; estimates for DMD and BMD) | Very low |
| •ACE inhibitor initiation at LVEF≥55% (vs. no treatment): β= 2.2; p = 0.240. | |||||||
| Myocardial fibrosis | •ACE inhibitor initiation at LVEF < 55% (vs. no treatment): β= –0.1; p = 0.550. | Low | |||||
| •ACE inhibitor initiation at LVEF≥55% (vs. no treatment): β= 1.5; p = 0.970. | |||||||
| Bonifati et al. (2006) [IT] [11] | Glucocorticoids (prednisone or deflazacort) | LoA | Cox proportional hazard model | •Age at treatment initiation (independent variable): HR = NR; p < 0.001. | Low | – | Low |
| Davidson et al. (2012) [AUS] [12] | Glucocorticoids (drugs NR) | LoA before 10 years of age | Logistic regression model | •Duration of glucocorticoid treatment (independent variable): OR = NR; p < 0.05. | Low | Very low (lack of information on exposure) | Very low |
| Duboc et al. (2005) [FR] [13] | ACE inhibitor (perindopril) or ACE inhibitor (perindopril) and β-blockers (drugs NR) | LVEF | Descriptive (mean change across follow-up) | •Mean LVEF at end of follow-up: 59% (early treatment) and 56% (late treatment); p > 0.05. | High | Moderate (inconsistency of results) | Moderate |
| Duboc et al. (2007) [FR] [14] | ACE inhibitor (perindopril) or ACE inhibitor (perindopril) and β-blockers (drugs NR) | Survival | Kaplan-Meier; log-rank test | •Cumulative incidence proportion at 10 years of follow-up: 93% (early treatment) and 66% (late treatment); p = 0.013. | High | Moderate (risk of bias due to confounding) | Moderate |
| Forst et al. (1995) [DE] [15] | Lower limb surgery | LoA | Descriptive (proportion ambulatory, by age; differences tested using the Wilcoxon test) | •Age when 60% were still ambulatory: 12 years (early surgery) and 10 years (late surgery); p-value NR. | Low | – | Low |
| Joint quotients | Descriptive (mean quotient; differences tested using the Wilcoxon test) | •Mean joint quotient by age 12: 2.3 (early surgery) and 2.5 (late surgery); p-value NR. | – | ||||
| Motor quotients | •Mean motor quotient by age 12: 7.8 (early surgery) and 5.5 (late surgery); p < 0.001. | – | |||||
| Kim et al. (2015) [USA] [16] | Glucocorticoids (prednisone and/or deflazacort) | LoA | Descriptive (mean age at LoA) | •Mean age at LoA: 9.5 years (short/late treatment) and 12.3 years (early/long treatment); p-value NR. | Low | – | Low |
| Kim et al. (2017) [USA] [17] | Glucocorticoids (deflazacort, prednisone, or prednisolone) | LoA | Cox proportional hazard model | •Early vs. late glucocorticoid treatment: HR = 1.0 (95% CI: 0.7 to 1.7); p = 0.880. | Low | – | Low |
| Cardiomyopathy | •Early vs. late glucocorticoid treatment: HR = 2.1 (95% CI: 1.2 to 3.5); p = 0.010. | – | |||||
| Scoliosis | •Early vs. late glucocorticoid treatment: HR = 1.7 (95% CI: 0.9 to 3.0); p = 0.080. | – | |||||
| Fracture | •Early vs. late glucocorticoid treatment: HR = 2.3 (95% CI: 1.4 to 3.7); p < 0.010. | – | |||||
| FVC | OLS | •Early vs. late glucocorticoid treatment: β= – 3.9; p < 0.010. | – | ||||
| Lamb et al. (2016) [USA] [18] | Glucocorticoids (deflazacort and/or prednisone) | Height Z-score | Mixed-effects regression model | •Age at deflazacort initiation: β= 0.04; p = 0.230. | Low | – | Low |
| •Age at prednisone initiation: β=0.11; p < 0.001. | |||||||
| Weight Z-score | •Age at deflazacort initiation: β = – 0.04; p = 0.390. | – | |||||
| •Age at prednisone initiation: β=0.02; p = 0.350. | |||||||
| BMI Z-score | •Age at deflazacort initiation: β = – 0.11; p = 0.002. | – | |||||
| •Age at prednisone initiation: β = – 0.08; p = 0.005. | |||||||
| Raman et al. (2017) [USA] [19] | Eplerenone | Left ventricular systolic strain | Descriptive (median change across follow-up) | •Median change in left ventricular systolic strain: – 4.4% (IQR: – 5.8% to – 0.9%) (young patients) and 0.2% (IQR: – 1.1% to 4.3%) (old patients). | Low | Very low (small sample size) | Very low |
| Ricotti et al. (2013) [UK] [20] | Glucocorticoids (prednisolone and deflazacort) | NSAA | Mixed-effects regression model | •Early treatment was associated with better NSAA total scores (p = 0.06). | Low | – | Low |
| Ruggiero et al. (2018) [IT] [21] | Ataluren | 6MWT | Descriptive (mean test results across follow-up) | •Early treatment was associated with better timed function test results (p = NR). | Very low | – | Very low |
| Timed 10 m run/walk | |||||||
| Timed four-stair ascend | |||||||
| Timed four-stair descend | |||||||
| Timed stand from supine |
Note: Forced vital capacity (FVC). Left ventricular ejection fraction (LVEF). Loss of ambulation (LoA). Not reported (NR). The North Star Ambulatory Assessment (NSAA). Hazard ratio (HR). Odds ratio (OR). Ordinary Least Square (OLS). Australia (AUS). Germany (DE). France (FR). Italy (IT). Japan (JP). United Kingdom (UK). United States of America (USA).
DISCUSSION
In this systematic review, encompassing a total of 12 studies involving 1,623 patients from seven countries, we synthesized and graded the body of evidence of the timing of clinical interventions in DMD. Taken together, our findings show that there is:
• Low quality evidence that earlier initiation of glucocorticoids is associated with prolonged ambulation in patients with DMD;
• Low quality evidence that earlier initiation of glucocorticoids is associated with decreased cardiac and respiratory health and function in patients with DMD;
• Very low quality evidence that earlier initiation of ACE inhibitors, or ACE inhibitors and β-blockers in combination, is associated with improved cardiac health and function in patients with DMD;
• Moderate quality evidence that earlier initiation of ACE inhibitors is associated with improved survival in patients with DMD;
• Low quality evidence that earlier initiation of lower limb surgery is associated with improved motor quotient and prolonged ambulation in patients with DMD.
• Very low quality evidence that earlier initiation of ataluren is associated with improved lower extremity and motor function in patients with nmDMD.
Accordingly, although there is a clear clinical rationale for prevention, early identification, and treatment of disease complications (as outlined by the international clinical care guidelines [2]), optimum timings of several principal pharmacological treatments in patients with DMD remain elusive. Our review also demonstrate that little is known of the impact of the timing of initiation of rehabilitative and psychological interventions, as well as novel therapies, such as ataluren and eteplirsen.
The synthesized evidence base should be interpreted in the context of several general methodological limitations expected to impact estimates of the impact of different treatment timings. One such source of bias is confounding by indication, which occurs when the clinical indication for selecting a particular treatment also affects the outcome [22]. For example, physicians would be expected to be more inclined to initiate early treatment with glucocorticoids in young boys with particularly aggressive phenotypes of the disease, or ACE inhibitors in patients with more pronounced cardiac involvement and therefore less favorable prognosis, on average. As a result, patients starting treatment early might have relatively poor disease outcomes later in life, not because of lack of treatment effect, but because of confounding. This could potentially help explain why patients initiating glucocorticoid treatment in early childhood have been observed to have lower respiratory function later in life (aside, for example, adverse effects due to growth suppression) [17]. One of few options to help mitigate this bias is to perform a randomized study, in which investigated timings of exposure are randomly distributed across patients. This, however, might raise ethical concerns, if there is a clinical justification for early intervention. Moreover, for most pharmacological therapies, in particular glucocorticoids in DMD [2], there is also a trade-off between benefits and harms, which naturally has a non-trivial impact on treatment decisions.
The second source of bias concerns the fact that rare disease research generally involves analyzing small sample populations and are therefore associated with low power and precision due to random error. This is an issue of particular relevance to studies of progressive diseases with heterogenous presentation, such as DMD, with notable inter- and intra-patient variability in outcomes and treatment response. For this reason, researchers may find it challenging from a methodological point of view to explore the effects of different treatment timings via, for example, stratification.
Finally, studies of the impact of timings of clinical interventions in DMD usually involve evaluating outcomes several years, or even decades, post treatment initiation. Examples found in this review include treatment with glucocorticoids and loss of ambulation, and ACE inhibitors and survival. The relatively long follow-up means that it is typically not feasible to study this topic in controlled settings, and also that it might be problematic to ensure that the sample populations compared are sufficiently homogeneous with respect to factors directly or indirectly associated with outcomes of interest over time. This issue is related to the fact that many rare diseases, including DMD, do not have a unique disease classification code, which makes it challenging to identify cases in administrative or clinical databases or registers. Therefore, it is typically not possible to perform effectiveness research in DMD using secondary data assets encompassing routinely collected information, which otherwise could have provided a cost-effective option to retrospectively evaluate the long-term impact of different treatment timings (although subject to confounding).
Strengths of our review include the unrestrictive review criteria, as well as our in-depth evidence grading. In terms of limitations, due to the many different timings of initiation, clinical interventions, and outcome measures identified, we were unable to perform a formal meta-analysis. In addition, our review did not encompass grey literature, which means that evidence for some clinical interventions might have not been fully synthesized.
In conclusion, we show that the evidence of the impact of the timing of clinical interventions in patients with DMD is scarce and of generally low quality. Further research of this topic is warranted to inform treatment guidelines in this indication.
CONFLICTS OF INTEREST
This study was funded by PTC Therapeutics. EL has acted as a consultant to PTC Therapeutics through his employment at ICON plc. and declares that he has no personal, commercial, academic, or financial conflicts of interest. NF has acted as a consultant to PTC Therapeutics through her employment at BresMed and declares that she has no personal, commercial, academic, or financial conflicts of interest. KB is an employee of PTC Therapeutics and may own stock/options in the company.
REFERENCES
[1] | Emery AE . The muscular dystrophies. Lancet. (2002) ;359: :687–95. |
[2] | Birnkrant DJ , Bushby K , Bann CM , et al. Diagnosis and management of Duchenne muscular dystrophy, part Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. (2018) ;17: (3):251–67. |
[3] | Landfeldt E , Lindgren P , Bell C , et al. Health-Related Quality of Life in Patients with Duchenne Muscular Dystrophy: A Multi-National, Cross-Sectional Study. Dev Med Child Neurol. (2016) ;58: (5):508–15. |
[4] | Landfeldt E , Edström J , Buccella F , et al. Duchenne muscular dystrophy and caregiver burden: A systematic review. Dev Med Child Neurol. (2018) ;60: (10):987–96. |
[5] | Landfeldt E , Lindgren P , Bell C , et al. The Burden of Duchenne Muscular Dystrophy: An International, Cross-Sectional Study. Neurology. (2014) ;83: :529–36. |
[6] | Landfeldt E , Thompson R , Sejersen T , et al. Life expectancy at birth in Duchenne muscular dystrophy: A systematic review and meta-analysis. Eur J Epidemiol.. (2020) ;35: (7):643–53. |
[7] | McDonald CM , Han JJ , Mah JK , Carter GT . Corticosteroids and Duchenne muscular dystrophy: Does earlier treatment really matter? Muscle Nerve. (2012) ;45: (6):777–9. |
[8] | Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) ;134: :178–89. |
[9] | Guyatt GH , Oxman AD , Schunemann HJ , Tugwell P , Knottnerus A . GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) ;64: (4):380–2. |
[10] | Aikawa T , Takeda A , Oyama-Manabe N , et al. Progressive left ventricular dysfunction and myocardial fibrosis in Duchenne and Becker muscular dystrophy: A longitudinal cardiovascular magnetic resonance study. Pediatr Cardiol. (2019) ;40: (2):384–92. |
[11] | Bonifati DM , Witchel SF , Ermani M , et al. The glucocorticoid receptor N363S polymorphism and steroid response in Duchenne dystrophy. J Neurol Neurosurg Psychiatry. (2006) ;77: (10):1177–9. |
[12] | Davidson ZE , Kornberg AJ , Ryan MM ,et al. Deletions in the dystrophin gene predict loss of ambulation before 10 years of age in boys with Duchenne muscular dystrophy. Neuromuscul Disord. (2012) ;22: :804–908. |
[13] | Duboc D , Meune C , Lerebours G , et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. (2005) ;45: (6):855–7. |
[14] | Duboc D , Meune C , Pierre B , et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up.Am Heart J.(2007) ;154: (3):596–602. |
[15] | Forst R , Forst J . Importance of lower limb surgery in Duchenne muscular dystrophy. Arch Orthop Trauma Surg. (1995) ;114: (2):106–11. |
[16] | Kim S , Campbell KA , Fox DJ , et al. Corticosteroid Treatments in Males With Duchenne Muscular Dystrophy: Treatment Duration and Time to Loss of Ambulation. J Child Neurol. (2015) ;30: (10):1275–80. |
[17] | Kim S , Zhu Y , Romitti PA , et al. Associations between timing of corticosteroid treatment initiation and clinical outcomes in Duchenne muscular dystrophy. Neuromuscul Disord. (2017) ;27: (8):730–737. |
[18] | Lamb MM , West NA , Ouyang L , et al. Corticosteroid Treatment and Growth Patterns in Ambulatory Males with Duchenne Muscular Dystrophy. J Pediatr. (2016) ;173: :207–13. |
[19] | Raman SV , Hor KN , Mazur W , et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: Results of a two-year open-label extension trial. Orphanet J Rare Dis. (2017) ;12: (1):39. |
[20] | Ricotti V , Ridout DA , Scott E , et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. (2013) ;84: (6):698–705. |
[21] | Ruggiero L , Iodice R , Esposito M , et al. One-year follow up of three Italian patients with Duchenne muscular dystrophy treated with ataluren: Is earlier better? Ther Adv Neurol Disord. (2018) ;11: :1756286418809588. |
[22] | Kyriacou DN , Lewis RJ . Confounding by Indication in Clinical Research. JAMA. (2016) ;316: (17):1818–9. |
Appendices
APPENDIX
Final search strings
Embase (via Ovid)
(duchenne muscular dystrophy and (prophylactic or early or late or delayed or timely or timing) and (treatment or therapy or intervention)).ab. OR (duchenne muscular dystrophy and (prophylactic or early or late or delayed or timely or timing) and (treatment or therapy or intervention)).ti.
PubMed
(duchenne muscular dystrophy[MeSH Major Topic] or duchenne muscular dystrophy[Title/Abstract]) AND (prophylactic[Title/Abstract] OR early[Title/Abstract] OR late[Title/Abstract] OR delayed[Title/Abstract] OR timely[Title/Abstract] OR timing[Title/Abstract]) AND (treatment[Title/Abstract] OR therapy[Title/Abstract] OR intervention[Title/Abstract])
Cochrane Database of Systematic Reviews (via Ovid)
(duchenne muscular dystrophy and (prophylactic or early or late or delayed or timely or timing) and (treatment or therapy or intervention)).ab. OR (duchenne muscular dystrophy and (prophylactic or early or late or delayed or timely or timing) and (treatment or therapy or intervention)).ti.




