Terence A Partridge: A career dedicated to pursuit of curiosity, mentorship, and secrets of skeletal muscle stem cells
In this special issue of the Journal of Neuromuscular Diseases we celebrate the illustrious career of Terence Partridge (Terry) (Fig. 1). Terry has not only helped lay the foundation of the field of muscle regeneration research, but over the decades has also been a thought leader, teacher, mentor, innovator and a friend of many in this field. Over his long and productive career, Terry has consistently been driven by curiosity. His curiosity about how our body uses satellite cells to maintain muscle health in the face of injury in healthy as well as myopathic conditions has led him to research involving muscle cell and tissue transplants. He has utilized his approach of meticulous observation coupled with rigorous quantitation to obtain fascinating insights and lay the foundations of regenerative muscle biology. His research has remained centered on the cell biology of skeletal muscle and has led to improving our understanding of muscular dystrophy and development of therapeutic approaches for Duchenne Muscular Dystrophy (DMD) that are now in clinic. Over his career Terry has conducted research across multiple countries, establishing labs in two continents. His efforts have broken new grounds in the field of basic and therapeutic muscle research and produced a number of accomplished mentees who took part in Terry’s significant contributions to the field of muscle research (Fig. 2). Many of these trainees and colleagues have participated in this special issue where, in line with Terry’s view of science, the articles presented here run the gamut from discussing the past to looking to future challenges.
Fig. 1
Terence A. Partridge. A recent picture of camera-shy Terry.

Fig. 2
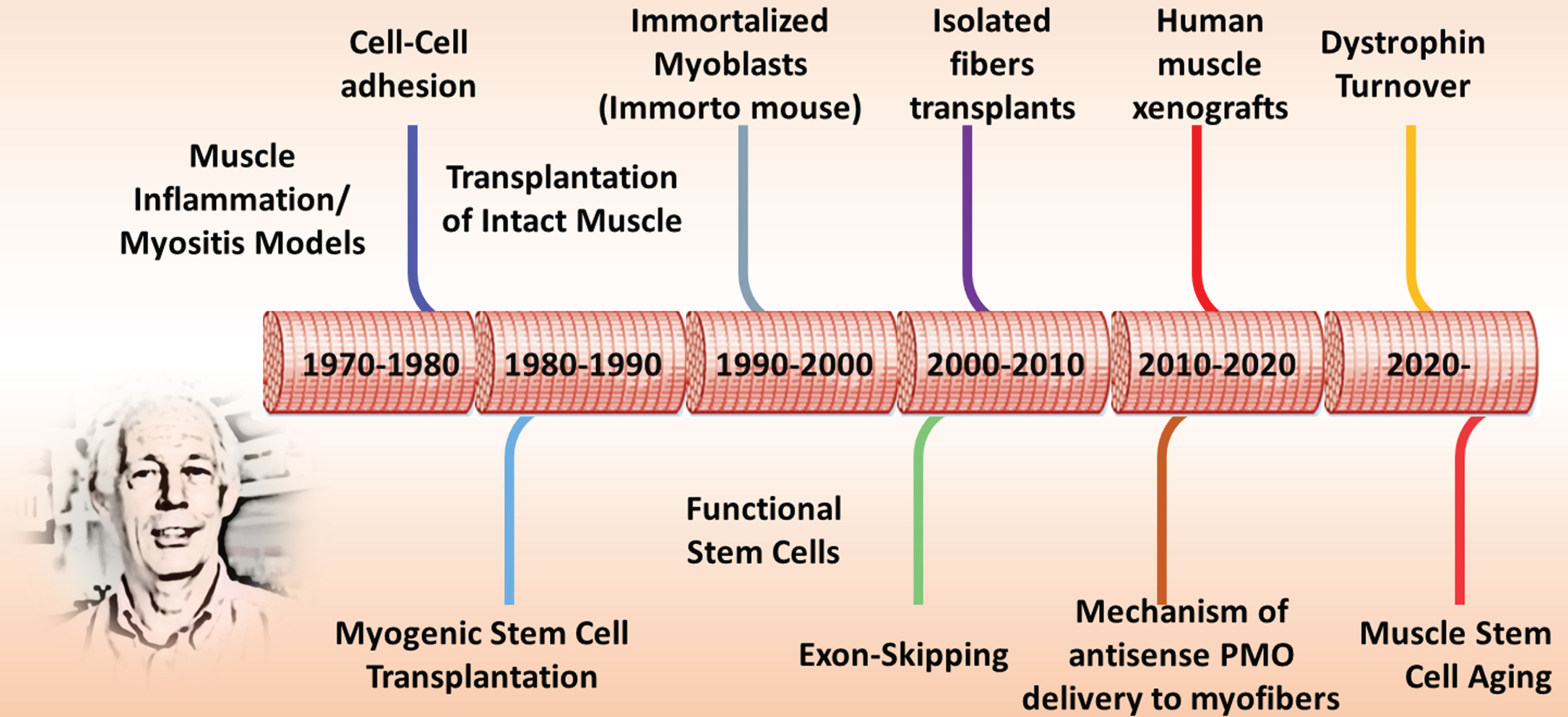
Timeline of Terry’s contributions to muscle biology. This timeline is a rough presentation of some of the technical and conceptual contributions Terry and his colleagues have made over the past 5 decades. Into the 6th decade, Terry continues to tackle new questions relevant to DMD therapy and muscle biology.
FORMATIVE YEARS AND EDUCATION
Born in East London during World War 2, Terry occasionally relays his memories of the war. Among his earliest memories are of being terrified in a London shelter as the night time air-raids involving the German ‘Doodlebug’ flying overhead, and meeting an American, who gave him a chewing gum. War also led to Terry be evacuated to Wales, where he spent some time and picked up speaking Welsh. It would seem that this and other such early events introduced Terry to other cultures, fueling his love for travel and exploration. After finishing schooling in London, Terry joined University College of London (UCL) in 1959 to pursue his undergraduate education in zoology. The scholastic environment at UCL suited Terry, and upon completing his undergraduate studies he joined the laboratory of Michael Abercrombie to pursue his PhD degree. While Terry has been a mentor to many, it appears Abercrombie was the most influential amongst Terry’s mentors. He instilled in Terry the healthy skepticism needed for scientific pursuit and the love for rigor and quantitative approach to science, that Terry has embodied and instilled in his own mentees. In the Abercrombie lab, Terry pursued the experimental work towards his PhD till 1965, generating a body of work that formed the basis for his PhD. However, Terry’s interests continued beyond this work and in 1965 having not yet completed and submitted his PhD thesis he went to the laboratory of the French parasitologist, Alain Chabaud at the Muséum National d’Histoire Naturelle in Paris. There Terry worked for over a year at isolating and characterizing strains of rodent malaria. Thereafter in 1967, he returned to the UK taking the position of assistant lecturer at the Department of Cell Biology in University of Glasgow. Aside from teaching, at Glasgow, Terry was also engaged in studying cell-cell adhesion using the limpet blood system. A series of publications that resulted from these studies provided insights into the importance of physical contact in regulating intercellular interactions [1, 2]. These studies were a prelude to his future work on cell-cell fusion that he has pursued in much of his later years. At Glasgow, he also completed writing up his PhD dissertation, receiving his PhD degree in 1970. The early education in zoology and his research in cell biology influenced Terry’s holistic view of biology and his keen eye for assessing the role of single cells as part of the whole tissue. His early scientific acumen has also guided Terry’s future choice of scientific questions and the approaches he utilized to pursue these questions.
A CAREER IN MUSCLE RESEARCH
Following the award of his PhD, Terry took on a research fellowship in the laboratory of John Chaplin Sloper at the Charing Cross Hospital Medical School in London. At first Terry pursued his studies with the cell-cell adhesion, and published this work in an article in Nature that described the role of cell-cell contact stabilization in fast versus slow adhesion of hemocytes [3]. Concomitantly, through work supported by the Muscular Dystrophy Group, Terry begun his long-standing affiliation with the field of muscle research. This interest aligned with the focus of the pathology lab of John Sloper that provided diagnostic service for muscle disease patients at the Hospital. Terry worked on the diagnostic histopathology, and utilized Abercrombie’s quantitative approach for his histopathology assessments. He had become interested in the basics of muscle tissue damage, and began asking questions regarding the mechanistic and immunological basis of the immune response observed in the polymyositis patients. Going beyond observational approach, Terry utilized the guinea-pig model of experimental polymyositis to conduct a series of studies that identified the role of muscle myofibrillar proteins in pathogenic activation of the immune system that causes polymyositis [4, 5]. Working with Pat Smith, Terry used an allergic model of myositis to study the predilection of lymphocytes to kill off existing and newly forming myotubes. By injecting guinea-pigs with rabbit muscles these studies identified the role of T lymphocytes in this process and showed that if the immune activation was stopped, the T lymphocyte response and the myositis went away [6, 7].
While these studies provided insights into the mechanisms of muscle degeneration, Terry had also embarked upon the issues pertinent to muscle regeneration through the use of muscle cell and tissue transplantation approaches. The question regarding regeneration of muscle from precursor (satellite) cells and whether the donor (grafted) cells can fuse with the host cells were vexing questions at that time. Together with John Sloper, Bruce Carlson and others at the time, as well as several subsequent students that joined his laboratory, Terry made used the approach of in vivo transplantation of intact muscle to answer these questions. His clever use of the dimeric enzymes as a tool showed that donor myoblasts from mice homozygous for one allelic form of the enzyme glucose-6-phopsphate isomerase (GPI) could fuse with host myoblasts with another allelic form of GPI to produce heterodimeric GPI enzymes. This discovery was reported in a Nature paper he published in the summer of 1978 [8]. This was the first in a series of other such meticulous studies on myogenesis that Terry pursued to establish the stem cell nature of the satellite cells and show that damaged muscle fibers regenerate by satellite cell fusion [9–15]. Smitten by the challenges posed by the interesting properties of the satellite cells, Terry continued working on tackling these challenges, starting as a junior lecturer in 1975 at Charing Cross Hospital Medical School at London and established a large muscle research group, before retiring as the head of the muscle cell biology group at the MRC Clinical Sciences Center at the Hammersmith Hospital in 2005 (Figs. 3, 4). He never wavered from his quest to elucidate the various facets of satellite cell identity, decision making ability and role in regenerating muscle. Over the years, the large body of work Terry produced has been replicated by many in the field and have not only stood the test of time, but formed the basis for the stem cells capturing the fascination of skeletal muscle and non-muscle biologists, as well as the public at large. Terry’s efforts also led to the successful careers of many of his students (see below), and helped lay the foundation of the work on the use of muscle regenerative therapies that started with Terry’s early work with the mdx mouse as a model for DMD [16].
Fig. 3
The silver jubilee. Picture of the Partridge lab around the turn of this century. From L to R the picture includes - Front row: Joanne Cousins, Sarah De Val, Pete Zammit, Jon Beauchamp. Back row: George Bou-Gharios, Terry Partridge, Jenny Morgan, Louise Heslop, TBN, Markella Ponticos, Jamie Morrison, Jacqueline Gross.

Fig. 4
All eyes on Terry. Partridge lab picture arranged by the UK DMD parent group around his retirement from MRC London. From L to R the picture includes - Front row: Jon Golding, Jacqueline Gross, Pete Zammit, Adam Rabinowitz. Back row 1: Karima Brimah, Jenny Morgan, Marjorie Serra, Catherine Alexakis, Emma Calderbank, Joanna Sales, Yosuke Nagata. Back row 2: Haifang Yin, Julia Alter, Terry Partridge, Ana Pérez Ruiz, Marie Therese Rached. Back row 3: Jon Beauchamp, Wendy Priest, Toshifumi Yokata, Charlotte Collins, Janine Ehrhardt.

TRANSLATING MUSCLE RESEARCH FROM BENCH TO BEDSIDE
The basic research on muscle repair and stem cells that Terry has pursued have all been conducted in the setting of one or the other hospital, perhaps pressing upon the need to translate these discoveries from lab to the hospital. His earlier research had already contributed to a better understanding of initiation and mechanism of myositis. However, it is his work on the regenerative role of satellite cells that raised the potential for cell transplants to grow lost muscles in the patients. Use of myogenic stem cell transplantation as a potential therapy for DMD began in 1987 as Terry initiated work with Eric Hoffman. At that time Eric worked in Louis Kunkel’s laboratory and had just identified dystrophin deficit as the genetic basis for DMD, showing mdx mouse lacks this protein. He had also generated the antibody to mark the dystrophin protein, allowing a reagent to look at the transplantation of healthy muscle in the mdx host. This ended up being a long-standing collaboration between Terry and Eric, with the first outcome reported in the 1989 Nature article [11]. This article showed that the myofibers of the mdx mice can be transformed from dystrophin-negative to dystrophin-positive by injecting healthy myoblasts that fuse with the host myofibers to enable dystrophin expression in the dystrophic muscle. This led to others adopting the use of myoblasts and coaxing other stem cells into becoming myogenic, for stem cell-based therapy for DMD and other diseases [17]. Despite seminal contributions to the genesis of this cell therapy approach for muscle diseases, true to his self-critical nature, Terry has also been a critic and a voice of reason for this field [18].
Another series of studies began by Terry and his mentee Qi Long Lu identifying revertant fibers in the mdx mice that spontaneously produced dystrophin protein. As discussed in his article in this issue, Qi Lu indicates how they suspected the revertant fibers may result from random epigenetic events that help the mutated exon to be skipped, restoring the reading frame of the mutated dystrophin gene [19]. Lu and Terry joined hands with Steve Wilton in Perth, who was also interested in this idea. Lu and Terry had developed the Mouse-On-Mouse method for antibody labeling to use murine monoclonal antibody for marking specific proteins in mouse tissue sections [20]. Working with Steve, Lu and Terry conducted in vivo exon skipping studies and demonstrated expression of functional dystrophin protein in situ in the mdx mouse model [21]. They went on to demonstrate the utility of the exon skipping approach to produce functionally useful amounts of dystrophin proteins and dystrophin expressing myofibers in dystrophic mouse muscle [22]. This 2003 study published in Nature Medicine was instrumental in paving the way for development of Phosphorodiamidate morpholino oligomers (PMO) antisense oligonucleotide-based exon skipping therapy, becoming the first genetic therapy approach to have reached the clinic for DMD. In 2005 as Terry retired, he moved across the pond from London’s Hammersmith Hospital to the Washington DC’s Children’s National Hospital. In the next 15 years Terry spent in DC, Terry’s collaborative work focused extensively on developing and translating the exon skipping based gene therapy in concert with his mentees, and by way of continuing or starting new collaborations with other labs. His initial work has already resulted in approval of four (Casimersen; Viltolarsen; Golodirsen; Eteplirsen) new drugs for DMD. This exemplifies Terry’s abilities to pursue basic science research ideas that have clear translational value to DMD patients. He continues to lead to new directions that raise the hope for future improvements of this therapy [23–27].
MENTORING THE FUTURE MUSCLE BIOLOGISTS
Starting from his earliest mentee Miranda Grounds, to his most recent ones, a number of accomplished researchers belong to the Partridge pedigree (Figs. 3, 4). Some of these include Peter Zammit, Jonathan Beauchamp, Jennifer Morgan, Diana Watt, George Bou-Gharios, David Rosenblatt, Jens Reimann, Qi Long Lu, Markella Ponticos, Sarah De Val, Toshifumi Yokota, Haifang Yin, William Duddy, Stephanie Duguez, Alyson Fiorillo, James Novak, and Davi Mazala, who have since continued to grow the field of muscle research and have contributed to this special issue [19, 25, 26, 28, 29]. Those who have had the chance to know and work with Terry have experienced his contagious passion for science. He revels in doing science in cohesive groups, where people get to know each other well and enjoy freedom of scientific pursuit. Known for being extremely rigorous he engendered a culture for his mentees to be self-critical. Just as with his students, Terry shares his views openly and honestly, without a thought for the consequences that he has faced but remained incorrigible. There is never a malice in his critiques and he is truly democratic in his interactions. His interactions do not change from a student to a professor or from a cleaner to the Royalty –all of whom Terry has interacted with. His scientific enthusiasm is paralleled by the depth of knowledge and a never-ending stream of fresh ideas. So much so, that his students did not find it possible to do all that he suggested and the unspoken rule in his lab was to not do an experiment until Terry had mentioned it three times. In mid 1990s Terry had become the beneficiary of a large donation though a private donor’s bequest. This helped Terry begin a line of research using isolated muscle fibers, and work that helped develop the immortomouse approach to study muscle by enabling generation of immortalized myoblasts from various mouse model [30]. During the late 1990s and early 2000s David Rosenblatt and Pete Zammit pursued this very productive line of investigation and Terry remains fond of such funding to pursue science [31–33]. Aside from the students who Terry has trained in his own laboratory, Terry has also been a mentor to many of his colleagues and collaborators. Several of these colleagues have benefited significantly from Terry’s insights. This became quite evident when during the peak of the pandemic in summer of 2020, Terry’s colleagues and mentees collected over Zoom from across 4 different continents and time zones to celebrate his 80th birthday. A common theme that emerged in everyone’s remarks at that gathering was their appreciation of the extent to which their research has benefited from Terry’s guidance, insights and eagerness to even travel to their laboratory to contribute and further these research work. One of the participants remarked “Of the many things, I learned from Terry one of the most valuable ones is engaging young people in Science, mentoring them to reach their full potential as scientists.” Terry’s exceptional level of mentorship and support is amongst what Terry considers as his greatest contributions to this field, the fruits of which is evident in the research presented by these colleagues in this issue dedicated to Terry [34–38].
EPILOGUE
A scientific discussion with Terry is never dull, as while he loves to discuss science, he has the knack of connecting that with humor, travel stories, and even history and literature. He is not only a great conversant, but also a great collaborator who freely shares his data and ideas, is exceptionally creative and has an open and incisive mind. He enjoys developing and understanding new techniques and is passionate about taking a rigorous mathematical approach to study biological questions [39]. So, it is no surprise that even after six decades of research career, having retired and successfully started his laboratory twice in different continents, he is still far from giving up his practice of science or take a back seat in pursuit of research questions. Never seeking limelight or working towards any honors, Terry has had his fair share of these becoming member of multiple societies and being elected Fellow of the Academy of Medical Science, Chair of Scientific Advisory Board of International Parent Project for Duchenne Muscular Dystrophy, and being Awarded Chaire International de Rechearche Blaise Pascal award. However, Terry considers his scientific work and mentorship as his accolades and remains dedicated in his efforts towards these. This year, while we celebrate 6th decade of satellite cell’s discovery [40] and the start of the 9th decade of Terry’s life, it is clear that just as his work showing age does not affect satellite cell’s abilities [32, 41, 42], age has not dampened Terry’s own research and mentoring abilities. He is still taking new courses, learning Matlab and mathematical modeling, and this Judo black belt can still do 10 straight pull ups. Whether it is due to his love for life or something special about his stem cells, one may never know. But what we do know is that with Terry’s effort and enthusiasm, his contributions to new research and researchers in the field of muscle biology is yet to find its horizon.
REFERENCES
[1] | Davies PS , Partridge T . Limpet haemocytes. I. Studies on aggregation and spike formation. J Cell Sci. (1972) ;11: (3):757–69. |
[2] | Partridge T , Davies PS . Limpet haemocytes. II. The role of spikes in locomotion and spreading. J Cell Sci. (1974) ;14: (2):319–30. |
[3] | Partridge T , Jones GE , Gillett R . Cytochalasin B inhibits stabilisation of adhesions in fast-aggregating cell systems. Nature. (1974) ;23: (4):632–4. |
[4] | Manghani D , Partridge TA , Sloper JC . The role of myofibrillar fraction of skeletal muscle in the production of experimental polymyositis. J Neurol Sci. (1974) ;23: (4):489–503. |
[5] | Partridge TA , Sloper JC . A host contribution to the regeneration of muscle grafts. J Neurol Sci. (1977) ;33: (3):425–35. |
[6] | Smith PD , Partridge TA . Macrophage migration inhibition studies of lymphocytes taken from guinea-pigs suffering from experimental polymyositis. Clin Exp Immunol. (1976) ;25: (1):133–8. |
[7] | Partridge TA , Smith PD . A quantitative test to detect lymphocytes sensitized against the surface of muscle cells. Clin Exp Immunol. (1976) ;25: (1):139–43. |
[8] | Partridge TA , Grounds M , Sloper JC . Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. (1978) ;273: (5660):306–8. |
[9] | Grounds MD , Partridge TA . Isoenzyme studies of whole muscle grafts and movement of muscle precursor cells. Cell Tissue Res. (1983) ;230: (3):677–88. |
[10] | Watt DJ , Morgan JE , Clifford MA , Partridge TA . The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol (Berl). (1987) ;175: (4):527–36. |
[11] | Partridge TA , Morgan JE , Coulton GR , Hoffman EP , Kunkel LM . Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. (1989) ;337: (6203):176–9. |
[12] | Blaveri K , Heslop L , Yu DS , Rosenblatt JD , Gross JG , Partridge TA , et al. Patterns of repair of dystrophic mouse muscle: Studies on isolated fibers. Dev Dyn. (1999) ;216: (3):244–56. |
[13] | Collins CA , Olsen I , Zammit PS , Heslop L , Petrie A , Partridge TA , et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. (2005) ;122: (2):289–301. |
[14] | Ehrhardt J , Brimah K , Adkin C , Partridge T , Morgan J . Human muscle precursor cells give rise to functional satellite cells in vivo . Neuromuscul Disord. (2007) ;17: (8):631–8. |
[15] | Lepper C , Partridge TA , Fan CM . An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. (2011) ;138: (17):3639–46. |
[16] | Coulton GR , Morgan JE , Partridge TA , Sloper JC . The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol. (1988) ;14: (1):53–70. |
[17] | Peault B , Rudnicki M , Torrente Y , Cossu G , Tremblay JP , Partridge T , et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. (2007) ;15: (5):867–77. |
[18] | Partridge TA . Stem cell therapies for neuromuscular diseases. Acta Neurol Belg. (2004) ;104: (4):141–7. |
[19] | Lu QL . Revertant Phenomenon in DMD and LGMD2I and Its Therapeutic Implications: A Review of Study Under Mentorship ofTerrence Partridge. J Neuromuscul Dis. 2021. |
[20] | Lu QL , Partridge TA . A new blocking method for application of murine monoclonal antibody to mouse tissue sections. J Histochem Cytochem. (1998) ;46: (8):977–84. |
[21] | Lu QL , Morris GE , Wilton SD , Ly T , Artem’yeva OV , Strong P , et al. Massive idiosyncratic exon skipping corrects the nonsense mutation in dystrophic mouse muscle and produces functional revertant fibers by clonal expansion. J Cell Biol. (2000) ;148: (5):985–96. |
[22] | Lu QL , Mann CJ , Lou F , Bou-Gharios G , Morris GE , Xue SA , et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. (2003) ;9: (8):1009–14. |
[23] | Takeda S , Clemens PR , Hoffman EP . Exon-Skipping in Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021. |
[24] | Li N , Parkes JE , Spathis R , Morales M , McDonald J , Kendra RM , et al. The Effect of Immunomodulatory Treatments on Anti-Dystrophin Immune Response After AAV Gene Therapy in Dystrophin Deficient mdx Mice. J Neuromuscul Dis. 2021. |
[25] | Benny Klimek ME , Vila MC , Edwards K , Boehler J , Novak J , Zhang A , et al. Effects of Chronic, Maximal Phosphorodiamidate Morpholino Oligomer (PMO) Dosing on Muscle Function and Dystrophin Restoration in a Mouse Model of Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021. |
[26] | Novak JS , Spathis R , Dang UJ , Fiorillo AA , Hindupur R , Tully CB , et al. Interrogation of Dystrophin and Dystroglycan Complex Protein Turnover After Exon Skipping Therapy. J Neuromuscul Dis. 2021. |
[27] | Novak JS , Hogarth MW , Boehler JF , Nearing M , Vila MC , Heredia R , et al. Myoblasts and macrophages are required for therapeutic morpholino antisense oligonucleotide delivery to dystrophic muscle. Nat Commun. (2017) ;8: (1):941. |
[28] | Deres F , Schwartz S , Kappes-Horn K , Kornblum C , Reimann J . Early Changes in Skeletal Muscle of Young C22 Mice, A Model of Charcot-Marie-Tooth 1A. J Neuromuscul Dis. 2021. |
[29] | Morgan S , Malatras A , Duguez S , Duddy W . Optimized Molecular Interaction Networks for the Study of Skeletal Muscle. J Neuromuscul Dis. 2021. |
[30] | Morgan JE , Beauchamp JR , Pagel CN , Peckham M , Ataliotis P , Jat PS , et al. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: A model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. (1994) ;162: (2):486–98. |
[31] | Zammit PS , Golding JP , Nagata Y , Hudon V , Partridge TA , Beauchamp JR ((2004) ) Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 166: (3):347–57. |
[32] | Bockhold KJ , Rosenblatt JD , Partridge TA . Aging normal and dystrophic mouse muscle: Analysis of myogenicity in cultures of living single fibers. Muscle Nerve. (1998) ;21: (2):173–83. |
[33] | Rosenblatt JD , Parry DJ , Partridge TA . Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation. (1996) ;60: (1):39–45. |
[34] | Phelps M , Yablonka-Reuveni Z . Female Outperformance in Voluntary Running Persists in Dystrophin-Null and Klotho-Overexpressing Mice. J Neuromuscul Dis. 2021. |
[35] | Thiruvengadam G , Sreetama SC , Charton K , Hogarth M , Novak JS , Suel-Petat L , et al. Anoctamin 5 Knockout Mouse Model Recapitulates LGMD2L Muscle Pathology and Offers Insight Into in vivo Functional Deficits. J Neuromuscul Dis. 2021. |
[36] | Goswami MV , Tawalbeh SM , Canessa EH , Hathout Y . Temporal Proteomic Profiling During Differentiation of Normal and Dystrophin-Deficient Human Muscle Cells. J Neuromuscul Dis. 2021. |
[37] | Elangkovan N , Dickson G . Gene Therapy for Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021. |
[38] | Morgan J , Muntoni F . Changes in Myonuclear Number During Postnatal Growth -Implications for AAV Gene Therapy for Muscular Dystrophy. J Neuromuscul Dis. 2021. |
[39] | Partridge TA . Enhancing Interrogation of Skeletal Muscle Samples for Informative Quantitative Data. J Neuromuscul Dis. 2021. |
[40] | Engquist EN , Zammit PS . The Satellite Cell at 60: The Foundation Years. J Neuromuscul Dis. 2021. |
[41] | Collins CA , Zammit PS , Ruiz AP , Morgan JE , Partridge TA . A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. (2007) ;25: (4):885–94. |
[42] | Novak JS , Mazala DAG , Nearing M , Hindupur R , Uapinyoying P , Habib NF , et al. Human muscle stem cells are refractory to aging. Aging Cell. (2021) ;20: (7):e13411. |




