Motor Responses in Pediatric Pompe Disease in the ADVANCE Participant Cohort
Abstract
Background:
ADVANCE (NCT01526785) presented an opportunity to obtain a more nuanced understanding of motor function changes in treatment-experienced children with Pompe disease receiving 4000L-production-scale alglucosidase alfa for 52 weeks.
Objective:
To estimate minimal detectable change (MDC) and effect size on Gross Motor Function Measure-88 (GMFM-88) after 52 weeks of 4000L alglucosidase alfa (complete data N = 90).
Methods:
The GMFM-88 mean total % score changes, MDC, and effect size were analyzed post hoc by Pompe Motor Function Level at enrollment, age groups at enrollment, and fraction of life on pre-study 160L-production-scale alglucosidase alfa.
Results:
Overall, participants aged < 2 years surpassed MDC at Week 52 (change [mean±standard deviation] 21.1±14.1, MDC range 5.7–13.3, effect size 1.1), whereas participants aged≥2 years did not attain this (change –0.9±15.3, MDC range 10.8–25.2, effect size –0.03). In participants aged < 2 years, improvements surpassed the MDC for walkers (change 17.1±13.3, MDC range 3.0–6.9, effect size 1.7), supported standers (change 35.2±18.0, MDC range 5.9–13.7, effect size 1.8) and sitters (change 24.1±12.1, MDC range 2.6–6.2, effect size 2.7). Age-independent MDC ranges were only attained by walkers (change 7.7±12.3, MDC range 6.4–15.0, effect size 0.4) and sitters (change 9.9±17.2, MDC range 3.3–7.7, effect size 0.9).
Conclusions:
These first GMFM-88 minimal-detectable-change estimates for alglucosidase alfa-treated Pompe disease offer utility for monitoring motor skills.
Trial registration:
ClinicalTrials.gov; NCT01526785; Registered 6 February 2012; https://clinicaltrials.gov/ct2/show/NCT01526785
INTRODUCTION
Pompe disease is an autosomal recessive glycogenosis resulting from lysosomal acid α-glucosidase (GAA) deficiency, progressively damaging cardiac, respiratory, skeletal, and smooth muscle [1]. Infantile-onset Pompe disease (IOPD) is defined by cardiomegaly or hypertrophic cardiomyopathy and onset at≤12 months of age [2]; untreated, it precludes most motor development and is fatal in infancy [1, 3]. Late-onset Pompe disease (LOPD) presents > 12 months of age (or earlier without cardiomyopathy) [2], with a spectrum of onset and progression [1, 4] marked by respiratory [5] and proximal muscle weakness [1, 6], leading to respiratory and motor disability [7, 8].
Alglucosidase alfa received US approval at the 160L manufacturing scale for Pompe disease in 2006 and then at the 4000L manufacturing scale in 2010 for patients≥8 years of age without cardiomyopathy. US approval was further expanded in 2014 to all patients with Pompe disease [9]. The open-label ADVANCE study (NCT01526785) of 4000L alglucosidase alfa in 113 US participants≥1 year of age previously treated with 160L alglucosidase alfa [9] supported this label expansion. Main efficacy and safety outcomes [9], genotypes and phenotypes [10] are published. In ADVANCE, 87 of 104 (83.7%) participants with Week 52 data were stable or improved on the composite primary study endpoint (free of any of the following clinical worsening events: death, new invasive-ventilator dependency, left ventricular mass z-score increase > 1 if participants had a Week 52 observed score > 2, forced vital capacity % predicted decrease≥15 percentage points, or Gross Motor Function Measure-88 [GMFM]-88 total % score decrease≥8 percentage points) [9]. Of the 17 participants who clinically worsened at Week 52, 12 met the GMFM-88 worsening criterion [9].
The motor component of ADVANCE’s primary composite endpoint (GMFM-88 total % score absolute decrease of≥8 percentage points) was based on published change ranges and estimates of minimal clinically important differences (MCID) in pediatric cerebral palsy (CP) populations, which had been correlated with parent, therapist, and masked video assessments of clinically important changes [11] as well as with changes in other motor scales [12]. While the clinical worsening threshold of 8 percentage points of GMFM-88 decline derived from CP studies was a starting approximation of potentially meaningful motor change for the ADVANCE composite endpoint, it did not account for potential differences in GMFM-88 performance between patients with CP and those with Pompe disease. It also may have over-detected decline in the most mobile ADVANCE participants and under-detected improvement in those with more impairment. A fuller understanding of GMFM-88 change in treated pediatric Pompe disease warrants the identification of disease-specific clinically meaningful differences that are tailored to motor function levels and age.
Functional change sensitivity of motor outcome measures needs to be distinguished from random or administration-related variation. One estimate of meaningful change is the MCID: the smallest outcome difference that informed participants or caregivers perceive as beneficial or harmful, and that would lead to a consideration of change in management [13–15]. MCID estimation methods may be anchor-based (referencing an external measure of change (e.g., patient/clinician-reported change in functional status or healthcare utilization) [16], or distribution-based (utilizing the statistical properties of the measure of interest within a population) [16, 17]. Minimal detectable change (MDC), a related concept, is the smallest change detectable beyond random error [16] or unlikely to be due to a chance variation in measurement [17]. MDC is a more appropriate term than MCID for statistically-based estimates not clinically anchored to other motor measurements [16].
Ko [12] estimated MCID ranges for GMFM-88 in pediatric CP with a distribution-based approach grounded on effect size (change divided by standard deviation [SD] at enrollment) of 6 months’ physical therapy, and determined its correlation to changes in GMFM-66 and Pediatric Evaluation of Disability Inventory mobility (PEDI) scores. GMFM-88 was developed [18] and validated [11] for CP, a nonprogressive motor disability, yet also has precedent (though not yet validation) for use in children and adults with Pompe disease [19, 20]. It complements the infant-specific motor measures used in the pivotal IOPD trials of 160L alglucosidase alfa [21, 22]. The Ko study’s distribution-based approach [12] invited adaptation to evaluate meaningful GMFM-88 change in Pompe disease. Current post hoc analyses of the ADVANCE study were patterned on Ko’s subgrouping by age and motor functional levels. A Pompe disease-specific motor function classification questionnaire was adapted from the GMFCS for use in ADVANCE: the Pompe Motor Function Levels questionnaire, which classified children into 5 levels (I, walkers, II, supported walkers, III, supported standers, IV, sitters, and V, restricted antigravity movement) and assessed motor decline in the 2 months prior to ADVANCE enrollment. A Pompe disease-specific GMFM-88 MDC estimate in age and Pompe Motor Function Level subgroups offers potential utility for study design and monitoring of motor responses to treatment.
The objectives of this ADVANCE post hoc analysis were to estimate MDC and effect size on GMFM-88 after 52 weeks of 4000L alglucosidase alfa in the largest pediatric Pompe disease population assessed with GMFM-88 to date, and to describe GMFM-88 dimension and item changes at the group and individual level based on age, Pompe Motor Function Levels, and prior 160L-scale alglucosidase alfa treatment duration at time of enrollment.
PARTICIPANTS AND METHOD
Study design
At 52 US centers, ADVANCE enrolled 113 participants,≥1 to 18.7 years of age with confirmed IOPD or pediatric LOPD. (See Supplementary Table 1 for a list of centers and their ethics committees or institutional review boards.) Details of design, ethics, inclusion and exclusion criteria, treatment, and assessments were published previously [9].
Treatment
Participants received 4000L alglucosidase alfa infusions for 52 weeks at the same stable dose and frequency as their pre-study 160L treatment (at physicians’ discretion); 81 (72%) of 113 treated participants received 20 mg/kg body weight every 2 weeks at enrollment. Of the 100 who remained on-study at Week 52, 68 (68%) were still receiving this dose [9]. Other dosing regimens at Week 52 were 20 mg/kg/week (n = 10); 30 mg/kg/2 weeks (n = 6); 30 mg/kg/week (n = 2); 40 mg/kg/2 weeks (n = 8); 40 mg/kg/week (n = 4); 10 mg/kg/week (n = 1); and 10 mg/kg/2 weeks (n = 1) [9]. On a monthly cumulative basis, 1 participant received 20 mg/kg, 69 received 40 mg/kg, 6 received 60 mg/kg, 18 received 80 mg/kg, 2 received 120 mg/kg, and 4 received 160 mg/kg.
Development of the Pompe Motor Function Levels Questionnaire
The GMFCS was originally designed to classify motor abilities for individuals with CP [23]; it was initially validated for those≤12 years of age [24–26] and was later extended/revised for those aged≤18 years [25]. The GMFCS does not necessarily reflect the clinical presentation of Pompe disease, a myopathy with differential weakness of proximal muscles and lower limbs [27], a progressive myopathy and evidence of lower motor neuron involvement [28], which may result in relatively preserved upper body function even in patients whose lower limbs cannot bear weight [27]. Therefore, the Pompe Motor Function Level questionnaire was developed by a physical therapy outcomes specialist with expertise in neuromuscular diseases, with the intent to classify motor function levels and assess existing motor decline in pediatric patients with Pompe disease before switching to 4000L alglucosidase alfa. The questionnaire classified participants into five levels analogous to GMFCS levels, but adapted for Pompe disease: Level I, walkers; Level II, supported walkers; Level III, supported standers (deemphasizing device-based ambulation relative to GMFCS level III, and allowing crawling for most indoor mobility); Level IV, sitters (omitting GMFCS IV’s optional ambulation items); Level V, restricted antigravity movement. Caregivers completed the questionnaire, which included age-specific criteria related to current motor level and questions regarding decline in level-specific motor skills over the 2-month period preceding enrollment. As with the GMFCS [23–25], the Pompe Motor Function Level questionnaire is a functional classifier and was only administered at enrollment; the new questionnaire was not intended as a validated outcome measure, but as a tool to stratify participants with potential for different motor responses on treatment in patients with pre-study motor decline.
Motor efficacy evaluation
GMFM-88 was administered at enrollment, Week 26, and Week 52 by neuromuscular-assessment-experienced and centrally trained physical therapists, with repeat central training if evaluators changed during the study. It comprises 88 skills typically attained by 5 years of age in children without motor impairments, in five dimensions (A: Lying/Rolling, B: Sitting, C: Crawling/Kneeling, D: Standing, E: Walking/Running/Jumping). Each item is scored from 0–3 points depending on the degree to which the task is attempted or achieved. Dimensional and total raw and % scores are calculable. The test is not norm-referenced.
This article describes previously unpublished detailed subgroup and dimensional GMFM-88 % score changes in ADVANCE in addition to post hoc analyses of GMFM-88 MDC range and effect size in subgroups classified by age and Pompe Motor Function Level.
Post hoc analysis of GMFM-88 MDC and effect size
GMFM-88 total % score changes at Week 52 are previously published as ADVANCE secondary efficacy endpoints [9]. To summarize, mean±SD total % score improved significantly in the overall group, from 46.3± 33.1 at enrollment to 50.8±36.0 at Week 52, with a mean change of 3.7 percentage points (95% confidence interval [CI]: 0.1–7.4 percentage points) [9]. In every dimension except for Lying/Rolling (0.0± 16.7), mean dimensional % scores overall improved (though with wide SDs reflecting the heterogeneity of the study): Sitting, 1.4± 21.4; Crawling/Kneeling, 4.1± 27.3; Standing, 7.0± 21.8; and Walking/Running/Jumping, 6.2± 18.8.
Starting GMFM-88 total % scores at ADVANCE entry had ranged from 0% to 100% [9, 10]. Across this heterogeneous cohort, mean total % scores and their changes (overall and by broad disease state groups) may tend to blunt individual differences in motor trajectories. In our post hoc analyses, we sought to define what differences were meaningful and to describe patterns of motor change stratified by Pompe Motor Function Levels to gain a more nuanced understanding of change on therapy in patients who began 4000L treatment at different ages and with different degrees of motor impairment.
GMFM-88 MDC and effect size were estimated by a distribution-based method similar to analyses in a CP population by Ko et al. [12]. Effect size was estimated by dividing change from enrollment to Week 52 by standard deviation at enrollment [12, 29–31] for each Pompe Motor Function Level and age group. MDC was determined as a range from 0.3 to 0.7 ×SD at enrollment, bracketing low and high effect sizes; as Haley and Fragala-Pinkham argue [17], a range may account better for variability in responses and be more informative than a single value of MCID or MDC [17]. We considered estimation of an MDC range rather than a point estimate especially important in this first study of the GMFM-88 MDC in Pompe disease on alglucosidase alfa, because it is a single study in the pediatric Pompe population of one country at one point in time, and because our patients varied widely in both their motor abilities at enrollment and their degree of response to 4000L therapy. A point MDC estimate would have imposed spurious precision on this natural variability.
To understand the effect of pre-study motor decline or stability on treatment responses within each Pompe Motor Function Level as categorized at enrollment, MDC ranges were then used to categorize Week 52 GMFM-88 changes (improved, stable, or worsened) for each Pompe Motor Function Level and age group, and responses with or without pre-study motor decline (as reported on the Pompe Motor Function Level questionnaire). For this post hoc analysis, improvement was defined as GMFM-88 total % score increase of at least the lower bound of the subgroup MDC range, and worsening was defined as a total % score decrease of at least the magnitude of the lower bound of the subgroup MDC range.
Additional post hoc analyses
Detailed motor change descriptions, not only total GMFM-88 % scores and their MDC ranges and effect sizes, are important in longitudinal monitoring [12]. To better understand subgroups and skill areas that may change, in which subgroups and skill areas these changes may be occurring, we also assessed GMFM-88 dimension and item change in age cohorts and Pompe Motor Function Levels. Proportions of participants with positive, negative, or no change in each GMFM-88 dimensional subscore were determined by Pompe Motor Function Levels, separately for those < 2 years of age (termed the younger cohort elsewhere in the text) and≥2 years of age (termed the older cohort). We also identified specific GMFM-88 items that improved in high proportions of each age and phenotype cohort.
To account for different durations of pre-study alglucosidase alfa therapy and their contributions to participants’ motor status at enrollment and responses during ADVANCE, each participant’s fraction of life on 160L alglucosidase alfa at ADVANCE enrollment (FoL) was determined by the following formula: (Age at first 4000L infusion –Age at first 160L infusion)/Age at first 4000L infusion). The median FoL in the total ADVANCE cohort (N = 113) was 0.79. GMFM-88 total % scores at enrollment, Week 26, and Week 52 were analyzed descriptively by age groups (<2, 2 to < 5, 5 to < 8, 8 to < 12, and≥12 years of age) and FoL below or at/above the median value as one measure of the effect of prior therapy on motor function.
RESULTS
Participants
Participant disposition was published previously [9]. Participants in the full analysis set (N = 113) received 4000L alglucosidase alfa; 100 completed Week 52. Two participants died on-study before Week 52, 2 more discontinued before Week 52 and then died, 1 was discontinued by physician decision, and 8 discontinued and were transitioned to commercial 4000L alglucosidase alfa prior to Week 52 evaluations when the study terminated at US label expansion. At enrollment, 110 participants had Pompe Motor Function Level data. GMFM-88 data were available for 108 participants at enrollment and for 90 participants at Week 52 (the demographics of this n = 90 motor analysis set are presented in Table 1).
Table 1
Demographics of ADVANCE Participants by GMFM-88 Data Status
| Parameter | ADVANCE Motor analysis set (N = 90) | ADVANCE participants without complete GMFM-88 data (i.e., missing either enrollment and/or Week 52) (N = 23)a |
| Sex, n (%) | ||
| Male | 47 (52.2) | 13 (56.5) |
| Female | 43 (47.8) | 10 (43.5) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 12 (13.3) | 6 (26.1) |
| Not Hispanic or Latino | 75 (83.3) | 17 (73.9) |
| Not Reported | 1 (1.1) | 0 |
| Unknown | 2 (2.2) | 0 |
| Race, n (%) | ||
| Asian | 7 (7.8) | 0 |
| Black | 19 (21.1) | 7 (30.4) |
| White | 58 (64.4) | 13 (56.5) |
| Not Reported | 0 | 2 (8.7) |
| Multiple | 6 (6.7) | 1 (4.3) |
| Age (years) at first infusion of 160L alglucosidase alfa | ||
| Mean±SD | 1.7±2.7 | 1.6±2.8 |
| Median | 0.6 | 0.5 |
| Min, max | 0.0, 11.4 | 0.1, 10.6 |
| Age group (years) at first infusion of 160L alglucosidase alfa, n (%) | ||
| <0.5 | 34 (37.8) | 12 (52.2) |
| 0.5 to <1 | 21 (23.3) | 5 (21.7) |
| 1 to <5 | 25 (27.8) | 4 (17.4) |
| 5 to <8 | 5 (5.6) | 0 |
| 8 to <12 | 5 (5.6) | 2 (8.7) |
| Age (years) at first infusion of 4000L alglucosidase alfa | ||
| Mean±SD | 5.2±3.6 | 3.1±3.9 |
| Median (min, max) | 4.6 (1.0, 15.5) | 1.4 (1.0, 18.7) |
| Age group (years) at first infusion of 4000L alglucosidase alfa | ||
| <2 | 19 (21.1) | 14 (60.9) |
| 2 to <5 | 30 (33.3) | 7 (30.4) |
| 5 to <8 | 25 (27.8) | 0 |
| 8 to <12 | 10 (11.1) | 1 (4.3) |
| ≥12 | 6 (6.7) | 1 (4.3) |
| Baseline genotype category, n (%) | 86 (95.6) | 20 (87.0) |
| Missense/Missense | 29 (32.2) | 5 (21.7) |
| Null/Missense | 25 (27.8) | 5 (21.7) |
| Null/Null | 10 (11.1) | 3 (13.0) |
| Missense/Intronic | 14 (15.6) | 1 (4.3) |
| Null/Intronic | 7 (7.8) | 6 (26.1) |
| Intronic/Intronic | 1 (1.1) | 0 |
| Disease phenotype, n | ||
| IOPD | 70 | 17 |
| LOPD | 20 | 6 |
| Age (years) at first symptoms | ||
| N | 89 | 23 |
| Mean±SD | 0.6±1.3 | 0.3±0.3 |
GMFM-88, Gross Motor Function Measure-88; IOPD, infantile-onset Pompe disease; LOPD, Late-onset Pompe disease; SD, standard deviation. aTen participants completed Week 52 of the ADVANCE but had incomplete GMFM-88 data: 4 had GMFM-88 measured at Week 52 but not at enrollment, 5 had GMFM-88 at enrollment but not at Week 52, and 1 had missing GMFM-88 data at both timepoints. Thirteen participants in ADVANCE discontinued before Week 52 [9] and were not included in the GMFM-88 analyses: 2 died on-study before Week 52, and 2 discontinued before Week 52 and then died. 1 discontinued by physician’s decision and 8 discontinued when the study was terminated at US label expansion of 4000L alglucosidase alfa. The full N = 113 analysis set demographics are published [9].
Immunogenicity was published previously [9]. Within the full analysis set, 77 participants had anti-alglucosidase alfa antibodies at enrollment; of the 36 starting seronegative, 12 seroconverted before Week 52. Median peak titer among the 89 ever antibody-positive was 1600. Five participants had sustained titers≥25,600, whose antibody trajectories and GMFM-88 score changes were published previously. Fourteen participants in the full analysis set had severe IOPD genotypes determined to be cross-reacting immunologic material (CRIM)-negative [9, 10]. CRIM status was not formally included in either the primary analysis protocol or the motor post hoc analysis, but we describe motor status and outcomes in this group of patients.
Pompe Motor Function Levels
Results of the Pompe Motor Function Levels questionnaire at the time of enrollment including participants’ functional levels and whether caregivers had reported pre-study motor decline are summarized in Table 2 for the 110 participants who received this initial assessment. This assessment was intended to identify participants who had actively worsening disease within the 2 pre-study months before 4000L therapy began. Overall, there were 37 occurrences of motor decline affecting 26 participants (as each decline question was scored as a separate occurrence, 1 participant could have multiple occurrences): there were seven occurrences among the younger cohort (1 LOPD, 6 IOPD) and 30 among the older cohort (6 LOPD, 24 IOPD).
Table 2
Baseline Pompe Motor Function Levels and motor decline within the 2 pre-study months
| Participant age < 2 years at baseline (n = 31) | IOPD (n = 27) | LOPD (n = 4) |
| Level I –Walkers | 9 | 3 |
| Increased fatigue during play time | 1 | 1 |
| Level II –Supported Walkers | 1 | 0 |
| Increased fatigue during or after play | 0 | 0 |
| Decreased play in standing | 0 | 0 |
| Loss of ability to step independently at furniture | 0 | 0 |
| Loss of ability to pull to stand at furniture or crib | 0 | 0 |
| Level III –Supported Standers | 2 | 0 |
| Increased fatigue during play | 0 | 0 |
| Decreased crawling and independent exploration | 0 | 0 |
| Loss of ability to take steps with hand-held assistance | 0 | 0 |
| Level IV –Sitters | 9 | 1 |
| Increased fatigue during play | 0 | 0 |
| Decreased or loss in ability to transition in and out of sitting without adult assistance | 0 | 0 |
| Decreased ability to take weight into legs in supported standing | 0 | 0 |
| Level V –Restricted Antigravity Movement | 6 | 0 |
| Increased fatigue during supported sitting with caregiver or in adaptive equipment | 2 | 0 |
| Decreased ability to grasp and manipulate toys | 2 | 0 |
| Decreased ability to hold head upright in supported sit | 1 | 0 |
| Participant age≥2 years at baseline (n = 79) | IOPD (n = 57) | LOPD (n = 22) |
| Level I –Walkers | 7 | 4 |
| Increased fatigue during or after walking | 1 | 0 |
| Level II –Supported Walkers | 15 | 9 |
| Increased fatigue during or after walking | 5 | 2 |
| Change in their ability to transition from floor to standing | 2 | 1 |
| Change in their ability to climb stairs | 3 | 2 |
| Level III –Supported Standers | 10 | 3 |
| Increased fatigue during crawling at home | 0 | 0 |
| Decreased ability to take weight into their legs in supported standing | 1 | 0 |
| Decreased ability to take steps with use of an assistive device | 0 | 0 |
| Level IV –Sitters | 7 | 4 |
| Increased fatigue during sitting activities at home | 3 | 1 |
| Decrease or loss in ability to transition in and out of sitting without adult assistance | 1 | 0 |
| Decrease in the ability to take weight into legs in supported standing | 2 | 0 |
| Level V –Restricted Antigravity Movement | 18 | 2 |
| Increased fatigue during supported sitting with caregiver or in adaptive equipment | 4 | 0 |
| Decrease in ability to grasp and manipulate toys | 2 | 0 |
| Decrease in ability to hold head upright in supported sit | 0 | 0 |
IOPD, infantile-onset Pompe disease; LOPD, late-onset Pompe disease. Bold type indicates Pompe Motor Function Levels. Italic type indicates motor decline within the 2 pre-study months.
GMFM-88 contributions to composite primary endpoint in dose-regimen subgroups
Dose-regimen subgroups on the composite primary endpoint have not been reported previously, and the contribution of GMFM-88 to this endpoint is the only motor outcome available for the dose-regimen subgroups. At Week 52, 63 (85%) of the 74 participants who received 20 mg/kg/2 weeks dosing were stable or improved on the composite primary endpoint; the other 11, 20 mg/kg/2 weeks recipients, clinically worsened on the primary endpoint, and of these 7 had GMFM-88 decreases of≥8 percentage points. Thirteen participants received 20 mg/kg/week; 9 (69%) of these were stable or improved and 4 clinically worsened on the composite primary endpoint, 3 of whom had GMFM-88 decreases of≥8 percentage points. Ten participants received 40 mg/kg/2 weeks; 8 (80%) of these were stable or improved on the composite primary endpoint and 2 clinically worsened, both with GMFM-88 decreases of≥8 percentage points. Other regimens had fewer participants who were all (100%) stable or improved and without GMFM-88 decreases≥8 percentage points on the composite primary endpoint: 2 on 40 mg/kg/week, 3 on 30 mg/kg/2 weeks, and 1 each on 30 mg/kg/week and 10 mg/kg/week.
Motor status and responses among CRIM-negative participants with IOPD
The details for 14 children with IOPD with CRIM-negative genotypes are shown in Supplementary Table 2, and include ambulatory status at enrollment, Pompe Motor Function Levels and any pre-study decline, and GMFM-88 total percent scores and Week 52 changes. Of note, 6 of these participants received concomitant immunomodulation as previously reported [9] and 2 more participants had a recorded history of immunomodulation; additional participants may have received pre-study immunomodulation not captured in the ADVANCE data. The CRIM-negative group included participants at all Pompe Motor Function Levels, including 5 independent walkers and 2 assisted walkers. Baseline GMFM-88 scores ranged from 4.7% to 91.5%, and their Week 52 changes ranged from –14.4 to +25.8 percentage points.
GMFM-88 MDC and effect size evaluated by age based on Pompe Motor Function Levels
When motor functional subgroups of the whole ADVANCE population were considered irrespective of age, walkers and sitters had GMFM-88 total percent score changes within or above the MDC range at Week 52 (Table 3), suggesting that these were meaningful beyond measurement error. In the younger cohort, all Motor Function Levels except the lowest functioning level, Level V (restricted antigravity movement) attained MDC at Week 52 (Table 3). Among older participants, no Pompe Motor Function Level subgroup had Week 52 changes achieving MDC ranges (Table 3).
Table 3
MDC and effect size estimates by Pompe Motor Function Levels at enrollment and Week 52
| Parameters (all ages) | Overall (N = 90) | Walkers (Level I, n = 17) | Supported walkers (Level II, n = 22) | Supported standers (Level III, n = 14) | Sitters (Level IV, n = 16) | Restricted antigravity movement (Level V, n = 20) |
| % score at enrollment, mean±SD | 47.0±33.3 | 76.5±21.5 | 77.8±18.4 | 49.2±9.4 | 22.5±11.0 | 5.2±5.3 |
| Week 52 % score, mean±SD | 50.8±36.0 | 84.2±18.2 | 81.4±16.1 | 46.6±24.3 | 32.3±21.0 | 4.4±5.2 |
| Week 52 change in % score, mean±SD | 3.7±17.5 | 7.7 ± 12.3 | 3.6±22.9 | –2.7±23.4 | 9.9 ± 17.2 | –0.8±1.9 |
| Week 52 effect size (mean change/SD at enrollment) | 0.11 | 0.36 | 0.20 | –0.29 | 0.90 | –0.15 |
| Total score MDC range (0.3–0.7×SD at enrollment) | 10.0–23.3 | 6.4–15.0 | 5.5–12.9 | 2.8–6.6 | 3.3–7.7 | 1.6–3.7 |
| Parameters (<2 years of age) | Overall (n = 19) | Walkers (Level I, n = 7) | Supported walkers (Level II, n = 0) | Supported standers (Level III, n = 2) | Sitters (Level IV, n = 8) | Restricted antigravity movement (Level V, n = 1) |
| % score at enrollment, mean±SD | 38.5±19.0 | 53.1±9.8 | – | 45.4±19.5 | 22.9±8.8 | 15.8 |
| Week 52 % score, mean±SD | 59.5±24.5 | 70.2±21.4 | – | 80.6±1.6 | 47.0±17.3 | 13.3 |
| Week 52 change in % score, mean±SD | 21.1 ± 14.1 | 17.1 ± 13.3 | – | 35.2 ± 18.0 | 24.1 ± 12.1 | –2.5 |
| Week 52 effect size (mean change/SD at enrollment) | 1.11 | 1.74 | – | 1.80 | 2.74 | – |
| Total score MDC range (0.3–0.7×SD at enrollment) | 5.7–13.3 | 3.0–6.9 | – | 5.9–13.7 | 2.6–6.2 | – |
| Parameters (≥2 years of age) | Overall (n = 71) | Walkers (Level I, n = 10) | Supported walkers (Level II, n = 22) | Supported standers (Level III, n = 12) | Sitters (Level IV, n = 8) | Restricted antigravity movement (Level V, n = 19) |
| % score at enrollment, mean±SD | 49.3±35.9 | 92.8±5.9 | 77.8±18.4 | 49.9±8.2 | 22.0±13.4 | 4.7±4.9 |
| Week 52 % score, mean±SD | 48.4±38.3 | 94.0±5.1 | 81.4±16.1 | 40.9±21.2 | 17.7±12.3 | 4.0±4.9 |
| Week 52 change in % score, mean±SD | –0.9±15.3 | 1.2±5.9 | 3.6±22.9 | –9.0±17.6 | –4.3±4.8 | –0.7±1.9 |
| Week 52 effect size (mean change/SD at enrollment) | –0.03 | 0.20 | 0.20 | –1.10 | –0.32 | –0.14 |
| Total score MDC range (0.3–0.7×SD at enrollment) | 10.8–25.2 | 1.8–4.1 | 5.5–12.9 | 2.5–5.7 | 4.0–9.4 | 1.5–3.4 |
MDC, minimal detectable change; SD, standard deviation. Bold type data denote Week 52 changes that attain the subgroup-specific MDC range.
Directions of GMFM-88 dimensional change
Detailed description of patients’ patterns of motor change is important, and these changes are expected to differ among specific skill sets and reflect age and starting motor status [12]. Therefore, we evaluated GMFM-88 dimensional changes to determine proportions of participants who improved, were unchanged, or worsened on each GMFM-88 dimension by age cohorts and Pompe Motor Function Levels.
Overall cohort comparison
When GMFM-88 dimensional changes were analyzed in age groups only (Fig. 1A), a majority of younger participants improved (the Standing and Sitting dimensions had the highest proportions of young improvers, both 83.3%), whereas more of the older participants showed stability in all dimensions. The dimensions with highest proportions of older improvers were the Lying/Rolling, Standing, and Walking/Running/Jumping dimensions (29.6%, 28.2%, and 36.6%, respectively), but these proportions were less than those improving in the younger group in the same dimensions (55.6%, 83.3%, and 66.7%, respectively). Fewer younger participants showed a negative change in the bed or mat mobility associated with Lying/Rolling, Sitting, and Crawling/Kneeling dimensions; other higher motor dimensions were improved or unchanged. In the older cohort, every dimension had some proportion of negative change; dimensions of lower motor skill requirements showed greater proportions with negative change (15.5% on Walking/Running/Jumping to 39.4% on Lying/Rolling).
Fig. 1
Percentages of participants with positive, none, or negative GMFM-88 dimension scores change after 52 weeks. Percentages of participants (who had Week 52 data) with positive change, no change, or negative change in GMFM-88 dimension scores after 52 weeks of 4000L alglucosidase alfa, by age and Pompe Motor Function Level. Pompe Motor Function Level was available for 89 of the 90 participants who had GMFM-88 data available at enrollment and Week 52. In the younger cohort there were no Level II (supported walkers).
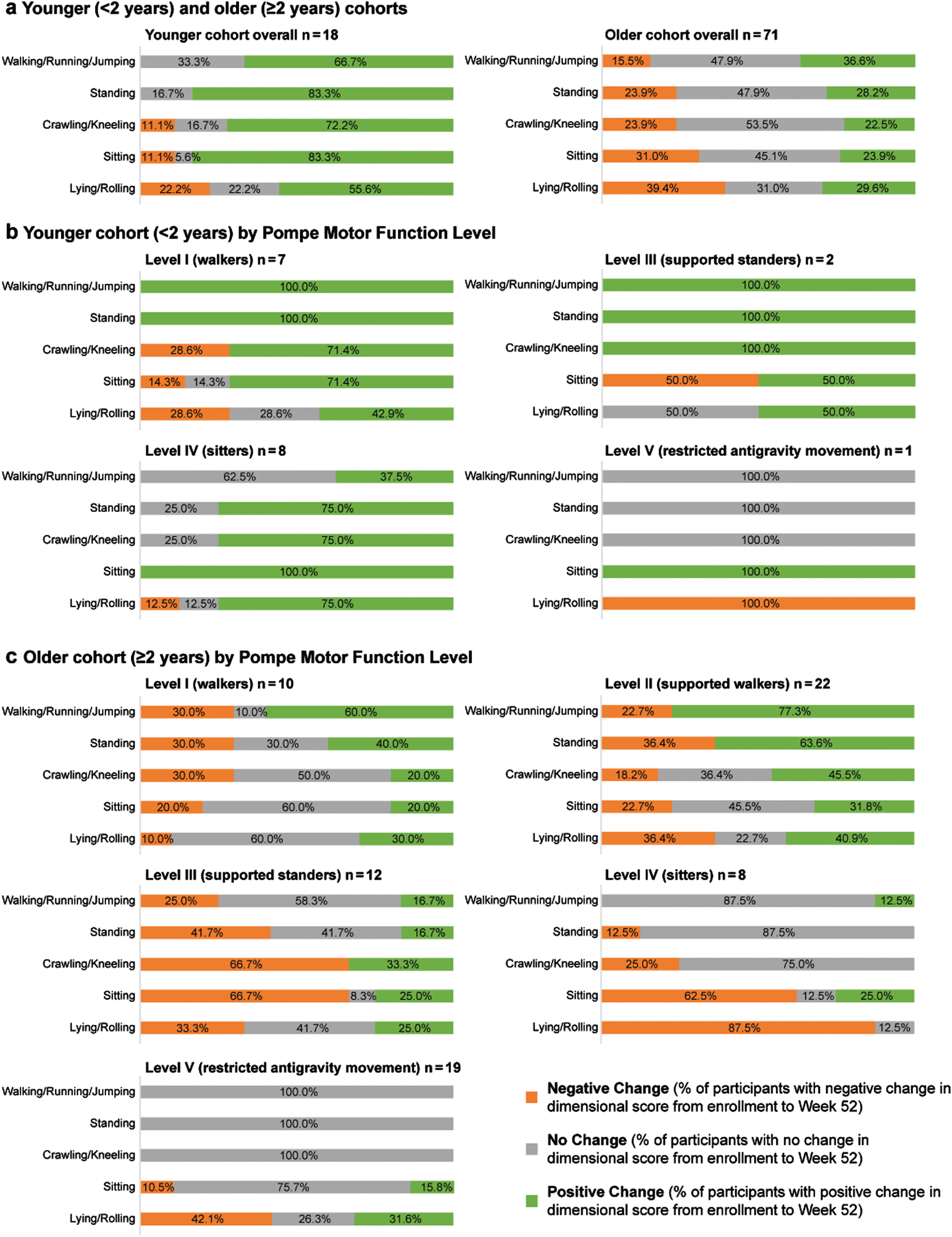
Younger cohort (<2 years of age)
Dimensional changes in the Pompe Motor Function Level subgroups of the younger cohort are shown in Fig. 1B. All walkers and supported standers (Levels I and III) in the younger cohort (there were no supported walkers, Level II, in this age group) improved on the Walking/Running/Jumping and Standing dimensions. All supported standers and 71.4% of walkers improved on the Crawling/Kneeling dimension. Half of supported standers improved on the Sitting and Lying/Rolling dimensions (both 50.0%), whereas, respectively, 71.4% and 42.9% of walkers did the same. All sitters (Level IV) and the 1 Level V participant improved in the Sitting dimension. Most sitters (75.0%) improved on Crawling/Kneeling and Standing dimensions and 37.5% on the Walking/Running/Jumping dimension, whereas these dimensions were unchanged in the restricted-antigravity-movement participant (Level V). The Lying/Rolling dimension improved in 75.0% of sitters but worsened in the participant with the lower functional level of restricted antigravity movement (Level V). Other negative changes in the younger group affected 14.3% to 28.6% of walkers (Level I) in the dimensions of Sitting, Lying/Rolling, and Crawling/Kneeling. Half of younger supported standers (Level III) had a negative change in the Sitting dimension.
Older cohort (≥2 years of age)
Dimensional changes in the older cohort by Pompe Motor Function Levels are shown in Fig. 1C. Older walkers and supported walkers (Levels I and II) had similar patterns of improvement on all dimensions, with the highest proportions improving on Walking/Running/Jumping and Standing dimensions and fewer on the transitional skill dimensions of Crawling/Kneeling, Sitting, and Lying/Rolling. Within each dimension, a larger proportion of older supported walkers (Level II) than independent walkers (Level I) improved. Among older supported standers (Level III), smaller proportions of improvement occurred, with a pattern favoring tasks not requiring standing (33.3% improved on Crawling/Kneeling, 25.0% each on Sitting and Lying/Rolling, and 16.7% each on Standing and Walking/Running/Jumping dimensions). Supported standers had proportions with negative changes ranging from 25.0% on the Walking/Running/Jumping dimension to 66.7% each on Crawling/Kneeling and Sitting dimensions. Older sitters and restricted-antigravity-movement participants (Levels IV and V, respectively) had similar high proportions of “no change” on Walking/Running/Jumping, Standing, and Crawling/Kneeling dimensions (skills they started without and did not acquire during ADVANCE, except for the 12.5% (1 of 8) sitters who showed any improvement on the Walking/Running/Jumping dimension; item review revealed that this participant scored 1 point each on the two Dimension E tasks of cruising to the left or right with both hands on a large bench, thus showing partial ability to take weight into the legs). Negative change was prevalent in older sitters (Level IV) on the Sitting and Lying/Rolling dimensions (62.5% and 87.5% respectively); older Level V participants (restricted antigravity movement) had respectively 10.5% and 42.1% negative change on the same two dimensions. Improvements in the Sitting dimension occurred for 25.0% of Level IV participants and 15.8% of Level V participants, 31.6% of whom also improved in the Lying/Rolling dimension.
GMFM-88 changes by Pompe Motor Function Level and pre-study decline status
Ninety participants had GMFM-88 data and 88 of these also had Pompe Motor Function Level data; 18 had pre-study motor decline (in 28 occurrences) and 70 had pre-study motor stability or improvement. When Week 52 GMFM-88 total % scores were categorized by Pompe Motor Function Levels and pre-study motor decline status, those with pre-study motor decline showed improvement within the MDC range at Week 52 only among walkers (Level I; 1/1, 100%) and supported walkers (Level II; 2/8, 25.0%; Supplementary Figure. 1). In non-ambulatory participants (levels III through V) with pre-study motor decline, two-thirds of those with restricted antigravity movement remained stable while only one-third of sitters showed stability on GMFM-88 by MDC range criteria at week 52. The other level IV and V participants with pre-study motor decline functionally worsened over the study period. Conversely, among those free of pre-study motor decline, every Pompe Motor Function Level had some participants with GMFM-88 improved within the MDC range (46.7% of walkers, 28.6% of supported walkers, 21.4% of supported standers, 53.8% of sitters, and 7.1% of those with restricted antigravity movement).
GMFM-88 changes by FoL on 160L alglucosidase alfa
The possibility that FoL on pre-ADVANCE 160L alglucosidase alfa treatment, reflecting how early treatment was initiated and how long it continued, affected motor response to 4000L alglucosidase alfa was also investigated. GMFM-88 total % scores were stratified through time by age groups and FoL (those who had received pre-study 160L alglucosidase alfa for < 0.79 or≥0.79 of their lives; 0.79 was the median FoL of the full analysis set, N = 113). Mean and median GMFM-88 total % scores increased from enrollment to Week 52 in participants < 2 years of age with FoL < 0.79; in the same age group, in participants with FoL≥0.79, means and medians also increased but less markedly. Among participants aged 2 to < 5 years, median total % scores appeared to increase with FoL < 0.79 and decrease with FoL≥0.79. In those aged 5 to < 8 years, median total % scores decreased with FoL < 0.79 and were roughly stable with FoL≥0.79. Among participants aged 8 to < 12 years, median total % scores appeared to increase with FoL < 0.79 (to be interpreted cautiously in these small subgroups), whilst those with FoL≥0.79 remained approximately stable. Participants≥12 years of age with FoL < 0.79 had low and roughly stable mean and median total % scores. The 1 participant in this age group with FoL≥0.79 began with high scores, which modestly declined through Week 52. Details are shown in Supplementary Table 3. Differences among age groups were consistent with MDC and effect size observations showing more improvement in younger ADVANCE participants.
GMFM-88 items frequently improved
To explore which motor skills ADVANCE participants acquired or improved during study treatment, we identified the individual GMFM-88 items that had improved in≥50% (or if no items reached 50%,≥20%) of each age group or IOPD/LOPD phenotype (Supplementary Table 4).
Thirteen individual GMFM-88 items improved in≥50% of the younger cohort (n = 19 with Week 52 item data), distributed among all GMFM-88 dimensions with a preponderance in the Standing dimension, including squatting, lifting one foot, or returning to erect standing after bending to pick up an object—skills often reported as difficult in Pompe disease. This indicates that over half of the younger cohort showed substantial improvement in 13 of the 88 functional motor skills that would be attained by 5 years of age in normally developing children without Pompe disease. Of note, “no change” did not distinguish between items already attained at study entry and those not attained at either study entry or Week 52.
Among the older cohort (n = 71 with Week 52 data) no single GMFM-88 item improved in≥50% of participants; only one item (lifting the foot in standing) improved in at least 20% of participants, indicating possible gains in lower limb stability.
Among the IOPD cohort (n = 70 with Week 52 data), no single GMFM-88 item improved in≥50% of the group. Ten items, five of them in the Standing dimension (including squatting, lifting one foot with or without hand support, and bending to pick up an object), improved in at least 20%. Frequently improved Walking/Running/Jumping items in IOPD were ascending and descending steps with alternate feet holding one railing.
Among the LOPD cohort (n = 20 with Week 52 data), no single GMFM-88 item improved in≥50% of participants. Sixteen items improved in at least 20%. The LOPD cohort’s most-improved items included supine antigravity tasks and advanced lower-limb skills such as jumping with both feet or stair use without a railing.
DISCUSSION
ADVANCE is the largest pediatric Pompe disease population evaluated to date with the GMFM-88. ADVANCE was a real-world study of pediatric patients who were treated in US practice with 160L alglucosidase alfa at the time of study recruitment prior to on-study treatment with 4000L alglucosidase alfa. The heterogeneities reflect a naturalistic cohort representing the diversity of pediatric Pompe presentations and practice in 2012–2014. This clinical study sought to address the challenges of capturing change in gross motor function in this very heterogeneous, previously 160L alglucosidase alfa-treated population with Pompe disease. Due to the heterogeneity of Pompe disease, we determined MDC values for different functional levels and age groups. Younger, higher-functioning participants with IOPD showed the most gains in GMFM-88 scores within or exceeding the MDC. Similar motor skill change patterns were seen using different assessments (e.g., Alberta Infant Motor Scale or Peabody Developmental Motor Scale) in previously published works, reflecting early initiation of treatment and motor development in young children with emerging motor skills [21, 32], which contrasts with the natural history of Pompe disease in untreated individuals [3]. Our results are consistent with those from a previous investigative GAA replacement therapy (from transgenic rabbit milk) that also showed more improvement in participants with IOPD who were < 2 years of age and had therapy initiation early in life than in those older and with later treatment initiations [33]. Ko and colleagues’ distribution-based MCID study of children with CP receiving physical therapy [12] also found greater GMFM-88 change in younger than older children, due to expected early developmental motor gains. While this ADVANCE post hoc analysis used an approach similar to the Ko study, it did not anchor or compare GMFM-88 changes with those of other motor scales or therapist or caregiver ratings of change.
Our results indicate that recipients of alglucosidase alfa produced at the 4000L scale improved their existing motor skills and attained new skills, especially for those younger participants who had good residual muscle strength at enrollment, particularly those who could walk, stand supported, or sit independently. Greater responsiveness in the < 2-year-old cohort likely was a combination of motor development, made possible by effects of therapy, that would have been impaired or precluded in untreated Pompe disease, especially infantile-onset phenotypes. Despite small subgroup effect sizes for the older cohort, older participants who could walk with or without support at enrollment had stability during the study (mean group positive changes, but less than the lower bound of the MDC range), contrasting with negative changes in older participants who started with less mobility, including supported standers and sitters, which may reflect continued disease progression in these older, more-impaired groups. Most motor changes were seen in the GMFM-88 dimensions related to Sitting and Lying/Rolling and supine activities, as the scale seems to be more sensitive to detect smaller changes associated with these activities as compared with the higher task dimensions of Walking/Running/Jumping, which would require greater effect in motor gains to detect changes.
These post hoc analyses did not subgroup motor MDC or effect sizes by anti-alglucosidase alfa antibody status, sex, or ventilator status (although primary-endpoint outcomes were published for those groups [9]). Primary and motor outcomes in the 5 participants with high sustained antibody titers (HSAT,≥25,600 and remaining within a≤2-fold dilution titer at the final measurement) were published in the primary efficacy paper [9]. Three of these achieved the primary composite endpoint, 1 worsened on the primary endpoint at Week 52, and 1 lacked Week 52 data and worsened on the primary endpoint at Week 26. HSAT was not invariably connected to either poor initial motor status or poor GMFM-88 response on treatment. The 3 HSAT participants with primary-endpoint success and GMFM-88 improvement or stability had peak titers not exceeding 25,600, whereas the 2 with primary-endpoint worsening (with or without GMFM-88 decline≥8 percentage points) had peak titers up to 102,800. Two HSAT participants with LOPD showed either substantial GMFM-88 improvement (baseline score 70%, +14.4 percentage points at Week 52) or stable maximum score (baseline score 100%, unchanged). Two HSAT participants with IOPD had GMFM-88 decreases of < 8 percentage points (ie, less than the primary-endpoint definition of motor worsening): baseline 4.7%, –2.3 percentage points at Week 52; baseline 1.2%, –0.8 percentage points at Week 26 and no data at Week 52). One HSAT participant with IOPD had Week 52 GMFM-88 decrease > 8 percentage points (motor worsening on the primary endpoint): baseline 19.8%, –14.4 percentage points. Further studies are needed to relate antibody responses to detailed motor responses and to identify other factors including impact of further longitudinal follow-up affecting motor outcomes.
While we did not analyze MDC or effect size by CRIM status, review of the 14 CRIM-negative children with IOPD showed that half of them were walking at ADVANCE enrollment. Among the CRIM-negative group, the highest baseline GMFM-88 total percent score was 91.5% and the highest GMFM-88 increase at Week 52 was +25.8 percentage points in a never ambulatory participant who started at a total percent score of 19.2%. In the alglucosidase alfa-experienced ADVANCE cohort, and especially with concomitant immunomodulation, CRIM-negative status did not necessarily portend either poor initial motor status or poor GMFM-88 response to 4000L alglucosidase alfa.
In all reported Week 52 dose regimen subgroups, a majority of participants were clinically stable or improved on the composite primary endpoint. Even with doses and/or frequencies higher than 20 mg/kg/2 weeks, some participants clinically worsened on the composite primary endpoint, and among those, GMFM-88 decline of≥8 percentage points contributed to this outcome.
Total % scores from baseline to Weeks 26 and 52 by FoL on pre-ADVANCE 160L alglucosidase alfa treatment suggested that the youngest participants with FoL below the population median of 0.79, who may have been those with IOPD with treatment initiations closer to ADVANCE enrollment, had more opportunity for improvement on 4000L alglucosidase alfa treatment before reaching school age. These participants started at lower scores but improved to higher Week 52 means than similar-aged participants with FoL at or above population median. Participants 8–12 years old with FoL below the median, who had higher starting scores and more marked increases on-study than their age peers with FoL at or above the median, may represent those with LOPD who came to clinical attention at school age.
The varied GMFM-88 total % scores in the ADVANCE cohort at time of enrollment reflect this heterogeneous population ranging over the entire spectrum of the scale from 0% to 100%. The participant(s) who had maximal tested skills at time of enrollment indicated a ceiling effect of the GMFM-88 and Pompe Motor Function Level assessments, suggesting a need for a scale that may measure higher level motor skills. Floor effects were also seen in the 2 of the least-mobility HSAT participants starting at respectively, 4.7% (decrease of 2.4 percentage points) and 1.2% (decrease of 0.8 percentage points), also indicating the need for valid measures on the lower end of the motor spectrum and patient or caregiver-reported outcomes such as the Pompe-PEDI. Sensitive measurement of motor decline will need to be based on Pompe functional subgroup levels. Dimensional proportions of improvement by functional levels at enrollment gave a more refined view of changes than total % score patterns. In the older cohort, improvement proportions were highest in those who could walk, with or without support, in the GMFM-88 dimension of Walking/Running/Jumping. Proportionally more supported walkers than independent walkers at this age improved on these dimensions because they had more room to improve. Large majorities of younger participants who were able to stand with support improved on lower-limb weight-bearing and quadruped dimensions with improvements towards independent standing. In contrast, many older participants who were able to stand with support showed no changes in weight-bearing skills while exhibiting declines in quadrupedal and sitting skills indicating decreased trunk and pelvic strength. Observations in older and less-mobile participants are consistent with previous reports of slowly progressive residual muscle weakness and severe motor delays alongside longer-term survival with treated IOPD [34]. Contributing factors associated with muscle imbalances and contractures may affect the degree of motor improvement attainable for those who are older and less mobile.
This analysis builds on work by van Capelle et al. [35], who constructed the Pompe Quick Motor Function Test (QMFT) (which draws on multiple sources including GMFM-88) by evaluating motor function in 91 children and adults with LOPD (5–76 years of age). This ADVANCE analysis expands understanding of dimensional and item-level responsiveness of the GMFM-88 for participants≤5 years of age with IOPD or LOPD and identified which GMFM-88 items were most frequently improved across age and disease phenotype. GMFM-88 detected change during 4000L alglucosidase alfa treatment in motor skills such as neck or hip/leg flexion from supine, transitions or stabilization against gravity, or stair climbing. Negative change may reflect numerous variables associated with development and disease progression, such as children’s refusal/behavior to perform these effort-dependent tests, joint contractures, and/or growth in body/limb size and weight requiring greater muscle strength for functional movement. However, this study did not capture reasons why participants may have refused or scored lower on individual test items.
Participants with IOPD had patterns of item improvement in the Standing or Walking/Running/Jumping dimensions including squatting, bending to pick up an object and returning to standing, and alternate stair climbing using a rail. Improvements in the Lying/Rolling dimension may translate to improved bed mobility and transitional mobility for patients with LOPD. Dimensional and item changes showed that all subgroups had concentrations of motor improvements in the Standing dimension and gross motor transitional activities, reflecting more trunk and pelvic girdle gains in strength. GMFM-88 does not assess upper limb or fine motor function, thus future studies should consider these skills in the most impaired group (Pompe Motor Function Level V), as gains may have occurred that were not captured as part of this study.
While GMFM-88 was not developed for Pompe disease, clinical experience shows that it is useful for monitoring participants with Pompe disease and not interchangeable with other gross motor assessments. GMFM-88 has more lying/rolling tasks than GMFM-66 [36, 37] and thus is potentially more informative for very young children and those who are non-ambulatory. Future studies assessing very young children with Pompe disease should consider norm-referenced tests to understand developmental motor maturation alongside a more specific test such as the GMFM-88, which is most informative when followed through time.
Although the QMFT (with 16 antigravity and proximal motor tasks similar to GMFM-88 items, chosen for their difficulty in Pompe disease) showed a significant difference after 1 year of alglucosidase alfa treatment in an LOPD cohort [35], QMFT is likely to capture fewer subtle changes than GMFM-88, especially for children in the developmental period of growth and those with lower functional motor abilities. For individuals with Pompe disease who have less mobility, even subtle motor improvements can have significant impact on independence and function, thus detailed measures such as GMFM-88 and Pompe-PEDI are still needed for motor monitoring.
We also found that categorization of Pompe motor functional levels was important in determining MDC values. ADVANCE is the first study that has modified the GMFCS into the Pompe Motor Function Levels tool to reflect levels of mobility. Towns et al. [23] argue that the GMFCS should not be applied outside CP populations without disease-specific validation and that different disorders need their own motor function classifiers. Functional levels and decline history as captured in the Pompe Motor Function Levels tool became important variables in understanding subcohort changes in MDC values with treatment. Our results show that those who were free of pre-study motor decline tended to show signs of stability or improvement compared to those with reported pre-study declines, thus making the decline questions on the Pompe Motor Function Level questionnaire a means to help identify participants who may be better responders on the GMFM-88 with treatment. We did not retrospectively collect participants’ GMFM-88 histories from diagnosis to ADVANCE enrollment, and the study was not set up to compare the slopes of change on 160L versus 4000L alglucosidase alfa enzyme therapy.
The ADVANCE study was not designed to compare different doses of 4000L enzyme replacement therapy. Though a majority of participants received 20 mg/kg/2 weeks throughout, the other regimens represented were diverse. Dose variation was one among many potential confounding patient-level variables, which also included disease phenotypes, ages of onset, diagnosis, and 160L treatment initiation, and ages and motor statuses at ADVANCE entry. Our analyses also did not evaluate effects of dose changes; therefore, we could not determine whether changes in GMFM-88 were less pronounced in participants who were on a stable dosage for a prolonged period.
The heterogeneous cohort comprising both IOPD and LOPD participants in this study is one of the primary limitations of the post hoc analyses despite its reflection of the real-world spectrum of Pompe disease. Additionally, our analyzed subgroups in some of the age groups and Pompe functional levels had very small group sizes. To fully understand meaningful clinical difference, future studies should combine anchor-based approaches with distribution-based estimations [15]) to understand changes that may impact clinical decision making and patient perception of change.
CONCLUSIONS
There has been little to no information heretofore on the responsiveness of the GMFM-88 and clinimetric properties of the GMFM-88 in Pompe disease. This study is, to our knowledge, the first estimation of MDC for GMFM-88 in Pompe disease to date. Due to the heterogeneity of the disease, MDC measures should be considered by Pompe Motor Function Levels and age for design of clinical trials and interpretation of results. Based on functional levels, walkers and sitters had changes within or above the MDC range at 52 weeks as well as younger participants with a Pompe Motor Function Level above Level V. Among older participants, regardless of subgroup level, none achieved MDC ranges. The Pompe Motor Function Level tool exemplifies a way of modifying an existing scale for the motor profile of Pompe disease and has provided an operational definition of functional groups to evaluate ADVANCE participants’ GMFM-88 responses. Stratification of starting motor status is important for evaluating treatment efficacy and does not strictly depend on a formally validated classification system.
This analysis identified subgroups who were high and low responders as well as the skill areas of greatest change. Gains in GMFM-88 skills on enzyme replacement therapy, even when subtle, can improve patients’ ability to move and function in their environment, contributing to overall independence. This study illustrates potential areas where functional change may be expected, based on age and functional level, that may be used to understand better responsiveness to change with medical treatment or rehabilitation interventions, and indicates the importance of measures complementary to the GMFM-88, such as Pompe-PEDI, to capture change in less-mobile patients and the need for tools to capture functional benefits in daily life.
The broad patterns of the data suggest that younger age, higher functional level at enrollment, and absence of pre-study motor decline were conducive to improvement on GMFM-88 dimensional scores and total percent score improvement within or exceeding the MDC. These findings support the importance of early diagnosis and treatment to improve opportunity for motor development in Pompe disease.
AVAILABILITY OF DATA AND MATERIALS
Qualified researchers may request access to participant-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Participant-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org.
ACKNOWLEDGMENTS
This study was supported by Sanofi. Medical writing assistance under the sole scientific control of the authors was provided by Kim Coleman Healy, PhD, CMPP and Jane M. Gilbert, BSc (Hons), CMPP, of Elevate Scientific Solutions, contracted by Sanofi for publication support services. Dawn Phillips, MSc, PhD, who consulted for Sanofi when the protocol was developed, designed the Pompe Motor Function Level modifications of GMFCS. Authors employed or contracted by the sponsor (KAH, MCF, SS, and CW) participated in study design, data collection, analysis, and interpretation, intellectually important input into the manuscript, final approval, accountability for accuracy and integrity of the work, and the decision to submit the manuscript.
CONFLICT OF INTEREST
TD reports Pompe-disease-related honoraria from Sanofi (advisory boards within and outside the scope of the study), membership of advisory boards for Roche, Scholar Rock, Cure SMA, Cytokinetics, Biogen, Novartis, Bristol Myers Squibb, and SMA Foundation, and service as a consultant for Roche, Audentes, Bristol Myers Squibb, Novartis, Trinds, ATOM International, and Scholar Rock. PSK reports grants from Amicus Therapeutics, Sanofi, and Valerion Therapeutics; consulting fees and honoraria from Amicus Therapeutics, Asklepios BioPharmaceuticals (AskBio), and Sanofi; membership of the Pompe and Gaucher Disease Registry Advisory Boards for Amicus Therapeutics, Baebies, and Sanofi; and equity with Asklepios BioPharmaceuticals (AskBio). JBG discloses study sponsorship and professional writing assistance from Sanofi during the conduct of the study and advisory board membership for Mallinckrodt Pharmaceuticals outside the submitted work. SHH reports personal fees from Alexion, employment by Seattle Children’s Hospital, and personal fees and grants from Sanofi outside the submitted work. RH has nothing to disclose. DK reports research funding from Sanofi and New York Medical College during the conduct of the study and is on the Sanofi speakers’ bureau for Pompe disease. NDL reports personal fees (honoraria for advisory board activities) and non-financial support (professional writing support) from Sanofi during the conduct of the study and outside the submitted work. LDMP discloses grant support and advisory board membership for AveXis, grant support from Biogen, Ionis, and Sanofi, advisory board membership for Roche/Genentech, and research support from Roivant, Inc. DWS is a member of the Sanofi Pompe Registry Advisory Board outside the submitted work and discloses grant support from Sanofi during the conduct of the study. PT discloses current employment by Quest Diagnostics. KAH, SS, and MCF disclose being employees of Sanofi and may hold stock and/or stock options in the company; CW discloses a contract with Sanofi for consulting work during the conduct of the study and services as a consultant for REGENXBIO. JWD reports personal fees from Audentes, grants from Ionis Pharmaceuticals, and grants and personal fees from AveXis, Biogen, Cytokinetics, Roche/Genentech, Sarepta, and Scholar Rock, outside the submitted work; he also has a patent 7442782 with royalties paid to Athena Diagnostics.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 ADVANCE investigators, centers, and institutional review boards (IRBs) or institutional ethics committees (IECs)
Supplementary Table 2 Ambulatory status, Pompe Motor Function Levels, and GMFM-88 total percent scores and Week 52 changes in ADVANCE participants with IOPD who were CRIM-negative (n = 14)
Supplementary Table 3 GMFM-88 total percent scores through time in the motor analysis set (N = 90) stratified by age groups and median fraction of life on 160L alglucosidase alfa (fraction of life; overall median 0.79 derived from the full analysis set [N = 113])
Supplementary Table 4 Individual GMFM-88 items frequently improved in subgroups defined by age or disease state (≥50% or, if no items attained 50%,≥20% of the participants in a group) within ADVANCE
Supplementary Figure 1 Patterns of GMFM-88 change from enrollment to Week 52 by Pompe Motor Function Level and motor decline status at baseline
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-210784.
REFERENCES
[1] | van der Ploeg AT , Reuser AJ . Pompe’s disease. Lancet. (2008) ;148: (5):372–53. doi: 10.1016/S0140-6736(08)61555-X. |
[2] | Kishnani PS , Amartino HM , Lindberg C , et al. Timing of diagnosis of patients with Pompe disease: Data from the Pompe registry. Am J Med Genet A. (2013) ;161A: (10):2431–43. doi: 10.1002/ajmg.a.36110. |
[3] | Kishnani PS , Hwu WL , Mandel H , et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. (2006) ;148: (5):671–6. doi: 10.1016/j.jpeds.2005.11.033. |
[4] | van Capelle CI , van der Meijden JC , van den Hout JM , et al. Childhood Pompe disease: Clinical spectrum and genotype in31 patients. Orphanet J Rare Dis. (2016) ;11: (1):65. doi: 10.1186/s13023-016-0442-y. |
[5] | Spiesshoefer J , Henke C , Kabitz HJ , et al. The nature of respiratory muscle weakness in patients with late-onset Pompe disease. Neuromuscul Disord. (2019) ;29: (8):618–27. doi: 10.1016/j.nmd.2019.06.011. |
[6] | van der Beek NA , de Vries JM , Hagemans ML , et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: A nationwide prospective observational study. Orphanet J Rare Dis. (2012) ;7: :88. doi: 10.1186/1750-1172-7-88. |
[7] | Hagemans ML , Winkel LP , Hop WC , Reuser AJ , Van Doorn PA , Van der Ploeg AT . Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. (2005) ;64: (12):2139–41. doi: 10.1212/01.WNL.0000165979.46537.56. |
[8] | Yang C-C , Chien Y-H , Lee N-C , et al. Rapid progressive course of later-onset Pompe disease in Chinese patients. Mol Genet Metab. (2011) ;104: (3):284–8. doi: 10.1016/j.ymgme.2011.06.010. |
[9] | Hahn SH , Kronn D , Leslie ND , et al. Efficacy, safety profile, and immunogenicity of alglucosidase alfa produced at the 4,000-liter scale in US children and adolescents with Pompe disease: ADVANCE, a phase IV, open-label, prospective study. Genet Med. (2018) ;20: (10):1284–94. doi: 10.1038/gim.2018.2. |
[10] | Kishnani PS , Gibson JB , Gambello MJ , et al. Clinical characteristics and genotypes in the ADVANCE baseline data set, a comprehensive cohort of US children and adolescents with Pompe disease. Genet Med. (2019) ;21: (11):2543–51. doi: 10.1038/s41436-019-0527-9. |
[11] | Russell DJ , Rosenbaum PL , Cadman DT , Gowland C , Hardy S , Jarvis S . The gross motor function measure: A means to evaluate the effects of physical therapy. Dev Med Child Neurol. (1989) ;31: (3):341–52. doi: 10.1111/j.1469-8749.1989.tb04003.x. |
[12] | Ko J . Sensitivity to functional improvements of GMFM-88, GMFM-66, and PEDI mobility scores in young children with cerebral palsy. Percept Mot Skills. (2014) ;119: (1):305–19. doi: 10.2466/03.25.PMS.119c14z1. |
[13] | Schünemann HJ , Guyatt GH . Commentary—goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res. (2005) ;40: (2):593–7. doi: 10.1111/j.1475-6773.2005.00374.x. |
[14] | Wright A , Hannon J , Hegedus EJ , Kavchak AE . Clinimetrics corner: A closer look at the minimal clinically important difference (MCID). J Man Manip Ther. (2012) ;20: (3):160–6. doi: 10.1179/2042618612Y.0000000001. |
[15] | Copay AG , Subach BR , Glassman SD , Polly DW Jr , Schuler TC . Understanding the minimum clinically important difference: A review of concepts and methods. Spine. (2007) ;7: (5):J.541–6. doi: 10.1016/j.spinee.2007.01.008. |
[16] | Turner D , Schünemann HJ , Griffith LE , et al. The minimal detectable change cannot reliably replace the minimal important difference. J Clin Epidemiol. (2010) ;63: (1):28–36. doi: 10.1016/j.jclinepi.2009.01.024. |
[17] | Haley SM , Fragala-Pinkham MA . Interpreting change scores of tests and measures used in physical therapy. Phys Ther. (2006) ;86: (5):735–43. |
[18] | Rosenbaum PL , Walter SD , Hanna SE , et al. Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. JAMA. (2002) ;288: (11):1357–63. doi: 10.1001/jama.288.11.1357. |
[19] | Winkel LP , Van den Hout JM , Kamphoven JH , et al. Enzyme replacement therapy in late-onset Pompe’s disease: A three-year follow-u. Ann Neurol. (2004) ;55: (4):495–502. doi: 10.1002/ana.20019. |
[20] | Bar-Yoseph R , Mandel H , Mainzer G , et al. Cardiopulmonary exercise test to quantify enzyme replacement response in pediatric Pompe disease. Pediatr Pulmonol. (2018) ;53: (3):366–73. doi: 10.1002/ppul.23830. |
[21] | Kishnani PS , Corzo D , Leslie ND , et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res. (2009) ;66: (3):329–35. doi: 10.1203/PDR.0b013e3181b24e94. |
[22] | Kishnani PS , Corzo D , Nicolino M , et al. Recombinant human acid [alpha]-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology. (2007) ;68: (2):99–109. doi: 10.1212/01.wnl.0000251268.41188.04. |
[23] | Towns M , Rosenbaum P , Palisano R , Wright FV . Should the Gross Motor Function Classification System be used for children who do not have cerebral palsy? Dev Med Child Neurol. (2018) ;60: (2):147–54. doi: 10.1111/dmcn.13602. |
[24] | Palisano R , Rosenbaum P , Walter S , Russell D , Wood E , Galuppi B . Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. (1997) ;39: (4):214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. |
[25] | Palisano RJ , Rosenbaum P , Bartlett D , Livingston MH . Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. (2008) ;50: (10):744–50. doi: 10.1111/j.1469-8749.2008.03089.x. |
[26] | Rosenbaum PL , Palisano RJ , Bartlett DJ , Galuppi BE , Russell DJ . Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. (2008) ;50: (4):249–53. doi: 10.1111/j.1469-8749.2008.02045.x. |
[27] | Case LE , Kishnani PS . Physical therapy management of Pompe disease. Genet Med. (2006) ;8: (5):318–27. doi: 10.1097/01.gim.0000217789.14470.c5. |
[28] | Tsai L-K , Hwu W-L , Lee N-C , Huang P-H , Chien Y-H . Clinical features of Pompe disease with motor neuronopathy. Neuromuscul Disord. (2019) ;29: (11):903–6. doi: 10.1016/j.nmd.2019.09.011. |
[29] | Oeffinger D , Bagley A , Rogers S , et al. Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Dev Med Child Neurol. (2008) ;50: (12):918–25. doi: 10.1111/j.1469-8749.2008.03150.x. |
[30] | Pardasaney PK , Latham NK , Jette AM , et al. Sensitivity to change and responsiveness of four balance measures for community-dwelling older adults. Phys Ther. (2012) ;92: (3):388–97. doi: 10.2522/ptj.20100398. |
[31] | Adair B , Said CM , Rodda J , Morris ME . Psychometric properties of functional mobility tools in hereditary spastic paraplegia and other childhood neurological conditions. Dev Med Child Neurol. (2012) ;54: (7):596–605. doi: 10.1111/j.1469-8749.2012.04284.x. |
[32] | Chien Y-H , Lee N-C , Chen C-A , et al. Long-term prognosis of patientswith infantile-onset Pompe disease diagnosed by newborn screeningand treated since birth. J Pediatr. (2015) ;166: (4):985–91e1-2. doi: 10.1016/j.jpeds.2014.10.068. |
[33] | Van den Hout JM , Kamphoven JH , Winkel LP , et al. Long-term intravenous treatment of Pompe disease with recombinant human alpha-glucosidase from milk. Pediatrics. (2004) ;113: (5):e448–57. doi: 10.1542/peds.113.5.e448. |
[34] | Prater SN , Banugaria SG , DeArmey SM , et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. (2012) ;14: (9):800–10. doi: 10.1038/gim.2012.44. |
[35] | van Capelle CI , van der Beek NA , de Vries JM , et al. The quick motorfunction test: A new tool to rate clinical severity and motorfunction in Pompe patients. J Inherit Metab Dis. (2012) ;35: (2):317–23. doi: 10.1007/s10545-011-9388-3. |
[36] | CanChild. FAQ’s about measures. [updated 2021; cited 2021, 5 Nov]. Available from: https://www.canchild.ca/en/diagnoses/cerebral-palsy/faq-s-about-measures. |
[37] | Beckers LW , Bastiaenen CH . Application of the Gross Motor Function Measure-66 (GMFM-66) in Dutch clinical practice: A survey study. BMC Pediatr. (2015) ;15: :146. doi: 10.1186/s12887-015-0459-8. |




