Adult North Star Network (ANSN): Consensus Document for Therapists Working with Adults with Duchenne Muscular Dystrophy (DMD) – Therapy Guidelines
BACKGROUND
The survival of people with Duchenne Muscular Dystrophy (DMD) significantly increased due to improvements in standards of care (SOC) [1]. Consequently, DMD has evolved from a paediatric disease to a severe, chronic, multisystem, adult condition. The published international standards of care advocate specialist multidisciplinary health monitoring through proactive, anticipatory approaches to slow down the effects of the disease and allow advanced, informed decision-making [1–3]. Therapy starts as soon as the diagnosis is made and plays a vital role in symptom management in individuals to improve function, participation and effective quality of life. Therapy interventions for management, differ depending on the setting in which the care is being provided, specifically in terms of the expertise within the teams and resources available within these settings.
People with DMD find that when they transition to adult services there is a dearth of expertise and limited access to therapy services. The survey conducted in the UK highlighted substantial differences between the care received by adults and children with the condition [2]. A large proportion of adults with DMD reported increased difficulties with access to professional physiotherapy, particularly at transition from childhood to adulthood. Additionally, having their functional abilities assessed regularly or receiving professional physiotherapy in general were both significantly more difficult to achieve within adult services in the UK. Furthermore, some of the major problems expressed by adults with DMD were mobility and transportation as well as, getting involved in leisure activities and work [3]. Therefore, while pediatric services are predominantly family-centred, after transition the paradigm of patient care changes towards individual-centred with focus on different therapy goals. Those become more tailored to the individuals’ needs, balancing quality of life and management options.This document is aimed at providing guidelines for physiotherapy, occupational therapy and speech and language considerations.
The ‘Adult North Star Network’ (ANSN) was founded in 2015 to advance care of adults with DMD living in the UK and to develop a prospective natural history database. There are currently 28 adult centres within the network, caring for at least 700 DMD patients. Transition age is varied depending on services and is generally between the ages of 16 to 18. There is a wide range of severity affecting people with DMD transitioned to adult services, those who are steroid naive will have been permanent wheelchair users for many years and have profound muscle weakness. On the other hand, steroid treated patients will most commonly have good upper limb function, and some maybe ambulant at the time of transition. Additionally the specific type of genetic mutation, compliance to therapy and environmental factors may play a role in disease progression and presentation at transition.
The aim of these guidelines is to support therapists working with adults with DMD with little or no experience to assist their clinical practice. Whilst the recommendations can be adopted by other health care systems in the world, we appreciate it will depend on resource availability.
METHODS
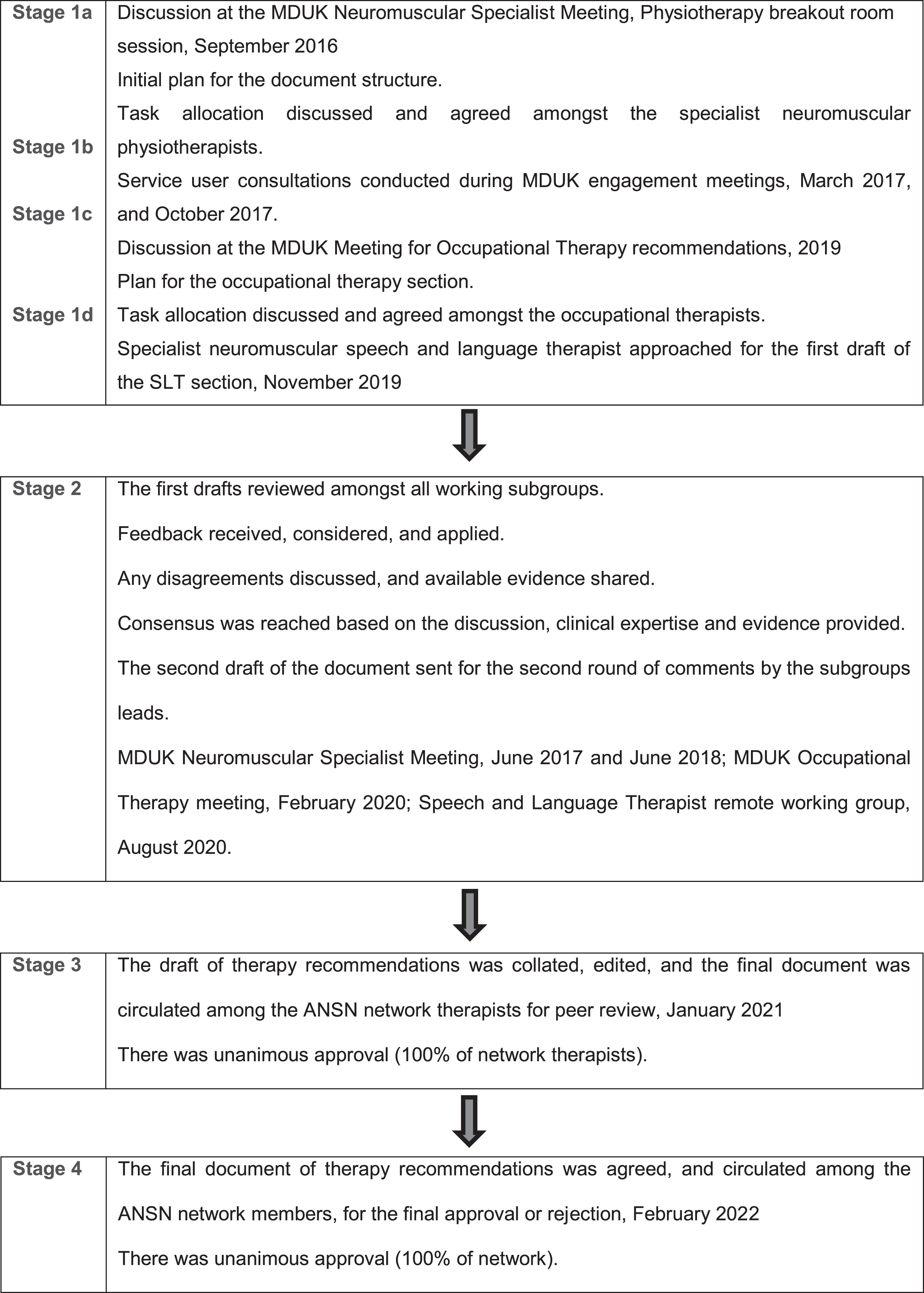
A step-by-step, structured process which included a multi-disciplinary-multi-professional approach, review of literature, subgroup discussions, and documentation of the discussions, agreements, and disagreements with a facilitator was used. The findings were reviewed by the group leads and at wider group meetings which involved multi-centre professionals. The final drafts were shared with the wider group and opportunities to raise concerns or comments were provided.
The ANSN consensus document for therapists working with the adults with DMD living in the UK was collated by Neuromuscular specialist therapists working in UK Centres of Excellence for neuromuscular diseases and rehabilitation therapists with a special interest in neuromuscular conditions. The working groups included physiotherapists, occupational therapists, speech and language therapists. The final document was collated and edited by two project lead therapists with clinical and research experience in neuromuscular conditions including DMD.
A detailed therapy manual was produced based on published literature and expert opinion to complement this article and is included in the supplementary materials. This document was published in conjunction with the ‘ANSN: Consensus Guideline For The Standard Of Care Of Adults With DMD. It will be reviewed and updated every 5 years.
Fig. 1
Consensus building process.
THERAPY RECOMMENDATIONS DIVIDED BETWEEN PHYSIOTHERAPY, OCCUPATIONAL THERAPY AND SPEECH AND LANGUAGE THERAPY
Physiotherapy considerations
Men with DMD require continuing support from Neuromuscular specialist physiotherapists, res-piratory physiotherapists and rehabilitation or community physiotherapists. In the UK, unlike in paediatric services where therapy input in the community is ongoing, adult community physiotherapy services provide a block of treatment lasting no longer than 6 weeks, following which individuals are usually discharged. This is not in the best interest of adults with DMD who have ongoing physiotherapy needs due to the progressive nature of the condition. Thus, for men with DMD, physiotherapy should be offered on a long-term continuing basis.
Respiratory physiotherapy management
The objective of respiratory physiotherapeutic interventions is to anticipate deterioration by maximising ventilation and airway clearance; to prevent, acute presentation to hospital [4, 6, 7].
An ineffective cough and poor ventilatory function can result in changes in aleveolar ventilation, atelectasis, mucus plugging and recurrent respiratory tract infections (RTI’s). Recurrent RTI’s along with oro-pharyngeal dysphagia (OPD) are the main causes of morbidity and mortality in patients with neuromuscular conditions [8–12].
Adults with DMD should be reviewed at least annually by a respiratory physiotherapist for support and advice on ventilation and airway clearance to both prevent and treat chest infections. Patients and their carers should be trained in airway clearance techniques such as assisted inspiration (e.g. breath stacking), Manual Insufflation Exsufflation devices and manual assisted cough. The respiratory physiotherapist should work closely with the Long Term Ventilation team to assess the indication and effectiveness of ventilation; advise on the use of other devices such as nebulisers, antimuscarinics and mucolytics [5–8].
Despite regular monitoring, optimising ventilation and secretion clearance management, individuals may still present with an acute hospital admission. The specialist respiratory physiotherapist should liaise with the admitting team, to support optimising management during admission and for discharge planning, with increase or change in care. Close collaboration with palliative care teams for symptom control maybe required. These supported conversations should be had, where possible, at earlier stages as the condition changes.
NEUROMUSCULAR SPECIALIST PHYSIOTHERAPY AND COMMUNITY / REHABILITATION PHYSIOTHERAPY MANAGEMENT
The community or rehabilitation physiotherapist has a key role to play in monitoring, assessing and offering treatments and support for adults with DMD. This role should be seen as a continuation of physiotherapy care accessed by boys with DMD in Children’s services. Community or rehabilitation physiotherapy should concentrate on several key areas including musculoskeletal management, orthoses, posture, seating and exercise for health and well-being.
Contracture management
For individuals with DMD, contractures are part of the natural history of the condition. There is a negative link between contractures and both long-term positioning and postural problems. Reduced and insufficient postural stability caused by weakened muscles, restricted joint movement can lead to increased fatigue, spinal abnormalities and kyphotic postures, as well as muscular contractures requiring further medical assistance [5].
Individuals with DMD should implement a daily stretching routine which takes a joint to a point of tightness being felt at end of range (rather than inducing pain); this will help preserve joint range of movement (ROM), provide relief from stiffness, ensure good positioning, maintain posture and function and help decrease pain and discomfort.
All individuals with DMD should have a contracture management plan which has been reviewed or provided by a Specialist Neuromuscular physiotherapist. This should include the use of passive stretches and static and/or dynamic splints [13]. The contracture management plan should also include postural management advice for 24/7 care [14].
Early implementation of a stretching program is an integral part of the physiotherapy management of DMD and begins in childhood. Good quality evidence to suggest that passive stretches change the length or properties of muscle/tendons is limited. However, some evidence is present to suggest that stretching in a normal muscle along with use of orthoses and other management strategies can improve the muscle tolerance to stretch [15]. Though the muscle pathology is affected in DMD, clinical consensus was in favour of a regular management programme that included stretching. In terms of time to hold stretches and frequency of stretches, the recommended time to hold a stretch is 15–30 seconds and 2–4 repetitions in healthy population [16].
Recommendations for a stretching program are as follows [17]:
• Stretching should be tailored to the individual and should focus on areas highlighted as tight or having reduced ROM within the assessment.
• Stretching should be performed daily or at least 4–6 times each week and ideally should be tailored into an individuals’ daily routine where possible.
• Stretches can be performed by carers, family or staff in educational institutions based on the programme designed by the Neuromuscular therapist.
• 24-hour positioning and equipment should be considered for use in conjunction with the stretching program to maximize the benefits of stretching.
• Standing programs are recommended when ambulation is no longer possible e.g. by aid of a standing frame or standing electric wheelchair, keeping in mind impact on cardiac function, particularly when an individual has not been standing for a while or has reduced cardiac output. Care must be taken when moving into an upright position and vitals need to be monitored when attempting a stand for the first time after a prolonged period.
Gastro soleus complex (ankle range of movement, ROM) is one of the first to be at risk of contractures. Stretches to gastrocnemius and soleus should be commenced from an early age continuing into adulthood. Resting splints (ankle-foot orthoses AFOs) are orthoses that can assist in the management of contractures at the ankle. These should be custom made, not “off the shelf” supplied. Daytime AFOs are not recommended for ambulant individuals with DMD [1].
Maintaining ROM at the ankle joint is extremely important for both ambulant and non-ambulant men. Tightness at the ankle, leading to a plantar flexed or supinated position of the foot can have an impact on the knee and hip including the Iliotibial band (ITB).
Tightness around the knee joint has an effect on hip position. It is important to maintain hamstring length to ensure the individual is still able to get into a comfortable sitting position with knees at 90 degrees with the pelvis / lumbar spine in a neutral position. To be able to maintain hygiene, prevent any pressures areas or skin breakdown in the popliteal fossa, good ROM at the knee is essential.
At the hip, psoas, hip flexors and ITB can become contracted making it difficult to fully extend the hip into a neutral position: this can make standing, positioning in bed, lying flat to roll, extremely difficult.
Maintaining upper limb ROM is extremely important in individuals with DMD to maintain independence with eating, drinking, gaming and for driving their powered wheelchair [18]. Resting hand splints should be discussed and prescribed for individuals with tight long digit flexors to maintain functional hand position.
Neck position and thoracic extension should be maintained as able, to help preserve an upright posture.
Postural management
Postural management requires a multi-disciplinary team (MDT) approach that has the potential to assist in enhancing and maximising an individual’s abilities in a wide variety of ways. It is important to manage the individual’s posture in order to:
• Improve and optimise the user’s ability of taking part in daily life –physically, mentally and socially.
• To communicate, breathe easily and be able to eat, drink and sleep in comfort
• Be as pain free as possible
• To support the individual proximally but also facilitate the individual move as freely as possible, with as much independent mobility as can be achieved
• Reduce the risk of injury e.g. development of pressure sores and further deterioration
• Preserve functional skills and participation
• Improve quality of life
Posture management requires a 24-hour approach involving the assessment and management of all positions that an individual uses i.e. lying in bed, sitting and standing, and is part of the condition management programme [1, 5, 14, 19]. This requires close working with Occupational therapy and specialist wheelchair services.
Wheelchair considerations
Individuals with DMD have specific needs that are often extremely limiting, causing users to become dependent upon others for all activities of daily living. Mobility is one of the few areas that, with appropriate provision, people can be fully independent whilst maintaining good postural alignment and this should be the primary goal. The DMD international Standard of Care guidelines state wheelchair provision should take place when the child is in the early late ambulatory phase [1].
Provision of the correct wheelchair and seating system is likely to be one of the most critical factors in helping to alleviate dependency and the sense of isolation that can result if mobility is restricted [20]. The disease course of DMD is well understood and anticipatory management is essential. Recent work developing consensus for powered mobility noted the importance of provision of powered mobility soon after the loss of the ability to walk 10metres independently [21].
As the condition progresses, individual needs may vary and regular reviews should be considered. The slow habitual patterns have a potential to severely restrict functional movements and ability in the future. It is therefore important to provide adequate assessment and support to make informed choices [22].
Specialist Neuromuscular services, should work closely with specialist wheelchair services, requesting reviews and liaise with regards to functions like seat elevation (riser function) in electric wheelchairs or assisted standing if appropriate.
Each stage of the progression of DMD is well documented. Attention to the sitting posture should start from the very first wheelchair and should be regularly reviewed and modified to meet changing needs. Foot positioning in neutral is recommended to avoid hip and knee pain and contractures, caused by external rotation at these joints. Centrally located footplates can encourage external rotation proximally. Careful assessment of optimum foot support is important. Fatigue should also be taken into consideration as posture deteriorates as the user becomes more tired.
It is also important to highlight that over time the user may need daytime ventilatory support. This may require equipment such as ventilator, suction machine, cough assist and oxygen. There needs to be space within the seating/wheelchair to accommodate this additional equipment following risk assessment. The user would need to be able to move independently in different environments with all vital equipment.
There are many different types of adaptations available. Among other adaptations it is important to adapt the joystick control with appropriate mounting and function of joystick. Light touch or sensitive joystick must be considered if indicated.
If adaptations are not available via the NHS in the area where the individual resides, charitable funding may be a financial source to help the user maximise their functional potential with the best possible sitting posture.
For further information please refer to the Wheelchair and Seating manual [23].
Exercise recommendations
Healthy individuals who exercise have greater VO2max (aerobic capacity) and exercise endurance, and greater energy levels [24]. Strength training, or weight-bearing exercise is also beneficial for healthy individuals and is recommended for vulnerable populations, including the elderly.
In DMD the muscles have a unique vulnerability which predisposes them to damage due to exercise [25]. There is progressive loss of contractile function, muscle membrane instability, physiological stress on blood vessels supplying active muscle, all contributing to muscle damage and muscle cell necrosis. Every consideration must be afforded to avoid overwork in exercise in DMD adults who are likely to be weaker and more prone to injury and fatigue than children or adolescents [26].
Therapists need to be aware of both overwork weakness as a result of exercise in DMD [27] and the long-term deleterious effects of de-conditioning and disuse associated with enforced sedentary behaviour, which can be significant on health and wellbeing. As DMD results in a progressive state of enforced immobility, individuals should be encouraged to break sedentary behaviour where possible.
It is therefore critical to ensure a balance between overuse and disuse in the DMD adult.
Exercise prescription
As there is marked heterogeneity within disease (particularly between steroid treated and steroid naive adults), individuals need to be assessed and individualised exercise or activity recommendations should be considered. Taking precautions into account exercise could prove beneficial in adults with DMD and the use of the FITT acronym; Frequency, Intensity, Type and Time may still be applicable for individualised exercise programmes. Physical activity sufficient to break sedentary behaviour and promote maintenance of whole-body fitness should be encouraged.
The risks of accelerated muscle damage associated with overwork in exercise are a more acute consideration in DMD adults. Therapists should be vigilant of the symptoms of overuse and appropriate modification of exercise should be implemented.
Occupational Therapy
Occupational Therapy (OT) is concerned with minimising the effects of occupational dysfunction, optimising occupational performance, helping people to regain occupational balance and develop a positive occupational identity [28–30].
Progressive loss of ability in adults with DMD results in declining participation in all performance areas –self-care, productivity and leisure [5, 31]. This is likely to result in a difficulty/inability to engage in age appropriate and meaningful roles, activities and occupations [32].
OTs use a person-centred approach to assess and evaluate physical, psychological and social needs. Their focus is to promote health and well-being by supporting individuals to maximise skills, increase competency and offering alternative ways to participate in valued meaningful every-day activities/occupations in all environments [33].
Physical needs
Assessment of function, as well as environmental barriers, will identify problem areas affecting participation in activities of daily living. Non-existent or limited upper limb function can affect an individual’s ability to manage personal care and basic nutritional needs. Muscle weakness, fatigue, anxiety, poor quality of sleep, sensory issues and reduced respiratory function will all have an impact on ability to engage in meaningful activity.
Occupational performance assessments to be carried out will include activity analysis, functional upper limb assessments and outcome measures such as the Performance of Upper Limb Assessment (PUL) [34], and the Canadian Occupational Performance Measure (COPM) [35].
As well as addressing participation in self-care activities (washing and bathing, toileting, grooming, dressing, eating and drinking, and sleep) consideration should be given to an individual’s ability to express emotions and intimacy. Body image, self-esteem, resilience, mood and parental/family attitude can all be factors. The importance of physical intimacy can be underestimated when an individual has regular physical contact for care needs.
Interventions will include equipment provision such as arm supports and assistive technology, positioning advice, fatigue management, splinting and home adaptations or re-housing. Education about the condition and how it affects their body and function is also important to help individuals develop compensatory strategies and access appropriate support. A manual handling care plan will address transfers. A referral for Continuing health Care or Personal Health Budget should be considered for personal assistant support. Referral to Psychology or counselling many also be indicated.
Psychological needs
Mental wellbeing is an important part of an individuals’ overall health and quality of life. It is important to recognise the link between feelings, thoughts and behaviours for an individual who has a progressive, life-limiting condition, which can have a negative impact on their capacity to participate in meaningful activities.
Support may be required to adjust and manage the feelings of grief related to the constant changes and loss of ability and skills, as well as to plan for future changing needs. It is also important to consider the impact on an individual’s self-esteem and resilience and the effect these feelings can have on the quality and type of relationships they have with others.
The importance of physical intimacy can be underestimated when an individual has regular physical contact for care needs. Individuals should be empowered to discuss their sexual health and well-being.
Some individuals may have additional learning needs or a deficit in cognitive function, particularly executive functioning skills. A cognitive assessment is required to determine any areas of difficulty/barriers for participation, so that relevant support can be provided. Education for the individual and their family/carers may be required so they understand the impact of a lack of dystrophin in the brain. Additional support may be required for an individual to pursue further academic education (e.g. referral to disability officers at university).
Social needs including sports and leisure
Engagement in work, sport, leisure and social activities can have physical, social and psychological benefits [36–42].
It is important to consider what an individual’s aspirations and interests are, and the value they place on their ability to participate in meaningful activities that contribute to wider society. Consideration should be given to educational opportunities, voluntary or paid work. Introducing individuals to groups in the voluntary sector can help initiate participation in campaigning, peer support, social activities and explore work/volunteering opportunities. Encouraging individuals to be physically and socially active should be included as part of the holistic condition management.
Physical barriers to participation might mean that individuals choose to engage in leisure activities that can be pursued at home, such as computer games, social media engagement and online campaigning, or playing musical instruments. These may require referral to specialist services for adaptations and assistive technology, such as eye gaze software or adapted controllers. Team members may need to think outside the box for available options to maximise engagement.
Leisure activities provide an individual the opportunity to control how they are spending their time. However, recognising family dynamics is important as individuals may need carer/family support for accessing leisure. Assessment should be carried out using a holistic family approach.
Individuals should be encouraged to participate in active leisure pursuits as they are more likely to engage in leisure activity than structured exercise programs. It gives them a chance to socialise and build a circle of friends. Accessibility is crucial to enable individuals to explore, engage and develop an enthusiasm for sport and leisure activities at any level.
Support should also be provided to enable accessible holiday planning, including insurance, fitness to fly, equipment at destination and handling of wheelchair.
Knowledge of local and national council accessible sports schemes is recommended (e.g. disabled sports organisations). Examples of accessible activities that could be explored are:
• Accessible water based activities
• Table top cricket
• Table tennis
• Wheelchair football
• Boccia
SPEECH AND LANGUAGE THERAPY
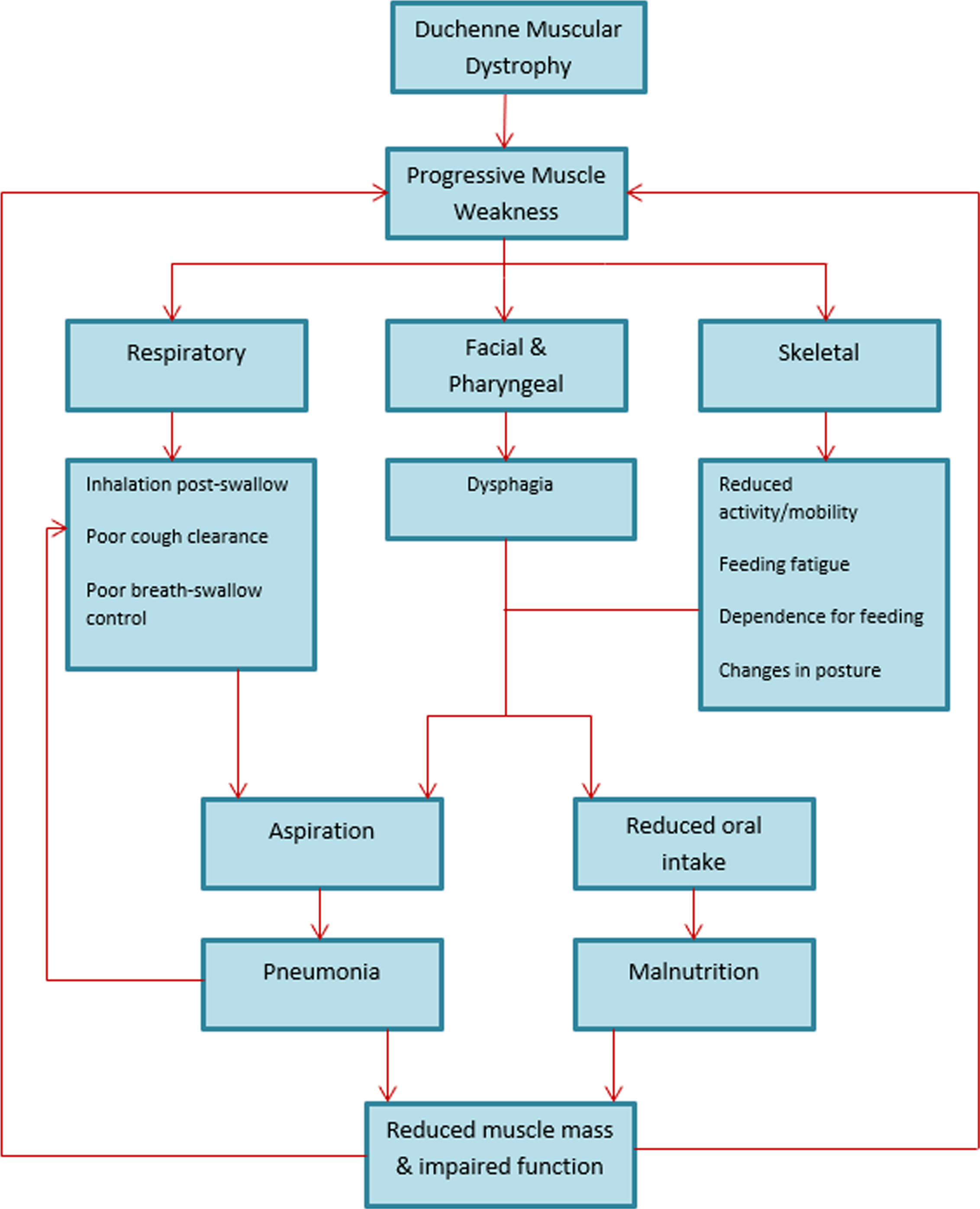
The identification and management of oro-phary-ngeal dysphagia (OPD) is vital to prevent respiratory complication and has thus become an integral part of the DMD pathway. The use of corticosteroids and developments in orthopaedic, respiratory and cardiac interventions have improved function, quality of life, health and longevity of DMD patients [1]. Increased lifespan has led to previously unidentified complications associated with swallowing [4, 43] such as aspiration pneumonia, weight loss, malnutrition and dehydration.
Whilst repeatable assessment tools for ortho-paedic, respiratory and cardiology monitoring have been identified [44–47], such an accepted tool for assessing and monitoring swallowing is yet to be considered [48].
Prevalence of OPD and natural history
Prevalence of OPD specific to DMD has never been examined, however there is enough evidence of existence to prompt expert panels to recommend routine input from speech and language therapists (SLT) [5].
There are no longitudinal studies of OPD to provide evidence of patterns or rate of progression in DMD. There is some agreement that pharyngeal residues increase with advancing age [49–51] as well as reports of increasing coughing or choking when eating and drinking [48, 50]. Worsening of these symptoms have also been reported in-line with deterioration in ambulation [53, 54]. More recently, researchers have identified correlations between respiratory function parameters and eating and digestive symptoms. Findings suggest that respiratory function rather than age may be a better predictor for swallowing and gastroenterological issues [55].
In the absence of longitudinal studies there is currently no evidence to guide recommendations for repeat OPD evaluation. The importance of patient education to understand the signs and symptoms of OPD in DMD is therefore paramount to allow patients to access repeat assessments in a timely and proactive manner. Six monthly reviews have been recommended for patients with signs and symptoms of OPD in the non-ambulatory stage [56].
Symptoms of OPD include prolonged meal times, drooling, difficulties in chewing, feeling of food sticking in the mouth or throat and/or coughing whilst eating and drinking and requiring dietary modifica-tions. These may become severe enough to preclude or reduce oral intake resulting in malnutrition, respiratory compromise and even mortality. Wider consequences of OPD include social isolation, depression, embarrassment, and poor quality of life [57]. Increased risk of infection and delayed recovery can lead to prolonged hospital admission, higher mortality and marked increase in public health spending [58].
Fig. 2
Impact of oropharyngeal dysphagia (OPD) in DMD.
Pathophysiology of OPD in DMD
Predisposing features and progression of OPD are not well understood in this population, however research relating to symptom and pathophysiology of swallow changes in DMD does exist [49–51, 53, 54, 59–66].
The pathophysiology of OPD in DMD is considered a result of underlying weakness in the oral and pharyngeal muscles. This weakness results in poor clearance of food and fluids through the mouth and throat, giving rise to post-swallow residues increasing the potential to aspirate.
A correlation between OPD and respiratory muscle failure has been investigated [65, 66] to understand the potential benefits of mechanical ventilation on swallow functioning and reduction of aspiration risk.
Whilst considered a primary motor disease, sensory components of OPD have been reported anecdotally via retrospective review of videofluoroscopic swallow studies (VFSS) [50, 69]. Impairment of pharyngeal sensation predisposes patients to silent aspiration; the mechanisms of which are currently unclear but could arise from unexplored interactions with the gastrointestinal tract such as gastro oesophageal reflux disease [70], central nervous system involvement [71], impact of hypercapnia [72], desensitisation secondary to slow progression or ventilation interfaces [73] and/or other reasons not yet considered.
Sensory changes in the pharynx should be borne in mind in relation to an individual’s ability to self-evaluate symptoms of OPD.
Identification of OPD
Early diagnosis of OPD identifies those requiring specialist intervention to reduce risk of future complications; to mitigate clinical and economic ill effects [4, 5, 74]. Symptom-specific questions should be incorporated into clinical screening interviews by a suitably qualified healthcare professional which may include the patients’ neurologist, GP, clinical nurse specialist or therapist. Questions and clinical history suggestive of weight loss and/or recent history of chest infections should also be incorporated.
Low weight /weight loss could be considered as a possible sign of undiagnosed OPD but could also contribute to a worsening profile of OPD.
Careful attention should be paid to changes in meal-time regimes such as lengthened meal-times, food avoidance and adaptations which may provide vital clues to emerging OPD.
Additional screening tools should be considered for individuals undergoing more comprehensive clinical assessment.
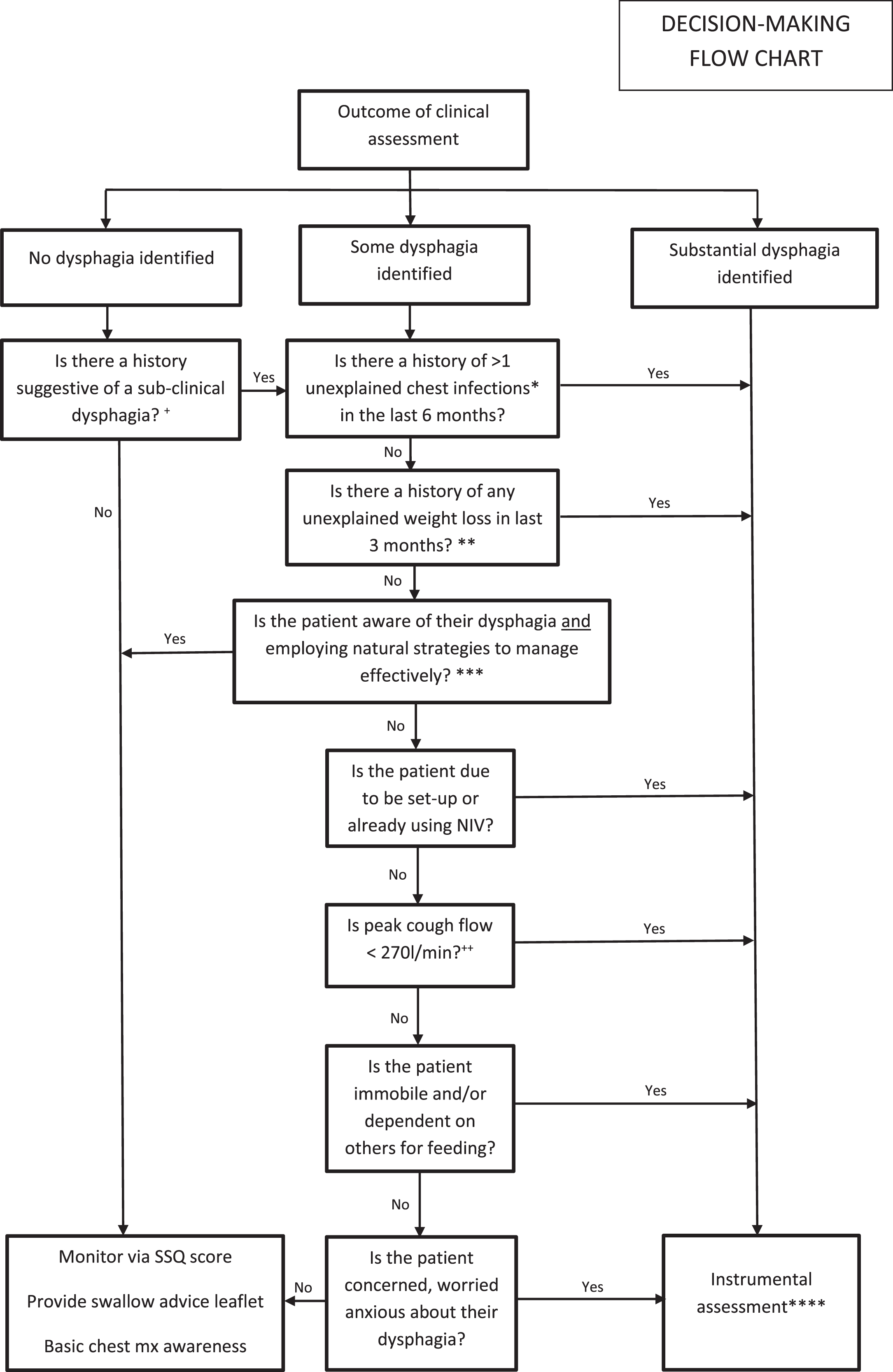
In the presence of signs or symptoms of OPD, individuals should be referred for assessment by a SLT, ideally with specialism or access to specialty advice in neuromuscular disease +/- respiratory care. Figure 3 demonstrates a flow chart to support clinical decision-making for instrumental swallowing assessment.
Fig. 3
*Any unexplained chest infection(s) resulting in either a hospital admission and intravenous antibiotic (abx) treatment or GP prescription of abx (ideally confirmed by consolidation on chest x-ray) with no other explanation e.g., no history of cold virus in the family or associated cold symptoms. **Weight loss of >5% that cannot easily be attributed to such things as mood, difficulties with preparing meals or gastroenterological symptoms. ***Answer no if patient is aware of their dysphagia but not employing any good compensations. ****When deciding between Videofluoroscopic Swallowing Study (VFSS) or Fibreoptic Endoscopic Evaluation of Swallowing (FEES), consider factors such as the need to assess secretion management, oesophageal clearance and glottic/supraglottic function for cough augmentation. Ask whether doing the assessment would ultimately change clinical management. Benefits of instrumental assessment may include additional information to support differential diagnosis regarding aetiology of chest infections, targeted prescription of abx treatment for chest infections,targeted dysphagia strategies and management (including biofeedback), selection and prescription of cough augmentation and chest clearance strategies. +This may include ≥10% weight loss over 3 months, any hospital admission with diagnosed chest infection in last three months and/or persistent use of domiciliary antibiotics. Patients reporting dysphagia symptoms but without bedside signs may also come into this category. ++If the patient is unable to participate in peak cough flow measurements, consider spirometry findings and/or measurements of peak expiratory flow to guide your decision making. Liaise with the respiratory physiotherapist and/or respiratory physician.
Aims of management
• Early identification of OPD should aim to support proactive management and advanced, informed decision-making to prevent avoidable hospital admissions secondary to consequences of undiagnosed OPD.
• Early, supported conversations should be encouraged, to prevent decisions being made on an emergency, acute basis.
• Any management decision should be patient-centred and focus on QoL as well as risk reduction.
• Whilst the emphasis of OPD assessment is on proactive, preventative identification and management, there is always the possibility that OPD maybe present /apparent only after emergency hospital admission.
• Given the potential for decompensation of the swallow at times of acute illness, attention should be paid to the cause of hospital admission and likelihood of any reversible issues, including alterations in swallow ability.
Self management
• Individuals tend to make dietary adaptations [50, 52, 59, 63]
• A move to small frequent calorie-rich diet with fluids washes is recommended [65].
• Given the issues with pharyngeal clearance, thickening of fluids is not recommended in routine practice.
Nutrition
The nutrition team should be familiar with the appropriate investigation, treatment and long term follow up of these patients and must be experienced and skilled in discussing the options for support with patients. Discussions should include the potential benefits, and any associated risks, of gastrostomy placement to support with nutrition and hydration for patients where this cannot be maintained orally. Dental or orthodontic referrals should be considered should such issues impact nutrition.
The literature is still inconclusive in defining what tests or parameters mandate OPD assessment and management. A shared decision-making framework as shown in Fig. 2 should be used alongside a team that is experienced and skilled in discussing the options for support.
SPEECH AND COMMUNICATION
Weakness in the oral, laryngeal and respiratory muscles can present problems with low speech volume. This can affect the ability of others to successfully understand what the person living with DMD is saying, particularly when there is background noise. This can be burdening in activities which involve social participation [2, 3]. Such issues should be considered as part of the therapy assessment process and steps put in place to minimise the impact of low speech volume on social participation. This may include:
• Management of superfluous background noise in conversation environments
• Encouraging conversation partners to adjust their height (for example sit rather than stand) to the same level of the person speaking or accessing wheelchair functions to raise the height of the chair to the same level as the conversation partner.
• Consideration of microphone or voice amplification devices
• Consideration of assistive technology to aid communication
ADULT NORTH STAR NETWORK
| Aleksandra Pietrusz | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Alice Roe | University Hospitals Birmingham NHS Foundation Trust |
| Andy Rose | Cambridge University Hospitals NHS Foundation Trust |
| Angela Reddy | Guy’s and St Thomas’ NHS Foundation Trust |
| Ann Morgan | South West Neuromuscular Operational Delivery Network |
| Anna Walker | Muscular Dystrophy Support Centre |
| Ashley Moore | Devon |
| Bobby Ancil | Muscular Dystrophy UK |
| Caroline Hutchings | University Hospitals Southampton NHS Foundation Trust |
| Charlotte Massey | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Dionne Moat | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Dr Adam Rochester | Royal Brompton and Harefield NHS Trust |
| Dr Adnan Manzur | Great Ormond Street Hospital for Children NHS Foundation Trust |
| Dr Adrian Morley-Davies | University Hospitals of North Midlands NHS Trust |
| Dr Ajit Thomas | University Hospitals of North Midlands NHS Trust |
| Dr Alanna Hare | Royal Brompton and Harefield NHS Trust |
| Dr Anna Mayhew | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Dr Andria Merrison | South West Neuromuscular Operational Delivery Network |
| Dr Antonis Pantazis | Royal Brompton and Harefield NHS Trust |
| Dr Ben Messer | Newcastle upon Tyne Hospitals NHS Foundation Trust |
| Dr Channa Hewamadduma | Sheffield Teaching Hospitals Foundation NHS Trust |
| Dr Charlotte Brierley | Cambridge University Hospitals NHS fFoundation Trust |
| Dr Charlotte F Dougan | The Walton Centre NHS Foundation Trust |
| Dr Chiara Marini-Bettolo | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Dr Clare Wood-Allum | South West Neuromuscular Operational Delivery Network |
| Dr David Shakespeare | Lancashire Teaching Hospitals NHS Foundation Trust |
| Dr Dispansu Ghosh | Leeds Teaching Hospitals NHS Trust |
| Dr Eleanor Marsh | Cardiff and Vale University Health Board |
| Dr Emma Husbands | Gloucestershire Hospitals Foundation Trust |
| Dr Fiona Norwood | King’s College Hospital NHS Foundation Trust |
| Dr Georgina Burke | University Hospitals Southampton NHS Foundation Trust |
| Dr Girija Sadalage | University Hospitals Birmingham NHS Foundation Trust |
| Dr Gita Ramdharry | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Dr James Lilleker | Salford Royal Teaching Hospital NHS Foundation Trust |
| Dr Jarod Wong | University of Glasgow |
| Dr Jatin Pattni | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Dr Javid Khan | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Dr Jennifer Spillane | Guy’s and St Thomas’ NHS Foundation Trust |
| Dr Jenny Thomas | Cardiff and Vale University Health Board |
| Dr John Bourke | Newcastle upon Tyne Hospitals NHS Foundation Trust |
| Dr John McConville | Belfast City Hospital |
| Dr Lynne Williams | Royal Papworth NHS Foundation Trust |
| Dr Mahalekshmi Desikan | National Hospital for Neurology and Neurosurgery |
| Dr Margaret Phillips | University Hospitals of Derby and Burton |
| Dr Maria Farrugia | Scottish Muscle Network |
| Dr Mark Busby | Bradford Teaching Hospitals NHS Foundation Trust |
| Dr Mark Roberts | Salford Royal Teaching Hospital NHS Foundation Trust |
| Dr Mark Rogers | Cardiff and Vale University Health Board |
| Dr Michael Davies | Davies Royal Papworth NHS Foundation Trust |
| Dr Michelle Chatwin | Royal Brompton and Harefield NHS Trust |
| Dr Patrick Murphy | Guy’s and St Thomas’ NHS Foundation Trust |
| Dr Priya Shanmugarjah | Leeds Teaching Hospital NHS Foundation Trust |
| Dr Rahul Mukhergee | Birmingham Heartlands Hospital |
| Dr Richa Kulshrestha | The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust |
| Dr Richard Keen | Royal National Orthopaedic Hospital NHS Trust |
| Dr Richard Walters | Morriston Hospital Swansea |
| Dr Ronan Astin | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Dr Saam Sedehizadeh | Nottingham University Hospitals NHS Foundation Trust |
| Dr Shagufay Mahendran | The Walton Centre NHS Foundation Trust |
| Dr Shelley Srivastava | Guy’s &St Thomas’ NHS Foundation |
| Dr Simon Baudoin | Newcastle upon Tyne Hospitals NHS Foundation Trust |
| Professor Nick Hart | Guy’s &St Thomas’ NHS Foundation |
| Dr Stam Kapetanakis | Guy’s &St Thomas’ NHS Foundation |
| Dr Stefen Brady | Oxford University Hospitals NHS Foundation Trust |
| Dr Tim Quinnell | Royal Papworth NHS Foundation Trust |
| Dr Venkataramanan Srinivasan | University Hospitals Birmingham NHS Foundation Trust |
| Emily Ballard | Guy’s and St Thomas’ NHS Foundation Trust |
| Emma Gallagher | University Hospitals Birmingham NHS Foundation Trust |
| Emma Manchester | Bradford Teaching Hospitals NHS Foundation Trust |
| Hayley Davis | Cardiff and Vale University Health Board |
| Heledd Tomos | Morriston Hospital Swansea |
| Helen Chase | University Hospitals Birmingham NHS Foundation Trust |
| Jane Freebody | Oxford University Hospitals NHS Foundation Trust |
| Janet McCay | South West Neuromuscular Operational Delivery Network |
| Jassi (Amritpal Singh) Sodhi | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Jill Davies | Cardiff and Vale University Health Board |
| Jo Reffin | King’s College Hospital NHS Foundation Trust |
| Jodi Allen | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Joni Cox | Norfolk Community Health and Care NHS Trust |
| Jonathon Slimming | School of Health Sciences, University of Liverpool |
| Julie Cassell | University Hopsitals of Derby and Burton |
| Kate Russell | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Kate Strachan | The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust |
| Kathryn Docherty | University Hospitals Dorset NHS Foundation Trust |
| Katie Nevin | Sheffield Teaching Hospitals NHS Foundation Trust |
| Katie Waller | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Kirstie Spencer | Nottingham University Hospitals |
| Lindsay Maidment | Sheffield Teaching Hospitals Foundation NHS Trust |
| Lisa Cutsey | Bradford Teaching Hospitals NHS Foundation Trust |
| Lynn Ward | MD Support Centre Coventry |
| Maria Patasin | National Hospital for Neurology and Neurosurgery |
| Marina Di Marco | Scottish Muscle Network |
| Meredith James | John Walton Muscular Dystrophy Research Centre, Newcastle |
| Michelle Ennis | The Walton Centre NHS Foundation Trust |
| Nicholas Emery | The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust |
| Nicola Grose | South West Neuromuscular Operational Delivery Network |
| Nicola Moorcroft | West Midlands Rehabilitation Centre |
| Nicola White | The Walton Centre NHS Foundation Trust |
| Paul Orme | The Neuromuscular Centre, Winsford |
| Paula Fenty | Nottingham University Hospitals NHS Foundation Trust |
| Phil Kelly | Salford Royal Teaching Hospital NHS Foundation Trust |
| Professor Anita Simonds | Royal Brompton and Harefield NHS Trust |
| Professor Anton Emmanuel | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Professor Derek Willis | Shrewsbury and Telford Hospital NHS Trust |
| Professor Nick Davies | University Hospitals Birmingham NHS Foundation Trust |
| Professor Ros Quinlivan | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Professor Tracey Willis | The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust |
| Rachel Ibbotson | Derby Neuromuscular Service |
| Rebecca Flesher | The Walton Centre NHS Foundation Trust |
| Rebecca Hart | Scottish Muscle Network |
| Sally Glover | University Hospitals Birmingham NHS Foundation Trust |
| Samantha Wood | Lancashire &South Cumbria NHS Foundation Trust |
| Sarah Gates | Morriston Hospital Swansea |
| Sarah Holmes | University College London Hospitals, National Hospital for Neurology and Neurosurgery |
| Sarah Mason | Belfast City Hospital |
| Siobhan Macauley | Belfast City Hospital |
| Siobhan Lamb | Belfast City Hospital |
| Sunitha Narayan | University Hospital Southampton NHS Foundation Trust |
| Tracey Adjei | Hull and East Yorkshire Hospitals NHS Trust |
| Yvonne Julian | University Hospitals of Leicester |
SUMMARY
Therapeutic interventions are an integral part of the holistic care of adults of people with DMD. Individualised symptom management process to improve function and increase participation is vital for maintaining and improving quality of life. Adults with DMD require a MDT approach including regular assessments completed by physiotherapists (neuromuscular, respiratory and rehabilitation), occupational therapists, speech and language therapists and dietitians.
All patients require regular monitoring of respiratory function and cough strength to anticipate deterioration and to prevent hospital admissions. Joint ROM should be monitored to preserve range, prevent contractures and ensure good positioning to provide relief from pain and stiffness. Postural management should be completed by an MDT with 24-hours approach involving the assessment and management of all positions used in everyday life. Customised wheelchairs should be prescribed to provide support while allowing functional mobility. As the condition progresses, needs may vary, and regular reviews are necessary to maintain independence. Considering a progressive state of enforced immobility because of DMD, individuals should be encouraged to break sedentary behaviour where possible. Physically active leisure pursuits can provide opportunities to socialise while engaging in physical activity.
OT assessment should be an integral part of routine MDT reviews. OT can facilitate engagement in age appropriate and meaningful roles, activities, and occupations. Assistive Devices and Technologies (ADT) may also be recommended to maintain or improve function and independence to enhance overall well-being.
Swallowing and nutritional status should be assessed regularly to maintain good nutrition and facilitate informed decisions.
ACKNOWLEDGMENTS
We are grateful to Muscular Dystrophy UK (MDUK) for funding this project and to Bobby Ancil from MDUK for his help in organising and co-ordinating the workshops. Dr Quinlivan is supported by the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre.
We would like to thank Dan Dean, Benjamin James, Hamada Hussain, Jon Hastie, Jordan Wright, Josh Howard, Josh Langley, Mahesh Malhotra, Matthew O’Sullivan, Mitch Coles, Mithan Soul, Phillippa Farrant, Phillip Carroll and Janet Bloor, Ravi Mehta, Shelley Simmonds, Tyran Hawthorne, Vivek Gohil, Zishan Kinoo for their input into this document from a service user perspective.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-210707.
REFERENCES
[1] | Birnkrant D , Bushby K , Bann C et al. Diagnosis and management of Duchenne muscular dystrophy, part diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritionalmanagement. Lancet Neurology. (2018) ;17: (3):251–67. |
[2] | Rodger S , Woods KL , Bladen CL , Stringer A , Vry J , Gramsch K , Kirschner J , Thompson J , Bushby K , Lochmüller H . Adult care for Duchenne muscular dystrophy in the UK. J Neurol. (2015) ;262: (3):629–41. DOI: 10.1007/s00415-014-7585-3 |
[3] | Pangalila RF , van den Bos GAM , Bartels B , Bergen MP , Kampelmacher MJ , Stam HJ , Roebroec ME . Quality of life of adult men with Duchenne muscular dystrophy in the Netherlands: implications for care. J Rehabil Med. (2015) ;47: (2):161–6. DOI: 10.2340/16501977-1898 |
[4] | Hull J , Aniapravan R , Chan E , Chatwin M , Forton J , Gallagher J , Gibson N , Gordon J , Hughes I , McCulloch R , Ross Russell R , Simonds A . British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. (2012) ;67: :i1–i40. |
[5] | Bushby K , Finkel R , Birnkrant DJ , Case LE , Clemens PR , Cripe L , Kaul A , Kinnett K , McDonald C , Pandya S and Poysky J . Diagnosis and management of Duchenne muscular dystrophy, part implementation of multidisciplinary care. The Lancet Neurology. (2010) ;9: (2):177–89. |
[6] | Chatwin M , Simonds AK . The addition of mechanical insufflation/exsufflation shortens airway-clearance sessions in neuromuscular patients with chest infection. Respiratory Care. (2009) ;54: (11):1473–9. |
[7] | Kravitz RM . Airway clearance in Duchenne muscular dystrophy. Pediatrics. (2009) ;123: (Suppl 4):S231–5. doi: 10.1542/peds.2008-2952G |
[8] | Chatwin M , Toussaint M , Gonçalves MR , Sheers N , Mellies U , Gonzales-Bermejo J , Sancho J , Fauroux B , Andersen T , Hov B , Nygren-Bonnier M , Lacombe M , Pernet K , Kampelmacher M , Devaux C , Kinnett K , Sheehan D , Rao F , Villanova M , Berlowitz D , Morrow BM . Airway clearance techniques in neuromuscular disorders: A state of the art review. Respiratory Medicine. (2018) ;136: :98–110. |
[9] | Rahbek J , Steffensen BF , Bushby K , de Groot IJ . 206th ENMC International Workshop: care for a novel group of patients –adults with Duchenne muscular dystrophy Naarden, The Netherlands, 23-25 May 2014. Neuromuscular Disorders. (2015) ;25: (9):727–38. |
[10] | Rutkowski A , Chatwin M , Koumbourlis A , Fauroux B , Simonds A , Consortium CMDRP . 203rd ENMC International Workshop: respiratory pathophysiology in congenital muscle disorders: implications for pro-active care and clinical research 13-15 December, 2013, Naarden, The Netherlands. Neuromuscular Disorders. (2015) ;25: (4):353–8. |
[11] | Tzeng AC , Bach JR . Prevention of Pulmonary Morbidity for patients with neuromuscular disease. Chest. (2000) ;118: :1390–6. |
[12] | Chatwin M , Simonds AK . The addition of mechanical insufflation/exsufflation shortens airway-clearance sessions in neuromuscular patients with chest infection. Respiratory Care. (2009) ;54: (11):1473–9. |
[13] | Skalsky AJ , McDonald CM . Prevention and management of limb contractures in neuromuscular diseases. Phys Med Rehabil Clin N. Am. (2012) ;23: (3):675–87. |
[14] | Farley R , Clark J , Davidson C , Evans G , MacLennan K , Michael S , Morrow M , Thorpe S . What is the evidence for the effectiveness of postural management? International Journal of Therapy and Rehabilitation. (2003) ;10: (10):449–55. |
[15] | Harvey LA , Katalinic OM , Herbert RD , Moseley AM , Lannin NA and Schurr K . Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev. (2017) ;2017: (1) doi: 10.1002/14651858.CD007455.pub3 |
[16] | Page P . Current concepts in muscle stretching for exercise and rehabilitation. Int J Sports Phys Ther. (2012) ;7: (1):109–19. |
[17] | Eagle M . Report on the Muscular Dystrophy Campaign workshop: Exercise in neuromuscular diseases. Neuromuscul Disord. (2002) ;12: (10):975–83. |
[18] | Wagner KR , Lechtzin N , Judge DP . Current treatment of adult Duchenne muscular dystrophy. Biochimica Biophysica Acta. (2007) ;1772: :229–37. |
[19] | Ham R , Aldersea P , Porter D . Wheelchair Users and Postural Seating A Clinical Approach. London: Churchill Living-stone; (1998) . |
[20] | Mortenson WB , Miller WC , Miller-Pogar J . Measuring Wheelchair Intervention Outcomes: Development of the Wheelchair Outcome Measure. Disabil Rehabil Assist Technol. (2007) ;2: (5):275–85. |
[21] | Schofield C , Evans K , Young H , Paguinto S-G , Carroll K , Townsend E , Kiefer M , McGuire M , Sodhi J , Bray P , Bayley K , Vorster NM , Downs J . The development of a consensus statement for the prescription of powered wheelchair standing devices in Duchenne Muscular Dystrophy. Disability and Rehabilitation. (2020) ;Sep 2: :1–9. doi: 10.1080/09638288.2020.1810786 |
[22] | Harpin P Adaptations Manual, Muscular Dystrophy Campaign 2nd ed. ISBN 0 903561 042; 2003 |
[23] | Muscular Dystrophy Campaign. Wheelchair Provision for Children and Adults with Muscular Dystrophy and other Neuromuscular Conditions, Best Practice Guidelines. London. Available at:https://www.musculardystrophyuk.org/wp-content/uploads/2015/02/wheelchair-guidelines.pdf; 2011 [Accessed on: 10 July 2020]. |
[24] | Anziska Y , Sternberg A . Exercise in Neuromuscular Disease. Muscle and Nerve. (2013) ;48: :3–20. |
[25] | Markert CD , Case LE , Carter GT , Furlong PA , Grange RW . Exercise and DMD Roundtable. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve. (2012) ;45: :746–51. |
[26] | Abresch RT , Carter GT , Han JJ , McDonald CM . Exercise inNeuromuscular Diseases. Physical Medicine and Rehabilitation Clinicsof North America. (2012) ;23: (3):653–73. |
[27] | Kimura S , Ikezawa M , Nomura K , Ito K , Ozasa S , Ueno H , Yoshioka K , Yano S , Yamashita T , Matuskura M , Miike T . Immobility reduces muscle fiber necrosis in dystrophin deficient muscular dystrophy. Brain Dev. (2006) ;28: :473–6. |
[28] | Creek J . Occupational therapy defined as a complex intervention: a 5-year review. British Journal of Occupational Therapy March. (2009) ;72: (3):105–15. |
[29] | Lindsay S , Cagliostro E , McAdam L . Meaningful occupations of young adults with muscular dystrophy and other neuromuscular disorders. Canadian Journal of Occupational Therapy. (2019) ;86: (4):277–88. |
[30] | Turner A . Occupation for Therapy. In: Turner A, Foster M, Johnson SE. Occupational therapy and physical dysfunction: principles, skills, and practice, Edinburgh; New York: Churchill Livingstone; (2002) , pp. 25–46. |
[31] | Brown G . Muscular Dystrophy. In: Turner A, Foster M, Johnson SE. Occupational therapy and physical dysfunction: principles, skills, and practice, Edinburgh; New York: Churchill Livingstone; (2002) , pp. 341–362. |
[32] | Stone K , Tester C , Blakeney J , Howarth A , McAndrew H , Traynor N , McCutcheon M , Johnston R . Occupational therapy and Duchenne muscular dystrophy. Chichester, England: John Wiley & Sons; (2007) . |
[33] | Duncan EAS . Foundations for practice in occupational therapy. Edinburgh: W.B. Saunders; (2012) . |
[34] | Pane M , Mazzone E , Fanelli L , De Sanctis R , Bianco F , Sivo S , D’Amico A , Messina S , Battinie R , Scutifero M , Petillo R , Frosini S , Scalise R , Vita G , Bruno C , Pedemonte M , Mongini T , Pegoraro E , Mercuri E . Reliability of the Performance of Upper Limb assessment in Duchenne muscular dystrophy. Neuromuscular Disorders [online]. (2014) ;24: (3):201–6. |
[35] | Law MC , Baptiste S , Carswell A , Mccoll MA , Polatajko HJ , Pollock N and Canadian Occupational Performance Measure. Canadian occupational performance measure. Ottawa, Ontario: Copm; (2019) . |
[36] | Chalder M , Wiles NJ , Campbell J , Hollinghurst SP , Haase AM , Taylor AH , et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ. (2012) ;344: :e2758. https://doi.org/10.1136/bmj.e2758 (Published 06 June 2012). |
[37] | Rimer J , Dwan K , Lawlor D , Greig C , McMurdo M , Morley W , et al. Exercise for depression. Cochrane Database of Systematic Reviews. 2012; 7; Art. No.:CD004366. DOI: 10.1002/14651858.CD004366.pub5 |
[38] | The Scottish Government. Mental Health Strategy for Scotland 2012-15. Edinburgh; (2012) . |
[39] | Department of Health PA, Health Improvement and Protection. Start Active, Stay Active: A report on physical activity from the four home countries. Chief Medical Officers. London: Department of Health; (2011) . |
[40] | Beddington J , Cooper CL , Field J , Goswami U , Huppert FA , Jenkins R , et al. The mental wealth of nations. Nature. (2008) ;455: (7216):1057–60. |
[41] | Heaney CA , Israel BA . Social networks and social support. In: Glanz K, Rimer BK,Viswanath K, editors. Health behavior and health education: theory, research, and practice. 4th ed. San Francisco: Jossey-Bass; (2008) , pp. 189–210. |
[42] | Morrow M . Duchenne muscular dystrophy –A biopsychosocial approach Physiotherapy. (2004) ;90: (3):145–50. |
[43] | Davidson ZE , Truby H . A review of nutrition in Duchenne muscular dystrophy. Journal of Human Nutrition and Dietetics. (2009) ;22: (5):383–93. |
[44] | Ishikawa Y , Bach JR , Minami R . Cardioprotection for Duchenne’s muscular dystrophy. American Heart Journal. (1999) ;137: (5):895–902. |
[45] | Toussaint M , Steens M , Soudon MP . Lung Function Accurately Predicts Hypercapnia in Patients With Duchenne Muscular Dystrophy. Chest. (2007) ;131: (2):368–75. |
[46] | Mazzone E , Messina S , Vasco G , et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscular Disorders. (2009) ;19: (7):458–61. |
[47] | Mazzone E , Martinelli D , Berardinelli A , et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscular Disorders. (2010) ;20: (11):712–6. |
[48] | Audag N , Goubau C , Toussaint M , Reychler G . Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: a systematic review. Ther Adv Chronic Dis. 2019; doi: 10.1177/2040622318821622 |
[49] | Nozaki S , Umaki Y , Sugishta S , Tatara L , Adachi K , Shinno S . Videofluorographic assessment of swallowing function in patients with Duchenne muscular dystrophy. Clinical Neurology. (2007) ;47: (7):407–12. |
[50] | Aloysius A , Born P , Kinali M , Davis T , Pane M , Mercuri E . Swallowing difficulties in Duchenne muscular dystrophy: Indications for feeding assessment and outcome of videofluroscopic swallow studies. European Journal of Paediatric Neurology. (2008) ;12: (3):239–45. |
[51] | Hanayama K , Liu M , Higuchi Y , Ishihara T . Dysphagia in patients with Duchenne muscular dystrophy evaluated with a questionnaire and videofluorography. Disability and Rehabilitation. (2008) ;30: (7):517–22. |
[52] | Pane M , Vasta I , Messina S , Sorleti D , Aloysius A , Sciarra F , Mangiola F , Kinali M , Ricci E , Mercuri E . () Feeding problems and weight gain in Duchenne muscular dystrophy. European Journal of Paediatric Neurology. (2006) ;10: (5-6):231–6. |
[53] | Jaffe KM , McDonald CM , Ingman E , Haas J . Symptoms of upper gastrointestinal dysfunction in Duchenne muscular dystrophy: case-control study. Archives of Physical Medicine and Rehabilitation. (1990) ;71: (10):742–4. |
[54] | Van Bruggen HW , van den Engel-Hoek L , Steenks MH , Bronkhorst EM , Creugers NHJ , de Groot IJM , Kalaykova SI . Predictive factors for masticatory performance in Duchenne muscular dystrophy. Neuromuscular Disorders. (2014) ;24: (8):684–92. |
[55] | Lee JW , Oh HJ , Choi Won Ah , Kim DJ , Kang SW . Relationship between Eating and Digestive Symptoms and Respiratory Function in Advanced Duchenne Muscular Dystrophy Patients. Journal of Neuromuscular Diseases. (2020) ;7: (2):101–7. |
[56] | Toussaint M , Davidson Z , Bouvoie V , Evenepoel N , Haan J , Soudon P . Dysphagia in Duchenne muscular dystrophy: practical recommendations to guide management. Disability and Rehabilitation. (2016) ;38: (20):2052–62. |
[57] | Ekberg O , Hamdy S , Woisard V , Wuttge-Hannig A , Ortega P . Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. (2002) ;17: (2):139–46. |
[58] | Altman KW , Yu G-P , Schaefer SD . Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. (2010) ;136: (8):784–9. doi: 10.1001/archoto.2010.129 |
[59] | Willig TN , Paulus J , Lacau Saint Guily J , Béon C , Navarro J . Swallowing problems in neuromuscular disorders. Archives of Physical Medicine and Rehabilitation. (1994) ;75: (11):1175–81. |
[60] | Lissoni A , Bianchi C , Ugolini M , Tufarulo L , Sopransi M , Del Favero C . Studio clinico e radiologico dei disturbi della deglutizione in pazienti affetti da distrofia muscolare di Duchenne e ripercussioni pneumologiche. Europa Medicophysica. (1996) ;32: (3):131. |
[61] | Kiliaridis S , Katsaros C . The effects of myotonic dystrophy and Duchenne muscular dystrophy on the orofacial muscles and dentofacial morphology. Acta Odeontaol Scand. (1998) ;56: (6):369–74. |
[62] | Shinonanga C , Fukuda M , Suzuki Y , Higaki T , Ishida Y , Ishii E , Hyodo M , Morimoto T , Sano N . Evaluation of swallowing function in Duchenne muscular dystrophy. Developmental Medicine and Child Neurology. (2008) ;50: (6):478–80. |
[63] | Umemoto G , Furuya H , Kitashima A , Sakai M , Arahata H , Kikuta T . Dysphagia in Duchenne Muscular Dystrophy versus Myotonic Dytrophy Type 1. Muscle and Nerve. (2012) ;46: :490–5. |
[64] | Archer SK , Garrod R , Hart N , Miller S . Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. International Journal of Language and Communication Disorders. (2013) ;48: (2):240–6. |
[65] | Van den Engel-Hoek L , Erasmus CE , Hendriks JC , Geurts ACH , Klein WM , Pillen S , Sie LT , de Swart BJM , de Groot IJM . Oral muscles are progressively affected in Duchenne muscular dystrophy: implications for dysphagia treatment. Journal of Neurology. (2013) ;260: (5):1295–303. |
[66] | Hamanaka-Kondoh S , Kondoh J , Tamine K , Hori K , Fujiwara S , Maeda Y , Matsumura T , Yasuk K , Fujimura J , Sakoda S , Ono T . Tongue pressure during swallowing is decreased in patients with Duchenne muscular dystrophy. Neuromuscular Disorders. (2014) ;24: (6):474–81. |
[67] | Garguilo M , Lejaille M , Vaugier I , Orlilowski D , Terzi N , Lofaso F , Prigent H . Noninvasive Mechanical Ventilation Improves Breathing-Swallowing Interaction of Ventilator Dependent Neuromuscular Patients: A Prospective Crossover Study. PLoS One. (2016) ;11: (3):e0148673. |
[68] | Terzi N , Orlikowski D , Aegerter P , Lejaille M , Ruquet M , Zalcman G , Fermanian C , Raphael JC , Lofaso F . Breathing–Swallowing Interaction in Neuromuscular Patients: A Physiological Evaluation. Am J Respir Crit Care Med. (2007) ;175: (3):269–76. |
[69] | Allen J , Gallager E , Mukherjee R . A Survey of Silent Aspiration in Duchenne Muscular Dystrophy. Journal of Oral Health and Dentistry. (2017) ;1: (s1):A006. |
[70] | Borreli O , Salvia G , Mancini V , Santoto L , Tagliente F , Romeo EF , Cucchiara S . Evolution of Gastric Electrical Features and Gastric Emptying in Children with Duchenne and Becker Muscular Dystrophy. The American Journal of Gastroenterology. (2005) ;100: (3):695–701. |
[71] | Emery AEH . Central nervous system. In Motulsky A.G., et al. (Eds) Duchenne Muscular Dystrophy 2. New York: Oxford University Press; (1987) , pp. 99–106. |
[72] | Katsura K , Kristian T , Smith ML , Siesjo BK . Acidosis induced by hypercapnia exaggerates ischemic brain damage. J Cereb Blood Flow Metab. (1994) ;14: (2):243–50. |
[73] | Leder SB . Incidence and type of aspiration in acute care patients requiring mechanical ventilation via a new tracheotomy. Chest. (2002) ;122: (5):1721–6. |
[74] | Finder JD , Birnkrant D , Carl J , Farber HJ , Gozal D , Iannaccone ST , Kovesi T , Kravitz RM , Panitch H , Schramm C , Schroth M , Sharma G , Sievers L , Silvestri JM , Sterni L , American Thoracic Society. Respiratory care of the patient with Duchenne muscular dystrophy: ATS Consensus Statement. American Journal of Respiratory Critical Care Medicine. (2004) ;170: (4):456–65. |




