Clinical Management of Duchenne Muscular Dystrophy in the Netherlands: Barriers to and Proposals for the Implementation of the International Clinical Practice Guidelines
Abstract
Background:
In order to successfully implement the international clinical care guidelines for Duchenne muscular dystrophy (DMD) in the Netherlands, it is essential to know what barriers are experienced by healthcare practitioners regarding guideline adherence and organization of care. In the Netherlands, academic medical centers provide follow up visits and work together with peripheral hospitals, rehabilitation centers, centers for home ventilation and primary care centers for treatment.
Objective:
To investigate perceived barriers to international clinical DMD guideline adherence and identify potential areas of improvement for implementation in the Dutch ‘shared care’ organization.
Methods:
Semi-structured in-depth interviews with healthcare practitioners of academic medical hospitals and questionnaires for healthcare practitioners of rehabilitation centers, based on the framework of Cabana.
Results:
The analyses identified 4 barriers for non-adherence to the DMD guideline: (i) lack of familiarity/awareness, (ii) lack of agreement with specific guideline, (iii) lack of outcome expectancy, (iv) external barriers.
Conclusions:
A heterogeneous set of barriers is present. Therefore, a multifaceted intervention strategy is proposed to overcome these barriers, including a clear division of roles, allowing for local (Dutch) adaptations per specialism by local consensus groups, and the facilitation of easy communication with experts/opinion leaders as well as between care professionals.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked recessive neuromuscular disorder with an estimated incidence of 1 in 4000–6000 newborn boys, and is caused by a mutation in the dystrophin gene [1]. In the absence of dystrophin, muscle dystrophic degeneration occurs, which leads to progressive muscle weakness [2]. The clinical course is characterized by a progressive loss of muscle strength, respiratory, orthopedic, cardiac complications and functional abilities. If treated with corticosteroids, the mean age of wheelchair dependency is around 12 years of age [3, 4]. The most common causes of death are respiratory failure and cardiomyopathy. Although no cure is available, the mean life expectancy has increased to the early thirties due to improved symptomatic treatments [5]. These include respiratory, cardiac, orthopedic, rehabilitation management and corticosteroid therapy [3, 6–8].
A complex disease such as DMD requires comprehensive, standardized and well-coordinated multidisciplinary care to recognize the multisystem primary manifestations and secondary complications. In the Netherlands, care for persons with DMD is organized according to the shared care principle. Seven academic medical centers provide follow up visits and work together with peripheral hospitals, rehabilitation centers, centers for home ventilation and primary care centers for treatment. When patients are treated at multiple centers, care practice may differ between centers. The quality of care might also depend on the experiences of a healthcare practitioner, or the number of patients with DMD who are seen in a center. Hence, it is important for all centers and healthcare practitioners to adhere to available guidelines to make the best possible quality of care accessible to all patients.
Clinical care guidelines can facilitate the reduction of undesirable variability, enhancing the probability that patients receive similar care regardless of the center, location, or clinician [9]. In 2010, the first comprehensive multidisciplinary international clinical care guideline for DMD was published [10, 11]. This guideline provides a framework for multidisciplinary care for boys and men with DMD worldwide. Although the guideline is freely accessible for both professionals and patients, literature shows that compliance to the guideline is not optimal and differs per country [12–15]. In the Netherlands, compliance to the guideline is unknown. In 2018, a renewed version of the guideline was published [16–18]. For the implementation of the renewed care guideline, it is very relevant to learn from bottlenecks that were encountered working with the initial version. In addition, publication of a clinical guideline, on its own, does not automatically result in implementation. Identification of perceived barriers is a crucial step in the process of guideline implementation [19].
Therefore, the primary objective of this study was to investigate barriers, as perceived by healthcare practitioners, to the implementation of the international DMD clinical practice guidelines in the Netherlands. The secondary objective was to describe the current organization of DMD care in the Netherlands and combined with findings from the first objective propose interventions for improvement.
MATERIALS AND METHODS
Study design and population
Healthcare practitioners at seven academic medical centers providing periodic follow-up of patients with DMD were invited to participate. The semi-structured interviews were designed to explore perceived barriers to the provision of DMD care in accordance with the international care guidelines. Centers were asked to appoint the professional(s) with the most pivotal care role for patients with DMD.
Additionally, professionals in rehabilitation centers were invited to fill in a web-based questionnaire about organization of care and guideline use. ‘Spierziekten Nederland’, a Dutch patient organization, distributed the questionnaire to 99 rehabilitation practitioners (e.g. physiotherapists, occupational therapists, orthotists, and psychologists) involved in the in- or- outpatient follow-up and care of individuals with neuromuscular diseases, including children and adults with DMD.
All participants provided consent. This study does not meet the criteria of Medical Research Involving Human Subjects Acts and was classified as exempt from ethical review.
Data collection and procedures – interviews
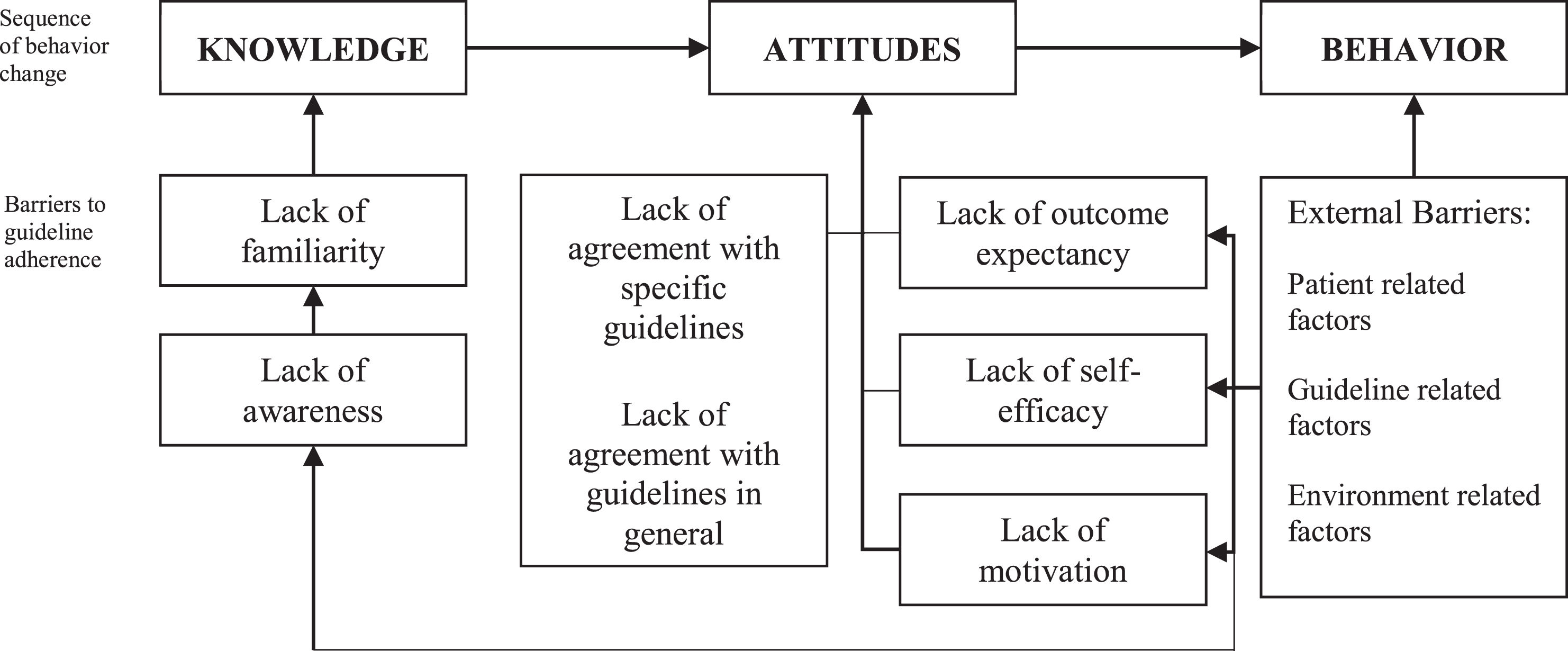
As basis for the interviews and questionnaire the model of Cabana [20] was used. In this model, barriers can be related to knowledge (e.g. lack of familiarity and awareness), attitudes (e.g. lack of motivation and agreement) or to external (e.g. environment- and patient-related) factors. The model is based on the principle of the knowledge-attitude-behavior framework, where knowledge shapes attitude and a change in attitude can influence behavior [21].
The in-depth interviews were structured around three main themes, each explored with a single, open-ended question: (1) Organization of care, ‘How is care for boys and men with DMD in your center organized?’, ‘Which healthcare practitioners are involved and which assessments are done during the follow up?’; (2) Barriers to guideline adherence, ‘What do you think of the DMD care guideline?’; and (3) The ideal situation, ‘What is, in your opinion, the ideal way to organize care for boys and men with DMD in the Netherlands?’
Responses to these questions were further explored with probes focused on for example: (1) ‘Are you satisfied with the care you provide?’, ‘What would you like to improve in your center?’, ‘What barriers do you experience?’; (2) ‘Do you work according to the guideline?’, ‘Why do you not adhere to aspects of the guideline?’; (3) ‘What would be the best way to organize the care for boys and men with DMD in the Netherlands?’, ‘What would be the best way to implement the renewed version of the care guideline in the Netherlands?’
The interviews lasted between 40 and 60 minutes. One investigator (LH) moderated the interviews. During data collection, the updated version of the clinical practice guidelines for DMD was published [16–18]. In the interviews, it was made clear to the participants that all questions referred to the first version of the clinical practice guidelines.
Data collection and procedures –questionnaire
The questionnaire consisted of a total of 28 questions, structured around the themes presented above (i.e. knowledge, attitude, and external factors). The questionnaire is provided as supplemental material. Theme (1), Organization of care for persons with DMD, consisted of questions about the healthcare team and most used interventions, and questions about possible bottlenecks and satisfaction with the provided care. Theme (2), Barriers to guideline use, started with an open question: ‘What do you think of the care guideline?’ In addition, we used the questionnaire of Taba (2006) to assess in which domains of the theoretic framework of Cabana (1999) barriers to adherence to the guideline existed [20, 22]. The questionnaire consisted of statements about the guideline with five response options on a Likert scale, ranging from ‘strongly agree’ to ‘strongly disagree’. Theme (3), The ideal situation, consisted of the open question: ‘What is, in your opinion the best way to organize care for patients with DMD in the Netherlands?’, and questions regarding the facilitation of adherence [22].
Additional to the themes also the organization of and the availability of care was inquired (see for the questions the supplement).
Data analysis
The interviews were audio-taped and transcribed for analysis with the constant comparative method of qualitative analysis. Independently, two investigators (LH and MG) read the transcripts to identify relevant information. In this first phase of the coding process, all relevant information for either organization of care, guideline adherence, or the ideal situation was labeled. The investigators compared transcripts and discussed discrepancies in coding until they reached consensus. In the second phase of coding, the two investigators independently searched to identify relations among all codes. Codes about barriers of guideline adherence were categorized in the domains of the framework of Cabana (Fig. 1). The categorization of codes was compared and discussed until consensus was reached. In the last phase of the coding process, investigators elaborated on the dominant themes. The median and interquartile range were used to describe the age and years of experience of the participants.
Fig.1
Cabana framework. Theoretical framework of Cabana is based on the principle of the knowledge-attitude-behavior framework, where knowledge shapes attitude and a change in attitude can influence behavior.
Quantitative data recorded via the questionnaires were summarized using medians and interquartile ranges, and categorical data using n (proportion %). Free-text replies were analyzed with the constant comparative method.
RESULTS
From the seven academic medical centers that provided periodic, structured follow-up for patients with DMD, eleven professionals were interviewed between February and November, 2018. Additionally, we received elven completed questionnaires from the rehabilitation centers/teams between August - November 2018. Descriptive characteristics are shown in Table 1. The majority of the participants was female and there was a wide variety in type of profession.
Table 1
Participant characteristics: interviews in academic hospitals, questionnaires for rehabilitation centers
| Interviews | Questionnaires | |
| Years of experience with DMD, median (IQR) | 11.0 (10.0) | 5.5 (4.5) |
| Gender (n) | ||
| Male | 2 | 3 |
| Female | 9 | 8 |
| Profession (n) | ||
| Neurologist | 1 | – |
| Pediatric neurologist | 3 | – |
| Rehabilitation physician | 1 | 8 |
| Pediatric rehabilitation physician | 2 | 2 |
| Care coordinator | 2 | – |
| Occupational therapist | – | 1 |
Theme 1 –Organization of the care for persons with DMD
All seven academic medical centers offered a structured follow-up for boys with DMD up to the age of 18 (four annually, three twice a year), during which they were seen by several specialists and underwent several assessments. Table 2 provides an overview of care components, as well as healthcare providers and clinical assessments/tests that were included in the follow-up or available on indication. The number of boys treated in the academic medical centers ranged from 10 to 75 patients (mean = 32.0, SD = 24.1).
Table 2
Overview of the availability of care providers and assessments/tests in the seven academic hospitals for DMD patients of age < = 18 years distinguished in regular follow up or on indication
| Care components | Available care providers | Assessments/tests | Care providers or tests in follow up program | Care providers or tests on indication |
| Diagnostics | Genetic counselor | NA | all | |
| Diagnostic examination | NA | all | ||
| Neuromuscular and rehabilitation management | Care coordinator | 2/7 | NA | |
| Neurologist | all | NA | ||
| Rehabilitation physician | all | NA | ||
| Physiotherapist | 5/7 | all | ||
| Occupational therapist* | 1/7 | 3/7 | ||
| Function, strength, ROM | all | NA | ||
| Posture and gait | all | NA | ||
| Functioning in daily life | all | NA | ||
| Corticosteroid regime and AE management | all | NA | ||
| Orthopedic management | Orthopedist | 1/7 | all | |
| Radiologist | 3/7 | all | ||
| ROM | all | NA | ||
| Spinal assessment | 6/7 | all | ||
| Spinal radiograph | 5/7 | all | ||
| Bone age | 1/7 | all | ||
| Bone densitometry | 3/7 | all | ||
| Pulmonary management | Pulmonologist | 3/7 | all | |
| Spirometry | 6/7 | all/CTB | ||
| Pulse oximetry | 3/7 | all/CTB | ||
| Capnography | 1/7 | all/CTB | ||
| PCF | 5/7 | all/CTB | ||
| MIP/MEP | 3/7 | all/CTB | ||
| BGA | 2/7 | all/CTB** | ||
| Cardiac management | Cardiologist | all | NA | |
| ECG | 6/7 | all | ||
| Echocardiogram | 7/7 | all | ||
| Holter | 0/7 | all | ||
| GI, speech and swallowing, nutrition management | Dietician* | 3/7 | 2/7 | |
| Speech-language therapist* | 1/7 | 2/7 | ||
| Gastroenterologist | 0/7 | all | ||
| Urologist | 1/7 | all | ||
| Endocrinologist | 0/7 | all | ||
| Weight measurement | all | all | ||
| Height measurement | all | all | ||
| Speech and language | 5/7 | all | ||
| Nutrition | 4/7 | all | ||
| Micturition and bowel | all | all | ||
| Psychosocial management | Psychosocial care | a/7 | all | |
| Screening neurocognitive problems | all*** | referral | ||
| Transition of care across the life span | Multidisciplinary out-patient ‘transition clinic’ | 5/7 | NA |
*An occupational therapist, a dietician, and a speech-language therapist are not available in every medical center. For these specializations, academic medical centers refer their patients to rehabilitation centers. **The academic medical centers refer patients to centers specialized in ventilation when indicated. ***All medical centers provide a screening for neurocognitive functions. When a patient is suspected of having a problem, they are referred within the hospital or to a specialized center. Abbreviations: BGA = blood gas analysis, AE = adverse events, ECG = electrocardiogram, MIP/MEP = maximal inspiratory/expiratory pressure, NA = not applicable, PCF = peak cough flow, ROM = range of motion.
For adults with DMD, two academic medical centers provided a multidisciplinary annual follow-up and one center offered a structured twice a year follow-up. In other centers patients were seen annually or twice a year by their neurologist or rehabilitation physician and by their cardiologist and were referred to other specialists if needed. The number of adult patients with DMD treated in the academic medical centers ranged from 10 to 58 (mean = 35, SD = 20). When patients needed ventilation, academic medical centers refer to specialized outpatient centers for the support of home ventilation.
In the rehabilitation centers, the healthcare team for patients with DMD consisted at a minimum of a rehabilitation physician, physiotherapist, occupational therapist, speech-language therapist, social worker, and psychologist. Additional professions available in some rehabilitation centers were creative therapist, music therapist, (pediatric-) orthopedic surgeon, plastic surgeon, orthopedic shoe technician, orthotist, and sports pedagogue. The care consisted of single consultations or intermittent to continuous care, depending on the stage of the disease and actual needs of the patient. Patients who attend a regular school or young patients living far away often received single consultations on indication. Some centers offered a periodic (multidisciplinary) consultation or follow-up, but actual treatment took place in primary care in their own region. Patients received intermittent care in case of a care demand or specific treatment goal. Treatment stopped when the goal was reached and could be interrupted during holidays. Patients can also receive intermittent consultation in a rehabilitation center, while receiving continuous treatment at a primary care center or specialized school. Some rehabilitation centers offered continuous treatment, for example weekly appointments with a physiotherapist. In the supplement an overview of targets of interventions per disease stage as reported by the rehabilitation centers can be found.
Theme 2 –Barriers to the implementation of the international clinical guidelines
Perceived barriers to the implementation of the international clinical practice guidelines concerned the following categories: ‘lack of familiarity/awareness’, ‘lack of agreement with specific guidelines’, ‘lack of outcome expectancy’, ‘external factors’ and ‘environmental factors’.
Lack of familiarity/awareness
At three academic medical centers, the professionals noted that care providers who were only involved on indication were unaware of or unfamiliar with the guideline. At the rehabilitation centers, one rehabilitation physician was unaware of the guideline. Table 3.1 shows that three rehabilitation professionals note that not all team members were aware or familiar with the guideline and the guideline is not fully implemented in all centers. One respondent noted that knowledge about the guideline among practitioners is low due to the limited number of patients.
Table 3
Responses to the survey questions by professionals in rehabilitation centers
| Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| 3.1 Lack of familiarity/awareness | |||||
| The guideline is completely implemented in our center | 0 (0) | 5 (45.5%) | 2 (18.2%) | 4 (36.4%) | 0 (0%) |
| All members of the healthcare team are aware of the guideline | 1 (9.1%) | 4 (36.4%) | 3 (27.3%) | 3 (27.3%) | 0 (0%) |
| 3.2 Lack of agreement with specific guidelines | |||||
| The guideline is evidence-based | 0 (0%) | 9 (81.2%) | 2 (18.2%) | 0 (0%) | 0 (0%) |
| The guideline is useful in daily clinical work and can improves the quality of treatment | 0 (0%) | 7 (63.6) | 3 (27.3%) | 1 (9.1%) | 0 (0%) |
| The guideline includes different aspects of a disease, and is a good tool for confirming diagnosis, starting initial treatment, and managing complications | 0 (0%) | 9 (81.2%) | 2 (18.2%) | 0 (0%) | 0 (0%) |
| The guideline is convenient and the information is easy to find | 1 (9.1%) | 4 (36.4%) | 1 (9.1%) | 5 (45.5%) | 0 (0%) |
| The guideline is not accessible | 0 (0%) | 2 (18.2%) | 2 (18.2%) | 6 (54.4%) | 1 (9.1%) |
| The guideline is too complicated and it is difficult to find the information | 0 (0%) | 2 (18.2%) | 3 (27.3%) | 3 (27.3%) | 3 (27.3%) |
| The guideline reduces doctors’ autonomy (a ‘cookbook’) | 0 (0%) | 0 (0%) | 3 (27.3%) | 4 (36.4%) | 4 (36.4%) |
| The guideline limits treatment options | 0 (0%) | 0 (0%) | 5 (45.5%) | 2 (18.2%) | 4 (36.4%) |
| The guideline limits flexibility and individual approach | 0 (0%) | 0 (0%) | 0 (0%) | 1 (9.1%) | 6 (54.4%) |
| There is no need for the guideline as treatment routines exist | 0 (0%) | 0 (0%) | 1 (9.1%) | 5 (45.5%) | 5 (45.5%) |
| 3.3 External barriers –patient related | |||||
| Patients do not want doctors to conform to treatment guidelines | 0 (0%) | 0 (0%) | 3 (27.3%) | 3 (27.3%) | 5 (45.5%) |
| The guideline is hard to implement in daily practice due to too much strain on or a lack of resources of patients | 0 (0%) | 3 (27.3%) | 3 (27.3%) | 4 (36.4%) | 1 (9.1%) |
| 3.4 External barriers –environmental factors | |||||
| The guideline is hard to implement in daily practice due to lack of medical resources | 0 (0%) | 5 (45.5%) | 3 (27.3%) | 1 (9.1%) | 2 (18.2%) |
| There is no time to search for information | 0 (0%) | 2 (18.2%) | 5 (45.5%) | 2 (18.2%) | 2 (18.2%) |
Lack of agreement with specific guidelines
At the academic medical centers, professionals found that the guideline provided a good overview of the different aspects of DMD care and was a useful tool in providing uniform care. However, the interpretation of the guideline could be ambiguous as it lacked specific information in certain areas. As a result, the interpretation and practical implementation of the guideline depended on the expertise of the professional. For inexperienced care professionals, parts of the guideline might be too concise. Topics that emerged from the interviews requiring more in-depth information were 1) how to accurately measure height (especially in the non-ambulant phase and when contractures are present), 2) the position in which spinal radiographs should be taken (seated versus supine), 3) attention for the later phases in life, and 4) specific information about joint mobility, contractures, and splints. Furthermore, parts of the guideline were based on expert opinion and there was a need for more scientific evidence. One particular topic that emerged was the need for more scientific evidence regarding corticosteroid regimens and their side effects.
Professionals in rehabilitation centers overall had a positive attitude towards the guideline, but some professionals found the guideline not accessible and too complicated (see Table 3.2). Furthermore, four professionals noted there is insufficient guidance and for clinical rehabilitation practice.
Lack of outcome expectancy
The guideline proposes numerous assessments or tools for each field of expertise. Although professionals at academic medical centers agreed with the idea that attention should be given to each field, they argued that not all suggested assessments or tools have added value for the patient. Specifically, professionals in the academic medical centers noted they had their doubts about the clinical relevance of the results some of the suggested neuromuscular assessments (specifically ‘Egen Klassification’, ‘Hammersmith’, ‘Motor Function Measure’ and step activity monitoring) and the annual DEXA scan.
External barriers –barriers based on patient characteristics
Professionals at academic medical centers noted that it might be difficult to implement the guideline in daily practice because it could cause too much strain on patients. The follow up visit often takes up a full day filled with lots of visits and assessments, which can be exhausting for patients. Furthermore, when patients grew older and reached a ‘steady state’, professionals experienced that patients simply did not want to go to all the different appointments, for example because they felt they would not hear anything new. This barrier is also present in rehabilitation centers (see Table 3.3).
External barriers –environmental factors
Communication
Overall, professionals at academic medical centers were satisfied with the inter-professional communication about DMD patient care. However, professionals also noted that (mis-) communication could be a barrier to guideline adherence. This could be present at different organizational levels. Within the organization, not all institutions actively discuss the guideline and its implementation with specialists involved on indication. In addition, communication between medical centers could act as a barrier when each center would have its own policy on how to implement the guideline. Although academic professionals were overall satisfied with the communication and collaboration between the rehabilitation centers and primary healthcare centers, they noted that their advice was not always adopted or implemented. In the rehabilitation centers, professionals noted that there was not enough room for innovation and collaboration with other centers.
Resources
Lack of resources caused by a lack of funding and, consequently, insufficient capacity, was a frequently perceived barrier in academic medical centers –lack of funding for a care coordinator was often mentioned. This leads to care not being coordinated in an optimal manner, forcing the rehabilitation physician or neurologist to handle coordination on top of their existing workload. In addition, while funding for outside specialists could be insufficient, this differed for each center. Lack of resources also acted as a barrier for rehabilitation centers (see Table 3.4), leading to waiting lists at some rehabilitation centers.
Logistics
Logistical issues and time constraints were barriers for the academic medical centers regarding the organization of the follow-up. In most centers, the follow-up took up one full day. However, professionals were not always able to organize it in such a way that all necessary appointments are included. Also, for adult patients, follow up is not provided in a structured visit. Furthermore, because adult patients were also treated in the centers for home ventilation, it was not always clear who held the coordination role.
Theme 3 –Ideal situation and facilitators
Overall, professionals at academic medical centers argued that DMD care should be organized in a way that expertise is available at each level of care in academic medical, rehabilitation, and primary care centers. They wanted to ensure uniform care throughout the country. A clear policy about the organization of the follow-up was considered necessary, including, for example, a guarantee that each center would provide a minimum of care. They also argued that the implementation of the renewed guideline should be tailored to suit the Dutch shared care organization, with input from Dutch healthcare professionals, before collaborative implementation. For example, the recommended twice a year visits to a neuromuscular specialist could take place once a year at an academic neuromuscular team and once a year at a rehabilitation center with a specialized neuromuscular team.
Furthermore, it was noted that there was room for improvement in local organization of the annual follow-up. Because time was a limitation in all centers, the annual follow-up should be organized efficiently. Academic medical centers could learn from each other by exchanging information, such as schedules and protocols. Furthermore, it was proposed that patients and parents could complete digital questionnaires in preparation of the annual follow-up, which was already practiced in some centers. In addition, the electronic patient filing systems should better facilitate multidisciplinary care.
Professionals at academic medical centers also argued that a center that provided a yearly follow-up should see enough patients to ensure expertise. If a center had too few patients, it should consider transferring the patients to another academic medical center. There was no consensus about the minimum number of patients nor were specific numbers mentioned. Additionally, centers offering a follow-up should be geographically spread throughout the country to limit the burden of traveling for the patients.
Professionals in the rehabilitation centers would like to see clear alignment and division of tasks between academic hospitals and rehabilitation centers. Treatment should be done locally near the patients’ home, annual follow-up and specialized procedures should be done at specialized centers. Also, professional want to have easy access to academic experts in the field, to ask questions and receive courses or coaching.
DISCUSSION
The objective of this study was to investigate barriers perceived by healthcare professionals, to the implementation of the international clinical practice guidelines for boys and men with DMD in the Netherlands Our results identified a heterogenous set of barriers for non-adherence to the guideline which will be discussed below.
‘Lack of familiarity or awareness’ was mainly present in professionals who were only involved on indication. Dissemination of the guideline is essential to raise awareness of and increase familiarity with it [23, 24]. Because the guideline is extensive, multidisciplinary, and international, a standard dissemination strategy (e.g. via e-mail) might not be effective. To increase familiarity, it is important to actively involve each discipline in the implementation process. Involving professionals from centers throughout the country would facilitate collaboration between centers and make care more uniform [19].
In all centers, the barriers revealed a ‘lack of agreement with specific guidelines’ and ‘lack of outcome expectancy.’ The professionals noted that the guideline should not simply be adopted, as it is (partly) based on expert opinion. Furthermore, because the guideline development did not involve Dutch experts, it was not clear whether it would be feasible to implement all care considerations into the Dutch shared care system.
At the external level, patient-related and environmental barriers were present. The environment-related barriers consisted of insufficient communication, a lack of funding and capacity, logistical issues, and time constraints. In the process of overcoming these barriers it is crucial to define clear roles across contexts. Further interventions for improvements are the standardization of processes and procedures and the development of protocols (for example, standardizing a care minimum).
The secondary objective was to describe the current organization of DMD care in the Netherlands and combined with findings from the first objective propose interventions for improvement. The respondents to our study expressed the desire for adaptation of the guidelines to the Dutch care system, which is based on shared care between the academic specialized teams and the specialized teams in the rehabilitation centers. In shared care communication is extremely important and clear agreement is necessary on who is doing what kind of assessments and timing and who is doing interventions, next to exchange of all data of a patient between the different sites. Shared care has as the advantage of care in the home environment of the patient, however as already stated above agreement between the sites and efficient communication is a prerequisite. Guideline implementation may improve through local (Dutch) consensus groups per specialization. Furthermore, professionals were worried that the guideline was oversimplified, hindering implementation for professionals with little experience. They expressed the need for clear cut-off points as indicators for treatment, and that the rehabilitation part of the guideline should offer more practical guidance to decide which intervention should be used at what time. At the external level, patient-related and environmental barriers were present. The environment-related barriers consisted of insufficient communication, a lack of funding and capacity, logistical issues, and time constraints. In the process of overcoming these barriers it is crucial to define clear roles across contexts. Further interventions for improvements are the standardization of processes and procedures and the development of protocols (for example, standardizing a care minimum).
This study has limitations. All academic hospitals involved in the care for DMD were interviewed, but the response rate for rehabilitation centers was only 11%. A possible explanation is that we invited professionals working with neuromuscular diseases and the main reason for non-response was that not all professionals treated patients with DMD. Still, with a low response rate, a reporting bias is expectable. Overall, we included the majority of centers treating patients with DMD. Also, professionals working in peripheral hospitals and primary care and home ventilation centers were not included.
Given the presence of barriers at the knowledge, attitudinal, and external levels, a multifaceted tailored intervention strategy is paramount. Such intervention strategy can overcome the reported barriers, including a clear division of roles, allowing for local adaptations per specialization by local consensus groups, and facilitating easy communication with experts/opinion leaders as well as between care professionals [19]. In the Netherlands, steps towards uniform care are taken by the Duchenne Center Netherlands, a collaboration between Leiden University Medical Center, Radboud University Medical Center, and Kempenhaeghe/Maastricht University Medical Center. Next to this, a network of all interested clinicians and scientists in DMD has been established, called ALADIN (All Against Duchenne In the Netherlands). In the attempt to provide patients with optimal and uniform care, it is essential to share the process of guideline implementation with all professionals involved in care for persons with DMD. The results from this study and the arguments for local adaptation are not unique for DMD. They can very well be applicable for other diseases with complex care demands.
ACKNOWLEDGMENTS
We thank Spieren voor Spieren for funding the Duchenne Center Netherlands and this study. We also thank all professionals who participated in this study. We thank Spierziekten Nederland for their help in distributing the questionnaire to the rehabilitation centers.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JND-200586.
REFERENCES
[1] | Mendell JR , Lloyd-Puryear M . Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle & Nerve. (2013) ;48: (1):21–6. DOI: 10.1002/mus.23810 |
[2] | Deconinck N , Dan B . Pathophysiology of duchenne muscular dystrophy: Current hypotheses. Pediatr Neurol. (2007) ;36: (1):1–7. DOI: 10.1016/j.pediatrneurol.2006.09.016 |
[3] | Eagle M , Baudouin SV , Chandler C , Giddings DR , Bullock R , Bushby K . Survival in Duchenne muscular dystrophy: Improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscular disorders: NMD. (2002) ;12: (10):926–9. DOI: 10.1016/S0960-8966(02)00140-2 |
[4] | Emery AE . The muscular dystrophies. Lancet. (2002) ;359: (9307):687–95. DOI: 10.1016/s0140-6736(02)07815-7 |
[5] | Landfeldt E , Thompson R , Sejersen T , McMillan HJ , Kirschner J , Lochmuller H . Life expectancy at birth in Duchenne muscular dystrophy: A systematic review and meta-analysis. Eur J Epidemiol. (2020) ;35: (7):643–53. |
[6] | Moxley RT 3rd , Pandya S , Ciafaloni E , Fox DJ , Campbell K . Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: Implications for management. Journal of Child Neurology. (2010) ;25: (9):1116–29. DOI: 10.1177/0883073810371004 |
[7] | Eagle M , Bourke J , Bullock R , Gibson M , Mehta J , Giddings D , et al. Managing Duchenne muscular dystrophy–the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscular disorders: NMD. (2007) ;17: (6):470–5. DOI: 10.1016/j.nmd.2007.03.002 |
[8] | McDonald CM . Limb contractures in progressive neuromuscular disease and the role of stretching, orthotics, and surgery. Phys Med Rehabil Clin N Am. (1998) ;9: (1):187–211. DOI: 10.1016/s1047-9651(18)30286-9 |
[9] | Woolf SH , Grol R , Hutchinson A , Eccles M , Grimshaw J . Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ. (1999) ;318: (7182):527–30. DOI: 10.1136/bmj.318.7182.527 |
[10] | Bushby K , Finkel R , Birnkrant DJ , Case LE , Clemens PR , Cripe L , et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. (2010) ;9: (1):77–93. DOI: 10.1016/S1474-4422(09)70271-6 |
[11] | Bushby K , Finkel R , Birnkrant DJ , Case LE , Clemens PR , Cripe L , et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. The Lancet Neurology. (2010) ;9: (2):177–89. DOI: 10.1016/S1474-4422(09)70272-8 |
[12] | Landfeldt E , Lindgren P , Bell CF , Schmitt C , Guglieri M , Straub V , et al. Compliance to Care Guidelines for Duchenne Muscular Dystrophy. J Neuromuscul Dis. (2015) ;2: (1):63–72. DOI: 10.3233/jnd-140053 |
[13] | Landfeldt E , Lindgren P , Guglieri M , Lochmuller H , Bushby K . Compliance to care guidelines for Duchenne muscular dystrophy in Italy. Neuromuscular disorders: NMD. (2018) ;28: (1):100. DOI: 10.3233/jnd-140053 |
[14] | Vry J , Gramsch K , Rodger S , Thompson R , Steffensen BF , Rahbek J , et al. European Cross-Sectional Survey of Current Care Practices for Duchenne Muscular Dystrophy Reveals Regional and Age-Dependent Differences. J Neuromuscul Dis. (2016) ;3: (4):517–27. DOI: 10.3233/JND-160185 |
[15] | Andrews JG , Conway K , Westfield C , Trout C , Meaney FJ , Mathews K , et al. Implementation of Duchenne Muscular Dystrophy Care Considerations. Pediatrics. (2018) ;142: (1). DOI: 10.1542/peds.2017-4006 |
[16] | Birnkrant DJ , Bushby K , Bann CM , Apkon SD , Blackwell A , Brumbaugh D , et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. The Lancet Neurology. (2018) ;17: (3):251–67. DOI: 10.1016/S1474-4422(18)30024-3 |
[17] | Birnkrant DJ , Bushby K , Bann CM , Alman BA , Apkon SD , Blackwell A , et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. The Lancet Neurology. (2018) ;17: (4):347–61. DOI: 10.1016/S1474-4422(18)30025-5 |
[18] | Birnkrant DJ , Bushby K , Bann CM , Apkon SD , Blackwell A , Colvin MK , et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. The Lancet Neurology. (2018) ;17: (5):445–55. DOI: 10.1016/S1474-4422(18)30026-7 |
[19] | Fischer F , Lange K , Klose K , Greiner W , Kraemer A . Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare (Basel). (2016) ;4: (3). DOI: 10.3390/healthcare4030036 |
[20] | Cabana MD , Rand CS , Powe NR , Wu AW , Wilson MH , Abboud PAC , et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Pediatr Res. (1999) ;45: (4):121a-a. |
[21] | Woolf SH . Practice guidelines: A new reality in medicine. III. Impact on patient care. Arch Intern Med. (1993) ;153: (23):2646–55. DOI: 10.1001/archinte.1993.00410230060008 |
[22] | Taba P , Rosenthal M , Habicht J , Tarien H , Mathiesen M , Hill S , et al. Barriers and facilitators to the implementation of clinical practice guidelines: A cross-sectional survey among physicians in Estonia. BMC Health Serv Res. (2012) ;12: :455. DOI: 10.1186/1472-6963-12-455 |
[23] | Barosi G . Strategies for dissemination and implementation of guidelines. Neurol Sci. (2006) ;27: Suppl 3:S231–4. DOI: 10.1007/s10072-006-0624-9 |
[24] | Francke AL , Smit MC , de Veer AJ , Mistiaen P . Factors influencing the implementation of clinical guidelines for health care professionals: A systematic meta-review. BMC Med Inform Decis Mak. (2008) ;8: :38. DOI: 10.1186/1472-6947-8-38 |




