The Position of Neuromuscular Patients in Shared Decision Making. Report from the 235th ENMC Workshop: Milan, Italy, January 19-20, 2018
Abstract
In the era of patient-centered medicine, shared decision-making (SDM) – in which healthcare professionals and patients exchange information and preferences and jointly reach a decision – has emerged as the gold standard model for the provision of formal healthcare. Indeed, in many geographical settings, patients are frequently invited to participate in choices concerning the design and delivery of their medical management. From a clinical perspective, benefits of this type of patient involvement encompass, for example, enhanced treatment satisfaction, improved medical compliance, better health outcomes, and maintained or promoted quality of life. Yet, although the theory and enactment of SDM in healthcare are well-described in the literature [1–3], comparatively less attention has been devoted to contextualizing questions relating to if, when, and how to include patients in decisions within medical research. In this context, patient involvement would be expected to be potentially relevant for and applicable to a wide range of activities and processes, from the identification of research priorities and development of grant applications, to the design of patient information and consent procedures, formulation of interventions, identification and recruitment of study sample populations, feasibility of a clinical trial, identification, selection, and specification of endpoints and outcomes in clinical trials and observational studies, data collection and analysis, and dissemination of results. To this end, 45 clinicians, healthcare professionals, researchers, patients, caregivers, and representatives from regulatory authorities and pharmaceutical companies from 15 different countries met to discuss the level of involvement of patients with neuromuscular diseases, specifically in the following settings of medical research for neuromuscular diseases: i) registries and biobanks; ii) clinical trials; and iii) regulatory processes. In this report, we present summaries of the talks that were given during the workshop, as well as discussion outcomes from the three topic areas listed above.
BACKGROUND
Involving patients in research has long been recognized as a key issue for many rare disorders, and patient contributions have been manifold: from (political) agenda setting, building networks, and sharing knowledge, to engaging with industry or, albeit to a lesser extent, actively participating in the planning and conduct of biomedical research (community engaged research) [4–9].
Shared Decision Making (SDM) is a model of communication between a health professional and a patient about the options of prevention, screening, diagnostic tests and treatment, including the option of not intervening; an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options and to achieve informed preferences [10, 11] in contrast to a simple informed consent [12]. The goals of SDM are: better-informed patients, more confidence in and satisfaction about the treatment and more compliance. The model consists of three steps: choice talk, option talk and decision talk. During the choice talk it is decided whether or not the patient will actively participate in the decision-making process: not all patients want this or are able to do so. In the option talk, the professional presents all options in a neutral fashion without indicating his or her own preference. During the decision talk, professional and patient decide about the options and arrange follow-up [13].
We refer to the terminology defined by the National Institute for Health Research (UK) regarding the use of following terms [14]:
Patient and public involvement is the development of partnerships between patients, carers or other members of public and researchers. Such partnerships are made in order to influence what research is done, how, and what happens to the results.
Patient and public engagement is the sharing of information and knowledge about research by professionals, such as at open days, science festivals or through newspapers or other media.
Patient and public participation is the recruitment of patients or others to take part in clinical trials or other research studies.
GOALS OF THE WORKSHOP
For years, ENMC has implemented a strict policy [15] to include all stakeholders among the workshops’ participants, in particular patients affected by neuromuscular diseases (NMD) and their representatives from patient organisations and support groups. ENMC itself is funded and governed by the NMD patient organisations of different European countries and has decided to contribute in applying the concept of SDM to wider areas of NMD-healthcare and – research than in the dual communication between professional and patient, by organizing a special workshop in occasion of ENMC’s 25th anniversary. The workshop was attended by 45 participants from 15 different countries: Belgium, Denmark, Finland, France, Germany, Greece, Hungary, Italy, The Netherlands, Poland, Spain, Sweden, Switzerland, UK and the USA. This group represented a wide range of experts: patients and parents, representatives from NMD organisations, clinicians, health care professionals, researchers, societal and policy researchers, psychologists, ethicists, representatives from regulatory authorities and pharmaceutical companies.
This special ENMC workshop aimed at implementing patient involvement in the field of NMD by taking on board the model of SDM; explore opportunities and improve the level and quality of patient involvement in areas that are closely related to disease, care and treatment, where decisions are traditionally made by health care professionals, as well as in areas related to research settings:
1. psycho-social support of families going through the processes of screening and diagnosis
2. transition from child, to adolescent to adult patient
3. research that has major impact on daily life (nutrition, pain, fatigue)
4. registries and biobanks
5. clinical trial design
6. regulatory and consenting processes
In the first part of the workshop the concepts of SDM and of patient involvement were introduced. In the second part, after presenting the six topics, patient organizations, professionals, industry, and regulators addressed the raised issues in small group discussions. The three SDM steps (option talk, choice talk, decision talk) were applied to structure the discussions in a way that examines i) the interest of the patients in increasing their engagement in a particular field; ii) the state of the art of patient involvement in that field (good examples, illustrated in the plenary talks) and opportunities to increase it, identifying challenges and barriers; iii) the consensus of the stakeholder community as to which improvements are wished for the near future. Finally, the conclusions obtained in each discussion group were presented and summarized in a table of recommendations (see Table 1).
Table 1
Recommendations of the ENMC workshop study group
| Educational changes (Patients organisations, institutions) | Cultural changes (all stakeholders) | Structural changes (professional stakeholders) | |
| Biobanks and registries | – The consequences in donating samples or clinical data to biobanks and registries should be fully understood by participating patients: possible long times before research delivers results, need of actualized data in registries, worldwide use of samples and data – The consent form should be updated to accommodate modern techniques applied to cell lines and biopsies, and written together with patient organisations to ensure understanding | – The position for patient organisations in the ladder of participation should be at a top level (collaboration and/or control) – Patients who donate data or samples are to be seen as partners in research and should be aware of this role – Use of samples and data should also reflect the needs and priorities of the patients | – Research information should go back to patients in form of regular newsletters and updates – Effort should be made to ensure that registries are global, not national / allow international linkage of national registries – Researchers and companies that make use of data from registries and biobanks should make the generated outcomes available to the community, even if a project is discontinued and results are not published. – Patients organisations should be represented in ethic committees; the representatives should be multiple and not always the same individuals |
| Clinical trials | – Patient organisations should take a leading role in ensuring that (several) patients are empowered for a competent collaboration in this effort and that they bring in the community’s and not individual concerns, for instance by promoting Eurordis patients expert courses (Eurordis’ Open Academy) – The consent form should be elaborated in collaboration with patient organisations | – Patient engagement should happen from the very first phases of trial planning and through the discussion on design options (inclusion and exclusion criteria, outcome measures, outcome assessment, recruitment, expectations’ management, results sharing, discontinuation) – The scientific rationale and rigor of the trial should be in any case guaranteed; clinical studies leaders should be responsible of explaining such rationales – If children are the study participants, their perspective and direct engagement in decisions should be addressed as with adult patients. Consider possibility of diverging opinions/priorities between patients and their carers | – Clinical studies leaders are also responsible for reporting study results back to patients, even if negative, and explain, in case, the reasons for study discontinuation – Part of the clinical trial budget and allocated planning time should be dedicated to the interaction with patents and patient organisations, conduct of surveys, evaluation of preferences |
| Regulatory processes | – Prepare patients to understand the specific situation of the trial with its regulatory issues – Consider conflict of interests | – Ensure that patients are aware of their role in the consultation: as individual patient or as representing of a patient group | – Ensure that the participation of disease-specific patients is guaranteed also at the later phase of national consultations for the reimbursement and pricing |
This workshop report will focus on the discussions and outcomes related to research settings, e.g. topics 4, 5 and 6. The discussions and outcomes of the quality of life-topics and health care, e.g. topics 1, 2 and 3 will be reported elsewhere; only a brief overview of the outcomes of this part is reported here for the sake of completeness.
INTRODUCTORY TALKS
Guus Schrijvers opened the workshop illustrating the traditional SDM concept. He highlighted that proper allocation of time for discussion and comprehension of the health problem by all stakeholders is a key factor for its success. He provided his personal experience in this field and concluded by addressing the potential of improving the level of patient involvement from the dual conversation with the doctor to the levels of political decisions and of definition of research priorities.
Ingeborg Meijer introduced another working model to describe the level of patient’s involvement in the decision processes that relate to health, called “the ladder of participation” [16] (Fig. 1). The participation ladder is about power structures in society and how they interact. Specifically, it is a guide to seeing who has power when important decisions are being made.
Fig.1
Levels of proactive patient involvement along the participation ladder (A. Ambrosini; modified from [17]).
![Levels of proactive patient involvement along the participation ladder (A. Ambrosini; modified from [17]).](https://content.iospress.com:443/media/jnd/2019/6-1/jnd-6-1-jnd180368/jnd-6-jnd180368-g001.jpg)
The actions represented by the ladder’s steps not necessarily refer to individual decisions on health care alone, but they could easily be transposed and explored regarding patient involvement in research. Meijer pointed out the need for a change in culture in many medical research disciplines in order to succeed in the empowerment of patients, their families and advocates, but this change should be adopted also by researchers, doctors, and all other professionals. She highlighted the timeliness of addressing this issue in the NMD field. In fact, in an era where evidence-based medicine does not provide the answers yet and the change from ‘one size fits all’ to personalised medicine is becoming increasingly actual, patient representatives become more and more professional, industry recognizes the high value of their contribution, and limitations of the reimbursement system of health care makes a high patient compliance more and more necessary.
George Padberg spanned the final bridge between the traditional SDM in the bilateral patient-doctor consultation to the broader range of professional activities around NMD, where a patient involvement at a higher level is not yet accomplished, or not yet at equal levels in different European countries. The paternalistic approach of an old-fashioned relationship between patient and professional is now developing into the mutual trust, responsibility and agreement, which is at the basis of SDM. The extension of the classical patient-physician-partnership to a patient-professional-partnership that covers a broad range of research aspects (like clinical trial readiness and implementation, registries and biobanks) is not only an ethical imperative but would serve the community and allow for faster progress. For instance, inventory and prioritization of patient preferences in the context of a clinical trial outcomes’ selection change over time and across cultures [18, 19] and influence the trial design, and inclusion/exclusion criteria and recruitment strategies may benefit from the patient perspective. Modern medicine faces new challenges: for instance, gene therapy promises a final cure but actually creates new phenotypes with possible new issues; personal and precision medicine is not safe from side effects; preventive medicine does not account for environmental factors. Therefore, therapies require permanent medical supervision and patients should take a lead in the discussion about when to treat, when to stop, whom to treat, and whom not. The described patient-professional-partnership outside the dual interaction in the doctor’s room requires that patients and doctors are organized in networks and can work together under umbrella organisations: the creation of a European Neuromuscular Trial Coordination Centre is suggested as a possible solution.
TOPICS 1, 2 AND 3: SDM AND PATIENT INVOLVEMENT IN QUALITY OF LIFE – RELATED NMD RESEARCH
In general, quality of life (QoL) is the individual perception of the quality of daily life, including all its emotional, social, and physical aspects. In health care, health-related quality of life (HRQoL) concerns the impact that a disease, disability or disorder may have on the individual’s well-being. Session 1 addressed HRQoL in NMD for topics related to ethical and psychological burden of people affected by neuromuscular conditions and their families at the point of diagnosis and for screening promotion, during transition to adulthood and throughout life, with special focus on the various European countries’ conditions. The SDM and ladder of participation models were adopted to investigate the quality of information received and the level of involvement as individuals and/or representatives of patient organisations with emphasis on patients’ contribution in the definition of research priorities.
Overall, the conclusions of this first session activity indicated that more/better quality dialogue between doctors and their patients is required. On the one side, this is essential to help families coping with the difficult moments of the diagnosis and adopting the best medical options and attitudes tailored to their needs. On the other side, involving people with a neuromuscular condition in clinical research on HRQoL is fundamental to address what really matters patients and incorporate their suggestions in trial design. Moreover, patient organisations may play a relevant role in creating awareness and engaging professionals and public to stimulate discussion on HRQoL topics at different societal level.
TOPIC 4: PATIENT INVOLVEMENT IN REGISTRIES AND BIOBANKS
Hanns Lochmüller illustrated how rare disease patients represent a paradigm for personalized medicine and cutting-edge therapeutic developments, since 70% of the diseases are monogenetic allowing more straightforward approaches to understand pathology and therapy targets, unlike more common diseases that are often etiologically complex. Today, many rare disease research centres address the whole translational pathway, from gene identification to proof-of-concept studies, from the clinical trials and natural history studies to back-translation of clinical results into mechanism understanding, and from research outcomes to therapy delivery. In this view, rare disease research centres offer an ideal setup to implement and integrate patient involvement at all levels. The International Rare Diseases Research Consortium (IRDiRC), launched in 2010, aims at supporting and accelerating the development of therapies for rare diseases, and despite having reached some of its milestones ahead of time, the work to be done remains huge [20]. Many areas of research are overlapping across diseases and could be re-used instead of re-invented, and many bottlenecks are reconducible to lack of data sharing opportunities. Several initiatives aim at bridging such gaps by offering the possibility to share more data and tools, in particular through sample biobanks and disease registries [21, 22]. One good example of patient co-creation in research program is the launching of patient-driven, professionally supported disease registries, like the UK myotonic dystrophy registry, where the patient initiates the registration and names a doctor that enters the clinical data. Criticized in the past for the risk of bias and of gaps in the data, such registries actually delivered the basis for risk stratification and drug evaluation studies and allowed, with large cohorts of well characterized patients, for a faster clinical trial readiness in a number of clinical trials and studies that followed (20-24). Based on the same principle of shared governance between clinicians and patient organisations, the TREAT-NMD global registries have been recognized by the IRDiRC as a valuable and important resource [23–27]. Another good example is the International Charter of principles for sharing bio-specimens and data, developed in collaboration with patient representatives and meant to offer a tool to overcome contradictory legal and ethical frameworks across national borders that obstacle effective sharing [28]. In general, despite not systematically applied, patient involvement led to positive results when integrated in research efforts. There are many opportunities to explore an increased level of patient involvement in the following issues: setting goals, acquiring, providing and allocating resources; distributing information; recruiting participants; providing access; oversight and governance; lobbying.
Panel Discussion: A model for patient involvement in registries and biobanks
Major activities of a registry that applies SDM are the incorporation of caregivers’ and patient involvement from the beginning and the setting of research priorities for registry-facilitated projects. This offers a chance to engage people of different ages and environments and identify their needs and their expectations from the registry and from research and educational efforts. Multi-stakeholder advisory committees, online asynchronous focus groups and surveys are useful tools to understand why people prioritize certain types of research. It is important to have a range of stakeholders participate in decision making, and to have the right plans, means and personnel for dissemination. At the same time, it should be avoided having always the same individuals involved in all projects and committees. Patient commitment is facilitated when registries and researchers commit to reporting research results back to the data providers, the patients and families. A challenge is educating scientists to be flexible and change their research protocols according to the priorities of the research participants. For registries that obtain longitudinal self-report data, updating of registry data can become an issue. One strategy to motivate patients and families to update their data is a closer exchange of information – in this way supporting data sharing instead of only data delivery, and further using the data collected to develop educational materials. Information can be presented in multiple formats to enhance access by Registrants with different information needs, as videos or infographics. Mike Snape (AMO Pharma) illustrated his positive experience with an early interaction with registries and individual patients, especially in identifying patient-relevant outcome measures for clinical trials. He also encountered a conflict of interests between family- and carer-priorities and patient priorities that, especially in case of diseases with cognitive involvement, often drive decisions away from the patient’s interest. In other cases, excessive information is not wished by the trial participant, and this needs to be respected. It was noted that one main goal worth following by the registries would be developing pressure on regulators to create global registries for a real support to drug development.
Similarly to registries, biobanks offer to researchers the opportunity to share rare samples and to patients the opportunity of participating in research. Challenges are the long times between donating a sample and learning what research discovered out of it. The different levels of regulations over the years and decades sometimes make samples unusable for certain research projects, simply because certain uses of samples (for instance creating induced pluripotent cell lines) was not yet known at the time of the written consent to use the specimen. The distribution of cells duplicated or cloned from the cell of a specific person needs to be regulated. The information back to a donor whose cells were genotyped and may reveal new risks or opportunities for that particular person is not yet structured. Similarly, the question arose about what to do with data generated from biobank samples during a research project, how long can they be considered property of the researcher/company and when should they be given back to the database. Especially in the case a project is stopped and not further developed, data generated should go back to the biobank. Publications of research on biobank samples should also be notified to the biobank, and effort should be invested by biobanks in making use of the samples and generate knowledge out of them. Another issue is the understanding of the content and consequences of written consents: patient organisations should be involved in formulating the consent forms in lay language. Ideally, they would also support patients on their journey through the research project to fully comprehend agreements. To ensure the involvement of patients in deciding how to overcome such problems, it is key to have a powerful and balanced patient representation also in ethics committees. In some cases, it may be useful to have separate committees for professionals and lay people, but the integration of knowledge from both parts is critical. Also, it should be defined under which circumstances a decision is considered shared (majority, complete consensus, other models), and at which level of the decision the consultation of all stakeholders is actually introduced.
The common consensus of this group was that there is definitely room for improved patient participation in registries and biobanks. The conclusions can be summarized into four main points: i) the patient’s consent and its consequences for research upon entering in a registry or donation to a biobank needs to be fully understood and improved; ii) the patients consider themselves as owners of their data and samples, therefore they should be appropriately represented in any decision making or ethics committee that advises or governs data usage; iii) information generated from registries and biobanks should primarily benefit the patient community either directly or indirectly, any other usage of these services needs to be well justified and agreed; iv) information should go back to patients in forms of newsletters and updates (see also Table 1).
TOPIC 5: PATIENT INVOLVEMENT IN CLINICAL TRIAL DESIGN
Baziel van Engelen presented his experience with patient participation in the clinical trial OPTIMISTIC (Observational Prolonged Trial In Myotonic Dystrophy type 1 to Improve Quality of Life Standards, a Target Identification Collaboration). The trial was set up to improve participation and activity and at the same time to create a “trial toolbox” to facilitate future trials: setting up clinical infrastructure for myotonic dystrophy trials, validating outcome measures in a time frame comparable to drug trials and identifying individual or composite biomarker profiles. In many aspects, the trial was designed similarly to a drug trial: comparable time frame, use of similar outcome measures (patient-reported and examiner-reported), application of lessons learnt from pilot studies, carefully checking for side effects, molecular signature of the treatment effect.
However, the planning of the trial did not follow the classical way from preclinical animal data to selection of patient outcome and clinical trial readiness, instead, it followed the “reversed engineering” approach: from the determinant of the health status of DM1 patients through changing the determinants by cognitive behavioural therapy, the selection of appropriate biomarker to the identification of a druggable target. From the beginning, patient involvement was key in identifying the determinants of health status, and hence the clinical trial design where every participant selected the determinants he/she wanted to change. The trial chose the cognitive behavioural therapy as a tool to improve initiative and physical activity; primary and secondary outcome measures were defined based on patient preferences but assessed with measurable scales. A questionnaire distributed to participants at the end of the trial revealed that many of them had been motivated to engage in the trial because of the extra time spent with doctors and because of the wish to help researchers in their work. The holistic approach therefore contributed greatly to the retention of the patients in the trial and the overall success of the trial, as compared to the standard molecular approach. The concept of promoting health (vs decreasing the disease) and treating the patient (vs treating the disease) led in this case to an impressive retention level throughout the study and to a very high satisfaction of the involved patients. Van Engelen underlined the importance for the neurologist to understand the concepts of recovery approach and capability when dealing with SDM. The recovery approach is the support of a person’s potential for recovery, where recovery is generally seen as a personal journey rather than a goal to be reached. The “capability”, a concept borrowed from the economic sciences, is the real opportunity to be and do what one has reason to value (‘potential achievements’) and depends on the real freedom in the assessment of a person’s advantage and on the availability of equity, i.e. equal chances instead of equal resources: equal resources do not necessarily imply the equal chance to use these resources [29] (Fig. 2).
Fig.2
Equal resources vs equal chances (from: http://muslimgirl.com/46703/heres-care-equity-equality/).
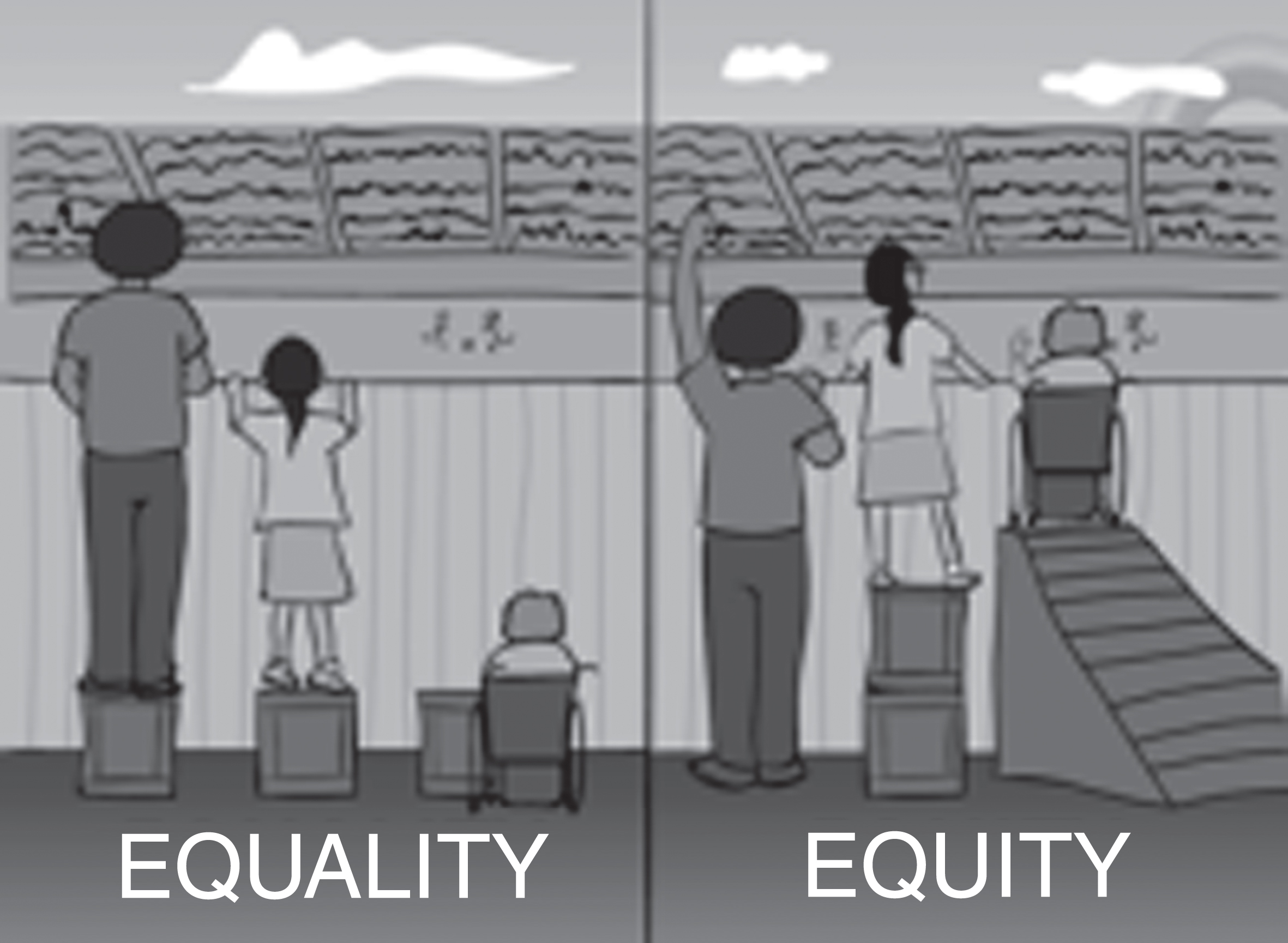
Discussion: A model for patient involvement in clinical trial design
Having the patient voice in a clinical trial is not only feasible, it should be a logical and natural thing. The quality of consultation between professionals and patients was addressed, highlighting that the perception of a good consultation may vary between the two parties. The involvement of persons with a NMD (or patients organisations) in clinical trials, as expert of the own disease, should happen in a proactive way from the beginning and through the discussion on trial design options. Not only on patient reported outcome measures (PROMS) the patient voice should be included. In other words, the final goal of the patient’s co-creation in clinical trials is to set up a sustainable patient-professionals-partnership with a clear communication strategy, expectations’ management and results sharing.
Patient organisations should be responsible to identify patients for the co-creation of a clinical trial design, to empower them with the necessary background information needed to fulfil this role, and to make sure that the involved patients bring in the community’s concerns and not only the own ones. This should be taken into consideration also with patients of young age. It is important also to balance the patient legitimate wishes and opinions with the scientific rationale; the responsibility of the researchers is to explain this rationale and if necessary explain why a certain study cannot be done, and to report back to patients the results of a study, even if negative.
Ideally, patients should be contacted before setting up the research plan of a clinical trial, be involved in all main phases (trial design, inclusion/exclusion criteria definition, outcomes definition, recruitment and preparation of the informed consent). Part of the budget should be devoted to this collaboration.
The conclusion of the group can be summarized in three points: i) patient participation in clinical trial design is desirable from the very first phases of trial design at the level of co-creation (partnership) and a dedicated budget should be reserved for this interaction by the clinical trial sponsors/PIs; ii) patient organisations should play an active role in identifying and promoting proactive individuals and in giving the necessary information and knowledge to fill their role in clinical trial design; iii) researchers should be responsible in sharing results (also if negative) with patients after the trial (see also Table 1).
TOPIC 6: PATIENT INVOLVEMENT IN THE REGULATORY AND CONSENTING PROCESSES
Patient preferences and priorities can be included in all steps of drug development, from upstream research to the post-approval phase (see for instance DIA Consideration Guide [30] and EMA lifecycle opportunities [31]. Today, several companies wish to assess patient preferences for their clinical trials and run patient preference studies. However, there is little consensus on the methodology to assess such preferences, on the design of such studies and even on the definition and role of patient preferences. Mats Hansson led this section and presented his experience as coordinator of the ongoing study PREFER, a 5-years public-private collaborative research project under the Innovative Medicines Initiative (IMI) that received contributions from the Horizon 2020 programme and from the European Federation of Pharmaceutical Industries and Associations. The PREFER project aims at identifying methods for preference elicitation, education and psychological measures in the literature, considering a set of criteria to determine which methods are suitable at different decision points in the drug life cycle. It addresses three main questions: What matters to patients? How much does it matter? What matters most? Answers to these questions depend greatly on the background information received by patients, but also on their condition and age. With the candidate methodologies and assessment criteria, patient preference studies will be run in three disease areas where patients and clinical research partners have been engaged: cancer, rheumatoid arthritis and NMD. These studies will evaluate at different decision points in the drug development process what patients deem relevant about their disease, which treatment options they prefer and their willingness to accept trade-offs between benefits and risks of their treatment. Finally, the project aims at developing evidence-based recommendations and best practices for conducting clinical case studies that offer industry, Regulatory Authorities and HTA bodies a valuable support in decision making.
Discussion: A model for patient involvement in regulatory and consenting processes
Participants in this working group discussed their experience of interaction with the Regulatory Authorities, and, in particular, with European Medicines Agency (EMA). Direct contacts occur at different stages of the development of medicines, from design of trial protocols, including patient related outcome measures, to pre-approval, evaluation and post-approval phases, and involve both professionals and patient representatives. All agreed that EMA has always been very open and proactive in listening to the different voices, with the aim of understanding both the clinical background of a disease and the burden that it puts on the daily life of patients, families, and caregivers. Experiences include involvement in discussions on risk-benefits analyses within the assessment of marketing authorisation applications for new therapeutics. Patient representatives found this experience extremely valuable, although very demanding, as they take important responsibilities towards their community. Particularly, the following challenges were identified: i) need for an understanding of the specific situation (disease natural history, drug efficacy and safety etc.), which implies that the patient has to be prepared to read some background information in relation to the clinical and regulatory issues; ii) independence from companies that have developed the product (if any), to avoid any conflict of interest; iii) if possible, good level of connection with the larger patient community, to also be able to share some wider perspectives, but this is not necessarily a requirement (depends on the particular activity), as there is always an intrinsic value even for individual patients to bring their own real life perspectives. Therefore, a right blend of “naïveté” (meant as original view of patient’s perspective) and some knowledge of the regulatory dynamics is considered optimal.
EMA relies very much on European patient organisations, such as EURORDIS and condition-specific patient organisations to identify the most suitable patient representatives, in addition to its own patient database (where individual patients can register). There are several initiatives in place to help achieve relevant competences such as the EURORDIS summer schools and EUPATI trainings, to support patients who want to take this challenge. Another area where patient involvement is required concerns the interaction with HTA bodies, where development and selection of HTA endpoints and the economic evaluation are yet to be defined. For instance, patients’ preferences in ease of use of device, ease of use of drug administration, caregivers burden should be considered. Issues concerning costs of healthcare derived from longer living and how this affects HTA should be discussed separately in ethics committees. It was noted that representatives of patient view in national ethics committees for rare diseases are rarely directly expert patients. A fair representation of patient preferences at both regulatory agencies and HTA bodies can be achieved by surveys. To this regard, the consensus of this group was that: i) methodologically well-planned surveys on patient preferences on the risk-benefit assessment and on the economical evaluation of a new medicine should be conducted with transparent processes, instruments, analysis and publication of results; ii) results of such surveys should flow into educational programs for expert patients; iii) expert patients or patient representatives should also be included in national ethics committees (see also Table 1).
The working group also encouraged a direct patient participation in the process of definition of the Informed Consent. As already noted in the context of consent forms for registries, biobanks, and clinical trials, these are often not easily understandable or too long. In case of a clinical trial, informed consent forms need to frame the expectations on the trial and provide realistic information. All agreed that the involvement of patients in the trial set-up and in the formulation of the consent form would be useful to increase readability and compliance/commitment. The information for generating the consent should also state clearly that a feedback on the study outcomes would be provided to all participants at the end of the trial. Also, the amendments during the trial should be re-discussed with the patients. Another important issue raised concerned children’s participation in the trials. Consent forms for children do exist but a major effort should be put in making sure that the consent also capture the effective child’s preferences, with respect to those of the parents. An additional observation was made in relation to “access to new medicines” which is an area bridging over from clinical research to health care. Across Europe, there is a single regulatory body (EMA) in charge of marketing authorization, but multiple national bodies in charge of reimbursement and pricing which determines whether patients receive such new medications. It was stressed that patients and patient organisations need to be fully involved in such consultation process.
CONCLUSIONS
With this workshop, ENMC aimed at looking at the position of patients with neuromuscular conditions in the decision processes regarding research and drug development. Considerable progress has been made in the last decades that resulted in a higher involvement of patients and patient organisations in clinical development. Several examples presented during the workshop showed that partnership and co-creation between professionals of neuromuscular disease and the patients – the real experts of the diseases – improve collaboration, compliance, and commitment on both sides. On the long term, this improves generation of positive research results and progress toward effective treatments and standards of care. However, the workshop identified some areas of research that need improvement and developed the recommendations summarized in Table 1. We identified three types of improvements, for which different stakeholders are in charge: i) Educational changes, to be implemented by patients organisations and institutions, comprise those changes needed to develop a better understanding of processes to allow for a full and competent patients involvement; ii) Cultural changes, to be implemented by all stakeholders, are those changes of basic societal attitudes that will facilitate the implementation of a higher patients involvement; iii) Structural changes, to be implemented by professional stakeholders, are changes in the work flow that will make space for an increased patient participation. With this report, ENMC wishes to contribute to the world-wide effort to improve patient position on the ladder of participation, with a particular focus on the gaps and needs of the neuromuscular community and hopes to offer a practical tool to implement awareness and discussions on the necessary changes.
CONFLICT OF INTERESTS
The authors have no conflict of interest to report
– Association Française contre les Myopathies (France)
– Deutsche Gesellschaft für Muskelkranke (Germany)
– Muscular Dystrophy UK (UK)
– Muskelsvindfonden (Denmark)
– Prinses Beatrix Spierfonds (The Netherlands)
– Schweizerische Stiftung für die Erforschung der Muskelkrankheiten (Switzerland)
– Telethon Foundation (Italy)
– Spierziekten Nederland (The Netherlands)
and Associated members:
– Finnish Neuromuscular Association (Finland) With special thanks to Audentes Therapeutics for supporting travel costs from non-ENMC member countries.
ACKNOWLEDGMENTS
This Workshop was made possible thanks to the financial support of the European Neuromuscular Centre (ENMC) and ENMC main sponsors:
REFERENCES
[1] | Elwyn G , Frosch D , Thomson R , Joseph-Williams N , Lloyd A , Kinnersley P , et al. Shared decision making: A model for clinical practice. Journal of General Internal Medicine. (2012) ;27: (10):1361–7. |
[2] | Elwyn G , Durand MA , Song J , Aarts J , Barr PJ , Berger Z , et al. A three-talk model for shared decision making: Multistage consultation process. BMJ. (2017) ;359: :j4891. |
[3] | Legare F , Adekpedjou R , Stacey D , Turcotte S , Kryworuchko J , Graham ID , et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. (2018) ;7: :CD006732. |
[4] | Lochmuller H , Torrent IFJ , Le Cam Y , Jonker AH , Lau LP , Baynam G , et al. The International Rare Diseases Research Consortium: Policies and Guidelines to maximize impact. European Journal of Human Genetics: EJHG. (2017) ;25: (12):1293–302. |
[5] | Geissler J , Ryll B , di Priolo SL , Uhlenhopp M . Improving Patient Involvement in Medicines Research and Development: A Practical Roadmap. Ther Innov Regul Sci. (2017) ;51: (5):612–9. |
[6] | EURORDIS Open Academy. https://wwweurordisorg/content/eurordis-open-academy. |
[7] | (DIA) DIA. Considerations guide to implementing patient-centric initiatives in health care product development. https://wwwdiaglobalorg/en/resources/areas-of-interest/patient-engagement. (2017) . |
[8] | Forsythe LP , Ellis LE , Edmundson L , Sabharwal R , Rein A , Konopka K , et al. Patient and Stakeholder Engagement in the PCORI Pilot Projects: Description and Lessons Learned. Journal of General Internal Medicine. (2016) ;31: (1):13–21. |
[9] | Witteman HO , Chipenda Dansokho S , Colquhoun H , Fagerlin A , Giguere AMC , Glouberman S , et al. Twelve Lessons Learned for Effective Research Partnerships Between Patients, Caregivers, Clinicians, Academic Researchers, and Other Stakeholders. Journal of General Internal Medicine. (2018) ;33: (4):558–62. |
[10] | Elwyn G , Edwards A , Kinnersley P . Shared decision-making in primary care: The neglected second half of the consultation. The British journal of general practice: The Journal of the Royal College of General Practitioners. (1999) ;49: (443):477–82. |
[11] | Charles C , Gafni A , Whelan T . Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc Sci Med. (1997) ;44: (5):681–92. |
[12] | Brehaut JC , Carroll K , Elwyn G , Saginur R , Kimmelman J , Shojania K , et al. Elements of informed consent and decision quality were poorly correlated in informed consent documents. Journal of Clinical Epidemiology. (2015) ;68: (12):1472–80. |
[13] | Makoul G , Clayman ML . An integrative model of shared decision making in medical encounters. Patient Education and Counseling. (2006) ;60: (3):301–12. |
[14] | National Institute of Health Research U. https://patientsactiveinresearch.org.uk/faqs/is-there-a-difference-between-patient-engagement-involvement-and-participation (2014) |
[15] | van Engelen B , Zollinger D , Pohlschmidt M , Ambrosini A , Rahbek J . The European NeuroMuscular Centre (ENMC): 20 years on. Neuromuscular Disorders: NMD. (2013) ;23: (4):375–6. |
[16] | Arnstein SR . Ladder of Citizen Participation. J Am I Planners. (1969) ;35: (4):216–24. |
[17] | de Wit MP , Kvien TK , Gossec L . Patient participation as an integral part of patient-reported outcomes development ensures the representation of the patient voice: A case study from the field of rheumatology. RMD Open. (2015) ;1: (1):e000129. |
[18] | Heatwole C , Bode R , Johnson N , Quinn C , Martens W , McDermott MP , et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1). Neurology. (2012) ;79: (4):348–57. |
[19] | Johnson NE , Quinn C , Eastwood E , Tawil R , Heatwole CR . Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle & Nerve. (2012) ;46: (6):951–3. |
[20] | Cutillo CM , Austin CP , Groft SC . A Global Approach to Rare Diseases Research and Orphan Products Development: The International Rare Diseases Research Consortium (IRDiRC). Advances in Experimental Medicine and Biology. (2017) ;1031: :349–69. |
[21] | Thompson R , Johnston L , Taruscio D , Monaco L , Beroud C , Gut IG , et al. RD-Connect: An integrated platform connecting databases, registries, biobanks and clinical bioinformatics for rare disease research. Journal of General Internal Medicine. (2014) ;29: (Suppl 3):S780–7. |
[22] | Mora M , Angelini C , Bignami F , Bodin AM , Crimi M , Di Donato JH , et al. The EuroBioBank Network: 10 years of hands-on experience of collaborative, transnational biobanking for rare diseases. European Journal of Human Genetics: EJHG. (2015) ;23: (9):1116–23. |
[23] | Ambrosini A , Calabrese D , Avato FM , Catania F , Cavaletti G , Pera MC , et al. The Italian neuromuscular registry: A coordinated platform where patient organizations and clinicians collaborate for data collection and multiple usage. Orphanet Journal of Rare Diseases. (2018) ;13: (1):176. |
[24] | Bladen CL , Rafferty K , Straub V , Monges S , Moresco A , Dawkins H , et al. The TREAT-NMD Duchenne muscular dystrophy registries: Conception, design, and utilization by industry and academia. Hum Mutat. (2013) ;34: (11):1449–57. |
[25] | Koeks Z , Bladen CL , Salgado D , van Zwet E , Pogoryelova O , McMacken G , et al. Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of Patients from the TREAT-NMD DMD Global Database. Journal of Neuromuscular Diseases. (2017) ;4: (4):293–306. |
[26] | Thompson R , Robertson A , Lochmuller H . Natural History, Trial Readiness and Gene Discovery: Advances in Patient Registries for Neuromuscular Disease. Advances in Experimental Medicine and Biology. (2017) ;1031: :97–124. |
[27] | Lochmuller H , Le Cam Y , Jonker AH , Lau LP , Baynam G , Kaufmann P , et al. ‘IRDiRC Recognized Resources’: A new mechanism to support scientists to conduct efficient, high-quality research for rare diseases. European Journal of Human Genetics: EJHG. (2017) ;25: (2):162–5. |
[28] | Mascalzoni D , Dove ES , Rubinstein Y , Dawkins HJ , Kole A , McCormack P , et al. International Charter of principles for sharing bio-specimens and data. European Journal of Human Genetics: EJHG. (2015) ;23: (6):721–8. |
[29] | Sen A . Development as freedom. In: Press ONYOU, editor. (2001) . pp. 291. |
[30] | http://engage.diaglobal.org/PatientEngagementConsiderationsGuide.html (2015) . |
[31] | EMA Oopiatmla. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/01/WC500199941.pdf |



