Cardiac Involvement Classification and Therapeutic Management in Patients with Duchenne Muscular Dystrophy
Abstract
Duchenne muscular dystrophy (DMD) is an inherited myogenic disorder due to mutations in the dystrophin gene on chromosome Xp21.1. The clinical picture included peripheral muscle weakness, cardiomyopathy and chronic respiratory insufficiency. In this paper, the authors review cardiac involvement in patients with DMD, propose a cardiac impairment classification and discuss therapeutic management options.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an inherited myogenic disorder due to mutations in the dystrophin gene on chromosome Xp21.1. It represents the most common and severe form of muscular dystrophy and occurs in 1 / 5000 male births [1]. The underlying gene mutations cause the absence of dystrophin, a protein located on the inner side of the skeletal and the cardiac muscle cells [2]. Symptoms include gait disturbances and difficulties in climbing stairs starting early in childhood with loss of ambulation around the age of twelve. The involvement of heart and respiratory function is classically observed in DMD and affects prognosis [2, 3]. Over the last few decades, mechanical ventilation (MV) has radically increased the survival of DMD patients by offering a way to improve respiratory functions [4, 5]. However, cardiac complications remain a serious issue impacting survival and thus requiring optimal management. In this manuscript, we review cardiac involvement in DMD patients and therapeutic management options.
PATHOPHYSIOLOGY
Dystrophin is the largest gene in the human genome with 79 exons. Mutation in the dystrophin gene causes the absence of dystrophin protein production because of a shift within the reading frame (out of frame) [2]. Dystrophin is a protein located in the sarcolemma and has a major structural role in muscle, as it links the internal cytoskeleton to the extracellular matrix [2]. The dystrophin contains four components: an amino-terminal domain that links the actin, a flexible rod domain, a cysteine-rich domain that links to cytoskeleton to the extracellular matrix and the carboxyl terminal domain [6]. The dystrophin protein plays a key role in the cellular stabilization [7]. It links the intracellular components (actin) with the membrane cell glycoprotein complex, giving a mechanical support during the cellular contraction [8]. In DMD, the lack of dystrophin leads to intracellular mechanical destabilization that weakens the sarcolemma and progressively causes cell degeneration. Cells degeneration mechanisms are complex and involve intracellular calcium overload related to tears in the cell membrane, calcium leakage, protease activation, production of reactive oxygen species and nitric oxide pathway impairment [9]. From a mechanistic point of view, the myocardial impairment begins in the inferolateral wall, due the dystrophin absence, and progressively affects the entire left ventricle (LV) at the end of the second decade [10]. Myocardial impairment progression is associated with myocardial fibrosis [11, 12]. As myocardial fibrosis increases, the LV dilates progressively which leads to an increase in the cardiac workload and an activation of the renin angiotensin system and the sympathetic nervous system. This process worsens the heart failure, creating a vicious circle. Moreover, the high heart rate (HR) associated with the autonomous system impairment in DMD [13] and the presence of a LV dyssynchrony may worsen the LV dysfunction overtime (Fig. 1).
CLINIC
Because of limited mobility, cardiomyopathy related symptoms are often absent in DMD. In the study by Nigro et al. [2], only 28% of patients aged <18 years disclosed related symptoms. Palpitations may be related to arrhythmia [13]. Dizziness is rare. Lipothymia, an incomplete transient loss of consciousness, is rare and may be associated with conduction abnormalities. In adult patients treated with mechanical ventilation because of respiratory insufficiency, peripheral edema and ascites are classical, as is pleural effusion in end-stage disease [14]. The presence of right-sided heart failure associated symptoms and peripheral edema in patients with chronic mechanical ventilation is related to the positive intra-thoracic pressures that impede the venous return [14].
ELECTROCARDIOGRAM (ECG)
ECG should be systematically included in the clinical management of DMD patients. ECG abnormalities that have been reported in DMD include sinus tachycardia, short PR intervals, and tall R wave in the right precordial leads, deep and narrow Q waves in inferolateral leads which are different from what is seen in myocardial ischemia, right bundle branch block and flat and inverted T waves [15, 16]. In a study that included 106 DMD patients, sinus tachycardia was present in 81 patients, V1 tall R waves in 79 patients and V5-V6 deep Q waves in 51 patients [17]. ECG in DMD patients may also show a Wolff Parkinson White (WPW) pattern [18]. Electrical right ventricular hypertrophy (RVH) is frequent, reaching 37% in the study by Takami et al. [16], without any correlation to LV dysfunction [19].
CARDIOMYOPATHY, ARRHYTHMIA AND CONDUCTION ABNORMALITIES
DMD is associated with a high prevalence of cardiomyopathy affecting the left ventricle and leading to chronic heart failure and heart rhythm disorders [20, 21]. Nigro et al. [2], in a pediatric population study that included 328 patients, reported that cardiomyopathy appeared as early as ten years with all DMD patients after the age of 18 years being affected. Echocardiography classically shows patterns of dilated cardiomyopathy. However, cases of left ventricle non-compaction have been reported in DMD [22]. Cardiac thrombus and cerebral infarction are rarely reported, mainly in DMD patients with severe heart failure [23]. Cardiac thrombus may be related to a significant activation of the coagulation system in DMD with heart failure [24]. Additional complications seen in DMD include arrhythmia and conduction disorder. Perloff et al. [13] reported atrial flutter in 5% of DMD patients and sinus pause in 5% of patients in a study that included 20 patients. Ventricular tachycardia was seen in 7% of patients in the study by Corrado et al. [25], reaching 16% in a study including DMD and BMD [26]. Complete atrioventricular blocks and sinus sick disease have been reported in DMD patients [27, 28]. Arrhythmia affects mainly patients with severe left ventricular dysfunction [24]. A LVEF <45% may predict the occurrence of adverse cardiac events in DMD [26].
DOPPLER ECHOCARDIOGRAPHY
Standard Doppler echocardiography should be performed according to the guidelines issued by the American Society of Echocardiography [29], using M-mode for the analysis of left atrial diameter, septal and posterior wall thickness and motion of the LV, LV end systolic and end diastolic diameters, calculation of the LV shortening fraction and LV ejection fraction (LVEF). 2D mode is used to assess cardiac anatomic structures and function by the assessment of the LV function a from 4 chambers apical view [29]. Doppler is used to assess the LV systolic function (LV aortic outflow tract systolic velocity) and the LV diastolic function (trans-mitral flow velocities, tissue Doppler imaging) and to estimate arterial pulmonary pressures [30]. However, assessment of the LV contractility by the classical LVEF analysis with standard echography is limited because in early stages, patients may have heart involvement with normal LVEF. 2D Strain imaging is a recent technology that assesses regional myocardial impairment and may detect early cardiac involvement in muscular dystrophy [31]. In early stages, subclinical diastolic function has been reported in DMD pediatric population, preceding the LV systolic dysfunction [32]. In DMD, regional myocardial abnormalities have been reported in early stages, affecting mainly the inferolateral region [33]. Mertens et al. [34] reported alteration of peak systolic and early diastolic myocardial velocities in the anterolateral and inferolateral walls in young DMD patients. In the adult population, echocardiography may show akinesia located in the LV inferobasal wall [35], LV dilation and LV systolic dysfunction. Mitral functional regurgitation may also be present, in relation with the LV and mitral annulus dilation. However, thorax deformities and difficulties to have optimal images in wheelchair- bound patients technically limit Doppler echocardiography.
CARDIAC MAGNETIC RESONANCE IMAGING
Cardiac magnetic resonance (CMR) imaging is used to assess LVEF and wall motion abnormalities. CMR may reveal early cardiac impairment in DMD with normal left ventricular ejection fraction in Doppler echocardiography [36]. Free wall segments are classically seen with late gadolinium enhancement (LGE) imaging [37]. LGE is used to assess myocardial fibrosis in CMR. In DMD, a transmural LGE pattern has been reported to be a prognostic factor in addition to LV systolic dysfunction [26]. In patients with LVEF≥55%, LGE was positive in 30% of patients, particularly in the LV free wall, reaching 84% in patients with LVEF <55% [37]. Cardiac CMR may help to predict ventricular arrhythmia and cardiac remodeling in DMD [34] and may be used to assess pharmacological approach targeting myocardial fibrosis [37]. Current researches in CMR are evaluating different myocardial sequencing in dystrophinopathies.
HEART INVOLVEMENT CLASSIFICATION
With the development of new targets in Heart Failure drug treatment, heart involvement in DMD needs to be better assessed. Researches in CMR will help clinicians to better understand pathophysiology and assess therapeutic efficacies [12, 38]. In patients with advanced heart failure, clinical presentation may be atypical because of loss of global muscle strength and limitations of wheelchair-bound patients even though in end-stage cardiomyopathy, right signs have been reported and anasarca is often present [14]. Diagnosis of dyspnea is hampered by MV use, which also protects against high LV filling. Peripheral edema may be seen as a consequence of muscle loss. Adult’s DMD patients may have subtle myocardial impairment whereas young DMD patients may have obvious heart failure.
Taking into account all the previous considerations, we have constructed a clinical-radiological classification of heart involvement in DMD, based on clinical, echocardiography and CMR findings (Table 1).
TREATMENT
Medical treatment
Drug treatment relies mainly on angiotensin-converting enzyme (ACE) inhibitors and beta blockers [40, 41], with the addition of aldosterone antagonists (potassium-sparing diuretic) in patients with chronic heart failure [42]. ACE inhibitors act by blocking the conversion of angiotensin I to angiotensin II, therefore leading to a decrease of arterial vascular resistance and an increase of stroke volume. In the general population, ACE inhibitors have been associated with a decrease morbidity and mortality in patients with chronic heart failure [42]. In DMD patients, ACE inhibitors (perindopril) have been shown to delay the onset of cardiomyopathy, which could be related to its anti-fibrotic properties [43, 44]. Beta-blockers act by blocking the beta-adrenergic receptors, reducing sympathetic activities, heart rate, heart contractility and relaxation. In the general population, beta blockers (bisoprolol, carvedilol, metoprolol) have been associated with a decrease morbidity and mortality in patients with chronic heart failure [42, 45, 46]. A beneficial effect of beta-blocker administration in association to ACE inhibitors has been reported for cardiac morbidity and mortality in DMD [38, 47]. According to the European guidelines [42], ACE inhibitors are recommended, in addition to a beta blocker, for symptomatic heart failure patients with reduced LVEF (class I, level A). Recently, in DMD pediatric population, a beneficial effect of eplerenone, an aldosterone antagonist, has been reported [48] when prescribed with either an ACE inhibitor or an angiotensin receptor blocker. Raman et al. [48], reported a lower LV circumferential strain decline after 12 months in DMD children with normal left ventricular ejection fraction and treated with eplerenone. According to the European guidelines, a MRA (mineralocorticoid receptor antagonist) is recommended in symptomatic heart failure patients with reduced LVEF despite a treatment including an ACE inhibitor and a beta blocker (class I, level A). Steroid therapy may also have positive impact on myocardial function, reducing mortality and new-onset cardiomyopathy [49]. Finally, idebenone administration has been associated with a slight increase of the peak systolic radial strain in the inferolateral wall of the LV that suggests a possible positive effect on LV contraction [50] using 2D strain echocardiography. Idebenone is not currently approved in this indication.
Instrumental Treatment
Instrumental treatment relies mainly on cardiac resynchronization therapy (CRT) and non-invasive ventilation. DMD patients exhibit restrictive respiratory failure, requiring long-term mechanical ventilation, which may influence cardiac function. Non-invasive ventilation (NIV) has been endorsed by the ATS consensus as the choice therapy in DMD for respiratory failure [48]. Positive pressure ventilation has a positive effect on LV function, resulting in a decrease in afterload [39]. In DMD, potential beneficial effects of chronic MV on cardiac function has been suggested [40]. Cardiac resynchronisation therapy (CRT) may be beneficial to patients with symptomatic heart failure despite optimal drug therapy and a QRS duration >130 ms [51]. According to the European guidelines, CRT is recommended for symptomatic heart failure patients with QRS duration≥150 ms, LBBB and LVEF≤35% despite optimal medical therapy (class I, level A) [42]. CRT is also recommended in symptomatic patients with QRS duration of 130–149 ms, LBBB and LVEF≤35% despite optimal medical therapy (class I, level B). However, Hor et al. [37] reported narrow QRS in the majority of DMD patients (97%), which reduces the number of DMD patients eligible for CRT implantation. Technical difficulties to insert CRT device and endocarditis risks also need to be taken into account [52]. An implantable cardiac defibrillator may be indicated in patients with severe LV dysfunction, particularly in case of ventricular arrhythmia events. Thus, only a minority of DMD patients may benefit from CRT. Recently, left ventricular device therapy has been proposed for DMD patients with end-stage cardiomyopathy [53] but requires further studies.
In conclusion, heart failure is a significant complication in DMD. Management relies on regular ECG, echocardiography and sometimes ECG Holter. 2D strain echocardiography and CMR may help clinicians to depict early cardiac involvement. According to the guidelines of the DMD Care Working Group, annual cardiac assessment including ECG and an echocardiography should be performed in all DMD patients aged >10 years. Pharmacological management relies mainly on ACE inhibitors, beta blockers and steroids. Electrical devices may be proposed in selected patients.
CONFLICTS OF INTEREST
The authors have no conflict to report.
ACKNOWLEDGMENTS
To Henri LELEU, MD, PhD, Paris for the English relecture of the manuscript.
REFERENCES
[1] | Mah JK , Korngut L , Fiest KM , Dykeman J , Day LJ , Pringsheim T , Jette N . A Systematic Review and Meta-analysis on the Epidemiology of the Muscular Dystrophies. Can J Neurol Sci. (2016) ;43: (1):163–77. |
[2] | Nigro G , Comi LI , Politano L , Bain RJ . The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. (1990) ;26: (3):271–7. |
[3] | Ashwath ML , Jacobs IB , Crowe CA , Ashwath RC , Super DM , Bahler RC . Left ventricular dysfunction in muscular dystrophy and genotype. Am J Cardiol. (2014) ;114: (2):284–9. |
[4] | Eagle M , Baudouin SV , Chandler C , Giddings DR , Bullock R , Bushby K . Survival in Duchenne muscular dystrophy: Improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. (2002) ;12: (10):926–9. |
[5] | Simonds AK , Muntoni F , Heather S , Fielding S . Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax. (1998) ;53: (11):949–52. |
[6] | Kaspar RW , Allen HD , Montanaro F . Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract. (2009) ;21: (5):241–9. |
[7] | Hoffman EP , Brown RH Jr , Kunkel LM . Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. (1987) ;51: (6):919–28. |
[8] | Pasternak C , Wong S , Elson EL . Mechanical function of dystrophin in muscle cells. J Cell Biol. (1995) ;128: (3):355–61. |
[9] | Verhaert D , Richards K , Rafael-Fortney JA , Raman SV . Cardiac involvement in patients with muscular dystrophies: Magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging. (2011) ;4: (1):67–76. |
[10] | Frankel KA , Rosser RJ . The pathology of the heart in progressive muscular dystrophy: Epimyocardial fibrosis. Hum Pathol. (1976) ;7: (4):375–86. |
[11] | Spurney CF . Cardiomyopathy of Duchenne muscular dystrophy: Current understanding and future directions. Muscle Nerve. (2011) ;44: (1):8–19. |
[12] | Silva MC , Magalhães TA , Meira ZM , Rassi CH , Andrade AC , Gutierrez PS , Azevedo CF , Gurgel-Giannetti J , Vainzof M , Zatz M , Kalil-Filho R , Rochitte CE . Myocardial Fibrosis Progression in Duchenne and Becker Muscular Dystrophy: A Randomized Clinical Trial. JAMA Cardiol. (2017) ;2: (2):190–199. |
[13] | Perloff JK . Cardiac rhythm and conduction in Duchenne’s muscular dystrophy: A prospective study of 20 patients. J Am Coll Cardiol. (1984) ;3: (5):1263–8. |
[14] | Fayssoil A , Ritzenthaler T , Luis D , Hullin T , Clair B , Annane D , Orlikowski D . Be careful about abdominal discomfort in adult patients with muscular dystrophy. Rev Neurol (Paris). (2014) ;170: (8-9):548–50. |
[15] | Manning GW , Cropp GJ . The electrocardiogram in progressive muscular dystrophy. Br Heart J. (1958) ;20: (3):416–20. |
[16] | Takami Y , Takeshima Y , Awano H , Okizuka Y , Yagi M , Matsuo M . High incidence of electrocardiogram abnormalities in young patients with duchenne muscular dystrophy. Pediatr Neurol. (2008) ;39: (6):399–403. |
[17] | Slucka C . The electrocardiogram in Duchenne progressive muscular dystrophy. Circulation. (1968) ;38: (5):933–40. |
[18] | Fayssoil A , Amara W , Annane D , Orlikowski D . Wolff-Parkinson-White syndrome in Duchenne muscular dystrophy. Int J Cardiol. (2013) ;167: (3):e53–4. |
[19] | Thrush PT , Edward N , Flanigan KM , Mendell JR , Allen HD . Precordial R wave height does not correlate with echocardiography findings in boys with Duchenne muscular dystrophy. Congenit Heart Dis. (2013) ;8: (6):561–7. |
[20] | Connuck DM , Sleeper LA , Colan SD , Cox GF , Towbin JA , Lowe AM , Wilkinson JD , Orav EJ , Cuniberti L , Salbert BA , Lipshultz SE . Pediatric Cardiomyopathy Registry Study GrouCharacteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: A comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. (2008) ;155: (6):998–1005. |
[21] | de Kermadec JM , Bécane HM , Chénard A , Tertrain F , Weiss Y . Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: An echocardiography study. Am Heart J. (1994) ;127: (3):618–23. |
[22] | Finsterer J , Stöllberger C , Feichtinger H . Non-compaction on autopsy in Duchenne muscular dystrophy. Cardiology. (2007) ;108: (3):161–3. |
[23] | Gimenez-Muñoz A , Capablo JL , Alarcia R , Torné L , Errea JM . Intracardiac thrombus and cerebral infarction in a patient with duchenne muscular dystrophy. J Clin Neuromuscul Dis. (2009) ;11: (2):79–80. |
[24] | Saito T , Yamamoto Y , Matsumura T , Nozaki S , Fujimura H , Shinno S . Coagulation system activated in Duchenne muscular dystrophy patients with cardiac dysfunction. Brain Dev. (2005) ;27: (6):415–8. |
[25] | Corrado G , Lissoni A , Beretta S , Terenghi L , Tadeo G , Foglia-Manzillo G , Tagliagambe LM , Spata M , Santarone M . Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol. (2002) ;89: (7):838–41. |
[26] | Florian A , Ludwig A , Engelen M , Waltenberger J , Rösch S , Sechtem U , Yilmaz A . Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. (2014) ;16: :81. |
[27] | Fayssoil A , Orlikowski D , Nardi O , Annane D . Complete atrioventricular block in Duchenne muscular dystrophy. Europace. (2008) ;10: (11):1351–2. |
[28] | Fayssoil A , Orlikowski D , Nardi O , Annane D . Pacemaker implantation for sinus node dysfunction in a young patient with Duchenne muscular dystrophy. Congest Heart Fail. (2010) ;16: (3):127–8. |
[29] | Cheitlin MD , Armstrong WF , Aurigemma GP , Beller GA , Bierman FZ , Davis JL , et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. (2003) ;16: (10):1091–110. |
[30] | Nagueh SF , Appleton CP , Gillebert TC , Marino PN , Oh JK , Smiseth OA , et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. févr. (2009) ;22: (2):107–33. |
[31] | Wissocque L , Brigadeau F , Richardson M , Boulé S , Kouakam C , Polge AS , Marquié C , Klug D . Impairment of Global and Regional Longitudinal Strains in patients with Myotonic Dystrophy type 1. Int J Cardiol. (2015) ;191: :46–7. |
[32] | Markham LW , Michelfelder EC , Border WL , Khoury PR , Spicer RL , Wong BL , Benson DW , Cripe LH . Abnormalities of diastolic function precede dilated cardiomyopathy associated with Duchenne muscular dystrophy. J Am Soc Echocardiogr. (2006) ;19: (7):865–71. |
[33] | Giatrakos N , Kinali M , Stephens D , Dawson D , Muntoni F , Nihoyannopoulos P . Cardiac tissue velocities and strain rate in the early detection of myocardial dysfunction of asymptomatic boys with Duchenne’s muscular dystrophy: Relationship to clinical outcome. Heart. (2006) ;92: (6):840–2. |
[34] | Mertens L , Ganame J , Claus P , Goemans N , Thijs D , Eyskens B , Van Laere D , Bijnens B , D’hooge J , Sutherland GR , Buyse G . Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr. (2008) ;21: (9):1049–54. |
[35] | Rapezzi C , Leone O , Biagini E , Coccolo F . Echocardiographic clues to diagnosis of dystrophin related dilated cardiomyopathy. Heart. (2007) ;93: (1):10. |
[36] | Silva MC , Meira ZM , Gurgel Giannetti J , da Silva MM , Campos AF , Barbosa Mde M , Starling Filho GM , Ferreira Rde A , Zatz M , Rochitte CE . Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol. (2007) ;49: (18):1874–9. |
[37] | Hor KN , Taylor MD , Al-Khalidi HR , Cripe LH , Raman SV , Jefferies JL , O’Donnell R , Benson DW , Mazur W . Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: Effect of age and left ventricular systolic function. J Cardiovasc Magn Reson. (2013) ;15: :107. |
[38] | Raman SV , Hor KN , Mazur W , Halnon NJ , Kissel JT , He X , Tran T , Smart S , McCarthy B , Taylor MD , Jefferies JL , Rafael-Fortney JA , Lowe J , Roble SL , Cripe LH . Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2015) ;14: (2):153–61. |
[39] | Pinsky MR , Matuschak GM , Klain M . Determinants of cardiac augmentation by elevations in intrathoracic pressure. J Appl Physiol. (1985) ;58: (4):1189–98. |
[40] | Bushby K , Finkel R , Birnkrant DJ , Case LE , Clemens PR , Cripe L , Kaul A , Kinnett K , McDonald C , Pandya S , Poysky J , Shapiro F , Tomezsko J , Constantin C . DMD Care Considerations Working GrouDiagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet Neurol. (2010) ;9: (2):177–89. |
[41] | Garg R , Yusuf S . Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. (1995) ;273: (18):1450–6. |
[42] | Ponikowski P , Voors AA , Anker SD , Bueno H , Cleland JG , Coats AJ , Falk V , González-Juanatey JR , Harjola VP , Jankowska EA , Jessup M , Linde C , Nihoyannopoulos P , Parissis JT , Pieske B , Riley JP , Rosano GM , Ruilope LM , Ruschitzka F , Rutten FH , van der Meer P . Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) :2016. |
[43] | Duboc D , Meune C , Pierre B , Wahbi K , Eymard B , Toutain A , Berard C , Vaksmann G , Weber S , Bécane HM . Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am Heart J. (2007) ;154: (3):596–602. |
[44] | Duboc D , Meune C , Lerebours G , Devaux JY , Vaksmann G , Bécane HM . Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. (2005) ;45: (6):855–7. |
[45] | Packer M , Coats AJ , Fowler MB , Katus HA , Krum H , Mohacsi P , Rouleau JL , Tendera M , Castaigne A , Roecker EB , Schultz MK , DeMets DL . Carvedilol Prospective Randomized Cumulative Survival Study GrouEffect of carvedilol on survival in severe chronic heart failure. N Engl J Med. (2001) ;344: (22):1651–8. |
[46] | Kajimoto H , Ishigaki K , Okumura K , Tomimatsu H , Nakazawa M , Saito K , Osawa M , Nakanishi T . Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J. (2006) ;70: (8):991–4. |
[47] | Ogata H , Ishikawa Y , Ishikawa Y , Minami R . Beneficial effects of beta blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol. (2009) ;53: (1):72–8. |
[48] | Finder JD , Birnkrant D , Carl J , Farber HJ , Gozal D , Iannaccone ST , Kovesi T , Kravitz RM , Panitch H , Schramm C , Schroth M , Sharma G , Sievers L , Silvestri JM , Sterni L . American Thoracic Society. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. (2004) ;170: (4):456–65. |
[49] | Schram G , Fournier A , Leduc H , Dahdah N , Therien J , Vanasse M , Khairy P . All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. (2013) ;61: (9):948–54. |
[50] | Buyse GM , Goemans N , van den Hauwe M , Thijs D , de Groot IJ , Schara U , Ceulemans B , Meier T , Mertens L . Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord. (2011) ;21: (6):396–405. |
[51] | Fayssoil A , Nardi O , Annane D , Orlikowski D . Successful cardiac resynchronisation therapy in Duchenne muscular dystrophy: A 5-year follow-up. Presse Med. (2014) ;43: (3):330–1. |
[52] | Fayssoil A , Lazarus A , Wahbi K , Ogna A , Nardi O , Lofaso F , Clair B , Orlikowski D , Annane D . Cardiac implantable electronic devices in tracheotomized muscular dystrophy patients: Safety and risks. Int J Cardiol. (2016) ;222: :975–7. |
[53] | Iodice F , Testa G , Averardi M , Brancaccio G , Amodeo A , Cogo P . Implantation of a left ventricular assist device as a destination therapy in Duchenne muscular dystrophy patients with end stage cardiac failure: Management and lessons learned. Neuromuscul Disord. (2015) ;25: (1):19–23. |
Figures and Tables
Fig.1
Pathophysiology of heart failure in DMD. LVEF: left ventricular ejection fraction. LV: left ventricle. RAA: renin angiotensin aldosterone.
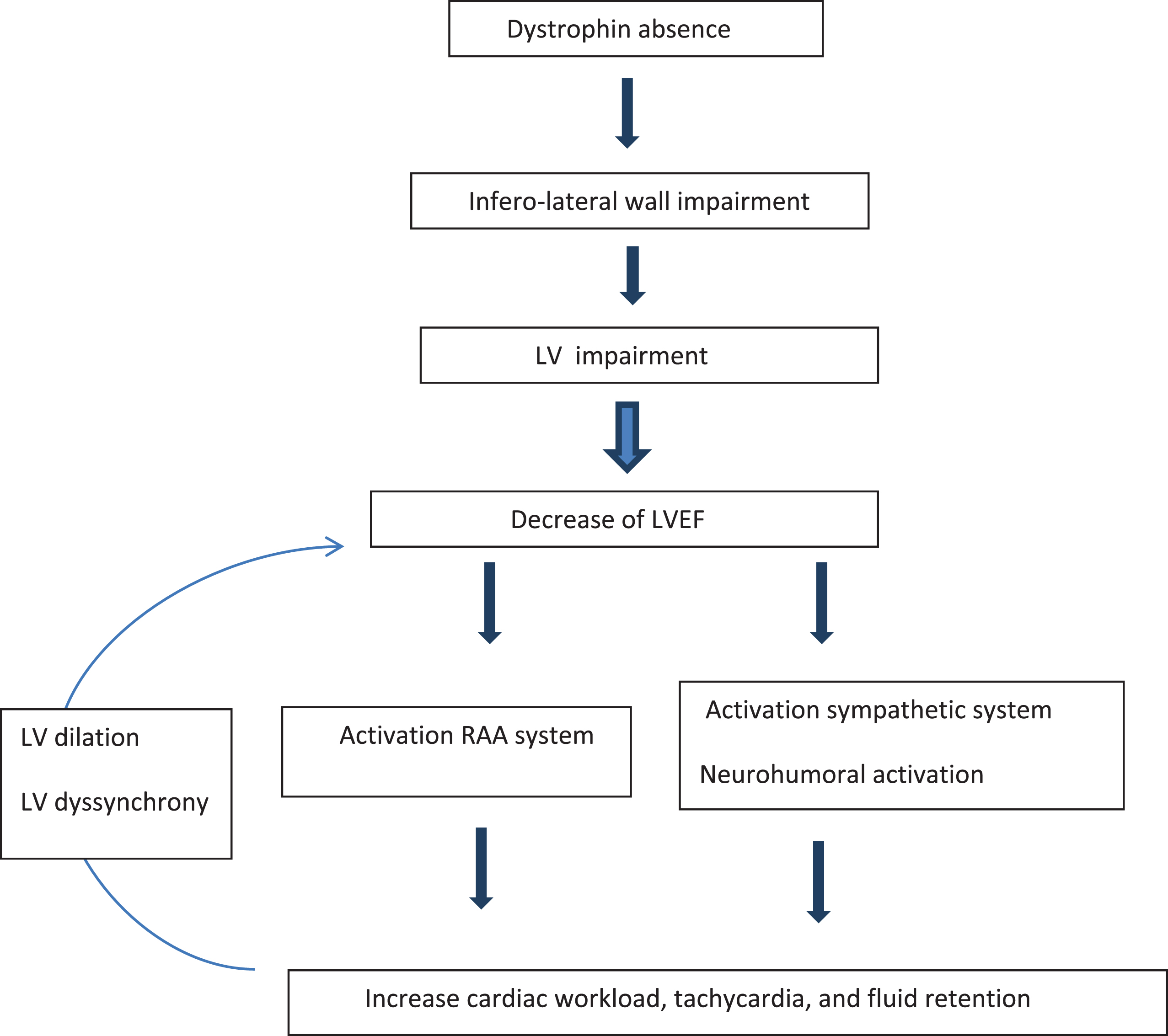
Table 1
Proposal for staging of heart involvement in DMD
| Stage | Clinic | Echocardiography CMR | Treatment |
| Stage 1 | Asymptomatic | Echo: | ACE inhibitors |
| LVEF>55% | |||
| Possible 2D strain abnormalities | |||
| CMR: | |||
| Myocardium LGE often negative | |||
| Stage 2 | Tachycardia | Echo: | ACE inhibitors |
| 45% <LVEF <55% | Beta blockers | ||
| 2D strain abnormalities | Mechanical ventilation at the end of the second decade | ||
| Infero-lateral wall impairment | |||
| CMR: | |||
| Myocardium with positive LGE | |||
| Stage 3 | Peripheral edema | Echo | ACE inhibitors |
| Sometimes Dyspnea | 35% <LVEF <45% | Beta blockers | |
| (patients without MV) | 2D strain abnormalities | Anti-aldosterone | |
| Tachycardia | CMR: | Diuretic (congestion) | |
| Myocardium with | Intermittent mechanical ventilation | ||
| Positive LGE+++ | |||
| Stage 4 | Anasarca | Echo: | ACE inhibitors |
| Peripheral edema | LVEF<35% | Beta blockers | |
| Ascites | 2D stain abnormalities | Anti-aldosterone | |
| Lipothymia | CRM: | Diuretic (congestion) | |
| Tachycardia | Diffuse myocardial LGE+++ | +- CRT | |
| or CRT-D | |||
| Permanent mechanical ventilation |
LVEF: left ventricular ejection fraction. CRT-D: cardiac resynchronization therapy defibrillator. CRT-P: cardiac resynchronization therapy pacemaker. ACE: angiotensin converting enzyme. LGE: late gadolinium enhancement. CMR: cardiac magnetic resonance.



