Changes in Communication between Muscle Stem Cells and their Environment with Aging
Abstract
Aging is associated with both muscle weakness and a loss of muscle mass, contributing towards overall frailty in the elderly. Aging skeletal muscle is also characterised by a decreasing efficiency in repair and regeneration, together with a decline in the number of adult stem cells. Commensurate with this are general changes in whole body endocrine signalling, in local muscle secretory environment, as well as in intrinsic properties of the stem cells themselves. The present review discusses the various mechanisms that may be implicated in these age-associated changes, focusing on aspects of cell-cell communication and long-distance signalling factors, such as levels of circulating growth hormone, IL-6, IGF1, sex hormones, and inflammatory cytokines. Changes in the local environment are also discussed, implicating IL-6, IL-4, FGF-2, as well as other myokines, and processes that lead to thickening of the extra-cellular matrix. These factors, involved primarily in communication, can also modulate the intrinsic properties of muscle stem cells, including reduced DNA accessibility and repression of specific genes by methylation. Finally we discuss the decrease in the stem cell pool, particularly the failure of elderly myoblasts to re-quiesce after activation, and the consequences of all these changes on general muscle homeostasis.
INTRODUCTION
Over the last 60 years, work performed on animal models, chiefly mouse, rat, and avian, and on human samples, has revealed and explored the capacity of adult stem cells - also called somatic stem cells – to self-renew and to differentiate into unipotent progeny within their residing tissue [1], generally for the purpose of repair. Resident stem cell populations have now been described in most tissues, including bone marrow [2], blood vessels [3], peripheral blood [4], skin [5], teeth [6], gut [7], liver [8], heart [9], brain [10] and skeletal muscle [11]. Once body growth has stopped and adulthood is reached, most of these stem cells become quiescent, and will only be activated for tissue turnover. Although this turnover can be very active as in circulating blood or gut epithelium in other tissues such as liver the stem cells usually remain unsolicited as hepatic damage rarely occurs in healthy adults [8]. Despite this heterogeneity, a decline in number and properties is universally observed in aged stem cells, a phenomenon which alters the maintenance of tissue homeostasis with aging. In aged skeletal muscle, a tissue with low turnover, this decline in the adult stem cell (also called satellite cell), which is responsible for muscle repair [12], is associated with muscle atrophy and muscle weakness [13–15], although their depletion in the mouse has differential effects depending on the muscle [12].
Muscle stem cells or satellite cells are localized beneath the basal lamina, peripheral to the muscle fibers [11], and express Pax7 [16] and Notch3 [17]. After muscle injury, satellite cells are driven out of their quiescent state, and start to proliferate. Most of the activated satellite cells rapidly co-express MyoD or Myf5 [16, 18]. The proliferating satellite cells - also called myogenic precursor cells or myoblasts - expand under the control of Notch3 [17] and Notch1/Hey1 pathways [19, 20]. They divide asymmetrically, with self-renewal of the stem cell pool being maintained by a minor population of myogenic precursor cells that down-regulate their expression of MyoD and Myf5 and return to a quiescent state [18, 21–23]. This asymmetrical division involves Numb, an antagonist of the Notch signalling pathway [19, 24]. Numb is asymmetrically localized during myoblast mitosis and it is the cell that has a high level of Numb that goes back to quiescence for self-renewal [19, 24–26]. After several rounds of proliferation, activated myoblasts decrease their expression levels of Pax7, Myf5 [16, 18] and Notch3 [17]. The Notch1 pathway is then repressed by Stra13 [20] through the CBF1 pathway [20, 27]. Simultaneously, the Wnt pathway is activated and promotes myoblast differentiation through β-catenin [28]. Myoblasts exit the cell cycle by expressing p57 [29], and then cyclin inhibitors - p21 and hypophosphorylated pRb [30–32] - together with higher levels MyoD followed by myogenin, a driver which triggers the expression of the differentiation genes [33, 34]. The myoblasts consequently undergo differentiation into myocytes, and fuse either with each other or with existing multi-nucleated myofibers in order to repair injured muscle [35, 36]. The differentiation and maturation process is regulated by MEF2, MEF3, and Mrf4 pathways [37–39], while other factors, such as Myomaker, are involved in fusion [40]. Muscle precursor cell proliferation, fusion and differentiation are tightly orchestrated by circulating hormones (e.g. growth hormone [41], testosterone [42, 43] and thyroid hormones [44, 45]), growth factors (e.g. IGF system [41], FGF system [46–48], TGF-b [49, 50]), G-CSF [51], chemokines (e.g. interleukines [52–55], MPC [55, 56]) and other secreted components (e.g. vesicles [57, 58]) present in the muscle stem cell environment.
Aged human [59] or murine [60–62] muscle can regenerate and repair, although the rate of regeneration declines [60–62]. This slower regeneration can be explained by: (1) changes in the muscle stem cell environment (growth factors, growth hormones, inflammation, and extracellular matrix content); (2) a lowered responsiveness of progenitor cells to repair stimuli; and (3) decrease in the number of muscle stem cells with aging. Each of these factors may impact on muscle homeostasis and each may both participate to, and be affected by, age-associated changes in intercellular communication. The subsequent sections will describe the different roles that intercellular communication may play in muscle aging, from hormonal and other circulating endocrine factors to local paracrine and autocrine secretory environment of the stem cell niche that may also modify the intrinsic properties of the stem cells themselves.
HORMONAL AND OTHER CIRCULATING FACTORS: CHANGE IN ENDOCRINE COMMUNICATION WITH AGING
The decline in muscle regenerative capacity with age [63] has been partly attributed to a decline in extrinsic environmental cues (see Fig. 1). Levels of circulating hormones, such as testosterone or IL-6 or growth hormone (GH) or IGF-1, are low in serum samples of aged subjects [64–66].
The endocrine hypothalamic-pituitary axis is altered with aging, leading to changes in hormone secretion that can contribute to cognitive decline or depression. Epidemiological studies have also shown a correlation between the decrease in growth hormone (GH) secretion and sarcopenia as well as other signatures of aging (e.g. intra-abdominal adiposity, osteopenia, etc.) [67]. GH is a stress hormone produced by the hypothalamus. It plays a key role in muscle mass maintenance through life [66]. It acts on myoblasts through its receptor GHR and activates NFATc2 that in turn stimulates the expression and secretion of IL-4 [41, 68, 69] - IL-4 being critical for myoblast fusion [68, 69]. GH also stimulates IGF-1 secretion by both liver and muscle [66]. IGF-1 and its splice variants - IGF-1Ea and IGF-1Eb - modulate myoblast proliferation [70] and differentiation [71] through MAPK and ERK1/2 signalling [70]. The latter regulates myogenesis, for example by interacting with p38α β MAPK and the asymmetric division and self-renewal of satellite cells [72].
These age-associated changes in the endocrine hypothalamic-pituitary axis can have further effects on the gonadotropic axis. Sex-steroid privation associated with age participates to, among other phenomena, loss of muscle mass [67]. The sex-steroid testosterone, secreted by the testis, has been extensively studied in muscle, and can be considered as a double-sided blade, acting both on myoblast proliferation and differentiation. It acts on myoblasts through the androgen receptor localized in the nucleus [73] or through G protein-coupled receptors [74]. It promotes myoblast proliferation through protein kinase C (PKC) signaling [74] - for instance through nPKCδ and extracellular signal-regulated kinases 1 and 2 (ERK1/2) activation [75]. Once ERK1/2 is phosphorylated, it is accompanied by an increase in cyclin E and Cdk2 –which are involved in myoblast proliferation [76]. Testosterone acts also on myoblast differentiation via protein kinase A (PKA) signaling [74] - PKA being required for myoblast fusion [77, 78]. Interestingly, oestrogens act similarly on the myogenic program through IGF-1signaling [79].
Aging is associated with an increase in low grade chronic and systemic inflammation, also called inflammaging [80]. Inflammaging could be due to microbial infection, cell debris, over-activated coagulation system, or an increase in cellular senescence with the associated changes in secretion [80]. This increased inflammation is generally attributed to a modified immune partner. Indeed, while young macrophages have been shown to have a beneficial effect to clear muscle debris after injury and stimulate myogenesis [81–83], aged macrophages can release a higher level of osteopontin that inhibits the muscle regeneration process [84]. Not only macrophages are involved in the muscle regeneration process, but also neutrophils, lymphocytes, dendritic cells, etc. These inflammatory cells secrete numerous chemokines and cytokines, but little is known about the impact of aging on cytokine secretion [85]. In the literature, it is described that IL-6 serum level is decreasing during aging [65]. IL-6 originates from the inflammatory cells, but also from the skeletal muscle itself [86]. It has been shown to be an important regulator of muscle stem cells [53], as it activates janus kinase 2 (Jak2) that will in turn phosphorylate STAT3 [52]. Once STAT3 is phosphorylated, it homodimerizes and translocates to the nucleus to bind to the γ-interferon activation sequence [87] in the promoter regions of genes involved in myoblast proliferation such as c-myc [52]. IL-6 not only regulates myoblast proliferation, but also promotes myoblast differentiation through the p38 MAPK pathway [88]. A decrease in IL-6 serum level could thus impact muscle regeneration efficacy.
The tissues from which circulating factors originate, such as muscle, hypothalamus, gonads, and liver, become atrophic and less active with age [8, 66, 89]. This change in body composition and activity with aging can thus participate to the decrease in circulating hormones (see Fig. 1). Consequently, when muscle damage occurs in an aged person, satellite cells be less prone to activation and differentiation, leading to a less efficient repair. Ten years ago, Conboy et al. elegantly showed that muscle regeneration could be partly rescued in aged mice exposed to serum from young mice through a parabiosis system [90]. Similarly, hormones released during pregnancy rescued the muscle regenerative capacity of aged female mice [91]. When aged subjects are trained, a rejuvenating effect is observed on muscle. This benefit effect could probably be due to a decrease of the inflammation for instance, as observed in exercised patients affected by myositis [92, 93]. When aged muscle stem cells were engrafted into young mice [94], their capacities to proliferate and differentiate were partly restored. Together these data suggest that circulating agents, which can originate from different tissues, impinge on muscle regeneration efficiency. Aging affects both the size and function of each tissue and consequently tissue secretory capacity. This alters the composition of circulating serum effecting intercellular communication atdistance.
SECRETORY ENVIRONMENT OF THE STEM CELL NICHE: CHANGE IN PARACRINE AND AUTOCRINE CELL-CELL COMMUNICATION WITH AGING
In addition to its classical role as a locomotive system, skeletal muscle has recently been shown to have a secretory activity. For instance, IL-6 [86] and musculin [95] have been identified to originate from and be secreted by skeletal muscle in vivo. In vitro, the secretome profile of C2C12 myotubes [55, 56], human myotubes [57] and rat muscle explants [96] suggest that muscle cells secrete numerous growth factors (e.g. follistatin like protein 1, IGF-2, TGF, etc) and cytokines. Secreted proteins - also named myokines [95] - may act in an autocrine/paracrine manner on neighboring muscle cells and contribute to muscle growth and regeneration. This local muscle secretome can be altered with aging. For instance, Chakkalakal et al. have shown that an increased secretion of FGF-2 by aged myofibers in mice inhibits sprouty1 expression in satellite cells, and consequently reduces their capacity to go back to quiescence and replenish the pool of the muscle stem cell [47]. The muscle secretome includes not only hormones, but also extracellular matrix components (ECM, e.g. TIMP2, fibronectin), miRNAs, and vesicles (exosomes and microvesicles) [57, 58, 97, 98]. Interestingly, exosomes originating from differentiated myocytes stimulate the myogenic program of proliferating myoblasts [58]. Myocyte exosomes contain miRNAs that inhibit Sirtuin expression, and thus stimulate the myoblast differentiation into myotubes [98]. A decrease in muscle mass with aging may thus reduce muscle secretory output. In a transcriptomic analysis performed on quadriceps muscle from young (15–24 years old) and elderly (72–80 years old) subjects, we indeed observed down-regulation of secretome markers in aged muscle [99]. However, little is known about the changes in the composition of the muscle secretome of aged muscle and further investigation is needed.
The local niche of muscle stem cells includes growth factors and cytokines secreted not only by the myofibers themselves, but also potentially by other cell types present within muscle, such as fibroblasts, endothelial or peri-endothelial cells [100, 101]. This local secretome can also be altered with aging (Fig. 2). For instance, fibroblasts present in aged skeletal muscle express a high level of TGF-β [101] –a growth factor that inhibits differentiation of myoblasts [102], and thus slows down the regeneration process. In addition, aging is described to be associated with an increase in senescent or pre-senescent cells in muscle and other tissue [103, 104]. During the last decade, the secretome of senescent cells from different tissues has been investigated and has been described to have an impact on the inflammatory response (by stimulating it in chronic obstructive pulmonary disease [105]) and to be instrumental in poor tissue regeneration (as observed in aged skin [106]). Altogether, these data suggest that the presence of senescent cells distinct from satellite cells within muscle tissue could alter these microenvironment of the satellite cells, and thus their behavior.
Muscle perfusion is decreased with aging [107], which may render myofibers and satellite cells less accessible to circulating hormones. This loss of perfusion may be maintained by the muscle loss itself. Indeed, sarcopenic muscle presents a disruption of the dystroglycan complex [108], leading to NOS-1 mis-localization, due to the link of NOS-1 to the dystrophin protein [109]. The mis-localization of NOS-1 results in decreased NO production, thereby diminishingmuscle perfusion [110]. A second effect of decreased NO production is a reduction in satellite cell activation [111].
Aged skeletal muscle presents a thickening of the ECM and a general increase in fibrosis [112]. Even if muscle fibers can secrete collagens and other components of the ECM [57, 97], little is known about their role in ECM thickening. A recent study shows that fibroblasts present in aged rat muscles express a higher level of collagen IVa2 and laminin 2 –which may participate in the thickening of the ECM [101]. This increase in the ECM thickness can interfere with the muscle regeneration process by modifying myoblast activation, proliferation and migration [48, 113]. Finally, a thickened ECM may act as a partial barrier, reducing the accessibility of the satellite cells to circulating growth factors, as observed for smooth muscle cells [114], and thus impair satellite cell activation and differentiation during musclerepair.
Together, these data suggest that the changes in the secretory composition of the muscle stem cell’s local niche with aging can slow down the regenerationprocess and decrease the replenishment of the pool of reserve cells. Repetitive iterations of this could contribute to the loss of muscle stem cells with aging.
CHANGE IN THE INTRINSIC PROPERTIES OF STEM CELLS WITH AGING
Exposure to a young environment by engraftment into young subjects or by parabiosis experiments only partly rescues the properties of aged satellite cells [90]. For instance their capacity to replenish the pool of reserve cells is not rescued (our unpublished data and [115]). These data suggest that some intrinsic properties of satellite cells are altered with aging and are not easily manipulated by external cues.
Intrinsic properties rely at least partly on DNA methylation, which may regulate gene expression in two ways [116]: (1) the accessibility of methylated enhancer regions to transcription factors is reduced, resulting in gene expression repression; (2) methyl-CpG-binding proteins bind to methylated DNA and alter the activity of histone deacetylases and methyltransferases. Consequently, local histones are hypermethylated, stabilizing the nucleosomes, so that DNA in methylated regions is tightly packed preventing binding of transcription factors or RNA polymerases. A recent study shows that histone methylation patterns are different between aged and young satellite cells in mice [117], and that the methylation profile can be modified by the presence of local growth factors such as FGF-2 [118]. The authors associated this histone methylation profile to a slower capacity of aged satellite cells to re-enter the cell cycle for aged satellite cells [117]. Interestingly, this study [117], as well as our own observations on culture of aged human muscle stem cells, show that once activated, aged satellite cells have a similar myogenic potential to young satellite cells. This indicates that muscle stem cells do not lose their differentiation potency with age, suggesting that the decrease with aging in the differentiation program during muscle regeneration is strongly related to changes in circulating factors.
DNA methylation has been shown to be increased in several tissues with aging, and the skeletal muscle is no exception [119, 120]. We have observed a higher level of DNA methylation in satellite cells of aged subjects (unpublished data). This hypermethylation could impact on the satellite cell fate and interfere with their capacity for self-renewal as observed in previously published studies (our data and [47, 115]). How DNA methylation is regulated with aging is not well known. Repeated stress over time can be one of the parameters implicated [121], involving for instance reactive oxygen species (ROS) [122]. Increased ROS production with age can be due to an increase in inflammation with aging [80] or to mitochondrial dysfunction in aged muscle [123, 124]. A decrease of circulating GH is also associated with a higher level of ROS and a lower level of anti-oxidants [125]. This overproduction of ROS could participate to increased DNA damage observed with aging [126]. Consequently, DNA methyltransferases (DNMT) are recruited to the DNA damage site, potentially inducing DNA silencing of the region nearby [127]. When we re-analyzed the transcriptome data available online (GSE9103), we indeed confirm a significant enrichment in the cellular response to oxidative stress in aged muscles (Fig. 3), suggesting a higher stress in aged muscle. ROS diffuse easily through membranes of fibers, thus potentially affecting DNA damage in neighboring satellite cells, and modifying their methylation status.
Factors discussed above - changes in the composition of circulating hormones in serum, as well as in the microenvironment - could also modify the epigenetic status of satellite cells and thus their behavior during regeneration, slowing satellite cell activation and decreasing their capacity to go back to quiescence
THE LOSS OF MUSCLE STEM CELLS WITH AGING AND ITS CONSEQUENCES ON MUSCLE HOMEOSTASIS
The number of muscle stem cells declines with age in mouse [13, 47, 90, 94] and humans [128, 129]. Although this loss can be caused by an increase in cell death, cellular senescence, or a deficiency in re-quiescence, apoptosis is rarely observed in aged murine and human muscle stem cells [94, 130, 131], suggesting that this cannot by itself explain the loss of muscle stem cells with aging. However, we cannot exclude the fact that apoptosis is a short punctual event that may be missed experimentally. Cellular senescence - also called replicative senescence - is defined as a phenomenon by which normal diploid cells cease to divide. It can be induced by telomere shortening that occurs during cell proliferation, and has been proposed to contribute to the loss of satellite cell function with aging [103]. However, shortened telomere length has not been reported in aged human satellite cells. Furthermore, there may be insufficient activation and turnover of satellite cells to allow senescence to be a major contributor to stem cell decline. Satellite cells are rarely activated in healthy adult human or mouse, and once muscle growth is complete in young human adults [14], subsequent myonuclear turnover is slow, being estimated at 15 years during adulthood [132]. In mouse models, myofiber growth by the addition of new nuclei through satellite cell fusion is completed by 21 days postnatally [133], and there is little evidence to suggest significant turnover. As discussed earlier, the capacity to re-quiesce through the sprouty1 pathway is decreased in aged stem cells [47, 117]. Consequently, when satellite cells are activated for muscle repair in elderly subjects, they do not replenish the pool of reserve cells. This failure of re-quiescence is a likely contributor to stem cell population decline.
The decrease in the number of satellite cells with aging can affect muscle homeostasis by altering the ECM composition. Indeed, aged muscle depleted in satellite cells in a Pax7CreER-DTA murine model shows an increase in fibrotic deposition [134], while fiber size was unaffected [135]. Resulting thickening of the ECM may increase myofiber fragility, and reduce the response of satellite cells to muscle damage, as discussed earlier. Therefore a loss of satellite cells could impinge directly upon muscle homeostasis, and exacerbate muscle fragility with aging.
CONCLUSION
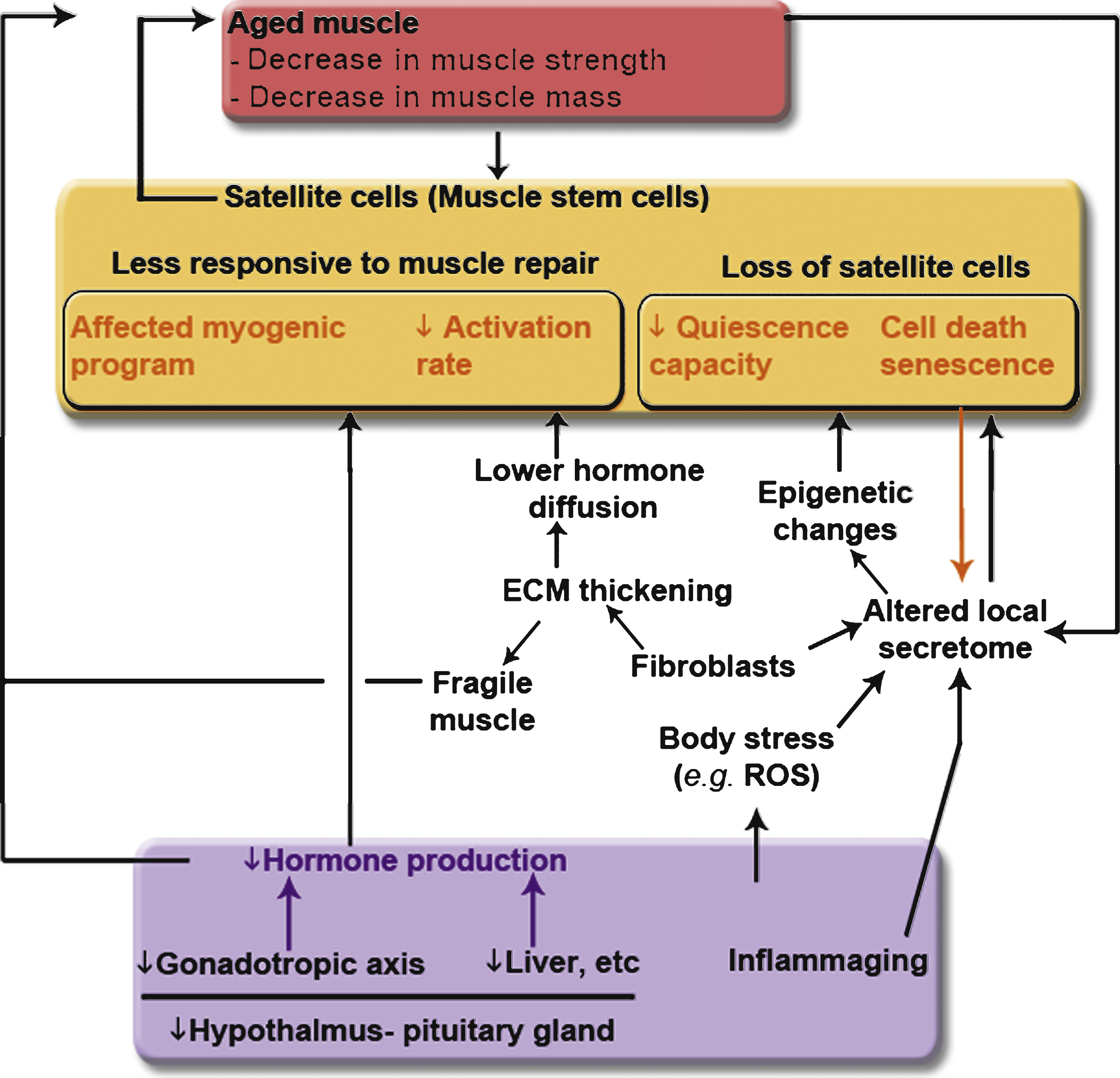
The interplay between whole-body tissue composition, the quantity and content of circulating serum hormones, and whole-body stress, such as ROS production, changes with age, and contributes to the decline in muscle mass and function (Fig. 4). Increased stress can act through epigenetic marking of satellite cells, changing their intrinsic properties with age - aged satellite cells show a decrease in their activation rate due to epigenetic changes. In addition, satellite cell number is decreased during aging, a loss that can contribute not only to a decreased regenerative capacity, but also to an increase in fibrotic deposition and ECM thickening. Increased muscle stiffness renders myofibers more fragile, requiring the activation of an already reduced and less responsive satellite cell population. Age-associated changes in the local signalling environment can affect the myogenic program causing a lower regeneration efficacy, a decrease in myonuclearturnover, and a failure in replenishing the pool of reserve cells, further contributing to the loss of muscle mass. Changes in the whole system of intercellular communication – both at the whole-body scale and in muscle microenvironment – may thus act as a vicious circle to exacerbate sarcopenia as the body ages. It is noteworthy that most studies on muscle aging in the literature have been done on muscle stem cells, rather than myofibers, and thus emphasize the role of satellite cells in muscle mass maintenance. The effect of hormones and cytokines and more generally the effect of aging on myofibers is difficult to assess directly and should not be neglected. The myofibers themselves comprise the bulk of the muscle mass and are clearly a key part of the maintenance of their own mass with aging, as indicated by disequilibrium of protein synthesis and degradation or the expression of micropeptides such as myoregulin that have a key role in muscle performance [138].
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was financed by the EU FP7 Programme project MYOAGE (contract HEALTH-F2-2009-223576), the ANR Genopath-INAFIB, the AFLD, the CNRS, INSERM, the University Pierre and Marie Curie Paris 6 and the AFM (Association Française contre les Myopathies).
REFERENCES
1 | Hosseinkhani M, Shirazi R, Rajaei F, Mahmoudi M, Mohammadi N, Abbasi M(2013) Engineering of the embryonic and adult stem cell nichesIran Red Crescent Med J15: 28392 |
2 | Thomas ED, Lochte HL, Cannon JH, Sahler OH, Ferrebee JW(1959) Supralethal whole body irradiation and isologous marrowtransplantation in manJ Clin Invest38: 17091716 |
3 | Baddour LM, Wilson WR, Bayer AS, Fowler VG, Bolger AF, Levison ME(2005) Infective endocarditis: Diagnosis, antimicrobial therapy,and management of complicationsCirculation111: 23e394e434 |
4 | McCredie KB, Hersh EM, Freireich EJ(1971) Cells capable of colonyformation in the peripheral blood of manScience171: 968293294 |
5 | Potten CS (1981) Cell replacement in epidermis (keratopoiesis) viadiscrete units of proliferationInt Rev Cytol69: 271318 |
6 | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG(2003) SHED: Stem cells from human exfoliated deciduous teethProc Natl Acad Sci U S A100: 1058075812 |
7 | Potten CS, Owen G, Booth D(2002) Intestinal stem cells protect theirgenome by selective segregation of template DNA strandsJ Cell Sci115: Pt 1123812388 |
8 | Dollé L, Best J, Mei J, Al Battah F, Reynaert H, van Grunsven LA(2010) The quest for liver progenitor cells: A practical pointof viewJournal of Hepatology117129 |
9 | Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S(2003) Adult cardiac stem cells are multipotent and supportmyocardial regenerationCell114: 6763776 |
10 | Altman J, Das GD(1965) Autoradiographic and histological evidence ofpostnatal hippocampal neurogenesis in ratsJ Comp Neurol124: 3319335 |
11 | MAURO A (1961) Satellite cell of skeletal muscle fibersJ Biophys Biochem Cytol9: 493495 |
12 | Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ(2015) Muscle stem cells contribute to myofibres in sedentaryadult miceNat Commun6: 7087 |
13 | Brack AS, Bildsoe H, Hughes SM(2005) Evidence that satellite celldecrement contributes to preferential decline in nuclear numberfrom large fibres during murine age-related muscle atrophyJ Cell Sci.118: Pt 2048134821 |
14 | Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC(2014) Satellite cells in human skeletal muscle; from birth to old ageAge (Dordr)36: 2545547 |
15 | Barberi L, Scicchitano BM, De Rossi M, Bigot A, Duguez S, Wielgosik A(2013) Age-dependent alteration in muscleregeneration: The critical role of tissue nicheBiogerontology14: 3273292 |
16 | Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR(2004) Muscle satellite cells adopt divergent fates: A mechanism forself-renewal?J Cell Biol166: 3347357 |
17 | Kitamoto T, Hanaoka K(2010) Notch3 null mutation in mice causes musclehyperplasia by repetitive muscle regenerationStem Cells28: 1222052216 |
18 | Yablonka-Reuveni Z(2011) The skeletal muscle satellite cell: Stillyoung and fascinating at 50J Histochem Cytochem59: 1210411059 |
19 | Conboy IM, Rando TA(2002) The regulation of Notch signaling controlssatellite cell activation and cell fate determination in postnatalmyogenesisDev Cell3: 3397409 |
20 | Sun H, Li L, Vercherat C, Gulbagci NT, Acharjee S, Li J(2007) Stra13 regulates satellite cell activation by antagonizing NotchsignalingJ Cell Biol177: 4647657 |
21 | Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA(2006) Pax7 and myogenic progression inskeletal muscle satellite cellsJ Cell Sci119: Pt918241832 |
22 | Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z(2007) Nestin-GFP reporter expression defines the quiescent state ofskeletal muscle satellite cellsDev Biol304: 1246259 |
23 | Gayraud-Morel B, Chrétien F, Jory A, Sambasivan R, Negroni E, Flamant P(2012) Myf5 haploinsufficiency reveals distinct cellfate potentials for adult skeletal muscle stem cellsJ Cell Sci125: Pt 717381749 |
24 | Shinin V, Gayraud-Morel B, Gomès D, Tajbakhsh S(2006) Asymmetricdivision and cosegregation of template DNA strands in adult musclesatellite cellsNat Cell Biol.8: 7677687 |
25 | Kuang S, Gillespie MA, Rudnicki MA(2008) Niche regulation of musclesatellite cell self-renewal and differentiationCell Stem Cell2: 12231 |
26 | George RM, Biressi S, Beres BJ, Rogers E, Mulia AK, Allen RE(2013) Numb-deficient satellite cells have regeneration andproliferation defectsProc Natl Acad Sci U S A110: 461854918554 |
27 | Zhou S, Fujimuro M, Hsieh JJ, Chen L, Miyamoto A, Weinmaster G(2000) SKIP, a CBF1-associated protein,interacts with the ankyrin repeat domain of NotchIC To facilitateNotchIC functionMol Cell Biol20: 724002410 |
28 | Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA(2008) A temporalswitch from notch to Wnt signaling in muscle stem cells isnecessary for normal adult myogenesisCell Stem Cell2: 15059 |
29 | Bigot A, Jacquemin V, Debacq-Chainiaux F, Butler-Browne GS, Toussaint O, Furling D(2008) Replicative aging down-regulatesthe myogenic regulatory factors in human myoblastsBiol Cell100: 3189199 |
30 | Duguez S, Sabido O, Freyssenet D(2004) Mitochondrial-dependentregulation of myoblast proliferationExp Cell Res299: 12735 |
31 | Ciavarra G, Zacksenhaus E(2010) Rescue of myogenic defects inRb-deficient cells by inhibition of autophagy or byhypoxia-induced glycolytic shiftJ Cell Biol191: 2291301 |
32 | Heron-Milhavet L, Franckhauser C, Fernandez A, Lamb NJ(2013) Characterization of the Akt2 Domain Essential for Binding Nuclearp21cip1 to Promote Cell Cycle Arrest during MyogenicDifferentiationPLoS One8: 10e76987 |
33 | Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R(1991) The myoD gene family: Nodal point during specification ofthe muscle cell lineageScience251: 4995761766 |
34 | Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H(1989) Expression of two myogenic regulatory factors myogeninand MyoD1 during mouse embryogenesisNature341: 6240303307 |
35 | Duguez S, Féasson L, Denis C, Freyssenet D(2002) Mitochondrialbiogenesis during skeletal muscle regenerationAm J PhysiolEndocrinol Metab.E282: 4802809 |
36 | Chargé SBP, Rudnicki MA(2004) Cellular and molecular regulation ofmuscle regenerationPhysiol Rev84: 1209238 |
37 | Naya FJ, Olson E(1999) MEF A transcriptional target for signalingpathways controlling skeletal muscle growth and differentiationCurrent Opinion in Cell Biology2: 683688 |
38 | Rhodes SJ, Konieczny SF(1989) Identification of MRF A new member ofthe muscle regulatory factor gene familyGenes Dev3: 12B20502061 |
39 | McGeachie AB, Koishi K, Andrews ZB, McLennan IS(2005) Analysis of mRNAs that are enriched in the post-synaptic domain of the neuromuscularjunctionMol Cell Neurosci30: 2173185 |
40 | Millay DP, O’Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R(2013) Myomaker is a membrane activator ofmyoblast fusion and muscle formation(Supp). Nature499: 7458301305 |
41 | Mavalli MD, DiGirolamo DJ, Fan Y, Riddle RC, Campbell KS, VanGroen T(2010) Distinct growth hormone receptor signaling modesregulate skeletal muscle development and insulin sensitivity inmiceJ Clin Invest.120: 1140074020 |
42 | Serra C, Tangherlini F, Rudy S, Lee D, Toraldo G, Sandor NL(2013) Testosterone improves the regeneration of old and youngmouse skeletal muscleJournals Gerontol - Ser A Biol Sci Med Sci68: 11726 |
43 | Sinha-Hikim I, Roth SM, Lee MI, Bhasin S(2003) Testosterone-inducedmuscle hypertrophy is associated with an increase in satellitecell number in healthy, young menAmerican Journal of Physiology Endocrinology and MetabolismE197E205 |
44 | Dentice M, Ambrosio R, Damiano V, Sibilio A, Luongo C, Guardiola O(2014) Intracellular inactivation of thyroid hormone is asurvival mechanism for muscle stem cell proliferation and lineageprogressionCell Metab20: 6103848 |
45 | Leal ALRC, Albuquerque JPC, Matos MS, Fortunato RS, Carvalho DP, Rosenthal D(2014) Thyroid hormones regulate skeletal muscleregeneration after acute injuryEndocrine233240 |
46 | Han D, Zhao H, Parada C, Hacia JG, Bringas P, Chai Y(2012) ATGF-Smad4-Fgf6 signaling cascade controls myogenic differentiationand myoblast fusion during tongue developmentDevelopment16401650 |
47 | Chakkalakal JV, Jones KM, Basson MA, Brack AS(2012) The aged nichedisrupts muscle stem cell quiescenceNature490: 7420355360 |
48 | Grounds MD (1998) Age-associated changes in the response of skeletalmuscle cells to exercise and regenerationAnn N Y Acad Sci854: 7891 |
49 | Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U(2003) Cross-talk between the Notch and TGF-β signaling pathways mediated by interaction of the Notch intracellular domain with Smad3J Cell Biol163: 4723728 |
50 | McFarlane C, Hui GZ, Amanda WZW, Lau HY, Lokireddy S, Xiaojia G(2011) Human myostatin negatively regulates human myoblast growthand differentiationAm J Physiol Cell Physiol301: 1C195C203 |
51 | Hara M, Yuasa S, Shimoji K, Onizuka T, Hayashiji N, Ohno Y(2011) G-CSF influences mouse skeletal muscle development andregeneration by stimulating myoblast proliferationJ Exp Med208: 4715727 |
52 | Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G(2011) IL-6 induced STAT3 signalling is associated with theproliferation of human muscle satellite cells following acutemuscle damagePLoS One6: 3e17392 |
53 | Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P(2008) Interleukin-6 is an essential regulatorof satellite cell-mediated skeletal muscle hypertrophyCellMetab7: 13344 |
54 | Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol A-C, Poron F(2003) Satellite cells attract monocytes and usemacrophages as a support to escape apoptosis and enhance musclegrowthJ Cell Biol163: 511331143 |
55 | Chan CYX, Masui O, Krakovska O, Belozerov VE, Voisin S, Ghanny S(2011) Identification of differentially regulated secretomecomponents during skeletal myogenesisMol Cell Proteomics10: 5M110.004804 |
56 | Henningsen J, Rigbolt KTG, Blagoev B, Pedersen BK, Kratchmarova I(2010) Dynamics of the skeletal muscle secretome during myoblastdifferentiationMol Cell Proteomics9: 1124822496 |
57 | Le Bihan M-C, Bigot A, Jensen SS, Dennis J, Rogowska-Wrzesinska A, Lainé J(2012) In-depth analysis of the secretome identifiesthree major independent secretory pathways in differentiating human myoblastsJ Proteomics77: 344356 |
58 | Forterre A, Jalabert A, Berger E, Baudet M, Chikh K, Errazuriz E(2014) Proteomic analysis of C2C12 myoblast and myotubeexosome-like vesicles: A new paradigm for myoblast-myotube crosstalk?PLoS One9: 1e84153 |
59 | Clarkson PM, Dedrick ME(1988) Exercise-induced muscle damage, repair,and adaptation in old and young subjectsJ Gerontol43: 4M91M96 |
60 | Gutmann E, Carlson BM(1976) Regeneration and transplantation of musclesin old rats and between young and old ratsLife Sci18: 1109114 |
61 | Sadeh M(1988) Effects of aging on skeletal muscle regenerationJ Neurol Sci87: 16774 |
62 | Smythe GM, Shavlakadze T, Roberts P, Davies MJ, McGeachie JK, Grounds MD(2008) Age influences the early events of skeletal muscleregeneration: Studies of whole muscle grafts transplanted betweenyoung (8 weeks) and old (13-21 months) miceExp Gerontol43: 6550562 |
63 | Carlson ME, Conboy IM(2007) Loss of stem cell regenerative capacitywithin aged nichesAging Cell6: 3371382 |
64 | Kojo G, Yoshida T, Ohkawa S, Odamaki M, Kato A, Takita T(2014) Association of serum total testosterone concentration withskeletal muscle mass in men under hemodialysisInt Urol Nephrol46: 5985997 |
65 | Gordon SE, Kraemer WJ, Looney DP, Flanagan SD, Comstock BA, Hymer WC(2014) The influence of age and exercise modality on growth hormone bioactivity in womenGrowth Horm IGF Res24: 2-395103 |
66 | Sattler FR(2013) Growth hormone in the aging maleBest Pract Res ClinEndocrinol Metab27: 4541555 |
67 | Veldhuis JD(2008) Aging and hormones of the hypothalamo-pituitary axis: Gonadotropic axis in men and somatotropic axes in men and womenAgeing Research Reviews189208 |
68 | Sotiropoulos A, Ohanna M, Kedzia C, Menon RK, Kopchick JJ, Kelly PA(2006) Growth hormone promotes skeletal muscle cell fusionindependent of insulin-like growth factor 1 up-regulationProc Natl Acad Sci USA103: 1973157320 |
69 | Horsley V, Jansen KM, Mills ST, Pavlath GK(2003) IL-4 acts as amyoblast recruitment factor during mammalian muscle growthCell113: 4483494 |
70 | Brisson BK, Barton ER(2012) Insulin-Like Growth Factor-IE-Peptide Activity Is Dependent on the IGF-I ReceptorPLoS One7: 9e45588 |
71 | Matheny RW, Nindl BC(2011) Loss of IGF-IEa or IGF-IEb impairs myogenicdifferentiationEndocrinology152: 519231934 |
72 | Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, Olwin BB(2012) Coordination of satellite cell activation and self-renewal bypar-complex-dependent asymmetric activation of p38??/?? MAPKCellStem Cell.11: 4541553 |
73 | Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S(2004) Androgen receptor in human skeletal muscle and cultured musclesatellite cells: Up-regulation by androgen treatmentJ Clin Endocrinol Metab.89: 1052455255 |
74 | Fu R, Liu J, Fan J, Li R, Li D, Yin J(2012) Novel evidence thattestosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblastcell lineJ Cell Physiol227: 198107 |
75 | Czifra G, Szöllösi A, Nagy Z, Boros M, Juhász I, Kiss A(2015) Protein kinase Cδ promotes proliferation andinduces malignant transformation in skeletal muscleJ Cell MolMed19: 2396407 |
76 | Wei C, Ren H, Xu L, Li L, Liu R, Zhang L(2015) Signals of Ezh2, Src, and Akt Involve in Myostatin-Pax7 Pathways Regulatingthe Myogenic Fate Determination during the Sheep MyoblastProliferation and DifferentiationPLoS One10: 3e0120956 |
77 | Mukai A, Hashimoto N(2008) Localized cyclic AMP-dependent proteinkinase activity is required for myogenic cell fusionExp CellRes314: 2387397 |
78 | Han SY, Park DY, Lee GH, Park SD, Hong SH(2002) Involvement of type Iprotein kinase A in the differentiation of L6 myoblast inconjunction with phosphatidylinositol 3-kinaseMol Cells14: 16874 |
79 | Ahtiainen M, Pöllänen E, Ronkainen PHA, Alen M, Puolakka J, Kaprio J(2012) Age and estrogen-based hormonetherapy affect systemic and local IL-6 and IGF-1 pathways inwomenAge (Omaha)34: 512491260 |
80 | Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F(2007) Inflammaging and anti-inflammaging: A systemic perspectiveon aging and longevity emerged from studies in humansMech AgeingDev128: 192105 |
81 | Zhang J, Xiao Z, Qu C, Cui W, Wang X, Du J(2014) CD8 T Cells AreInvolved in Skeletal Muscle Regeneration through FacilitatingMCP-1 Secretion and Gr1high Macrophage InfiltrationJ Immunol193: 1051495160 |
82 | Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK(2014) Altered macrophage phenotype transition impairs skeletal muscleregenerationAm J Pathol184: 411671184 |
83 | Chazaud B(2014) Macrophages: Supportive cells for tissue repair andregenerationImmunobiology219: 3172178 |
84 | Paliwal P, Pishesha N, Wijaya D, Conboy IM(2012) Age dependent increasein the levels of osteopontin inhibits skeletal muscleregenerationAging (Albany NY).4: 8553566 |
85 | Ebersole JL, Steffen MJ, Pappo J(1988) Secretory immune responses inageing ratsII. Phenotype distribution of lymphocytes insecretory and lymphoid tissues. Immunology64: 2289294 |
86 | Pedersen BK, Febbraio M(2005) Muscle-derived interleukin-6–a possiblelink between skeletal muscle, adipose tissue, liver, and brainBrain Behav Immun19: 5371376 |
87 | Ivanova AV, Ivanov SV, Zhang X, Ivanov VN, Timofeeva OA, Lerman MI(2004) STRA13 interacts with STAT3 and modulates transcription of STAT3-dependent targetsJ Mol Biol340: 4641653 |
88 | Baeza-Raja B, Muñoz-Cánoves P(2004) p38 MAPK-induced nuclearfactor-kappaB activity is required for skeletal muscledifferentiation: Role of interleukin-6Mol Biol Cell15: 420132026 |
89 | Nilwik R, Snijders T, Leenders M, Groen BBL, van Kranenburg J, Verdijk LB(2013) The decline in skeletal muscle mass withaging is mainly attributed to a reduction in type II muscle fibersizeExp Gerontol.48: 5492498 |
90 | Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA(2005) Rejuvenation of aged progenitor cells by exposure to ayoung systemic environmentNature433: 7027760764 |
91 | Falick Michaeli T, Laufer N, Sagiv JY, Dreazen A, Granot Z, Pikarsky E, Bergman Y, Gielchinsky Y(2015) The rejuvenating effect ofpregnancy on maternal regenerationAging Cell14: 4698700 |
92 | Nader GA, Lundberg IE(2009) Exercise as an anti-inflammatory intervention to combat inflammatory diseases of muscleCurr Opin Rheumatol21: 6599603 |
93 | Lundberg IE, Nader GA(2008) Molecular effects of exercise in patients with inflammatory rheumatic diseaseNat Clin Pract Rheumatol4: 11597604 |
94 | Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA(2007) Apopulation of myogenic stem cells that survives skeletal muscleagingStem Cells25: 4885994 |
95 | Engler D(2007) Hypothesis: Musculin is a hormone secreted by skeletalmuscle, the body’s largest endocrine organ. Evidence for actionson the endocrine pancreas to restrain the beta-cell mass and toinhibit insulin secretion and on the hypothalamus to co-ordinatethe neuroendocrine and appetite responses to exerciseActa Biomed78 Suppl 1: 156206 |
96 | Roca-Rivada A, Al-Massadi O, Castelao C, Senín LL, Alonso J, Seoane LM(2012) Muscle tissue as an endocrine organ: Comparativesecretome profiling of slow-oxidative and fast-glycolytic ratmuscle explants and its variation with exerciseJ Proteomics75: 1754145425 |
97 | Duguez S, Duddy W, Johnston H, Lainé J, Le Bihan MC, Brown KJ(2013) Dystrophin deficiency leads to disturbance ofLAMP1-vesicle-associated protein secretionCell Mol Life Sci70: 1221592174 |
98 | Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A(2014) Myotube-derived exosomal miRNAs downregulate Sirtuin1 inmyoblasts during muscle cell differentiationCell Cycle13: 17889 |
99 | Baraibar MA, Gueugneau M, Duguez S, Butler-Browne G, Bechet D, Friguet B(2013) Expression and modification proteomics during skeletal muscle ageingBiogerontology14: 3339352 |
100 | Abou-Khalil R, Mounier R, Chazaud B(2010) Regulation of myogenic stemcell behavior by vessel cells: The “ménage à trois” of satellite cells, periendothelial cells and endothelial cellsCell Cycle9: 5892896 |
101 | Zwetsloot KA, Nedergaard A, Gilpin LT, Childs TE, Booth FW(2012) Differences in transcriptional patterns of extracellular matrix,inflammatory, and myogenic regulatory genes in myofibroblasts,fibroblasts, and muscle precursor cells isolated from old male ratskeletal muscle using a novel cell isolation procedureBiogerontology13: 4383398 |
102 | Liu D, Black BL, Derynck R(2001) TGF-β inhibits muscledifferentiation through functional repression of myogenictranscription factors by Smad3Genes Dev15: 2229502966 |
103 | Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V(2014) Geriatric muscle stem cells switchreversible quiescence into senescenceNature506: 7488316321 |
104 | Fukada S, Ma Y, Uezumi A(2014) Adult stem cell and mesenchymalprogenitor theories of agingFront Cell Dev Biol2: March19 |
105 | Kumar M, Seeger W, Voswinckel R(2014) Senescence-associated secretoryphenotype and its possible role in chronic obstructive pulmonarydiseaseAm J Respir Cell Mol Biol51: 3323333 |
106 | Demaria M, Desprez PY, Campisi J, Velarde MC(2015) Cell Autonomous and Non-Autonomous Effects of Senescent Cells in the SkinJ Invest Dermatol17221726 |
107 | Behringer EJ, Segal SS(2012) Spreading the signal for vasodilatation:Implications for skeletal muscle blood flow control and theeffects of ageingJ Physiol590: Pt 2462776284 |
108 | Leiter JRS, Upadhaya R, Anderson JE(2012) Nitric oxide and voluntaryexercise together promote quadriceps hypertrophy and increasevascular density in female 18-mo-old miceAJP: Cell PhysiologyC1306C1315 |
109 | Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C(2009) Dystrophinscarrying spectrin-like repeats 16 and 17 anchor nNOS to thesarcolemma and enhance exercise performance in a mouse model ofmuscular dystrophyJ Clin Invest119: 3624635 |
110 | Song W, Kwak HB, Kim JH, Lawler JM(2009) Exercise training modulatesthe nitric oxide synthase profile in skeletal muscle from oldratsJournals Gerontol-Ser A Biol Sci Med Sci64: 5540549 |
111 | Anderson JE(2000) A role for nitric oxide in muscle repair: Nitricoxide-mediated activation of muscle satellite cellsMol BiolCell11: 518591874 |
112 | Goldspink G, Fernandes K, Williams PE, Wells DJ(1994) Age-relatedchanges in collagen gene expression in the muscles of mdxdystrophic and normal miceNeuromuscul Disord4: 3183191 |
113 | Ferreira MM, Dewi RE, Heilshorn SC(2015) Microfluidic analysis ofextracellular matrix-bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxisIntegr Biol569579 |
114 | Fannon M, Forsten-Williams K, Zhao B, Bach E, Parekh PP, Chu CL(2012) Facilitated diffusion of VEGF165 through descemet’smembrane with sucrose octasulfateJ Cell Physiol227: 113693700 |
115 | Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter Ta, Olwin BB(2014) p38 MAPK signaling underlies a cell-autonomous loss of stemcell self-renewal in skeletal muscle of aged miceNat Med20: 3265271 |
116 | Saini A, Mastana S, Myers F, Lewis M(2013) “From death, Lead me toimmortality”- mantra of ageing skeletal muscleCurr Genomics14: 4256267 |
117 | Liu L, Cheung TH, Charville GW, Hurgo BMC, Leavitt T, Shih J(2013) Chromatin modifications as determinants of muscle stem cellquiescence and chronological agingCell Re4: 1189204 |
118 | Li J, Han S, Cousin W, Conboy IM(2015) Age-Specific FunctionalEpigenetic Changes in p21 and p16 in Injury-Activated SatelliteCellsStem Cells33: 3951961 |
119 | Horvath S(2013) DNA methylation age of human tissues and cell typesGenome Biol14: 10R115 |
120 | Bocker MT, Hellwig I, Breiling A, Eckstein V, Ho AD, Lyko F(2011) Genome-wide promoter DNA methylation dynamics of humanhematopoietic progenitor cells during differentiation and agingBlood117: 19e182e189 |
121 | Blaze J, Roth TL(2015) Evidence from clinical and animal model studiesof the long-term and transgenerational impact of stress on DNAmethylationSemin Cell Dev BiolS1084S9521 |
122 | Cencioni C, Spallotta F, Martelli F, Valente S, Mai A, Zeiher AM(2013) Oxidative stress and epigenetic regulation in ageing andage-related diseasesInt J Mol Sci14: 91764317663 |
123 | Claflin DR, Jackson MJ, Brooks SV(2015) Age affects thecontraction-induced mitochondrial redox response in skeletalmuscleFront Physiol6 |
124 | Liu D, Sartor M, Nader G, Pistilli EE, Tanton L, Lilly C(2013) Microarray analysis reveals novel features of the muscle agingprocess in men and womenJ Gerontol A Biol Sci Med Sci68: 910351044 |
125 | Brioche T, Kireev R a, Cuesta S, Gratas-Delamarche A, TresguerresJ a, Gomez-Cabrera MC(2013) Growth hormone replacement therapyprevents sarcopenia by a dual mechanism: Improvement of proteinbalance and of antioxidant defensesJ Gerontol A Biol Sci MedSci.7113 |
126 | Signer RAJ, Morrison SJ(2013) Mechanisms that regulate stem cell agingand life spanCell Stem Cell.12: 2152165 |
127 | Zampieri M, Ciccarone F, Calabrese R, Franceschi C, Bürkle A, Caiafa P(2015) Reconfiguration of DNA methylation in agingMech Ageing Dev.pii:S0047-6374(15)00007-X. |
128 | Renault V, Thornell L-E, Eriksson P-O, Butler-Browne G, Mouly V, Thorne L-E(2002) Regenerative potential of human skeletal muscle duringagingAging Cell1: 2132139 |
129 | Malmgren LT, Fisher PJ, Jones CE, Bookman LM, Uno T(2000) Numericaldensities of myonuclei and satellite cells in muscle fiber typesin the aging human thyroarytenoid muscle: An immunohistochemicaland stereological study using confocal laser scanning microscopyOtolaryngol Head Neck Surg123: 4377384 |
130 | Cousin W, Ho ML, Desai R, Tham A, Chen RY, Kung S(2013) Regenerative capacity of old muscle stem cells declines withoutsignificant accumulation of DNA damagePLoS One8: 5e63528 |
131 | Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SDR(2013) Primary human muscle precursor cells obtained fromyoung and old donors produce similar proliferative,differentiation and senescent profiles in cultureAging Cell12: 3333344 |
132 | Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J(2005) Retrospective birth dating of cells in humansCell122: 1133143 |
133 | White RB, Biérinx A-S, Gnocchi VF, Zammit PS(2010) Dynamics ofmuscle fibre growth during postnatal mouse developmentBMC Dev Biol10: 21 |
134 | Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F(2015) Agedmuscle demonstrates fiber-type adaptations in response tomechanical overload, in the absence of myofiber hypertrophy,Independent of satellite cell abundanceJ Gerontol A BiolSci Med Sci.17 |
135 | Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F(2014) Inducible depletion of satellite cells in adult, sedentary miceimpairs muscle regenerative capacity without affecting sarcopeniaNat Med7680 |
136 | Takeuchi F, Yonemoto N, Nakamura H, Shimizu R, Komaki H, Mori-Yoshimura M(2013) Prednisolone improves walking in JapaneseDuchenne muscular dystrophy patientsJ Neurol260: 1230233029 |
137 | Hussein MRA, Abu-Dief EE, Kamel NF, Mostafa MG(2010) Steroid therapy isassociated with decreased numbers of dendritic cells andfibroblasts, and increased numbers of satellite cells, in thedystrophic skeletal muscleJ Clin Pathol63: 9805813 |
138 | Anderson DM, Anderson KM, Bassel-duby R, Olson EN, Mcanally JR, Kasaragod P(2015) Article a micropeptide encoded by a putativelong noncoding RNA regulates muscle performance article amicropeptide encoded by a putative long noncoding RNA regulatesmuscle performanceCell112 |
139 | Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M(2013) NCBI GEO: Archive for functional genomicsdata sets - UpdateNucleic Acids Res.41: D1 |
140 | Clark NR, Hu KS, Feldmann AS, Kou Y, Chen EY, Duan Q(2014) Thecharacteristic direction: A geometrical approach to identify differentially expressed genesBMC Bioinformatics15: 179 |
141 | Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV(2013) Enrichr: Interactive and collaborative HTML5 gene list enrichmentanalysis toolBMC Bioinformatics14: 1128 |
Figures and Tables
Fig.1
Age alters serum composition and thereby affects intercellular communication at distance. The endocrine hypothalamic-pituitary axis is altered with aging, affecting the composition of circulating hormones in the serum. For instance, the secretion of growth hormone is decreased, leading to loss of muscle mass. In addition, the lower level of growth hormone will also stimulate less the secretion of IGF-1 - IGF-1 being involved in muscle mass maintenance and in the satellite cell myogenic program. The endocrine hypothalamic-gonadotropic axis is also affected, leading to a decrease of sex steroids such as Testosterone, another hormone involved in muscle mass maintenance. Similarly, a decrease in oestrogen can act on the myogenic program through IGF-1 signaling. The decrease in circulating hormones affects the capacity of the satellite cells to respond to muscle damage. Aging is also associated with an increase in inflammation. The cytokines secretion by aged inflammatory cells as well as their ROS production is modified and can also affect the capacity of the satellite cells to respond to muscle damage. The modification of the entire serum composition with aging has negative effects on muscle mass and on muscle regeneration capacity.
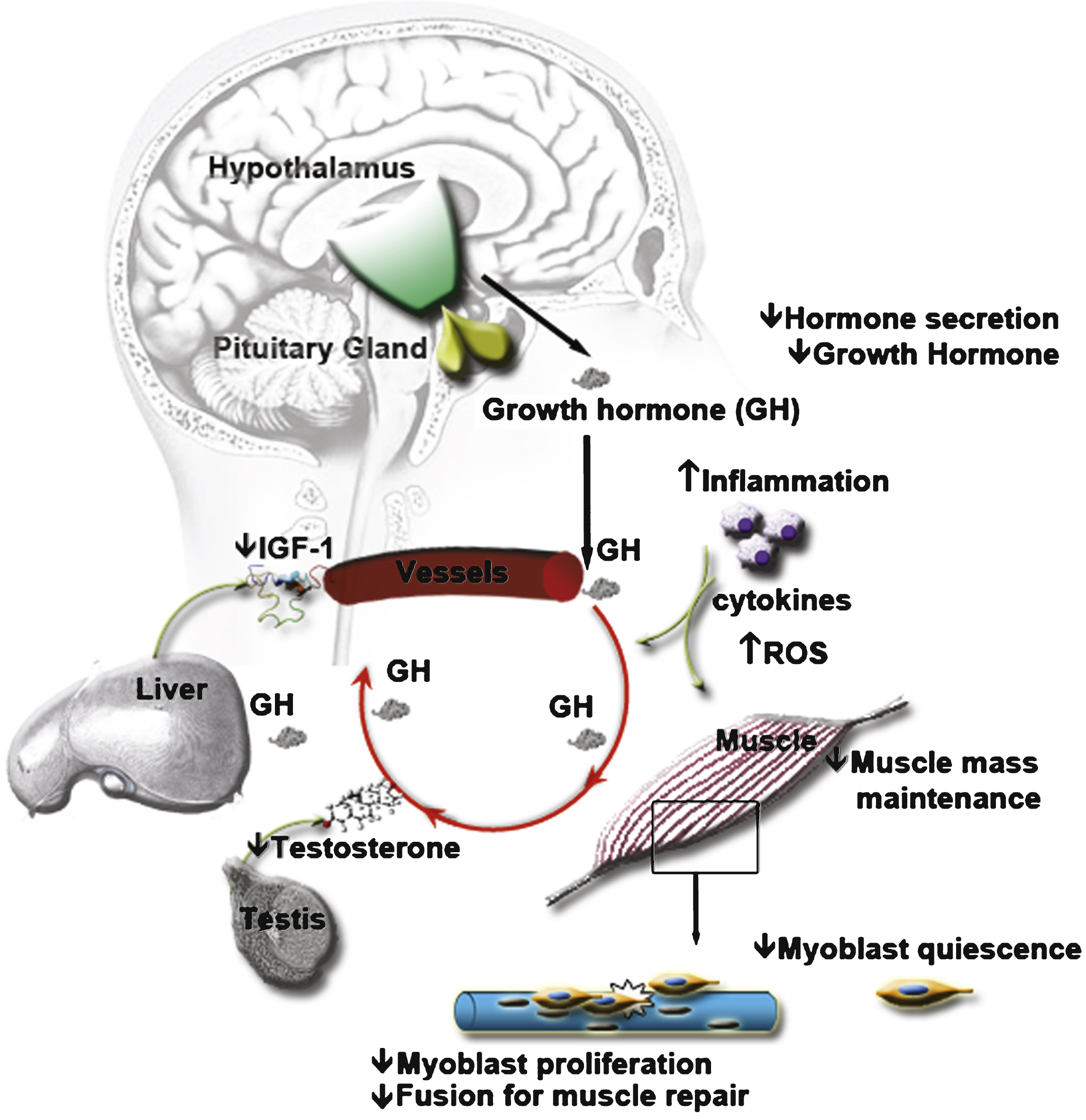
Fig.2
Aging changes the microenvironment of the satellite cell. Decreased muscle mass can be accompanied by a decrease in myokines and vesicles secreted into the microenvironment of the satellite cells. Aged myofibers produce more ROS and FGF-2, factors that can change epigenetic marking of the satellite cells and shut down their myogenic program and their capacity to re-quiesce. They also release less NO into their environment, stimulating vasoconstriction which may inhibit serum tissue perfusion. Aged fibroblasts present in the muscle can secrete more fibrous proteins, thickening the ECM. In turn, this decreases the diffusion of growth factors toward the satellite cells and thus their responsiveness to muscle repair cues. Increase in senescent cells with age can secrete factors that inhibit tissue regeneration. The microenvironment of the satellite cells is thus altered and affects their capacity to respond to any muscle damage.
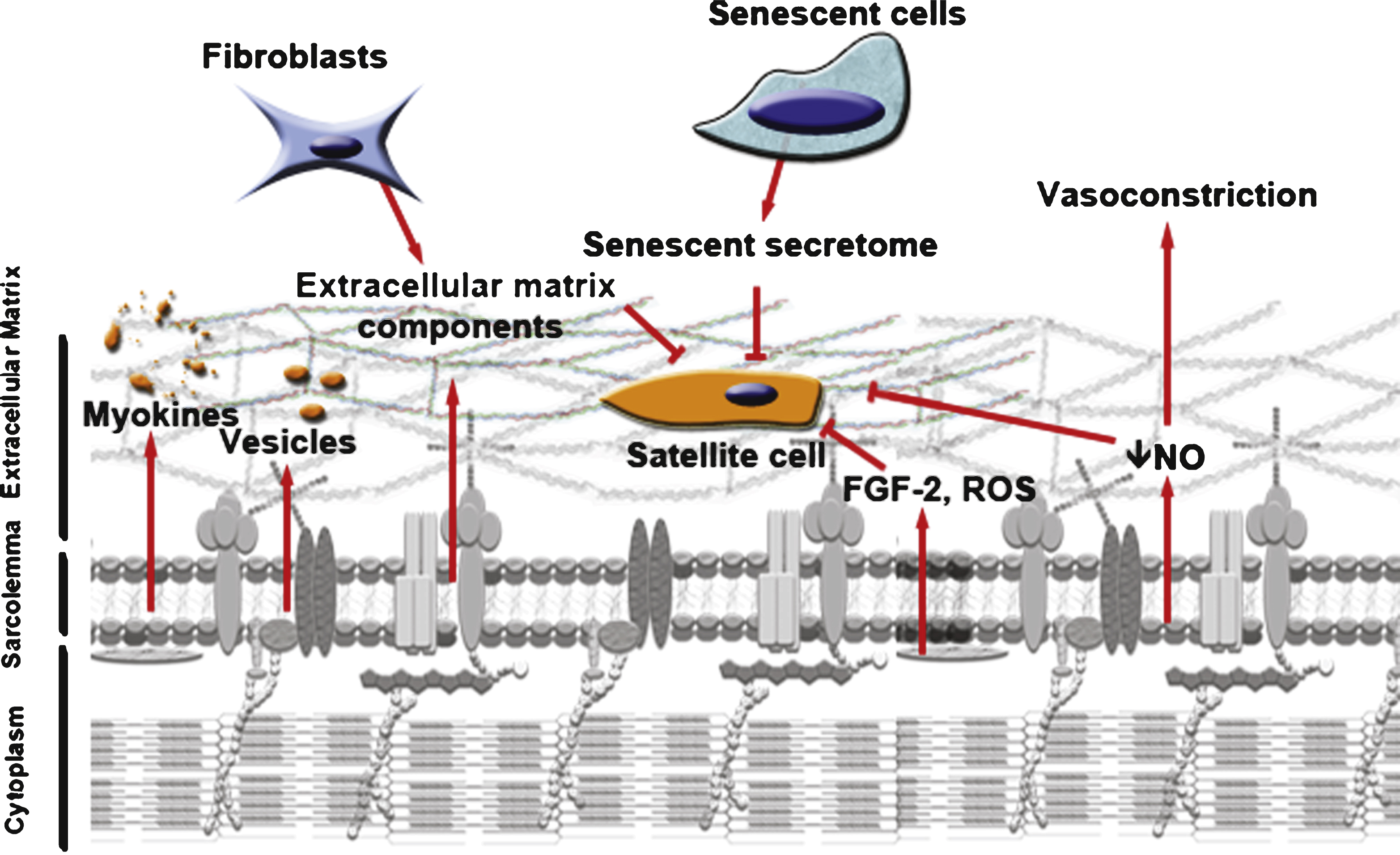
Fig.3
Increase of oxidative stress response in aged muscles. For this analysis we retrieved the gene expression matrix of old and young muscles of sedentary subjects (series GSE9103 from the Gene Expression Omnibus [139]). We discarded three samples as they did not pass the quality control threshold. To identify differentially expressed genes we used the characteristic direction method [140] followed by gene set enrichment with Enrichr [141]. The top figure depicts a bar graph from the top 10 up-regulated GO Biological Processes (combined score: p-value multiplied by z-score) and the bottom figure a network of the same Processes, where each node represents the enriched term and the edges represent the gene content similarity between the nodes.
![Increase of oxidative stress response in aged muscles. For this analysis we retrieved the gene expression matrix of old and young muscles of sedentary subjects (series GSE9103 from the Gene Expression Omnibus [139]). We discarded three samples as they did not pass the quality control threshold. To identify differentially expressed genes we used the characteristic direction method [140] followed by gene set enrichment with Enrichr [141]. The top figure depicts a bar graph from the top 10 up-regulated GO Biological Processes (combined score: p-value multiplied by z-score) and the bottom figure a network of the same Processes, where each node represents the enriched term and the edges represent the gene content similarity between the nodes.](https://content.iospress.com:443/media/jnd/2015/2-3/jnd-2-3-jnd150097/jnd-2-3-jnd150097-g003.jpg)
Fig.4
Links between whole-body composition changes, the decline of muscle size and function, and the loss of muscle stem cells and their functions with aging. Modifications with age of the endocrine systems (hypothalamus-pituitary system, gonad glands, liver, etc.) affect the quantity and the content of circulating serum hormones and impact muscle mass maintenance (atrophy of the muscle fibers and decrease in the regenerative capacity of the muscle). Increased inflammation with age - also called inflammaging - affects whole body stress level and is accompanied by modification in secreted cytokine content. Increased oxidative stress of the muscle leads to DNA damage and epigenetic changes, and consequently affects the regenerative capacity of the muscle. The composition of the microenvironment of the satellite cells is affected with age, through the presence of aged fibroblasts, of senescent cells, and aged myofibers. These local changes contribute to the fragility of the myofibers and to a decrease in the regenerative potency.