Orthopedic Management of Scoliosis by Garches Brace and Spinal Fusion in SMA Type 2 Children
Abstract
Background:
Scoliosis is the most debilitating issue in SMA type 2 patients. No evidence confirms the efficacy of Garches braces (GB) to delay definitive spinal fusion.
Objective:
Compare orthopedic and pulmonary outcomes in children with SMA type 2 function to management.
Method:
We carried out a monocentric retrospective study on 29 SMA type 2 children who had spinal fusion between 1999 and 2009. Patients were divided in 3 groups: group 1-French patients (12 children) with a preventive use of GB; group 2-French patients (10 children) with use of GB after the beginning of the scoliosis curve; and group 3-Italian patients (7 children) with use of GB after the beginning of the scoliosis curve referred to our centre to perform orthopedic preoperative management.
Results:
Mean preoperative and postoperative Cobb angle were significantly lower in the group 1 of proactively braced than in group 2 or 3 (Anova p = 0.03; Kruskal Wallis test p = 0.05). Better surgical results were observed in patients with a minor preoperative Cobb angle (r = 0.92 p < 0.0001). Fewer patients in the group 1 proactively braced required trunk casts and/or halo traction and an additional anterior fusion in comparison with patients in the group 2 and 3. Moreover, major complications tend to be less in the group 1 proactively braced. No significant differences were found between groups in pulmonary outcome measures.
Conclusions:
A proactive orthotic management may improve orthopedic outcome in SMA type 2. Further prospective studies comparing SMA management are needed to confirm these results.
Level of Evidence:
Therapeutic Level III. See Instructions to Authors on jbjs.org for a complete description of levels of evidence (Retrospective comparative study).
INTRODUCTION
Spinal Muscular Atrophy (SMA) is an autosomal recessive disease characterized by loss of lower motor neurons resulting in progressive muscle wasting and weakness. There is a consolidated agreement that SMA manifestations have three main clinical severity variants based on of age of onset and maximal motor function achieved: severe (type 1); intermediate(type 2) and mild (type 3). Adult-onset type 4 has been added to include very mild disease [1]. Children with the earliest and most severe form of SMA (type 1) have a very poor life expectancy due to the extensive motor involvement beginning in the first months of life and associated respiratory failure that rapidly leads to death in the absence of mechanical ventilation. Children that achieve sitting (type 2) or walking abilities (type 3) usually do not have severe pulmonary complications during the first years of life, however they develop a progressive kyphoscoliosis [2] that worsens respiratory function later in childhood and may cause problematic pelvic obliquity in non-walking patients [3]. Prevalence, age at onset and severity of the scoliosis curve are related to the severity of the disease; nearly all patients with SMA type 1 and 2, and approximately 50% of SMA type 3 patients develop scoliosis [4, 5]. Children with SMA type 2 usually develop scoliosis at an average of 3 age years [5, 6] with a progressive course even after spinal growth is completed [7].
Spinal fusion is the only definitive treatment, however outcomes of spinal fusion in the prepubescent period remain uncertain [8]. International recommendations regarding SMA standard of care did not suggest the use of trunk orthosis before surgery [9, 7] not only because respiratory impairment discourages the use of some types of braces that decrease chest wall movements that further impairs pulmonary function [10–12], but also because of differences in management of these patients between countries.
In France, for many years, a rigid plexidur brace called Garches type brace (GB) has been used for the management of SMA patients [13] or other early onset neuromuscular diseases characterized by scoliosis and respiratory failure [14]. This type of brace facilitates transfers and the sitting position in such hypotonic patients and the advantage of the GB in comparison with others braces is the absence of thoracic compression and the multi modular aspect that allows for setting adjustments [15].
Our aims were to describe orthopedic and pulmonary outcomes in children with SMA type 2 proactively treated by GB in comparison with SMA type 2 patients treated by GB after the onset of spinal deformity.
PATIENTS AND METHODS
Study design
The study is a retrospective, monocentric, observational study.
Settings
The study took place in the “Service de Pédiatrie et Réanimation Neurorespiratoire” at the Raymond-Poincaré Hospital (Garches, France).
Patients
All patients included in the study were SMA type 2 patients, with a confirmed genetic diagnosis of SMN1 homozygous deletion and with at least one year of post-surgical follow-up. These patients were:
(1) French children from the active list of our centre, who underwent spinal fusion occurred between 1999 and 2008 and with regular (at least once a year) follow-up in our centre in at least the last 5 years before surgery.
(2) Italian children referred to our team for preoperative orthopaedic management and scoliosis surgery between 2000 and 2009, with a preoperative follow-up in our centre ranging from 5 to 38 months.
Garches type brace (GB)
The GB is a very specific type of back brace. Developed at the Garches (France) hospital, it was originally designed to treat paralytic scoliosis mainly produced by the polyomyelitis epidemics. This brace is made of hard thermoplastic (methacrylate), it is moulded in the supine position with head and hip gentle traction (Cotrel derotation technique)(Fig. 1), and combines several pieces that move relative to each other with hinges. The weight of the body is supported by the hip and the trunk is maintained in the upright position by pre-humeral supports that are attached to the brace laterally so that thoracic expansion is free. It includes a neck piece which adds a cervical traction and allows head rest. Other than its superb hold, it is well-known for being relatively easy to put on.
The brace opens and closes like a book, and is not only used to fight against the progression of the spinal deformity but also to improve sitting position, head support and allows the child to maintain a standing position (by the attachment of leg orthosis from the pelvis to the feet), even if the person has no autonomy (Fig. 1) [15].
Care procedures
Children were advised to wear their GB for at least 8 hours daily when sitting but allowing periods of free movements (i.e. during physical therapy, playing, swimming). When the GB was not expected to be sufficiently corrective (i.e. for severe deformity above 50° in supine position), or if a rapid curve progression occurred in spite of using the GB (i.e. more than 10° per year), other conservative treatment such as trunk Abbott casts and cephalic halo-traction were performed to improve scoliotic curve and pelvic obliquity, in order to delay spinal fusion or to ameliorate surgical results [16]. Pulmonary care included the use of insufflation therapy by an intermittent positive-pressure breathing (IPPB) device, the Alpha 200 (Taema, Anthony, France) (15–30 min minutes per day in one or more sessions) [17]. The intent was to improve chest wall and lung development, to reduce ribs and sternal deformities [18] and to increase lung ventilation [19]. In case of bronchial obstruction or infections manual respiratory therapy and percussive ventilation were carried out. Respiratory care and pulmonary monitoring were intensified before spinal fusion to optimize the respiratory status at baseline and in the postoperative phase due to the high risk of postoperative complications and prolonged mechanical ventilation [20]. Finally, on weekdays physical therapy by stretching was regularly performed. All of these practices have been summarized in 2001 in a French SMA consensus Conference on Rehabilitation of Neuromuscular disease [21].
Surgical procedures
Surgical correction of scoliosis was considered on the base of the patient?s curve progression, pulmonary function and bone maturity.
All children had posterior fusion with Cotrel-Dubousset (CD) [22, 23] or Luque [24] technique. An additional anterior procedure was carefully performed in children with severe early onset scoliosis and vertebral rotation [25] taking into account the negative effect on respiratory function, the increased time of postoperative hospitalization and the higher risk of complications related to surgery [26]. All patients had pelvic fixation [27].
Data collection
The following data were collected from the patients’ medical records: age at diagnosis of disease, age at unaided sitting position, age at diagnosis of scoliosis, age at starting bracing and at spinal surgery and the pattern of scoliosis. A Cobb angle of 10° in supine position was considered as the minimum angulation to define scoliosis. The curve progression was evaluated by measuring the Cobb angle [28] in anterior-posterior X-ray in supine position and without the brace. Cobb angles of all available examinations were collected in each patient, with a minimum of 3 measurements (at diagnosis of scoliosis, before and after spine fusion) in the supine position. Supine was indeed considered the most adequate and reliable position to measure the Cobb angle because it was not dependent on daily dynamic factors and/or gravity, and it was the only position common to all the patients, those able to hold sitting and those that had lost this ability. Finally supine position allowed for an appreciation of the residual flexibility of the spine. Additional non-operative treatments before fusion, the surgical techniques used, the correction obtained (Cobb angle in supine position in the immediate postoperative period or in the 3 following months) and the complications related to surgery were also collected. Data regarding pulmonary function included measurements of forced vital capacity (FVC) performed in supine position without the brace, before and 6–12 months after surgery. Treatment of respiratory insufficiency by mechanical ventilation was also considered.
Statistical analysis
Quantitative variables are expressed as means ± standard deviation (SD), and qualitative variables as number and percentage. To analyse progression of the scoliosis curve we used a mixed model to study the variation of Cobb angle with age. For the comparison of quantitative variables, Wilcoxon rank sum test and Student’s test were used for two groups, and analysis of variance (Anova) and Wallis test were performed for more than two groups. For qualitative variables chi-square analysis, or Fisher’s test when necessary, were performed. All tests were two sided. A p value of 0.05 was considered statistically significant. The various analyses were performed using R (http://www.R-project.org) software.
RESULTS
All patients were clinically classified as type 2 SMA, achieving the seated position at the mean age of 8 months (SD 2.5). Their mean age at diagnosis of the disease was 15 months (SD 4). The mean age of last visit was 13.6 years (SD 1.4).
Patients were divided in 3 groups to compare 3 different standards of care. The Table 1 summarizes main orthopedic characteristics and managements of the 3 groups of children:
– Group 1: composed of 12 French patients (5 girls and 7 boys) who were treated with GB proactively, before the onset of scoliosis (as early as possible in infancy even in the absence of scoliosis, when trunk support was poor showing collapse (kyphosis) or had signs of cervical weakness) at a mean age of 2 years (range 1–2.9). Patients of this group had worn the GB at least for the last four years before spinal fusion. Diagnosis of scoliosis averaged 5.3 years (SD 2.3) with a mean Cobb angle of 10° (SD 3.1). Regarding the pattern of scoliosis, a great majority of patients in this group presented a double curve pattern (10/12).
– Group 2: composed of 10 French patients (6 girls and 4 boys) who were treated with GB after the onset of scoliosis at the mean age of 4.4 years (range 1.9–7.4). Patients of this group had worn the GB at least for the last four years before spinal fusion. Diagnosis of scoliosis averaged 4.4 years (SD 1.9) with a mean Cobb angle of 19° (SD 9.1). Most of these patients (8/10) showed a single long C shaped thoraco-lumbar curve.
– Group 3: composed of 7 Italian patients (6 girls and 1 boy) who were referred to our centre because of evolving scoliosis and to perform orthopedic preoperative management and spine fusion. They were all treated with polypropylene underarm brace when scoliosis was 20° in sitting position and for a mean age of 5 years (range 4–7.5 y). The mean age at the first visit to our centre was 10.5 years (SD 0.9), scoliosis during this first visit ranged from 30° to 105°, with a mean Cobb angle of 66°. All these patients started to wear GB after their first visit to our center and for a mean duration of 1.9 year (SD 1.7).
Additional preoperative treatments
Among group 1 and 2, thirteen patients required trunk Abbott casts and/or halo traction, six (50%) of the group 1 and seven (70%) of the group 2 (Table 1). Most of the cases required the casts between 9 and 12 years of age, at the time of the growth spurt. One or more serial trunk casts were performed until no further correction was obtained. Then a GB was moulded in the new corrected posture and used during the day, cast could be also worn at night for a period of 1 to 3 months to enhance the corrective effect. Only one child of group 2 reported atelectasis during casting that recovered after standard treatments. Cephalic halo-traction was performed during weeks or last months (range 1 week to 3 months) preceding surgery or in between anterior and posterior procedures. In the group 3, 71% of the patients needed several treatments by trunk Abbott casts and/or cephalic halo traction before surgery (Table 1).
Bracing and scoliosis progression
The trend of the progression of the spinal deformity is represented for each patient of group 1 and 2 in Fig. 2 The analysis of the progression of scoliotic curve with age showed a mean increase of Cobb angle of 4.2° per year in group 1 and 8.4° per year in group 2 with a significant different (p = 0.02) between groups as shown in Fig. 3. Regarding Italian children data about age and Cobb angle at onset of scoliosis were not available in the medical charts, thus it was not possible to estimate the progression rate of the curve in this group.
Spinal surgery
Twenty-eight patients had CD fusion, and one patient underwent Luque technique. Twelve children required an additional anterior procedure, four children (30%) of the group 1, five (50%) of the group 2 and 3 (43%) in the group 3 (Table 1). All patients had pelvic screw fixation.
Mean age at fusion was 13.2, 11.7 and 12.4 years for group 1, group 2 and group 3 respectively and this difference resulted statistically significant (Anova p = 0,03). Moreover, mean age at fusion was statistically different between group 1 and group 2 (t-test p = 0,007), whereas there was not a statistically significant difference between group 2 and group 3 (t-test p = 0,3).
Mean preoperative and postoperative Cobb angles for each group are shown in the Table 1. Mean preoperative Cobb angle were significantly lower in the group 1 of proactively braced than in group 2 and 3:54° in group 1 versus 79° in group 2 and 74 in group 3 (Anova p = 0.03; Kruskal Wallis test p = 0.05, Fig. 4). Mean postoperative Cobb angle was 28° (SD 10) in group 1, 41 °(SD 15) in group 2 and 39° (SD 11) in group 3. The percentage of correction was similar for the three groups (respectively 48% , for group 1, 48% for group 2 and 47% for group 3). Better surgical results in terms of postoperative Cobb angle were observed in patients with a minor preoperative angle value (r=0.92 p <0.0001), so as expected in the group 1 (Anova p = 0.03; Kruskal Wallis test p = 0.06, Fig. 4).
Regarding functional outcomes,15 patients were able to sit independently before and after surgery, 2 patients were not able to sit unaided before surgery but they were able after fusion (before surgery the deformity was very severe and they could not be seated without support because of a marked heap imbalance); 8 patients could not be seated independently before and after surgery; 3 patients were able to sit independently before surgery but they were not able after spinal fusion.
Pulmonary issues
Whereas all patients in group 1 and 2 started IPPB treatment early, in the group 3, only two children started regular IPPB treatment 2 years before spine fusion. Endotracheal intubation was maintained until the patient recovered at least 80% of preoperative FVC values (mean 7 days, range 2–21 days). Following extubation, all the patients underwent non invasive positive pressure ventilation (NIPPV) and intensive respiratory rehabilitation therapy by IPPB with the Alpha 200 (R) device (at least 30 minutes three times a day). The loss of FVC observed immediately after surgery was progressively regained in most cases: the values returned to at least 90% of baseline in 82% of patients and 46% had a FVC higher than the preoperative level by the 6–12 months follow-up visit. Preoperative and postoperative FVC were respectively 45% and 40% in group 1, 47% and 46% in group 2 and 51% and 47% in group 3 (Table 1). At time of last visit, 6 patients (23%) were on nocturnal non invasive ventilation (NNIV). One patient refused the indication to start NNIV. A boy with severe respiratory compromise underwent a tracheostomy at 10.5 years of age, 3 years before fusion. We compared pulmonary results, for the three groups (Table 1) and we did not find any significant difference in preoperative (Anova p = 0.9; Kruskal Wallis test p = 0.5) and postoperative FVC (Anova p = 0.8; Kruskal Wallis test p = 0.6).
Complications related to surgery
No deaths were reported. Major complications were respiratory, neurological and infective problems, feeding difficulties and reduced functional abilities of the upper limbs. Minor complications were transient sensory disturbances at lower limbs, superficial wound infection, sacral/hip pain, mild lumbar torsion and sacral screw displacement without lost of correction. Eleven major complications were observed, three (25% of patients) occurring in group 1 (1 atelectasis, 1 progressive feeding difficulties with severe weight loss and 1 reduction of functional abilities on upper limbs), four (40% of patients) in group 2 (2 atelectasis, 1 septicemia and 1 complete intraoperative spinal cord lesion with paraplegia) and four (57% of patients) in group 3 (1 pleural effusion after the anterior procedure, 1 progressive neck muscles weakness associated with reduced coughing capability, 1 deep wound infection and 1 sacral screw displacement with loss of correction in pelvic obliquity and need of a revision surgery). Respiratory complications and septicemia reported in group 2 developed after the anterior surgical procedure. Minor complications were observed in 50% of patients of both the group 1 and 2 and 57% of patients in group 3.
DISCUSSION
Scoliosis is one of the most important and unresolved problem in SMA type 2 children. Spinal fusion is the definitive treatment to correct the deformity and to stabilize the spine but there is no international consensus on the best orthopedic care, particularly in the preoperative phase. Our study suggested a proactive orthotic management may improve orthopedic outcomes in SMA type 2 in terms of rate of scoliosis progression and success of the surgical procedure with a reduced number of severe complications.
Few studies reported in the literature describe scoliosis progression and treatment in SMA children [7, 29]. Merlini et al. reported the course of scoliosis in a series of type II and type 3 SMA patients [7]. Those children started wearing an underarm plastic orthosis when kyphosis was over 50° or when Cobb angle was over 20° in sitting position. An annual increment of the deformity of 8°, 3° and 0.6° was respectively described in 24 SMA type 2, in 13 SMA type 3 who had lost the ability to walk and in 10 type 3 SMA still ambulant patients [7]. In our proactively braced children we observed that scoliosis progressed at rate of 4.2° per year, suggesting that the brace did slow the progression of scoliosis in this population and supports its preventive use. However, in contrast to previous studies, the Cobb angles measured in our study were done in supine position to allow comparison in all the patients, independently of their ability to maintain unsupported seated position. Also, we found supine examinations of higher value to assess the progression of the fixed deformity because in supine position there is not contribution of other confounding factors such as gravity and axial muscles strength. The measurement of the fixed deformity is the most meaningful for the surgical outcome because the surgeon stabilize the spine in a degree close to that of the fixed deformity. Finally supine examination may give indirect information about the residual spinal flexibility in patients able to sit independently, indeed in these patients, the difference between the angles in seated and in supine position allows appreciating indirectly the degree of flexibility.
Regarding pulmonary outcomes, a literature review showed no agreement on the effects of scoliosis surgery on pulmonary function in SMA children. Some studies report on a continuous decline in pulmonary function in children with type II and type 3 SMA after spinal fusion [30, 31] though the rate of decline is less marked than observed in the preoperative phase [32, 33]. Other authors describe no change in pulmonary function [34, 7] or an increased predicted FVC at last visit [35]. Our results suggest that the severity of the curve does not significantly correlate with pulmonary function. It is difficult to compare precisely pre and postoperative CV measurements due to the changes in height. Our results regarding the postoperative decline of pulmonary function may be limited because of the short duration of follow up (only 12 months). A longer observational period is required to assess the long-term effects of the surgical scoliosis correction on pulmonary function.
One limitation of this study is its retrospective collection of data. However, the results are interesting particularly about the beneficial effects of proactive administration of Garches Brace besides the required surgical procedure for all SMA type 2 patients. Further possible prospective studies comparing different management worldwide would be necessary to confirm these findings.
Over the past few years newer surgical treatments have been developed for the management of severe scoliosis in skeletally immature patients prior to definitive spinal fusion. Growing-rods [36] or Vertical Expandable Prosthetic Titanium Rib (VEPTR) [37, 38] may be utilized to prevent the progression of the curve in young children when bracing isn’t successful. These techniques have shown to be effective in controlling progressive early onset scoliosis prior to definitive spine fusion [39–41] but the GB in our experience remain an interesting complementary approach in this population. This is especially in the youngest or weakest children in whom the growing-rod technique may be not give satisfactory results (limits of rod distraction; head instability). The Garches Brace could also be useful after the definitive spinal fusion even if the scoliotic curve is under control, either to reinforce the stability of the material or in case of marked neck weakness. Thanks to chin and head support, the GB allows for adequate and secure maintenance of the head in seated position, as some children will need these supports especially during transfers and transports. It allows stable sitting and standing positions for long periods of time, what has multiple functional benefits for the everyday living of children. In contrast, growing-rods may be a more acceptable approach at later ages due to the lower efficacy of the bracing during the rapid growth period and poorer compliance of prepubertal children to wear the brace, particularly with regard to the use of the head and chin support, which reduces the efficacy of thedevice.
CONCLUSIONS
Our experience suggests that an early proactive orthotic treatment may be useful to improve orthopaedic outcome in SMA type 2 patients and improve outcomes after spinal without harming pulmonary function.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
We are grateful for a grant from the European project TREAT-NMD number ISTRI6PQa1. We thank CORNEMUS Federation and FILNEMUS French Network, Françoise Hatton and Charlotte Monsaingeon for her previous work on the use of brace in SMA patients. To Pr Duval Beaupère and Pr Annie Barois for her clinical advise. Jean-Louis Neut for his technical expertise and work. The heads of the orthopaedic surgery teams, Pr Reinhard Zeller and Pr Christophe Glorion.
REFERENCES
1 | Lunn MR, Wang CH (2008) Spinal Muscular Atrophy Lancet 371: 9630 2120 2133 |
2 | Schwentker EP, Gibson DA (1976) The orthopaedic aspects of spinal muscular atrophy J Bone Joint Surg Am 58: 1 32 38 |
3 | Duval-Baupère G, Barois A, Quinet I, Estournet B (1985) Respiratory, spinal and thoracic problems in children with prolonged spinal muscular atrophy Arch Fr Pediatr 42: 625 623 |
4 | Mullender M, Blom N, De Kleuver M, Fock J, Hitters W, Horemans A, Kalkman C, Pruijs J, Timmer R, Titarsolej P, Van Haasteren N, Jager MV, Van Vught A, Van Royen B (2008) A Dutch guideline for the treatment of scoliosis in neuromuscular disorders Scoliosis 3: 14 |
5 | Evans GA, Drennan JC, Russman BS (1981) Functional classification and orthopaedic management of spinal muscular atrophy J Bone Joint Surg Br 63B 4 516 522 |
6 | Bono R, Inverno M, Botteon G, Iotti E, Estienne M, Berardinelli A, Lanzi G, Fedrizzi E (1995) Prospective study of gross motor development in children with SMA type II Ital J Neurol Sci 16: 4 223 230 |
7 | Merlini L, Granata C, Bonfiglioli S, Marini ML, Cervellati S, Savini R (1989) Scoliosis in Spinal Muscular Atrophy: Natural history and management Dev Med and Child Neurol 31: 4 501 508 |
8 | Fujak A, Ingenhorst A, Heuser K, Forst R, Forst J (2005) Treatment of scoliosis in intermediate spinal muscular atrophy (SMA type II) in childhood Ortop Traumatol Rehabil Apr 30 7: 2 175 179 |
9 | Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, Aloysius A, Morrison L, Main M, Crawford TO (2007) Trela A; Participants of the International Conference on SMA Standard of Care. Consensus Statement for Standard of Care in Spinal Muscular Atrophy J of Ch Neurol 22: 8 1027 1049 |
10 | Tangsrud SE, Carlsen KC, Lund-Petersen I, Carlsen KH (2001) Lung function measurements in young children with spinal muscle atrophy; a cross sectional survey on the effect of position and bracing Arch Dis Child 84: 6 521 524 |
11 | Morillon S, Thumerelle C, Cuisset JM, Santos C, Matran R, Deschildre A (2007) Effect of thoracic bracing on lung function in children with neuromuscular disease Ann Readapt Med Phys 50: 8 645 650 |
12 | Noble-Jamieson C M, Heckmatt JZ, Dubowitz V, Silverman M (1986) Effects of posture and spinal bracing on respiratory function in neuromuscular disease Arch Dis Child 61: 2 178 181 |
13 | Cuisset JM, Estournet B (2012) Recommendations for the diagnosis and management of typical childhood spinal muscular atrophy; French Ministry of Health Rev Neurol (Paris) 168: 12 902 909 |
14 | Wang CH, Bonnemann CG, Rutkowski A, Sejersen T, Bellini J, Battista V, Florence JM, Schara U, Schuler PM, Wahbi K, Aloysius A, Bash RO, Béroud C, Bertini E, Bushby K, Cohn RD, Connolly AM, Deconinck N, Desguerre I, Eagle M, Estournet-Mathiaud B, Ferreiro A, Fujak A, Goemans N, Iannaccone ST, Jouinot P, Main M, Melacini P, Mueller-Felber W, Muntoni F, Nelson LL, Rahbek J, Quijano-Roy S, Sewry C, Storhaug K, Simonds A, Tseng B, Vajsar J, Vianello A, Zeller RInternational Standard of Care Committee for Congenital Muscular Dystrophy (2010) Consensus statement on standard of care for congenital muscular dystrophies J Child Neurol 25: 12 1559 1581 |
15 | Touzeau Breniére C (1995) Use of the Garches brace in very young children. [Intêret du corset en plexidur type garchois chez le tout-petit enfant (ou corset de la 2eme génération)] Rachis 7: 1 23 30 |
16 | Cotrel Y, Morel G (1964) The elongation-derotation-flexion technique in the correction of scoliosis Rev Chir Orthop Reparatrice Appar Mot 50: 59 75 |
17 | Ioos C, Leclair-Richard D, Mrad S, Barois A, Estournet-Mathiaud B (2004) Respiratory capacity course in patients with infantile spinal muscular atrophy Chest 126: 3 831 837 |
18 | Bach JR, Bianchi C (2003) Prevention of pectus excavatum for children with spinal muscular atrophy type 1 Am J Phys Med Rehabil 82: 10 815 819 |
19 | Guérin C, Vincent B, Petitjean T, Lecam P, Luizet C, Rabilloud M, Richard JC (2010) The short-term effects of intermittent positive pressure breathing treatments on ventilation in patients with neuromuscular disease Respir Care 55: 7 866 872 |
20 | Udink ten Cate FE, van Royen BJ, van Herde M, Roerdink D, Plötz FB (2008) Incidence and risk factors of prolonged mechanical ventilation in neuromuscular scoliosis surgery J pediatr orthop B 17: 4 203 206 |
21 | Consensus conference, Neuromuscular diseases Rehabilitations. Recommendations, modalités, indications, limites de la rééducation dans les pathologies neuromusculaires non acquises Haute Autorité de Santé Sept 2001 |
22 | Cotrel Y, Dubousset J, Guillaumat M (1988) New universal instrumentation in spinal surgery Clin Orthop Relat Res 227: 10 23 |
23 | Dubousset J, Cotrel Y (1991) Application technique of Cotrel-Dubousset Instrumentation for scoliosis deformities Clin Orthop Relat Res 264: 103 111 |
24 | Luque ER (1982) Segmental spinal instrumentation for correction of scoliosis Clin Orthop Relat Res 163 192 198 |
25 | Dohin B, Dubousset JF (1994) Prevention of the crankshaft phenomenon with anterior spinal epiphysiodesis in surgical treatment of severe scoliosis of the younger patient Eur Spine J 3: 3 165 168 |
26 | Jules-Elysee K, Urban MK, Urquhart BL, Susman MH, Brown AC, Kelsey WT (2004) Pulmonary complications in anterior-posterior thoracic lumbar fusions Spine J 4: 3 312 316 |
27 | Miladi LT, Ghanem IB, Draoui MM, Zeller RD, Dubousset JF (1997) Iliosacral screw fixation for pelvic obliquity in neuromuscular scoliosis. A long term follow-up study Spine 22: 15 1722 1729 |
28 | Cobb JR (1948) Outline for the study of scoliosis. Instructional Course lectures Am Acad Orthop Surg 5: 261 275 |
29 | Sucato DJ (2007) Spine Deformity in Spinal Muscular Atrophy J Bone Joint Surg Am 89: 1 148 154 |
30 | Brown JC, Zeller JL, Swank SM, Furumasu J, Warath SL (1989) Surgical and functional results of spine fusion in spinal muscular atrophy Spine 14: 7 763 770 |
31 | Piasecki JO, Mahinpour S, Levine DB (1986) Long-term follow-up of spinal fusion in spinal muscular atrophy Clin Orthop Relat Res 207: 44 54 |
32 | Chng SY, Wong YQ, Hui JH, Wong HK, Ong HT, Goh DY (2003) Pulmonary function and scoliosis in children with spinal muscular atrophy types II and III J Paediatr Child Health 39: 9 673 676 |
33 | Aprin H, Bowen R, MacEwen GD, Hall JE (1982) Spine fusion in patients with Spinal Muscular Atrophy J Bone and Joint Surg Am 64: 8 1179 1187 |
34 | Granata C, Cervellati S, Ballestrazzi A, Corbascio M, Merlini L (1993) Spinal surgery in spinal muscular atrophy: Long term results Neuromus Disord 3: 3 207 215 |
35 | Robinson D, Galasko CSB, Delaney C, Williamson JB, Barrie JL (1995) Scoliosis and lung function in spinal muscular atrophy Eur Spine J 4: 5 268 273 |
36 | Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA (2005) Dual growing rod technique for the treatment of progressive early-onset scoliosis: A multicenter study Spine (Phila Pa 1976) 30: 17 Suppl S46 S57 |
37 | Emans JB, Caubet JF, Ordonez CL, Lee EY, Ciarlo M (2005) The treatment of spine and chest wall deformities with fused ribs by expansion thoracostomy and insertion of vertical expandable prosthetic titanium rib: Growth of thoracic spine and improvement of lung volumes Spine (Phila Pa 1976) 30: 17 Suppl S58 S68 |
38 | Hell AK, Campbell RM, Hefti F (2005) The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children J Pediatr Orthop B 14: 4 287 293 |
39 | Thompson GH, Akbarnia BA, Campbell RMJr (2007) Growing rod techniques in early-onset scoliosis J Pediatr Orthop 27: 3 354 361 |
40 | Chandran S1, McCarthy J, Noonan K, Mann D, Nemeth B, Guiliani T (2011) Early treatment of scoliosis with growing rods in children with severe spinal muscular atrophy: A preliminary report J Pediatr Ortho 31: 4 450 454 |
41 | McElroy MJ, Shaner AC, Crawford TO, Thompson GH, Kadakia RV, Akbarnia BA, Skaggs DL, Emans JB, Sponseller PD (2011) Growing rods for scoliosis in spinal muscular atrophy: Structural effects, complications, and hospital stays Spine (Phila Pa 1976) 36: 16 1305 1311 |
Figures and Tables
Fig.1
The Garches Brace
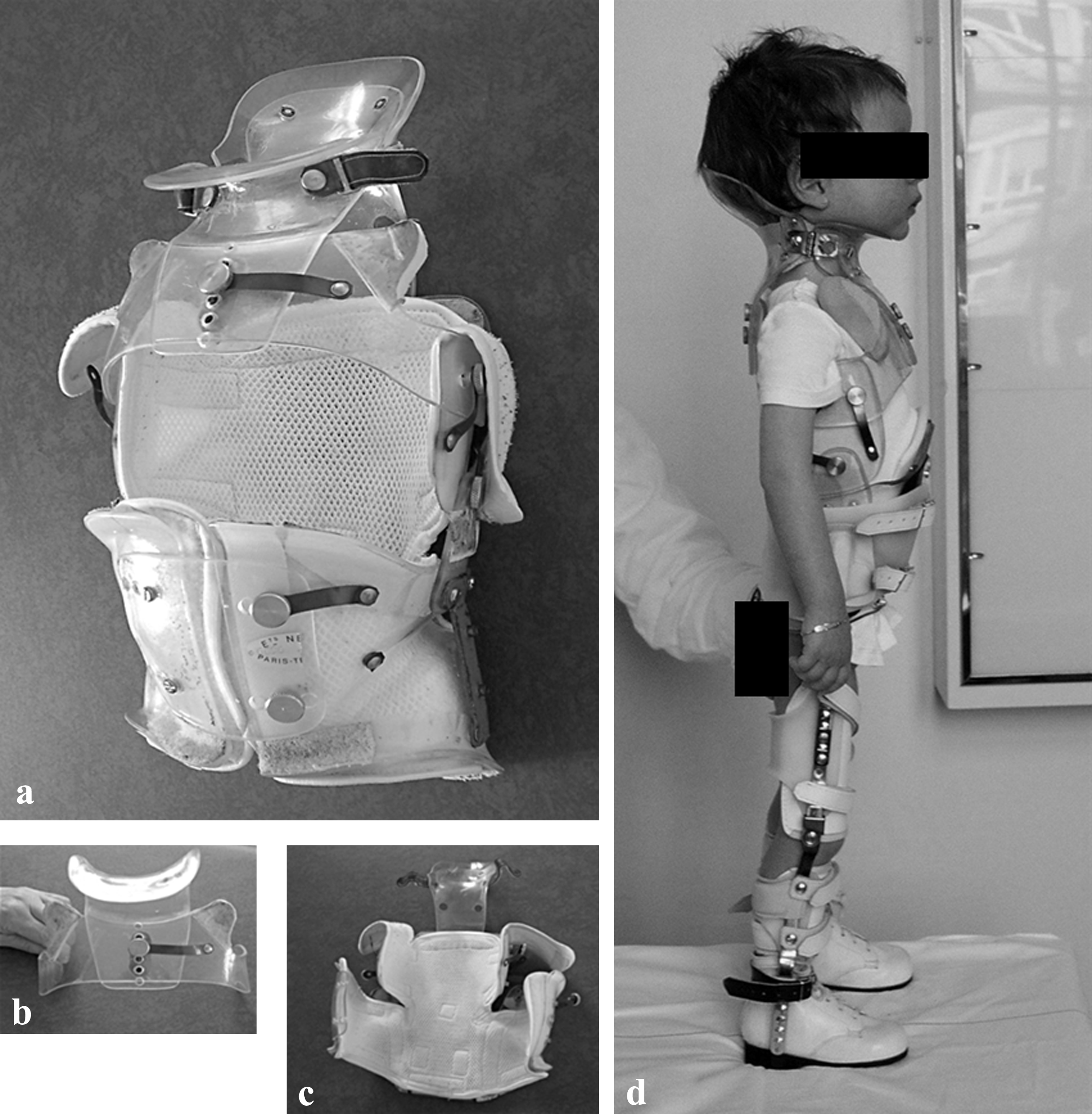
Fig2
Spaghetti plots representing the trend of progression of the scoliotic curve for each patient of group 1 and group 2. Preoperative values are represented by empty circles, postoperative values are represented by black triangles.
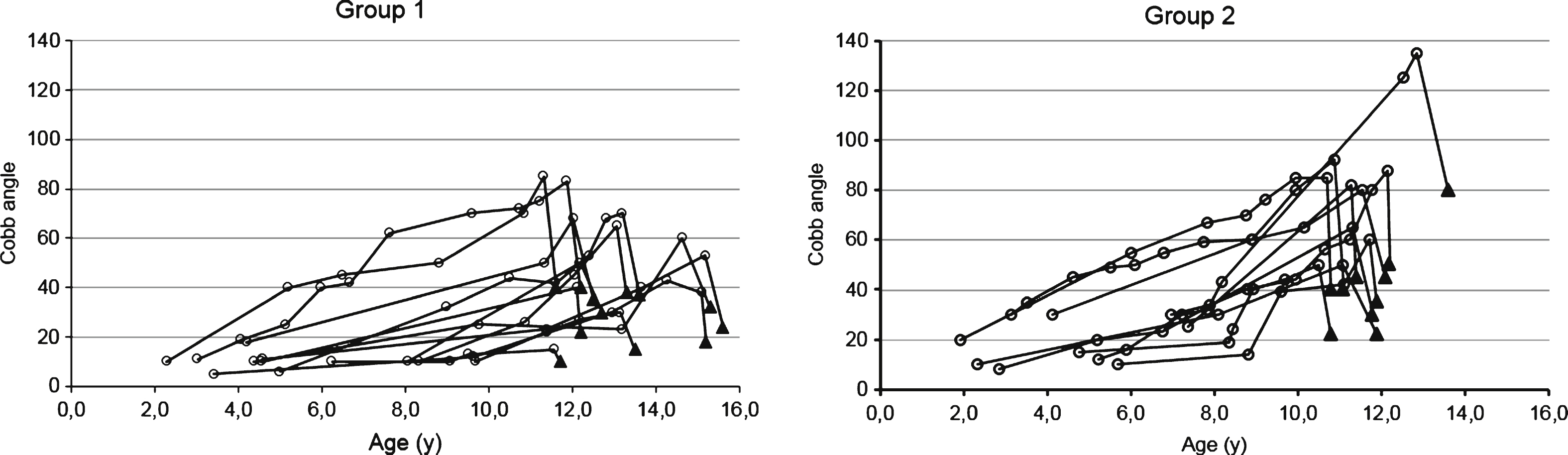
Fig.3
Evolution of Cobb angle function to age for each patient from group 1 (black circle) and from group 2 (empty circle) patients.
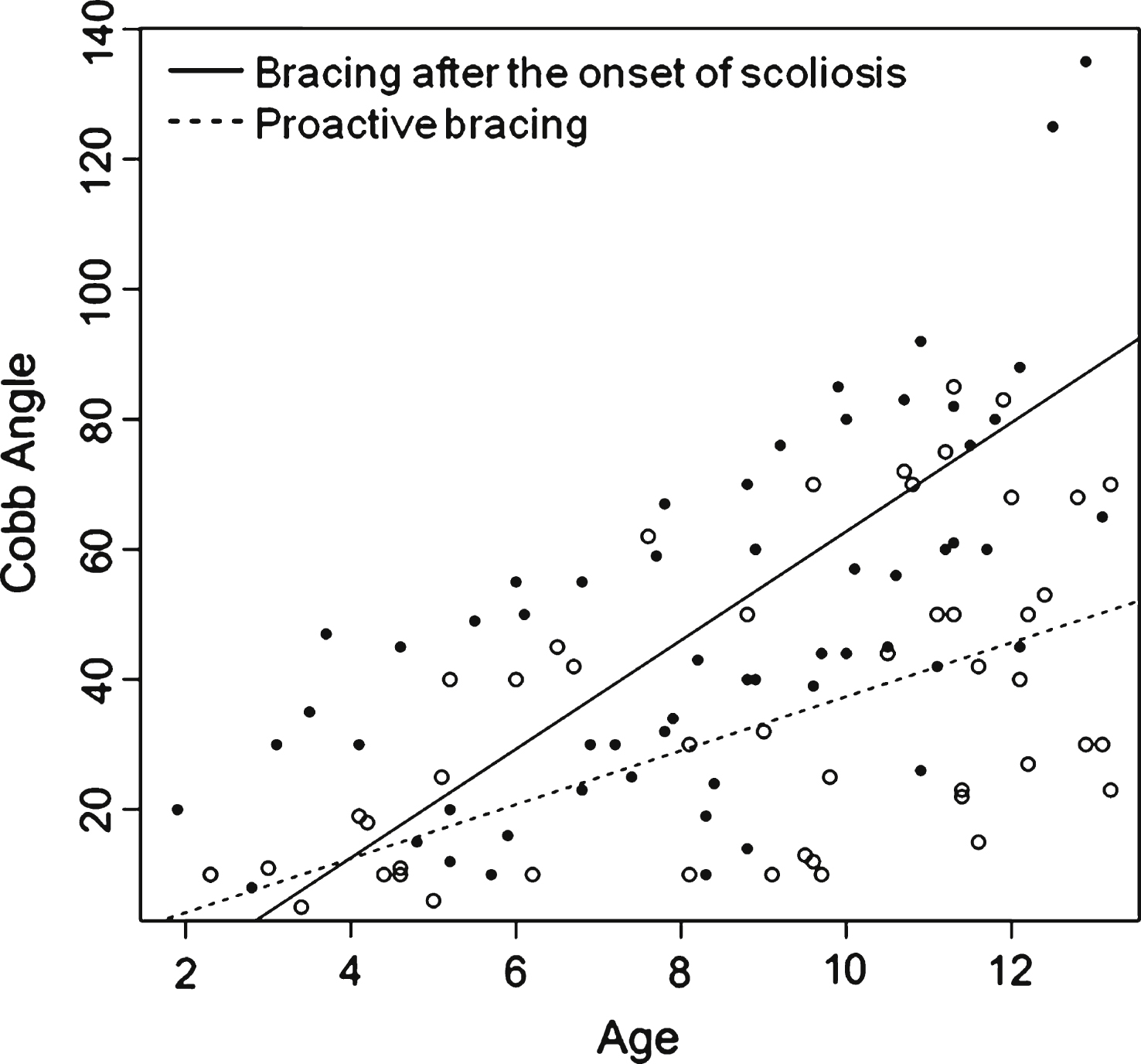
Fig4
Boxplots representing comparison between preoperative and post-operative Cobb angle in the three groups. T0 preoperative Cobb angle value; T1 postoperative Cobb angle value.
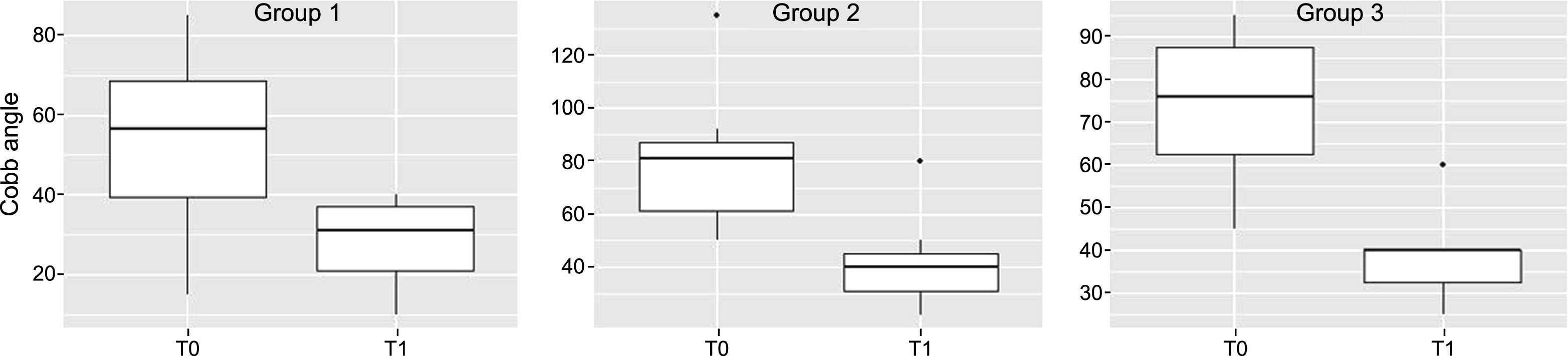
Table 1
Descriptive characteristics, orthopaedic management, preoperative and postoperative outcomes in each group
| Group 1 (n=12) | Group 2 (n=10) | Group 3 (n=7) | p Anova | p Kruskal Wallis | |
| Age at diagnosis of scoliosis | |||||
| mean | 5.3 | 4.4 | m.d. | ||
| SD | 2.3 | 1.9 | m.d. | ||
| range | 2.3–9.7 | 1.9–7.4 | m.d. | ||
| Age at starting brace (y) | |||||
| mean | 2.0 | 5.6 | 5.0* | ||
| SD | 0.6 | 1.4 | 1.2 | ||
| range | 1–2.9 | 4–7.8 | 3.5–7.5 | ||
| Trunk casts/cephalic halo traction | |||||
| number of patients (%) | 6/12 (50%) | 7/10 (70%) | 5/7 (71%) | ||
| Age at spinal fusion (y) | |||||
| mean | 13.3 | 11.7 | 12.4 | ||
| SD | 1.4 | 0.8 | 1.4 | ||
| range | 11.6–15.6 | 10.8–13.6 | 11.1–14.8 | ||
| Additional anterior fusion | |||||
| number of patients (%) | 4/12 (30%) | 5/10 (50%) | 3/7 (43%) | ||
| Preoperative Cobb angle | |||||
| mean | 54° | 79° | 74° | ||
| SD | 21 | 26 | 19 | 0,03 | 0,05 |
| range | 15–85° | 45–135° | 55–95° | ||
| Postoperative Cobb angle | |||||
| mean | 28° | 41° | 39° | ||
| SD | 10 | 16 | 11 | 0,05 | 0,06 |
| range | 10–40° | 30–75° | 25–60° | ||
| Change in pre- versus postoperative Cobb angle | |||||
| absolute value | 26 | 38 | 35 | ||
| percentage (%) | 48% | 48% | 47% | ||
| Preoperative predicted FVC | |||||
| mean | 45% | 47% | 51% | ||
| SD | 26 | 27 | 18 | 0.9 | 0.5 |
| range | 17–114% | 22–109% | 22–75% | ||
| Postoperative predicted FVC | |||||
| mean | 40% | 46% | 47% | ||
| SD | 24 | 25 | 20 | 0.8 | 0.6 |
| range | 11–99% | 29–105% | 17–81% |
n, number of subjects; SD, standard deviation; m.d. missing data; *age at starting polypropylene underarm brace; p value of 0.05 was considered statistically significant.



