Psychological Interventions for People with Huntington’s Disease: A Call to Arms
Abstract
Background:
Although Huntington’s disease (HD) can cause a wide range of psychological difficulties, no review has ever been carried out on the range of psychological interventions adopted with this population.
Objective:
To scope the literature on psychological interventions for psychological difficulties in people affected by HD.
Methods:
A systematic scoping review was performed across MEDLINE, PsycINFO, CINAHL, Academic Search Ultimate, and Cochrane Library up to 1 March 2020.
Results:
From an initial return of 1579 citations, a total of nine papers were considered eligible for review. These included a qualitative investigation, three case studies, two case series, two uncontrolled pretest-posttest designs, and only one randomised control trial (RCT). Despite the wide range of psychological difficulties which can be experienced by people affected by the HD gene expansion, the adopted interventions only accounted for five main psychological outcomes (anxiety, apathy, depression, irritability, and coping). Further discussion and suggestions for future research are provided for each outcome.
Conclusion:
The current literature on psychological interventions in people affected by HD is extremely limited both in terms of methods and addressed clinical outcomes. Consequently, no conclusions can be offered yet as to which psychological therapy may help this population. As further more comprehensive research is urgently needed for this group, the ultimate aim of the present review is to act as a call to arms for HD researchers worldwide to help shed light on the most effective way to translate psychological theory into practice for the benefit of people affected by HD.
INTRODUCTION
Huntington’s disease (HD) is a hereditary autosomal-dominant neurodegenerative disease characterised by variable prevalence worldwide, with estimates ranging from 5.96 to 13.7 per 100,000 in North America, Europe, and Australia, and lower figures among Asian countries [1]. It is caused by a CAG expansion on the huntingtin gene (HTT) on chromosome 4, resulting in substantial damage to the basal ganglia and their pathways (especially the corpus striatum, but also the globus pallidus and substantia nigra), as well as generalised atrophy of the cerebral cortex, with a particular involvement of frontal and prefrontal areas [2].
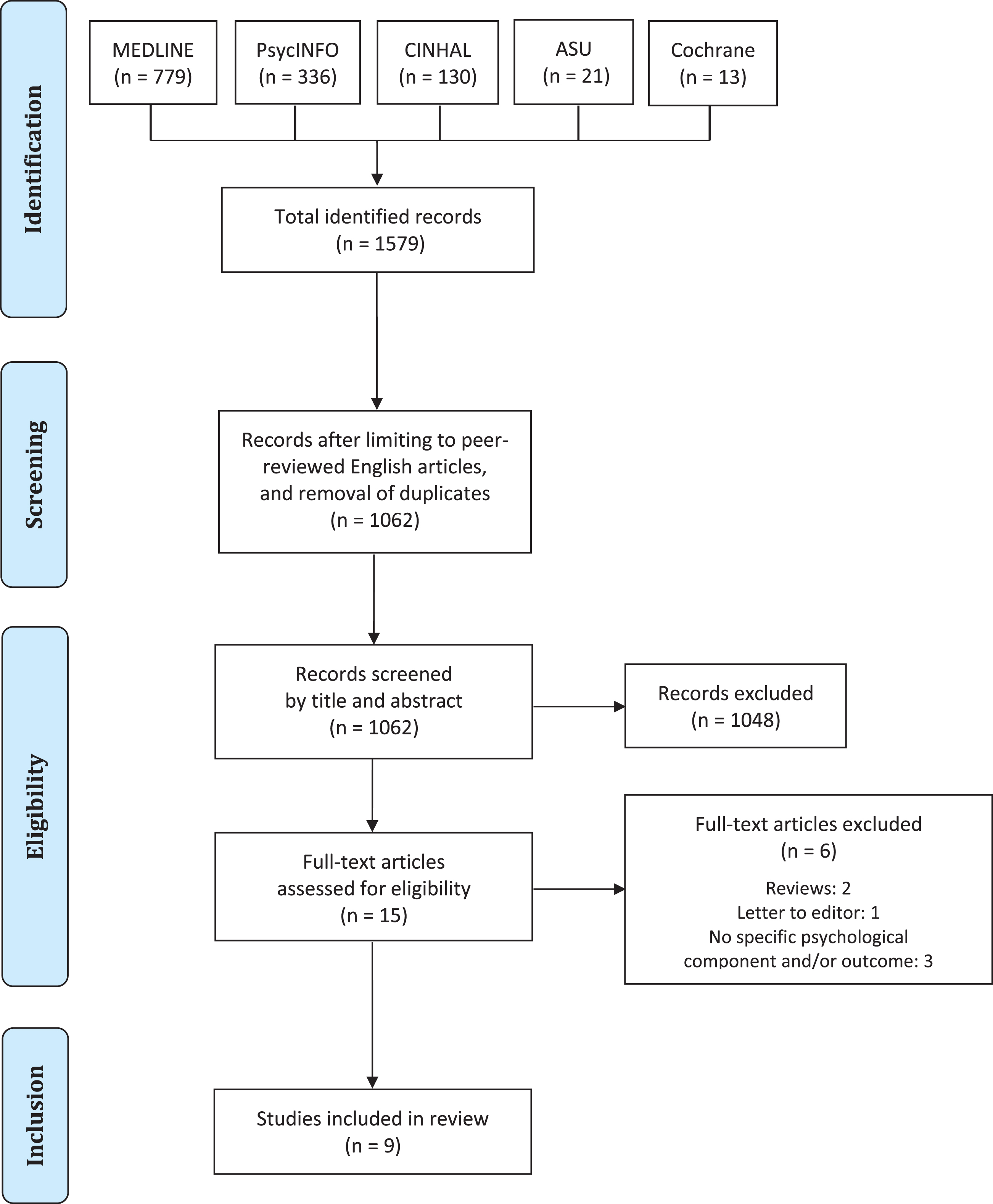
Fig. 1
PRISMA flow diagram of study selection.
Typical neurological symptoms include involuntary movements (chorea), difficulties with coordination, dystonia, bradykinesia, dysarthria, and dysphagia [3]. Genetic testing can confirm whether someone will at some point develop the condition, but not the exact time of onset, which is typically around age 35 to 45, although juvenile onset (i.e., before 20 and as early as 2) can also occur [4]. No cure has been identified so far and the mean life expectancy after the diagnosis is around 15–20 years [5]. People with positive testing for HD without a formal diagnosis are usually referred to as ‘gene carriers’, ‘presymptomatic’ or ‘premanifest’ individuals [6, 7], while those with family history of the disease who have not undergone genetic testing are usually defined as ‘at-risk’ [8]. For the purpose of the present review, the abbreviation ‘pwHD’ (people with HD) has been used generally when referring to individuals with the expanded Huntingtin gene at any stage of their disease trajectory.
Along with motor difficulties, people with symptomatic HD also face a wide range of cognitive changes, which can affect memory, psychomotor speed, executive functioning and language, and lead to full dementia at later stages [6]. In addition, while no changes are usually observed in premanifest individuals in terms of language and memory functioning, early deficits of executive processes and working memory may become apparent prior to receiving a formal diagnosis [9, 10].
Psychological difficulties in pwHD
While many pwHD typically experience a combination of motor impairments and cognitive deterioration [6, 7, 9–18], they can also experience significant psychological difficulties [2]. Of these, the most frequently reported are depression, mood extremes, irritability and aggressiveness, anxiety, agitation, compulsions, and apathy [3, 4, 19–23]. It is estimated that around 5 to 52% and up to 75% of pwHD may also show obsessive-compulsive and perseverative behaviours respectively [21, 24], while delusions and hallucinations are usually rarer [3, 4]. In addition, an increased risk of suicide has often been reported in both premanifest and symptomatic individuals [25]. Considering the hereditary nature of the disease, predictive testing represents a particular source of psychological distress in pwHD [26], which reflects on the overall low uptake among at-risk individuals, ranging between 3% and 24% worldwide [27, 28]. However, research on premanifest people has often reported inconsistent findings, as some show good adjustment after one year [29, 30], while others may develop regret, negative views of their future, and suicidal thoughts [31, 32].
With the exception of predictive genetic testing—which has traditionally seen the adoption of psychological approaches to the aftermath of a positive test result—the current understanding of psychological difficulties in pwHD is widely dominated by a neuropsychiatric framework. For example, the notion that anosognosia and lack of awareness in general may represent a barrier to benefitting from psychological interventions has a long history within the context of neuropsychiatric approaches to neurodegenerative conditions [33–35]. Accordingly, in the case of HD this has often translated into the idea that anosognosia is “a very important part of the patient presentation, and something that has to be assessed accurately and then taken into account in patient treatment and rehabilitation” [36] (p. 155). In turn, this may have been limiting the amount of research carried out so far on psychological interventions for pwHD. However, anosognosia has also shown to affect psychological manifestations of HD in heterogenous and sometimes selective ways [37, 38], and this lack of generalisation for problems with awareness should in fact represent a further incentive to explore targeted psychological approaches with this population.
The predominant neuropsychiatric view also reflects on the subjective experience of pwHD themselves, with evidence suggesting that affected individuals tend to develop a strictly disease-focused interpretation of the difficulties they face, possibly due to the neuropsychiatric explanations conveyed by many medical and clinical professionals [39]. Therefore, this approach appears not only to affect the paradigm in which most of the current research is conducted [23], but also the beliefs and perceptions of pwHD around their own condition, which in turn represent a pivotal factor in psychological adjustment to chronic illness [40–42]. This issue appears to be even more relevant due to evidence suggesting that psychological difficulties and the potential lack of expert advice around them may represent more significant determinants of quality of life in affected individuals compared to motor symptoms or pain [43–48]. Consistently, further evidence also suggests that premanifest people seem to express interest in engaging with psychological interventions [39], while an international committee of 11 multidisciplinary experts on HD has recently highlighted that psychological interventions should be offered whenever possible to early stage pwHD [49].
As a consequence, the current findings around psychological distress in pwHD support the case for the development of alternative, more psychologically-informed frameworks of the difficulties caused by the condition. However, as no literature review has been carried out to date on the range of psychological interventions adopted so far with pwHD, little is currently known on the ways and areas in which psychological approaches can be best implemented in this population. Thus, the aim of the present review was to answer the following research question: what psychological interventions have been explored and adopted so far with pwHD?
METHODS
Methodological approach
A systematic scoping review was adopted, following the guidance outlined by the Joanna Briggs Institute [50]. This approach was chosen due to the heterogenous nature of the targeted research (i.e., both quantitative and qualitative), and since it allowed the use of a systematic and replicable search strategy without specifying a narrowly defined research question, as usually required by traditional systematic reviews [51, 52]. In addition, it was considered particularly suitable to the aim of the current study due to the paucity of research and lack of comprehensive reviews currently available in the literature on psychological factors and interventions for pwHD [50, 53].
A combination of free text terms was used to perform a comprehensive search across five major databases (MEDLINE, PsycINFO, CINAHL, Academic Search Ultimate, and Cochrane Library) up until 1 March 2020. Hand-searches were also performed in reference lists of included studies and key reviews in order to identify further relevant citations. Table 1 shows the logic grid for the search strategy, while Table 2 provides an overview of the adopted search terms.
Table 1
Logic grid for search strategy
| Population | Interventions | |
| Huntington* disease | Acceptance and commitment therapy | Motivational interviewing |
| Behavio* therapy | Narrative therapy | |
| Cognitive analytic therapy | Person cent* therapy | |
| Cognitive behavio* therapy | Psychoanal* | |
| Cognitive therapy | Psychodynamic therapy | |
| Compassion* focused therapy | Psychoeducati* | |
| Counsel* | Psychological intervention | |
| Couple* therapy | Psychotherap* | |
| Dialectical behavioral therapy | Rational emotive behavio* therapy | |
| Emotion focused therapy | Schema therapy | |
| Emotive behavio* therapy | Self-management | |
| Eye movement desensiti* and reprocessing | Solution focused therapy | |
| Family therapy | Systemic therapy | |
| Gestalt therapy | ||
| Group* therapy | ||
| Integrative therapy | ||
| Interpersonal therapy | ||
| Meditat* | ||
| Metacognitive therapy | ||
| Mindfulness | ||
| Mindfulness-based cognitive therapy | ||
| Mindfulness-based stress reduction |
*truncation searching.
Table 2
Overview of adopted search terms and identified items per database
| Search terms |
| (Huntington* disease AND Acceptance and commitment therapy) OR (Huntington* disease AND Behavio* therapy) OR (Huntington* disease AND Cognitive analytic therapy) OR (Huntington* disease AND Cognitive behavio* therapy) OR (Huntington* disease AND Cognitive therapy) OR (Huntington* disease AND Compassion* focused therapy) OR (Huntington* disease AND Counsel*) OR (Huntington* disease AND Couple* therapy) OR (Huntington* disease AND Dialectical behavioral therapy) OR (Huntington* disease AND Emotion focused therapy) OR (Huntington* disease AND Emotive behavio* therapy) OR (Huntington* disease AND Eye movement desensiti* and reprocessing) OR (Huntington* disease AND Family therapy) OR (Huntington* disease AND Gestalt therapy) OR (Huntington* disease AND Group* therapy) OR (Huntington* disease AND Integrative therapy) OR (Huntington* disease AND Interpersonal therapy) OR (Huntington* disease AND Meditat*) OR (Huntington* disease AND Metacognitive therapy) OR (Huntington* disease AND Mindfulness) OR (Huntington* disease AND Mindfulness-based cognitive therapy) OR (Huntington* disease AND Mindfulness-based stress reduction) OR (Huntington* disease AND Motivational interviewing) OR (Huntington* disease AND Narrative therapy) OR (Huntington* disease AND Person cent* therapy) OR (Huntington* disease AND Psychoanal*) OR (Huntington* disease AND Psychodynamic therapy) OR (Huntington* disease AND Psychoeducati*) OR (Huntington* disease AND Psychological intervention) OR (Huntington* disease AND Psychotherap*) OR (Huntington* disease AND Rational emotive behavio* therapy) OR (Huntington* disease AND Schema therapy) OR (Huntington* disease AND Self-management) OR (Huntington* disease AND Solution focused therapy) OR (Huntington* disease AND Systemic therapy) |
*truncation searching.
Inclusion criteria
To be included in the present review, studies had to present the following characteristics: a) be related to people with premanifest or symptomatic HD; b) involve participants aged 18 or above; c) describe the delivery of any form of psychological intervention with pwHD. Systematic reviews, reviews, commentaries, conference proceedings, editorials, letters, as well as studies not adhering to the concept under investigation, not published in full in English, and involving participants aged 17 or under were excluded. As the focus of this study was on interventions for specific psychological outcomes in the people experiencing the condition themselves, interventions developed solely for family members and/or carers were also excluded. Similarly, interventions directly targeting only cognitive functions have not been included. While both these types interventions can be of pivotal importance to promote effective multidisciplinary care of pwHD, they were deemed to be outside the scope of the present review.
Quality appraisal
Since a formal quality appraisal is not a typical component of a scoping review [50, 53], this was not performed on the identified studies. However, any methodological and clinical limitations of the reviewed studies were highlighted whenever possible.
RESULTS
The database searches returned a total of 1579 studies, which were reduced to 1062 after deduplication and filtering for the English language. Following screening of titles and abstracts, a total of 15 full-text articles were left to assess for inclusion. Of these, two were reviews and one a letter to the editor, while three did feature a specific psychological component in the intervention (e.g., mainly physical interventions) and/or did not focus on specific psychological constructs among their outcome measures. Thus, nine studies were eventually included in the present review, of which one was a qualitative investigation, five were either single cases or case series [54–58], two were uncontrolled pretest-posttest designs [59, 60], and only one [61] consisted of a pilot randomised controlled trial (RCT). Table 3 summarises the key characteristics and results of the included studies, while Table 4 lists the excluded full-texts along with the rationale for exclusion. The findings of each paper are presented below categorised by study design.
Table 3
Key characteristics of included studies
| Citation | Design | Sample | Intervention | Outcome | Key results |
| [54] | Single case | Manifest: 1 | Behaviour support and pharmacological | Irritability | Reduction of delirium after pharmacological intervention.Reduction of challenging behaviours after medication with behaviour support. |
| [55] | Case studies | Manifest: 2 | Bespoke sensory modulation intervention | Irritability | Participant 1: reduced agitation and wandering, fewer complaints about food and improved ability to engage in activities.Participant 2: reduced aggression, reduced vocalisation, and greater ability to engage in occupations such as reading. |
| [56] | Single case experimental design | Manifest: 1 | Sensory modulation and behaviour support modification | Irritability | Sensory modulation and behaviour support associated with a reduction in recorded rates of all types of aggression. Decreased aggression at trend level with sensory modulation alone. |
| [57] | Case study | Premanifest: 1 | CBT | Anxiety Depression | Decreases in anxiety and depression from moderate to minimal, benefits sustained at 6-month follow-up |
| [58] | Case series | Manifest: 6 | Remotivation therapy (RmT) | Apathy | Reported improvements in interest, awareness, attention span, frustration tolerance, reading ability, oral communication, and overall participation.No quantitative data available. |
| [59] | Uncontrolled pre-post | Manifest: 29Premanifest: 12 | Psychoeducation with CBT components | DepressionAnxietyCoping | Significant improvements for symptomatic pwHD on behavioural problems, anxiety, and specific components of coping.Significant improvement in seeking social support as a coping strategy in premanifest pwHD.Significant improvement in mood (analogue scale) for both symptomatic and premanifest pwHD. |
| [60] | Uncontrolled pre-post | Manifest: 2 | Behavioural relaxation training | Irritability | Significant improvements in behavioural ratings of relaxation and heart-rate reductions in both within- and between-subject comparisons at post-test. |
| [61] | Pilot RCT | Manifest: 6Control (HD): 6 | Multi-sensory environment (MSE) | Irritability | No significant differences between groups between sessions. Evidence of some positive effects within-sessions for the MSE group.No sustained therapeutic effect for MSE, although it appears more effective than relaxation as a leisure activity. |
| [62] | Qualitative | Premanifest: 6 | Group genetic counselling session based on narrative therapy | N/A | Positive results regarding feasibility, acceptability, reduced feelings of loneliness, recognition of extant coping strategies and the discovery of new ones. |
N/A, not applicable; HD, Huntington’s disease; pwHD, people with HD.
Table 4
Articles excluded following full-text review
| Study | Design | Type of intervention | Reason for exclusion |
| [107] | Feasibility RCT | Physical Activity Self-Management Coaching | No specific psychological component and/or outcome |
| Social Interaction | |||
| [108] | Qualitative | Social support group | No specific psychological component and/or outcome |
| [109] | Single case | Not specified | Letter to editor |
| [110] | Uncontrolled pre-post | Multidisciplinary rehabilitation programme | No specific psychological component and/or outcome |
Excluded reviews not listed.
Qualitative studies
Stopford et al. [62] carried out a qualitative evaluation of a group genetic counselling intervention for premanifest individuals based on narrative therapy. The study enrolled six patients along with two carers and adopted a thematic framework analysis to explore their impressions after one 2-hour group narrative exercise. The results showed that the intervention was feasible and acceptable, with participants valuing the group format in particular as well as praising the opportunity to share common experiences, reduce feelings of loneliness, and recognise and rediscover coping strategies.
Single case studies
Silver [57] explored the adoption of standard cognitive behavioural therapy (CBT) for anxiety and depression with a 44-year-old woman with premanifest HD. The author reported a decrease from moderate to minimal difficulties on the Beck Anxiety Inventory (BAI; 60) and the Beck Depression Inventory (BDI; 61), as well as maintenance of benefits at 6-month follow-up. However, the study did not include any information on the statistical significance of such changes.
Blass and colleagues [54] reported the adoption of a combination of pharmacological therapy with a behavioural management plan based on applied behaviour analysis [65] to target challenging behaviours in a 32-year-old man with advanced HD. The results showed a reduction of challenging and inappropriate behaviours (e.g., inappropriate touching) at post-intervention. However, the behavioural difficulties reappeared four months following discharge after the treatment, which led the participant to be readmitted, and no follow-up data were provided following the second inpatient stay.
Fisher and Brown [56] carried out a single case experimental design adopting sensory modulation with adjunct behaviour modification to treat physical and verbal aggression with a 50-year-old man with symptomatic HD. The results showed that the combined approaches appeared to work well in tandem, with sensory modulation techniques showing to be effective at de-escalating early agitation, and behaviour modification leading to a reduction of the frequency of behavioural triggers. A trend towards aggression decrease was also noticed with sensory modulation alone.
Case series
Sullivan et al. [58] carried out a case series with six people with symptomatic HD to target constructs akin to apathy, such as levels of interest, awareness, oral communication, and overall participation. The intervention consisted of 12 sessions of remotivation therapy (RmT), a single group technique focused on motivating pwHD to think about reality in relation to themselves as opposed to their disabilities [66]. The authors reported improvements in interest, awareness, and overall participation at post-intervention, but no quantitative data were provided to evidence such benefits.
Brown and Fisher [55] investigated the effectiveness of a bespoke sensory modulation treatment to reduce behavioural difficulties in pwHD. The sample consisted of a man and a woman aged 22 and 31 who had received a diagnosis of juvenile HD prior to age 20. The authors reported reduced agitation and wandering and improved ability to engage in activities for participant one, as well as reduced aggression and vocalisation and better engagement in occupations such as reading for participant two. However, no details were provided on the specifics of the sensory modulation intervention.
Uncontrolled pretest-posttest designs
A’Campo and colleagues [59] developed an uncontrolled pretest-posttest study in which they enrolled 29 symptomatic and 12 premanifest pwHD to explore the effectiveness of a Participant Education Program for HD (PEP-HD) on anxiety, depression, and coping. The program consisted of eight 2-weekly sessions of 90 minutes each based on psychoeducation combined with CBT components and adapted from a manualised intervention already available for people with Parkinson’s disease [67, 68]. The results showed significant improvements in depression and specific components of coping (e.g., seeking social support and reduced use of passive reactions) for both premanifest and symptomatic participants, and in anxiety for symptomatic individuals only. However, the significant findings on depression were based on a 100-point visual analogue scale, while the score comparison with a standardised measure (Hospital Anxiety and Depression Scale, HADS; [69]) showed no significant difference in either group between pre- and post-intervention. In addition, the study generally suffered from a high dropout rate (up to 25% for symptomatic participants) and, as no follow-up assessments were conducted, it is unclear whether the observed improvements were maintained over time.
Fecteau et al. [60] adopted an uncontrolled pretest-posttest design with two symptomatic pwHD to explore the effectiveness of seven sessions of behavioural relaxation on challenging behaviours and physiological outcomes (e.g., heartrate, EMG activity). The results showed significant improvements in behavioural ratings of relaxation and heart-rate reductions in both within- and between-subject comparisons at post-intervention. However, these benefits were not retained at the 2-week follow-up.
Randomised controlled trials (RCTs)
Leng et al. [61] carried out a pilot RCT to compare the effectiveness on mood of a multi-sensory stimulation intervention against simple relaxation in people with mid-to-late stage HD. The intervention was based on a multisensory environment consisting of a quiet room arranged to stimulate the five primary sensory modalities, while the relaxation condition included activities such as listening to music and being read to. Both conditions lasted four weeks with two 30-minute sessions per week. Psychological outcomes were measured at baseline, post-intervention, and 4-week follow-up with the combined Behaviour and Mood Disturbance Scale (BMD) [70]. The results found no difference between groups on any of the main outcome variables, showing that the multisensory environment had no sustained therapeutic effect, aside from some positive “leisure” benefits being observed within sessions. However, the study may have struggled to detect effects due to the small size of the enrolled samples (i.e., 12).
DISCUSSION
The findings from the present review show that the current literature on psychological interventions for pwHD is severely limited both in terms of methods and clinical outcomes. Alongside a small number of case studies and qualitative or uncontrolled pretest-posttest designs, only one small pilot RCT was identified [61]. Moreover, the identified citations only targeted five main psychological outcomes, namely anxiety, apathy, depression, irritability, and coping. These are discussed below, along with suggestions for future research.
Anxiety
Although anxiety represents one of the most common and debilitating psychological difficulties in pwHD [22], to date no trial studies have been carried out for the psychological management of anxiety in this population. This review identified some preliminary evidence from single case and uncontrolled pretest-posttest studies suggesting that CBT may be effective to reduce anxiety [57, 59]. However, further evidence is required to shed light on its efficacy and feasibility with people with premanifest and symptomatic HD, and inspiration may be drawn from approaches used with other motor neurodegenerative conditions with some level of defined genetic risk, such as motor neuron disease/amyotrophic lateral sclerosis (MND/ALS). In particular, the current evidence for psychological approaches to anxiety in people with MND/ALS suggests that tailored mindfulness-based stress reduction (MBSR) and CBT based on the stress-coping model may represent ideal candidate for adoption with pwHD [71–73].
Apathy
Apathy is one of the most prevalent psychological difficulties in people with HD, affecting up to 70% of symptomatic individuals [74]. The current conceptualisation and assessment of apathy in HD relies heavily on a neuropsychiatric framework, which sees it solely as the result of neurodegeneration [75]. However, as patients’ understanding of their difficulties has been shown to represent a significant determinant of apathy in people with Parkinson’s disease [76], similar adaptive reasons have also been recently suggested for HD [77]. Nonetheless, no investigations have been carried out so far to address apathy in pwHD with a psychological intervention. The present review identified only a case series which described improvements in interest, awareness, and overall participation in symptomatic individuals after adopting remotivation therapy (RmT), but failed to provide any form of quantitative evidence [58]. Thus, further exploration is currently warranted on alternative conceptualisations of apathy in pwHD, as well as the best way to address them with psychological interventions. Preliminary explorations of the latter may take the form of CBT or behavioural activation techniques (e.g., activity scheduling and monitoring), both of which have shown to be effective when adopted with people with Parkinson’s disease [78, 79].
Depression
As with anxiety, depression is among the most commonly observed psychological difficulties in pwHD [3, 4], with over a third of individuals reporting clinical levels of low mood [80]. Moreover, depression can often be experienced by premanifest people well before a formal diagnosis is reached [81], especially in relation to predictive testing [31], and an increased risk of suicide has been linked to both premanifest [25] and at-risk individuals [82]. Nevertheless, no RCT has so far been carried out for psychological interventions for depression in pwHD, while smaller studies have shown partially positive effects of CBT-informed interventions in reducing low mood in this population [57, 59]. As a consequence, further more comprehensive insight into the applications of CBT for depression in pwHD is urgently needed, possibly adopting variations based on the stress-coping model which have proved to be effective to treat depression in people with MND/ALS [71]. In addition, based on positive evidence involving the same population [72, 73], tailored MBSR interventions may also be considered.
Irritability
Irritability may be described as a tendency to react excessively to negative stimuli, consisting of an affective component (anger) as well as a behavioural one (aggression) [83, 84]. It represents a common difficulty in pwHD, estimated to affect over 70% of individuals [85]. While to date no psychological study has been carried out with pwHD to address irritability specifically, the present review identified a number of case studies targeting aggression and other challenging behaviours. Promising results showing reduced aggressive behaviour in symptomatic individuals are available from studies using sensory modulation alone [55], or combined with behavioural support based on applied behaviour analysis [54, 56]. Data from an uncontrolled pretest-posttest study with two people with advanced HD also suggest that behavioural relaxation might be effective in reducing arousal in the short term, although not in the long run [60]. As more methodologically rigorous studies are needed to explore the concept of aggression [86], the consideration of alternative psychologically-informed models is currently warranted more widely to increase our understanding of irritability in pwHD. Inspiration for this may be drawn from the general literature on psychological interventions for people with anger and aggression, which currently identifies cognitive behavioural approaches as the most effective to address these difficulties (for a recent review, see [87]).
Coping
Despite not representing a symptom like the other psychological outcomes mentioned above, coping is widely recognised as a significant predictor of psychological distress and adjustment in chronic conditions [88, 89], and the successful operationalisation of coping strategies is considered to play a pivotal role for pwHD [90]. In particular, dysfunctional or ineffective coping in this population has been shown to carry the risk of developing strategies characterised by avoidance, denial, and isolation [91]. However, no RCT to date has addressed coping with pwHD directly. Preliminary quantitative evidence is currently only available from an uncontrolled pretest-posttest study, which found a significant effect of CBT-informed interventions on specific components of coping such as seeking social support and reduced use of passive reactions [59]. In addition, qualitative evidence is also available on the potential of narrative approaches to group genetic counselling in promoting the recognition of extant coping strategies and the discovery of new ones. Further explorations are therefore required to clarify whether coping interventions are effective for pwHD, and whether the effect differs between premanifest and symptomatic individuals.
Areas not yet addressed in the literature
Despite extensive evidence showing that obsessive-compulsive symptoms and perseverative behaviours are highly prevalent among premanifest and especially symptomatic pwHD [21, 24], no psychological interventions have been carried out so far to address them specifically. However, based on a rich literature highlighting that both face to face and telephone-delivered CBT are effective at reducing obsessive-compulsive symptoms and perseverative behaviours in the general population [92, 93]—as well as that they are feasible with other motor neurodegenerative diseases like multiple sclerosis and Parkinson’s disease [94, 95]—the adoption of such approach for premanifest and early-stage pwHD should be explored by future studies.
Psychotic experiences such as delusions and hallucinations are rare in pwHD [3, 4, 96], but may still occur at any stage of the disease [97] and cause significant difficulties and concerns in both affected individuals and their relatives [98]. However, to date no psychological interventions could be identified which may address psychotic experiences in this population. While this appears to be the case of various other primary neurodegenerative conditions (e.g., Lewy body dementia, [99]; MND/ALS, [71]), limited evidence is currently available on the positive effect of multidimensional interventions for dementia combining music therapy with recreational activities such as painting sessions and physical exercises like group ball games [100, 101]. Therefore, if adapted to take into consideration the impact of motor symptoms, this may represent a potential avenue for future explorations of psychotherapeutic approaches to psychosis in pwHD.
Further broader work is also needed focusing on people who are at-risk of having the gene expansion and choose not to be tested, as evidence suggests that they may also develop significant psychological difficulties [26]. With regards to this, the adoption of family therapy models (e.g., focused on attachment styles [102, 103]) for children and younger individuals affected by HD may be helpful, in particular due to recent evidence showing that the clinical presentation of juvenile HD may be more heterogeneous than previously thought [104]. In addition, as one of the qualitative studies identified by the present review showed preliminary positive results for a group genetic counselling session based on narrative therapy [62], similar forms of support may be explored and adapted for individuals at risk of HD not wishing to undergo genetic testing,
Finally, more consideration should be given to investigating how psychological approaches may be adopted across the entire clinical trajectory of HD, from the premanifest phase to stage 5 [105]. Indeed, as severe cognitive deterioration tends to characterise HD’s intermediate to advanced stages (e.g., 3–5), especially in the domains of language and executive functioning, the opportunity for effective psychological interventions may appear to be relatively limited to prodromal and early stages (e.g., 1–2). However, current evidence also shows that cognitive and behavioural techniques may be effective in treating psychological difficulties in people with moderate dementia, especially when carers are actively involved [106], and one of the uncontrolled pretest-posttest studies identified by the present review showed positive short-term results for behavioural relaxation with people with advanced HD [60]. As a consequence, tailored CBT-based approaches should also be explored in the future with people with intermediate to advanced stage HD, in order to assess differences in feasibility and acceptability compared to earlier stages as well as potentially inform the development of longitudinal psychotherapeutic plans which may adapt dynamically to pwHD across the entire trajectory of the disease.
Conclusion
Given the current severe paucity of research on psychological interventions in HD, no specific conclusions can be offered yet as to which psychological therapy may benefit this specific population. Moreover, since the current view of psychological difficulties in the condition is mainly neuropsychiatric, the adoption of psychologically-informed models for behavioural and affective difficulties should be promoted, along with further education for healthcare staff. Ultimately, the overarching aim of the present review is to act as a call to arms for HD researchers worldwide to help shed light on the most effective way to translate psychological theory into practice for the benefit of people with HD.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
The present study was funded by the British Psychological Society as part of ‘Minds & Movement’, (https://www.lancaster.ac.uk/health-and-medicine/dhr/minds-and-movement/) a project aimed at producing UK-based national guidance on psychological approaches to the understanding and treatment of psychological difficulties in adults with motor neurodegenerative conditions.
REFERENCES
[1] | Baig SS , Strong M , Quarrell OW . The global prevalence of Huntington’s disease: A systematic review and discussion. Neurodegener Dis Manag. (2016) ;6: :331–43. https://doi.org/10.2217/nmt-2016-0008 |
[2] | Novak MJU , Tabrizi SJ . Huntington’s disease. BMJ. (2010) ;340: :34–40. https://doi.org/10.1136/bmj.c3109 |
[3] | Roos RA . Huntington’s disease: A clinical review. Orphanet J Rare Dis. (2010) ;5: :40. https://doi.org/10.1186/1750-1172-5-40 |
[4] | Walker FO . Huntington’s disease. Semin Neurol. (2007) ;27: :143–50. https://doi.org/10.1055/s-2007-971176 |
[5] | Folstein S . Huntington’s disease: A disorder of families. Maryland: The Johns Hopkins University Press; (1989) . |
[6] | Dumas EEM , van den Bogaard SJA , Middelkoop HAM , Roos RAC . A review of cognition in Huntington’s disease. Front Biosci (Schol Ed). (2013) ;5: :1–18. |
[7] | Zarotti N , Simpson J , Fletcher I , Squitieri F , Migliore S . Exploring emotion regulation and emotion recognition in people with presymptomatic Huntington’s disease: The role of emotional awareness. Neuropsychologia. (2018) ;112: :1–9. https://doi.org/10.1136/jnnp-2018-ehdn.151 |
[8] | Chisholm LZ , Flavin KT , Paulsen JS , Ready R . Psychological well-being in persons affected by Huntington’s disease: A comparison of at-risk, prodromal, and symptomatic groups. J Health Psychol. (2013) ;18: :408–18. https://doi.org/10.1177/1359105312444646 |
[9] | You SC , Geschwind MD , Sha SJ , Apple A , Satris G , Wood KA , et al. Executive functions in premanifest Huntington’s disease. Mov Disord. (2014) ;29: :405–9. https://doi.org/10.1002/mds.25762 |
[10] | Dumas EM , Say MJ , Jones R , Labuschagne I , O’Regan AM , Hart EP , et al. Visual working memory impairment in premanifest gene-carriers and early Huntington’s disease. J Huntingtons Dis. (2012) ;1: :97–106. https://doi.org/10.3233/JHD-2012-120010 |
[11] | Milders M , Crawford JR , Lamb A , Simpson SA . Differential deficits in expression recognition in gene-carriers and patients with Huntington’s disease. Neuropsychologia. (2003) ;41: :1484–92. https://doi.org/10.1016/S0028-3932(03)00079-4 |
[12] | Labuschagne I , Jones R , Callaghan J , Whitehead D , Dumas EM , Say MJ , et al. Emotional face recognition deficits and medication effects in pre-manifest through stage-II Huntington’s disease. Psychiatry Res. (2013) ;207: :118–26. https://doi.org/10.1016/j.psychres.2012.09.022 |
[13] | Johnson SA , Stout JC , Solomon AC , Langbehn DR , Aylward EH , Cruce CB , et al. Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain. (2007) ;130: :1732–44. https://doi.org/10.1093/brain/awm107 |
[14] | Robotham L , Sauter DA , Bachoud-Levi AC , Trinkler I , Bachoud-Lévi A-C , Trinkler I . The impairment of emotion recognition in Huntington’s disease extends to positive emotions. Cortex. (2011) ;47: :880–4. https://doi.org/10.1016/j.cortex.2011.02.014 |
[15] | Henley SMD , Novak MJU , Frost C , King J , Tabrizi SJ , Warren JD . Emotion recognition in Huntington’s disease: A systematic review. Neurosci Biobehav Rev. (2012) ;36: :237–53. https://doi.org/10.1016/j.neubiorev.2011.06.002 |
[16] | Bates G , Tabrizi S , Jones L . Huntington’s disease. New York: Oxford University Press; (2014) . |
[17] | Zarotti N , Fletcher I , Simpson J . New perspectives on emotional processing in people with symptomatic Huntington’s disease: Impaired emotion regulation and recognition of emotional body language. Arch Clin Neuropsychol. (2019) ;34: (5):610–24. https://doi.org/10.1093/arclin/acy085 |
[18] | Kipps CM , Duggins AJ , McCusker EA , Calder AJ . Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J Cogn Neurosci. (2007) ;19: :1206–17. https://doi.org/10.1162/jocn.2007.19.7.1206 |
[19] | Beglinger L , Paulsen J , Watson DB , Wang C , Duff K , Langbehn DR , et al. Obsessive and compulsive symptoms in prediagnosed Huntington’s disease. J Clin Psychiatry. (2008) ;69: :1732–44. |
[20] | van Duijn E , Kingma EM , van der Mast RC . Psychopathology in verified Huntington’s disease gene carriers. J Neuropsychiatry Clin Neurosci. (2007) ;19: :441–8. https://doi.org/10.1176/appi.neuropsych.19.4.441 |
[21] | van Duijn E , Craufurd D , Hubers AAM , Giltay EJ , Bonelli R , Rickards H , et al. Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry. (2014) ;85: :1411–8. https://doi.org/10.1136/jnnp-2013-307343 |
[22] | Dale M , van Duijn E . Anxiety in Huntington’s disease. J Neuropsychiatry Clin Neurosci. (2015) ;27: :262–71. https://doi.org/10.1176/appi.neuropsych.14100265 |
[23] | Simpson J , Dale M , Theed R , Gunn S , Zarotti N , Eccles FJR . Validity of irritability in Huntington’s disease: A scoping review. Cortex. (2019) ;120: :353–74. https://doi.org/10.1016/j.cortex.2019.06.012 |
[24] | Oosterloo M , Craufurd D , Nijsten H , Van Duijn E . Obsessive-compulsive and perseverative behaviors in Huntington’s disease. J Huntingtons Dis. (2019) ;8: :1–7. https://doi.org/10.3233/JHD-180335 |
[25] | Hubers AA , Reedeker N , Giltay EJ , Roos RA , van Duijn E , van der Mast RC . Suicidality in Huntington’s disease. J Affect Disord. (2012) ;136: :550–7. https://doi.org/10.1016/j.jad.2011.10.031 |
[26] | Crozier S , Robertson N , Dale M . The psychological impact of predictive genetic testing for Huntington’s disease: A systematic review of the literature. J Genet Couns. (2014) ;24: :29–39. https://doi.org/10.1007/s10897-014-9755-y |
[27] | Laccone F , Engel U , Holinski-Feder E , Weigell-Weber M , Marczinek K , Nolte D , et al. DNA analysis of Huntington’s disease: Five years of experience in Germany, Austria, and Switzerland. Neurology. (1999) ;53: :801–6. |
[28] | Harper P , Lim C , Craufurd D . Ten years of presymptomatic testing for Huntington’s disease: The experience of the UK Huntington’s Disease Prediction Consortium. J Med Genet. 2000:567–71. |
[29] | Broadstock M , Michie S , Marteau T . Psychological consequences of predictive genetic testing: A systematic review. Eur J Hum Genet. (2000) ;8: :731–8. https://doi.org/10.1038/sj.ejhg.5200532 |
[30] | Duisterhof M , Trijsburg RW , Niermeijer MF , Roos RA , Tibben A . Psychological studies in Huntington’s disease: Making up the balance. J Med Genet. (2001) ;38: (12):852–61. doi: 10.1136/jmg.38.12.852. |
[31] | Hagberg A , Bui T-H , Winnberg E . More appreciation of life or regretting the test? Experiences of living as a mutation carrier of Huntington’s disease. J Genet Couns. (2011) ;20: :70–9. https://doi.org/10.1007/s10897-010-9329-6 |
[32] | Robins Wahlin TB . To know or not to know: A review of behaviour and suicidal ideation in preclinical Huntington’s disease. Patient Educ Couns. (2007) ;65: :279–87. https://doi.org/10.1016/j.pec.2006.08.009 |
[33] | Koltai DC , Welsh-Bohmer KA , Schmechel DE . Influence of anosognosia on treatment outcome among dementia patients. Neuropsychol Rehabil. (2001) ;11: :455–75. https://doi.org/10.1080/09602010042000097 |
[34] | Orfei MD , Varsi AE , Blundo C , Celia E , Casini AR , Caltagirone C , et al. Anosognosia in mild cognitive impairment and mild Alzheimer’s disease: Frequency and neuropsychological correlates. Am J Geriatr Psychiatry. (2010) ;18: :1133–40. https://doi.org/10.1097/JGP.0b013e3181dd1c50 |
[35] | Orfei MD , Assogna F , Pellicano C , Pontieri FE , Caltagirone C , Pierantozzi M , et al. Anosognosia for cognitive and behavioral symptoms in Parkinson’s disease with mild dementia and mild cognitive impairment: Frequency and neuropsychological/neuropsychiatric correlates. Parkinsonism Relat Disord. (2018) ;54: :62–7. https://doi.org/10.1016/j.parkreldis.2018.04.015 |
[36] | Tranel D , Paulsen JS , Hoth KF . Anosognosia in Huntington’s disease. In: Prigatano GP, editor. The Study of Anosognosia, New York: Oxford University Press; 2010, p. 147–58. |
[37] | Chatterjee A , Anderson KE , Moskowitz CB , Hauser WA , Marder KS . A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington’s disease. J Neuropsychiatry Clin Neurosci. (2005) ;17: :378–83. https://doi.org/10.1176/jnp.17.3.378 |
[38] | Ho AK , Robbins AOG , Barker RA . Huntington’s disease patients have selective problems with insight. Mov Disord. (2006) ;21: :385–9. https://doi.org/10.1002/mds.20739 |
[39] | Theed R , Eccles FJR , Simpson J . Understandings of psychological difficulties in people with the Huntington’s disease gene and their expectations of psychological therapy. Psychol Psychother. (2018) ;91: :216–31. https://doi.org/10.1111/papt.12157 |
[40] | Leventhal H , Brissette I , Leventhal E . The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, eds. The Self-Regulation of Health and Illness Behaviour. London: Routledge, 2003;42–65. |
[41] | Dempster M , Howell D , McCorry NK . Illness perceptions and coping in physical health conditions: A meta-analysis. J Psychosom Res. (2015) ;79: :506–13. https://doi.org/10.1016/j.jpsychores.2015.10.006 |
[42] | Hagger MS , Koch S , Chatzisarantis NLDD , Orbell S , Arat S , Araújo-soares V , et al. The common sense model of self-regulation: Meta-analysis and test of a process model. Psychol Bull. (2017) ;143: :1117–54. https://doi.org/10.1037/bul0000118 |
[43] | McCabe MP , Firth L , O’Connor E . A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings. (2009) ;16: :355–62. https://doi.org/10.1007/s10880-009-9168-5 |
[44] | Ho A , Hocaoglu M . Impact of Huntington’s across the entire disease spectrum: The phases and stages of disease from the patient perspective. Clin Genet. (2011) ;80: :235–9. https://doi.org/10.1111/j.1399-0004.2011.01748.x |
[45] | Ho AK , Gilbert AS , Mason SL , Goodman AO , Barker RA . Health-related quality of life in Huntington’s disease: Which factors matter most? Mov Disord. (2009) ;24: :574–8. https://doi.org/10.1002/mds.22412 |
[46] | Underwood M , Bonas S , Dale M . Huntington’s disease: Prevalence and psychological indicators of pain. Mov Disord Clin Pract. (2017) ;4: :198–204. https://doi.org/10.1002/mdc3.12376 |
[47] | Banaszkiewicz K , Sitek EJ , Rudzińska M , Sołtan W , Sławek J , Szczudlik A . Huntington’s disease from the patient, caregiver and physician’s perspectives: Three sides of the same coin? J Neural Transm. (2012) ;119: :1361–5. https://doi.org/10.1007/s00702-012-0787-x |
[48] | Smith S , D’Cruz G , Gray R , Flaherty H , Ivanecka A , Deane KHO . A concept map of what helps people with HD live with their condition. J Huntingtons Dis. (2015) ;4: :260–70. https://doi.org/10.3233/JHD-150161 |
[49] | Anderson KE , Van Duijn E , Craufurd D , Drazinic C , Edmondson M , Goodman N , et al. Clinical management of neuropsychiatric symptoms of huntington disease: Expert-based consensus guidelines on agitation, anxiety, apathy, psychosis and sleep disorders. J Huntingtons Dis. (2018) ;7: :355–66. https://doi.org/10.3233/JHD-180293 |
[50] | Peters MDJ , Godfrey CM , Khalil H , McInerney P , Parker D , Soares CB . Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. (2015) ;13: :141–6. https://doi.org/10.1097/XEB.0000000000000050 |
[51] | Arksey H , O’Malley L . Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. (2005) ;8: :19–32. https://doi.org/10.1080/1364557032000119616 |
[52] | Mays N , Roberts E , Popay J . Synthesising research evidence. In: Fulop N, Allen P, Clarke A, Black N, eds. Methods for studying the delivery and organisation of health services. London: Routledge; 2001, p. 188–219. |
[53] | Pham MT , Rajić A , Greig JD , Sargeant JM , Papadopoulos A , Mcewen SA . A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res Synth Methods. (2014) ;5: :371–85. https://doi.org/10.1002/jrsm.1123 |
[54] | Blass D , Steinberg M , Leroi I , Lyketsos C . Successful multimodality treatment of severe behavioral disturbance in a patient with advanced Huntington’s disease. Am J Psychiatry. (2001) ;158: :1966–72. |
[55] | Brown A , Fisher C . Optimising occupational performance through sensory modulation interventions: Case reports of two young adults diagnosed with Juvenile Huntington’s disease. Br J Occup Ther. (2015) ;78: :767–71. https://doi.org/10.1177/0308022615569249 |
[56] | Fisher CA , Brown A . Sensory modulation intervention and behaviour support modification for the treatment of severe aggression in Huntington’s disease. A single case experimental design. Neuropsychol Rehabil. (2017) ;27: :891–903. https://doi.org/10.1080/09602011.2015.1091779 |
[57] | Silver A . Cognitive-behavioural therapy with a Huntington’s gene positive patient. Patient Educ Couns. (2003) ;49: :133–8. https://doi.org/10.1016/S0738-3991(02)00070-8 |
[58] | Sullivan FR , Bird ED , Alpay M , Cha JH . Remotivation therapy and Huntington’s disease. J Neurosci Nurs. (2001) ;33: :136–42. https://doi.org/10.1097/01376517-200106000-00005 |
[59] | A’Campo LEI , Spliethoff-Kamminga NGA , Roos RAC . The Patient Education Program for Huntington’s Disease (PEP-HD). J Huntingtons Dis. (2012) ;1: :47–56. https://doi.org/10.3233/JHD-2012-120002 |
[60] | Fectau G , Boyne J , Fecteau GW , Boyne J . Behavioural relaxation training with Huntington’s disease patients: A pilot study. Psychol Rep. (1987) ;61: :151–7. https://doi.org/10.2466/pr0.1987.61.1.151 |
[61] | Leng TR , Woodward MJ , Stokes MJ , Swan AV , Wareing LA , Baker R . Effects of multisensory stimulation in people with Huntington’s disease: A randomized controlled pilot study. Clin Rehabil. (2003) ;17: :30–41. https://doi.org/10.1191/0269215503cr582oa |
[62] | Stopford C , Ferrer-Duch M , Moldovan R , MacLeod R . Improving follow up after predictive testing in Huntington’s disease: Evaluating a genetic counselling narrative group session. J Community Genet. (2020) ;11: :47–58. https://doi.org/10.1007/s12687-019-00416-9 |
[63] | Beck AT , Epstein N , Brown G , Steer RA . An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. (1988) ;56: :893–7. doi: 10.1037//0022-006x.56.6.893. |
[64] | Beck A , Ward C , Mendelson M , Mock J , Erbaugh J . An inventory for measuring depression. Arch Gen Psychiatry. (1961) ;4: :561–71. |
[65] | Lundervold DA , Jackson T . Use of applied behavior analysis in treating nursing home residents. Hosp Community Psychiatry. (1992) ;43: :171–3. https://doi.org/10.1176/ps.43.2.171 |
[66] | Williams I . Remotivation-therapy: What is it? Hosp Adm Can. (1975) ;17: :68–9. |
[67] | Smith Pasqualini M , Simons G . Patient education for people with Parkinson’s disease and their carers: A manual. Chichester: John Wiley & Sons; (2006) . |
[68] | Macht M , Gerlich C , Ellgring H , Schradi M , Rusinol AB , Crespo M , et al. Patient education in Parkinson’s disease: Formative evaluation of a standardized programme in seven European countries. Patient Educ Couns. (2007) ;65: :245–52. https://doi.org/10.1016/j.pec.2006.08.005 |
[69] | Zigmond AS , Snaith RP . The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. (1983) ;67: :361–70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x |
[70] | Greene JG , Smith R , Gardiner M , Timbury GC . Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: A factor analytic study. Age Ageing. (1982) ;11: :121–6. https://doi.org/10.1093/ageing/11.2.121 |
[71] | Zarotti N , Mayberry E , Ovaska-stafford N , Eccles F , Simpson J . Psychological interventions for people with motor neuron disease: A scoping review. Amyotroph Lateral Scler Front Degener. (2020) ;0: :1–11. https://doi.org/10.1080/21678421.2020.1788094 |
[72] | Pagnini F , Marconi A , Tagliaferri A , Manzoni GM , Gatto R , Fabiani V , et al. Meditation training for people with amyotrophic lateral sclerosis: A randomized clinical trial. Eur J Neurol. (2017) ;24: :578–86. https://doi.org/10.1111/ene.13246 |
[73] | Marconi A , Gragnano G , Lunetta C , Gatto R , Fabiani V , Tagliaferri A , et al. The experience of meditation for people with amyotrophic lateral sclerosis and their caregivers - a qualitative analysis. Psychol Health Med. (2016) ;21: :762–8. https://doi.org/10.1080/13548506.2015.1115110 |
[74] | Krishnamoorthy A , Craufurd D . Treatment of apathy in Huntington’s disease and other movement disorders. Curr Treat Options Neurol. (2011) ;13: :508–19. https://doi.org/10.1007/s11940-011-0140-y |
[75] | Starkstein SE , Leentjens AFG . The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. (2008) ;79: :1088–92. https://doi.org/10.1136/jnnp.2007.136895 |
[76] | Simpson J , McMillan H , Leroi I , Murray CD . Experiences of apathy in people with Parkinson’s disease: A qualitative exploration. Disabil Rehabil. (2015) ;37: :611–9. https://doi.org/10.3109/09638288.2014.939771 |
[77] | Bachoud-Lévi AC , Ferreira J , Massart R , Youssov K , Rosser A , Busse M , et al. International guidelines for the treatment of Huntington’s disease. Front Neurol. (2019) ;10: :1–18. https://doi.org/10.3389/fneur.2019.00710 |
[78] | Butterfield LC , Cimino CR , Salazar R , Sanchez-Ramos J , Bowers D , Okun MS . The Parkinson’s Active Living (PAL) Program. J Geriatr Psychiatry Neurol. (2017) ;30: :11–25. https://doi.org/10.1177/0891988716673467 |
[79] | Berardelli I , Bloise MC , Bologna M , Conte A , Pompili M , Lamis DA , et al. Cognitive behavioral group therapy versus psychoeducational intervention in Parkinson’s disease. Neuropsychiatr Dis Treat. (2018) ;14: :399–405. https://doi.org/10.2147/NDT.S152221 |
[80] | Slaughter JR , Martens MP , Slaughter KA . Depression and Huntington’s disease: Prevalence, clinical manifestations, etiology, and treatment. CNS Spectr. (2001) ;6: :306–8, 325-6. https://doi.org/10.1017/S109285290002201X |
[81] | Vaccarino AL , Sills T , Anderson KE , Bachoud-Lévi A-C , Borowsky B , Craufurd D , et al. Assessment of depression, anxiety and apathy in prodromal and early huntington disease. PLoS Curr. (2011) ;3: :RRN1242. https://doi.org/10.1371/currents.RRN1242 |
[82] | Arciniegas DB , Anderson CA . Suicide in neurologic illness. Curr Treat Options Neurol. (2002) ;4: :457–68. https://doi.org/10.1007/s11940-002-0013-5 |
[83] | Buss AH , Durkee A . An inventory for assessing different kinds of hostility. J Consult Psychol. (1957) ;21: :343–9. |
[84] | Caprara GV , Cinanni V , D’Imperio G , Passerini S , Renzi P , Travaglia G . Indicators of impulsive aggression: Present status of research on irritability and emotional susceptibility scales. Pers Individ Dif. (1985) ;6: :665–74. https://doi.org/10.1016/0191-8869(85)90077-7 |
[85] | van Duijn E . Treatment of irritability in Huntington’s disease. Curr Treat Options Neurol. (2010) ;12: :424–33. https://doi.org/10.1007/s11940-010-0088-3 |
[86] | Fisher CA , Sewell K , Brown A , Churchyard A . Aggression in Huntington’s disease: A systematic review of rates of aggression and treatment methods. J Huntingtons Dis. (2014) ;3: :319–32. https://doi.org/10.3233/JHD-140127 |
[87] | Glancy G , Saini MA . An evidenced-based review of psychological treatments of anger and aggression. Br Treat Cris Interv. (2005) ;5: :229–48. https://doi.org/10.1093/brieftreatment/mhi013 |
[88] | Jordan C , Sin J , Fear NT , Chalder T . A systematic review of the psychological correlates of adjustment outcomes in adults with inflammatory bowel disease. Clin Psychol Rev. (2016) ;47: :28–40. doi: 10.1016/j.cpr.2016.06.001. |
[89] | Meijer SA , Sinnema G , Bijstra JO , Mellenbergh GJ , Wolters WHG . Coping styles and locus of control as predictors for psychological adjustment of adolescents with a chronic illness. Soc Sci Med. (2002) ;54: :1453–61. https://doi.org/10.1016/S0277-9536(01)00127-7 |
[90] | Kaptein AA , Scharloo M , Helder DI , Snoei L , van Kempen GMJ , Weinman J , et al. Quality of life in couples living with Huntington’s disease: The role of patients’ and partners’ illness perceptions. Qual Life Res. (2007) ;16: :793–801. https://doi.org/10.1007/s11136-007-9194-4 |
[91] | Lowit A , van Teijlingen ER . Avoidance as a strategy of (not) coping: Qualitative interviews with carers of Huntington’s Disease patients. BMC Fam Pract. (2005) ;6: :38. https://doi.org/10.1186/1471-2296-6-38 |
[92] | Lovell K , Cox D , Haddock G , Jones C , Raines D , Garvey R , et al. Telephone administered cognitive behaviour therapy for treatment of obsessive compulsive disorder: Randomised controlled non-inferiority trial. BMJ. (2006) ;333: :883–6. https://doi.org/10.1136/bmj.38940.355602.80 |
[93] | Öst LG , Havnen A , Hansen B , Kvale G . Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993–2014. Clin Psychol Rev. (2015) ;40: :156–69. https://doi.org/10.1016/j.cpr.2015.06.003 |
[94] | Cosio D , Jin L , Siddique J , Mohr DC . The effect of telephone-administered cognitive-behavioral therapy on quality of life among patients with multiple sclerosis. Ann Behav Med. (2011) ;41: :227–34. https://doi.org/10.1007/s12160-010-9236-y |
[95] | Ghielen I , Rutten S , Boeschoten RE , Houniet-de Gier M , van Wegen EEHH , van den Heuvel OA , et al. The effects of cognitive behavioral and mindfulness-based therapies on psychological distress in patients with multiple sclerosis, Parkinson’s disease and Huntington’s disease: Two meta-analyses. J Psychosom Res. (2019) ;122: :43–51. https://doi.org/10.1016/j.jpsychores.2019.05.001 |
[96] | Craufurd D , Thompson JC , Snowden JS . Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol. (2001) ;14: :219–26. https://doi.org/. |
[97] | Anderson KE , Marder KS . An overview of psychiatric symptoms in Huntington’s disease. Curr Psychiatry Rep. (2001) ;3: :379–88. https://doi.org/10.1007/s11920-996-0030-2 |
[98] | Ding J , Gadit AM . Psychosis with Huntington’s disease: Role of antipsychotic medications. BMJ Case Rep. 2014:2013–5. https://doi.org/10.1136/bcr-2013-202625 |
[99] | Connors MH , Quinto L , Mckeith I , Brodaty H , Allan L , Bamford C , et al. Non-pharmacological interventions for Lewy body dementia: A systematic review. Psychol Med. (2018) ;48: :1749–58. https://doi.org/10.1017/S0033291717003257 |
[100] | Brodaty H , Arasaratnam C . Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. (2012) ;169: :946–53. |
[101] | Chen RC , Liu CL , Lin MH , Peng LN , Chen LY , Liu LK , et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: A prospective cohort study in Taiwan. Geriatr Gerontol Int. (2014) ;14: :440–6. https://doi.org/10.1111/ggi.12126 |
[102] | Van der Meer L , Timman R , Trijsburg W , Duisterhof M , Erdman R , Van Elderen T , et al. Attachment in families with Huntington’s disease. A paradigm in clinical genetics. Patient Educ Couns. (2006) ;63: :246–54. https://doi.org/10.1016/j.pec.2005.11.019 |
[103] | van der Meer LB , van Duijn E , Giltay EJ , Tibben A . Do attachment style and emotion regulation strategies indicate distress in predictive testing? J Genet Couns. (2015) ;24: :862–71. https://doi.org/10.1007/s10897-015-9822-z |
[104] | Fusilli C , Migliore S , Mazza T , Consoli F , De Luca A , Barbagallo G , et al. Biological and clinical manifestations of juvenile Huntington’s disease: A retrospective analysis. Lancet Neurol. (2018) ;17: :986–93. https://doi.org/10.1016/S1474-4422(18)30294-1 |
[105] | Shoulson I , Fahn S . Huntington disease: Clinical care and evaluation. Neurology. (1979) ;29: :1–3. |
[106] | Tay KW , Subramaniam P , Oei TP . Cognitive behavioural therapy can be effective in treating anxiety and depression in persons with dementia: A systematic review. Psychogeriatrics. (2019) ;19: :264–75. https://doi.org/10.1111/psyg.12391 |
[107] | Busse M , Quinn L , Drew C , Kelson M , Trubey R , McEwan K , et al. Physical activity self-management and coaching compared to social interaction in Huntington disease: Results from the ENGAGE-HD Randomized, Controlled Pilot Feasibility Trial. Phys Ther. (2017) ;97: :625–39. https://doi.org/10.1093/ptj/pzx031 |
[108] | Dipple H , Evans B . The Leicestershire Huntington’s disease support group: A social network analysis. Heal Soc Care Community. (1998) ;6: :286–9. https://doi.org/10.1046/j.1365-2524.1998.00132.x |
[109] | Lee HM , Chen ST , Chen SJ . Non-pharmacological treatments in a patient with dementia due to Huntington’s disease. J Neuropsychiatry Clin Neurosci. (2010) ;22: :2010. https://doi.org/10.1176/jnp.2010.22.2.247.e17 |
[110] | Piira A , van Walsem MR , Mikalsen G , Nilsen KH , Knutsen S , Frich JC . Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLoS Curr. 2013;5. https://doi.org/10.1371/currents.hd.9504af71e0d1f87830c25c394be47027 |




