Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review
Abstract
Background:
A number of studies evaluating physical therapy and exercise interventions in Huntington’s disease have been conducted over the past 15 years. However, an assessment of the quality and strength of the evidence in support of these interventions is lacking.
Objective:
The purpose of this systematic review was to investigate the effectiveness of physical therapy and exercise interventions in people with Huntington’s disease, and to examine the perceptions of patients, families and caregivers of these interventions.
Methods:
This mixed-methods systematic review utilized the Joanna Briggs Institute (JBI) approach and extraction tools to evaluate the literature from January 2003 until May 2016. The review considered interventions that included exercise and physical therapy interventions, and included both quantitative and qualitative outcome measures.
Results:
Twenty (20) studies met the inclusion criteria, including eighteen (18) that had quantitative outcome measures and two (2) that utilized qualitative methods. JBI Levels of evidence for the 18 quantitative studies were as follows: Eight studies were at evidence Level 1, seven were at Level 2, two were at Level 3, and one was at Level 4.
Conclusions:
Our review suggests that there is preliminary support for the benefits of exercise and physical activity in Huntington’s disease in terms of motor function, gait speed, and balance, as well as a range of physical and social benefits identified through patient-reported outcomes. Variability in mode of intervention as well as outcome measures limits the interpretability of these studies, and high-quality studies that incorporate adaptive trial designs for this rare disease are needed.
INTRODUCTION
Physical therapy and exercise are promising interventions for those with neurodegenerative diseases including Huntington’s disease (HD) [1, 2]. Over the last 15–20 years, there has been a significant increase in the number of research studies devoted to evaluating both the feasibility and efficacy of physical therapy and exercise interventions in individuals with neurodegenerative diseases highlighting the potential to not only improve daily activity performance, function, cognition and quality of life, [3–6] but also to slow disease progression [7, 8].
Current available pharmacologic treatments for HD are aimed at decreasing involuntary choreic movements, however no interventions to date have demonstrated an ability to alter the progression of the disease or effectively manage motor symptoms. Exercise interventions aimed at improving the range of motor and cognitive impairments in people with HD may provide a long-term beneficial effect to maximize functional abilities, maintain independence over a longer period and potentially impact the progression of the disease. Indeed, loss of independent mobility and care dependency have been shown to be important predictors of nursing home admissions in people with HD and those with dementia [9, 10].
In 2003, a systematic review on the effectiveness of physiotherapy, occupational therapy and speech therapy in HD was published; [11] however, at the time there was little in the published literature that could support the use of physical therapy for addressing disease-specific impairments in HD. In the past 10 years, there has been a significant increase in the number and quality of physical therapy and exercise studies in HD [12–18]. These studies have ranged from the evaluation of short-term exercise programs, [13, 14, 18] video-game home interventions, [15] as well as inpatient multidisciplinary rehabilitation programs [12, 16, 17]. However, despite the increase in published studies and trials, a search of the Joanna Briggs Institute (JBI) and Cochrane Library highlighted no further published systematic reviews of physiotherapy and Huntington’s disease. One protocol evaluating non-pharmacologic health-related behaviors in HD was identified in Prospero [19], but to date no results have been published.
In 2009, a Physiotherapy Guidance Document was developed by the European Huntington’s Disease Network (EHDN) [20] based on a structured search of the available literature and expert consensus at the time. The aim of the Guidance Document was to provide, where possible, a scientific evidence-based document to inform the optimal, individualized physical therapy management of people with HD. Treatment-based classifications were further developed in 2012, aimed at guiding clinical decision-making across the life course of the disease [21]. While the EHDN Guidance Document and subsequent treatment-based classifications were an important first step for providing information about patient management in this relatively rare disease, we are now at a point where more rigorous clinical guidelines need to be developed. Importantly, such guidelines should be informed by a complete and systematic review of the existing literature.
This mixed-methods systematic review utilized the JBI approach and extraction tools to evaluate the available literature. By combining quantitative and qualitative syntheses in the same review, we set out to answer the following questions: 1) what is the effectiveness of physical therapy and therapeutic exercise interventions in people with HD? and 2) what are patients, families and caregivers’ perceptions of these interventions?
MATERIALS AND METHODS
The protocol for this systematic review was developed in accordance with guidelines from JBI (http://joannabriggs.org/assets/docs/sumari/ReviewersManual-2014.pdf; accessed 06/07/2017), and has been previously published [22]. We considered both experimental and epidemiological study designs including randomized controlled trials (RCTs), non-randomized controlled trials, quasi-experimental studies, before and after studies, prospective and retrospective cohort studies, case control studies and analytical cross-sectional studies. The qualitative component of the review considered studies that focused on qualitative data including designs such as phenomenology, grounded theory, ethnography, action research and feminist research.
Search strategy
A three-step search strategy was utilized for each component in this review. An initial limited search of PubMed and CINAHL was undertaken followed by an analysis of the text words contained in the titles and abstracts, and of the article index terms. A second, comprehensive search using all identified keywords and index terms was undertaken across seven databases and grey literature sources. Third, the references from all selected reports and articles were manually searched for additional studies. Studies published in English between January 2003 and May 2016 were considered for inclusion in this review. This date was chosen as our start date for our search as this was the completion date for the previous systematic review [11].
The databases searched were: CINAHL, PEDro, MEDLINE via PubMed, Cochrane Library, Scopus, SPORTDiscus, and PsycINFO. In addition to the relevant references from selected articles, sources of additional grey literature included: Google, Web of Science (Books, proceedings, other), ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), ISRCTN Registry, Prospero, National Guideline Clearinghouse (NGC), resources from professional organizations such as: Huntington Study Group (HSG), EHDN, and the American Physical Therapy Association (APTA). Primary keywords used in the searches were: huntington* disease, physical therap*, physical activit*, exercise, and physiotherapy*. Additional text words included the specific forms of exercise and mobility intervention (see Interventions section below).
Interventions
This review considered studies that evaluated physical therapy and exercise interventions such as aerobic exercise, strengthening exercises, gait or walking training, treadmill training, balance training, yoga, Pilates, Tai-chi (and variants), relaxation, technology-delivered exercise, dance, aquatics, daily living strategies, sensory stimulation, cueing (i.e., visual, verbal or physical prompts including attentional strategies with internal cues), chaining (i.e., breaking down the task), dual-task training (i.e., motor-cognitive training), task-specific training, education, flexibility range of motion (ROM) exercises, breathing exercises, wheelchair evaluation, seating, wheelchair mobility training, positioning, splinting, posture (alignment and exercises), manually assisted cough, and non-invasive ventilation.
Outcome measures
The quantitative component of this review considered studies that included outcome measures for physical and cognitive function. Outcome measures of physical function included: balance, fitness (cardiovascular function), goal attainment, motor function and performance, muscle strength, number of falls, physical activity, pulmonary function, rate of chest infections, ulcer staging, spatiotemporal and kinematic parameters of gait and balance, walking ability and endurance. Outcome measures of cognitive function included cognition and psychological measures (depression, anxiety, and apathy).
The qualitative components of this review considered studies that identified patient, family or caregiver perceptions of physical therapy and exercise interventions including patient and family/caregiver experiences, perceived improvement and satisfaction where these have been explored using qualitative methods.
Context
This review considered studies that investigated physical therapy and exercise interventions that were carried out in the community, hospital settings, clinics, rehabilitation centers, or patient’s homes.
Data extraction and assessment of methodological quality
Data were extracted from papers included in the review using standardized data extraction tools from the JBI (JBI Meta-Analysis of Statistics Assessment and Review Instrument: JBI-MAStARI; JBI Qualitative Assessment and Review Instrument: JBI-QARI; JBI Institute Narrative, Opinion and Text Assessment and Review Instrument: JBI-NOTARI). The data extracted included specific details about the population, setting, context, methods and outcome, related to the review questions and specific objectives. Papers selected for retrieval were assessed by two independent reviewers for methodological validity prior to inclusion in the review. Following completion of the appraisal instruments, all articles were verbally discussed in a group setting, and any disagreements were resolved through discussion.
Data synthesis
As the experimental studies included in this review used a range of different types of interventions to address a variety of outcomes, it was not possible to pool the results using the statistical meta-analysis process embedded in JBI-MAStARI. Quantitative findings from the experimental and descriptive observational studies are therefore presented in a narrative form. As only two qualitative studies were included in the final review, meta-synthesis was not undertaken and the results are presented in narrative form. Narrative, opinion and textual documents are not included in this review.
RESULTS
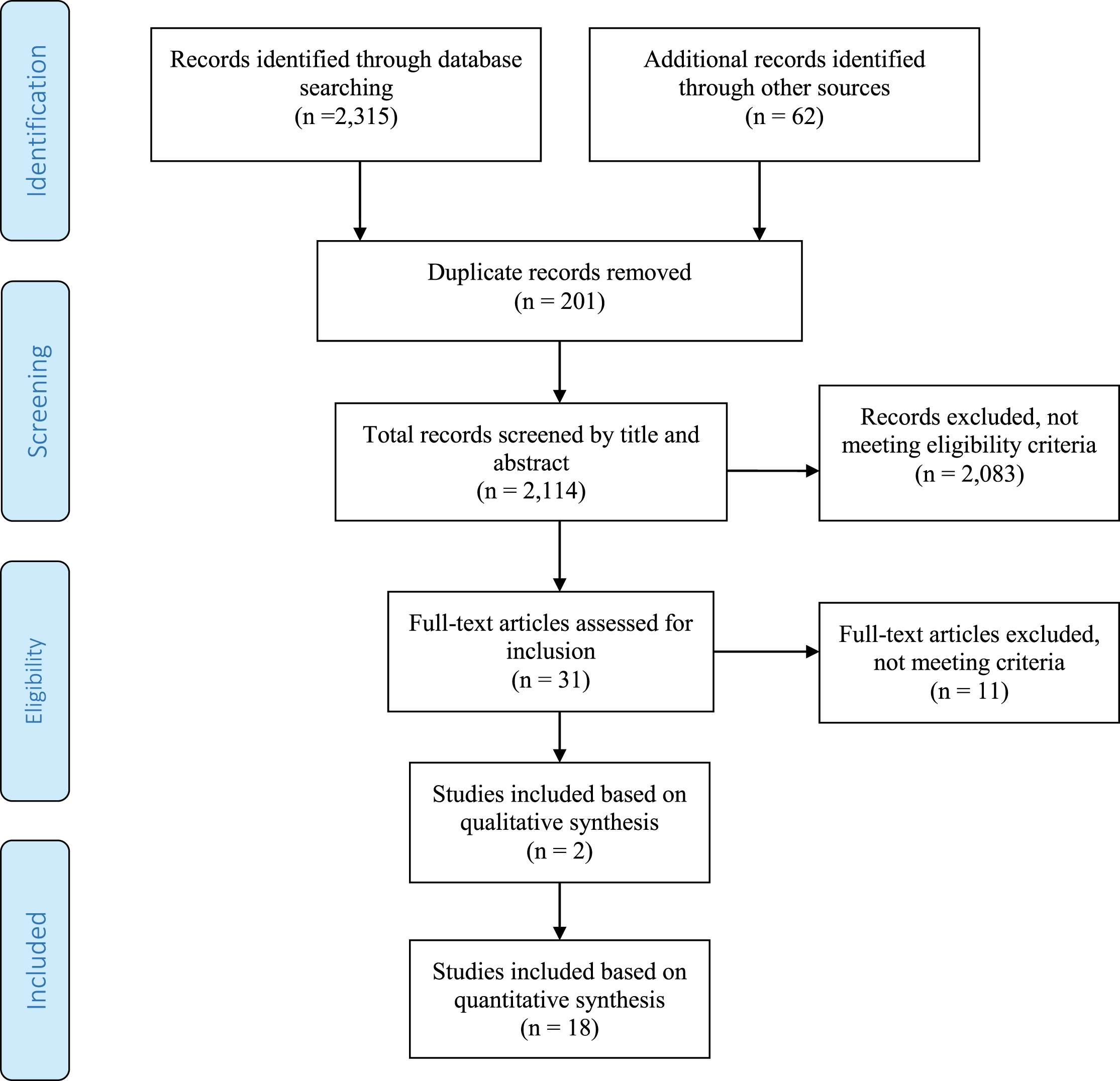
As shown in the PRISMA flowchart (Fig. 1), a systematic literature search across all sources returned 2,315 items, including 62 found in references and other grey literature sources. Following the removal of 201 duplicates, 2,114 unique records remained. The title and abstracts of these records were reviewed with 2,083 resources excluded due to not meeting inclusion criteria (the majority were drug or non-investigative studies). Finally, the full-text of 31 papers was scrutinized further with 11 papers assessed as not meeting inclusion criteria. A consensus between all authors was reached to identify the final 20 papers included in this review; two papers were included based on qualitative synthesis, 18 were included based on quantitative synthesis. All 20 studies met the criteria, which included that they were published in 2002 or later, were written in English, and were intervention studies of physical therapy, physical activity or exercise. Combined, the 20 papers assess physical therapy interventions and outcomes for 441 study participants. The majority of the studies, 14, were conducted in Europe (U.K., Italy, Norway, Germany, and Poland) while Australia and the United States were each home to three studies. Seventeen of the 20 papers were published between 2013 and 2016.
Fig.1
PRISMA Flowchart for identifying studies for systematic review.
Quantitative studies
The 18 quantitative studies are summarized in Table 1. Results are summarized below according to outcomes related to the International Classification of Functioning, Disability and Health (body structure and function, activity limitations and participation restrictions). JBI levels of evidence for the studies were as follows: Eight studies were at evidence Level 1, seven were at Level 2, two were at Level 3, and one was at Level 4.
Table 1
Summary of eighteen quantitative studies included in systematic review of exercise and physical therapy interventions in people with Huntington’s disease (HD)
| Study author & year | JBI Level of Evidence | Inclusion criteria &number of participants | Intervention of interest | Outcomes | Number of treatments | Session duration | Program duration | Results |
| Bohlen et al. [24] | 2.d-Pre-test – post-test control group study | 12 adults with manifest Huntington’s disease; mean age 50.0±17 years (42% men). | Physical therapy at outpatient clinic focused on transfer and gait training, balance and posture exercises and motor coordination tasks. | UHDRS-TMS, Spatiotemporal gait measures (GAITRite), TUG, BBS, and Force Plate | 2 times per week | 60 minutes | 6 weeks | Excellent compliance with attendance of 90% PT sessions. Significant changes in % double support, stride length, gait velocity, BBS, and TUG. No significant changes for stride length and gait velocity in the fast speed condition. Force Plate UHDRS-TMS measures showed no significant differences after therapy. |
| Busse et al. [14] | 1.c- randomized controlled trial | 31 adults with confirmed HD and able to walk independently; early to middle stages; 16 in intervention group, 15 in control group; mean age 53.3±12.5 (50% men). | Supervised gym sessions of stationary cycling and resistance exercises and unsupervised home-based walking program consisting of 1) aerobic training at 55% – 75% age-predicted maximal HR &moderate to hard levels of exertion on Borg RPE (4–6); 2) strength training for trunk/LE muscles progressed to 2 sets of 8–12 reps at 60–70% of participant’s 1 repetition max and 3) walking at moderate to somewhat hard (3-4 Borg scale) intensities; control group did usual care. | Feasibility (retention/adherence rates), acceptability, safety; UHDRS mMS; 10MWT, 30-s chair stand test, Romberg test, submaximal exercise test (HR/perceived exertion at minute 9); daily step counts, % of sedentary time, % time in moderate/high physical activity (activity monitors); self-reported 7-day physical activity recall; 6MWT; SF-36; UHDRS cognitive scales. | 1 time per week (gym); 2 times per week (home) | 30 minutes (gym and home) | 12 weeks | Intervention was feasible, safe, and acceptable. Seven individuals achieved 150 minutes of moderate physical activity for each week of the intervention. Intervention group had a significant improvement in SF-36 Mental Component Summary score (ES = 0.53) and non-significant improvements in UHDRS cognitive scores (0.40), 6MWT (0.44); 30 s chair stand test (0.25), and HR at minute 9 of exercise test (-0.25). |
| Ciancarelli et al. [32] | 2.d- Pre-test – post-test control group study | 34 adults with genetically confirmed HD who could understand tests and exercise sequences and were able to self-ambulate; early to middle stages; mean age 42±7.0 (35% men). | Intensive inpatient multidisciplinary neurorehabilitation program consisting of conventional neuromotor rehabilitation (balance, coordination, gait, posture, and strengthening exercises), light aerobics (cycling/walking), and OT for hand dexterity and fine motor function exercises. | Barthel Index, Tinetti Scale, Physical Performance Test, total Functional Capacity Scale | 2 times per day (neuro-motor rehab and OT); 1 time per day (aerobics) | 2 hours per day (neuro-motor rehab and OT); 20 minutes per day (aerobics) | 3 weeks | Significant (p < 0.001) improvements in all outcome measures in all participants after completion of neurorehabilitation program. |
| Clark et al. [28] | 4.c- Case series | 2 adults with manifest HD and 1 at risk for Huntington’s disease (1 man and 2 women; mean age 28.0±1.5 years). | Outpatient program with 4 key features: 1) com-munity-based group format for individuals with HD, caregivers, and those at-risk for HD; 2) individualized prescription within the group design; 3) circuit training; and 4) use of outcome measures. | 10MWT, BBS, Fatigue Impact Scale, and TUG. | Once a week | 60 minutes | 8 weeks | All 3 individuals improved on the TUG, 10MWT. One individual also improved on the BBS and the Fatigue Impact Scale. This individual had the highest disease burden. |
| Cruickshank et al. [31] | 2.d- Pre-test – post-test control group study | 15 adults with manifest Huntington’s disease; mean age 52.5±6.6 (53% men). | Multidisciplinary rehabilitation program of aerobic and resistance exercises in clinic, home-based exercise program, and OT focused on cognitive rehabilitation. | Structural Magnetic Resonance Imaging of gray matter volume; Hopkins verbal learning test; Trail Making test; Color Word interference test; SDMT. | 1 time per week (clinic), 3 times per week (home), every other week (OT) | 60 minutes | 9 months | Significant increases in GM volume in right caudate and bilaterally in the DLPFC after multidisciplinary rehab. Volumetric increases in GM were accompanied by significant improvements in Hopkins Verbal Learning-Test. GM volume increases in the DLPFC correlated with performance on verbal learning and memory. |
| Dawes et al. [29] | 3.e- Observa-tional study without a control group | 13 adults with confirmed HD in early and middle stages (9 analyzed); 20 age and gender-matched healthy controls; mean age 51.0±11 years (55% men). | Supervised gym sessions of stationary cycling and resistance exercises and unsupervised home-based walking program consisting of 1) aerobic training at 55% – 75% age-predicted maximal HR &moderate to hard levels of exertion on Borg RPE (4–6); 2) strength training for trunk/LE muscles progressed to 2 sets of 8–12 reps at 60–70% of participant’s 1 repetition max and 3) walking at moderate to somewhat hard (3-4 Borg scale) intensities; control group did usual care. | Blood pressure; work rate (watts) phase a (unloaded cycling for 3 mins); HR (beats per minute) phase a minute 3; Borg RPE phase a minute 3; work rate (watts) phase b (65–75% heart rate reserve); HR (beats per minute) phase b minute 9; Borg RPE phase b minute 9. | 1 time per week (gym); 2 times per week (home) | aerobic training 30 minutes followed by strength-ening exercises; home-based walking program 30 minutes per day | 12 weeks | 4 participants did not complete the intervention; no observable group training effects were found on any of the outcome measures in the 9 participants that completed the intervention; however, there was a wide variability in responses with some individuals showing a large training response and others none. |
| Jones et al. [30] | 1.d-Pseudo- randomized controlled trial | 20 adults with confirmed HD and stable meds; premanifest and manifest stages; 10 in intervention group; 10 in placebo group. | Home-based resistive inspiratory muscle training at 50% of maximal inspiratory pressure (MIP) using the POWERbreathe®K3 device; resistance was 10 cmH2O for placebo group. | SNIP; cough efficacy measured with PCF; Adherence (number of training sessions completed). | 2 sessions per day | 30 breaths | 6 weeks | Nonsignificant increases in SNIP and PCF for both groups; pooled data showed small effect sizes for SNIP (ES 0.36) and PCF (0.37); adherences were 70.67±26.35% for the intervention group and 74.53±21.03% for placebo group. |
| Khalil et al. [13] | 1.c- randomized controlled trial | 25 individuals mild/moderate HD; 13 exercise group; 12 in control group. Mean age 52.5±13.5 years. | Supervised home exercise program using a DVD and a walking program. One home visit to teach the program and then weekly follow up phone calls. | UHDRS-mMS; Gait analysis measures using GaitRite including gait speed and spatiotemporal measures of gait, SF-36. | 3 times a week (DVD exercises); 1 time a week (walking) | 45 min DVD; 30 minutes walking | 8 weeks | Intervention safe and feasible; Experimental group had significant improvements in gait (velocity ES 1.7), &UHDRS-mMS (ES 1.1). No significant changes in step time, step time CV, and the SF-36. Balance improved (BBS ES 1.4); not blind rated. |
| Kloos et al. [43] | 3.e- Observa-tional study without a control group | 21 adults with confirmed diagnosis of HD able to walk a minimum of 10 m without an AD or assist and follow instructions on UHDRS cognitive tests; early to middle stages; mean age 49.3±11 (52% men). | Forward walking at a comfortable pace across a GAITRite walkway and around two obstacles in a figure-of-eight pattern using no AD and with each of 6 different ADs. | Regular forward walking: gait spatiotemporal and variability (coefficients of variation) measures; Figure-of-eight: gait speed, observed numbers of stumbles and falls | Regular forward walking: 4 trials each condition; Figure-of-eight: 2 trials each condition | 1.5 hours | 1 day | Across devices, walking with the four-wheeled walker produced a gait pattern with the lowest double support time and variability in step to step measures compared to other devices. Figure-of-eight: faster completion times and less stumbles and falls using four-wheeled walker than all other devices except for the three-wheeled walker. |
| Kloos et al. [15] | 1.c- randomized controlled trial | 24 adults with HD able to walk 10 m without assistance, wide disease severity; 13 intervention; 11 control (18 analyzed); mean age 50.7±14.7 years (40% men). | Playing the video game Dance Dance Revolution with therapist supervision in homes; control group played a handheld video game unsupervised. | Feasibility (ability to play game, adherence), acceptability, and safety (vitals, adverse events); spatiotemporal gait parameters; FSST, Tinetti Mobility Test, ABC scale; WHO QOL-Bref scale | 2 days per week | 45 minutes | 6 weeks | 6 participants couldn’t receive intervention for medical reasons; feasible (game play improved, 100% adherence), acceptible (17 out of 18 participants stated they liked playing Dance Dance Revolution and safe; significant decrease in double support percentage in forward walking and backward walking; no significant changes in FSST, ABC scale, and WHO QOL Bref. |
| Mirek et al. [25] | 2.d- Pre-test – post-test control group study | 30 individuals with early-mid stage HD. (TFC I-III); mean age 43.4±13.8 years. | PNF intervention in an outpatient clinic focused on balance and gait. | 10 m walk, 20 m walk, TUG, Tinetti Gait Test, Functional Reach, BBS, Pastor Test. | 3 times a week | 90 minutes | 3 weeks | Statistically significant improvement in balance and gait, as measured by 10MWT, 20 m walk test, TUG, Tinetti Gait test, Functional Reach, BBS and Pastor Test. |
| Piira et al. [16] | 2.d- Pre-test – post-test control group study | 37 adults with early-mid stage Huntington’s disease (TFC stages I-III); mean age 52.4±13.1 (49% men). | Multi-disciplinary inpatient rehabilitation program focused on physical exercise, social activities, and group/teaching sessions. | TUG; 10MWT; 6MWT; BBS; ABC Scale; Barthel Index; MMSE; UHDRS Cognitive Assessments; HADS; Short Form-12; and BMI | 15 sessions repeated 3 times a year | 8 hours of multi-disciplinary therapy a day | 3 weeks repeated 3 times a year | There were significant improvements in gait measures from baseline through stay 2 and 3 to evaluation stay with mean changes as follows: TUG – 1.32 seconds, 10MWT – 0.27 m/s and 6MWT +68.71 m. Balance improved with mean BBS change baseline to evaluation stay of +1.0 (p < 0.03). No significant changes in mean UHDRS cognitive scores. Anxiety and depression (HADS) were significantly reduced (3.54 points, p < 0.001). Significant improvement in SF-12 physical component scores, but not in the mental component score. Participants gained some weight during the project period, with a change in BMI of 0.72 units (p < 0.024) from baseline to evaluation stay. No change was seen in ADL function (Barthel Index). |
| Piira et al. [27] | 2.d- Pre-test – post-test control group study | 10 adults with early-mid stage Huntington’s disease (TFC stages I-III): mean age 50±14.0 (50% men). | Multi-disciplinary inpatient rehabilitation program focused on physical exercise, social activities, and group/teaching sessions. | TUG; 10MWT; 6MWT; BBS; ABC Scale; Barthel Index; MMSE; UHDRS Cognitive Assessments; HADS; Short Form-12; and BMI. | 15 sessions repeated 3 times a year | 8 hours of multi-dis-ciplinary therapy a day | 3 weeks at 3 times a year | After 24 months individuals who participated in the program 3 times per year showed small, non-significant declines in gait, balance, and cognition (except MMSE). Anxiety and depression (HADS) improved, while BMI and quality of life (Short Form-12) did not change. |
| Quinn et al. [23] | 1.c- randomized controlled trial | 30 adults with mid-stage HD; 15 in intervention; 13 in control group; mean age 57.0±10.1 years (46% men). | Home-based task specific intervention program delivered by a physical therapist, up to a maximum of 15 sessions. Participants wore HR monitors during sessions. Programs were individualized to participants’ specific activity limitations in walking, sit-to-stand transfers, and standing ability and modified to their home environments. Control group continued usual care. | Goal Attainment Scale; Physical Performance Test; UHDRS-TMS; UHDRS cognitive; BBS; Gait Speed; Fast Gait Speed; 30 second Chair Rise; TUG; Vitality Score; HADS; HDQoL; EQ5D Health Index; | 2 times a week | 60 minutes | 8 weeks | The study demonstrated feasibility of doing task specific activities in clients with HD who agree to participate. Adherence was 96.9% in the experimental group. Of note almost half of those approached were not interested which may reflect low interest in exercise or home based therapy (as noted by the authors). On the Goal Attainment Scale 92% met their goals; all other measures had small and non-significant effect sizes. |
| Quinn et al. [18] | 1.c- randomized controlled trial | 32 adults with confirmed HD in early to middle stages. Adults on stable meds, able to speak English, able to ride exercise bike, and not currently exercising; 17 in intervention group; 15 in control group; mean age 53.0±11 years (53% men). | The intervention group participated in a 12-week exercise program performed in a gym or at home. Health professionals delivered the intervention, and monitored exercise dose, progression and safety. Support by trainers was tapered. Sessions followed a set program: 5-min warm up and up to 25 min on the bike within an aerobic zone, 10–15 min of strengthening (LE and core activities), 5 min of stretching. Control group continued usual care. | Feasibility (retention and adherence rates), acceptability, and safety; VO2 max, UHDRS mMS, 3-minute walk test, finger tapping, IPAQ, simple and complex dual task, trailmaking A &B, Stroop, word fluency, SDMT, HADS, EQ5D Health Index and weight. | 3 times a week | 50 minutes | 12 weeks | The intervention was safe and feasible. Significant improvements in VO2 max (ES = 0.73) and UHDRS-mMS (0.43); significantly lower weight in experimental group (0.13). Non-significant improvement in 3 minute walk (0.08), finger tapping, IPAQ (0.42), simple dual task walk time (0.06), complex dual task walk time (0.18), SDMT (0.01), verbal fluency (0.2), Stroop color naming (0.31), word reading (0.26), interference (0.08), trailmaking A, trailmaking B (0.02), HADS, and EQ5D Health Index (0.34). |
| Reyes et al. [26] | 1.c- randomized controlled trial | 18 adults with genetically confirmed HD with verified disease expression, and ability to understand and respond to instructions; 9 in the intervention group; 9 in control group; mean age was 56±10.2 (61% men). | Home-based resistive inspiratory and expiratory muscle training. Intervention group: progressively increased resistance from 30% to 75% of each patient’s maximum respiratory pressure; Control group: fixed resistance of 9 cmH2O. | MIP, MEP, spirometry (slow vital capacity, FVC, MVV, forced expiratory FEV1, PEF and the ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC)), 6MWT, dyspnea, water-swallowing test, SWAL Qol questionnaire | 6 days per week | 5 sets of 5 reps of inspiratory and expira-tory exercise | 4 months | There were greater improvements in MIP (ES = 2.8), MEP (1.5), FVC (0.80), FEV1 (0.90) and PEF (0.80) for the intervention group compared to the control group after 4 months of training; small positive effects seen in swallowing function, dyspnea sensation, and six minute walking for the intervention group. |
| Thompson et al. [17] | 1.d- pseudo- randomized controlled trial | 20 adults with confirmed HD, clinical disease diagnosis, able to follow verbal instruction and perform sub-maximal exercise; early to middle stages; 9 in intervention group; 11 in the control group (mean age 53.8±2.9 years). | Outpatient clinic exercise program (supervised group sessions including 5 minute warm-up, 10 minute aerobic exercise, 40 minute resistance exercise, 5 minute cool-down), home-based exercise program, and OT program focused on deficits identified by psychologists. | Primary outcome measure: UHDRS-TMS; Secondary: body composition, SOT, ABC Scale-UK, strength, SDMT, Hopkins Verbal Learning Test-R, Color Word Interference Test, Trail Making Trials, Beck Depression Inventory-II; Goal Attainment Scale; SF-36; and Huntington’s-Disease-Quality-of-Life-Battery-for Carers. | 1 time per week (clinic); 3 times per week (home); once every 2 weeks (OT) | 1 hour (clinic, home, and OT) | 9 months (clinic); 6 months (home program and OT) | Compared to control participants, the intervention group exhibited reduced motor (UHDRS-TMS chorea and tandem walking) and postural stability (Sensory Organization Test) deterioration, and significantly increased fat mass, fat-free mass, and lower/upper body strength, decreased written errors (SDMT), and increased performance on the ABC Scale-UK walking-up-and-down-stairs. |
| Zinzi et al. [12] | 2.d- Pre-test – post-test control group study | 40 adults with early-mid stage Huntington’s disease (TFC stages I-III); mean age 53.6±14.7 (42.5% men). | Multi-disciplinary inpatient rehabilitation program including respiratory, occupational, and physical therapy. | Performance Oriented Mobility Assessment; Physical Performance Test; MMSE; Zung Scale; and Barthel Index | 18 sessions repeated 6 times a year | 8 hours of multi-disciplinary therapy a day 5 days a week and 4 hours on the week-end | 3 weeks repeated 6 times a year | Of the 40 participants, only 11 completed the study (6th admission). Intensive rehabilitation improved gait and balance. No changes seen in cognition and ADLs. Limited carryover effects of intervention seen. |
ABC scale, Activities-specific Balance Confidence scale; AD, assistive device; ADL, activities of daily living; BBS, Berg Balance Scale; BMI, body mass index; CV, coefficient of variability; DLPFC, dorsolateral prefrontal cortex; DVD, digital video disc; EQ5D, 5-item EuroQoL; ES, effect size; FEV1, forced expiratory volume in one second; forced vital capacity; GM, grey matter; HADS, Hospital Anxiety and Depression Scale; HDQoL, Huntington’s Disease Health-Related Quality of Life; HR, heart rate; FSST, four square step test; IPAQ, International Physical Activity questionnaire; LE, lower extremity; MEP, maximum expiratory pressure; MIP, maximum inspiratory pressure; MMSE, Mini Mental State Examination; MVV, maximal voluntary ventilation; PCF, peak cough flow; PNF, proprioceptive neuromuscular facilitation; RPE, rating of perceived exertion; OT, occupational therapy; PEF, peak expiratory flow; SF-36, 36-item Short Form Health Survey; SDMT, symbol digit modalities test; 6MWT, six minute walk test; SNIP, sniff nasal inspiratory pressure; SOT, Sensory Organization Test; 10MWT, ten meter walk test; SWAL QoL, Swallow Quality of Life questionnaire; TFC, Total Functional Capacity; TUG, Timed Up and Go; UHDRS, Unified Huntington’s disease rating scale; UHDRS-mMS, UHDRS modified motor score; UHDRS-TMS, Unified Huntington’s Disease Rating Scale-total motor score; VO2 max, peak oxygen uptake; WHO QOL-Bref, World Health Organization Quality of Life-Bref scale.
Body function and structure
Physical function
Motor function and performance (United Huntington’s Disease Rating Scale-Total Motor Score (UHDRS-TMS) or modified Motor Score (mMS)). Five studies evaluated the effects of physical therapy or exercise on overall motor function measured by the UHDRS-TMS or mMS [13, 14, 17, 18, 23]. All five studies were randomized pilot or feasibility trials. Three studies used the modified motor score as their primary outcome measure, which is a composite of items related to voluntary movement [13, 14, 18]. Three studies reported a statistically significant improvement in motor score [13, 17, 18].
Spatiotemporal and kinematic parameters of walking (gait speed). Seven studies examined the effect of physical therapy or exercise on gait speed [13–16, 23–25]. Three of the studies were RCTs, [13, 14, 23] one study used a randomized cross-over design, [15] and three studies had a pre-post design [16, 24, 25]. Gait speed was measured either with the 10-meter walk test [14, 16, 23, 25] or with the 5-meter long GAITRite instrumented mat [13, 15, 24]. Four studies demonstrated statistically significant improvement in gait speed, three of which were pre-post design studies [16, 24, 25] and one was a RCT [13]. The other four studies did not demonstrate improvement in gait speed.
Walking endurance. Five studies measured walking endurance as a secondary outcome [14, 16, 18, 26, 27]. Individuals in both the intervention and control groups demonstrated improvements on the 3-minute walk test [18] or 6 minute walk test (6MWT) [14, 16, 26], however differences between control and intervention groups were not statistically significant in four of the five studies. The exception was the pre-post design study [16], which reported statistically significant improvement in walking endurance.
Balance. Seven studies examined balance [14, 16, 23–25, 27, 28] and four studies examined balance confidence [15–17, 27] as secondary outcomes. Busse et al. [14] demonstrated greater improvements on the Romberg test in the intervention compared to the control group. Following an intervention, individuals with HD maintained [23] or improved scores [16, 24, 25] on the Berg Balance Scale, though individuals in the control group [23] also demonstrated small improvements [24]. In one study, balance confidence on both walking and stairs measured by the Activities Balance Confidence scale improved in the intervention group but declined in the control group [17]. Kloos et al. [15] reported no significant difference between the intervention group and the control group following a video-game balance intervention.
Muscle strength (includes chair stand test). Three RCTs reported on muscle strength as an outcome [17, 18, 23]. Strength was assessed with the 15-repetition chair stand test in one study [18] with the 30-second chair stand test in another study [23]. The third study did not provide details of strength assessment [17]. Only one study reported statistically significant improvement in strength after physical therapy/exercise [17].
Fitness. Two studies, utilizing data from the same study population, examined fitness as a primary outcome [18, 29]. Predicted VO2 max measurements were calculated before and after a 12-week cycling and strength training program or usual care [18] or before and after 12 weeks of cycling and strength training [29]. Individuals in both intervention groups demonstrated improvements in actual and predicted VO2 max [18, 29]. Quinn et al. [18] demonstrated that the intervention group significantly improved predicted VO2 max compared to the control group.
Body weight. Three studies examined weight or body mass index (BMI) as a secondary outcome [16–18]. In one study, the intervention group had lower body weight following a 12-week exercise intervention than the control group [18]. Thompson et al. [17] demonstrated that individuals in the exercise intervention increased their total mass while maintaining their bone mineral density, while individuals in the control group had an average loss in weight over the course of the study.
Pulmonary function. Two studies [26, 30] evaluated pulmonary function as a primary outcome. Although the intervention type and duration differed (i.e., resisted inspirations for 6 weeks [30] vs. progressive resisted inspiratory and expiratory training for 4 months [26]), both studies reported improvements in inspiratory pressure. Jones et al. [30] reported improvements in sniff nasal inspiratory pressure and peak cough flow in both the control group (breathing exercises without resistance) and the intervention group with moderate effect sizes (d = 0.36 and 0.37, respectively), suggesting that regular breathing exercises over a short time frame of 6 weeks could improve respiratory muscle strength and cough efficiency, regardless of added resistance in persons with HD. Four months of progressive resisted inspiratory and expiratory training resulted in greater improvements in maximal inspiratory (d = 0.70 intervention, d = 0.02 control) and expiratory pressures (d = 0.47 intervention, d = –0.23 control) as well as forced vital capacity, and peak expiratory flow compared to fixed-resistance training [26]. This suggests that increasing the resistance with breathing exercises over time may produce robust improvements in persons with HD.
Rate of chest infections. Rate of chest infections was not included in any study as a primary or secondary outcome.
Ulcer Staging. Ulcer staging was not included in any study as a primary or secondary outcome.
Cognitive and psychological function
Cognition. Many studies examined cognition as a secondary outcome; [14, 16–18, 23] only one examined cognition as a primary outcome [31]. The most commonly used outcomes were the Symbol Digit Modalities Test (SDMT), Stroop Test, Hopkins Verbal Learning Test-Revised (HVLT-R), and the UHDRS Cognitive battery, which comprises the SDMT, Stroop and Verbal Fluency tests.
One study examined cognition and associated grey matter volume as a primary outcome of exercise training [31]. This study noted significant improvements in delayed recall and retention (i.e. memory) on the HVLT-R that were associated with significantly increased grey matter volume in the dorsolateral prefrontal cortex [31]. Thompson et al. [17] also examined HVLT-R performance following a similar intervention (i.e., multidisciplinary rehabilitation) and found that both the intervention group and the control group performed worse following an exercise training program.
The UHDRS Cognitive battery was evaluated in two RCTs [14, 23] and calculated for one [18]. On average, both the intervention and control groups improved performance, with the control group improving to a greater degree than the intervention group.
On average, the control and intervention groups demonstrated maintenance of performance or slight declines on the SDMT and Stroop components (i.e., color, word, interference) [16–18]. Clinically meaningful changes in SDMT and Stroop performance have not been established for people with HD.
Depression and Anxiety. Four studies [16–18, 23] examined depression as a secondary outcome. Only one study, which utilized a repeated measures design and did not include a control group, [16] reported significant reductions in depression scores (i.e., improvement) on the Hospital Anxiety and Depression Scale.
Apathy. Apathy was not included in any study as a primary or secondary outcome.
Activity limitations
Functional abilities. The Physical Performance Test was used in three studies [12, 23, 32] to measure physical activity. Only one study reported significant improvements following exercise [32].
Physical activity. Two studies examined self-reported physical activity as a secondary outcome [14, 18]. There were no significant improvements in self-reported physical activity in either study.
Number of falls. Number of falls was not reported as an outcome measure in any of the studies.
Goal attainment. Only one RCT examined goal attainment, [23] in which participants identified 2–5 goals. The most common goal was related to walking or stair climbing. Results of this study demonstrated that by the end of intervention 92% of the goals had been achieved.
Participation restrictions
Quality of life. Seven studies examined quality of life as a secondary outcome; outcome tools varied widely, including the World Health Organization Quality of Life scale, [15] European Quality of Life-5 Dimension scale, [18, 23] Huntington’s disease Quality of Life scale, [23] 36-Item Short Form Health Survey (SF-36), [13, 14] 12-Item Short Form Health Survey (SF-12), [16] and Swallowing Quality of Life Questionnaire, [26] which is specific to quality of life related to swallowing. Three studies reported significant improvements in quality of life following training. Busse et al. [14] demonstrated significant improvement on the mental component of the SF-36 following training, while Piira et al. [16], which did not include a control group, demonstrated significant improvements on the physical subscale of the SF-12 after training. Finally, Reyes et al. [26] noted moderate effect sizes in both the intervention (d = 0.54) and control groups (d = 0.38) after respiratory muscle training.
Qualitative studies
We identified three papers that included qualitative analyses for exercise and physical therapy interventions [33–35]. Zinzi et al. [35] was excluded following data extraction as the analyses were not deemed to utilize appropriate qualitative methods. Frich et al. [33] and Khalil et al. [34] included 26 participants in the early-late stages of HD. Qualitative methods utilized were in-depth [33] and semi-structured [34] interviews, and results were analyzed using systematic text condensation [33] and content analysis [34]. Results could be categorized into two overriding themes: perceived benefits and barriers, and facilitators to participation (see Fig. 2).
Fig.2
Summary of results from qualitative studies evaluating exercise and physical therapy interventions in Huntington’s disease (HD).
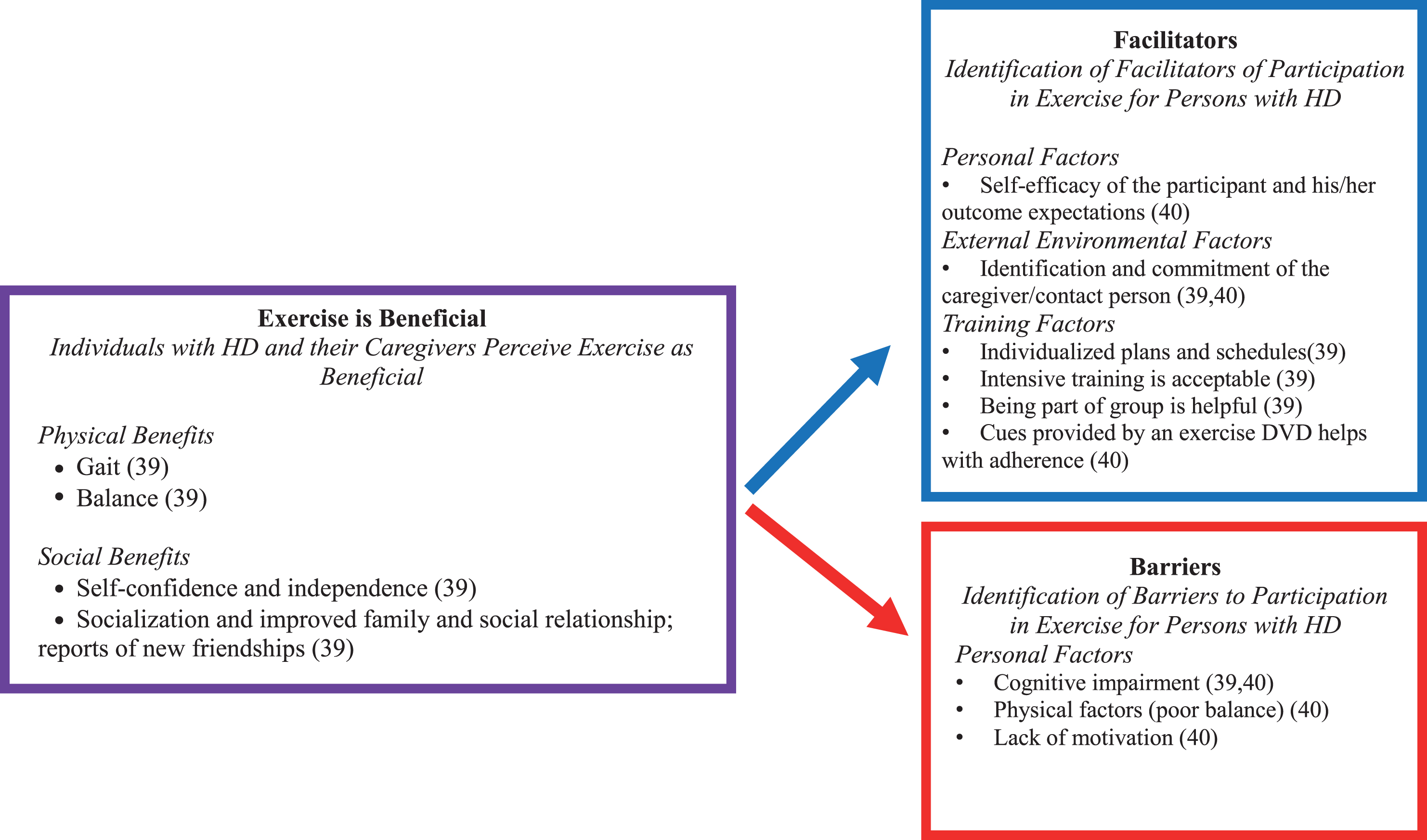
Perceived benefits. Results suggest that both individuals with HD and their caregivers perceive exercise as beneficial. Improvements were noted in both physical (i.e., walking, balance, speech/swallowing, and motor control, with associated reduction in falls), and social (i.e., self-confidence, independence, socialization with family and friends, new friendships, mood, well-being, and reduced apathy) domains [33] Caregivers perceived that these benefits lasted for greater than 1 month.
In addition to the perceived benefit of exercise, individuals with HD and their caregivers also identified a number of barriers and facilitators to participation in exercise programs. Barriers included lack of motivation, cognitive impairment and physical factors, such as poor balance [33, 34]. Many of these barriers may be overcome by identified facilitators such as identification of a contact person; [33] indeed, committed caregiver support resulted in improved outcomes in one study [34]. Further facilitators to participation in exercise training included individualized training, intensive/challenging training, and group training and cues to improve adherence (Fig. 2) [33, 34].
DISCUSSION
Our review of eighteen studies with quantitative outcomes suggests that exercise and physical activity may be beneficial for individuals with HD in terms of motor function, gait speed, and balance. Two studies with qualitative outcomes provide preliminary evidence for patient-reported outcomes, including a range of physical and social benefits, as well as identifying barriers and facilitators to exercise engagement. In line with the recently published CONSORT extension for randomized feasibility and pilot studies, [36] most studies were appropriately focused on feasibility, acceptability and preliminary efficacy to provide support for larger scale trials. Large sample RCTs in this population remain to be implemented, as do evaluation of interventions in people at the prodromal and advanced stages of HD.
Seven treatment-based classifications (TBCs) have been previously identified to assist physical therapy treatment planning for people with HD according to motor symptoms and physical functioning, [21] and work is currently underway to establish their validity [37]. Table 2 lists these classifications along with a summary of outcome and intervention recommendations. Six studies examined interventions for the treatment-based classification of Exercise Capacity and Performance [14, 16–18, 27, 29]. Interventions utilized in these studies included supervised gym or clinic-based aerobic (i.e., stationary cycling) and resistive strengthening exercise programs with or without additional unsupervised home-based walking and/or strengthening exercises, [14, 17, 18, 29] and three-week bouts of multi-disciplinary inpatient rehabilitation three times a year that included unspecified physical activities in a gym and swimming pool [16, 27]. In general, the aerobic and resistive exercises were found to be safe and feasible when performed 3-4 times per week for 30–50 minutes for at least 12 weeks at a moderate intensity [14, 17, 18, 29]. Following the interventions, significant improvements were reported in aerobic capacity/endurance (VO2 max, 6MWT), [16, 18] motor function (UHDRS-modified Motor Score), [18] muscle strength, [17] body composition measures, [16, 17] and mental health [14, 16, 27]. Taken together these findings provide good evidence that aerobic and strengthening exercise programs may be beneficial for improving exercise capacity and performance in people with HD in the early to middle stages. Future studies to determine optimal dosages and the long-term effects of aerobic and strengthening exercise programs on functional capacity in individuals with HD are needed.
Table 2
Treatment-based classifications for exercise and rehabilitation interventions in Huntington’s disease [21]
| Classification | Description | Stage | Intervention Recommendations |
| A. Exercise capacity and performance | Absence of motor impairment or specific limitations in functional activities; potential for cognitive and/or behavioral issues | Pre-manifest/early | Aerobic and resistance exercises |
| B. Planning and sequencing of tasks (including bradykinesia) | Presence of apraxia or impaired motor planning; slowness of movement and/or altered force generation capacity resulting in difficulty and slowness in performing functional activities | Early-mid | Task specific training including strategy training, sensory stimulation, cueing and chaining |
| C. Mobility, balance and falls risk | Ambulatory for community and/or household distances; impairments in balance, strength or fatigue resulting in mobility limitations and increased falls risk | Early-mid | Balance and gait training; task specific practice |
| D. Secondary and adaptive changes and deconditioning | Musculoskeletal and/or respiratory changes resulting in physical deconditioning, and subsequent decreased participation in daily living activities, or social/work environments | Early-mid | Patient and caregiver education, maintenance exercise program, gait and balance training |
| E. Impaired postural control and alignment in sitting | Altered alignment due to adaptive changes, involuntary movement, muscle weakness and incoordination resulting in limitations in functional activities in sitting | Mid-late | Handling and falls risk assessment; positioning schedule; seating and wheelchair evaluation |
| F. Respiratory dysfunction | Impaired respiratory function and capacity; limited endurance; impaired airway clearance resulting in restrictions in functional activities and risk for infection | Mid-late | Functional exercise and ADL training; positioning; breathing exercises; airway clearance techniques; relaxation |
| G. Palliative care | Active and passive range of motion limitations and poor active movement control resulting in inability to ambulate; dependent for most activities of daily living; difficulty maintaining upright sitting position | Late | Positioning; range of motion; active movement exercises |
Overall there were few studies examining interventions for the TBC of Planning and Sequencing of Tasks. Studies in this area were primarily focused on the impact of exercise on cognition. Two studies examined the inter-relationship of cognition and exercise [18, 31]. Cruikshank et al. [31] found changes in the gray matter volume in the caudate and dorsolateral prefrontal cortex after 9 months of 4 hours per week of exercise and cognitive therapy for an hour every other week. In addition, there were significant improvements in verbal learning and memory as measured by the HVLT-R. There were no significant improvements in cognitive function as measured by the UHDRS cognitive battery. A shorter duration aerobic and strengthening program found improvements in motor skills with no significant change in cognitive function as measured by the UHDRS cognitive battery [18]. It is difficult to draw conclusions based on these limited and diverse studies. Future studies may wish to incorporate a measure of verbal learning and memory such as the HVLT-R to compare to and build on the findings of Cruikshank et al. [31]. This area of the TBC remains largely unexplored; studies to examine impact of planning and sequencing deficits on function and participation as well as interventions to best treat these deficits are needed.
The majority of studies focused on interventions for the TBC of Mobility, Balance and Fall Risk, with 13 studies in this area. Interestingly, none of the measures utilized falls as an outcome measure. The most common outcome measures were gait speed (10 studies), Berg Balance Scale (6 studies), Timed Up and Go (5 studies), UHDRS-TMS and Tinetti Mobility Test (4 studies) and a variety of measures of cognition (6 studies). Interventions included aerobic exercise with strengthening, aerobic exercise with functional training, aerobic, strengthening, balance and functional exercises, video-game based exercise and assistive device training. A majority of studies utilized aerobic and strengthening exercise interventions in combination. Exercise interventions were found to be safe and feasible in this population across all studies, and there is preliminary evidence to suggest that exercise programs can lead to improvements in gait and balance. There were a few studies that demonstrated some improvement in one or more elements of quality of life [14, 16] while others saw no improvement [13, 15, 23, 27]. In general sample sizes were small and control groups generally continued usual care. Due to the diversity of interventions and outcome measures it is difficult to draw conclusions across studies. It is unclear if any benefits seen were due to any one type of exercise (aerobic, strengthening or balance) or best achieved through a combination of any or all of the types of exercise that have been used to date. Several studies demonstrated improvement or maintenance of motor function over 9 months or longer, which is notable in a degenerative condition [16, 17, 27, 31]. Future research should examine long-term benefits of exercise in HD. This will be challenging in a degenerative condition where slowing rate of decline or maintenance of current condition could be an appropriate therapeutic goal. It may be necessary to establish typical rates of decline in gait, balance and other domains that are usually addressed with exercise in order to better establish that programs can be successful in slowing rate of decline or maintaining function.
Two small randomized controlled studies examined interventions for the TBC of respiratory dysfunction [26, 30]. After home-based interventions of resistive inspiratory and expiratory respiratory muscle training, small to moderate improvements were found in pulmonary function measures, [26, 30] swallowing, [26] dyspnea, [26] walking endurance, [26] and cough efficacy [30]. Improvements in maximum inspiratory and expiratory pressures and forced vital capacity for the training group greatly exceeded the control group [26]. These preliminary findings suggest that resistive respiratory muscle training is feasible and may be beneficial for individuals with HD. Larger studies are needed to determine the best training protocols to achieve optimal pulmonary and swallowing function across all stages of the disease.
All studies stated that exercise training in individuals with HD was feasible. The majority of studies focused on individuals in the early-middle stages of disease, although one study reported feasibility of training even in the late stage of the disease [18]. A recent case series in late-stage HD also supports the feasibility and benefits of exercise training [37].
Five studies [12, 18, 23, 24, 32] included follow-up assessments after the primary intervention period. These follow-ups ranged in length from six to 24 weeks (average 12.6 weeks) and were either telephone interviews [18, 32] or in-person visits [12, 23, 24]. The results of follow-up visits were variable; studies utilizing telephone interviews reported that individuals with HD returned to lower levels of self-reported activity (International Physical Activity Questionnaire) while maintaining general health ratings (European Quality of Life scale-5 Dimensions) [18] and that improvements in independence on the Barthel Index were lost at a 12 week follow-up [32]. Interestingly, Zinzi et al. reported maintenance of motor function over 2 years as measured by physical performance test [12]. Maintenance of motor function in individuals with HD represents an important clinical outcome of exercise training, as the natural course of HD is marked by progressive decline.
There are several limitations to this review that are important to highlight. The lack of standard outcome measures across studies limited our ability to conduct a meta-analysis, and this would clearly be an important next step for future studies. In addition, future work should utilize hypothesis-driven outcomes assessment targeted at specific cognitive or behavioral aspects of HD. This is particularly important because the effect of exercise on cognitive function is not clear. In addition, the benefit of exercise and physical activity on other health-related outcomes such as sleep, bowel function, and blood pressure should be explored. The lack of control groups in the multidisciplinary research studies limited the interpretability of the intervention efficacy compared to natural disease progression and to tease out the active ingredients of the interventions when they were so multi-faceted. The heterogeneity of disability levels and disease stages across the studies also affected our ability to make meaningful comparisons of effectiveness across disease stages. Better incorporation of staging criteria and clearly defined inclusion criteria is essential in future studies.
It is important to establish the mode of exercise that has the best chance of facilitating disease modification or attenuating disease progression. All studies we evaluated had relatively small sample sizes, which may be largely due to the low prevalence of HD and the time commitment and effort required for exercise and physical therapy interventions. Importantly, involvement of caregivers may improve adherence to exercise programs [33, 34, 38]. Strategies to facilitate recruitment to exercise studies should be implemented across networks such as Huntington Study Group (US) and the European Huntington Disease Network (Europe). Furthermore, incorporating future interventions as sub-studies of current longitudinal registry datasets (e.g. Enroll-HD) will be particularly useful for comparative analysis on disease progression measures and to minimize subject burden.
In order to facilitate implementation of exercise interventions into clinical practice, studies with longer follow up duration are needed. Studies of at least 6 months duration would be able to elucidate the potential for exercise and physical activity to have a disease modifying effect in HD. Such longer term studies would also likely require the utilization of more reliable measures of exercise adherence, such as the use of wearable digital devices to track physical activity as well as other health-related outcomes (e.g. sleep and sedentary behaviors).
While the review may highlight the need for high quality RCTs of exercise interventions, it is important to consider recent recommendations for intervention studies in rare diseases when planning definitive evaluations [39–42]. In small sample studies, random subject assignment may not always balance out subject characteristics due to the inherent heterogeneity. An alternative is to broaden subject inclusion criteria at the risk of increasing within-group variability and in this respect, it may then be useful to consider alternative approaches such as cross-over or within-subject repeated measures designs. Multi-center collaborations, although imposing logistical and regulatory challenges, are critical to achieving sufficient study power and alongside this the use of either less stringent α levels or one-sided tests particularly when there are clear a-priori directional hypotheses could also be considered [42]. The prospective evaluation of well-considered covariate factors may also improve precision and increase statistical power. However, additional consensus would be needed to inform meaningful evaluation of outcomes in this respect [39]. Adaptive designs, which allow modification of design elements including re-estimation of sample size or modification of the randomization ratio based on accumulating data may also improve overall efficiency.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
This review was funded by the Huntington Study Group, the European Huntington Disease Network and the Griffin Foundation. Dr. Busse received funding from the Welsh Government through Health and Care Research Wales.
REFERENCES
[1] | Petzinger GM , Fisher BE , McEwen S , Beeler JA , Walsh JP , Jakowec MW . Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. (2013) ;12: (7):716–26. |
[2] | Mo C , Hannan AJ , Renoir T . Environmental factors as modulators of neurodegeneration: Insights from gene-environment interactions in Huntington’s disease. Neurosci Biobehav Rev. (2015) ;52: , 178–92. |
[3] | Hoffmann K , Sobol NA , Frederiksen KS , Beyer N , Vogel A , Vestergaard K , et al. Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: A randomized controlled trial. J Alzheimers Dis. (2016) ;50: (2):443–53. |
[4] | Baker LD , Frank LL , Foster-Schubert K , Green PS , Wilkinson CW , McTiernan A , et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch Neurol. (2010) ;67: (1):71–9. |
[5] | Latimer-Cheung AE , Pilutti LA , Hicks AL , Martin Ginis KA , Feuta AM , MacKibbon KA , Motl RW . Effects of exercise training on fitness, mobility, fatigue and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch Phys Med Rehabil. (2013) ;94: (9):1800–28. |
[6] | Paillard T , Rolland Y , de Souto Barreto P . Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: A narrative review. J Clin Neurol. (2015) ;11: (3):212–9. |
[7] | Frazzitta G , Bertotti G , Riboldazzi G , Turla M , Uccellini D , Boveri N , et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in Parkinsonian patients: A randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair. (2012) ;26: (2):144–50. |
[8] | Frazzitta G , Maestri R , Bertotti G , Riboldazzi G , Boveri N , Perini M , et al. Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. (2015) ;29: (2):123–31. |
[9] | Wheelock VL , Tempkin T , Marder K , Nance M , Myers RH , Zhao H , et al. Predictors of nursing home placement in Huntington disease. Neurology. (2003) ;60: (6):998–1001. |
[10] | Afram B , Stephan A , Verbeek H , Bleijlevens MHC , Suhonen R , Sutcliffe C , et al. Reasons for institutionalization of people with dementia: Informal caregiver reports from 8 European countries. J Am Med Dir Assoc. (2014) ;15: (2):108–16. |
[11] | Bilney B , Morris ME , Perry A . Effectiveness of physiotherapy, occupational therapy, and speech pathology for people with Huntington’s disease: A systematic review. Neurorehabilitation Neural Repair. (2003) ;17: (1):12–24. |
[12] | Zinzi P , Salmaso D , De Grandis R , Graziani G , Maceroni S , Bentivoglio A , et al. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: A pilot study. ClinRehabil. (2007) ;21: (7):603–13. |
[13] | Khalil H , Quinn L , van Deursen R , Dawes H , Playle R , Rosser A , et al. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin Rehabil. (2013) ;27: (7):646–58. |
[14] | Busse M , Quinn L , Debono K , Jones K , Collett J , Playle R , et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther. (2013) ;37: (4):149–58. |
[15] | Kloos AD , Fritz NE , Kostyk SK , Young GS , Kegelmeyer DA . Video game play (Dance Dance Revolution) as a potential exercise therapy in Huntington’s disease: A controlled clinical trial. Clin Rehabil. (2013) ;27: (11):972–82. |
[16] | Piira A , van Walsem MR , Mikalsen G , Nilsen KH , Knutsen S , Frich JC . Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLoS Curr. (2013) , Sep 20;5. |
[17] | Thompson JA , Cruickshank TM , Penailillo LE , Lee JW , Newton RU , Barker RA , et al. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: A pilot study. Eur J Neurol. (2013) ;20: (9):1325–9. |
[18] | Quinn L , Hamana K , Kelson M , Dawes H , Collett J , Townson J , et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat Disord. (2016) ;31: , 46–52. |
[19] | Wallace M , Downing N , Brogue R , Williams J , Paulsen J . Non-pharmacologic health-related behaviors associated with disease progression in Huntington disease: A systematic review protocol [Internet]. Crd.york.ac.uk 2014 [cited 19 April 2017] Available from https//www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013001 |
[20] | Quinn L , Busse M , on behalf of the Physiotherapy Working Group of the European Huntington Disease Network. Physiotherapy Guidance Document. European Huntington’s Disease Network; (2010) . |
[21] | Quinn L , Busse M . Development of physiotherapy guidance and treatment-based classifications for people with Huntington’s disease. Neurodegener Dis Manag. (2012) ;2: (1):11–9. |
[22] | Quinn L , Busse M , Carrier J , Fritz N , Harden J , Hartel L , et al. Physical therapy and exercise interventions in Huntington’s disease. JBI Database Syst Rev Implement Reports. (2017) ;15: (7):1783–99. |
[23] | Quinn L , Debono K , Dawes H , Rosser AE , Nemeth AH , Rickards H , et al. Task-specific training in Huntington disease: A randomized controlled feasibility trial. Phys Ther. (2014) ;94: (11):1555–68. |
[24] | Bohlen S , Ekwall C , Hellström K , Vesterlin H , Björnefur M , Wiklund L , et al. Physical therapy in Huntington’s disease–toward objective assessments? Eur J Neurol. (2013) ;20: (2):389–93. |
[25] | Mirek E , Filip M , Banaszkiewicz K , Rudzińska M , Szymura J , Pasiut S , et al. The effects of physiotherapy with PNF concept on gait and balance of patients with Huntington’s disease - pilot study. Neurol Neurochir Pol. (2015) ;49: (6):354–7. |
[26] | Reyes A , Cruickshank T , Nosaka K , Ziman M . Respiratory muscle training on pulmonary and swallowing function in patients with Huntington’s disease: A pilot randomised controlled trial. Clin Rehabil. (2015) ;29: (10):961–73. |
[27] | Piira A , vanWalsem M , Mikalsen G , Øie L , Frich J , Knutsen S . Effects of a two-year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: A prospective intervention study. PLOS Curr Huntingt Dis. (2014) ;1. |
[28] | Clark D , Danzl MM , Ulanowski E . Development of a community-based exercise program for people diagnosed and at-risk for Huntington’s disease: A clinical report. Physiother Theory Pract. (2016) ;3985: (April):1–8. |
[29] | Dawes H , Collett J , Debono K , Quinn L , Jones K , Kelson M , et al. Exercise testing and training in people with Huntington’s disease. Clin Rehabil. (2015) ;29: (2):196–206. |
[30] | Jones U , Busse M , Enright S , Rosser AE , Bates G , Ross C , et al. Respiratory decline is integral to disease progression in Huntington’s disease. Eur Respir J. (2016) ;6: (10):766–73. |
[31] | Cruickshank TM , Thompson JA , Domínguez DJF , Reyes AP , Bynevelt M , Georgiou-Karistianis N , et al. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain Behav. (2015) ;5: (2):n/a-n/a. |
[32] | Ciancarelli I , Tozzi Ciancarelli MG , Carolei A . Effectiveness of intensive neurorehabilitation in patients with Huntington’s disease. Eur J Phys Rehabil Med. (2013) ;49: (2):189–95. |
[33] | Frich JC , Røthing M , Berge AR . Participants’, caregivers’, and professionals’ experiences with a group-based rehabilitation program for Huntington’s disease: A qualitative study. BMC Health Serv Res. (2014) ;14: (1):395. |
[34] | Khalil H , Quinn L , van Deursen R , Martin R , Rosser A , Busse M . Adherence to use of a home-based exercise DVD in people with Huntington disease: Participants’ perspectives. Phys Ther. (2012) ;12: (1):69–82. |
[35] | Zinzi P , Salmaso D , Frontali M , Jacopini G . Patient’s and caregivers’ perspectives: Assessing an intensive rehabilitation programme and outcomes in Huntington’s disease. J Public Health (Bangkok). (2009) ;17: (5):331–8. |
[36] | Eldridge SM , Chan CL , Campbell MJ , Bond CM , Hopewell S , Thabane L , et al. CONSORT statement: Extension to randomised pilot and feasibility trials. BMJ. (2016) ;355. |
[37] | Fritz NE , Busse M , Jones K , Khalil H , Quinn L , Members of the Physiotherapy Working Group of the European Huntington’s Disease Network. A classification system to guide physical therapy management in Huntington disease. J Neurol Phys Ther. (2017) ;41: (3):156–63. |
[38] | Quinn L , Busse M , Khalil H , Richardson S , Rosser A , Morris H . Client and therapist views on exercise programmes for early-mid stage Parkinson’s disease and Huntington’s disease. Disabil Rehabil. (2010) ;32: (11):917–28. |
[39] | Parmar MKB , Sydes MR , Morris TP . How do you design randomised trials for smaller populations? A framework. BMC Med. (2016) ;14: (1):183. |
[40] | Gagne JJ , Thompson L , O’Keefe K , Kesselheim AS . Innovative research methods for studying treatments for rare diseases: Methodological review. BMJ. (2014) ;349: :g6802. |
[41] | Hilgers R-D , Roes K , Stallard N , IDeAl, Asterix and InSPiRe project groups. Directions for new developments on statistical design and analysis of small population group trials. Orphanet J Rare Dis. (2016) ;11: (1):78. |
[42] | Tudur Smith C , Williamson PR , Beresford MW . Methodology of clinical trials for rare diseases. Best Pract Res Clin Rheumatol. (2014) ;28: (2):247–62. |
[43] | Kloos A , Kegelmeyer D , Kostyk S . The effects of assistive devices on gait measures in Huntington’s disease. PLoS One. (2012) ;7: (2):e30903. |




