Bacterial production and purification of immunoreactive paraneoplastic neurological syndrome autoantigen Ma2
Abstract
Paraneoplastic neurological syndromes (PNS) are caused by an immune response against neuronal proteins upon their ectopical expression in tumor cells. Ma2 belongs to the protein family of paraneoplastic Ma antigens (PNMA). Detection of Ma2-specific autoantibodies is relevant for diagnostics of anti-Ma2 PNS and an underlying tumor such as germ testicular cancer, small cell lung cancer or breast cancer. Thus, early tumor treatment should improve the outcome for PNS therapy either.
Dot blot immunoassay based on recombinantly expressed and purified autoantigens could offer a sensitive method for identification of paraneoplastic autoantibodies from sera of PNS patients. Here we present purification with IMAC and FPLC of human Ma2 autoantigen upon its recombinant expression in E.coli. Furthermore, we provide evidence that dot blot immunoassays with purified Ma2 autoantigen can be used for detection of Ma2-specific autoantibodies from sera of PNS patients.
1Introduction
Paraneoplastic syndromes (PNS) are clinically defined by the appearance of symptoms indirectly caused by an underlying tumor, but those symptoms are not dependent on the local presence of the tumor in the affected tissue or organ. PNS typically encompass certain cancers characterized by tumor cell secretion of (hormone) peptides or autoimmune reactions directed against tumor (neo)antigens and neurological structures of the host [1]. The latter is also known as neurological PNS. In many cases, autoantibody formation and subsequent neurological symptoms such as encephalitis take place before clinical onset of the tumor disease. Thus, detection of autoantibodies is important for both differential diagnostics of the neurological syndrome and for early tumor diagnosis.
Ma2 belongs to the protein family of paraneoplastic Ma antigens (PNMA) with to date five identified members (PNMA1-5). Ma2 (PNMA2) has a molecular size of 41.5 kDa and is selectively expressed in neurons. More precisely, it has been described that Ma2 shows nuclear and cytoplasmic distribution in neurons of the brain, spinal cord, dorsal root ganglia, intestinal autonomic neurons and adrenal medullary ganglia [2]. PNS auto antigen Ma2 is expressed by most small intestine neuroendocrine tumors, whereby about 50% of those tumor patients develop anti-Ma2 autoantibodies [3]. Small intestine neuroendocrine tumors have a high risk for metastases and a poor prognosis [4]. Anti-Ma2 encephalitis can also be found with testicular germ cell tumors, predominantly in men younger than 50 years [5]. Tumor sizes are often microscopically small and thus difficult to detect. The biological function of Ma proteins is not completely known, but it has been identified that these proteins exhibit a homology to the recently discovered MAP-1 protein. Therefore, Ma proteins might function in promoting apoptosis [6, 7]. Autoimmunity to Ma2 antigen is predominantly associated with limbic and brain stem encephalitis [2]. Both associated indispositions can appear separately or in parallel. About 40% of the patients with anti-Ma2 auto antibodies do also develop anti-Ma1 auto antibodies [8].
In general, antineuronal antibodies against Ma proteins are of increasing clinical relevance because of their predictive value concerning an associated neoplasia [9]. Moreover, such antineuronal antibodies are considered as early diagnostic tumor marker [10] which can be used in standardised tests to detect a tumor before its clinical manifestation [11]. In 50–60% of cases, antineuronal antibodies are detectable in serum as well as in the liquor [12, 13]. Concerning PNS, the diagnostic sensitivity of antineuronal antibodies was reported to be at 50–60% while the specificity was found to be at 95–98% [14, 15]. Hence, verification of autoantibodies in a PNS patient almost counts as evidence for the existence of a neoplastic disease, even when the tumor is not discovered yet.
2Material and methods
2.1Ma2 cDNA cloning
Ma2 (PNMA2) cDNA (NCBI: NM_007257) obtained from Labomics SA, Nivelles, Belgium, was cloned into E.coli T7 expression vector pPSG to obtain pPSG-Ma2-IBA33 (C-terminal His-tag) and pPSG- Ma2-IBA35 (N-terminal His-tag), respectively.
2.2Protein expression
Plasmids were transformed into chemocompetent E.coli BL21 (DE3) (Agilent Technologies, Santa Clara, USA). One single colony of transformed bacteria was transferred into 50 ml 2x YT-Medium (Sigma Aldrich Chemie GmbH, Taufkirchen, Germany) with 100μg/ml carbenicillin (AppliChem, Darmstadt, Germany). Recombinant bacteria were pre-cultivated at 37°C and 220 rpm to an OD600 of maximal 3.0. This pre-culture was diluted 1 : 100 in 600 ml 2xYT-Medium containing carbenicillin. Main culture was cultivated at 37°C and 220 rpm in a rotation shaker until an OD600 of 0.6–0.8. Then, culture was chilled on ice to stop cell proliferation. Protein production was induced by adding 0.5 mM IPTG (Roth, Karlsruhe, Germany). Incubation was carried out at 18°C and 220 rpm for 16–20 h. Cell pellets were harvested by centrifugation at 4’000xg for 15 min at 4°C and stored at –20°C or used directly. Cell pellets were resuspended in 20 ml ice-cold lysis buffer (10 mM Na2HPO4, 10 mM NaH2PO4, 500 mM NaCl, 20 mM imidazole [Roth], 10 mM MgCl2, 500μg DNAse [Fermentas, St. Leon Roth, Germany], 2 mg/ml lysozyme [Sigma], 1 mM PMSF [Sigma]). Cell disruption was carried out by using French Press Cell SimAminco (Urbana, USA) for 3 times followed by centrifugation at 15,000xg and 4°C for 20 min. Supernatant (lysate) was purified by FPLC.
2.3Purification using IMAC and FPLC
Proteins from bacterial cell extracts were mixed with PMSF (1 mM) and diluted to a final volume of 50 ml with binding buffer (10 mM Na2HPO4; 10 mM NaH2PO4; 500 mM NaCl; 20 mM imidazole [pH 7.4]) followed by loading onto a HisTrap™ FF crude column (GE Healthcare Europe GmbH, Freiburg, Germany) as an IMAC stationary phase. Proteins were separated by ÄKTA™ Laboratory-scale FLPC Chromatography Systems (GE Healthcare) using a flow velocity of 1 ml/min. Elution was carried out by rinsing the column with elution buffer (10 mM Na2HPO4; 10 mM NaH2PO4; 500 mM NaCl; 500 mM imidazole [pH 7.4]) with a constant flow velocity of 1 ml/min.
2.4Dialysis
To remove imidazole from FPLC-purified proteins, dialysis was initially performed at 100 rpm and room temperature for 1 h using a volume of 700 ml-1 L dialysis buffer (300 mM NaCl; 25 mM Tris/HCl [pH 7.4]) and continued for 12 h and 4°C.
2.5Protein quantification
BCA assay was performed using PierceTM BCA assay kit according to the informations of the supplier (Thermo Fisher Scientific, Waltham, USA). Protein detection was performed using a FLUOstar OMEGA Microtiter plate reader (BMG Labtech GmbH, Ortenberg, Germany) and data were evaluation by integrated MARS Data Analysis Software.
2.6SDS-PAGE and Coomassie staining
Following discontinuous SDS-PAGE [16], gels were stained with Coomassie solution (10% ammonium sulfate, 0.1% Coomassie brilliant blue G-250 [Roth], 3% ortho-phosphoric acid, 20% ethanol) or used for Western blotting.
2.7Western blotting and dot blot assay
Western blotting onto PVDF membrane (Roth) was performed at 0.8 mA/cm2 for 45 min at room temperature by using semidry blotting system V20-SDB (VWR, Darmstadt, Germany). PVDF membranes were incubated in blocking solution (5% dry milk, 0.1% Tween-20 in 1x Tris-buffered saline [TBS: 20 mM Tris-HCl, 150 mM NaCl, pH 7.5]) for 1 h at room temperature. Thereafter, membranes were incubated with primary antibody (Anti-PNMA2, Abcam, Cambridge, UK) diluted 1 : 3,000 in blocking solution for overnight at 4°C. After washing with TBS containing 0.1% Tween-20 (TBS-Tween), protein detection was done by incubation with secondary peroxidase-coupled antibody (Jackson ImmunoResearch Europe, Suffolk, UK), diluted 1 : 10,000 for 1 h at room temperature followed by incubation with ECL solution (GE Healthcare). Evaluation was performed with Lumi Imager F1 (Roche Diagnostics Deutschland GmbH, Mannheim, Germany) and data were documented by LumiAnalyst 3.1 Software.
For dot blot assays, purified recombinant Ma2 protein (1μg or 0.1μg) diluted in 0.1% BSA/TBS-Tween was directly transferred onto nitrocellulose membranes. After blocking incubation with 5% BSA/TBS-Tween for 60 min, membranes were incubated for 60 min at room temperature with PNS patient sera diluted 1 : 1,000 in 0.1% BSA/TBS-Tween. Secondary antibody reaction was performed with HRP-conjugated anti-human IgG (Seramun Diagnostica GmbH, Heidesee) diluted 1 : 5,000 in 0.1% BSA/TBS-Tween for 60 min at room temperature followed by staining with tetramethylbenzidine (TMB, Seramun Diagnostica GmbH) as chromogenic substrate.
3Results
We aimed to recombinantly express and purify PNS autoantigen Ma2 as a tool for diagnostic testing of anti-Ma2 encephalitis. Expression of Ma2 was performed in E.coli BL21 (DE3) upon transformation with T7 expression vector pPSG-Ma2-IBA35. Recombinant Ma2 was purified with IMAC and FPLC as described in Materials and Methods. Starting with 600 ml bacterial culture, a final volume of 2 ml purified Ma2 protein with an average concentration of about 400μg/ml was obtained. Figure 1 shows that we could highly purify a protein with an estimated mass of about 45 kDa which appears slightly more than the expected mass of Ma2. This increase of protein mass could be due to posttranslational modifications, but those have not been described yet. This protein shows strong immunoreactivity with a commercial antibody against Ma2 (Fig. 2). A preliminary experiment showed that 1μg of purified Ma2 antigen was sufficient for autoantibody detection in patient sera using dot blot assay (Fig. 3). Based on this, five different sera from PNS patients, previously tested to be seropositive for anti-Ma2 autoantbodies, were analyzed by dot blot immunoassays on purified Ma2 protein. As negative controls we used sera from healthy blood donors and a serum from a patient with systemic lupus erythematosus (SLE). Table 1 demonstrates that all tested PNS sera were positive in contrast to all negative controls. This indicates that E.coli-expressed Ma2 protein can be used for immune diagnostics of anti-Ma2 encephalitis.
Fig.1
SDS-PAGE analysis of purified recombinant Ma2. E.coli BL21 (DE3) bacteria were transformed with pPSG-Ma2 expression plasmid for T7 promoter-controlled recombinant protein production. Induced by IPTG, protein expression was performed for overnight at 18°C. Proteins were purified with IMAC and FPLC as described in Materials and Methods. Samples were separated by SDS-PAGE and visualized by Coomassie silver blue staining.
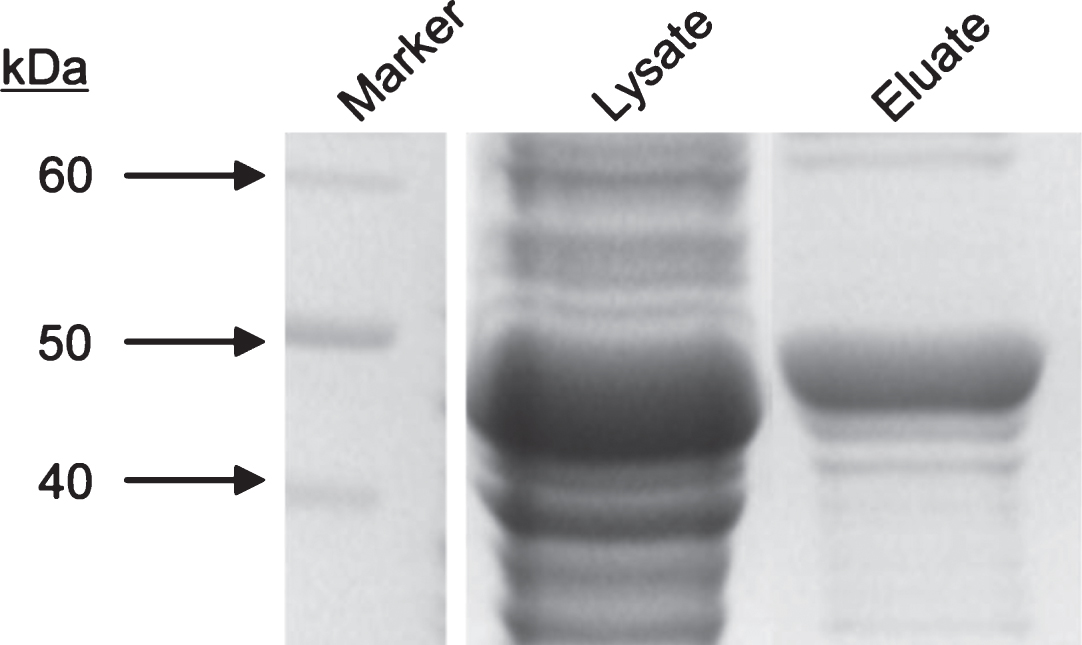
Fig.2
Western blot analysis of purified recombinant Ma2. E.coli BL21 (DE3) bacteria were transformed with pPSG-Ma2 expression plasmid for T7 promoter-controlled recombinant protein production. Protein expression was induced by IPTG for overnight at 18°C. Protein extracts were prepared, separated by SDS-PAGE and further processed for Western blot analysis. The membrane was incubated with primary anti-PNMA2 antibody and HRP-conjugated secondary antibodies. Western blot was developed using enhanced chemiluminescence (ECL), exposure time was 30 sec. Housekeeping gene product glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as loading control. Magic Mark Western Protein Standard was applied as size marker.
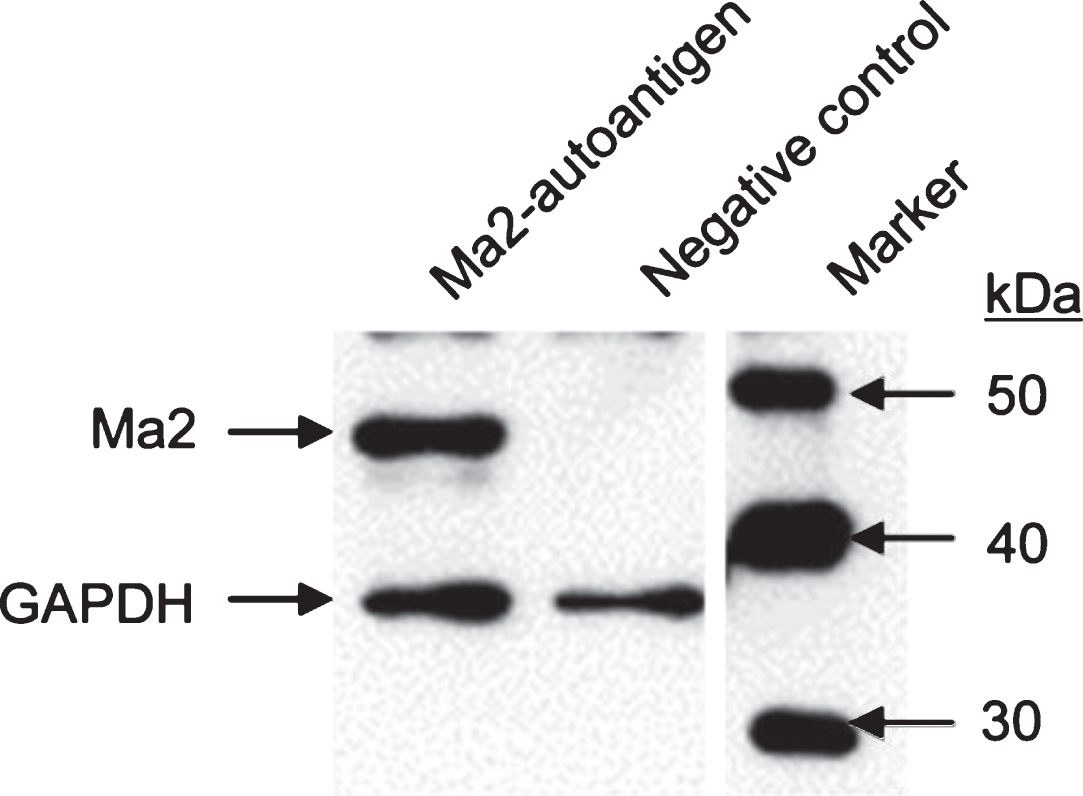
Fig.3
Experimental set-up of dot blot immunoassay for anti-Ma2 autoantibody detection. Purified Ma2 autoantigen (1μg) or 1μl of diluted human serum, respectively, were transferred onto a nitrocellulose membrane and incubated with human sera from PNS patients or control groups as indicated. Secondary antibody incubation was performed with HRP-conjugated anti-human IgG followed by staining with tetramethylbenzidine (TMB).
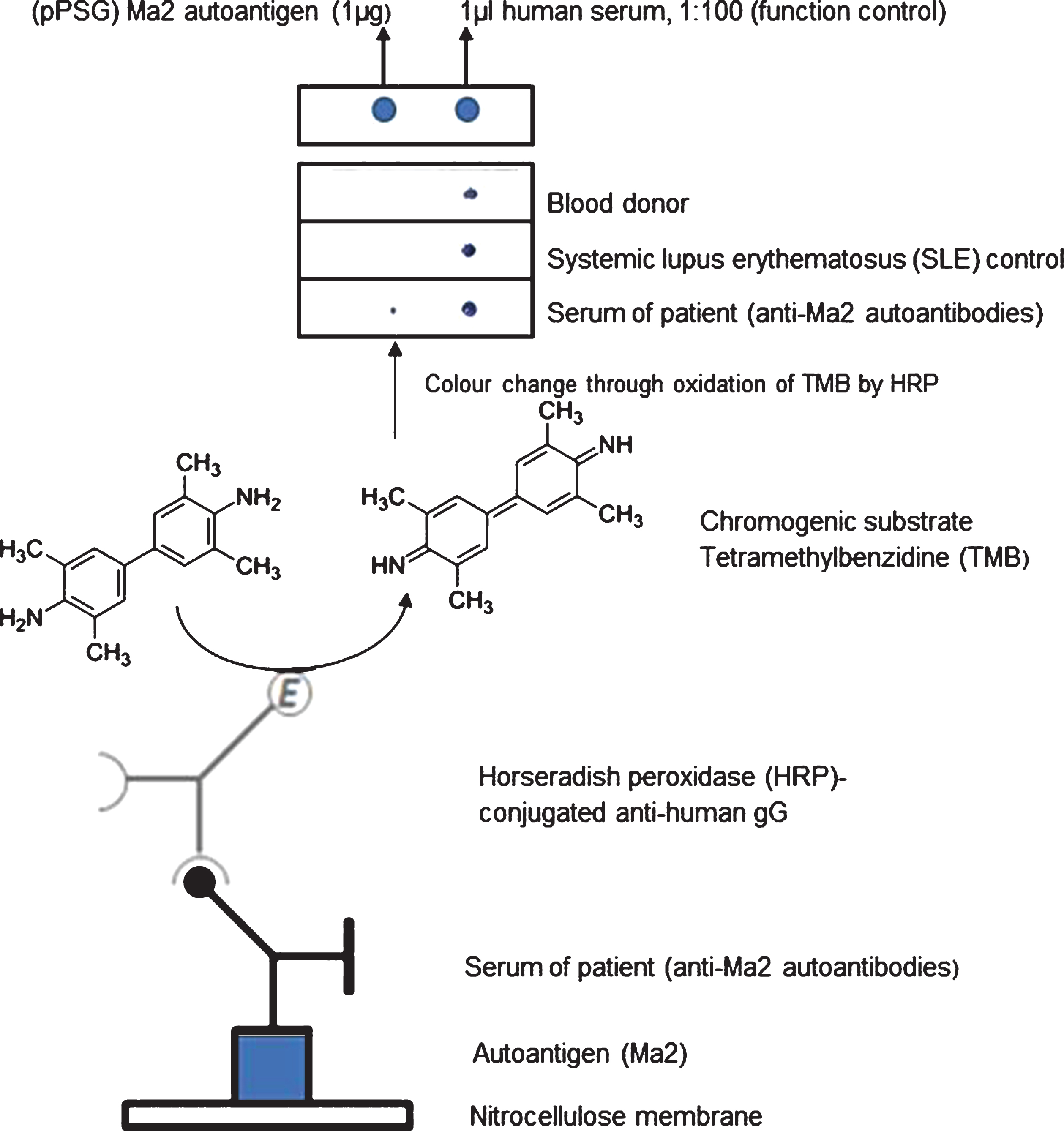
Table 1
Evaluation of recombinant Ma2 seroreactivity
| Tested sera | Positive | Negative |
| PNS patient sera (anti-Ma2 autoantibodies) | 5 | 0 |
| Blood donor | 0 | 6 |
| SLE control | 0 | 6 |
Recombinant Ma2 protein (1μg or 0.1μg) was spotted onto nitrocellulose membranes. Human serum (1μl diluted 1 : 100 in 0.1% BSA/TBS-Tween) was used as positive control for anti-human IgG binding. Membranes were incubated with sera from anti-Ma2-positive PNS patients and evaluated with HRP-conjugated anti-human IgG. Five out of five tested sera did recognize recombinant Ma2 protein while none of the samples from healthy blood donors or from the SLE patient showed seroreactivity with Ma2.
4Discussion
PNS can be initiated by an immune response against a neuronal protein such as Ma2 upon its ectopical expression in tumor cells. To date six different onconeuronal proteins, i.e. HuD, CDR62 (Yo), amphiphysin, CRMP-5 (CV2), NOVA-1 (Ri) and Ma2, respectively, are considered to be the most relevant ones in PNS [17].
Thus, proof of PNS-associated autoantibodies could allow for pre-symptomatic detection of a dormant germ cell tumor or carcinoma in situ. It can be expected that early treatment of that tumor can also improve the outcome of PNS therapy.
It is thought that anti-Ma2 antibodies are part of an effective anti-tumor immune response, which would be an explanation for the very small tumor sizes [18]. In men younger than 50 years, anti-Ma2 encephalitis is almost always associated with testicular germ cell tumors that can remain undiscovered for years [19]. In addition, anti-Ma2 antibodies can also be found in patients with small intestine neuroendocrine tumors, small cell lung cancer, breast cancer, Hodgkin’s lymphoma and thymoma [3, 19, 20].
For better diagnosis of PNS as well as for early diagnosis of the associated tumors we developed a reliable procedure for production of recombinant Ma2 protein to be used in dot blot immunoassays which are a cost-efficient multiplex-compatible diagnostic system. We could show that His-tag-based protein purification using a HisTrap™ FF crude column and FPLC is a useful method for purification of recombinant Ma2 antigens from E.coli lysates. Albeit our data with a restricted number of patient sera indicate that purified recombinant Ma2 could be used for sensitive and specific autoantibody detection in PNS patients, further studies with increased sample numbers are required. In addition, we will compare the performance of anti-Ma2 dot blot immunoassays with cell-based assays such as previously developed for diagnostics of Myasthenia gravis.
Acknowledgments
This work was funded by Zentrales Innovationsprogramm Mittelstand (ZIM) project KF2088016.
References
[1] | Pelosof LC , Gerber DE . Paraneoplastic syndromes: An approach to diagnosis and treatment. Mayo Clin Proc (2010) ;85: (9):838–54. |
[2] | Voltz R , Gultekin SH , Rosenfeld MR , Gerstner E , Eichen J , Posner JB , et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med (1999) ;340: (23):1788–95. |
[3] | Cui T , Hurtig M , Elgue G , Li SC , Veronesi G , Essaghir A , et al. Paraneoplastic antigen Ma2 autoantibodies as specific blood biomarkers for detection of early recurrence of small intestine neuroendocrine tumors. PLoS One (2010) ;5: (12):e16010. |
[4] | Hughes MS , Azoury SC , Assadipour Y , Straughan DM , Trivedi AN , Lim RM , et al. Prospective evaluation and treatment of familial carcinoid small intestine neuroendocrine tumors (SI-NETs). Surgery (2016) ;159: (1):350–6. |
[5] | Mathew RM , Vandenberghe R , Garcia-Merino A , Yamamoto T , Landolfi JC , Rosenfeld MR , et al. Orchiectomy for suspected microscopic tumor in patients with anti-Ma2-associated encephalitis. Neurology (2007) ;68: (12):900–5. |
[6] | Lee YH , Pang SW , Poh CL , Tan KO . Distinct functional domains of PNMA5 mediate protein-protein interaction, nuclear localization, and apoptosis signaling in human cancer cells. J Cancer Res Clin Oncol (2016) ;142: (9):1967–77. |
[7] | Lee YH , Pang SW , Tan KO . PNMA2 mediates heterodimeric interactions and antagonizes chemo-sensitizing activities mediated by members of PNMA family. Biochem Biophys Res Commun (2016) ;473: (1):224–9. |
[8] | Yamamoto T , Tsuji S . Anti-Ma2-associated encephalitis and paraneoplastic limbic encephalitis. Brain Nerve (2010) ;62: (8):838–51. |
[9] | Kaiser R . Paraneoplastic neurologic syndromes. Diagnostic and pathogenetic significance of autoantibodies. Nervenarzt (1999) ;70: (8):688–701. |
[10] | Verschuuren J , Chuang L , Rosenblum MK , Lieberman F , Pryor A , Posner JB , et al. Inflammatory infiltrates and complete absence of Purkinje cells in anti-Yo-associated paraneoplastic cerebellar degeneration. Acta Neuropathol (1996) ;91: (5):519–25. |
[11] | Graus F , Delattre JY , Antoine JC , Dalmau J , Giometto B , Grisold W , et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry (2004) ;75: (8):1135–40. |
[12] | Moll JW , Antoine JC , Brashear HR , Delattre J , Drlicek M , Dropcho EJ , et al. Guidelines on the detection of paraneoplastic anti-neuronal-specific antibodies: Report from the Workshop to the Fourth Meeting of the International Society of Neuro-Immunology on paraneoplastic neurological disease, held October 22-23, in Rotterdam, The Netherlands. Neurology (1995) ;45: (10):1937–41. |
[13] | Antoine JC , Mosnier JF , Absi L , Convers P , Honnorat J , Michel D . Carcinoma associated paraneoplastic peripheral neuropathies in patients with and without anti-onconeural antibodies. J Neurol Neurosurg Psychiatry (1999) ;67: (1):7–14. |
[14] | Blaes F . Immunotherapeutic approaches to paraneoplastic neurological disorders. Expert Opin Biol Ther (2002) ;2: (4):419–30. |
[15] | Moll JW , Henzen-Logmans SC , Splinter TA , van der Burg ME , Vecht CJ . Diagnostic value of anti-neuronal antibodies for paraneoplastic disorders of the nervous system. J Neurol Neurosurg Psychiatry (1990) ;53: (11):940–3. |
[16] | Laemmli UK . Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (1970) ;227: (5259):680–5. |
[17] | Maat P , Brouwer E , Hulsenboom E , VanDuijn M , Schreurs MW , Hooijkaas H , et al. Multiplex serology of paraneoplastic antineuronal antibodies. J Immunol Methods (2013) ;391: (1-2):125–32. |
[18] | Hoffmann LA , Jarius S , Pellkofer HL , Schueller M , Krumbholz M , Koenig F , et al. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J Neurol Neurosurg Psychiatry (2008) ;79: (7):767–73. |
[19] | Morita A , Kamei S . Limbic encephalitis with antibodies against intracellular antigens. Brain Nerve (2010) ;62: (4):347–55. |
[20] | Sahashi K , Sakai K , Mano K , Hirose G . Anti-Ma2 antibody related paraneoplastic limbic/brain stem encephalitis associated with breast cancer expressing Ma1, Ma2, and Ma3 mRNAs. J Neurol Neurosurg Psychiatry (2003) ;74: (9):1332–5. |
[21] | George S , Noack M , Vanek M , Rentzsch J , Rober N , Conrad K , et al. Expression of nicotinic acetylcholine receptor subunits in HEp-2 cells for immunodetection of autoantibody specificities in sera from Myasthenia gravis patients. Clin Hemorheol Microcirc (2015) ;61: (2):385–96. |
[22] | George S , Georgi M , Roggenbuck D , Conrad K , Kupper JH . A strategy for cell-based multiplex diagnostics of Myasthenia gravis and autoimmune encephalitis by modifying the subcellular localization of cell membrane autoantigens. Clin Hemorheol Microcirc (2014) ;58: (1):211–28. |
[23] | George S , Paulick S , Knutter I , Rober N , Hiemann R , Roggenbuck D , et al. Stable expression of human muscle-specific kinase in HEp-2 M4 cells for automatic immunofluorescence diagnostics of myasthenia gravis. PLoS One (2014) ;9: (1):e83924. |



