Maqui, Calafate, and Blueberry fruits extracts treatments suppress the pathogenic interaction amongst human adipocytes and macrophages
Abstract
BACKGROUND:
Obesity occurs due to a positive energy imbalance, leading to the expansion of adipose tissue. This phenomenon triggers a chronic low-grade inflammatory state, which is associated with comorbidities development. It is, therefore, of great interest to investigate new counteracting nutritional strategies. In this regard, polyphenol-rich Chilean native fruits, Aristotelia chilensis (Maqui) and Berberis microphylla (Calafate), and also the non-Chilean Vaccinium corymbosum (Blueberry), have been associated with antioxidant and anti-inflammatory features.
OBJECTIVE:
To evaluate Maqui, Calafate, and Blueberry aqueous extracts treatments on the pathogenic response of human activated macrophages and visceral adipocytes.
METHODS:
THP-1 monocyte human cell line and differentiated human visceral preadipocytes were activated (with lipopolysaccharide and TNF-α, for 48 and 24 h, respectively), and treated with the aqueous extracts. Inflammation and oxidative stress markers were assessed.
RESULTS:
Lower NO and IL-6 secretion, and inhibited apoptosis in activated macrophages, were observed. Also, decreased gene expression of MCP-1 and secretion of IL-6, inhibited apoptosis, and increased levels of GSH in activated adipocytes were detected.
CONCLUSIONS:
Maqui, Calafate, and Blueberry extracts showed anti-inflammatory and antioxidant responses in human macrophages and adipocytes.
1.Introduction
Obesity is one of the major concerns for public health systems around the globe. This pathology of multifactorial cause is characterized by an excessive accumulation of body fat, in detriment of population health [1]. As a consequence, there is an increase of the triglyceride deposits in white adipose tissues (WAT), mediated by hyperplasia and hypertrophy of adipocytes [2]. Additionally, the WAT increases lead to increased macrophage infiltration, angiogenesis, and extracellular matrix overproduction, all of which are determinant in tissue remodeling [3]. Also, this tissue expansion promotes endoplasmic reticulum stress, cell hypoxia, and oxidative stress by overproduction of reactive oxygen species (ROS) [4]. Alternatively, the endocrine function of WAT is modified by macrophage infiltration, especially by the ones that exhibit an M1 polarization (cytotoxic and pro-inflammatory phenotype), which is a common trait in obesity [5]. These changes in the expression and secretion profiles of adipokines in WAT lead to an increase of the production of inflammatory factors, such as tumor necrosis factor-alpha (TNF-α), IL-6, nitric oxide (NO) and monocyte chemoattractant protein 1 (MCP-1), and a decrease of anti-inflammatory factors like adiponectin and IL-10 [6, 7]. In this way, the macrophage-adipocyte interaction causes an inflammatory state, which enhances the concomitant hypertrophy, lipolysis and inhibits adipogenesis in WAT, setting the distinctive low grade, chronic pro-inflammatory state in obesity that plays a pivotal role in insulin resistance development [8, 9]. Oxidative stress and inflammation are critical factors in the development of metabolic diseases. Thus, new therapeutic strategies by the use of anti-inflammatory agents could prevent adverse obesity-associated consequences, like insulin resistance. In this context, it has been described that polyphenols, mainly anthocyanins, exhibit high antioxidant capacity, and cardioprotective, anti-cancer, anti-inflammatory and anti-diabetic effects as well [10–13]. Additionally, it is documented that the anti-inflammatory effect is caused, e.g., through decreased NO production and inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) expression in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages [14]. Also, inhibition of adipogenesis and lipidic accumulation in 3T3-L1 adipocytes in vitro have been reported [14]. Some polyphenol-rich food matrices have been shown to present these features. For example, manuka honey has been observed to block LPS stimulation over inflammation (TNF-α, IL-1β, and IL-6, related to NF-κB activation) and oxidative stress-related (NO and ROS, related to inhibition of antioxidant enzymes and Keap1-Nrf2 signaling pathway) markers, on RAW264.7 cells [15]. Moreover, a strawberry extract has been shown to present important antioxidant properties on 3T3-L1 cells, by suppressing ROS production, decreasing lipoperoxidation, and inducing the activity of antioxidant enzymes [16].
On the other hand, native Chilean fruits, such as Maqui (MA, Aristotelia chilensis) and Calafate (CA, Berberis microphylla) contain high anthocyanin content [17]. Also, a widely revised specie, Blueberry (BL, Vaccinium corymbosum), it has been described to contain higher amounts of these compounds within its fruits [18]. Extracts of MA have been observed to present anti-inflammatory effects on human colorectal cancer cells by suppressing NO release and inhibition of iNOS, NF-κB, and COX-2 protein expression [19]. Moreover, in vitro studies in mice macrophage and adipocytes revealed that treatments with MA and CA extracts were able to block oxidative stress inflammation and insulin resistance development [20, 21]. Also, anthocyanin-enriched fractions from fermented blueberry beverages have shown similar results in the same in vitro model [22]. Aiming to replicate the latter results in an in vitro study with human cells, the objective of this work is to assess the inhibition of the inflammatory response mediated by the adipocyte-macrophage interaction in obesity and insulin-resistance development, by treatment with berry extracts.
2.Materials and methods
2.1.Fruit extracts preparation and characterization
Fruits of MA, CA, and BL were obtained from SAAUTCHILE (Valdivia, Chile). The extracts were obtained by methanol:water (1 : 1) extraction, followed by a concentration step in a rotary evaporator. Then, the rota-evaporated product was resuspended in water. The total polyphenol content of the extracts was measured by Folin-Ciocalteu colorimetric method [23] and expressed as gallic acid equivalents (GAE). Anthocyanin content was assessed by differential pH method [24]. Absorbance was recorded at 515 and 700 nm with pH 1.0 and 4.5 buffers (respectively), using a molar extinction coefficient of 26,900. Results were expressed as mg cyanidin-3-glucoside equivalents (C3GE). Finally, total antioxidant capacity was determined by the ferric reducing ability of plasma (FRAP) [25], at 593 nm. Results are expressed as mmol Fe +2.
2.2.Human macrophage cell line culture and treatments
THP-1 monocyte human cell line was obtained from the Immunology Department of the Biomedical Sciences (ICBM, Universidad de Chile, Santiago, Chile). Monocytes were cultured in RPMI 1640 media supplemented with fetal bovine serum (FBS) 10% v/v, penicillin (100 U/mL), and streptomycin (100 μg/mL) until reaching 1×106 cells per well. Monocyte differentiation and macrophage activation methods were based on the work of Harrison et al. and Wang et al. [26, 27]. Briefly, THP-1 cells were differentiated by incubation on 100 nM phorbol-12-myristate-13-acetate, at 37° C and 5% v/v CO2 for 48 hours. Differentiation efficiency was assessed by the adhesion of macrophages to the culture plate. Then, cells were pre-treated with 100 μM total polyphenols of each extract for 2 hours. This concentration was already tested before but on murine cells [20, 21]. The treatment with this concentration means the incorporation of (per ml): 0.99 mg (MA), 1.40 mg (CA), 1.53 mg (BL), of each extract. Finally, cells were treated with 5 μg/mL LPS for 48 hours. A no-LPS control was included. At the end of the treatment, cells were stored and processed for cytokine gene expression and caspase-3 activity assays. The culture media was stored and processed for assessment of NO release, cytokine secretion, and cell viability.
2.3.Human adipocyte cell line culture and treatments
Human visceral preadipocytes (Lonza, New Jersey, USA) were isolated from visceral adipose tissue from a 92 years old female donor, with a BMI of 19 kg/m2. Culture procedures were performed according to [28], and manufacturer indications. Pre-adipocytes were cultured in PBM-2 growth medium, supplemented with 10% v/v FBS, 2 mM L-glutamine and 37 ng/mL GA-1000 SingleQuots™, until 5×104 cells per well were reached. Preadipocyte differentiation was achieved by stimulation with differentiation medium, which contains PBM-2, PGM™ SingleQuots™, supplemented with indomethacin, 3-isobutyl-1-methylxanthine, dexamethasone, and h-insulin, using supplier-recommended concentrations. We differentiate adipocytes for 9 days, and then were pre-treated with 100 μM total polyphenols from each extract for 1 hour, and finally with 4 ng/mL of TNF-α for 24 hours. At the end of the treatment, cells were stored and processed for adipokine gene expression, glutathione levels, antioxidant enzymes, and caspase-3 activity assays. The culture media was stored and processed for assessment of adipokine secretion and cell viability.
2.4.Gene expression assays
Procedures were performed according to a previously published protocol [20]. Total RNA was obtained by using Trizol (Invitrogen, Paisley, UK) using manufacturer indications. The concentration of RNA (ng/ μL) was determined by 260 nm absorbance measurement in a NanoQuant, infinite M200PRO spectrophotometer (TECAN, Männedorf, Switzerland). Then, samples were treated with the DNA-Free™ Kit (Ambion, Austin, USA). cDNA was synthesized by reverse transcription with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, USA), by supplier specifications. Real-time PCR was performed in a Stratagene Mx3000P System (Agilent Technologies, Santa Clara, USA). RT-PCR reactions were made in duplicates following gene expression assays protocols from TaqMan ® probes (Applied Biosystems, Carlsbad, USA) (supplementary data: Table S1). Gene expression was normalized with β-actin expression, and the relative expression of each gene was determined by the 2–ΔΔCt method [29].
2.5.Cell viability and apoptosis assay
Cell viability was determined with lactate dehydrogenase activity (LDH), which is a cytotoxicity marker. Levels of LDH activity were measured by the LDH Cytotoxicity Assay kit (Cayman Chemical Company, Ann Arbor, USA), a colorimetric assay. Apoptosis was determined with a modified Caspase-3 fluorescence assay kit (Cayman Chemical Company, Ann Arbor, USA), following supplier instructions, and as we performed previously in our laboratory [20].
2.6.Determination of oxidative stress/status
Reduced glutathione (GSH), oxidized glutathione (GSSG) and GSSG/GSH ratio were determined using trypsinized cells washed with 5% sulfosalicylic acid. Total glutathione (TG), GSSG, and GSH were determined by Griffith kinetic method [30]. GSSG was measured after treatment with 4-vynilpyridine [31]. GSH was determined by the calculation of the difference between TG and GSSG.
2.7.Determination of antioxidant enzymes
The activity of superoxide dismutase (SOD) and catalase (CAT) antioxidant enzymes were determined using the colorimetric Superoxide Dismutase Assay and Catalase Assay kits, respectively, following manufacturer indications (Cayman Chemical Company, Michigan, USA).
2.8.Release of nitric oxide
The nitrite NO2– levels were determined through the Griess colorimetric assay, following supplier indications, and as previously performed in our laboratory [21]. 200 μL of 1 : 1 solution was loaded considering culture medium and Griess reagent (Sigma-Aldrich Chemical Co., San Luis, USA) at 4% w/v, and incubated for 15 minutes in the dark to read at 540 nm using spectrophotometry (NanoQuant, infinite M200PRO, TECAN).
2.9.Analysis of protein secretion of adipokines and cytokines: Multiplex assays
MCP-1, adiponectin and IL-6 secretion were determined using MILLIPLEX ® MAP Human Adipocyte Magnetic Bead Panel (Merck Millipore, Darmstadt, Germany), following supplier instructions and as previously performed by our group [20], using Luminex ® xMAP ® technology (Virology Department of Biomedical Sciences, ICBM, University of Chile).
2.10.Statistical analysis
Data are presented as means±SD. Differences were evaluated by one-way ANOVA, followed by Tukey posthoc test. All analyses were performed on Graphpad Prism 6.0 (GraphPad Software Inc., San Diego, USA).
3.Results and discussion
3.1.Berries extracts characterization
Total polyphenols concentrations were: MA: 1906.5±73.2; CA: 1344.2±10.5; BL: 1229.6±20.9 mg GAE/100 g of dry weight. The levels observed were lower than the ones observed in previous works [32–34]. This difference could be due to the use of a non-acidified extraction. Anthocyanin content was MA: 72.7±0.1; CA: 31.5±0.8; BL: 20.1±1.2 mg C3GE/100 g of dry weight. According to previous data, the total anthocyanin content of these fruits ranges between 0.122–0.87 g/100 g fresh weight [17]. Considering a 5-times concentration estimation from fresh to dry matter, our data are above 16× of this previous report. Again, this difference could be attributable to differences in the extraction procedure and the raw material source. Total antioxidant capacity was: MA: 38.9±1.7; CA: 11.7±1.8; BL: 5.9±0.1 mmol Fe +2/100 g dry weight. Lower values of FRAP has been reported before [35]. This difference could be attributable to the experimental procedures, e.g., extraction duration. Specific anthocyanins presented on all three extracts were determined in a previously published work by our group [20], and match with the data reported by others [36].
3.2.In vitro effect of berries extracts on LPS-activated human macrophages: Gene expression and protein secretion of inflammation markers
The NO release, corrected by cell viability, did not show any differences in CA nor BL treatments related to the active condition, but MA treatment did. MA was able to counteract LPS-induced NO release (Fig. 1A). When the effects of the fruits extracts over gene expression of inflammatory markers were assessed, it was not observed any change in any of the treatments (Fig. 1B and 1C), excepting IL-10 gene expression, which showed a reduction in the BL treatment in regards of the LPS-activated condition (Fig. 1D). Additionally, there was a significant increment in IL-6 secretion in LPS-activated condition, which was reversed by CA and BL treatments (Fig. 1E), achieving control-like levels.
Fig. 1.
Anti-inflammatory effects of berries extract on in vitro LPS-activated human macrophages. THP-1 monocyte human cells were differentiated and then pre-treated with 100 μM total polyphenols of each extract for 2 hours. Finally, they were treated with 5 μg/mL LPS for 48 hours. Cells and culture media were saved for further determinations. (A) Nitric oxide (NO) release, (B) TNF-α gene expression, (C) IL-6 gene expression, (D) IL-10 gene expression, and (E) IL-6 release, were assayed. Data (n = 5–6) were expressed as mean±SD and analyzed with one-way ANOVA followed by Tukey posthoc tests. NO, nitric oxide; C, control; LPS, lipopolysaccharide; MA, Maqui; CA, Calafate; BL, Blueberry. Different letters showed a statistical significance of at least p < 0.05.
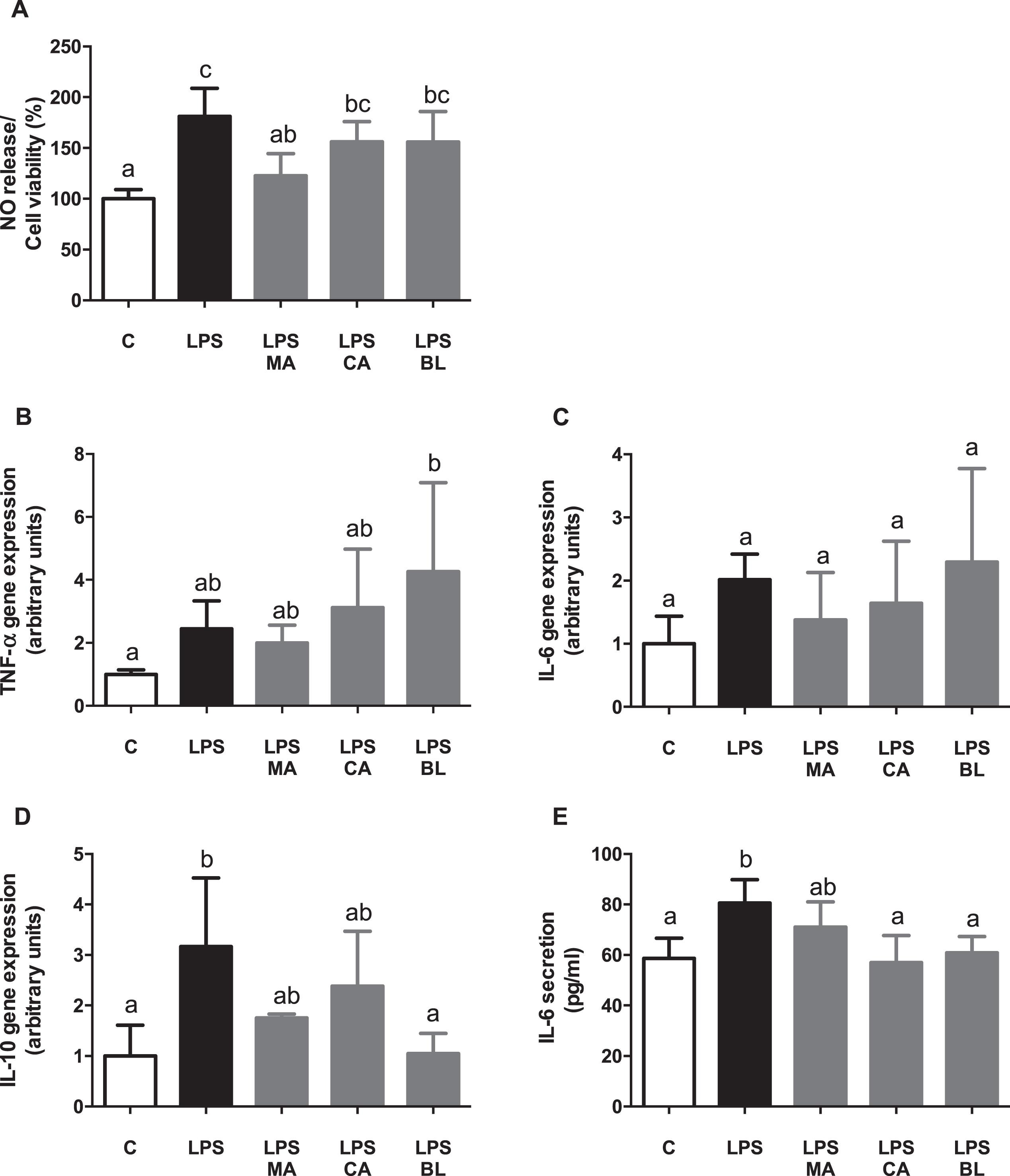
Thus, fruit extracts present an anti-inflammatory effect in LPS activated human macrophages in vitro. Similar anti-inflammatory effects were observed in previous studies using CA, MA, and BL extracts, with LPS activated RAW264.7 murine macrophages, mouse 3T3-L1 adipocyte conditioned media, and co-culture between these cells in vitro [21]. Moreover, macrophages can acquire different polarization phenotypes, each with a distinctive expression pattern of cytokines, surface markers, and metabolic enzymes, depending on the activating stimuli [5]. Healthy adipose tissue contains M2 macrophages, which secretes anti-inflammatory cytokines, such as IL-10 [37]. In the obesity state, when lipid accumulation occurs in the adipose tissue, macrophages switch their polarization pattern, from M2 to M1 types, in which pro-inflammatory cytokines are secreted, such as TNF-α, and IL-6. Also, it produces reactive nitrogen species, like NO, by the activation of iNOS [5].
Regarding analyzed cytokines, TNF-α gene expression did not present significant variations when control or pre-treatments with LPS activated conditioned media were compared. However, in CA or BL presence, an increase of its expression regarding control was observed, but displaying significant data dispersion, which hinders its analysis. It is proposed that TNF-α could be regulated at the post-transcriptional level [38], as a compensatory mechanism of endotoxemia [39]. However, this finding needs further research. Although there were no changes observed on IL-6 transcript levels, an increase in the secretory levels of IL-6 by treatment with LPS, which was counteracted by CA treatment, was detected, demonstrating an anti-inflammatory response.
Regarding IL-10, if the profile of M1 macrophages is considered, a decrease of its levels is expected, due to its anti-inflammatory action. However, the results showed a surprising LPS-induced augmented expression of IL-10, which was countered by BL presence, and partly by MA and CA. On this subject, an inflammatory effect of IL-10 in humans, induced by endotoxemia [40, 41], and a switch in macrophage polarization produced by high fat diet-induced inflammation in mice [42], were reported. This polarization is an inflammatory M2b type, different from the classic M1 activation, which presents high IL-10 secretion [43]. The latter could explain the presented results, as the switch of macrophage polarization to M2b type releases pro-inflammatory IL-10. According to the above, the BL effect could be anti-inflammatory in the presence of LPS. Summarizing, there is an apparent inflammatory phenotype induced by LPS on human macrophages, and an anti-inflammatory effect mediated by MA in terms of cell death and viability, and also mediated by MA in terms of NO secretion and regulated by CA and BL in terms of IL-6 secretion.
3.3.In vitro effect of berries extracts on LPS-activated human macrophages: Cell viability and apoptosis
LPS treatment decreased macrophages cell viability, an effect reversed by the treatment with MA and BL extracts (Fig. 2A). Caspase-3 activity was increased in LPS-activated macrophages, and all extracts treatments were able to prevent this increment (Fig. 2B). Besides, a significant negative correlation (r = –0.6519; p = 0.0002) between cell viability and caspase-3 activity was detected (Fig. 2C).
Fig. 2.
Berries treatments prevent LPS-induced caspase-3 increased activity. THP-1 monocyte human cells were differentiated and then pre-treated with 100 μM total polyphenols of each extract for 2 hours. Finally, they were treated with 5 μg/mL LPS for 48 hours. Cells and culture media were saved for further determinations. (A) Cell viability determined by LDH activity, (B) Caspase-3/total protein, and (C) Cell viability vs. Caspase-3 activity, were assayed. Data (n = 4–6) were expressed as mean±SD and analyzed with one-way ANOVA followed by Tukey posthoc tests. C, control; LPS, lipopolysaccharide; MA, Maqui; CA, Calafate; BL, Blueberry; r, Pearson correlation coefficient. Different letters showed a statistical significance of at least p < 0.05.
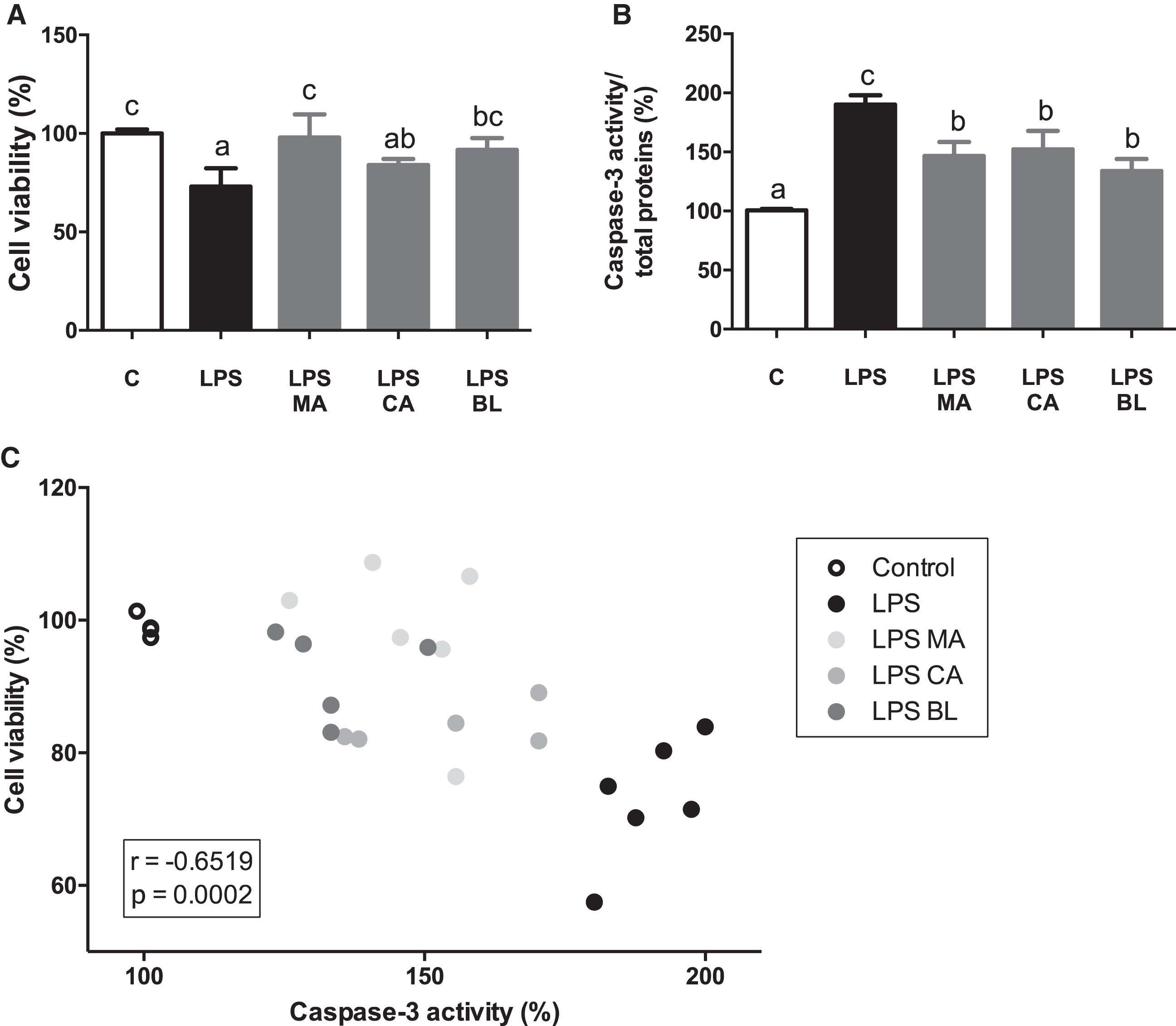
It is reported that there is a link between macrophage activation processes and apoptotic events modulation. Activation of iNOS and the subsequent liberation of NO has been linked with apoptosis induction in macrophages [44–46]. A negative linear correlation between cell viability and apoptosis were observed. The latter results can hint that there is a loss of cell viability by NO-mediated apoptosis, and the presence of the extracts counters that effect. In this regard, a protective cell viability effect by CA has been observed before in leukocytes against ROS production [47].
3.4.In vitro effects of berries extracts in TNF-α activated human adipocytes: Gene expression and protein secretion of inflammatory markers
TNF-α-activated adipocytes showed an increase in MCP-1 gene expression and secretion. When the treatment effects were assessed, BL was the only extract which reverted the MCP-1 gene expression increase entirely (Fig. 3A), while MA and CA did it partially. Moreover, all three extracts prevented MCP-1 secretion partially (Fig. 3B). TNF-α also incremented IL-6 expression and secretion. No significant preventing effect by the extracts was observed on its gene expression (Fig. 3C), but all treatments did it on its secretion (Fig. 3D). No effects were observed either by TNF-α or extracts on adiponectin gene expression and secretion (Fig. 3E and 3F).
Fig. 3.
In vitro anti-inflammatory effects of berries extracts in TNF-α activated human adipocytes. Human visceral preadipocytes were differentiated, pre-treated with 100 μM total polyphenols from each extract for 1 hour, then primed 24 hours with 4 ng/mL of TNF-α. Cells and culture media were saved for further determinations. (A) MCP-1 gene expression, (B) and release, (C) IL-6 gene expression, (D) and release, (E) Adiponectin gene expression, (F) and release, were assayed. Data (n = 4) were expressed as mean±SD and analyzed with one-way ANOVA followed by Tukey posthoc tests. C, control; MA, Maqui; CA, Calafate; BL, Blueberry. Different letters showed a statistical significance of at least p < 0.05.
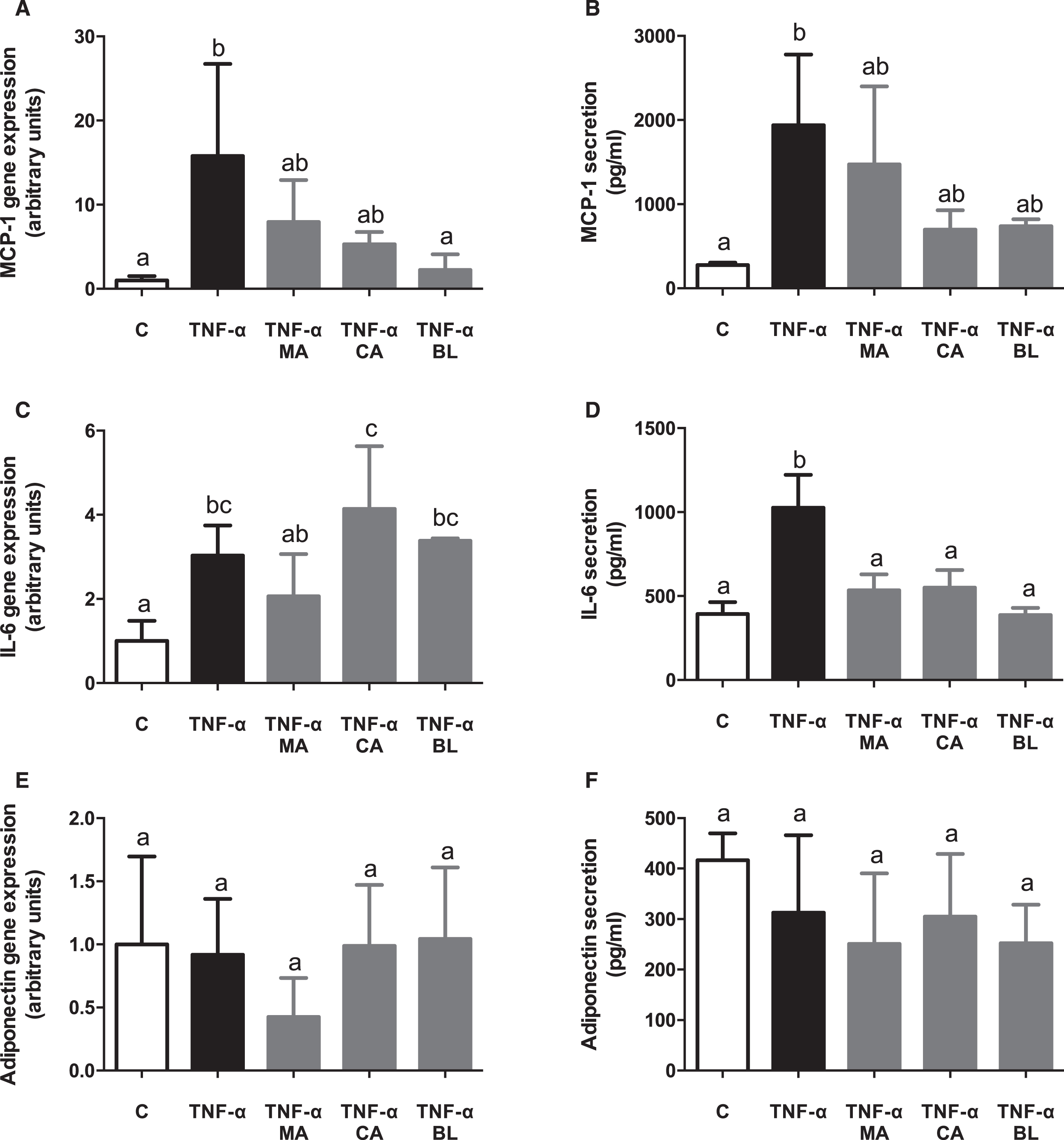
It has been reported that while adipocyte hypertrophy progresses through obesity, there is an increase in metabolic stress, induction of apoptosis, secretion of inflammatory mediators (MCP-1 and IL-6), and decreased adiponectin secretion, all of which stimulates macrophage infiltration on the adipose tissue [48]. These conditions boost the inflammatory response, either through NF-κB-dependent or independent pathways [49]. In the presented experimental model of in vitro TNF-α activated human adipocytes, MCP-1 shows an increased expression and secretion upon TNF-α stimulus, which was not alleviated by the fruit extracts presence, except for BL in gene expression. Besides, the expression and secretion of IL-6 were induced by TNF-α significantly, lowering its secretion upon pre-treatment with all extracts. Finally, expression and secretion of adiponectin did not show any variations between TNF-α stimulation, control, and berries extracts exposure. Results regarding adipokines could be contrasted with another study in which the expression of mitogen-activated protein kinase 1 (MKP-1) was compared with the expression of proposed inflammatory markers at different times of 3T3-L1 adipocyte hypertrophy (8, 15 and 21 days) [50]. It is deducted that expression and secretion of adiponectin did not present changes because the hypertrophy induction time with the inflammatory stimulus was too short (10 days) to be detected. Thus, it does not mean that TNF-α does not participate in its modulation. In that study, a significant decrease in adiponectin expression after 15–21 days of hypertrophy induction, and a significant decrease in its secretion after 8–15 days of hypertrophy induction [50] were observed, which correlates with MKP-1 inhibition. Adding to the above, previous results obtained by our group with a co-culture model with RAW264.7 macrophages and 3T3-L1 adipocytes proved that hypertrophy could be induced in a shorter period to observe a decrease in adiponectin expression in regards of control. However, it requires the presence of higher pro-inflammatory stimuli [21]. The graphed data shows high dispersion in expression and secretion of adiponectin, which can be linked with a premature measurement.
3.5.In vitro anti-inflammatory effects of berries extracts in TNF-α activated human adipocytes: Cell viability and apoptosis
There were no changes observed on adipocytes viability (Fig. 4A). Nonetheless, there was detected a decrease in caspase-3 activity induced by all treatments, reverting to the control levels (Fig. 4B). Finally, no significant correlations were observed between cell viability and caspase-3 activity (r = –0.284; p = 0.254) (Fig. 4C).
Fig. 4.
Effects of berries extracts on adipocytes viability and death. Human visceral preadipocytes were differentiated, pre-treated with 100 μM total polyphenols from each extract for 1 hour, then primed 24 hours with 4 ng/mL of TNF-α. Cells and culture media were saved for further determinations. (A) Cell viability determined by LDH activity, (B) Caspase-3/total protein, and (C) Cell viability vs. caspase-3 activity, were assayed. Data (n = 3–4) were expressed as mean±SD and analyzed with one-way ANOVA followed by Tukey posthoc tests. C, control; MA, Maqui; CA, Calafate; BL, Blueberry; r, Pearson correlation coefficient. Different letters showed a statistical significance of at least p < 0.05.
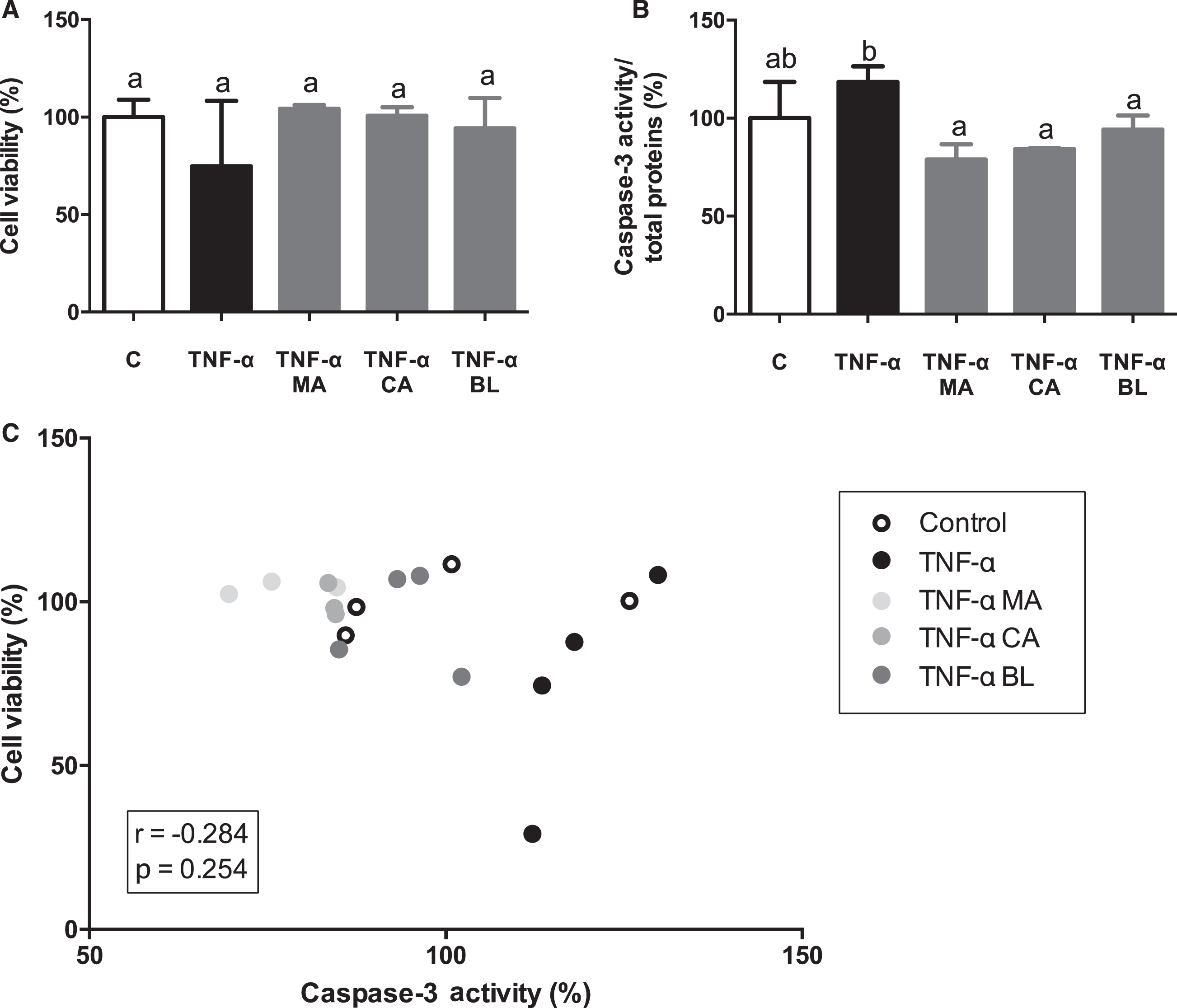
A study in which human preadipocytes and adipocytes with TNF-α in different concentrations, at 24 hours maximum, showed an increase in apoptosis levels (5–25%) compared to non-activated control (0–2.3%) [51]. Thus, a decrease in cell viability and an increase in apoptosis should be expected in the present study. However, cell viability results did not present significant changes when cells were activated with TNF-α related to control or pre-treatment with extracts, suggesting that there is no cell death in regard to TNF-α stimulus, neither activation of survival mechanism in the presence of the extracts. Induction of apoptosis by TNF-α stimulation is observed, which is reversed by pre-treatment with all extracts, in concordance with literature [51]. Hence, MA, CA, and BL had anti-inflammatory effects in terms of apoptosis, IL-6, and MCP-1 secretion. In this regard, it has been described before important anti-inflammatory features of anthocyanins on mice adipocytes [52, 53], that match with previously published ones from our laboratory [20]. Now we can confirm these outcomes, but on human cell lines.
3.6.In vitro anti-inflammatory effects of berries extracts in TNF-α activated human adipocytes: Antioxidant defenses
In order to assess if the berries fruit extracts had an effect over antioxidant variables on an in vitro model, both reduced/oxidized glutathione ratio and SOD and catalase CAT activity were measured. An increment in GSSG levels in active condition versus control condition was observed, without changes regarding extracts treatments (Fig. 5A). GSH levels in CA treatment was increased regarding control (Fig. 5B). Additionally, the GSSG/GSH ratio decreased in TNF-α active condition and all native fruit extracts (Fig. 5C). There were no significant changes detected in SOD activity (Fig. 5D), although CAT activity decreased on all extract treatments (Fig. 5E).
Fig. 5.
In vitro antioxidant effect of berries extracts on TNF-α activated human adipocytes. Human visceral preadipocytes were differentiated, pre-treated with 100 μM total polyphenols from each extract for 1 hour, then primed 24 hours with 4 ng/mL of TNF-α. Cells and culture media were saved for further determinations. (A) GSSG/total protein, (B) GSSG/total protein (C) GSSG/GSH ratio, (D) SOD activity/total protein, and (E) CAT activity/total protein Data (n = 3–4) were expressed as mean±SD and analyzed with one-way ANOVA followed by Tukey posthoc tests. GSSG, oxidized glutathione; GSH, reduced glutathione; SOD, superoxide dismutase; CAT, catalase; C, control; MA, Maqui; CA, Calafate; BL, Blueberry. Different letters showed a statistical significance of at least p < 0.05.
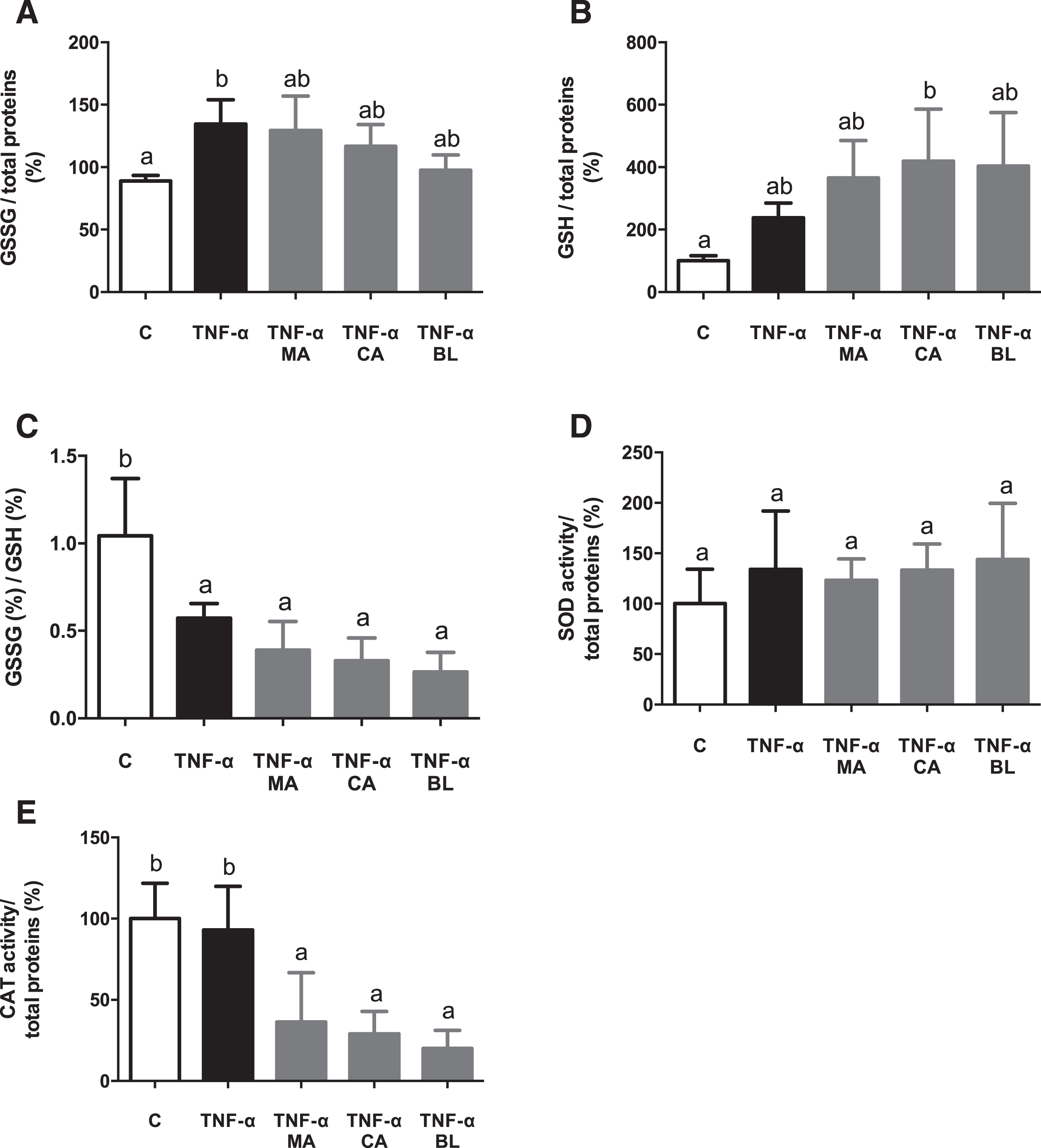
It has been described that antioxidant defense power (SOD, CAT, GPx) is lesser in obese people than in healthy or overweight patients [54–57]. The latter evidence led this study to measure SOD and CAT activity, and GSH, GSSG, and GSSG/GSH ratio in human cells. Values regarding GSSG/GSH ratio indicate low levels of oxidative stress, and high GSH availability for hydrogen peroxide, and lipoperoxides reduction, as an action by the glutathione peroxidase/glutathione reductase (GPx/GR) antioxidant system. Thus, the berries fruit extracts present an antioxidant effect, augmenting the GSH levels. Nevertheless, the lower levels of CAT enzymatic activity observed could be explained as GPx/GR antioxidant system does not act in parallel with the SOD/CAT system. It has been reported that CAT intervenes as an antioxidant defense when the hydrogen peroxide concentrations are high enough; meanwhile, in low concentrations, the GPx/GR system is activated. In other words, there are inversely related in a hydrogen peroxide concentration-dependent manner [58]. Similar results were reported before by our group [20], specifically in the glutathione system, but on mice cells. These outcomes also match with the ones described by Albrecht et al [47], on CA over leukocytes treated with chloramphenicol, and some other reports regarding MA [59, 60], even one describing protective effects over postprandial oxidative stress on humans [61]. Thus, the present results came to support the potential of the treatment with these berries in a human setting.
4.Conclusions
Aristotelia chilensis, Berberis microphylla, and Vaccinium corymbosum extracts were able to inhibit inflammatory and oxidative response on in vitro inflammation models of human adipocytes and macrophages. These findings would lead to considering the Chilean native fruits Maqui and Calafate for the development of future therapeutic strategies to battle the obesity-associated inflammation comorbidities. Also, the present results add some novel insights regarding the positive effects of Blueberry over inflammatory settings. However, it is necessary further research, especially at the in vivo level, to consolidate these healthy outcomes.
Funding
This work was supported by the National Commission for Scientific and Technological Research (CONICYT, Chile; FONDECYT Grant 11110219).
Conflict of interest
The authors have no conflict of interest to report
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JBR-200576.
Acknowledgments
The authors have no acknowledgments.
References
[1] | Goossens GH . The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obesity Facts. (2017) ; 10: (3): 207–15. |
[2] | Ferris WF , Crowther NJ . Once fat was fat and that was that: our changing perspectives on adipose tissue. Cardiovasc J Afr. (2011) ; 22: (3): 147–54. |
[3] | Lee MJ , Wu Y , Fried SK . Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. (2010) ; 13: (4): 371–6. |
[4] | Okuno Y , Fukuhara A , Hashimoto E , Kobayashi H , Kobayashi S , Otsuki M , et al. Oxidative Stress Inhibits Healthy Adipose Expansion Through Suppression of SREBF1-Mediated Lipogenic Pathway. Diabetes. (2018) ; 67: (6): 1113–27. |
[5] | Lumeng CN , Bodzin JL , Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. (2007) ; 117: (1): 175–84. |
[6] | Bray GA . Medical consequences of obesity. The Journal of Clinical Endocrinology & Metabolism. (2004) ; 89: (6): 2583–9. |
[7] | Weisberg SP , McCann D , Desai M , Rosenbaum M , Leibel RL , Ferrante AW , Jr. Obesity is associated with macrophage accumulation in adipose tissue . J Clin Invest (2003) ; 112: (12): 1796–808. |
[8] | Ros Perez M , Medina-Gomez G . [Obesity, adipogenesis and insulin resistance]. Endocrinologia y Nutricion. (2011) ; 58: (7): 360–9. |
[9] | Xu H , Barnes GT , Yang Q , Tan G , Yang D , Chou CJ , et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. (2003) ; 112: (12): 1821–30. |
[10] | Tsuda T . Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. Journal of Agricultural and Food Chemistry. (2008) ; 56: (3): 642–6. |
[11] | Wang J , Mazza G . Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW 264. 7 macrophages. Journal of Agricultural and Food Chemistry. (2002) ; 50: (4): 850–7. |
[12] | Zafra-Stone S , Yasmin T , Bagchi M , Chatterjee A , Vinson JA , Bagchi D . Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. (2007) ; 51: (6): 675–83. |
[13] | Afrin S , Giampieri F , Gasparrini M , Forbes-Hernandez TY , Cianciosi D , Reboredo-Rodriguez P , et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol Adv. (2020) ; 38: , 107322. |
[14] | Schreckinger ME , Wang J , Yousef G , Lila MA , Gonzalez de Mejia E . Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. Journal of Agricultural and Food Chemistry. (2010) ; 58: (16): 8966–76. |
[15] | Gasparrini M , Afrin S , Forbes-Hernandez TY , Cianciosi D , Reboredo-Rodriguez P , Amici A , et al. Protective effects of Manuka honey on LPS-treated RAW 264. 7 macrophages. Part Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem Toxicol. (2018) ; 120: , 578–87. |
[16] | Forbes-Hernandez TY , Afrin S , Cianciosi D , Manna PP , Zhang J , Gasparrini M , et al. Strawberry extract attenuates oxidative stress in 3T3-L1 cells. Journal of Berry Research. (2018) ; 8: (3): 193–203. |
[17] | Ruiz A , Hermosin-Gutierrez I , Mardones C , Vergara C , Herlitz E , Vega M , et al. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. Journal of Agricultural and Food Chemistry. (2010) ; 58: (10): 6081–9. |
[18] | Miller K , Feucht W , Schmid M . Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients. (2019) ; 11: (7). |
[19] | Cespedes-Acuña CL , Xiao J , Wei ZJ , Chen L , Bastias JM , Avila JG , et al. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. Journal of Berry Research. (2018) ; 8: (275–296). |
[20] | Reyes-Farias M , Vasquez K , Fuentes F , Ovalle-Marin A , Parra-Ruiz C , Zamora O , et al. Extracts of Chilean native fruits inhibit oxidative stress, inflammation and insulin-resistance linked to the pathogenic interaction between adipocytes and macrophages. Journal of Functional Foods. (2016) ; 27: , 69–83. |
[21] | Reyes-Farias M , Vasquez K , Ovalle-Marin A , Fuentes F , Parra C , Quitral V , et al. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J Med Food. (2015) ; 18: (5): 601–8. |
[22] | Garcia-Diaz DF , Johnson MH , de Mejia EG . Anthocyanins from fermented berry beverages inhibit inflammation-related adiposity response in vitro. J Med Food. (2015) ; 18: (4): 489–96. |
[23] | Singleton V , Rossi J . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. (1965) ; 16: , 144–58. |
[24] | Wrolstad R . Color and pigment analyses in fruit products. Oregon Agr Expt Sta Bul. (1976) ; 624: , 1–17. |
[25] | Benzie IF , Strain JJ . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. (1996) ; 239: (1): 70–6. |
[26] | Harrison LM , van den Hoogen C , van Haaften WC , Tesh VL . Chemokine expression in the monocytic cell line THP-1 in response to purified shiga toxin 1 and/or lipopolysaccharides. Infection and Immunity. (2005) ; 73: (1): 403–12. |
[27] | Wang M , Chen Y , Zhang Y , Zhang L , Lu X , Chen Z . Mannan-binding lectin directly interacts with Toll-like receptor 4 and suppresses lipopolysaccharide-induced inflammatory cytokine secretion from THP-1 cells. Cell Mol Immunol. (2011) ; 8: (3): 265–75. |
[28] | Fujita K , Iwama H , Oura K , Tadokoro T , Hirose K , Watanabe M , et al. Metformin-suppressed differentiation of human visceral preadipocytes: Involvement of microRNAs. Int J Mol Med. (2016) ; 38: (4): 1135–40. |
[29] | Livak KJ , Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method . Methods (2001) ; 25: (4): 402–8. |
[30] | Griffith OW . Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. (1980) ; 106: (1): 207–12. |
[31] | Suzuki H , Robinson MK , Rounds JD , Gatzen C , Wilmore DW . Glutathione deficiency accentuates hepatocellular fluid accumulation after ischemia-reperfusion. J Surg Res. (1994) ; 57: (5): 632–9. |
[32] | Mariangel E , Reyes-Diaz M , Lobos W , Bensch E , Schalchli H , Ibarra P . The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc Investig Agrar. (2013) ; 40: , 161–70. |
[33] | Rubilar M , Jara C , Poo Y , Acevedo F , Gutierrez C , Sineiro J , et al. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz. ): sources of antioxidant compounds and alpha-Glucosidase/alpha-Amylase inhibitors. J Agric Food Chem. (2011) ; 59: (5): 1630–7. |
[34] | Torri E , Lemos M , Caliari V , Kassuya CA , Bastos JK , Andrade SF . Anti-inflammatory and antinociceptive properties of blueberry extract (Vaccinium corymbosum). J Pharm Pharmacol. (2007) ; 59: (4): 591–6. |
[35] | Araya H , Clavijo C , Herrera C . Capacidad antioxidante de frutas y verduras cultivados en Chile. Arch Latinoam Nutr. (2006) ; 56: , 361–5. |
[36] | Bustamante L , Pastene E , Duran-Sandoval D , Vergara C , Von Baer D , Mardones C . Pharmacokinetics of low molecular weight phenolic compounds in gerbil plasma after the consumption of calafate berry (Berberis microphylla) extract. Food Chem. (2018) ; 268: , 347–54. |
[37] | Sica A , Mantovani A . Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) ; 122: (3): 787–95. |
[38] | Giambelluca M , Rollet-Labelle E , Bertheau-Mailhot G , Laflamme C , Pouliot M . Post-transcriptional regulation of tumour necrosis factor alpha biosynthesis: Relevance to the pathophysiology of rheumatoid arthritis. OA Inflammation. (2013) ; 1: , 3. |
[39] | Han J , Brown T , Beutler B . Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. (1990) ; 171: (2): 465–75. |
[40] | Lauw FN , Pajkrt D , Hack CE , Kurimoto M , van Deventer SJ , van der Poll T . Proinflammatory effects of IL-10 during human endotoxemia. The Journal of Immunology. (2000) ; 165: (5): 2783–9. |
[41] | Mocellin S , Panelli MC , Wang E , Nagorsen D , Marincola FM . The dual role of IL-10. Trends Immunol. (2003) ; 24: (1): 36–43. |
[42] | Lefevre L , Gales A , Olagnier D , Bernad J , Perez L , Burcelin R , et al. PPARgamma ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS One. (2010) ; 5: (9): e12828. |
[43] | Mantovani A , Sica A , Sozzani S , Allavena P , Vecchi A , Locati M . The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) ; 25: (12): 677–86. |
[44] | Albina JE , Cui S , Mateo RB , Reichner JS . Nitric oxide-mediated apoptosis in murine peritoneal macrophages. The Journal of Immunology. (1993) ; 150: (11): 5080–5. |
[45] | Messmer UK , Brune B . Nitric oxide (NO) in apoptotic versus necrotic RAW 264. 7 macrophage cell death: the role of NO-donor exposure, NAD+ content, and p53 accumulation. Archives of Biochemistry and Biophysics. (1996) ; 327: (1): 1–10. |
[46] | Sarih M , Souvannavong V , Adam A . Nitric oxide synthase induces macrophage death by apoptosis. Biochemical and Biophysical Research Communications. (1993) ; 191: (2): 503–8. |
[47] | Albrecht C , Pellarin G , Rojas MJ , Albesa I , Eraso AF . Beneficial effect of Berberis buxifolia Lam, Ziziphus mistol Griseb and Prosopis alba extracts on oxidative stress induced by chloramphenicol. Medicina. (2010) ; 70: (1): 65–70. |
[48] | Suganami T , Nishida J , Ogawa Y . A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arteriosclerosis, Thrombosis, and Vascular Biology. (2005) ; 25: (10): 2062–8. |
[49] | Suganami T , Ogawa Y . Adipose tissue macrophages: their role in adipose tissue remodeling. Journal of Leukocyte Biology. (2010) ; 88: (1): 33–9. |
[50] | Ito A , Suganami T , Miyamoto Y , Yoshimasa Y , Takeya M , Kamei Y , et al. Role of MAPK phosphatase-1 in the induction of monocyte chemoattractant protein-1 during the course of adipocyte hypertrophy. The Journal of Biological Chemistry. (2007) ; 282: (35): 25445–52. |
[51] | Prins JB , Niesler CU , Winterford CM , Bright NA , Siddle K , O’Rahilly S , et al. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. (1997) ; 46: (12): 1939–44. |
[52] | Muscara C , Molonia MS , Speciale A , Bashllari R , Cimino F , Occhiuto C , et al. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytother Res. (2019) ; 33: (7): 1888–97. |
[53] | Luna-Vital D , Weiss M , Gonzalez de Mejia E . Anthocyanins from Purple Corn Ameliorated Tumor Necrosis Factor-alpha-Induced Inflammation and Insulin Resistance in 3T3-L1 Adipocytes via Activation of Insulin Signaling and Enhanced GLUT4 Translocation. Mol Nutr Food Res. (2017) ;. 61: (12). |
[54] | Albuali WH . Evaluation of oxidant-antioxidant status in overweight and morbidly obese Saudi children. World Journal of Clinical Pediatrics. (2014) ; 3: (1): 6–13. |
[55] | Amirkhizi F , Minaie S , Djalali M , Rahimi A , Chamari M . Is obesity associated with increased plasma lipid peroxidation and oxidative stress in women? ARYA Atherosclerosis Journal (2007) ; 2: , 189–92. |
[56] | Boşnak M , Yel S , Koçyiğit Y , şen V , Ece A . Oxidative Stress in Marasmic Children: Relationships with Leptin. European Journal of General Medicine. (2010) ; 7: , 1–8. |
[57] | Viroonudomphol D , Pongpaew P , Tungtrongchitr R , Phonrat B , Supawan V , Vudhivai N , et al. Erythrocyte antioxidant enzymes and blood pressure in relation to overweight and obese Thai in Bangkok. Southeast Asian J Trop Med Public Health. (2000) ; 31: (2): 325–34. |
[58] | Kang S , Song J , Kang H , Kim S , Lee Y , Park D . Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. European Journal of Endocrinology. (2003) ; 148: (1): 147–55. |
[59] | Miranda-Rottmann S , Aspillaga AA , Perez DD , Vasquez L , Martinez AL , Leighton F . Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. Journal of Agricultural and Food Chemistry. (2002) ; 50: (26): 7542–7. |
[60] | Cespedes C , El-Hafidi M , Pavon N , Alarcon J . Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chemistry. (2008) ; 107: , 820–9. |
[61] | UrquiagaI, AvilaF, EcheverriaG, PerezD, TrejoS, LeightonF. A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxidative Medicine and Cellular Longevity. (2017) , 8361493. |




