Hypolipidaemic, hypoglycaemic and antioxidant effects of a tropical highland blackberry beverage consumption in healthy individuals on a high-fat, high-carbohydrate diet challenge
Abstract
BACKGROUND:
Blackberries have a high content of bioactive compounds such as anthocyanins and ellagitannins, which are associated with health benefits against cardiovascular diseases, cancer, diabetes, and other inflammatory conditions.
OBJECTIVE:
This study evaluated the effect of a tropical highland blackberry (Rubus adenotrichos Schltdl.) beverage (50% v/v) on lipids, glucose and antioxidant parameters of healthy individuals.
METHODS:
Thirteen healthy individuals of both sexes were assigned into two groups in a randomized crossover design. Each participant was subjected to a high fat and high carbohydrate diet challenge and drank 250 mL of either blackberry beverage or water with every meal, three times a day for 14 days. Total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, glucose level, superoxide dismutase (SOD), and catalase (CAT) enzymatic activities were assessed from plasma.
RESULTS:
Plasma levels of triglycerides, total cholesterol, and glucose levels significantly decreased (p < 0.05) after consuming the blackberry beverage. Changes in LDL and HDL cholesterol levels were not statistically significant (p > 0.05). CAT and SOD enzymatic activities increased slightly, although not statistically significant (p > 0.05).
CONCLUSION:
Drinking a beverage from a blackberry micro-filtered juice improved plasma lipid and glucose profiles, as well as CATand SOD enzymatic activities in healthy participants.
1Introduction
Healthy development and aging are directly influenced by the kind of food and eating habits people have throughout their lives. One of the most drastic changes in the second half of the 20th century was the so-called “nutritional transition”, which changed macronutrients composition of diets around the globe. Most populations shifted from diets rich in complex carbohydrates, cereals, vegetables, and fruits, to a diet composed of highly processed and energy dense meals [1–6]. This change in eating habits is responsible for the parallel increase in the incidence of chronic diseases such as obesity, cardiovascular diseases, cancer and diabetes [2, 7, 8]. Among these pathologies, cardiovascular diseases have risen to one of the main causes of death worldwide. This high mortality has been associated with changes in cardiovascular risk factors, especially alterations of plasma lipid markers [9–11].
Risk of cardiovascular events can be reduced with diets that are rich in bioactive compounds, such as those contained in many fruits and vegetables [12–16]. This type of diets reduces oxidative stress, regulates blood pressure and homeostasis and improves plasma lipid and glucose profiles [16–19]. The Mediterranean Diet is one example of diet that has proven to decrease the risk of cardiovascular complications. It is characterized by high intake of foods rich in minerals, vitamins, polyphenols and other nutrients. The bioactive compounds in these diets, such as catecols, flavonols, anthocyanins, flavanols, stilbenes, and other polyphenols, neutralize reactive oxygen species (ROS), and minimize the deleterious effects of free radicals in living organisms, protecting against lipid peroxidation and positively influencing antioxidant enzyme levels [15, 16, 20–27].
Tropical fruits are good sources of bioactive compounds. Among them, the Rubus genus is composed of thousands of species of blackberries and raspberries grown worldwide [28]. The tropical highland blackberry Rubus adenotrichos Schltdl. has a natural distribution from Mexico to Ecuador, it is widely cultivated in Latin America [29] and it has traditionally been used to produce blackberry beverages in countries like Colombia, Ecuador, and Costa Rica [30]. This blackberry is rich in anthocyanins and ellagitannins, and to a lesser extent in conjugated forms of ellagic acid, gallic acid, and hydroxycinnamic acid [29–35]. Many processed products of Rubus adenotrichos have been developed by microfiltration [36, 37], ultrafiltration [38, 39], nanofiltration [40], pasteurization [41] and spray drying [42]. These products and the raw fruit have been analyzed to determine their phenolic content, antioxidant capacity, anti-inflammatory activity, as well as the antimicrobial, hypoglycaemic and hypolipidaemic effects in vitro and in vivo [29, 43–45]. To our knowledge, this is the first study to evaluate the potential health benefits of a tropical highland blackberry beverage in humans taking into account the amount and type of polyphenols in this drink. This clinical trial evaluated the effect of consuming tropical highland blackberry from a micro-filtered juice on plasma lipid and glucose profiles, as well asspecific biomarkers of oxidative stress of healthy individuals exposed to a high-fat, high-carbohydrate diet challenge.
2Material and methods
2.1Blackberry beverage
2.1.1Fruit material
Cultivated, fully ripe, tropical highland blackberries (Rubus adenotrichos Schltdl. commercial cultivar ‘vino’) were harvested from Cartago, Costa Rica (altitude 1864–2517 m, latitude 09° 39′ 57.1′′N –09°44′40.3′′N, longitude 83°53′32.1′′W –84°00′06.3′′W). Blackberries were stored in plastic bags and frozen at –20°C until beverage preparation.
2.1.2Beverage preparation
The following method, previously described by Vaillant et al. [46], was used to prepare the micro-filtered blackberry juice. Briefly, blackberries were crushed, and treated for 1 h with 250 ppm of a commercial enzymatic preparation with cellulases and pectinases (Klerzyme® 150, DSM Food Specialties, Heerlen, Netherlands) applying constant agitation at 35°C. Subsequently, the homogenate was pressed for 20 min at 50 psi using a hydro press (Enotecnica Pillan SRL, Italy). The juice was micro-filtered by a tubular ceramic membrane (Membralox® 1 P19–40, Pall Exekia, Bazet, France) with a pore size of 0.2μm. A 50% v/v beverage was prepared with tap water, which was later packaged in plastic bags and stored at –20°C until used.
2.1.3Beverage characterization
The physicochemical properties of the beverage were analyzed following AOAC (Association of Official Agricultural Chemists) reference methods. Moisture content was determined by desiccation to constant weight at 100°C, estimating weight loss due to water evaporation on a stove (AOAC 920.151). Total soluble solids were measured by a digital refractometer (Fisher Scientific Japan Ltd., Japan) with temperature control. Values were reported as °Brix (AOAC 932.12). pH was measured using a pH-meter (Thermo Fisher Scientific, Waltham, MA, U.S.A.) (AOAC 981.12). Total acidity was determined by titration of 1 mL of beverage (diluted with deionized water to 20 mL final volume) using 0.1 M NaOH (AOAC 942.15) and expressed as malic acid equivalents.
The total polyphenol content was assessed by the Folin Ciocalteu assay modified by Georgé et al. [47]. Briefly, Folin Ciocalteu reagent solution (1 : 10 in water) was added to the beverage and incubated for two minutes at room temperature. After incubation, 2.0 mL of sodium carbonate (75 g/L) were added to the mixture and it was heated at 50°C for 15 min. The resulting mixture was immediately cooled in an ice-cold water bath. Absorbance was measured with a spectrophotometer (Pharmaspec UV-1700 Shimadzu, Kyoto, Japan) at 760 nm. Results were expressed as milligrams of gallic acid equivalent (GAE) per 100 mL.
Anthocyanins and ellagitannins were analyzed by HPLC according to Mertz et al. [29] Acosta-Montoya et al. [31] and Soto et al. [35]. The HPLC quantitative analysis was carried out on a Shimadzu liquid chromatography system equipped with a SPD-M20A photodiode array detector (Shimadzu Manufacturing, Inc., Canby, OR, USA) and coupled to Shimadzu EZ Start software (v. 7.4 SP1). This procedure used a reversed-phase ACE 300A C18 column (125×2.1 mm, 3μm) (AIT, Houilles, France). Polyphenols were quantified by calibration curves of cyanidin-3-glucoside as standard for anthocyanins and ellagic acid for ellagitannins.
2.1.4Oxygen radical absorbance capacity (ORAC)
The ORAC assay was performed as described by Ou et al. [48] and Gancel et al. [30]. Briefly, we performed the 2,2′-azobis (2-methylpropionamide)-dihydrochloride (AAPH) induced oxidation assay by measuring fluorescein signal in a spectrofluorometer (Biotek Instruments Inc, Winooski, USA)at 520 nm. ORAC values were reported in micromol of Trolox equivalents (μmol TE/100 mL).
2.2Subjects and study design
The Bioethics Committee for Human Investigation of the University of Costa Rica approved the study protocol (CEC #117-06). Each participant signed an informed consent to participate.
All participants met the following eligibility criteria: 1. No clinical record of cardiovascular, hepatic, gastrointestinal, or renal disease; 2. No alcohol or drug abuse; 3. No use of vitamins or minerals supplements during the 6 weeks previous to the study; 4. No blood donation in the prior four weeks to the study; 5. No history of surgery (abdominal, thoracic, etc.) in the six months before the study; 6. No self-reported high consumption of stimulant drinks (more than 5 cups a day of coffee, tea or caffeine-rich beverages); 7. No use of medications during the 15 days previous to the study. Pregnant and lactating women were excluded.
Participants underwent physical examination and completed a medical history questionnaire to determine their health status. Fasting plasma glucose, lipids, hepatic, renal, and hematologic profiles were obtained from blood samples.
2.2.1Phase I of the clinical study
Participants were randomly assigned in two groups, according to a crossover design (Fig. 1). One group received 250 mL of blackberry beverage sweetened with sugar ad-libitum and the other group had water with the option of sweetening it with sugar.
Fig. 1
Blackberry beverage clinical trial crossover design.
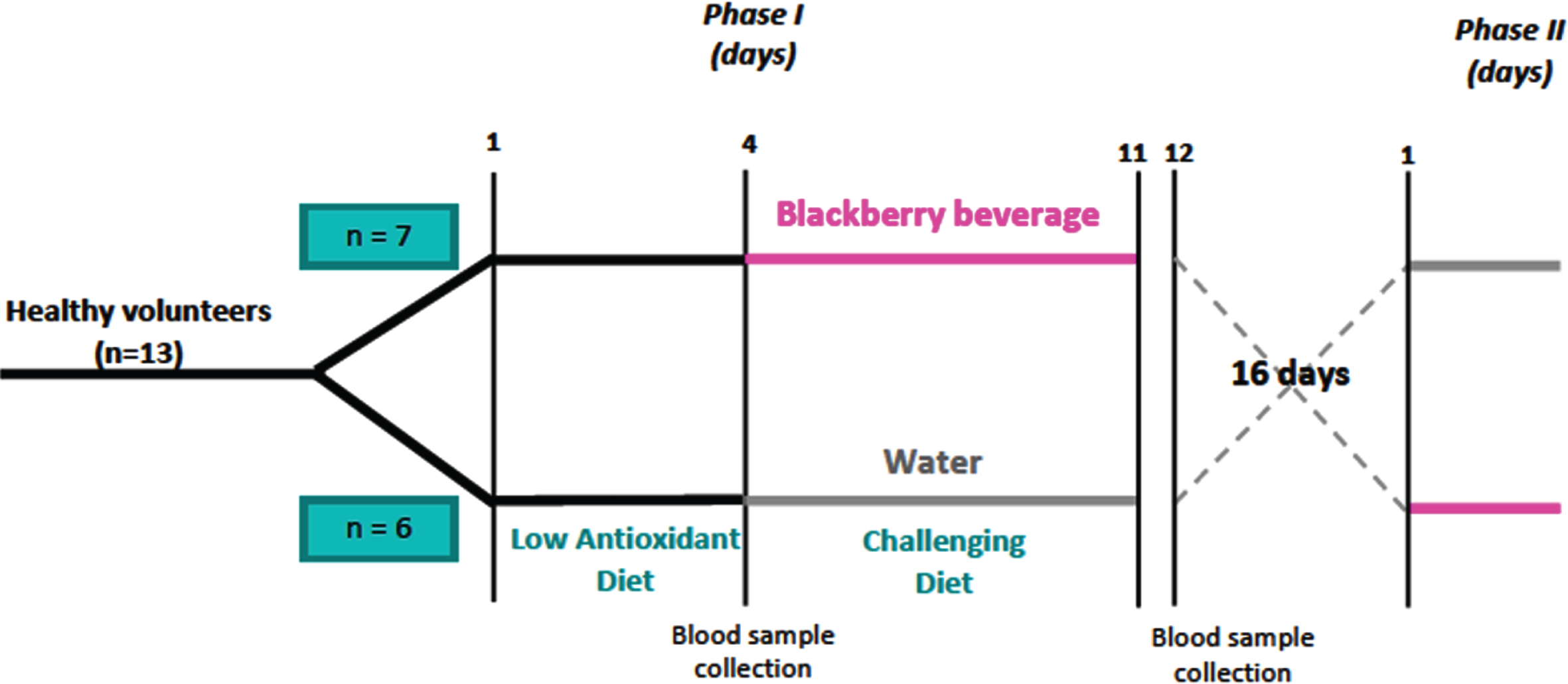
For three days, the 13 participants were asked to consume a low antioxidant diet (low in fruits, vegetables, wine, coffee, tea, chocolate, or derivative products). On day four, after an overnight fasting period (12 h), a blood sample was collected and used as a baseline for all biomarkers assessed.
From day 4 to day 11, participants were assigned to a high-fat, high-carbohydrate diet challenge for breakfast, lunch, and dinner. This diet contained approximately 4000 Kcal/day and was low in fruits and vegetables. Participants drank 250 mL of either blackberry beverage or water with every meal for a total of 750 mL a day. Participants could drink water or other beverages (not from fruit sources) during the rest of the day. In order to enhance compliance, participants had breakfast and lunch at the investigation site, along with the corresponding drink (blackberry or water). A full dinner and 250 mL of blackberry beverage were provided in a cooler container to be taken out and consumed at home. Participants in the water-drinking group were provided with their takeout dinner only. On Fridays, participants in the blackberry arm received a cooler container with seven bags, each containing 250 ml of blackberry beverage. Instructions were given to consume 250 mL of the blackberry beverage with Friday’s dinner and each meal during Saturday and Sunday. All participants received the indication to eat fast food for breakfast, lunch and dinner and no fruits or vegetables during the weekend.
On day 12, another blood sample was collected to assess total cholesterol, triglycerides, and cholesterol-LDL/HDL and oxidative stress enzymes (superoxide dismutase and catalase) in plasma. Participants had a free choice living period of 16 days before the next phase of the study.
2.2.2Phase II of the clinical study
During this phase, participants were switched between arms (Fig. 1) and given the drink they had not drunk during phase I. On day one of Phase II and, after an overnight fasting period (12 h), a blood sample was collected and used as a baseline for all biomarkers selected. From day 1 to day 7, all participants were assigned again to the carbohydrate and fat-rich diet challenge for breakfast, lunch, and dinner. Participants drank 250 mL of either blackberry beverage or water with every meal for a total of 750 mL a day. Participants could drink water or other beverages (not from fruit sources) during the rest of the day. In order to enhance compliance, participants had breakfast and lunch at the investigation site, along with the corresponding drink (blackberry or water). A full dinner and 250 mL of blackberry beverage were provided in a cooler container to be taken out and consumed at home. Participants in the water-drinking group were provided with their takeout dinner only. On Fridays, participants in the blackberry arm received a cooler container with seven bags, each containing 250 ml of blackberry beverage. Instructions were given to consume 250 mL of the blackberry beverage with Friday’s dinner and each meal during Saturday and Sunday. All participants received the indication to eat fast food for breakfast, lunch and dinner and no fruits or vegetables during the weekend.
On day eight of Phase II, another blood sample was collected to assess lipid profile, glucose levels, and oxidative stress enzymes.
Diets were designed by a certified nutritionist. Table 1 shows the nutritional information of each diet.
Table 1
Nutritional information for both low antioxidant and high fat and high carbohydrate diets
| Type of nutrient | Low antioxidant diet | High fat and high carbohydrate diet |
| Energy (kcal) | 1713* | 3362* |
| Carbohydrate (g) | 256.7 | 385.7 |
| Fat (g) | 54.81 | 158.9 |
| Saturated (g) | 13.15 | 48.99 |
| Monounsaturated (g) | 16.99 | 51.41 |
| Polyunsaturated (g) | 15.16 | 28.64 |
| Cholesterol (g) | 228.1 | 466.3 |
| Protein (g) | 64.58 | 121.4 |
| Fiber (g) | 13.62 | 27.75 |
| Ca (mg) | 478.8 | 888.8 |
| K (mg) | 2337 | 2887 |
| Mg (mg) | 253 | 321.8 |
| Fe (mg) | 11.7 | 22.45 |
| Zn (mg) | 8.95 | 15.65 |
| Mn (mg) | 2.73 | 1.88 |
| Se (μg) | 48.19 | 78.94 |
| B1 (mg) | 1.37 | 1.64 |
| B2 (mg) | 1.21 | 7.67 |
| B6 (mg) | 8.13 | 2.72 |
| B12 (μg) | 8.72 | 17.81 |
| Folate (μg) | 275.5 | 358.2 |
| Niacin (mg) | 17.43 | 26.33 |
| Retinol (μg) | 212.2 | 936 |
| Vit C (mg) | 110.7 | 19.26 |
| Vit D (μg) | 1.87 | 45.57 |
| Vit E (mg) | 10.19 | 19.4 |
*Approximate values since snacks for both diets were not considered (cookies, pastries, bread, saltines, pretzels, chips were to be taken as snacks); fast food taken on weekends for the high fat, high carbohydrate diet was not considered. Food was cooked with soy or sunflower oil. No fruits were permitted. Only artificial juices without added vitamins were allowed. Intakes were determined using Funiber Nutriber software (version 1.1.1.r5).
2.2.3Blood sample collection
The clinical laboratory from the Health Office of the University of Costa Rica collected the blood samples (10 mL). They were centrifuged at 2500×g for 10 min at 20°C. Plasma was stored at –70°C until analysed.
2.2.4Lipid profile, glucose level, and antioxidant capacity assay
Serum total cholesterol (TC), triglycerides (TG), LDL-cholesterol (LDL), high-density lipoprotein cholesterol (HDL) and plasma glucose levels were measured using commercial kits (Cobas® Roche Diagnostics GmbH, Mannheim, Germany), on a Cob as analyzer (Roche/Hitachi GmbH, Mannheim, Germany). Results were expressed as mg/dL.
Plasma antioxidant enzyme activities were determined using commercial kits for superoxide dismutase (SOD) and catalase (CAT) (Cayman Chemical Co., Detroit, MI). Enzyme activities were expressed as U/mL (International Units per millilitre) for SOD and nmol/min/mL for CAT.
2.3Statistical analysis
Mean values with standard deviations were calculated for all variables. Statistical analysis for cholesterol, triglycerides, enzymes biomarkers, and glucose (before and after the blackberry beverage drinking periods) were performed using a one-tail paired t-Student analysis; p < 0.05 values were considered statistically significant.
Normal distribution was tested with the Kolmogorov-Smirnov test and homoscedasticity was determined by the Levene test. All statistical analyses were performed using R Studio statistical software (version 3.5.1) with Stats and Plotly packages for graphics.
3Results
3.1Chemical characterization of the blackberry beverage
Table 2 shows polyphenol amounts, sugar content, and other chemical parameters, including the ORAC value of the beverage. In general, the blackberry beverage had high levels of the bioactive compounds of our interest, as reflected by the values of total polyphenols, ellagitannins, anthocyanins and ORAC shown in Table 2.
Table 2
Chemical characterization of Rubus adenotrichos Schltl. beverage 50% v/v
| Character | Value |
| Total polyphenols* | 260.0±29.0 |
| Ellagitannins† | 64.7±6.5 |
| Anthocyanins‡ | 51.8±9.1 |
| H-ORAC¥ | 1341.7±63.1 |
| pH | 2.69±0.04 |
| Soluble solids (∘Brix) | 7.5±0.7 |
| Moisture contentY | 91.9±0.5 |
| Total acidity§ | 3.12±0.02 |
Data are expressed as mean±SD; n = 3. H-ORAC: hydrophilic oxygen radical absorbance capacity. *mg gallic acid equivalents/100 mL; †mg ellagic acid equivalents/100 g; ‡mg cyanidin-3-glucoside equivalents/100 g; ¥micromol Trolox equivalents/100 mL; Yg/100 g; §mg malic acid equivalents/100 g.
3.2Clinical trial
The main characteristics of study participants are shown in Table 3. No statistical differences were found for baseline cholesterol levels (p = 0.30), triglycerides (p = 0.55), LDL (p = 0.35) and HDL (p = 0.83). All 13 participants completed the two phases of the clinical trial.
Table 3
Characteristics of the study participants
| Character | Value |
| Age* | 25±3 |
| Weight† | 57.4±7 |
| Height‡ | 1.6±0.1 |
| BMI¥ | 22.4±2.3 |
| Total cholesterol § | 171.2±23.5 |
| Triglycerides § | 82.0±23.4 |
| LDL§ | 95.0±22.6 |
| HDL§ | 59.8±9.6 |
| Cardiovascular risk factor | 3.0±0.6 |
Data are expressed in mean±SD, n = 13. BMI, body mass index; HDL: High-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; *years; †kg; ‡m; ¥kg/m2; §mg/Dl.
3.3Blood chemistry
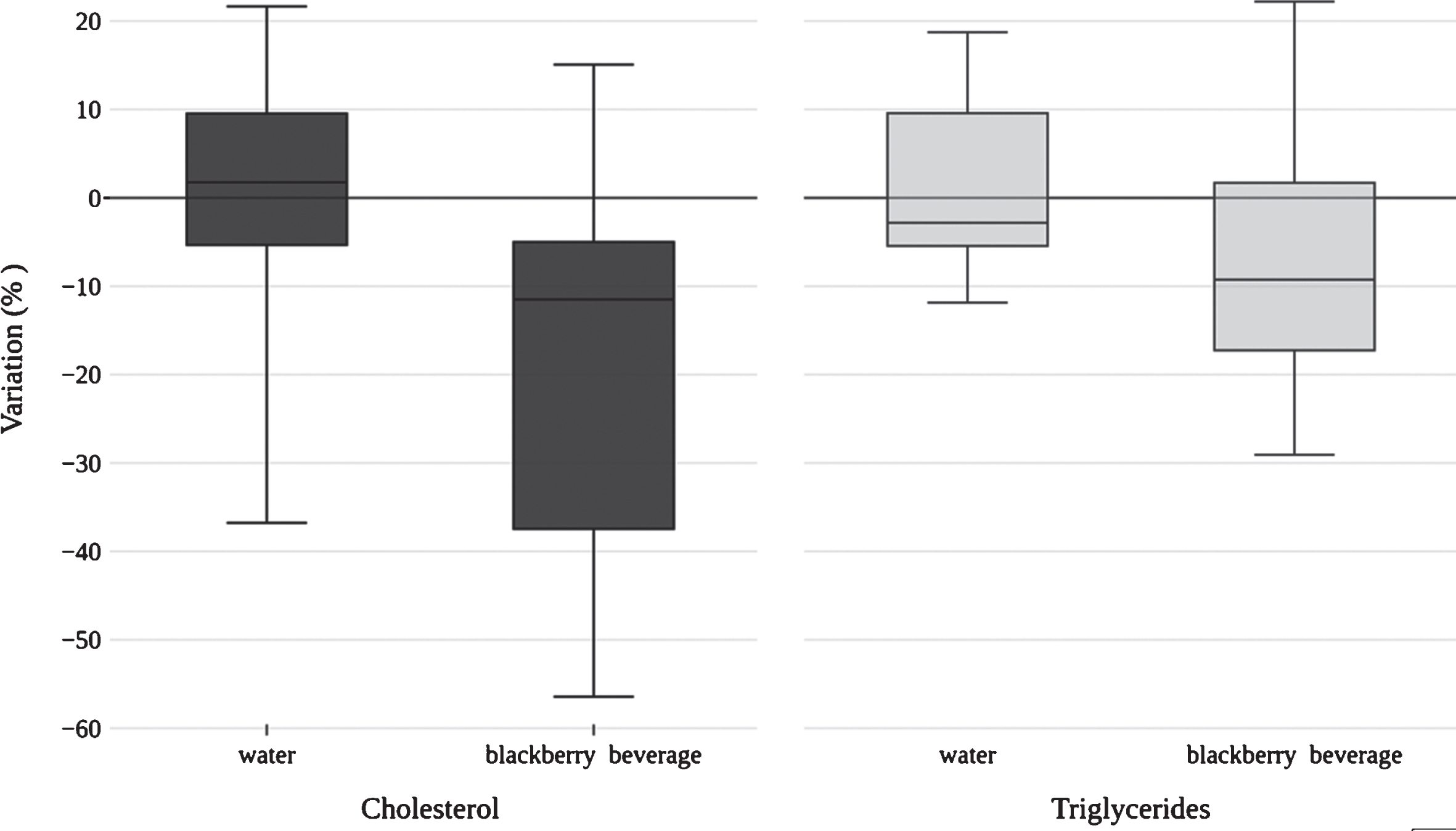
Total cholesterol and triglycerides levels significantly decreased (8.10%, p = 0.040 and 26.33%, p = 0.010, respectively) after drinking blackberry beverage (Table 4). Figure 2 shows the percentage of variation on total cholesterol and triglycerides. No effect on LDL or HDL levels was observed.
Table 4
Effect of blackberry beverage consumption on lipid profile of study participants
| Parameter (mg/dL) | Sweetened water | Blackberry beverage | ||
| Before | After | Before | After | |
| Total cholesterol | 173.2±22.7 | 173.9±24.9 | 184.2±29.2 | 169.2±24.4* |
| Triglycerides | 83.1±29.2 | 80.6±19.5 | 90.8±33.6 | 66.9±12.3* |
| LDL | 101.6±22.5 | 102.1±53.7 | 110.2±16.5 | 101.8±52.3 |
| HDL | 54.9±58.1 | 55.6±9.6 | 55.6±6.9 | 54.2±10.2 |
HDL: High-density lipoprotein cholesterol; LDL: Low-density lipoprotein cholesterol. All values are expressed as mean±S.D. *p < 0.05.
Fig. 2
Variation on total cholesterol and triglycerides levels of study participants after drinking blackberry beverage.
As shown in Fig. 3, there was a significant decrease (p = 0.014) in fasting glucose levels one week after the end of the clinical trial when compared to baseline levels at the beginning of the trial (Table 5).
Fig. 3
Effect of highland blackberry beverage on plasma glucose levels of study participants.
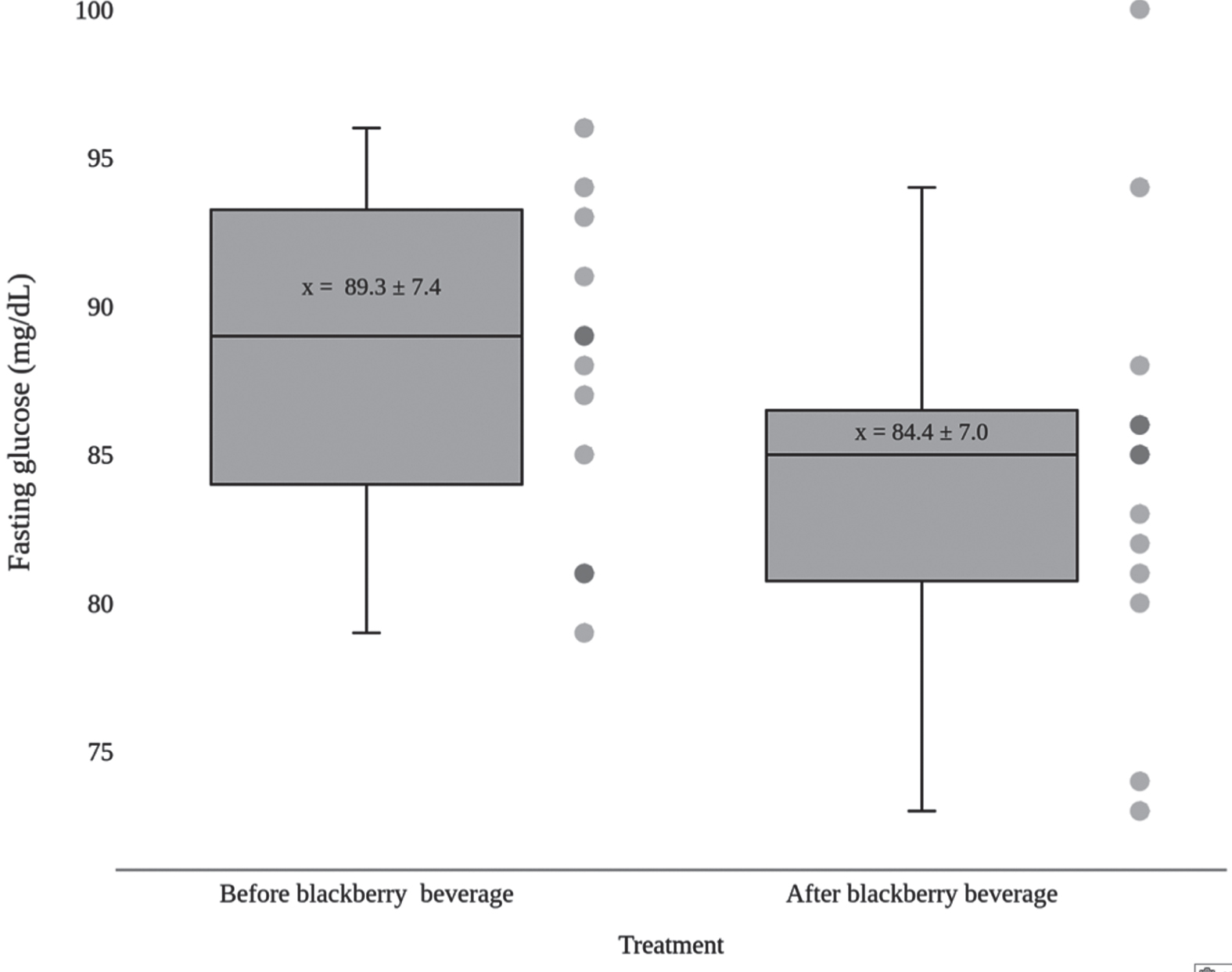
Table 5
Effects of blackberry beverage consumption on fasting serum glucose levels
| Participant | Fasting glucose level | Variation (%) | |
| Before | After | ||
| 1 | 88 | 81 | –8.0 |
| 2 | 94 | 85 | –9.6 |
| 3 | 87 | 94 | +8.0 |
| 4 | 108 | 100 | –7.4 |
| 5 | 96 | 86 | –10.4 |
| 6 | 79 | 73 | –7.6 |
| 7 | 91 | 74 | –18.7 |
| 8 | 81 | 88 | +8.6 |
| 9 | 89 | 86 | –3.4 |
| 10 | 93 | 80 | –14.0 |
| 11 | 85 | 83 | –2.4 |
| 12 | 89 | 85 | –4.5 |
| 13 | 81 | 82 | +1.2 |
| Mean | 89.3 | 84.4 | –5.2* |
| SD | 7.4 | 7.0 | 7.6 |
Data expressed as mg/dL; *p < 0.05.
The enzymatic activity of superoxide dismutase and catalase after consuming the blackberry beverage is detailed on Table 6. There was a very slight but statistically non-significant increase in these parameters.
Table 6
Effect of blackberry beverage consumption on SOD and CAT enzymatic activities
| Enzyme | Sweetened water | Blackberry beverage | ||
| Before | After | Before | After | |
| SOD (U/mL) | 0.15±0.04 | 0.15±0.03 | 0.12±0.03 | 0.13±0.04 |
| CAT (nmol/min/mL) | 17.2±3.7 | 17.0±6.4 | 14.6±7 | 16.0±3.8 |
Data are expressed as a mean±SD; CAT, catalase; SOD, superoxide dismutase.
4Discussion
Based on our review of the literature, this is the first clinical study to evaluate the effects of a tropical highland blackberry beverage from a micro-filtered juice on plasma lipid and glucose profiles, as well as antioxidant enzyme activities. We found that drinking a tropical highland blackberry beverage reduces total cholesterol, triglycerides and glucose levels of healthy participants subjected on a high-fat, high-carbohydrate diet challenge. This was an exploratory study of a processed product of Rubus adenotrichos Schltl., so we chose to work with a small number of participants. Several clinical report similar enrolments and statistically significant results on most of the measured parameters in our study [49–56]. A small number of participants allowed us a close follow up to obtain a high compliance from participants, although our results may not necessarily be generalized to all populations.
The high content of bioactive compounds in Rubus adenotrichos Schltl. has been previously reported by Mertz et al., 2007 [29], Acosta-Montoya et al., 2010 [31], Lee et al., 2012 [57], and Kaume et al., 2012 [58]. Also, four main polyphenols have been identified in a beverage made with this fruit: cyanidin-3-glucoside, cyanidin-3-malonyl glucoside, lambertianin C and sanguiin H-6 [59].
The ORAC value of the blackberry beverage we produced (Table 2) is likely the result of the high polyphenols contents in the fruit [30, 31], which could also explain the biological effects observed throughout this study.
Oxidative stress has been reported in animals fed on a diet high on fats [60]. Unexpectedly, we did not observe an effect of the fat and carbohydrates-rich diet challenge on the lipid profile and glucose levels of the participants when they drank water. Since this diet challenge was mainly devoid of fruits and vegetables, reductions on total plasma cholesterol, triglycerides, and glucose levels of participants could be attributed to the consumption of the blackberry beverage, most likely due to the anthocyanins and ellagitannins contained in it [29, 32, 33, 35]. These polyphenols have a positive impact on lipid metabolism, both in vitro and in vivo, through prevention of free radical-mediated peroxidation of membrane lipids, as well as by acting as powerful antioxidants [61–68].
Qin et al. [56] established that an effective dose of anthocyanins to reduce lipid levels in a 70 kg subject falls between 100 to 335 mg/day. According to Table 2, each participant drank 750 mL per day of the beverage, corresponding to a total amount of 1950 mg/day of polyphenols and 388.5 mg/day of anthocyanins. These quantities are similar to those reported in other studies evaluating lipid-lowering effects of fruits and they fall within the effective dose range proposed by Qin [54–56, 69].
Consumption of different types berries has shown to reduce total cholesterol and triglyceride levels in animal models and clinical studies [32, 36, 54, 70]. In this study, reductions in plasma lipid profiles are similar to those observed in healthy individuals whose diet was supplemented with strawberries (triglycerides and total cholesterol levels decreased on 20.8 % and 8.8 % respectively) [54]. The biological effect observed in the strawberry study can also be related to the content of anthocyanins and ellagic acid in that fruit [61, 70–73].
Anthocyanins and ellagitannins metabolites, urolithins, can be absorbed from the gastrointestinal tract in animals and humans [33, 58, 65, 74–78]. We did not determine plasma polyphenols after consuming the blackberry beverage, but it is possible that both urolithins and anthocyanins in our beverage were absorbed to some extent and could be responsible for the observed lipid-lowering effects. Further studies should be carried out to assess whether there is an association between these effects and the presence of the blackberries’ bioactive compounds or their metabolites in plasma and urine.
The bioactive compounds in Rubus adenotrichos Schltdl. could also have helped to reduce participants’ plasma glucose levels (Table 5). Consumption of fruits with similar phytochemical profiles to blackberry (strawberries, bilberries, cranberries, blackcurrants, blueberries and pomegranate) lower the glucose plasma levels in animal models and healthy volunteers [36, 50, 79–85] through different mechanisms. Bioactive compounds can bind to digestive enzymes α-glucosidase and α-amylase and inhibits them. These compounds also can interfere with glucose transport through the intestinal walls and absorption, modulate postprandial sugar metabolism, insulin sensitivity and improve glucose tolerance [50, 79, 81–84, 86, 87]. As reported by Kaume et al. [58], blackberry anthocyanins, let alone Rubus adenotrichos Schltdl. anthocyanins and ellagitannins, have not been studied thoroughly concerning plasma glucose levels. It is well known that some polyphenols and especially tannins can bound to proteins and inhibit digestive enzymes [88, 89], thus further research is necessary to determine if this mechanism of action applies to Rubus adenotrichos Schltdl. and to identify the bioactive compounds accountable for this effect.
During normal cellular metabolism, cells produce hydrogen peroxide (H2O2), superoxide ion (O2–), and hydroxide radical (OH–). On the other hand, superoxide dismutases (SOD), catalases (CAT), and glutathione peroxidases (GPx) are the main antioxidant enzymes in our endogenous antioxidant system [90–92]. SOD is widely distributed throughout the body [60] and metabolizes the superoxide anion to hydrogen peroxide, which is reduced by catalases and GPx to water and oxygen [60, 93].
There is evidence that antioxidant enzymes activities can be modulated by polyphenols. Low polyphenol diets decrease the plasma antioxidant capacity by diminishing the activity of endogenous enzymes, such as CAT and SOD [94, 95]. In vitro and in vivo studies in animal models have shown that polyphenols can modulate the antioxidant plasma status by scavenging ROS and modifying the antioxidant activity of enzymes. Demonstrating the opposite effect, consumption of polyphenols from strawberries, licorice, grape seed, chokeberry, jaboticaba peel, and other fruits have demonstrated to increase the enzymatic activity of CAT and SOD, as well as other relevant enzymes, in a similar manner to what we found in our research [60, 94–98].
The tropical highland blackberry is particularly rich in different polyphenols that can help by scavenging oxygen reactive species. Doronicheva et al. [99] suggested that polyphenols, especially flavonoids bind to CAT and enhance the enzyme’s activity. In our study, CAT enzymatic activity remained unchanged in the participants while consuming water but showed a non-statistically significant increase after consuming blackberry beverage. Consumption of dietary polyphenols, such as flavanols and anthocyanins, restores and enhances the redox homeostasis activities of antioxidant enzymes, partly by binding directly to the heme group or proteins in CAT structure, partly by directly scavenging and decreasing the ROS levels. This fact could explain the biological effect observed for CAT in our study [18, 24, 27, 91, 99, 100]. Plasma CAT enzymatic activity increases in obese rats consuming a jaboticaba juice, with a similar phytochemical profile of our Rubus adenotrichos Schltdl. beverage. A similar pattern is observed in healthy volunteers consuming a blackberry juice [91, 96]. These results agree with our investigation.
Animal studies have found an increase in plasma SOD enzymatic activity after consumption of anthocyanins and ellagitannins [92, 99]. Rats consuming an aqueous extract, an anthocyanin-enriched fraction, and the ellagitannin-enriched fraction of blackberry (Rubus fruticosus) have increased plasma SOD enzymatic activity [101].
In human interventions, three out of eight studies on the effect of berries on endogenous antioxidant enzymes found a significant increase in SOD levels [102]. Our results showed the same pattern for CAT. As reported shown in Table 6, CAT enzymatic activity remained the same while participants drank water and increased after participants consumed blackberry beverage, although, not statistically significant.
Because the present study was an exploratory assessment, the activity of the blackberry beverage should be tested on a wider range of plasma antioxidant enzymes, such as glutathione peroxidase (GPx) and glutathione reductase, which together with CAT and SOD is part of our antioxidant enzyme system.
5Conclusion
This clinical trial showed that healthy participants consuming a highland blackberry beverage from a micro-filtered juiced for 7 days while subjected to a high-fat, high-carbohydrate diet challenge had a significant reduction in total cholesterol (8.10%; p = 0.040) and triglycerides levels (26.33%; p = 0.010). A five-point reduction (mg/dL) on fasting glucose levels and a slight but not significant increase in SOD and CAT enzyme activities were observed. This latter result also suggests that the blackberry beverage had an antioxidant effect. We can assume that the protective antioxidant activity observed could be attributed to the different polyphenols contained in the beverage.
Supplementation with a polyphenol-rich highland blackberry beverage from a micro-filtered juice may have a beneficial impact on the cardiovascular well-being of healthy humans. Further research should be conducted to determine the clinical effects of such supplementation on dyslipidaemic individuals and patients with altered glucose metabolism. Likewise, more research is needed to see if these benefits can be observed in other processed foods from the tropical highland blackberry.
Funding
The authors report no funding.
Conflicts of interest
The authors have no conflict of interest to report.
Author contributions
Quesada-Morúa: Investigation, resources, visualization, writing: original draft preparation, reviewing and editing.
Hidalgo: Data curation, visualization, writing: original original draft preparation, reviewing and editing.
Morera: formal data analysis (statistics).
Rojas: conceptualization, methodology.
Pérez: resources, funding acquisition, validation, writing: reviewing and editing.
Vaillant: methodology, validation, writing: reviewing and editing.
Fonseca: supervision, project administration.
Acknowledgments
This work was supported by the European Commission FP6 (PAVUC project INCO contract 015279), and the Vicerrectoría de Investigación from the University of Costa Rica (project No. 817-A6-329).
References
[1] | Baker P , Friel S . Processed foods and the nutrition transition: Evidence from Asia: Processed foods and nutrition transition in Asia. Obes Rev. (2014) ;15: :564–77. doi: 10.1111/obr.12174 |
[2] | Monteiro CA , Levy RB , Claro RM , et al. Increasing consumption of ultra-processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutr. (2010) ;14: :5–13. doi: 10.1017/S1368980010003241 |
[3] | Rampersaud GC , Pereira MA , Girard BL , et al. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. (2005) ;105: :743–60. doi: 10.1016/j.jada.2005.02.007 |
[4] | Stuckler D , McKee M , Ebrahim S , et al. Manufacturing epidemics: The role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. (2012) ;9: :e1001235. doi: 10.1371/journal.pmed.1001235 |
[5] | Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, Weltgesundheitsorganisation, FAO (eds). Diet, nutrition, and the prevention of chronic diseases: Report of a WHO-FAO Expert Consultation; [JointWHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland]. Geneva:World Health Organization, 2003. ISBN: 978-92-4-120916-8 |
[6] | Yang Q , Zhang Z , Gregg EW , et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. (2014) ;174: :516. doi: 10.1001/jamainternmed.2013.13563 |
[7] | Jones PJ . Clinical nutrition: 7. Functional foods — more than just nutrition. CMAJ Can Med Assoc J. (2002) ;166: :1555–63. PMCID: PMC113804 |
[8] | Moubarac J-C , Martins APB , Claro RM , et al. Consumption of ultra-processed foods and likely impact on human health. Evidence from Canada. Public Health Nutr. (2013) ;16: :2240–8. doi: 10.1017/S1368980012005009 |
[9] | Al Sifri SN , Almahmeed W , Azar S , et al. Results of the Dyslipidemia International Study (DYSIS)-Middle East: Clinical perspective on the prevalence and characteristics of lipid abnormalities in the setting of chronic statin treatment. PLoS One. (2014) ;9: :e84350. doi: 10.1371/journal.pone.0084350 |
[10] | Nordestgaard BG , Varbo A . Triglycerides and cardiovascular disease. The Lancet. (2014) ;384: :626–35. doi: 10.1016/S0140-6736(14)61177-6 |
[11] | Rauber F , Campagnolo PDB , Hoffman DJ , et al. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr Metab Cardiovasc Dis. (2015) ;25: :116–22. doi: 10.1016/j.numecd.2014.08.001 |
[12] | Boeing H , Bechthold A , Bub A , et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. (2012) ;51: :637–63. doi: 10.1007/s00394-012-0380-y |
[13] | Gan Y , Tong X , Li L , et al. Consumption of fruit and vegetable and risk of coronary heart disease: A meta-analysis of prospective cohort studies. Int J Cardiol. (2015) ;183: :129–37. doi: 10.1016/j.ijcard.2015.01.077 |
[14] | Wang X , Ouyang Y , Liu J , et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. (2014) ;349: :g4490–g4490. doi: 10.1136/bmj.g4490 |
[15] | Battino M , Forbes-Hernández TY , Gasparrini M , et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit Rev Food Sci Nutr. (2019) ;59: :893–920. doi: 10.1080/10408398.2018.1526165 |
[16] | Rangel-Huerta OD , Pastor-Villaescusa B , Aguilera CM , et al. A Systematic review of the efficacy of bioactive compounds in cardiovascular disease: Phenolic compounds. Nutrients. (2015) ;7: :5177–216. doi: 10.3390/nu7075177 |
[17] | Alissa EM , Ferns GA . Dietary Fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. 2015;00-00. doi: 10.1080/10408398.2015.1040487 |
[18] | Amiot MJ , Riva C , Vinet A . Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes Rev Off J Int Assoc Study Obes. (2016) ;17: :573–86. doi: 10.1111/obr.12409 |
[19] | Ponzo V , Goitre I , Fadda M , et al. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J Transl Med. (2015) ;13: :218. doi: 10.1186/s12967-015-0573-2 |
[20] | Akhtar MJ , Ahamed M , Alhadlaq HA , et al. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim Biophys Acta BBA –Gen Subj. (2017) ;1861: :802–13. doi: 10.1016/j.bbagen.2017.01.018 |
[21] | Cho BO , Ryu HW , Jin CH , et al. Blackberry extract attenuates oxidative stress through up-regulation of nrf2-dependent antioxidant enzymes in carbon tetrachloride-treated rats. J Agric Food Chem. (2011) ;59: :11442–8. doi: 10.1021/jf2021804 |
[22] | Kasote DM , Katyare SS , Hegde MV , et al. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. (2015) ;11: :982–91. doi: 10.7150/ijbs.12096 |
[23] | Koca I , Karadeniz B . Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci Hortic. (2009) ;121: :447–50. doi: 10.1016/j.scienta.2009.03.015 |
[24] | Pisoschi AM , Pop A . The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. (2015) ;97: :55–74. doi: 10.1016/j.ejmech.2015.04.040 |
[25] | Sariburun E , Şahin S , Demir C , et al. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J Food Sci. (2010) ;75: :C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x |
[26] | Wolfe KL , Kang X , He X , et al. Cellular antioxidant activity of common fruits. J Agric Food Chem. (2008) ;56: :8418–26. doi: 10.1021/jf801381y |
[27] | Zhang H , Tsao R . Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. (2016) ;8: :33–42. doi: 10.1016/j.cofs.2016.02.002 |
[28] | Schulz M , Chim JF . Nutritional and bioactive value of Rubus berries. Food Biosci. (2019) ;31: :100438. doi: 10.1016/j.fbio.2019.100438 |
[29] | Mertz C , Cheynier V , Günata Z , et al. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J Agric Food Chem. (2007) ;55: :8616–24. doi: 10.1021/jf071475d |
[30] | Gancel A-L , Feneuil A , Acosta O , et al. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res Int. (2011) ;44: :2243–2251. doi: 10.1016/j.foodres.2010.06.013 |
[31] | Acosta-Montoya Ó , Vaillant F , Cozzano S , et al. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl. ) during three edible maturity stages. Food Chem. (2010) ;119: :1497–501. doi: 10.1016/j.foodchem.2009.09.032 |
[32] | Azofeifa G , Quesada S , Boudard F , et al. Antioxidant and anti-inflammatory in vitro activities of phenolic compounds from tropical highland blackberry (Rubus adenotrichos). J Agric Food Chem. (2013) ;61: :5798–804. doi: 10.1021/jf400781m |
[33] | García-Muñoz C , Hernández L , Pérez A , et al. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) juice. Food Res Int. (2014) ;55: :161–9. doi: 10.1016/j.foodres.2013.10.049 |
[34] | Paredes-López O , Cervantes-Ceja ML , Vigna-Pérez M , et al. Berries: improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum Nutr. (2010) ;65: :299–308. doi: 10.1007/s11130-010-0177-1 |
[35] | Soto M , Pérez AM , Cerdas M del M , et al. Physicochemical characteristics and polyphenolic compounds of cultivated blackberries in Costa Rica. J Berry Res. (2019) ;9: :283–96. doi: 10.3233/JBR-180353 |
[36] | Azofeifa G , Quesada S , Navarro L , et al. Hypoglycaemic, hypolipidaemic and antioxidant effects of blackberry beverage consumption in streptozotocin-induced diabetic rats. J Funct Foods. (2016) ;26: :330–7. doi: 10.1016/j.jff.2016.08.007 |
[37] | Azofeifa G , Quesada S , Pérez AM , et al. Effect of an in vitro digestion on the antioxidant capacity of a microfiltrated blackberry juice (Rubus adenotrichos). Beverages. (2018) ;4: :30. doi: 10.3390/beverages4020030 |
[38] | Calvo-Castro L , Syed DN , Chamcheu JC , et al. Protective effect of tropical highland blackberry juice (Rubus adenotrichos Schltdl. ) against UVB-mediated damage in human epidermal keratinocytes and in a reconstituted skin equivalent model. Photochem Photobiol. (2013) ;89: :1199–207. doi: 10.1111/php.12104 |
[39] | Acosta O , Vaillant F , Pérez A , et al. Potential of ultrafiltration for separation and purification of ellagitannins in blackberry (Rubus adenotrichus Schltdl. ) juice. Sep Purif Technol. (2014) ;125: :120–5. doi: 10.1016/j.seppur.2014.01.037 |
[40] | Acosta O , Vaillant F , Pérez AM , et al. Concentration of polyphenolic compounds in blackberry (Rubus Adenotrichos Schltdl. ) juice by nanofiltration. J Food Process Eng. (2017) ;40: :e12343. doi: 10.1111/jfpe.12343 |
[41] | Azofeifa G , Quesada S , Pérez A , et al. Pasteurization of blackberry juice preserves polyphenol-dependent inhibition for lipid peroxidation and intracellular radicals. J Food Compos Anal. (2015) ;42: :56–62. doi: 10.1016/j.jfca.2015.01.015 |
[42] | Farias-Cervantes VS , Chávez-Rodríguez A , García-Salcedo PA , et al. Antimicrobial effect and in vitro release of anthocyanins from berries and Roselle obtained via microencapsulation by spray drying. J Food Process Preserv. (2018) ;42: :e13713. doi: 10.1111/jfpp.13713 |
[43] | Cuevas-Rodríguez EO , Dia VP , Yousef GG , et al. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J Agric Food Chem. (2010) ;58: :9542–8. doi: 10.1021/jf102590p |
[44] | Araya M , Carvajal Y , Alvarez V , et al. Polyphenol characterization of three varieties of Blackberry fruits (Rubus adenotrichos), cultivated in Costa Rica. J Berry Res. (2017) ;7: :97–107. doi: 10.3233/JBR-170150 |
[45] | Soto M , Acosta O , Vaillant F , et al. Effects of mechanical and enzymatic pretreatments on extraction of polyphenols from blackberry fruits. J Food Process Eng. (2016) ;39: :492–500. doi: 10.1111/jfpe.12240 |
[46] | Vaillant F , Pérez AM , Acosta O , et al. Turbidity of pulpy fruit juice: a key factor for predicting cross-flow microfiltration performance. J Membr Sci. (2008) ;325: :404–12. doi: 10.1016/j.memsci.2008.08.003 |
[47] | Georgé S , Brat P , Alter P , et al. Rapid determination of polyphenols and vitamin C in plant-derived products. J Agric Food Chem. (2005) ;53: :1370–3. doi: 10.1021/jf048396b |
[48] | Ou B , Hampsch-Woodill M , Flanagan J , et al. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J Agric Food Chem. (2002) ;50: :2772–7. doi: 10.1021/jf011480w |
[49] | Mertens-Talcott SU , Rios J , Jilma-Stohlawetz P , et al. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart. ) in human healthy volunteers. J Agric Food Chem. (2008) ;56: :7796–802. doi: 10.1021/jf8007037 |
[50] | Törrönen R , Sarkkinen E , Tapola N , et al. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br J Nutr. (2010) ;103: :1094–7. doi: 10.1017/S0007114509992868 |
[51] | Damasceno NRT , Pérez-Heras A , Serra M , et al. Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis NMCD. (2011) ;21: Suppl 1:S14–20. doi: 10.1016/j.numecd.2010.12.006 |
[52] | Cerdá B , Espín JC , Parra S , et al. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. (2004) ;43: :205–20. doi: 10.1007/s00394-004-0461-7 |
[53] | Jensen GS , Wu X , Patterson KM , et al. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem. (2008) ;56: :8326–33. doi: 10.1021/jf8016157 |
[54] | Alvarez-Suarez JM , Giampieri F , Tulipani S , et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J Nutr Biochem. (2014) ;25: :289–94. doi: 10.1016/j.jnutbio.2013.11.002 |
[55] | Basu A , Wilkinson M , Penugonda K , et al. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr J. (2009) ;8: :43. doi: 10.1186/1475-2891-8-43 |
[56] | Qin Y , Xia M , Ma J , et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. (2009) ;90: :485–92. doi: 10.3945/ajcn.2009.27814 |
[57] | Lee J , Dossett M , Finn CE . Rubus fruit phenolic research: the good, the bad, and the confusing. Food Chem. (2012) ;130: :785–96. doi: 10.1016/j.foodchem.2011.08.022 |
[58] | Kaume L , Howard LR , Devareddy L . The Blackberry Fruit: a review on its composition and chemistry, metabolism and bioavailability, and health benefits. J Agric Food Chem. (2012) ;60: :5716–27. doi: 10.1021/jf203318p |
[59] | Azofeifa G , Quesada S , Pérez A-M . Effect of the microfiltration process on antioxidant activity and lipid peroxidation protection capacity of blackberry juice. Rev Bras Farmacogn. (2011) ;21: :829–34. doi: 10.1590/S0102-695X2011005000133 |
[60] | Hong Y-K , Wu H-T , Ma T , et al. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int J Biol Macromol. (2009) ;45: :61–4. doi: 10.1016/j.ijbiomac.2009.04.001 |
[61] | Giampieri F , Forbes-Hernandez TY , Gasparrini M , et al. The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases: strawberry and disease prevention. Ann N Y Acad Sci. (2017) ;1398: :62–71. doi: 10.1111/nyas.13373 |
[62] | Hassan HA , Yousef MI . Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol. (2009) ;47: :2332–7. doi: 10.1016/j.fct.2009.06.023 |
[63] | He J , Giusti MM . Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. (2010) ;1: :163–87. doi: 10.1146/annurev.food.080708.100754 |
[64] | Ishimoto H , Shibata M , Myojin Y , et al. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg Med Chem Lett. (2011) ;21: :5901–4. doi: 10.1016/j.bmcl.2011.07.086 |
[65] | Kosmala M , Zduńczyk Z , Juśkiewicz J , et al. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J Agric Food Chem. (2015) ;63: :2989–96. doi: 10.1021/acs.jafc.5b00648 |
[66] | Riso P , Klimis-Zacas D , Del Bo’ C , et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. (2013) ;52: :949–61. doi: 10.1007/s00394-012-0402-9 |
[67] | Yang Y , Andrews MC , Hu Y , et al. Anthocyanin extract from black rice significantly ameliorates platelet hyperactivity and hypertriglyceridemia in dyslipidemic rats induced by high fat diets. J Agric Food Chem. (2011) ;59: :6759–64. doi: 10.1021/jf201079h |
[68] | Yu Y-M , Chang W-C , Wu C-H , et al. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem. (2005) ;16: :675–81. doi: 10.1016/j.jnutbio.2005.03.013 |
[69] | Toscano LT , Silva AS , Toscano LT , et al. Phenolics from purple grape juice increase serum antioxidant status and improve lipid profile and blood pressure in healthy adults under intense physical training. J Funct Foods. (2017) ;33: :419–24. doi: 10.1016/j.jff.2017.03.063 |
[70] | Forbes-Hernández T , Giampieri F , Gasparrini M , et al. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients. (2017) ;9: :621. doi: 10.3390/nu9060621 |
[71] | Afrin S , Gasparrini M , Forbes-Hernandez TY , et al. Promising health benefits of the strawberry: a focus on clinical studies. J Agric Food Chem. (2016) ;64: :4435–49. doi: 10.1021/acs.jafc.6b00857 |
[72] | Shi N , Clinton S , Liu Z , et al. Strawberry Phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients. (2015) ;7: :1696–715. doi: 10.3390/nu7031696 |
[73] | Winardiantika V , Lee YH , Park NI , et al. Effects of cultivar and harvest time on the contents of antioxidant phytochemicals in strawberry fruits. Hortic Environ Biotechnol. (2015) ;56: :732–9. doi: 10.1007/s13580-015-0052-y |
[74] | Cortés-Martín A , García-Villalba R , González-Sarrías A , et al. The gut microbiota urolithin metabotypes revisited: the human metabolism of ellagic acid is mainly determined by aging. Food Funct. (2018) ;9: :4100–6. doi: 10.1039/C8FO00956B |
[75] | Hollands W , Brett GM , Dainty JR , et al. Urinary excretion of strawberry anthocyanins is dose dependent for physiological oral doses of fresh fruit. Mol Nutr Food Res. (2008) ;52: :1097–105. doi: 10.1002/mnfr.200700372 |
[76] | Tomás-Barberán FA , García-Villalba R , González-Sarrías A , et al. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem. (2014) ;62: :6535–8. doi: 10.1021/jf5024615 |
[77] | Seeram NP , Adams LS , Hardy ML , et al. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem. (2004) ;52: :2512–7. doi: 10.1021/jf0352778 |
[78] | Kay CD . Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev. (2006) ;19: :137–46. doi: 10.1079/NRR2005116 |
[79] | Balisteiro DM , Araujo RL de , Giacaglia LR , et al. Effect of clarified Brazilian native fruit juices on postprandial glycemia in healthy subjects. Food Res Int. (2017) ;100: :196–203. doi: 10.1016/j.foodres.2017.08.044 |
[80] | Al-Awwadi NA , Araiz C , Bornet A , et al. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J Agric Food Chem. (2005) ;53: :151–7. doi: 10.1021/jf048919f |
[81] | Balisteiro DM , Alezandro MR , Genovese MI . Characterization and effect of clarified araçá (Psidium guineenses Sw. ) juice on postprandial glycemia in healthy subjects. Ciênc E Tecnol Aliment. (2013) ;33: :66–74. doi: 10.1590/S0101-20612013000500011 |
[82] | Johnson MH , de Mejia EG , Fan J , et al. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol Nutr Food Res. (2013) ;57: :1182–97. doi: 10.1002/mnfr.201200678 |
[83] | Juśkiewicz J , Król B , Kosmala M , et al. Physiological properties of dietary ellagitannin-rich preparations obtained from strawberry pomace using different extraction methods. Pol J Food Nutr Sci. (2015) ;65: :199–209. doi: 10.1515/pjfns-2015-0007 |
[84] | Kerimi A , Nyambe-Silavwe H , Gauer JS , et al. Pomegranate juice, but not an extract, confers a lower glycemic response on a high–glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am J Clin Nutr. (2017) ;106: :1384–93. doi: 10.3945/ajcn.117.161968 |
[85] | García-Conesa M-T , Chambers K , Combet E , et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int J Mol Sci. (2018) ;19: :694. doi: 10.3390/ijms19030694 |
[86] | Jennings A , Welch AA , Spector T , et al. Intakes of Anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J Nutr. (2014) ;144: :202–8. doi: 10.3945/jn.113.184358 |
[87] | Stull A . Blueberries’ impact on insulin resistance and glucose intolerance. Antioxidants. (2016) ;5: :44. doi: 10.3390/antiox5040044 |
[88] | Bandyopadhyay P , Ghosh AK , Ghosh C . Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. (2012) ;3: :592. doi: 10.1039/c2fo00006g |
[89] | Kato CG , Gonçalves G de A , Peralta RA , et al. Inhibition of α -amylases by condensed and hydrolysable tannins: Focus on kinetics and hypoglycemic actions. Enzyme Res. (2017) ;2017: :1–12. doi: 10.1155/2017/5724902 |
[90] | Seifried HE , Anderson DE , Fisher EI , et al. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. (2007) ;18: :567–79. doi: 10.1016/j.jnutbio.2006.10.007 |
[91] | Hassimotto NMA , Pinto MDS , Lajolo FM . Antioxidant status in humans after consumption of blackberry (Rubus fruticosus L. ) juices with and without defatted milk. J Agric Food Chem. (2008) ;56: :11727–33. doi: 10.1021/jf8026149 |
[92] | Pervin M , Hasnat MA , Lee YM , et al. Antioxidant activity and acetylcholinesterase inhibition of grape skin anthocyanin (GSA). Mol Basel Switz. (2014) ;19: :9403–18. doi: 10.3390/molecules19079403 |
[93] | Kehrer JP , Klotz L-O . Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Crit Rev Toxicol. (2015) ;45: :765–98. doi: 10.3109/10408444.2015.1074159 |
[94] | Fernández-Pachón MS , Berná G , Otaolaurruchi E , et al. Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J Agric Food Chem. (2009) ;57: :6578–83. doi: 10.1021/jf901863w |
[95] | Noguer MA , Cerezo AB , Donoso Navarro E , et al. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol Res. (2012) ;65: :609–14. doi: 10.1016/j.phrs.2012.03.003 |
[96] | Batista ÂG , Lenquiste SA , Cazarin CBB , et al. Intake of jaboticaba peel attenuates oxidative stress in tissues and reduces circulating saturated lipids of rats with high-fat diet-induced obesity. J Funct Foods. (2014) ;6: :450–61. doi: 10.1016/j.jff.2013.11.011 |
[97] | Kedzierska M , Olas B , Wachowicz B , et al. Changes of platelet antioxidative enzymes during oxidative stress: the protective effect of polyphenol-rich extract from berries of Aronia melanocarpa and grape seeds. Platelets. (2011) ;22: :385–9. doi: 10.3109/09537104.2010.545151 |
[98] | Alvarez-Suarez JM , Dekanski D , Ristić S , et al. Strawberry Polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One. (2011) ;6: :e25878. doi: 10.1371/journal.pone.0025878 |
[99] | Doronicheva N , Yasui H , Sakurai H . Chemical structure-dependent differential effects of flavonoids on the catalase activity as evaluated by a chemiluminescent method. Biol Pharm Bull. (2007) ;30: :213–7. doi: 10.1248/bpb.30.213 |
[100] | Masella R , Di Benedetto R , Varì R , et al. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. (2005) ;16: :577–86. doi: 10.1016/j.jnutbio.2005.05.013 |
[101] | Hassimotto NM , Lajolo FM . Antioxidant status in rats after long-term intake of anthocyanins and ellagitannins from blackberries. J Sci Food Agric. (2011) ;91: :523–31. doi: 10.1002/jsfa.4216 |
[102] | Del Bo’ C , Martini D , Porrini M , et al. Berries and oxidative stress markers: an overview of human intervention studies. Food Funct. (2015) ;6: :2890–917. doi: 10.1039/C5FO00657K |




