Strawberry extract attenuates oxidative stress in 3T3-L1 cells
Abstract
BACKGROUND:
High levels of reactive oxygen species (ROS) within the adipose tissue promote a disturbed redox balance and influence its function, impairing adipogenesis, inducing insulin resistance and stimulating adipocyte hypertrophy. Supplementation with antioxidant rich foods can reverse some of these effects. Strawberry is well known as a good source of phytochemicals; however, whether strawberry suppresses increased oxidative stress in 3T3-L1 cells remain unclear.
OBJECTIVE:
The purpose of the present work was to determine the antioxidant potential of a strawberry extract in 3T3-L1 mouse embryo fibroblast cell line.
METHODS:
3T3-L1 pre-adipocytes were induced to differentiate into adipocytes in the presence or absence of different concentrations of the strawberry extract. At the end of the differentiation period, intracellular ROS production, thiobarbituric acid reactive substances (TBARS) content, as well as superoxide dismutase (SOD) and catalase (CAT) activities and gene expressions were evaluated.
RESULTS:
In this study, we confirmed that strawberry extract markedly inhibited increased-oxidative stress in 3T3L1 cells by suppressing intracellular ROS production and decreasing TBARS content. Likewise, SOD and CAT activities and gene expressions were increased.
CONCLUSIONS:
This paper provides evidence that strawberry extract is able to scavenging free radicals and activate endogenous defense systems, highlighting its potential capacity to modulate obesity induced- inflammatory states.
Abbreviations
ROS | reactive oxygen species |
DMEM | Dulbecco’s Modified Eagle’s Medium |
FBS | fetal bovine serum |
MTT | 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide |
TBARS | thiobarbituric acid reactive substances |
TBA | thiobarbituric acid |
SOD | superoxide dismutase |
CAT | catalase |
1Introduction
White adipose tissue is mainly constituted by adipocytes (fat cells) and their precursor cells (pre-adipocytes) which differentiate into mature adipocytes through a complex process named adipogenesis [1, 2]. Redox environment determined by the state of the major redox pairs in the cells (e.g. GSSG/2GSH and NAD+/NADH) and reactive oxygen species (ROS) have been recognized as important modulators of this process [2]. ROS, including hydrogen peroxide (H2O2), superoxide anion (
Increased oxidative stress in adipocytes is considered an important pathogenic mechanism of obesity-associated metabolic syndrome [7]. It has been associated with insulin resistance and adipocyte hypertrophy which stimulates inflammation, altered metabolism and dysregulated adipokines secretion [2]. ROS are also required for adipocytes differentiation by regulating mitotic clonal expansion during adipogenesis [6, 7].
Conversely, supplementation with antioxidant compounds that reduce ROS levels, either by inhibiting ROS formation or by inducing antioxidant enzymes, has been demonstrated to improve insulin sensitivity, lipid metabolism and glucose homeostasis in different experimental models [2, 8]. In that sense, strawberries are recognized as a good source of phytochemicals compounds [9, 10] which present several biological activities including antioxidant [11, 12], antiatherogenic [13], anti-inflammatory [14–16] and anticarcinogenic [17–20]. However, to date the effects of strawberry in adipocytes remain unknown. Thus, the purpose of the present study was to determine the antioxidant potential of a strawberry extract in 3T3-L1 mouse embryo fibroblast cell line, one of the most well characterized and reliable models for studying adipocytes differentiation.
2Materials and methods
2.1Strawberry extract preparation
Strawberry fruits Fragaria x ananassa were collected in the experimental fields of the Agricultural Faculty of the Universitá Politecnica delle Marche (UNIVPM, Italy). The strawberry variety selected was “Romina”, a cultivar released in 2011 as a result of the breeding program of UNIVPM [21]. For the extract preparation, 10 g of fruits were added to 100 mL of the extraction solution consisting of methanol/MilliQ water/formic acid (80:20:0.1 v/v) and homogenized using an Ultraturrax T25 homogenizer (Janke & Kunkel, IKA Labortechnik). Then, the mixture was centrifuged at 2400×g for 15 min in two sequential times and supernatants were filtered through a 0.45μm Minisart filter (PBI International). For cellular treatment, the extract was further concentrated through a rotary evaporator and stored in aliquots at –80°C.
2.2Cell culture and differentiation
3T3-L1 pre-adipocytes were purchased from the American Type Culture Collection (ATCC ® CL-173TM) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% bovine calf serum, 100 IU/mL penicillin and 100μg/mL streptomycin. To induce differentiation into mature adipocytes, 2-day postconfluent 3T3-L1 cells (designated as Day 0) were incubated in DMEM containing 10% fetal bovine serum (FBS), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 1μM dexamethasone and 1μg/mL insulin for 2 days. The differentiation medium was then replaced with DMEM containing 10% FBS and 1μg/mL insulin, which was changed every 48 h for another 8 days. Strawberry extract was added at Day 0 and maintained during cell differentiation until Day 10.
2.3Cell viability (MTT assay)
For cell viability assessment, 3T3-L1 pre-adipocytes were seeded at a density of 5×103 cells/well into 96-well plates and treated with increasing concentrations (from 0 to 1 mg/mL) of the strawberry extract for 24, 48 and 72 h. After treatment, 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (2 mg/mL) were added and cells were incubated for 2 h. The formazan crystals were dissolved in 100μL of dimethyl sulfoxide and the absorbance was read at 590 nm [22, 23] on a microplate reader (Thermo Scientific Multiskan EX).
2.4Assessment of intracellular ROS production by the Tali® Image-Based cytometer
Intracellular ROS levels were determined by the CellROX® Oxidative Stress Kit (Invitrogen TM, Life Technologies, Milan, Italy) according to the manufacturer’s instructions. Briefly, cells were seeded in 6-well plates at a density of 8×104 cells/well and incubated with different concentrations (10, 50 and 100μg/mL) of the strawberry extract for 24 h or throughout the differentiation period (Day 0 to Day 10). The strawberry extract concentrations used were chosen according to the MTT assay. After treatment, cells were trypsinized and centrifuged at 556×g for 10 min at 4 °C. The pellet was then re-suspended in 1 mL of complete medium containing the CellROX® Orange Reagent at a final concentration of 5μM. Samples were incubated for 30 min at 37 °C, centrifuged and re-suspended again in PBS. After labeling with CellROX® Orange Reagent, cells were analyzed with the Tali® Image-Based cytometer (Thermo Fisher Scientific, Milan, Italy).
2.5Cells’ lysates preparation
Cells were seeded in 6-well plates at a density of 8×104 cells/well and incubated with different concentrations (10, 50 and 100μg/mL) of the strawberry extract for 24 h or throughout the differentiation period (Day 0 to Day 10). The strawberry extract concentrations were chosen according to the MTT assay. After treatment, cells were lysed in a buffer containing 20 mM Tris–HCl (pH 7.5), 0.2% Triton X-100, 0.9% NaCl and 1% of the protease inhibitor cocktail (Sigma-Aldrich, Milan, Italy) for further western blot analysis or were lysed in the RIPA buffer (Sigma-Aldrich, Milan, Italy) for the determination of lipid peroxidation and antioxidant enzymes activity.
2.6Determination of lipid peroxidation
Lipid peroxidation was determined by the thiobarbituric acid reactive substances (TBARS) assay according to the method proposed by Ohkawa et al. [24]. Briefly, 300μL of cellular lysate were mixed with the thiobarbituric acid (TBA) reagent (TBA, 0.37% in 0.2 M HCl) and 15% trichloroacetic acid and heated at 95 °C for 20 min. Then, the mixture was chilled, centrifuged at 1200×g for 15 min at 4 °C and the absorbance was measured at 532 nm.
2.7Determination of antioxidant enzymes activity
Superoxide dismutase (SOD) activity was evaluated according to the method proposed by Kakkar et al. [25]. Briefly, 10μL of the cellular lysate were added to the assay mixture consisted of 1.2 mL of sodium pyrophosphate buffer (0.025 M), 100μL of phenazine methosulfate (186μM), 300μL of nitroblue tetrazolium (300μM), 190μL of PBS and 1 mL of water. The reaction was then initiated by the addition of 10μL of NADH, and the samples were incubated at 30 °C for 90 s. Subsequently, 1 mL of glacial acetic acid was added and the reaction mixture was stunned with 2 mL of n-butanol, allowed to stand for 10 min and centrifuged at 1300×g for another 10 min. The absorbance was measured at 540 nm in a microplate reader (Thermo Scientific, Multiskan® EX, Monza, Italy).
Catalase (CAT) activity was assayed according to the method proposed by Aebi, [26]. Briefly, 10μL of the cellular lysate were added to the assay mixture consisted of 990μL of sodium phosphate buffer (50 mM) and 500μL of H2O2 (30%). The absorbance diminution caused by H2O2 degradation was monitored at 240 nm after 10 to 70 s of reaction.
2.8Western blotting analysis
Equal amounts of cellular lysates (75μg of protein) were subjected to 10% acrylamide sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE), transferred to nitrocellulose membranes and immunoblotted with antibodies to SOD (sc-11407), CAT (sc-271803) and GADPH (sc-25778) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Protein bands were visualized using Immobilon Western Chemiluminescent Substrate (Millipore Corporation, Billerica, MA, USA) and the protein signals were detected by a Lycor C-Digit Blot Scanner. Quantification of protein expression was made using the software (Image Studio 3.1) provided by the manufacturer of the Blot Scanner (LI-COR Biotechnology, Bad Homburg, Germany).
2.9Statistical analysis
Statistical analyses were performed using STATISTICA software (Statsoft Inc., Tulsa, OK, USA). Data were subjected to one-way analysis of variance for mean comparison and significant differences among treatments were calculated according to Tukey’s HSD (honest significant difference) multiple range test. Data was reported as mean±standard deviation (SD). Differences at p < 0.05 were considered statistically significant. All the analyses were performed in triplicate.
3Results
The strawberry extract used in the present study was previously characterized by our group [27]. Pelargonidin 3-O-glucoside (29.30±0.59 mg/g) represented the main bioactive compound (about 80% of the total anthocyanins content), while ellagic acid derivatives (1.74±0.12 mg/g) and flavonols/dihydroflavonols (0.26±0.01 mg/g) were quantified in lower amounts. It presented a high antioxidant capacity as determined by the ferric-reducing antioxidant power (168.25±3.95μmol Trolox equivalent/g) and the trolox equivalent antioxidant capacity (35.51±0.06μmol Trolox equivalent/g) methods.
3.1Cytotoxic effects of the strawberry extract
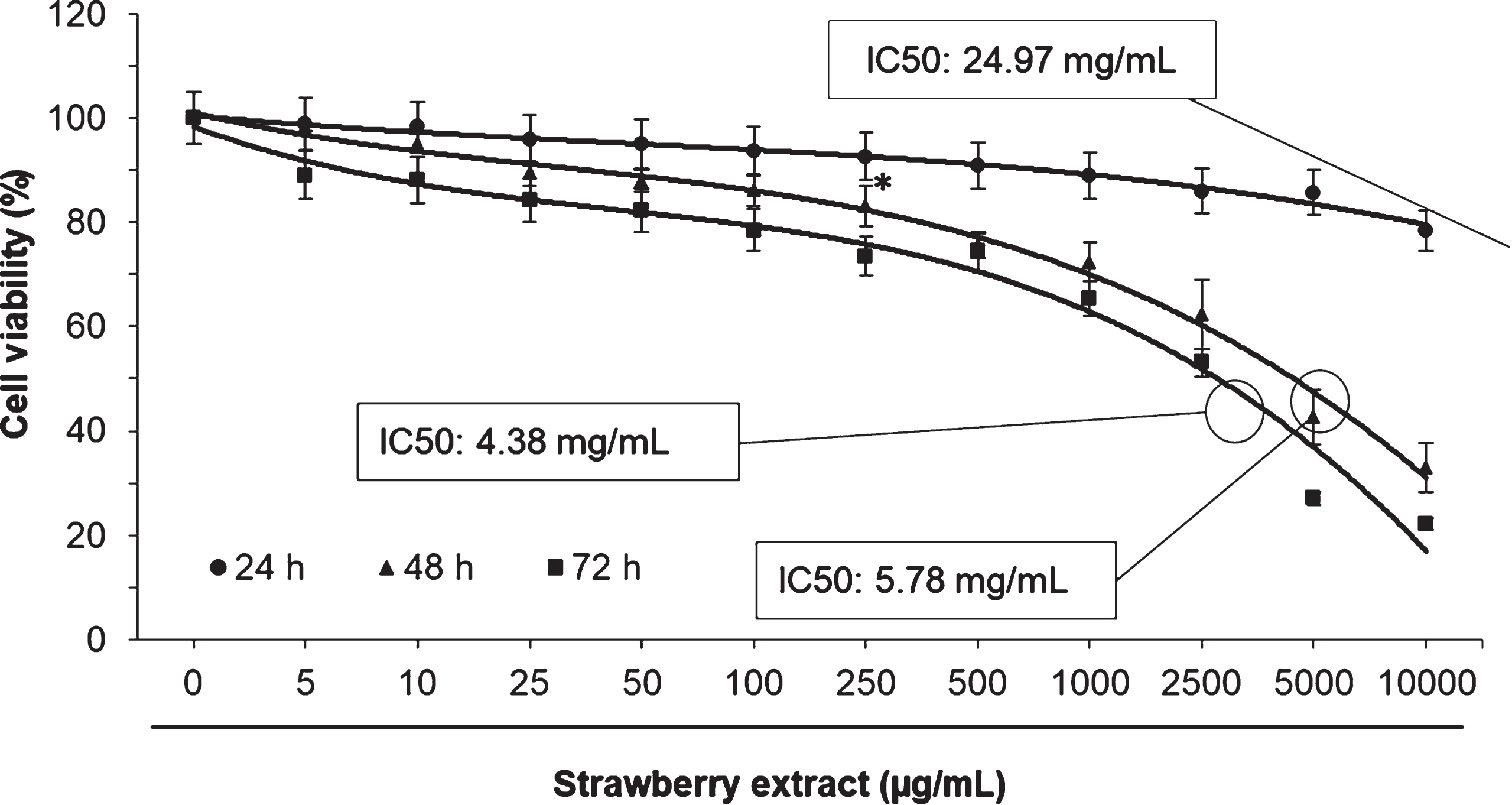
In order to evaluate the cytotoxic effects of the strawberry extract on 3T3-L1 cells a MTT assay was performed. As shown in Fig. 1, the strawberry extract did not cause significant effects (p < 0.05) on cell viability at concentrations lower than 100μg/mL. In all the evaluated times, the IC50 was greater than 1 mg/mL. Therefore, the concentrations 10, 50, and 100μg/mL were used in subsequent experiments.
Fig. 1
Effects of strawberry extract on 3T3-L1 cells viability as measured by the MTT assay. Cells were treated with the indicated concentration for 24, 48 or 72 h. IC50 indicates the concentration of strawberry extract which reduces the cells viability about 50%. Values are expressed as mean±SD of three independent experiments (n = 3). The asterisk indicates the concentrations from which significant differences (p < 0.05) were observed compared to the control.
3.2Effects of the strawberry extract on intracellular ROS production and lipid peroxidation
Once demonstrated that strawberry extract did not affect cell proliferation at concentrations ≤100μg/mL, the effects on intracellular ROS production and lipid peroxidation were evaluated.
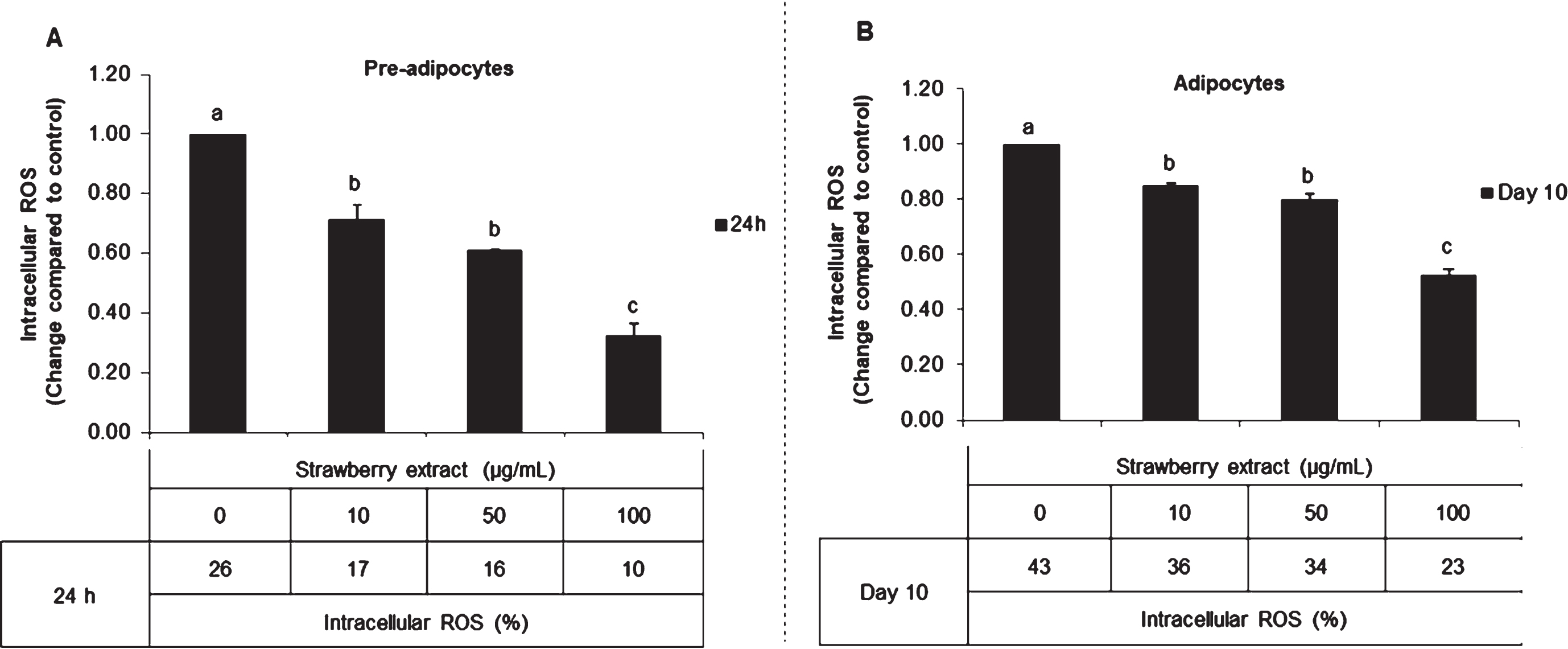
In untreated mature adipocytes, the intracellular ROS content was 0.6 times higher compared to pre-adipocytes without treatment. In both conditions, strawberry supplementation reduced the ROS levels. In pre-adipocytes (Fig. 2A), strawberry extract decreased ROS production up to 0.65, 0.61 and 0.38 fold compared to control when applied for 24 h at 10, 50, and 100μg/mL, respectively. Also in mature adipocytes (Fig. 2B), strawberry extract significantly (p < 0.05) decreased ROS production up to 0.85, 0.80 and 0.53 fold compared to control in correspondence with 10, 50, and 100μg/mL, respectively.
Fig. 2
Effects of strawberry extract on intracellular ROS production in 3T3-L1 cells as quantified by the Tali® Image-Based Cytometer. 3T3-L1 pre-adipocytes were incubated with the indicated concentrations of strawberry extract for 24 h (A) or induced to differentiate into mature adipocytes in the presence or absence of the strawberry extract as described above (B). The concentration of 0μg/mL corresponds to the control (untreated cells). Values are expressed as mean±SD of three independent experiments (n = 3). Different superscript letters for the same set of data indicate significant differences (p < 0.05).
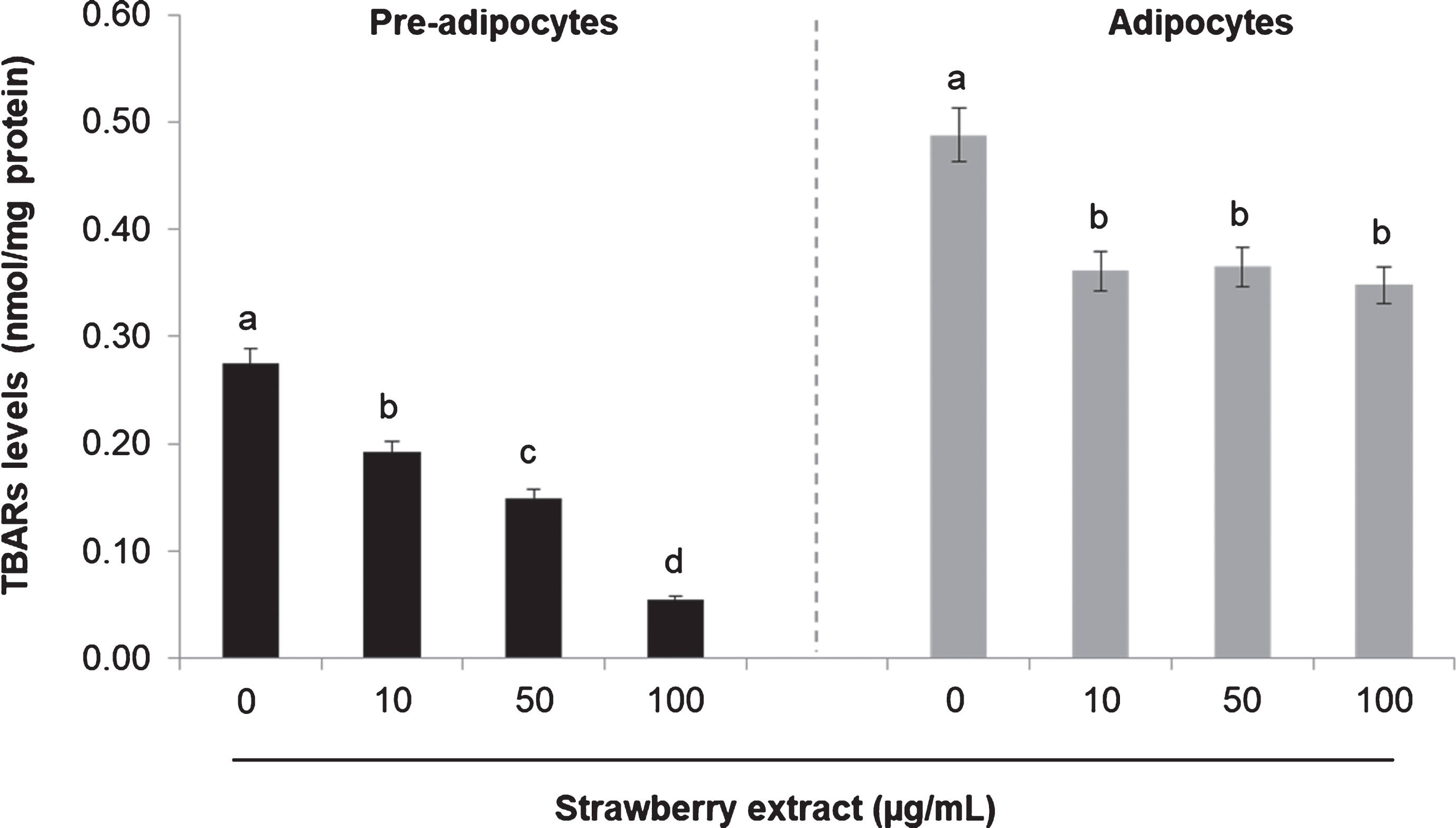
Treatment with strawberry extract also reduced lipid peroxidation as can be noticed in Fig. 3. In pre-adipocytes the highest concentration of the strawberry extract significantly (p < 0.05) decreased TBARs levels from 0.27±0.06 nmol/mg protein (in control) to 0.05±0.04 nmol/mg protein, while in mature adipocytes the same strawberry extract concentration caused a reduction from 0.48±0.04 nmol/mg protein (in control) to 0.34±0.02 nmol/mg protein. Also in this case, the basal level in mature adipocytes was considerably higher compared to undifferentiated cells prior to strawberry treatment (Fig. 3).
Fig. 3
Effects of strawberry extract on lipid peroxidation in 3T3-L1 cells as measured by the TBARs Assay. 3T3-L1 pre-adipocytes were incubated with the indicated concentrations of strawberry extract for 24 h or induced to differentiate into mature adipocytes in the presence or absence of the strawberry extract as described above. The concentration of 0μg/mL corresponds to the control (untreated cells). Values are expressed as mean±SD of three independent experiments (n = 3). Different superscript letters for the same set of data indicate significant differences (p < 0.05).
3.3Effects of the strawberry extract on antioxidant enzymes activity and protein expression
The activity and the protein expression of the antioxidant enzymes SOD and CAT were also evaluated. In mature adipocytes SOD (Fig. 4A) and CAT (Fig. 5A) activities was considerably lower compared to undifferentiated cells (considering the untreated cells).
Fig. 4
Effects of strawberry extract on superoxide dismutase (SOD) activity (A) or protein expression (B) in 3T3-L1 cells. The enzyme activity was measured by a colorimetric method while the protein expression was evaluated by western blotting. The protein signals were detected by a Lycor C-Digit Blot Scanner and quantification was made using the software Image Studio 3. 3T3-L1 pre-adipocytes were incubated with the indicated concentrations of strawberry extract for 24 h or induced to differentiate into mature adipocytes in the presence or absence of the strawberry extract as described above. The concentration of 0μg/mL corresponds to the control (untreated cells). Values are expressed as mean±SD of three independent experiments (n = 3). Different superscript letters for the same set of data indicate significant differences (p < 0.05).
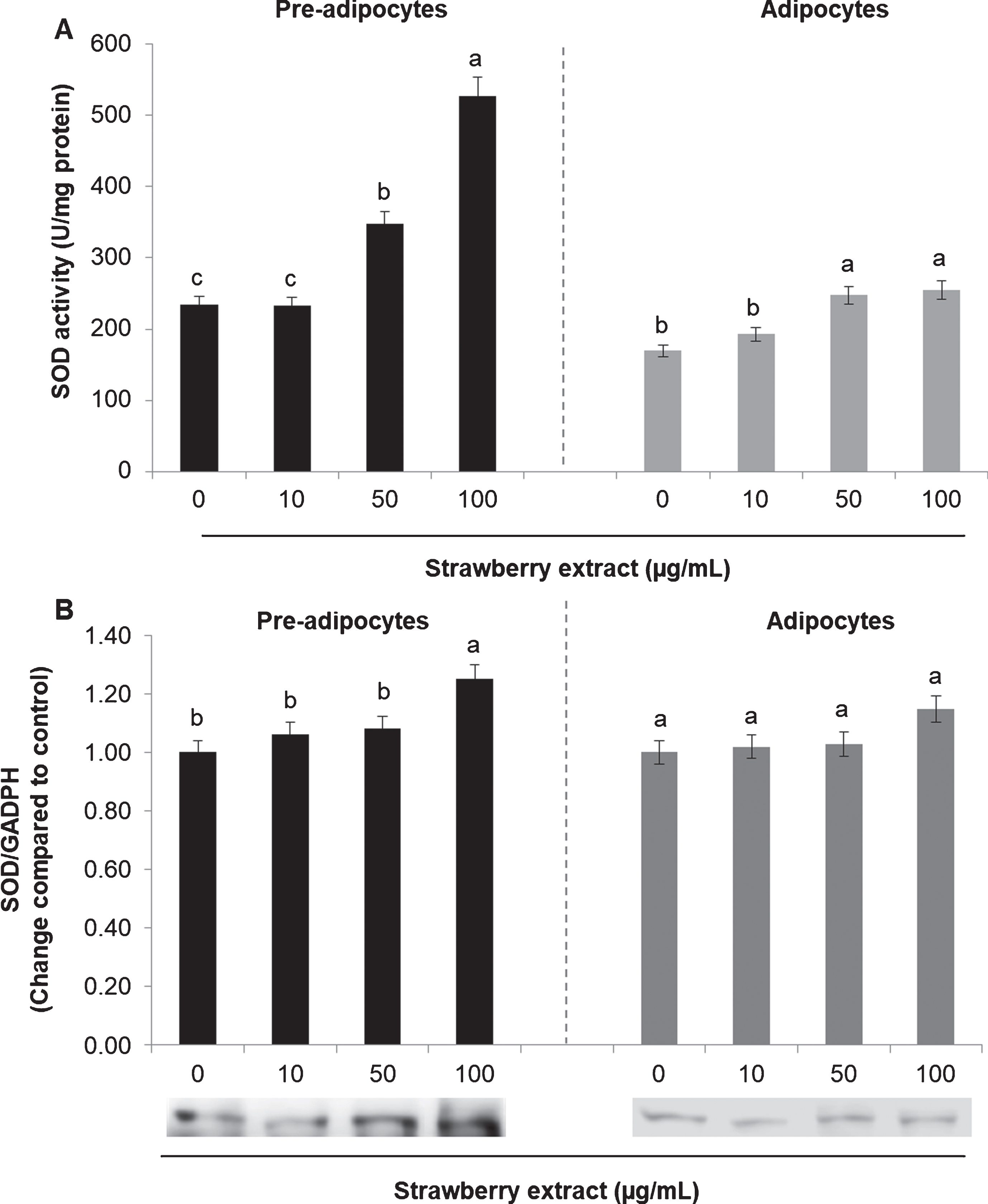
Fig. 5
Effects of strawberry extract on catalase (CAT) activity (A) or protein expression (B) in 3T3-L1 cells. The enzyme activity was measured by a colorimetric method while the protein expression was evaluated by western blotting. The protein signals were detected by a Lycor C-Digit Blot Scanner and quantification was made using the software Image Studio 3. 3T3-L1 pre-adipocytes were incubated with the indicated concentrations of strawberry extract for 24 h or induced to differentiate into mature adipocytes in the presence or absence of the strawberry extract as described above. The concentration of 0μg/mL corresponds to the control (untreated cells). Values are expressed as mean±SD of three independent experiments (n = 3). Different superscript letters for the same set of data indicate significant differences (p < 0.05).
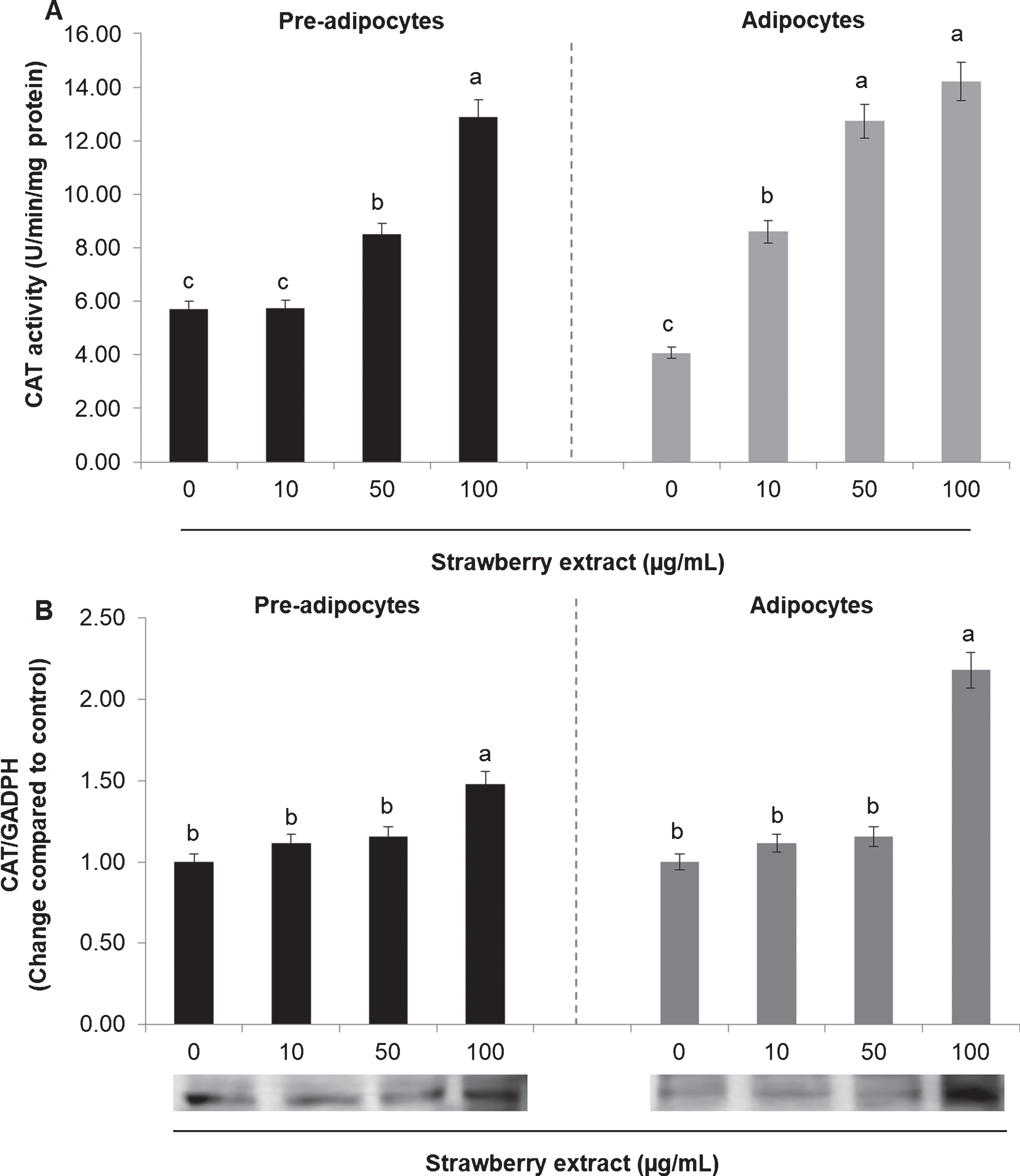
In both cases (either in pre-adipocytes and adipocytes) strawberry extract significantly increased (p < 0.05) the SOD activity when applied at 50 or 100μg/mL (Fig. 4A) while it didn’t cause significant (p < 0.05) effects in the protein expression in mature adipocytes. In pre-adipocytes the strawberry treatment increased the SOD expression only when applied at the higher concentration (100μg/mL) (Fig. 4B).
Regarding the CAT, strawberry extract caused an augmentation in the enzyme activity in a dose-dependent manner (Fig. 5A) while it increased the protein expression only at the highest concentration (Fig. 5B) for both pre-adipocytes and mature adipocytes.
4Discussion and conclusions
Oxidative stress has been associated to several metabolic diseases, including type 2 diabetes, insulin resistance, cardiovascular diseases and obesity. In adipocytes, ROS overproduction has been associated with multiple forms of insulin resistance [2, 7] and with some modifications in endoplasmic reticulum, mitochondrial functionality and cell signaling, that lead to inflammatory conditions [28]. The molecular mechanism involved could be based -at least in part- on ROS-induced production of the inflammatory cytokine tumor necrosis factor alpha (TNFα) which plays an essential role in insulin resistance through the down-regulation of insulin-stimulated glucose uptake and insulin receptor auto-phosphorylation [28]. In this work we demonstrated that strawberry treatment significantly decreased intracellular ROS production in a dose-dependent mode and it would be interesting to confirm in future studies if it capable of inhibiting the mentioned molecular pathways.
Likewise, increased biomarkers of lipid peroxidation such as TBARS and 4-hydroxynonenal protein adducts in adipose tissue has been significantly correlated with the body mass index in different animal and human models [2]. Also the inhibition of antioxidant enzymes has been related with increased ROS production and inflammatory states [28–31]. By contrast, mitochondrial-targeted antioxidant as well as overexpression of SOD and CAT have been associated to insulin sensitivity in mice fed a high-fat diet [2, 8]. In that sense, the observed diminution in TBARS levels as well as the stimulation of the antioxidant enzymes after strawberry treatment render this fruit a good candidate for the treatment and/or prevention of diabetes and obesity.
In conclusion, we demonstrated that strawberry supplementation significantly decreased ROS production and lipid peroxidation while increased antioxidant enzymes activities and expression in both pre-adipocytes and matures adipocytes. These effects may be attributable to strawberry polyphenols ability to scavenging free radicals and activate endogenous defense systems, highlighting its potential capacity to modulate obesity induced- inflammatory states.
Acknowledgments
Patricia Reboredo-Rodríguez is supported by a post-doctoral contract from Xunta de Galicia.
References
[1] | Li KK , Liu CL , Shiu HT , Wong HL , Siu WS , Zhang C , Han XQ , Ye CX , Leung PC , Ko CH . Cocoa tea (Camellia ptilophylla) waterextract inhibits adipocyte differentiation in mouse 3T3-L1 preadipocytes. Scientific Reports. (2017) ;6: :20172. doi: 10.1038/srep20172 |
[2] | Castro JP , Grune T , Speckmann B . The two faces of reactive oxygenspecies (ROS) in adipocyte function and dysfunction. Biol Chem. (2016) ;397: (8):709-24. doi: 10.1515/hsz-2015-0305 |
[3] | Halliwell B . Reactive species and antioxidants. Redox biology is afundamental theme of aerobic life. Plant Physiol. (2006) ;141: :312-22. doi: 10.1104/106.077073 |
[4] | Sies H . Role of metabolic H2O2 generation:Redox signaling and oxidative stress. J Biol Chem. (2014) ;289: :8735-874. doi: 10.1074/jbc.R113.544635 |
[5] | Alvarez-Suarez JM , Giampieri F , Cordero M , Gasparrini M , Forbes-Hernández TY , Mazzoni L , Afrin S , Beltrán-Ayala P , González-Paramás AM , Santos-Buelga C , Varela-Lopez A , LuisQuiles JL , Battino M . Activation of AMPK/Nrf2 signalling by Manukahoney protects human dermal fibroblasts against oxidative damage byimproving antioxidant response and mitochondrial function promotingwound healing. Journal of Functional Foods. (2016) ;25: :38-49. 10.1016/j.jff.2016.05.008 |
[6] | Han CY . Roles of reactive oxygen species on insulin resistance inadipose tissue. Diabetes Metab J. (2016) ;40: (4):272-9. doi: 10.4093/dmj.2016.40.4.272 |
[7] | Lee H , Lee YJ , Choi H , Ko EH , Kim JW . Reactive oxygen speciesfacilitate adipocyte differentiation by accelerating mitotic clonalexpansion. J Biol Chem. (2009) ;284: (16):10601-9. doi: 10.1074/jbc.M808742200 |
[8] | Anderson EJ , Lustig ME , Boyle KE , Woodlief TL , Kane DA , Lin CT , Price JW , Kang L , Rabinovitch PS , Szeto HH . Mitochondrial H2O2 emission and cellular redox state linkexcess fat intake to insulin resistance in both rodents and humans. J Clin Invest. (2009) ;119: :573-81. doi: 10.1172/JCI37048 |
[9] | Giampieri F , Alvarez-Suarez JM , Mazzoni L , Romandini S , Bompadre S , Diamanti J , Capocasa F , Mezzetti B , Quiles JL , Ferreiro MS , Tulipani S , Battino M . The potential impact of strawberry on human health. Natural Product Research. (2013) ;27: (4-5):448-455. doi: 10.1080/14786419.2012.706294 |
[10] | Mazzoni L , Perez-Lopez P , Giampieri F , Alvarez-Suarez JM , Gasparrini M , Forbes-Hernandez TY , Quiles JL , Mezzetti B , Battino M . Thegenetic aspects of berries: From field to health. J Sci Food Agric. (2015) ;96: (2):365-71. doi: 10.1002/jsfa.7216 |
[11] | Giampieri F , Alvarez-Suarez JM , Mazzoni L , Forbes-Hernandez TY , Gasparrini M , González-Paramás AM , Santos-Buelga C , Quiles JL , Bompadre S , Mezzetti B , Battino M . An anthocyanin-richstrawberry extract protects against oxidative stress damage andimproves mitochondrial functionality in human dermal fibroblastsexposed to an oxidizing agent. Food Funct. (2014) ;5: :1939. doi: 10.1039/c4fo00048j |
[12] | Giampieri F , Alvarez-Suarez JM , Cordero M , Gasparrini M , Forbes-Hernández TY , Afrin S , Santos-Buelga C , González-Paramás AM , Astolfi P , Rubini C , Zizzi A , Tulipani S , Quiles JL , Mezzetti B , Battino M . Strawberry consumption improvesaging-associated impairments, mitochondrial biogenesis andfunctionality through the AMP-activated protein kinase signalingcascade. Food Chemistry. (2017) ;234: :464-71. doi: 10.1039/c4fo00048j |
[13] | Forbes-Hernández TY , Giampieri F , Gasparrini M , Afrin S , Mazzoni L , Cordero M , Mezzetti B , Quiles JL , Battino M . Lipid accumulationin HepG2 cells is attenuated by strawberry extract through AMPKactivation. Nutrients. (2017) ;9: :621. doi: 10.3390/nu9060621 |
[14] | Calder PC , Ahluwalia N , Brouns F , Buetler T , Clement K , Cunningham K , Esposito K , Jonsson LS , Kolb H , Lansink M , Marcos A , Margioris A , Matusheski N , Nordmann H , O’Brien J , Pugliese G , Rizkalla S , Schalkwijk C , Tuomilehto J , Warnberg J , Watzl B , Winklhofer-Roob BM . Dietary factors and low-grade inflammation in relation to overweightand obesity. Br J Nutr. (2011) ;106: :S5-78. doi: 10.1017/S0007114511005460 |
[15] | Gasparrini M , Forbes-Hernandez TY , Giampieri F , Afrin S , Alvarez-Suarez JM , Mazzoni L , Mezzetti B , Quiles JL , Battino M . Anti-inflammatory effect of strawberry extract against LPS-inducedstress in RAW 264.7 macrophages Food and Chemical Toxicology. (2017) ;102: :1e10. doi: 10.1016/j.fct.2017.01.018 |
[16] | Gasparrini M , Giampieri F , Forbes-Hernandez TY , Afrin S , Cianciosi D , Reboredo-Rodriguez P , Varela-Lopez A , Zhang JJ , Quiles JL , Mezzetti B , Bompadre S , Battino M . Strawberry extracts efficientlycounteract inflammatory stress induced by the endotoxinlipopolysaccharide in Human Dermal Fibroblast. Food and ChemicalToxicology. (2018) ;S0278-6915: (18)30103-0. doi: 10.1016/j.fct.2018.02.038 |
[17] | Balansky R , Ganchev G , Iltcheva M , Kratchanova M , Denev P , Kratchanov C , Polasa K , D’Agostini F , Steele V E , De Flora S . Inhibition of lung tumor development by berry extracts in miceexposed to cigarette smoke. Int J Cancer. (2012) ;131: :1991-1997. doi: 10.1002/ijc.27486 |
[18] | Afrin S , Gasparrini M , Forbes-Hernandez TY , Reboredo-Rodriguez P , Mezzetti B , Varela-Lopez A , Giampieri F , Battino M . Promising HealthBenefits of the Strawberry: A Focus on Clinical Studies. J AgricFood Chem. (2016) ;64: :4435-49. doi: 10.1021/acs.jafc.6b00857 |
[19] | Forbes-Hernandez TY , Gasparrini M , Afrin S , Bompadre S , Mezzetti B , Quiles JL , Giampieri F , Battino M . The Healthy Effects of StrawberryPolyphenols: Which Strategy behind Antioxidant Capacity? Crit RevFood Sci Nutr. (2016) ;56: (Suppl 1):S46-59. doi: 10.1080/10408398.2015.1051919 |
[20] | Afrin S , Giampieri F , Gasparrini M , Forbes-Hernandez TY , Varela-López A , Quiles JL , Mezzetti B , Battino M . Chemopreventive and therapeutic effects of edible berries: A focuson colon cancer prevention and treatment. Molecules. (2016) ;21: (2):169. doi: 10.3390/molecules21020169 |
[21] | Diamanti J , Mazzoni L , Balducci F , Cappelletti R , Capocasa F , Battino M , Dobson G , Stewart D , Mezzetti B . Use of wild genotypes inbreeding program increases strawberry fruit sensorial andnutritional quality. Journal of Agricultural and Food Chemistry. (2014) ;62: (18):3944-3953. doi: 10.1021/jf500708x |
[22] | Moongkarndi P , Srivattana A , Bunyapraphatsara N , Puthong S , Laohathai K . Cytotoxicity assay of hispidulin and quercetin usingcolorimetric technique. Mahidol University Journal of PharmaceuticalSciences. (1991) ;18: :25-3. |
[23] | Studzinski GP . Cell Growth and Apoptosis a Practical Approach. Oxford University Press: Oxford, UK, (1995) ; ISBN 9780199635696. |
[24] | Ohkawa H , Ohishi N , Yagi K . Assay for lipid peroxides in animaltissues by thiobarbituric acid reaction. Anal Biochem. (1979) ;95: :351-8. 10.1016/0003-2697(79)90738-3 |
[25] | Kakkar P , Das B , Viswanathan PN . A modified spectrophotometric assayof superoxide dismutase. Indian J Biochem Biophys. (1984) ;21: :130-2. PMID: 6490072. |
[26] | Aebi H . Catalase in vitro. Methods Enzymol. (1984) ;105: :121-6. 10.1016/S0076-6879(84)05016-3 |
[27] | Forbes-Hernández TY , Gasparrini M , Afrin S , Cianciosi D , González-Paramás AM , Santos-Buelga C , Mezzetti B , Quiles JL , Battino M , Giampieri F , Bompadre S . Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int J Mol Sci. (2017) ;18: :1149. doi: 10.3390/ijms18061149 |
[28] | Marimoutou M , Le Sage F , Smadja J , Lefebvre d’Hellencourt C , Gonthier MP , Robert-Da Silva C . Antioxidant polyphenol-rich extractsfrom the medicinal plants Antirhea borbonica, Doratoxylonapetalum and Gouania mauritiana protect 3T3-L1 preadipocytesagainst H2O2, TNFα and LPSinflammatory mediators by regulating the expression of superoxidedismutase and NF-κB genes. Journal of Inflammation. (2015) ;12: :10. doi: 10.1186/s12950-015-0055-6 |
[29] | Seo MS , Kim JH , Kim HJ , Chang KC , Park SW . Honokiol activates theLKB1–AMPK signaling pathway and attenuates the lipidaccumulation in hepatocytes. Toxicology and Applied Pharmacology. (2015) ;284: :113-24. doi: 10.1016/j.taa2015.02.020 |
[30] | Dong SF , Yasui N , Negishb H , Kishimoto A , Sun JN , Ikeda K . Increasedoxidative stress in cultured 3T3L1 cells was attenuated by berberinetreatment. Nat Prod Commun. (2015) ;10: (6):8957. PMID: 2619751. |
[31] | Yoo SR , Seo CS , Kim OS , Shin HK , Jeong SJ . Anti-adipogenic andantioxidanteffects of the traditional Korean herbalformulaSamchulgeonbi-tang: An in vitro study. Int J Clin Exp Med. (2015) ;8: (6):8698-708. PMID: 26197511. |




