Inhibition of the development of N-nitrosomethylbenzylamine-induced esophageal tumors in rats by strawberries and aspirin, alone and in combination
Abstract
BACKGROUND:
Esophageal squamous cell carcinoma (ESCC) is one of two subtypes of esophageal cancer, with high incidence and mortality rates in developing countries.
OBJECTIVE:
The current study investigated the potential chemoprotective effects of strawberries and aspirin against the development of rat esophageal papillomas, the precursors to ESCC.
METHODS:
Using a prevention model, we administered study diets to rats before, during, and after N-nitrosomethylbenzylamine (NMBA) treatment. The effects of the four diets were evaluated: the control diet, 5% strawberry powder in the control diet, 0.01% aspirin in the drinking water, and the combination of strawberries and aspirin. At week 25, we euthanized all the rats and collected their esophagi to quantify tumor incidence, multiplicity, and burden, as well as for molecular analysis.
RESULTS:
Both strawberries and aspirin significantly decreased esophageal tumor multiplicity, with the combination causing the most robust suppression. Aspirin alone and the combination decreased the total tumor burden in the esophagus. None of the diets had a significant effect on tumor incidence or the expression of COX-1 and COX-2. Strawberries and aspirin, alone and in combination, significantly suppressed squamous epithelial cell proliferation (PCNA).
CONCLUSIONS:
Strawberries, aspirin, and their combination exhibit chemoprotective effects against NMBA-induced esophageal tumors in rats.
1Introduction
Esophageal cancer (EC) is the eighth most common cancer worldwide [1]. In 2012, there were approximately 456,000 new cases (accounting for 3% of all cancers) and 0.4 million EC-related deaths (accounting for 5% of all cancer deaths) [1]. In the United States, EC is more common among men than among women, and is the seventh leading cause of cancer-related death in men [2]. Overall, approximately 17,290 new cases (13,480 in men and 3,810 in women) and 15,850 EC-related deaths (12,850 in men and 3,000 in women) were estimated to occur in the US in 2018 [2].
EC patients can be asymptomatic or present with dysphagia, chest pain, weight loss, breastbone pressure, and cough [3]. Currently, imaging technologies, such as endoscopy, endoscopic ultrasonography, esophagoscopy, and computed tomography are widely used to diagnose and stage EC [4]. As for many other cancers, surgery, radiotherapy, and chemotherapy are the standard therapies. Unfortunately, esophagectomy itself has a high degree of morbidity and mortality, with 2% –25% of patients dying within 30 days after surgery [5, 6]. Also, more than half of patients present with distant metastases at the time of diagnosis, and so are not candidates for surgical resection [7]. Due to these challenges, the overall 5-year survival rate for EC patients is only about 18% [8].
There are two main subtypes of EC: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Developing countries have a relatively higher incidence of ESCC, while EAC is on the rise in developed countries [1, 9, 10]. In the US, there has been a steady decline in the incidence of ESCC, which might be attributed to decreased tobacco and alcohol use [11, 12]. However, the increased prevalence of gastroesophageal reflux disease and obesity might have contributed to the rise of EAC over the past three decades [7].
High-dose aspirin (325–600 mg/day) has analgesic effects, while low-dose aspirin (75–81 mg/day) is used to prevent cardiovascular disease [13], colorectal cancer, and lung cancer [14–16]. Observational studies also indicate a lower risk of EC (ESCC and EAC combined) with daily intake of low-dose aspirin [17–21]. For example, a meta-analysis by the Corley group that included 2 cohort studies and 7 case-control studies, a total of 1,813 cancer cases, suggested that routine aspirin intake correlated with a 42% reduction in the odds of developing ESCC (odds ratio = 0.58; 95% confidence interval (CI): 0.43–0.78) [17]. Similarly, the Bosetti group analyzed 139 studies and observed an overall 39% reduction in the risk of ESCC among regular aspirin users (risk ratio = 0.61; 95% CI: 0.50–0.76, p < 0.001) [19]. However, one British study of two independent population-based cohorts found that low-dose aspirin use did not increase the survival of patients with EC [22].
The most common etiological factors in both China and Western countries are tobacco and alcohol use. Other factors include a low intake of fruit and vegetables and a diet lacking in micronutrients, specifically in some regions of China where ESCC is very common [9]. In the Chinese-US cooperative Linxian Nutrition Intervention Trials (LNIT), the diets of 29,584 adults (aged 40–69 years) were supplemented with various micronutrients, including (A) retinol and zinc, (B) riboflavin and niacin, (C) ascorbate and molybdenum, (D) α-tocopherol, β-carotene, and selenium, and also these agents in combination as AB, AC, BC, AD, BD, CD, and ABCD, as well as placebo, for 63 months. Ten-year follow-up studies found that supplementation with α-tocopherol, β-carotene, and selenium reduced mortality from ESCC by 17% in subjects who began the supplementation at age ≤55 years [23]. These results suggest potential chemoprotective effects of micronutrients against ESCC prior to initiation or during the early stages of these disease. However, supplementation increased ESCC mortality by 14% in patients 55 years or older [23], which raises concerns regarding the optimized timing of administration of micronutrient supplement for preventing ESCC.
Berries, such as strawberries, blueberries, blackberries, red raspberries, and black raspberries, are widely consumed worldwide and present many health benefits [24–28]. For example, strawberries contain abundant vitamins, minerals, and phytochemicals that have shown to be chemoprotective through their regulation of key cellular pathways involved in the initiation and progression of cancer. These studies describe the anti-proliferative effects of strawberries in prostate cancer, cervical cancer, ovarian cancer, hepatoma, leukemia, and melanoma [29].
One focus of our laboratory is the chemopreventive effects of berries against ESCC [30–41]. Carlton, et al. previously demonstrated that both 5% and 10% strawberries reduced the multiplicity of rat esophageal tumors induced by the carcinogen N-nitrosomethylbenzylamine (NMBA) [42]. More importantly, they observed a dose-dependent inhibition as 10% strawberries produced more robust suppression than 5% strawberries and also decreased tumor incidence [42]. In a post-initiation protocol, in which study diets were initiated after NMBA treatment, they observed that both 5% and 10% strawberries inhibited tumor multiplicity but saw no dose-dependent effects [42]. The relevance of these observations by Carlton, et al. is that NMBA is found in many foods, including grilled/smoked meat, pickled and preserved vegetables [43], and moldy corn in the high-incidence regions of China [9]. It is also the most potent N-nitrosamine carcinogen for the rat esophagus [44].
To date, the evidence that aspirin might protect against ESCC comes largely from epidemiological studies. Therefore, our current study aimed to investigate the chemoprotective abilities of strawberries and aspirin during the complete carcinogenic process, including both tumor initiation and promotion/progression [32]. Our results confirmed the observations of Carlton et al. on the chemopreventive effects of strawberries in the rat esophagus. For the first time, they also provided experimental evidence of aspirin’s chemopreventive effects in rat esophagus. Moreover, our results indicate that the combination of strawberries and aspirin produces stronger suppression of esophageal tumor development in rats than either agent alone.
2Materials and methods
2.1Animals and reagents
All animal protocols followed the institutional guidelines for animal care and were approved by the Medical College of Wisconsin Animal Care and Use Committee. Three-to-five-weeks old male Fisher-344 (F-344) rats obtained from Harlan Sprague-Dawley (Indianapolis, IN) were housed two per cage under standard conditions (20±2°C, 50% ±10% relative humidity, and 12-hour light/dark cycles). Diet and water were available ad libitum. The hygienic condition of each cage was examined daily, and the bedding was changed twice a week, according to the recommendations of the American Association of Laboratory Animal Care (AALAC).
NMBA was purchased from Ash Stevens (Detroit, MI), and aspirin was purchased from Sigma-Aldrich (St. Louis, MO). All reagents were prepared immediately prior usage.
2.2Diet preparation
A Hobart mixer was used to prepare each diet. The American Institute of Nutrition-76A (AIN-76A) synthetic diet was purchased from Dyets, Inc. (Bethlehem, PA) for use as the control diet. Strawberries of the Commander variety were obtained from Driscoll Farms (Watsonville, CA), and were picked ripe, washed, frozen, and shipped to Van Drunen Farms (Momence, IL) for freeze-drying. Strawberry powder was added to the control diet at 5% of the final diet mixture. The control diet and the 5% strawberry diet were stored in a –20°C freezer.
Aspirin was first dissolved in 20% DMSO, and then diluted to a concentration of 0.01% in water.
2.3Animal experiments
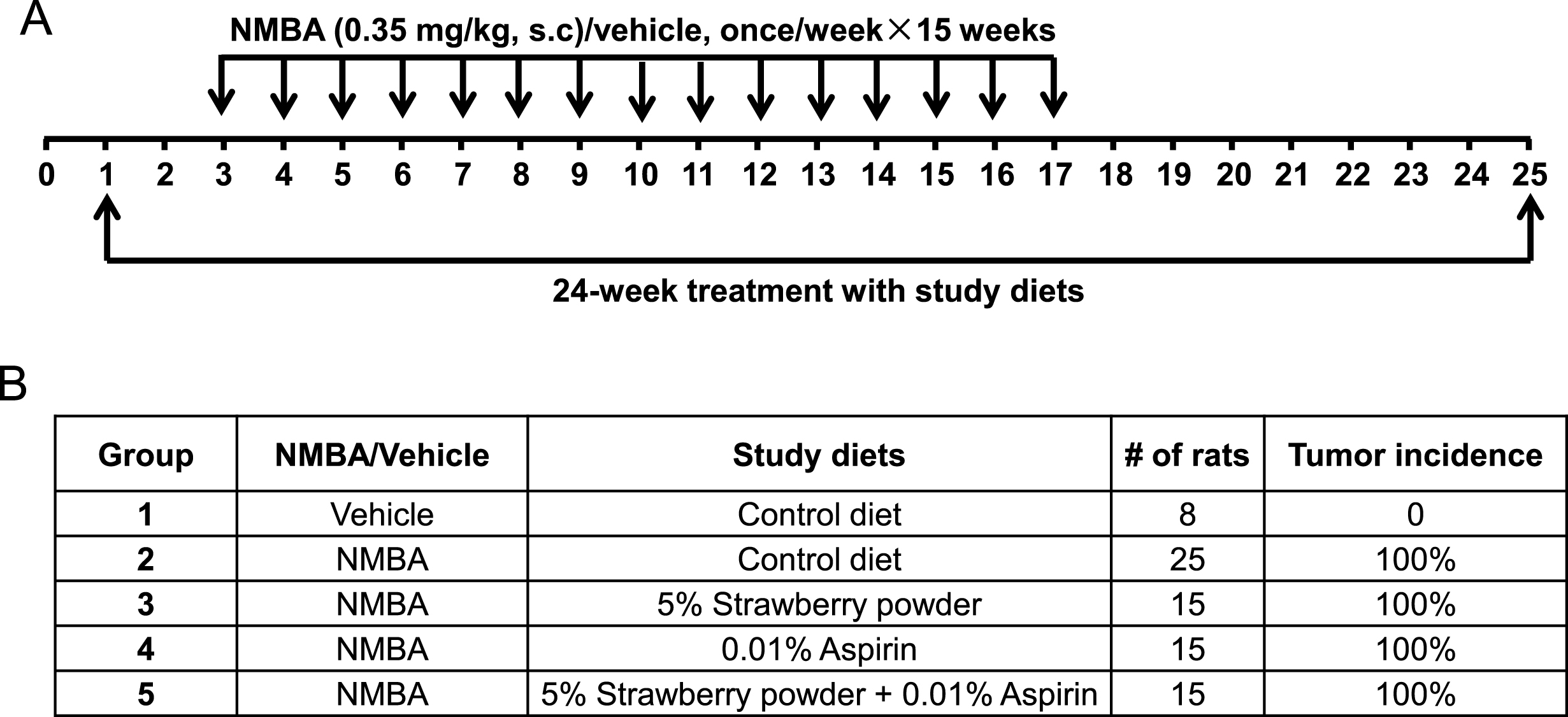
When the rats arrived at our animal facility, they were placed on the AIN-76A control diet to acclimate for 1 week and then changed to their corresponding diets until the end of the study (Fig. 1A). They were randomly assigned to five groups: group 1, vehicle injection and control diet; group 2, NMBA injection and control diet; group 3, NMBA injection and control diet supplemented with 5% strawberry powder; group 4, NMBA injection, control diet, and 0.01% aspirin in the drinking water; group 5, NMBA injection, control diet supplemented with 5% strawberry powder and 0.01% aspirin in the drinking water (Fig. 1B). After 2 weeks on their respective diets, the rats were injected subcutaneously with either vehicle control (20% DMSO in water) or NMBA (0.35 mg/kg body weight dissolved in 20% DMSO) once per week for 15 weeks. They continued to receive their respective diets during the 15-week injection period and for 7 subsequent weeks. At week 25, all the rats were euthanized, and their esophagi were collected to quantify tumor incidence, multiplicity, and burden (tumor burden = tumor multiplicity×average tumor size), and for histological and molecular analyses.
Fig.1
A prevention rat esophagus model was used to investigate the chemoprotective abilities of strawberry powder and aspirin. (A) Study protocol for the prevention model. Rats were subcutaneously injected with NMBA at 0.35 mg/kg body weight or vehicle control once per week for 15 weeks. Different study diets were administered 2 weeks prior to the initial injection with NMBA, during the 15 weeks of injections, and for an additional 7 weeks after the last injection. (B) Group assignment of the different study diets.
2.4Western blot analysis
Protein lysates of rat esophagi were used for western blot analysis. Antibodies for COX-1 (#9896; dilution, 1:1000), COX-2 (#12282; dilution, 1:500), PCNA (#13110; dilution, 1:500), cyclin D1 (#2978; dilution, 1:500), cyclin A2 (#4656; dilution, 1:500), and CDK4 (#12790; dilution, 1:500) were purchased from Cell Signaling Technology (Danvers, MA) and used to quantify their respective levels in the esophageal lysates. The antibody against β-actin (691001; dilution, 1:5000) was purchased from MP Biomedical (Santa Ana, CA). Anti-rabbit (#7074S; dilution, 1:3000) and anti-mouse (#7076S; dilution, 1:3000) secondary antibodies were purchased from Cell Signaling Technology (Danvers, MA). Blots were quantified by densitometry, using ImageJ software, and levels of the proteins of interest were normalized to levels of β-actin.
2.5Statistical analysis
Tumor data and western blot data were compared, using one-way ANOVA and Tukey’s post-hoc test (SigmaPlot, San Jose, CA). A p value <0.05 was considered statistically significant.
3Results
3.1Strawberries and aspirin significantly suppress esophageal tumor development
As indicated above in Materials and Methods, all rats were euthanized at week 25 and their esophagi were collected. Tumor incidence, multiplicity, and burden were evaluated. None of the rats on the control diet (group 1) developed esophageal tumors. All the rats in the carcinogen control group (group 2) developed tumors (100% incidence). The study diets had no effect on tumor incidence in groups 3–5 (Fig. 1B). However, all three study diets (groups 3–5) decreased tumor multiplicity, with the diet given to group 5 (strawberries in the diet and aspirin in the drinking water) being the most effective (Fig. 2A). This suggests that the combination of strawberries and aspirin inhibits esophageal tumor development more strongly than either agent alone. Furthermore, aspirin alone or in conjunction with strawberries significantly suppressed the tumor burden (Fig. 2B). Overall, these results suggest that strawberries and aspirin, alone and in combination, suppress the development of esophageal tumors.
Fig.2
Strawberries and aspirin significantly suppressed esophageal tumor development. (A) All three study diets significantly reduced tumor multiplicity. Strawberries and aspirin in combination showed a stronger effect. (B) Tumor burden was significantly reduced by aspirin alone and the combined diet. *p < 0.05, **p < 0.01, ***p < 0.001.
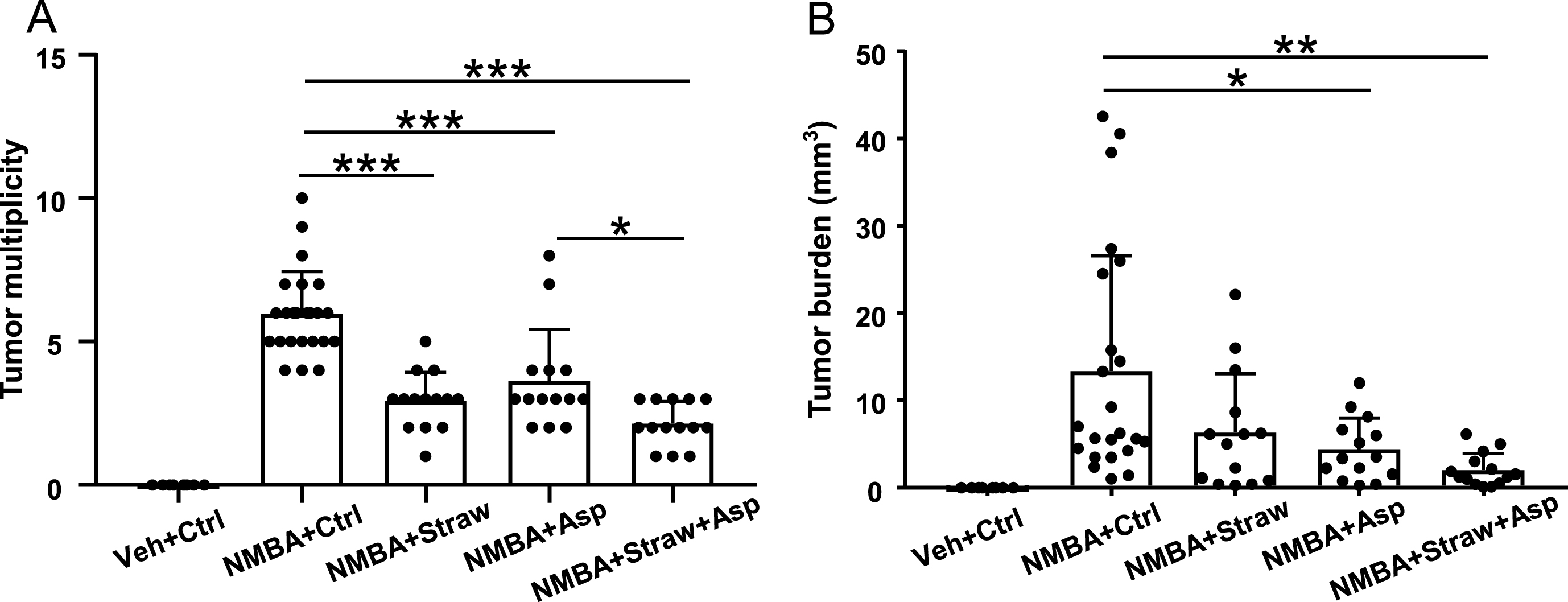
3.2Strawberries and aspirin do not affect cyclooxygenase expression
Our previous studies demonstrated that dietary 5% black raspberries suppress the expression of cyclooxygenase (COX)-2 at week 30 in the NMBA-induced rat esophageal tumor model [38]. In addition, a human clinical trial involving patients with esophageal dysplasia indicated that oral consumption of 60 g per day of freeze-dried strawberry powder in water for 6 months led to a 62.9% reduction (p < 0.001) in the levels of COX-2 protein expression in the esophagus [45]. Based on this observation and the fact that aspirin is an irreversible inhibitor for both COX-1 and COX-2 [46], we examined whether 5% strawberries, aspirin, or a combination of the two could inhibit COX-1 and COX-2 expression in NMBA-treated rat esophagus. However, our results revealed no overall increase in COX-2 expression in the esophagi of rats that were injected with the carcinogen NMBA compared with those on the control diet (Fig. 3). Therefore, it was not possible to evaluate any potential effects of the strawberry and aspirin treatment. These results are inconsistent with our previous study in which NMBA-treated esophagi had significantly enhanced COX-2 expression in whole esophagus, and to a greater extent in papilloma tissues, than in untreated control esophagus [38].
Fig.3
Strawberries and aspirin do not affect cyclooxygenase expression. Rat esophagi were collected and analyzed with western blotting to examine the expression of COX-1 and COX-2. No significant changes were observed in either the control groups or the groups on the study diets. n = 4–6.
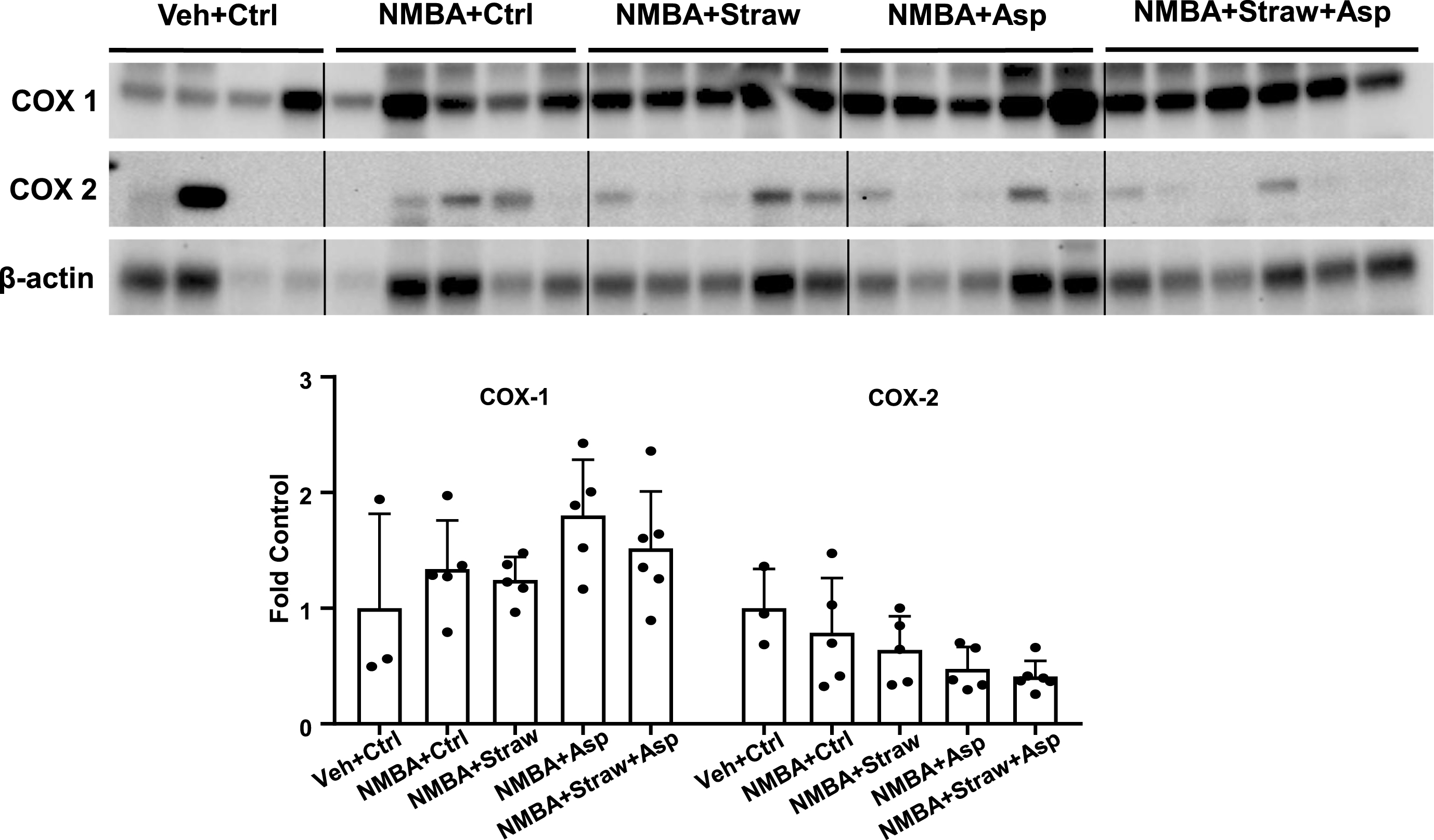
3.3Strawberries and aspirin suppress esophageal tumor cell proliferation
Given that all three study diets significantly decreased tumor multiplicity, we examined the diets’ effects on the proliferation of esophageal tumor cells. A significant decrease in the protein expression of proliferating cell nuclear antigen (PCNA) associated with each of the three study diets (Fig. 4), which may have directly contributed to the decreased tumor multiplicity (Fig. 2A). We further investigated the potential effects of strawberries and aspirin on the expression of cyclins and cyclin dependent kinases (CDK), and observed no significant changes in the protein expression of cyclin D1, cyclin A2, or CDK4 (Fig. 4), though aspirin showed a trend toward decreasing the expression of cyclin D1 and CDK4. We also attempted to examine the effects of these agents on caspase expression, but cleaved caspase-3 expression was not detectable in normal or treated esophagi (data not shown).
Fig.4
Strawberries and aspirin suppressed esophageal tumor cell proliferation. Rat esophagi were collected and analyzed with western blotting to examine the expression of PCNA, cyclin D1, cyclin A2, and CDK4. All three study diets significantly suppressed cell proliferation but had no significant effects on cell cycle regulation. n = 4–6, *p < 0.05, **p < 0.01, ***p < 0.001.
4Discussion
Chemoprevention research has gained more attention over the past decades in part because many large-scale epidemiological studies have demonstrated an inverse association between healthy diets and the risk of cancer. These results provide a convincing rationale for a diet rich in fruits, vegetables, and fiber.
Our laboratory focuses on chemoprevention of colorectal cancer [47–57] and ESCC [30–41], using different types of berries. Strawberries have been protective against a number of cancers, including prostate cancer, cervical cancer, ovarian cancer, hepatoma, leukemia, and melanoma [29]. Moreover, Carlton, et al. [42] demonstrated their protective effects in the rat esophagus. It is also known that low-dose aspirin protects against multiple human cancers such as colorectal cancer and lung cancer [58] as well as lowering the risk of cardiovascular diseases. Our study is unique because it provided the first experimental evidence for aspirin’s ability to reduce NMBA-tumorigenesis in the rat esophagus. It also suggests that the combination of strawberries plus aspirin is more effective than either agent alone. In the current study, tumor multiplicity was more strongly suppressed by the combination of 5% strawberries and 0.01% aspirin. The mechanism underlying this collaboration warrants further investigation.
Aspirin is an irreversible inhibitor of both COX-1 and COX-2, and Chen, et al showed that strawberries are capable of inhibiting COX-2 expression in the human esophagus [45]. However, we did not observe any significant effect of either strawberries or aspirin on COX-1 or COX-2 expression in NMBA-treated rat esophagus. One possible reason for this discrepancy might be the length of our study period. Our previous studies that examined the effects of black raspberries on esophageal tumor development [38] showed overexpression of COX-2 after NMBA treatment at week 30, which is 5 weeks later than the time point in the current study (week 25). In addition, 5% black raspberries suppressed cox-2 mRNA expression more strongly at week 35 than at weeks 15 and 25 [40]. These results suggest that a longer course of berry treatment might produce stronger suppression of COX-1 and COX-2. Another potential reason might be the relatively low dose of aspirin. We used 0.01% aspirin in the drinking water, which is equivalent to about 35 mg per day for human adults. At this low dose, aspirin exhibited chemoprotective effects against papilloma tumor development but did not change COX-2 expression. These results suggest that a higher dose of aspirin (equivalent to 75–81 mg per day for human adults) might be needed to inhibit COX-2 expression.
Given that the study diets reduced tumor multiplicity and burden, we examined their potential effects on esophageal cell proliferation. All three study diets significantly inhibited the expression of PCNA, which may directly contribute to decreased tumor multiplicity. Furthermore, we determined if the study diets suppressed cell proliferation by affecting cell cycle regulators, but did not observe any significant changes in the protein expression of cyclin D1, cyclin A2, or CDK4. Interestingly, cyclin D1, but not cyclin D2 or D3, is overexpressed in many human ESCC cell lines [59], and its expression associates with CDK4 but not PCNA [59]. In addition, one population-based study showed that NSAID use could lower the odds ratio of developing ESCC, regardless of the status of cyclin D1 [60]. These results suggest that mechanisms independent of cell cycle regulation might underlie the chemoprotective effects of strawberries and aspirin.
5Conclusions
In summary, the current study provided the first experimental evidence of aspirin’s ability to inhibit the development of NMBA-induced esophageal tumors in rats. It also revealed that the combination of strawberries plus aspirin has a stronger chemoprotective effect than either agent alone. Both strawberries and aspirin suppressed cell proliferation independently of changes in cell cycle regulation. Future studies should investigate the potential underlying mechanisms and whether strawberries and aspirin have similar effects in the post-initiation model of esophageal tumor development in rats. If this were the case, these agents might serve as adjuvant treatments to chemo/radiotherapy for human ESCC.
Conflict of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
Study design, acquisition of data, analysis, and interpretation of data: Pan Pan, Daniel S. Peiffer, Yi-Wen Huang, Kiyoko Oshima, Gary D. Stoner and Li-Shu Wang.
Manuscript writing and revising: Pan Pan, Daniel S. Peiffer, Gary D. Stoner and Li-Shu Wang.
Final approval: Li-Shu Wang and Gary D. Stoner.
Acknowledgments
We are grateful for financial support from NIH grants 5 R01 CA103180 (to G. Stoner) and 5 R01 CA148818 (to L.-S. Wang) as well as Advancing a Healthier Wisconsin grant 5520197.
References
[1] | StewartBW WC . World Cancer Report 2014. Available at: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014 |
[2] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2018. (2018) ;68: (1):7–30. doi:10.3322/caac.21442 |
[3] | Tan C , Qian X , Guan Z , Yang B , Ge Y , Wang F , etal. Potential biomarkers for esophageal cancer. Springerplus. (2016) ;5: , 467. doi:10.1186/s40064-016-2119-3 |
[4] | Ohashi S , Miyamoto S , Kikuchi O , Goto T , Amanuma Y , Muto M . Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. (2015) ;149: (7):1700–15. doi:10.1053/j.gastro.2015.08.054 |
[5] | Liu Y , Xiong Z , Beasley A , D’Amico T , Chen XL . Personalized and targeted therapy of esophageal squamous cell carcinoma: An update. Ann N Y Acad Sci. (2016) 1381: (1):66–73. doi:10.1111/nyas.13144 |
[6] | Kang X , Chen K , Li Y , Li J , D’Amico TA , Chen X . Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. (2015) ;21: (25):7648–58. doi:10.3748/wjg.v21.i25.7648 |
[7] | Alsop BR , Sharma P . Esophageal Cancer. Gastroenterol Clin North Am. (2016) ;45: (3):399–412. doi:10.1016/j.gtc.2016.04.001 |
[8] | Siegel RL , Miller KD , Jemal A . Cancer statistics, 2017. CA Cancer J Clin. (2017) ;67: (1):7–30. doi:10.3322/caac.21387 |
[9] | Yang CS , Chen X , Tu S . Etiology and prevention of esophageal cancer. Gastrointest Tumors. (2016) ;3: (1):3–16. doi:10.1159/000443155 |
[10] | Holmes RS , Vaughan TL . Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. (2007) ;17: (1):2–9. doi:10.1016/j.semradonc.2006.09.003 |
[11] | Kamangar F , Chow WH , Abnet CC , Dawsey SM . Environmental causes of esophageal cancer. Gastroenterol Clin North Am. (2009) ;38: (1):27–57, vii. doi:10.1016/j.gtc.2009.01.004 |
[12] | Freedman ND , Abnet CC , Leitzmann MF , Mouw T , Subar AF , Hollenbeck AR , etal. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. (2007) ;165: (12):1424–33. doi:10.1093/aje/kwm051 |
[13] | Antithrombotic Trialists C , Baigent C , Blackwell L , Collins R , Emberson J , Godwin J , etal. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) ;373: (9678):1849–60. doi:10.1016/S0140-6736(09)60503-1 |
[14] | Pan P , Huang YW , Oshima K , Yearsley M , Zhang J , Yu J , etal. Could aspirin and diets high in fiber act synergistically to reduce the risk of colon cancer in humans? Int J Mol Sci (2018) ;19: (1). doi:10.3390/ijms19010166 |
[15] | Nishihara R , Lochhead P , Kuchiba A , Jung S , Yamauchi M , Liao X , etal. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. (2013) ;309: (24):2563–71. doi:10.1001/jama.2013.6599 |
[16] | Hall MN , Chavarro JE , Lee IM , Willett WC , Ma J . A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. http://cebp.aacrjournals.org/content/17/5/1136.long. Cancer Epidemiol Biomarkers Prev. (2008) ;17: (5)1136–43. doi:10.1158/1055-9965.EPI-07-2803 |
[17] | Corley DA , Kerlikowske K , Verma R , Buffler P . Protective association of aspirin/NSAIDs and esophageal cancer: A systematic review and meta-analysis. Gastroenterology. (2003) ;124: (1):47–56. doi:10.1053/gast.2003.50008 |
[18] | Rothwell PM , Fowkes FG , Belch JF , Ogawa H , Warlow CP , Meade TW . Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. Lancet. (2011) 377: (9759):31–41. doi:10.1016/s0140-6736(10)62110-1 |
[19] | Bosetti C , Rosato V , Gallus S , Cuzick J , La Vecchia C . Aspirin and cancer risk: A quantitative review to 2011. Ann Oncol. (2012) ;23: (6):1403–15. doi:10.1093/annonc/mds113 |
[20] | Ratnasinghe LD , Graubard BI , Kahle L , Tangrea JA , Taylor PR , Hawk E . Aspirin use and mortality from cancer in a prospective cohort study. Anticancer research. (2004) ;24: (5b):3177–84. |
[21] | Jayaprakash V , Menezes RJ , Javle MM , McCann SE , Baker JA , Reid ME , etal. Regular aspirin use and esophageal cancer risk. Int J Cancer. (2006) ;119: (1):202–7. doi:10.1002/ijc.21814 |
[22] | Spence AD , Busby J , Johnston BT , Baron JA , Hughes CM , Coleman HG , etal. Low-dose Aspirin Use Does Not Increase Survival in 2 Independent Population-based Cohorts of Patients With Esophageal or Gastric Cancer. Gastroenterology (2017) . doi:10.1053/j.gastro.2017.10.044 |
[23] | Qiao YL , Dawsey SM , Kamangar F , Fan JH , Abnet CC , Sun XD , etal. Total and cancer mortality after supplementation with vitamins and minerals: Follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. (2009) ;101: (7):507–18. doi:10.1093/jnci/djp037 |
[24] | Giampieri F , Tulipani S , Alvarez-Suarez JM , Quiles JL , Mezzetti B , Battino M . The strawberry: Composition, nutritional quality, and impact on human health. Nutrition (Burbank, Los Angeles County, Calif). (2012) ;28: (1):9–19. doi:10.1016/j.nut.2011.08.009 |
[25] | Giampieri F , Alvarez-Suarez JM , Mazzoni L , Romandini S , Bompadre S , Diamanti J , etal. The potential impact of strawberry on human health. Natural Product Research. (2013) ;27: (4-5):448–55. doi:10.1080/14786419.2012.706294 |
[26] | Giampieri F , Alvarez-Suarez JM , Battino M . Strawberry and human health: Effects beyond antioxidant activity. J Agric Food Chem. (2014) ;62: (18):3867–76. doi:10.1021/jf405455n |
[27] | Giampieri F , Forbes-Hernandez TY , Gasparrini M , Alvarez-Suarez JM , Afrin S , Bompadre S , etal. Strawberry as a health promoter: An evidence based review. Food Funct. (2015) ;6: (5):1386–98. doi:10.1039/c5fo00147a |
[28] | Pan P , Skaer C , Yu J , Zhao H , Ren H , Oshima K , etal. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J Berry Res. (2017) ;7: (3):147–61. doi:10.3233/JBR-170159 |
[29] | Giampieri F , Forbes-Hernandez TY , Gasparrini M , Afrin S , Cianciosi D , Reboredo-Rodriguez P , etal. The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases. Ann N Y Acad Sci. (2017) ;1398: (1):62–71. doi:10.1111/nyas.13373 |
[30] | Stoner GD , Wang LS , Zikri N , Chen T , Hecht SS , Huang C , etal. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. (2007) ;17: (5):403–10. doi:10.1016/j.semcancer.2007.05.001 |
[31] | Stoner GD , Wang LS , Casto BC . Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. (2008) ;29: (9):1665–74. doi:10.1093/carcin/bgn142 |
[32] | Stoner GD , Chen T , Kresty LA , Aziz RM , Reinemann T , Nines R . Protection against esophageal cancer in rodents with lyophilized berries: Potential mechanisms. Nutr Cancer. (2006) ;54: (1):33–46. doi:10.1207/s15327914nc5401_5 |
[33] | Stoner GD , Wang LS , Chen T . Chemoprevention of esophageal squamous cell carcinoma. Toxicol Appl Pharmacol. (2007) ;224: (3):337–49. doi:10.1016/j.taap.2007.01.030 |
[34] | Lechner JF , Reen RK , Dombkowski AA , Cukovic D , Salagrama S , Wang LS , etal. Effects of a black raspberry diet on gene expression in the rat esophagus. Nutr Cancer. (2008) ;60: (Suppl 1):61–9. doi:10.1080/01635580802393118 |
[35] | Reen RK , Nines R , Stoner GD . Modulation of N-nitrosomethylbenzylamine metabolism by black raspberries in the esophagus and liver of Fischer 344 rats. Nutr Cancer. (2006) ;54: (1):47–57. doi:10.1207/s15327914nc5401_6 |
[36] | Stoner GD , Dombkowski AA , Reen RK , Cukovic D , Salagrama S , Wang LS , etal. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. (2008) ;68: (15):6460–7. doi:10.1158/0008-5472.can-08-0146 |
[37] | Zikri NN , Riedl KM , Wang LS , Lechner J , Schwartz SJ , Stoner GD . Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr Cancer. (2009) ;61: (6):816–26. doi:10.1080/01635580903285148 |
[38] | Wang LS , Hecht SS , Carmella SG , Yu N , Larue B , Henry C , etal. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res (Phila). (2009) ;2: (1):84–93. doi:10.1158/1940-6207.CAPR-08-0155 |
[39] | Stoner GD , Wang LS , Seguin C , Rocha C , Stoner K , Chiu S , etal. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharmaceutical Research. (2010) ;27: (6):1138–45. doi:10.1007/s11095-010-0102-1 |
[40] | Peiffer DS , Zimmerman NP , Wang LS , Ransom BW , Carmella SG , Kuo CT , etal. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila). (2014) ;7: (6):574–84. doi:10.1158/1940-6207.capr-14-0003 |
[41] | Peiffer DS , Wang LS , Zimmerman NP , Ransom BW , Carmella SG , Kuo CT , etal. Dietary consumption of black raspberries or their anthocyanin constituents alters innate immune cell trafficking in esophageal cancer. Cancer Immunol Res. (2016) ;4: (1):72–82. doi:10.1158/2326-6066.cir-15-0091 |
[42] | Carlton PS , Kresty LA , Siglin JC , Morse MA , Lu J , Morgan C , etal. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. (2001) ;22: (3):441–6. |
[43] | Jakszyn P , Gonzalez CA . Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J Gastroenterol. (2006) ;12: (27):4296–303. |
[44] | Siglin JC , Khare L , Stoner GD . Evaluation of dose and treatment duration on the esophageal tumorigenicity of N-nitrosomethylbenzylamine in rats. Carcinogenesis. (1995) ;16: (2):259–65. |
[45] | Chen T , Yan F , Qian J , Guo M , Zhang H , Tang X , etal. Randomized phase II trial of lyophilized strawberries in patients with dysplastic precancerous lesions of the esophagus. Cancer Prev Res (Phila). (2012) ;5: (1):41–50. doi:10.1158/1940-6207.CAPR-11-0469 |
[46] | Flower RJ . The development of COX2 inhibitors. Nature Reviews Drug Discovery. (2003) ;2: (3):179–91. doi:10.1038/nrd1034 |
[47] | Pan P , Skaer CW , Stirdivant SM , Young MR , Stoner GD , Lechner JF , etal. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev Res (Phila). (2015) ;8: (8):743–50. doi:10.1158/1940-6207.CAPR-15-0065 |
[48] | Pan P , Lam V , Salzman N , Huang YW , Yu J , Zhang J , etal. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr Cancer. (2017) ;1–9. doi:10.1080/01635581.2017.1340491 |
[49] | Pan P , Kang S , Wang Y , Liu K , Oshima K , Huang Y-W , etal. Black raspberries enhance natural killer cell infiltration into the colon and suppress the progression of colorectal cancer. Frontiers in Immunology.. (2017) ;8: (997). doi:10.3389/fimmu.2017.00997 |
[50] | Pan P , Skaer CW , Wang HT , Stirdivant SM , Young MR , Oshima K , etal. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: Relation to metabolite profiles. Carcinogenesis. (2015) ;36: (10):1245–53. doi:10.1093/carcin/bgv117 |
[51] | Pan P , C WS , Wang HT , Oshima K , Huang YW , Yu J , etal. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis. (2017) ;38: (1):86–93. doi:10.1093/carcin/bgw122 |
[52] | Wang LS , Arnold M , Huang YW , Sardo C , Seguin C , Martin E , etal. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin Cancer Res. (2011) ;17: (3):598–610. doi:10.1158/1078-0432.CCR-10-1260 |
[53] | Wang LS , Burke CA , Hasson H , Kuo CT , Molmenti CL , Seguin C , etal. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev Res (Phila). (2014) ;7: (7):666–74. doi:10.1158/1940-6207.CAPR-14-0052 |
[54] | Pan P , Skaer CW , Wang HT , Kreiser MA , Stirdivant SM , Oshima K , etal. Systemic metabolite changes in wild-type C57BL/6 mice fed black raspberries. Nutr Cancer. (2017) ;69: (2):299–306. doi:10.1080/01635581.2017.1263748 |
[55] | Wang LS , Kuo CT , Cho SJ , Seguin C , Siddiqui J , Stoner K , etal. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr Cancer. (2013) ;65: (1):118–25. doi:10.1080/01635581.2013.741759 |
[56] | Wang LS , Kuo CT , Stoner K , Yearsley M , Oshima K , Yu J , etal. Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis. Carcinogenesis. (2013) ;34: (12):2842–50. doi:10.1093/carcin/bgt310 |
[57] | Wang LS , Kuo CT , Huang TH , Yearsley M , Oshima K , Stoner GD , etal. Black raspberries protectively regulate methylation of Wnt pathway genes in precancerous colon tissue. Cancer Prev Res (Phila). (2013) ;6: (12):1317–27. doi:10.1158/1940-6207.CAPR-13-0077 |
[58] | Pasche B , Wang M , Pennison M , Jimenez H . Prevention and treatment of cancer with aspirin: Where do we stand? Semin Oncol. (2014) ;41: (3):397–401. doi:10.1053/j.seminoncol.2014.04.012 |
[59] | Nakagawa H , Zukerberg L , Togawa K , Meltzer SJ , Nishihara T , Rustgi AK . Human cyclin D1 oncogene and esophageal squamous cell carcinoma. Cancer. (1995) ;76: (4):541–9. |
[60] | Gammon MD , Terry MB , Arber N , Chow WH , Risch HA , Vaughan TL , etal. Nonsteroidal anti-inflammatory drug use associated with reduced incidence of adenocarcinomas of the esophagus and gastric cardia that overexpress cyclin D A population-based study. Cancer Epidemiol Biomarkers Prev. (2004) ;13: (1):34–9. |




