Free radical scavenging activity and cytotoxicity assay of Cissus sicyoides berries
Abstract
BACKGROUND:
The leaves of Cissus sicyoides has been utilized in traditional medicine. The berries however are underutilized and do not appear to be widely consumed.
OBJECTIVE:
This study was undertaken to further evaluate the antioxidant properties of C. sicyoides berries.
METHODS:
The free radical scavenging activity and IC50 value (inhibitory concentration) of Cissus sicyoides berries was determined utilizing the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Aqueous two-phase partitioning was utilized for preliminary purification of phenylalanine ammonia-lyase (PAL). PAL activity was determined using an endpoint spectrophotometric assay. The cytotoxicity of the berries was assessed by use of the Brine shrimp assay.
RESULTS:
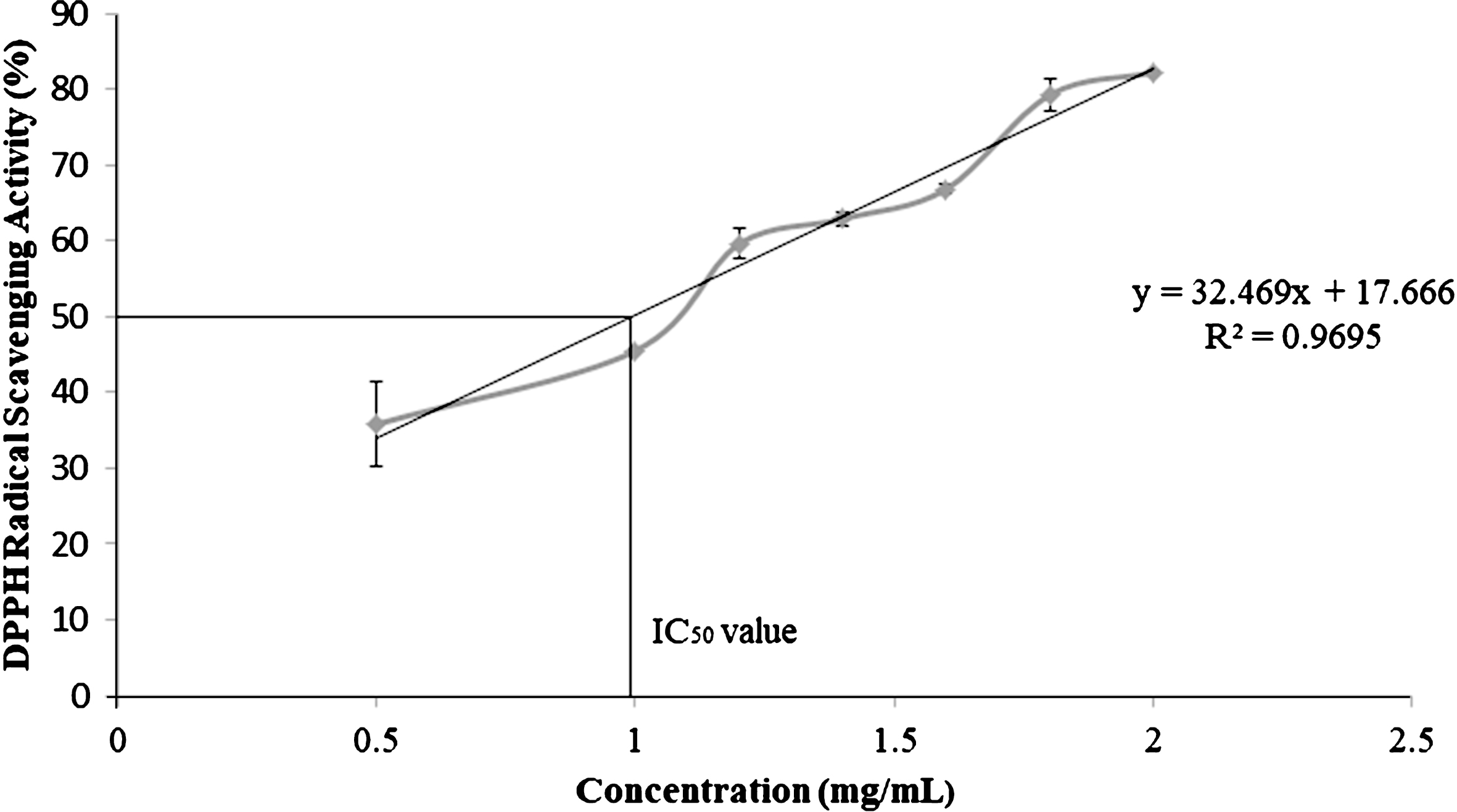
C. sicyoides berries exhibit excellent free radical scavenging activity with an IC50 value of 0.99 mg/mL which is intermediate to that of raspberries and blackberries. The berries are a potential source of PAL with enzyme activity of 0.10±0.01 U/mg protein.
CONCLUSION:
Preliminary investigations suggest that the fruits are non toxic and may be utilized as a source of antioxidants. It is evident that the plant is a good source of bioactive compounds with significant potential for food, beverage and medical applications.
1Introduction
Cissus sicyoides L (Cissus verticillata), a member of the Vitacea family is commonly found growing uncultivated in Jamaica but is not locally recognized as edible or a source of bioactive compounds. Commonly known as princess vine, curtain ivy and millionaire vine [1] the plant is also widely distributed in other Caribbean countries, Central America and South America where vegetative plant preparations have been traditionally used in folk medicine as a diuretic, anti-inflammatory, and anti-diabetic treatment [2]. Anxiolytic, anti-convulsant and sedative properties have also been reported [2]. These medicinal properties could be as a result of flavonoids, linalool and α-tocopherols present in the leaves [2]. Two flavonoids, quercetin 3-α-rhamnoside and kaempferol 3-α-rhamnoside have been identified in the leaves [3, 4]. Other chemical constituents of the leaves include stilbenes, steroids, coumarin, triterpenes, tannins and saponins [2, 5]. There is currently limited information in the literature regarding the secondary metabolites present in C. sicyoides berries.
Phenylalanine ammonia-lyase (PAL) is a key enzyme in the synthesis of secondary metabolites catalyzing the first step of the phenylpropanoid pathway which converts L-phenylalanine to trans-cinnamic acid [6]. Trans-cinnamic acid is the precursor of a variety of phenylpropanoids such as lignins, flavonoids and coumarins [7]. PAL activity is associated with the accumulation of anthocyanins and other phenolic compounds in fruit tissue of several species, which is active during the development and ripening of fruits [8]. Extensive studies have been conducted on this enzyme due to its role in plant development and response to environmental stimuli [9].
The aim of this study was to evaluate the potential application of C. sicyoides as a nutraceutical, functional food or functional food ingredient. The study investigated the phenylalanine ammonia-lyase activity, free radical scavenging activity and cytotoxicity of C. sicyoides berries.
2Materials and methods
C. sicyoides berries were freshly harvested from the Mona Campus of the University of the West Indies, Kingston, Jamaica.
2.1Phenylalanine ammonia-lyase (PAL) assay
The PAL activity of C. sicyoides berries was determined utilizing an end point spectrophotometric assay [10]. Samples were homogenized in extraction buffer (50 mM Tris-HCl, 1 mM EDTA, 10 mM 2-mercaptoethanol and 2.5% (w/v) polyvinylpolypyrrolidone) in a 1:2 ratio (fresh weight basis: buffer). After homogenization, samples were refrigerated (30 min) and then centrifuged (4000 rpm, 15 min, 20°C). The resulting supernatant (2 mL) was partitioned with polyethylene glycol 6000 (PEG6000, 11%)/magnesium sulphate (MgSO4, 14%), polyethylene glycol 6000 (PEG6000, 11%)/sodium sulphate (Na2SO4, 14%), polyethylene glycol 4000 (PEG4000, 11%)/magnesium sulphate (MgSO4, 14%) or polyethylene glycol 4000 (PEG4000, 11%)/sodium sulphate (Na2SO4, 14%) [11]. The reaction mixture consisted of reaction buffer (100 mmol/L Tris HCl, 400μL), substrate (40 mmol/L phenylalanine, 200μL) and enzyme extract (200μL). Enzyme assays were incubated at 25°C for 5 min and the reaction terminated by the addition of trichloroacetic acid (25%, 200μL). Samples were centrifuged (IKA Mini G Portable Centrifuge, 6000 rpm, 10 min) and the absorbance of trans cinnamic acid measured at 290 nm (Genesys 10S UV-Vis spectrophotometer). Protein concentration was determined by use of the Bradford assay [12].
2.21,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
C. sicyoides berry samples were oven dried (70°C, 1 days, Gallenkamp Laboratory Oven OV-330, England), ground and extracted with ethanol (80%) containing hydrochloric acid (1%) at room temperature. The free radical scavenging activity of berry extracts (1 mg/mL– 10 mg/mL) was determined by utilizing the DPPH assay according to the method of Brand-Williams [13]. The reaction mixture consisted of berry extract (0.5 mL), absolute ethanol (3 mL) and DPPH (0.5 mM, 0.3 mL). The reaction was allowed to proceed for 60 min in the dark after which the absorbance was measured at 517 nm using a spectrophotometer (Thermo Scientific Genesys 10S UV-Vis). A mixture of ethanol (3.3 mL) and berry extract (0.5 mL) served as the blank. A control solution was prepared by mixing ethanol (3.5 mL) with the DPPH radical solution (0.3 mL). Gallic acid was used as a positive control. The DPPH scavenging effect (%) was calculated by using the formula:
(1)
Where:
A1 = Absorbance of sample at 517 nm
A0 = Absorbance of control at 517 nm
The IC50 value of berry extracts was calculated from the linear regression equation of a dose response curve (R2 = 0.97).
2.3Fourier transform infrared spectroscopy
A Bruker Vector 22 Fourier Transform Infra Red (FTIR) Spectrometer was utilized to record the infrared spectra of ethanolic extracts of C. sicyoides berries. FTIR spectra were recorded between 4000 and 500 cm–1. Each spectrum was obtained by averaging 20 scans recorded at a resolution of 2 cm–1. Spectra were baseline-corrected. OPUS software was used to acquire and manipulate the spectral data.
2.4Brine shrimp assay
Aqueous extracts of C. sicyoides berries were prepared by a modified method described by Hamid et al. (2011) [14]. Brine shrimp eggs were allowed to hatch (48 h) in brine solution (40 g/L) after which they were placed in a test tube to which berry extract was added at the desired concentrations. The percentage mortality of the shrimps at each concentration was calculated after 24 h.
2.5Data analyses
Samples were analysed in triplicate. Means and standard deviations of the data are presented.
3Results and discussion
3.1Phenylalanine ammonia-lyase
PAL is a key enzyme of the phenylpropanoid pathway providing a link between the primary metabolism of aromatic amino acids and secondary metabolites. The enzyme serves a regulatory role controlling the biosynthesis of phenylpropanoid products. Enhanced production of phenylpropanoid compounds is associated with increased PAL activity [15]. The enzyme exists in different tetrameric forms with slight differences in molecular masses, iso-electric points (pI) and Michaelis-Menten constant [16]. The pI for PAL is usually found to be in the acid range (2.5 to 6.3) and most are considered hydrophobic [7]. In raspberries, PAL is encoded by a family of two genes (RiPAL1 and RiPAL2). RiPAL1 is associated with the early stages of fruit ripening whereas RiPAL2 is involved in later stages of flower and fruit development [17].
PAL activity has been detected in the skin of blueberries, cranberries and grapes [18, 19]. In cardinal grapes, PAL activity was limited to the epidermal cell layer and was undetectable in acetone extracts or nitrogen pulverized whole or peeled berries [19]. The utilization of polyvinylpolypyrrolidone (PVPP) or polyethylene glycol (PEG) 4000 was indispensable for the detection of PAL activity in Vitis vinifera [19]. The use of PVPP and aqueous two-phase partitioning (ATPP) was therefore investigated for the extraction of PAL from C. sicyoides berries. PEG4000, PEG6000, sodium sulphate and magnesium sulphate were utilized to prepare the ATPP systems in the ratio of PEG (11%)/salt (14%) based on the optimum partitioning system reported by Wang et al. (2010) [11]. ATPP represents an economical and versatile method of liquid-liquid extraction that can be utilized in the preparation of enzyme extracts [20].
The PAL enzyme was detected in whole extracts of C. sicyoides berries and readily partitioned into the upper layer of the PEG6000 salt system. Better results were obtained with PEG6000 compared to PEG4000 (Table 1). The PAL activity of the berry extract was 0.10±0.03 U/mg protein. In a study conducted by Goldson et al. (2007), over twenty plant sources inclusive of fruits, legumes and grains and were screened for PAL activity. Of the 26 plant sources screened, red spring wheat had the highest PAL activity with fruits exhibiting lower PAL activity [21]. C. sicyoides berries are a potential source of PAL that can be further explored.
Table 1
Aqueous two-phase partitioning of C. sicyoides berry extracts
| Aqueous two-phase system | PAL activity (U/mg protein) |
| PEG6000/MgSO4 | 0.10±0.03 |
| PEG6000/Na2SO4 | 0.05±0.01 |
| PEG4000/MgSO4 | *ND |
| PEG4000/Na2SO4 | *ND |
1 U of activity = 1μmoL of released trans-cinnamic acid per min. *ND: Not detected.
Possible commercial applications of PAL include its use in enzyme substitution therapy for the treatment of phenylketonuria [22– 24]. Phenylketonuria is a genetic disorder in which persons are unable to digest phenylalanine. Accumulation of phenylalanine can lead to mental disorder [24]. The enzyme may also be utilized in the microbial synthesis of phenylalanine [25].
3.2DPPH free radical scavenging activity
DPPH is a stable free radical which loses its characteristic deep purple colour in solution upon accepting hydrogen from an available donor. C. sicyoides berries exhibit excellent free radical scavenging activity. The IC50 value which is the concentration at which 50% inhibition of the free radical activity is observed was determined to be 0.99 mg/mL (Fig. 1). In a study conducted by Hangun-Balkir and McKenney (2012) the IC50 values for several berries was reported (blueberry 0.70 mg/mL; raspberry 0.80 mg/mL; blackberry 1.40 mg/mL; strawberry 5.60 mg/mL; acai berry >10 mg/mL) [26]. The deep blue colour of C. sicyoides berries is due to the presence of delphinidin and cyanidin-type anthocyanin. Delphinidin-3-rutinoside, cyanidin-3-rhamnosyl-arabinoside and delphinidin-3-rhamnoside have been identified as the major anthocyanins in C. sicyoides berries [27]. Anthocyanins contribute to the antioxidant activity of the fruit and are known to exhibit a myriad of health benefits [28]. Anthocyanin rich fractions of strawberry (cv. Rominia) extracts were found to improve the lipid profile and antioxidant status of human hepatocellular carcinoma (HepG2) cells [29]. There was a decline in lipid peroxidation, triglyceride and low density lipoprotein (LDL) content [29]. At the onset of arteriosclerosis oxidized LDL is absorbed by cells. Lower levels of triglycerides and LDL are desirable to limit the progression of arteriosclerosis. The main anthocyanin identified in strawberry extracts was pelargonidin 3-O-glucoside [29]. C. sicyoides berries are expected to exhibit similar health benefits as observed in strawberries given its high free radical scavenging activity. Further investigation of anthocyanin rich extracts from the berries could provide further insight as to their potential health benefits.
Fig.1
DPPH free radical scavenging activity of ethanolic extracts of C. sicyoides berries.
The consumption of berries may also be beneficial in reducing the risk of type 2 diabetes mellitus [30]. The anti-diabetic properties of the berries are believed to be due to the presence of polyphenolics which enhance insulin release and regulate glucose metabolism resulting in maintained glucose homeostasis [30]. C. sicyoides berries have a total phenolic content of 2.7±0.2 mg gallic acid/g dry weight [31].
3.3Fourier transform infrared spectroscopy (FTIR)
Analysis of grapes and wines typically involves assessing quality parameters such as °Brix, titratable acidity and pH. These parameters have been previously reported for C. sicyoides berries [32]. Total anthocyanin content and the total phenolic content of the berries have significant impact on the quality of value added products such as wines produced from grapes. Quantification of phenolic compounds can be very time consuming. Protocols typically utilized involve the use of spectroscopy [33– 35] and high performance liquid chromatography [35]. In South Africa, FTIR has been widely utilized in routine laboratory procedures for the quantification of wine and grape parameters [36]. FTIR represents a rapid method of analysis. There is however need for commercial calibration models for proper quantification of the phenolic compounds present [36].
FTIR was used to analyze ethanolic extracts of C. sicyoides berries. The presence of phenolic compounds was confirmed by FTIR with a broad band being observed at 3261 cm–1 (Fig. 2). Carbon-oxygen (C-O) stretch vibrations which are due to the presence of carbohydrates were observed at 1022 and 1033 cm–1 (Fig. 2, Table 2). Prior research confirmed the presence of reducing sugars in the berries [31]. In a study conducted by Adina et al., (2010), absorption peaks of 991, 1033, 1078, 1107 and 1149 cm–1 were reported for glucose with maximum absorption being observed at 1033 cm–1 [37]. An absorption peak was observed at 1033 cm–1 in berry extracts therefore suggesting that the major carbohydrate present in C. sicyoides berries may be glucose. The berries are a limited source of proteins with bands occurring at 1400 and 1624 cm–1 due to the amide functionality. The mineral phosphorus is also present with a peak being observed at 619 cm–1 which is indicative of the presence of phosphates [38]. A peak at 2906 cm–1 is due to stretching vibrations of CH which could be due to methyl or methylene functionalities. A study investigating the spoilage of strawberries utilizing FTIR, suggested that a peak at 2899 cm–1 may be due to ethylene which is produced during the ripening of fruits [39]. Smaller peaks were observed resonating at 667, 769, 783, 827, 891 cm–1 which may be attributable to ring vibrations, specifically, the aromatic C-H functionalities present in polyphenolic compounds. Peaks associated with the presence of gallic acid have been reported as resonating at 669, 763, 1025, 1100 and 1654 cm–1 [40].
Fig.2
Infrared spectrum of C. sicyoides berries ethanolic extract.
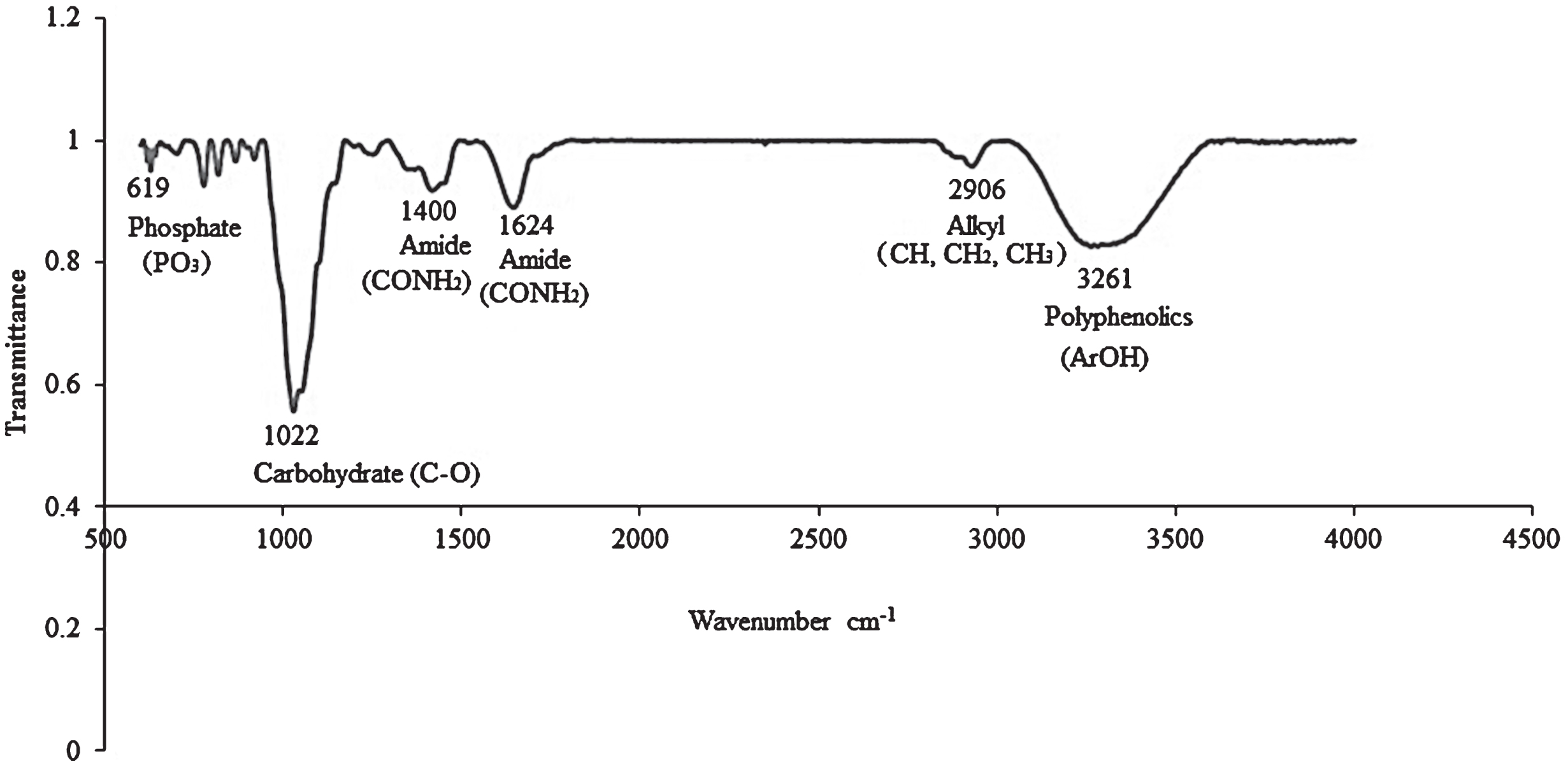
Table 2
FTIR spectral data for Cissus sicyoides berry ethanolic extracts
| Functionality | cm–1 |
| Phosphates: P-O | 619 (w) |
| Carbohydrates: C-O stretch vibrations (endocyclic &exocyclic) | 1022 (s); 1033 (sh) |
| Polyphenolics: Aromatic C-H functionalities | 667, 769, 783, 827, 891 (w) |
| Proteins: Amide functionality | 1400 (m); 1624 (m) |
| Stretching vibrations of CH (CH3 or CH2) groups | 2906 (m) |
| Polyphenolics: OH | 3261(b) |
†b: broad m: medium sh: shoulder s: sharp w: weak.
FTIR is a rapid experimental technique which requires minimal sample preparation, is environmentally friendly and does not require the use of toxic chemicals. In combination with other experimental techniques such as Principal Component Analysis and Artificial Neural Networks, a non-linear statistical data modeling tool, FTIR may prove invaluable in the quantification of phenolic components of berries and other fruits [40].
3.4Brine shrimp assay
Preliminary cytoxicity studies were performed on C. siycoides berry extracts utilizing the Brine shrimp lethality (Artemia salina Leach) assay [41– 43]. This bioassay is a preliminary toxicity screening that is widely utilized in determining the toxicity of plant extracts [44]. According to a study conducted by Meyer et al. (1982) extracts with LD50 values >1000μg/ml are considered as non toxic [44]. Extracts may also be classified as strongly toxic (LC50 <100μg/ml), moderately toxic (LC50 100– 500μg/ml) or weakly toxic (LC50 500– 1000μg/ml). In a study conducted by Karchesy et al. (2016), 211 methanolic plant extracts were evaluated which was inclusive of berry extracts [45]. Some berries were non toxic while others were moderately toxic. Non toxic berry extracts included blue elderberries (Sambucus nigra subsp. caerulea), snowberry (Symphoricarpos albus), juniper (Juniperus occidentalis), Pacific madrone (Arbutus menziesii), Kinnikinnick (Arctostaphylos uva-ursi), black hawthorn (Crataegus douglasii) and Indian-plum (Oemleria cerasiformis). Berry samples which were moderately toxic included Devil’s club berries (Oplopanax horridum, LC50 239μg/ml), Tall Oregon grape (Berberis aquifolium, LC50 305μg/ml), soapberry (Shepherdia canadensis, LC50 value 387μg/ml) and Mountain ash, (Sorbus scopulina, LC50 318μg/ml) [45].
The results from the present study suggests that C. sicyoides berries are non toxic with a LD50 value >1000μg/ml. At a concentration of 1,000μg/ml no cytotoxicity was observed in aqueous or methanolic extracts of the berries (Table 3). At 20,000μg/ml, 59% mortality was observed in aqueous extracts and 65% mortality in ethanolic extracts. No visible symptoms or signs of toxicity were observed in mice treated with ethanolic extracts of the C. sicyoides leaves [46]. Recent studies on the Cissus tiliacea berries harvested in Mexico concluded that the fruit liquor may be acceptable for food application [47]. C. sicyoides berries have similar physicochemical properties to Cissus tiliacea berries further substantiating that they may be considered for food applications [31].
Table 3
Percentage mortality of Brine shrimps exposed to C. sicyoides berry extracts
| Cissus sicyoides extracts | μg/mL | Mortality (%) |
| Aqueous extract | 1,000 | 0 |
| 10,000 | 29 | |
| 20,000 | 59 | |
| Ethanol extract | 1,000 | 0 |
| 10,000 | 15 | |
| 20,000 | 65 | |
| Potassium dichromate (Positive control) | 100 |
4Conclusion
C. sicyoides berries are non toxic and possess high free radical scavenging activity which is intermediate to that of raspberries and blackberries. The berries are also a good source of the PAL enzyme which has biotechnology and medicinal applications.
Conflict of interest
The authors have no conflicts of interest to report.
References
[1] | Fernandes G , Banu J . Medicinal properties of plants from the genus Cissus: A review. J Med Plants Res. (2012) ;6: (16):3080–86. doi:10.5897/JMPR11.1637 |
[2] | De Almeida ER , De Oliveira Rafael KR , Couto GBL , Ishigami AB . Anxiolytic and anticonvulsant effects on mice of flavonoids, linalool, and α-tocopherol presents in the extract of leaves of Cissus sicyoides L. (Vitaceae). J Biomed Biotechnol. (2009) ;Article ID 274740. doi:10.1155/2009/274740 |
[3] | Beltrame FL , Ferreira AG , Cortez DA . Coumarin glycoside from Cissus sicyoides . Nat Prod Lett. (2002) ;16: (4):213–16. doi:10.1080/10.575630290015736 |
[4] | De Paula Ferreira MP , Nishijima CM , Seito LN , Dokkedal AL , Lopes-Ferreira M , Di Stasi LC , Vilegas W , Hiruma-Lima CA . Gastroprotective effect of Cissus sicyoides (Vitaceae): Involvement of microcirculation, endogenous sulfhdryls and nitric oxide. J Ethnopharmacol. (2008) ;117: (1):170–74. doi:10.1016/j.je2008.01.008 |
[5] | Khalil NM , Pepato MT , Brunetti IL . Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: Bauhinia forficata and Cissus sicyoides . Biol Res. (2008) ;41: (2):165–71. doi:S0716-97602008000200006 |
[6] | Whetten RW , Sederoff RR . Phenylalanine ammonia-lyase from loblolly pine: Purification of the enzyme and isolation of complementary DNA clones. Plant Physiol. (1992) ;98: (1):380–86. doi: https://doi.org/10.1104/98.1.380 |
[7] | Hyun MW , Yun YH , Kim JY , Kim SH . Fungal and plant phenylalanine ammonia-lyase. Mycobiology. (2011) ;39: (4):257–65. doi:10.5941/MYCO.2011.39.4.257 |
[8] | Flores G , De la Peña Moreno F , Blanch GP , Del Castillo ML . Phenylalanine ammonia-lyase, flavanone 3β-hydroxylase and flavonol synthase enzyme activity by a new in vitro assay method in berry fruits. Food Chem. (2014) ;153: :130–33. doi:10.1016/j.foodchem.2013.12.034 |
[9] | Jones DH . Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry. (1984) ;23: (7):1349–59. doi: https://doi.org/10.1016/S0031-9422(00)80465-3 |
[10] | Abell CW , Shen R . Phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis . Methods Enzymol. (1987) ;142: :242–53. |
[11] | Wang WY , Yue HY , Yuan QP , Wang WC . Partitioning of phenylalanine ammonia-lyase from Rhodotorula glutinis in aqueous two-phase systems of PEG/salts. Chem Biochem Eng Q. (2010) ;24: (4), 459–65. |
[12] | Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. (1976) ;72: (1-2):248–54. doi: https://doi.org/10.1016/0003-2697(76)90527-3 |
[13] | Brand-Williams W , Cuvelier M , Berset C . Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. (1995) ;28: (1):25–30. doi: https://doi.org/10.1016/S0023-6438(95)80008-5 |
[14] | Hamid K , Sultana S , Urmi KF , Ullah MO , Zulfiker AH , Hossain MA . In vitro free radical scavenging and brine shrimp lethality bioassay of aqueous extract of Ficus racemosa seed. JJBS. (2011) ;4: (1), 51–54. |
[15] | Ozeki Y , Komamine A . Changes in activities of enzymes involved in general phenylpropanoid metabolism during the induction and reduction of anthocyanin synthesis in a carrot suspension culture as regulated by 2,4-D. Plant Cell Physiol. (1985) ;26: (5):903–11. 10.1093/oxfordjournals.pca076985 |
[16] | Appert C , Loggeman E , Hahlbrock K , Schmid J , Amrhein N . Structural and catalytic properties of the four phenylalaanine ammonia-lyase isoenzymes from parsley (Petroselinum crispum nym). Eur J Biochem. (1994) ;225: (1):491–99. doi:10.1111/j.1432-1033.1994.00491.x |
[17] | Kumar A and Ellis BE . The phenylalanine ammonia-lyase gene family in raspberry. structure, expression, and evolution. Plant Physiol. (2001) ;127: (1):230–39. doi: https://doi.org/10.1104/127.1.230 |
[18] | Sapers GM , Matulaitis RM , Beck JA . Detection of phenylalanine ammonia-lyase in the skin of blueberry and cranberry fruits. J Food Sci. (1987) ;52: (1):155–58. doi:10.1111/j.1365-2621.1987.tb13994.x |
[19] | Roubelakis-Angelakis KA , Kliewer WM . Phenylalanine ammonia-lyase in berries of Vitis vinifera L. : Extraction and possible sources of error during assay. Am J Enol Vitic. (1985) ;36: :314–15. |
[20] | Glyk A , Scheper T , Beutel S . PEG– salt aqueous two-phase systems: An attractive and versatile liquid– liquid extraction technology for the downstream processing of proteins and enzymes. Appl Microbiol Biotechnol. (2015) ;99: (16):6599–616. doi:10.1007/s00253-015-6779-7 |
[21] | Goldson A , Lam M , Scaman CH , Clemens S , Kermode A . Screening of phenylalanine ammonia-lyase in plant tissues, and retention of activity during dehydration. J Sci Food Agric. (2008) ;88: (4):619–25. doi:10.1002/jsfa.3126 |
[22] | Şirin S , Aydaş SB , Aslim B . Biochemical evaluation of phenylalanine ammonia- lyase from endemic plant Cyathobasis fruticulosa (Bunge) Aellen for the dietary treatment of phenylketonuria. Food Technol Biotechnol. (2016) ;54: (3):296–03. doi:10.17113/ftb.54.03.16.4519 |
[23] | Strisciuglio P , Concolino D . New strategies for the treatment of phenylketonuria (PKU). Metabolites. (2014) ;4: :1007–017. doi:10.3390/metabo4041007 |
[24] | Sarkissian CN , Gámez A . Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol Genet Metab. (2005) ;86: (1):S22–6. doi:10.1016/j.ymgme.2005.06.016 |
[25] | Kong JQ . Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv. (2015) ;5: :62587–2603. doi:10.1039/C5RA08196C |
[26] | Hangun-Balkir Y , McKenney ML . Determination of antioxidant activities ofberries and resveratrol. Green Chem Lett Rev. (2012) ;5: (2):147–53. 10.1080/17518253.2011.603756 |
[27] | Toledo MCF , Reyes FGR , Iaderoza M , Francis FJ , Draetta IS . Anthocyanins from anil trepador (Cissus sicyoides, Linn). J Food Sci. (1983) ;48: (4):1368–69. doi:10.1111/j.1365-2621.1983.tb09238.x |
[28] | Lila MA . Anthocyanins and human health: An in vitro investigative approach. J Biomed Biotechnol. (2004) ;5: :306–13. doi:10.1155/S111072430440401X |
[29] | Forbes-Hernández TY , Gasparrini M , Afrin S , Cianciosi D , González-Paramás AM , Santos-Buelga C , Mezzetti B , Quiles JL , Battino M , Giampieri F , Bompadre S . Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int J Mol Sci. (2017) ;18: :1149. doi:10.3390/ijms18061149 |
[30] | Edirisinghe I , Burton-Freeman B . Anti-diabetic actions of berry polyphenols – Review on proposed mechanisms of action. J Berry Res. (2016) ;6: (2):237–50. doi:10.3233/JBR-160137 |
[31] | Goldson Barnaby A , Reid R , Warren D . Phenylalanine ammonia-lyase activity, antioxidant properties, fatty acid profile, mineral content and physiochemical analyses of Cissus sicyoides berries. Journal of Berry Research. (2017) ;7: (2):117–27. doi:10.3233/JBR-170148 |
[32] | Aleixandre-Tudo JL , Nieuwoudt H , Olivieri A , Aleixandre JL , Toit W . Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control. (2018) ;85: :11–22. doi: https://doi.org/10.1016/j.foodcont.2017.09.014 |
[33] | Boulet JC , Ducasse MA , Cheynier V . Ultraviolet spectroscopy study of phenolic substances and other major compounds in red wines: Relationship between astringency and the concentration of phenolic substances. Aust J Grape Wine Res. (2017) ;23: (2):193–99. doi:10.1111/ajgw.12265 |
[34] | Ferrer-Gallego R , Hernández-Hierro JM , Rivas-Gonzalo JC , Escribano-Bailón MT . Determination of phenolic compounds of grape skins during ripening by NIR spectroscopy. LWT Food Sci Technol. (2011) ;44: (4):847–53. doi: https://doi.org/10.1016/j.lwt.2010.12.001 |
[35] | Gómez-Alonso S , García-Romero E , Hermosín-Gutiérrez I . HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J Food Compos Anal. (2007) ;20: (7):618–26. doi: https://doi.org/10.1016/j.jfca.2007.03.002 |
[36] | LochnerE . The evaluation of Fourier transform infrared spectroscopy (FT-IR) for the determination of total phenolics and total anthocyanins concentrations in grapes. Stellenbosch University, (2006) . |
[37] | Adina C , Florinela F , Abdelmoumen T , Carmen S . Application of FTIR spectroscopy for a rapid determination of some hydrolytic enzymes activity on sea buckthorn substrate. Rom Biotech Lett. (2010) ;15: (6), 5738–44. |
[38] | El-BahyGMS . FTIR and Raman spectroscopic study of fenugreek (Trigonella foenum graecum L.) seeds. J Appl Spectrosc. (2005) ;72: (1):111–16. doi:10.1007/s10812-005-0040-6 |
[39] | Dong D , Zhao C , Zheng W , Wang W , Zhao X , Jiao L . Analyzing strawberry spoilage via its volatile compounds using longpath fourier transform infrared spectroscopy. Sci Rep. (2013) ;3: :2585. doi:10.1038/srep02585 |
[40] | Agatonovic-Kustrin S , Morton DW , Yusof AP Md . The use of Fourier transform infrared (FTIR) spectroscopy and artificial neural networks (ANNs) to assess wine quality. Mod Chem Appl. (2013) ;1: :110. doi:10.4172/2329-6798.1000110 |
[41] | Michael AS , Thompson CG , Abramovitz M . Artemia salina as a test organism for a bioassay. Science. (1956) ;123: (3194):464. doi:10.1126/science.123.3194.464 |
[42] | Sleet RB , Brendel K . Improved methods for harvesting and counting synchronous populations of Artemia nauplii for use in developmental toxicology. Ecotoxicol Environ Saf. (1983) ;7: (5):435–46. doi: https://doi.org/10.1016/0147-6513(83)90082-9 |
[43] | Vanhaecke P , Persoone G , Claus C , Sorgeloos P . Proposal for a short-term toxicity test with Artemia nauplii . Ecotoxicol Environ Saf. (1981) ;5: :382–87. 10.1016/0147-6513(81)90012-9 |
[44] | Meyer BN , Ferrigni NR , Putnam JE , Jacobsen LB , Nichols DE , McLaughlin JL . Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. (1982) ;45: (5):31–34. doi:10.1055/s-2007-971236 |
[45] | Karchesy YM , Kelsey RG , Constantine G , Karchesy JJ . Biological screening of selected Pacific Northwest forest plants using the brine shrimp (Artemia salina) toxicity bioassay. Springer Plus. (2016) ;5: :510. doi: https://doi.org/10.1186/s40064-016-2145-1 |
[46] | Beserra FP , Santos RdeC , Perico LL , Rodrigues VP , Kiguti LRA , Saldanha LL , Pupo AS , da Rocha LRM , Dokkedal AL , Vilegas W , Hiruma-Lima CA . Cissus sicyoides: Pharmacological mechanisms involved in the anti-inflammatory and antidiarrheal activities. Int J Mol Sci. (2016) ;17: (2):149/1–149/15. doi:10.3390/ijms17020149 |
[47] | Franco-Mora O , Morales P , Aaran A , Mirelles V , Alma D , Castaneda-Vildozola A , Sanchez-Pale JR . Searching alternative uses for Cissus tiliacea kunth fruit in central Mexico: Seed oil and fruit liquor. Genet Resour Crop Ev. (2016) ;63: (1):141–49. doi: https://doi.org/10.1007/s10722-015-0343-2 |




