Structure-affinity relationship of dietary anthocyanin–HSA interaction
Abstract
BACKGROUND:
Dietary anthocyanins are plant pigments which occur with different chemical structures, being widely present in fruits and in many vegetables, are claimed to be beneficial for human health. The bioavailability of anthocyanins is the key factor influencing their health benefits.
OBJECTIVE:
Herein, the molecular structure-affinity relationship of anthocyanin–human serum albumin interaction was investigated.
METHODS:
Fluorescence quenching method was applied to determine the binding affinities of anthocyanins for human serum albumin.
RESULTS:
Demethylation of the methoxyl groups in anthocyanins enhanced the binding affinities. The number and position of the hydroxyl groups on ring [B] affect the affinities of anthocyanins for human serum albumin. The glycosylation of hydroxyl groups on ring [C] enhanced their binding affinities for human serum albumin.
CONCLUSIONS:
Anthocyanidins and anthocyanins, show different characteristics for their binding to human serum albumin when the methoxyl groups on the ring B are demethylated or hydroxylated.
1Introduction
Numerous cell culture, animal, and human subject studies have declared the health benefit of various phenolic-rich foods on human health. At present, the strongest evidence for the efficacy of phenolics against chronic disease exists for anthocyanins [1] and flavonoids [2–5] rich foods. The anthocyanidin structure is based on a three ring C6-C3-C6 system consisting of two aromatic rings ([A], [B]) attached to an oxygen-containingheterocyclic ring [C]. The anthocyanins are the glucosidic derivatives of the anthocyanidins, and these are widespread in natureas 3-monosides, 3-biosides, and 3,5- and 3,7-diglucosides. In nature, the 3-glucoside derivatives are 2.5-fold more prevalent than the 3,5-diglucosides [6]. The most abundant anthocyanins are the glucoside derivatives of cyanidin (Cy), delphinidin (Dp), and pelargonidin (Pg). These are non-methylatedanthocyanidins [6], with the most common being Cy-3-glucoside (Cy-3-glc) [7]. The anthocyanins are essential pigments in higher plants (mainly vascular plants), and are present in almost all plant organs, including fruit, roots, stems, leaves, and flowers [8]. Depending on the pH and their different substitution patterns, these pigments provide a variety of color to plant tissues, ranging from red to blue and purple [9].
Previous studies have determined the relationships between anthocyanin intake from dietary polyphenols and their human health benefits. These benefits have been demonstrated over multiple experimental platforms and with different disease targets [10], with various bioactivities including, cardio-protective effect [11], modulatory effect on vascular reactivity [12], anti-bacterial activity [13], anti-diabetic activity [14], anti-tumor activity [15], and so on.
The structural differences among anthocyanins relate to glycosylation of ring [C], and the number and distribution of hydroxyl and methoxyl groups on ring [B] and hydroxyl groups on ring [A]. These structural differences significantly influence their chemical and physical properties, as well as their bio-availability.
In general, hardly any polyphenols exist in their glycosylated forms in foods. In their natural forms, usually polyphenols cannot be absorbed, but following their hydrolysis in the intestinal tract, their absorption increases. However, anthocyanins are an exception in this regard, since the natural glycosides cannot only be absorbed, but can also be detected in the circulation [16]. Therefore, studies into the interactions between serum proteins and anthocyanins are essential and necessary.
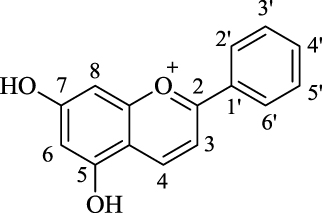
In recent years, reports have focused on the interactions between flavonoids and proteins [17, 18]. However, only a few reports have concerned the binding affinities of anthocyanins for human serum albumin(HSA), among which one study investigated the influence of pH and structural differences on their binding affinities [19]. The present study focuses on the relationships between the molecular properties of anthocyanins and their affinities for HSA. In nature, among the 17 known anthocyanidins only six are ubiquitous: Cy, Dp, malvidin (Mv), petunidin (Pt), peonidin (Pn), and Pg. In this report, we studied all of these except Pg, and also as their mono-glycosylated anthocyanins, Cy-3-glc, Dp-3-glc, Mv-3-glc, Pt-3-glc, and Pn-3-glc (Table 1).
Table 1
Chemical structures of the anthocyanidins and anthocyanins and their affinities for HSA in vitro
| Subclass | Name | Substitutions | lgKa | n | |||
| -OH | -OCH3 | -O-glucoside | |||||
| Anthocyanidins | Cyanidin chloride | Cy | 3, 5, 7, 4′, 5′ | — | — | 4.856 | 0.9965 |
 | Delphinidin chloride | Dp | 3, 5, 7, 3′, 4′, 5′ | — | — | 5.563 | 1.1090 |
| Malvidin chloride | Mv | 3, 5, 7, 4′ | 3′, 5′ | — | 4.605 | 1.0030 | |
| Peonidin chloride | Pn | 3, 5, 7, 4′ | 3′ | — | 4.480 | 0.9506 | |
| Petunidin chloride | Pt | 3, 5, 7, 4′, 5′ | 3′ | — | 4.830 | 1.0140 | |
| Anthocyanins | Cyanidin 3-glucoside chloride | Cy-3-glc | 3, 5, 7, 4′, 5′ | — | 3 | 4.012 | 0.8994 |
 | Delphinidin 3-glucoside chloride | Dp-3-glc | 3, 5, 7, 3′, 4′, 5′ | — | 3 | 4.542 | 0.9984 |
| Malvidin 3-glucoside chloride | Mv-3-glc | 3, 5, 7, 4′ | 3′, 5′ | 3 | 4.088 | 0.9483 | |
| Peonidin 3-glucoside chloride | Pn-3-glc | 3, 5, 7, 4′ | 3′ | 3 | 5.145 | 1.1210 | |
| Petunidin 3-glucoside chloride | Pt-3-glc | 3, 5, 7, 4′, 5′ | 3′ | 3 | 4.935 | 1.0590 | |
2Materials and methods
2.1Apparatus and reagents
Fluorescence emission spectra were recorded on a fluorescence spectrophotometer (Cary Eclipse; Varian Medical Systems, Inc., California, USA). Anthocyanidins and anthocyanins (as listed in Table 1) were from Tauto Biotech Co. Ltd. (Shanghai, China). HSA(96–99%, lyophilized powder) was provided from Solarbio Life Sciences Co. Ltd. (Beijing, China). The stock solutions of anthocyanins in methanol were prepared at a concentration of 1.0×10 - 3 mol/L. The stock solution of HSA in double distilled water was at a concentration of 1.0×10 - 5 mol/L. All of the sample solutions and reagents were freshly prepared just prior to use.
2.2Fluorescence emission spectra
Fluorescence emission spectra of HSA (Fig. 1) were measured in the wavelength range from 290 nm to 420 nm after titration with anthocyanin solutions with concentrations ranging f from 1×10 - 6 mol/L to 1×10 - 5 mol/L. Aliquots (2.5 μL) of anthocyanin solution was added to HSA (2.5 mL) in a cuvette using a micropipette, and the spectra were recorded at 25°C. The excitation wavelength was 280 nm for HSA. The fluorescence emission intensity was read at 346 nm. The experiments were performed at pH 7.0.
3Results and discussion
3.1Binding constants and number of binding sites
The main structural differences among the Cy, Dp, Mv, Pt, Pn chloride anthocyanidins are at positions 3′ and 5′ on ring [B] (Tables 1, 2). The differences among these that was focused on in this study were: (1) numbers and positions of hydroxyl groups on the basic skeleton; (2) numbers and the positions of methoxyl groups on the basic skeleton; and (3) presence or absence of glucoside attached to the 3 position. Here, the chemical structures of these investigated compounds can be divided into two groups: those with sugar moieties (anthocyanins) and those without sugar moieties (anthocyanidins).
Table 2
Effects of hydroxylation on ring [B] of the anthocyanidins on the affinities for HSA
| Anthocyanidin | Abbreviation | Ring [B] | Position | Effect |
| Cyanidin chloride | Cy | 4′, 5′ | — | — |
| Delphinidin chloride | Dp | 3′, 4′, 5′ | 3′H⟶OH | ↑5.09 |
| Malvidin chloride | Mv | 4′ | 5′OH⟶OMe | ↑1.78 |
| Peonidin chloride | Pn | 4′ | 5′OH⟶OMe | ↑2.38 |
| Petunidin chloride | Pt | 4′, 5′ | — | No effect (1.06) |
According to the double-logarithm equation [20, 21], the binding constant (Ka) can be calculated through Equation (1):
(1)
where F0 and F are the fluorescence intensities of HSA in the absence and presence of anthocyanins, respectively, Ka is the binding constant, n is the number of binding sites, and [Q] is the anthocyanin concentration. The summarized data are given in Table 1. The P value of the relationship between lgKa and n is significant (Fig. 2), which indicates that Equation (1) is a good model for the study of interactions between anthocyanins and HSA.
3.2Effects of Demethylation of Methoxyl Groups on Affinities for HSA
Demethylation of the methoxyl groups in anthocyanins enhanced the binding affinities, as shown in Fig. 3. The demethylation of some anthocyanins significantly affected their binding affinities for HSA, while the effects of others were small. In particular, the affinity of Dp chloride was nine-fold higher than that of Mv chloride, the affinity of Pt-3-glc chloride was seven-fold higher than that of Mv-3-glc chloride, the affinity of Mv-3-glc chloride was seven-fold lower than that of Pt-3-glc chloride, and the affinity of Dp-3-glc chloride was less than two-fold higher than that of Pt-3-glc chloride. For Mv, this has fewer hydroxyl groups than Dp, where the 3′,5′-methoxyl groups in Mv are hydroxyl groups in Dp. Xenobiotic metabolism for anthocyanin breakdown includes demethylation and deglucuronidation, which can occur in the intestinal wall through the colon microbiota [10]. Flores et al. found that whenever there was demethylation in the C3’ and C5’ of ring [B], the phenolic acid was produced by the gut microflora [22]. This aspect modulates the composition of the gut microbial community, and it has been proposed that anthocyanins influence human health in this manner [23].
Fig.1
Quenching effect of Dp chloride and Mv-3-glc chloride on human serum albumin fluorescence spectra at excitation wavelength of 280 nm. HSA, 10 μmol/L; a-k: 0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0×10 - 6 mol/L Dp chloride (A) and Mv-3-glc chloride (B).
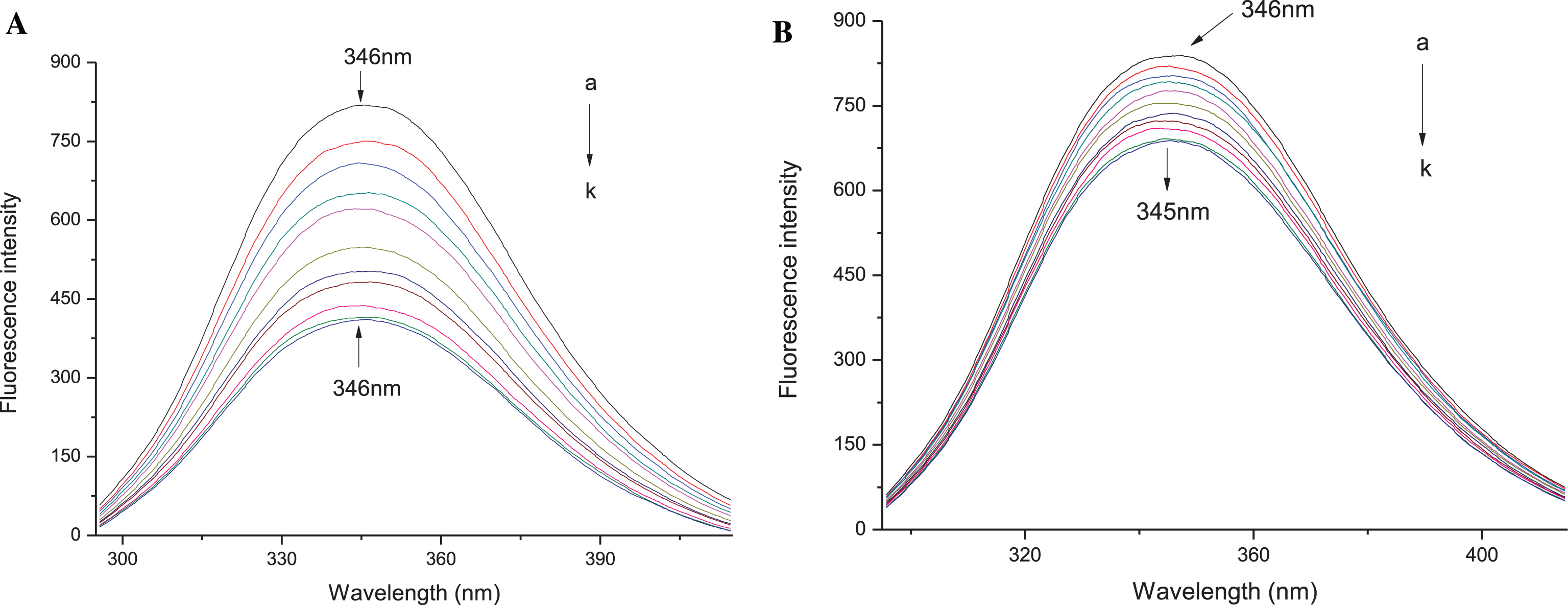
Fig.2
Fit between affinities (lgKa) and number of binding sites (n) for HSA and anthocyanins.
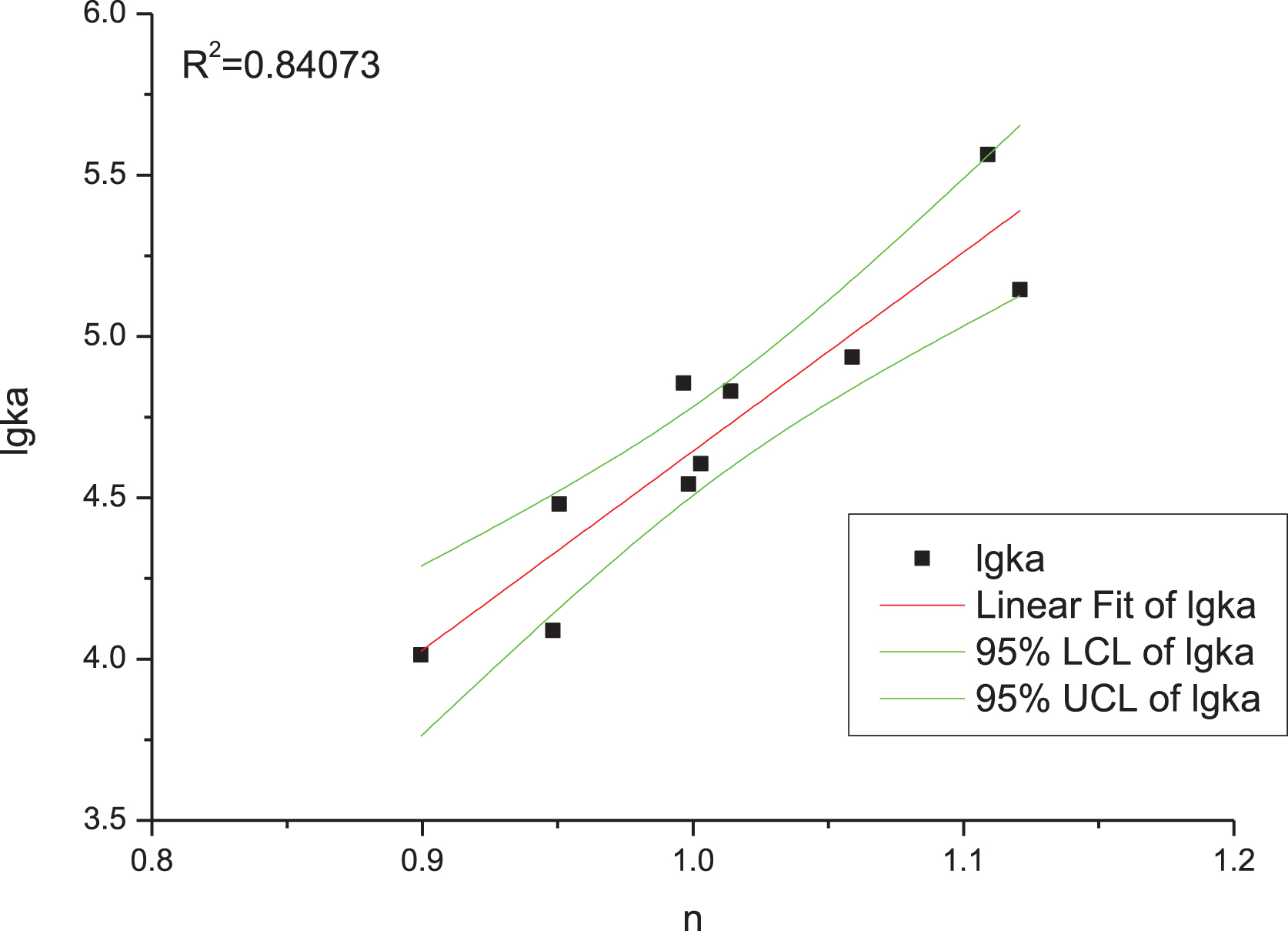
Fig.3
Effects of demethoxylation of anthocyanins and anthocyanidins on the affinity for HSA.
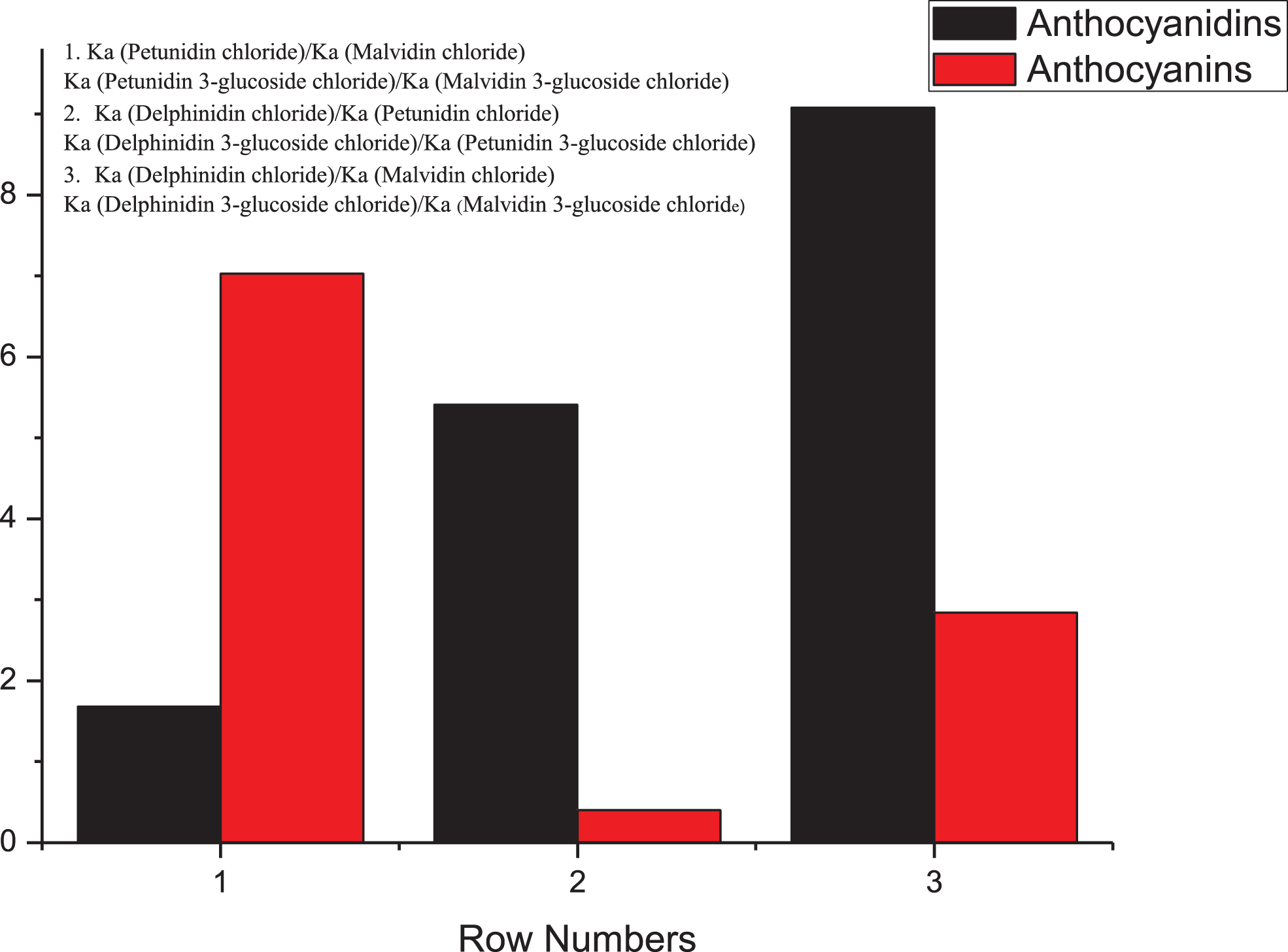
3.3Hydroxylation on Ring [B]
The hydroxylation and the position of the hydroxyl groups on ring [B] affect the affinities of anthocyanins in terms of their binding to HSA. We separated these compounds into two groups, and studied the relationships between their binding affinities and the hydroxylation of ring [B] for anthocyanins and anthocyanidins. Cy chloride was defined as the standard against which the other anthocyanidins were compared, and similarly for Cy-3-glc as standard for the anthocyanins. The data obtained are given in Tables 2 and 3.
Table 3
Effects of hydroxylation on ring [B] of anthocyanins on the affinities for HSA
| Anthocyanin | Abbreviation | Ring [B] | Position | Effect |
| Cyanidin 3-glucoside chloride | Cy-3-glc | 4′, 5′ | — | — |
| Delphinidin 3-glucoside chloride | Dp-3-glc | 3′, 4′, 5′ | 3′H⟶OH | ↑3.39 |
| Malvidin 3-glucoside chloride | Mv-3-glc | 4′ | 5′OH⟶OMe | No effect (1.19) |
| Peonidin 3-glucoside chloride | Pn-3-glc | 4′ | 5′OH⟶OMe | ↑13.58 |
| Petunidin 3-glucoside chloride | Pt-3-glc | 4′, 5′ | — | ↑8.38 |
3.3.1Hydroxylation on anthocyanidins
As shown in Table 2, the number of hydroxyl groups was related to the binding affinities for HSA. Cy chloride and Pt chloride have similar hydroxylation, and their binding affinities were also similar. With the increased number of hydroxyl groups in Dp chloride, the binding affinities were enhanced more than five-fold, while when the hydroxyl group was methylated, the shift in the affinities was not so obvious.
3.3.2Hydroxylation on anthocyanins
As shown in Table 3, no regular correlation exists between the numbers of hydroxyl groups on the anthocyanins with the binding affinities for HSA. For example, Cy chloride and Pt chloride share similar hydroxylation and binding affinities; in contrast, Pt-3-glc chloride had an over eight-fold higher affinity than Cy-3-glc chloride. The 5’-hydroxyls are methylated in Mv-3-glc chloride and Pn-3-glc chloride; however, the effects on their affinities were different.
3.4Effects of Glycosylation of Anthocyanins on their Affinities for HSA
Studies that have investigated anthocyanin bioavailability have all reported on the intact anthocyanin glycosides. In this respect, there appears to be an essential difference with the absorption of other flavonoids’classes [24]. Intact anthocyanins (which have the sugar moieties) can be detected in portal plasma after their introduction into the stomachs of rats [25].
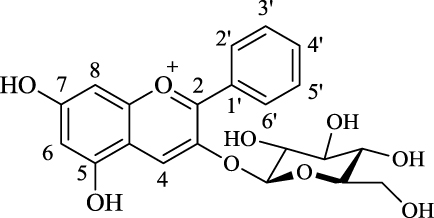
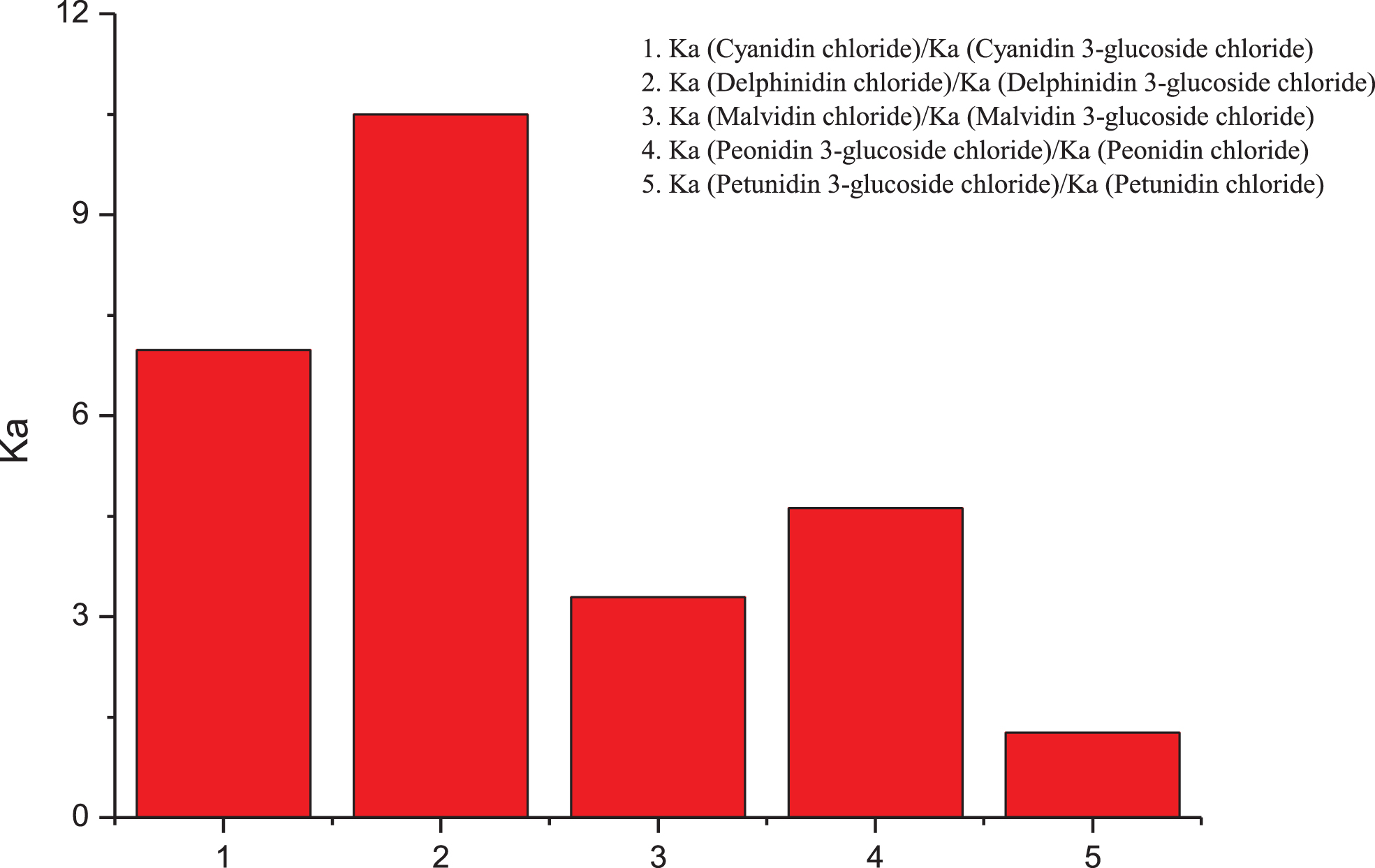
The anthocyanins and anthocyanidins in the current study are compounds that are glycosylated (anthocyanins) or are present inaglycone form (anthocyanidins). All anthocyanins are O-glycosides [26], and the C-3 position is the most common glycosylation position [27]. For these C-3 glucoside anthocyanins that were chosen as the targets in the present study, as shown in Fig. 4, there were differences in their binding affinities for HSA. Some of these glycosylated compounds showed decreased binding affinities (i.e., Cy-3-glc, Dp-3-glc, Mv-3-glc), while for the others, the glycosylation enhanced their binding affinities (i.e., Pn-3-glc, Pt-3-glc). On the one hand, the binding affinity of Dp chloride was 10-fold higher than for Dp-3-glc chloride, while on the other hand, the binding affinity of Pn-3-glc chloride was four-fold higher than for Pn chloride.
Fig.4
Effects of glycosylation of different aglycones on the affinity for HSA.
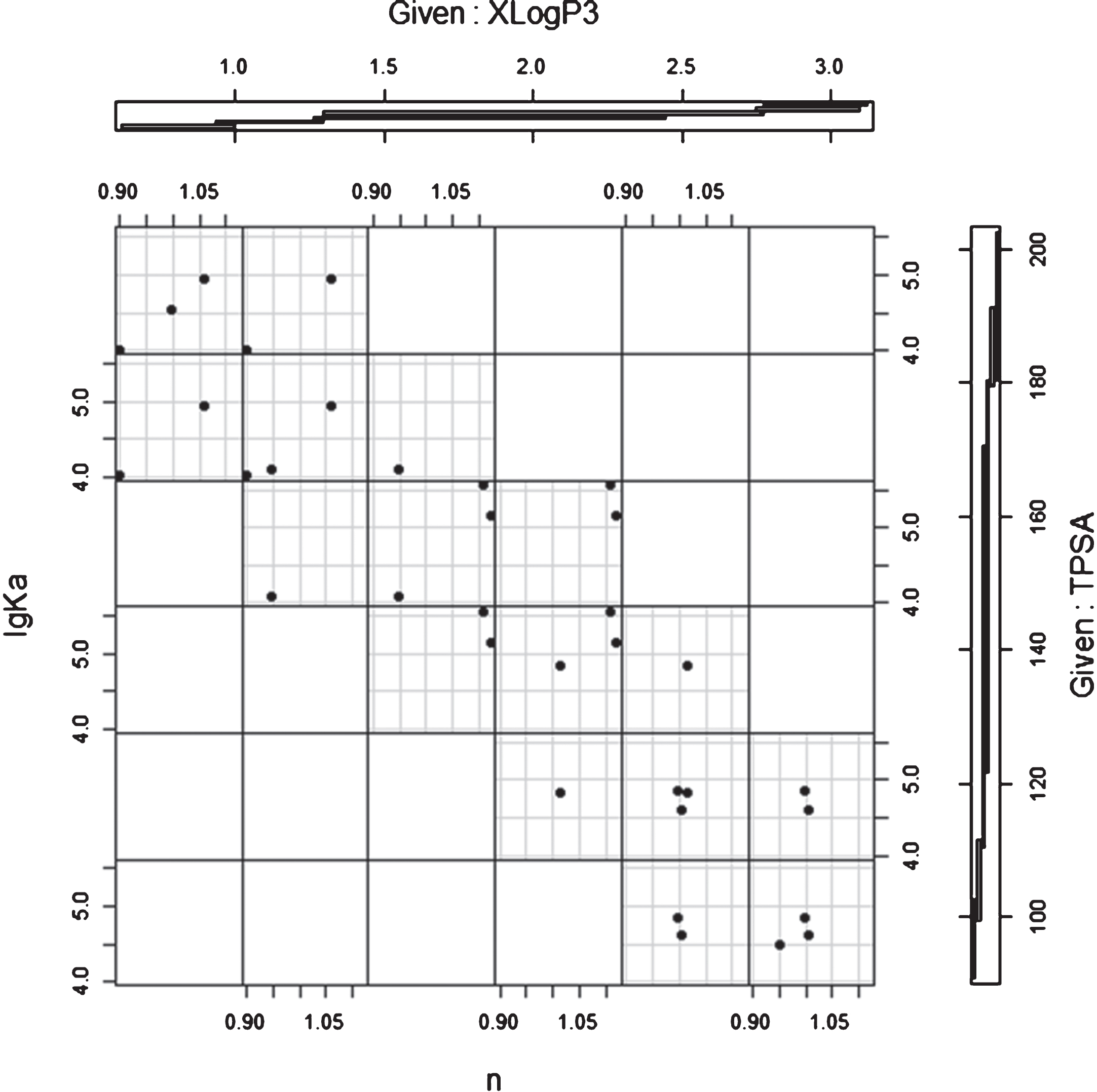
3.5Relationships among Partition Coefficients, Topological Polar Surface Areaand Affinity for HSA
The partition coefficients (XLogP3) and topological polar surface areas (TPSAs) are two of the six molecular descriptors. XLogP3 has been used in the prediction of octanol/water partitioning of different compounds to explore their oral absorption. TPSA is based on O, N, S, and H bonded to any of these atoms, and this is often used to predict molecular absorption. In the present study, we also selected these two descriptors to assess the relationships between them and the lgKa values. Figure 5 was drawn using the R language, and this illustrates the relationships between the number of binding sites (n) and the affinities (lgKa) using XLogP3 and TPSA as factors. As mentioned above, there was a strong relationship between the number of binding sites (n) and the affinity (lgKa) (R square = 0.8584), where Fig. 4 illustrates this relationship between these with XLogP3 and TPSA as factors.
Fig.5
Relationship between the number of binding sites (n) and affinities (lgKa) using XLogP3 and TPSA as factors.
3.6Relationships between Partition Coefficients of Anthocyanidins and their Affinities for HSA
The XLogP3 values were calculated by the Computational Chemical Biology group in Shanghai Institute of Organic Chemistry (http://www.sioc-ccbg.ac.cn/?p=42&software=xlogp3). The linear regression using the Graph Pad Prism 6.0 software was calculated according to Equation (2) with a R2 of 0.9323.
(2)
The affinities of anthocyanidins increased with decreasing XLogP3, although there were no significant relationships between the anthocyanin XLogP3 and their affinities for HAS (P > 0.5).
3.7Relationships between Topological Polar Surface Area (TPSA) of anthocyanidins and their affinities for HSA
As indicated above, TPSA is commonly used to measure the permeability of cells for a compound, and it has been shown to be a good descriptor for drug absorption. The TPSAs here were obtained from the PubChem public chemical database (http://pubchem.ncbi.nlm.nih.gov/), while the linear regression using Graph Pad Prism 6.0 followed Equation (3) with a R2 of 0.8653:
(3)
The anthocyanidins that have high TPSAs show tight binding in comparison to those with low TPSAs. On the other hand, there were still no significant relationships for anthocyanins between TPSA and the binding affinities for HAS (P > 0.5).
3.8Relationships between Binding Site Number and Partition Coefficient and Topological Polar Surface Area
The linear regression for anthocyanidins with XLogP3, again using Graph Pad Prism 6.0, followed Equation (4) with a R2 of 0.8075.
(4)
The linear regression for anthocyanidins with TPSA followed Equation (5) with a R2 of 0.9115.
(5)
However, there were no significant relationships between anthocyanins and XLogP3 (P > 0.5) or TPSA (P > 0.5).
4Concluding remarks
The anthocyanidins and anthocyanins belong to the flavonoidgroup,occuring as polyhydroxyl and polymethoxylderivates of flavilium salts, and they having a C6-C3-C6 skeleton. Their structural differences lead to variations in mean that their bioactivity and bioavailability.Ichiyanagi et al. compared the half times for the removal of these compounds from plasma [28], where the order was: Dp > Cy>Pt = Pn>Mv for anthocyanins carrying the same sugar moiety. In the present study, the order of the binding affinities of anthocyanidins to HSAwas: Dp chloride > Cy chloride > Pt chloride > Mv chloride > Pn chloride, thus demonstrating similar results. However, the binding affinities of anthocyanins with the glucoside moiety to HSA were irregular, regardless of the effects of hydroxylation of ring [B] and the effects of demethylation of the methoxyl groups.The limitations of current study is the method for determining the binding affinity with HSA, which is different from the real environment in vivo (Ulrih, 2017).The mechanisms for absorption of anthocyanins might involve the hexose transport pathway, which might mean that the factors for anthocyanin transportation and bioavailability are complex [29]. Indeed, here there is the need for a detailed comparative study involving anthocyanidins and anthocyanins in vivo, which would provide further information and give better direction to further research for in vivo situations.
References
[1] | Teng H , Fang T , Lin Q , Song H , Liu B , Chen L . Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends in Food Science Technology. (2017) ;66: :153–65. |
[2] | Miron A , Aprotosoaie AC , Trifan A , Xiao JB . Flavonoids as modulators of metabolic enzymes and drug transporters. Annals of the New York Academy of Sciences. (2017) ;1398: :152–67. |
[3] | Xiao JB . Dietary flavonoid aglycones and their glycosides: What show better biological benefits? Critical Reviews in Food Science and Nutrition. (2017) ;57: :1874–905. |
[4] | Xiao JB , Capanoglu E , Jassbi AR , Miron A . Advance on the flavonoid C-glycosides and health benefits. Critical Reviews in Food Science and Nutrition. (2016) ;56: :S29–S45. |
[5] | Chen L , Teng H , Xie ZL , Cao H , Cheang WS , Skalicka-Woniak K , Georgiev MI , Xiao JB . Modifications of dietary flavonoids towards improved bioactivity: An update on structure-activity relationshiCritical Reviews in Food Science and Nutrition (2017) ;57: :DOI: 10.1080/10408398.2016.1196334 |
[6] | Dey PM , Harborne JB . Plant biochemistry. London: Academic Press; (1997) . |
[7] | Kong JM , Chia LS , Goh NK , Chia TF , Brouillard R . Analysis and biological activities of anthocyanins. Phytochemistry. (2003) ;64: :923–33. |
[8] | Wallace TC , Giusti MM , eds. Anthocyanins in Health and Disease, pp. 13–90 (CRC Press, Boca Raton, Florida), (2014) . |
[9] | Riaz M , Zia-Ul-Haq M , Saad B . Introduction to anthocyanins. In Anthocyanins and human health: Biomolecular and therapeutic aspects. Springer. (2016) . pp. 21–33. |
[10] | Lila MA , Burton-Freeman B , Grace M , Kalt W . Unraveling Anthocyanin Bioavailability for Human Health. Annual Review of Food Science and Technology. (2016) ;7: :375–93. |
[11] | Burton-Freeman B , Linares A , Hyson D , Kappagoda T . Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. Journal of the American College of Nutrition. (2010) ;29: :46–54. |
[12] | Edwards M , Czank C , Woodward GM , Cassidy A , Kay CD . Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. Journal of Agricultural and Food Chemistry. (2015) ;63: :2423–31. |
[13] | Brown E , Gill C , Stewart D , McDougall G . Lingonberries (Vaccinium vitis-idaea L) and blueberries (Vaccinium corymbosum) contain discrete epicatechin anthocyanin derivatives linked by ethyl bridges. Journal of Berry Research. (2016) ;6: :13–23. |
[14] | Li D , Wang P , Luo Y , Zhao M , Chen F . Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition. (2017) ;57: :1729–41. |
[15] | Ding B , Lv Y , Zhang YQ . Anti-tumor effect of morusin from the branch bark of cultivated mulberry in Bel-7402 cells via the MAPK pathway. RSC Advances. (2016) ;6: :17396–404. |
[16] | Nurmi T , Mursu J , Heinonen M , Nurmi A , Hiltunen R , Voutilainen S . Metabolism of berry anthocyanins to phenolic acids in humans. Journal of Agricultural and Food Chemistry. (2009) ;57: :2274–81. |
[17] | Tang F , Xie YX , Cao H , Yang H , Chen XQ , Xiao JB . Interaction between resveratrol analogues with fetal bovine serum: Structure-affinity relationship, and influence on the stability and free radical scavenging activity. Food Chemistry. (2017) ;219: :321–8. |
[18] | Xiao JB , Kai GY , Yang F , Liu CX , Xu XC , Yamamoto K . Molecular structure-affinity relationship of natural polyphenols for bovine gamma -globulin. Molecular Nutrition and Food Research. (2011) ;55: :S86–S92. |
[19] | Cahyana Y , Gordon MH . Interaction of anthocyanins with human serum albumin: Influence of pH and chemical structure on binding. Food Chemistry. (2013) ;141: :2278–85. |
[20] | Xiao JB , Chen TT , Cao H , Chen LS , Yang F . Molecular property-affinity relationship of flavanoids and flavonoids for HSA in vitro. Molecular Nutrition and Food Research. (2011) ;55: :310–17. |
[21] | Xiao JB , Cao H , Wang YF , Yamamoto K , Wei XL . Structure-affinity relationship of flavones on binding to serum albumins: Effect of hydroxyl groups on ring A. Molecular Nutrition and Food Research. (2010) ;54: :S253–S260. |
[22] | Flores G , Ruiz del Castillo ML , Costabile A , Klee A , Bigetti Guergoletto K , Gibson GR . In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. Journal of Functional Foods. (2015) ;16: :50–57. |
[23] | Forester SC , Waterhouse AL . Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2, 4, 6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. Journal of Agricultural and Food Chemistry. (2010) ;58: :5320–7. |
[24] | McGhie TK , Walton MC . The bioavailability and absorption of anthocyanins: Towards a better understanding. Molecular Nutrition and Food Research. (2007) ;51: :702–13. |
[25] | Passamonti S , Vrhovsek U , Vanzo A , Mattivi F . The stomach as a site for anthocyanins absorption from food. FEBS Letters. (2003) ;544: :210–3. |
[26] | da Costa CT , Horton D , Margolis SA . Analysis of anthocyanins in foods by liquid chromatography, liquid chromatography–mass spectrometry and capillary electrophoresis. Journal of Chromatography A. (2000) ;881: :403–10. |
[27] | Bakowska-Barczak A . Acylated anthocyanins as stable, natural food colorants-a review. Polish Journal of Food and Nutrition Sciences. (2005) ;14: :107–16. |
[28] | Ichiyanagi T , Shida Y , Rahman MM , Hatano Y , Konishi T . Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. Journal of Agricultural and Food Chemistry. (2006) ;54: :6578–87. |
[29] | McGhie TK , Ainge GD , Barnett LE , Cooney JM , Jensen DJ . Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. Journal of Agricultural and Food Chemistry. (2003) ;51: :4539–48. |




