Antioxidant capacity of blueberry extracts: Peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants
Abstract
BACKGROUND:
Multiple oxidants oxidize biological molecules in vivo to induce their non-regulated oxidative modification and consequently antioxidants against multiple oxidants are required to cope with oxidative stress.
OBJECTIVE:
To determine the antioxidant capacity of blueberry extracts for peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by peroxyl radical, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen.
METHODS:
The capacity of blueberry extracts to scavenge peroxyl radical was assessed by competition with pyranine. Mouse plasma was oxidized by the above-mentioned oxidants and the lipid hydroperoxide formation was measured by the fluorescence intensity of diphenylpyrenylphosphine (DPPP) oxide produced by the stoichiometric reaction of lipid hydroperoxides and DPPP. The antioxidant capacity was assessed by the effects on DPPP oxide formation.
RESULTS:
Blueberry extracts by water and DMSO exerted potent antioxidant effects for scavenging peroxyl radicals and inhibition of plasma lipid oxidation induced by biologically relevant multiple oxidants. Blueberry skin was more effective than fruit and DMSO extracts were more potent than water extracts.
CONCLUSIONS:
Blueberry contains multiple antioxidants, which inhibit plasma lipid oxidation induced by diverse oxidants. The present methods may be applied for the assessment of antioxidant capacity of foods, beverages, and synthetic compounds.
1Introduction
We aerobic organisms are continuously exposed to reactive oxidants and under threat of oxidative stress [1, 2]. Lipids, proteins, and nucleic acids are randomly oxidized in vivo by multiple oxidants to give diverse products, giving rise to various disorders and diseases. In fact, it has been widely observed that the levels of oxidation products of biological molecules produced by multiple oxidants and progress of diseases are associated, implying that such oxidation plays an important role in the pathogenesis of many diseases including atherosclerosis, neurological disorders, cancer, and aging [2, 3].
It is now generally accepted that fruits and vegetables exert beneficial effects on the maintenance of health and prevention of diseases. Among others, blueberries, one of the most widely consumed fruits in the world, contain abundant and diverse bioactive compounds including flavonols, anthocyanins, carotenoids, and ascorbic acid, which act as antioxidant in vivo to protect biological molecules from unregulated oxidative modification [4–7]. The antioxidant compounds contained in blueberries have been measured and total antioxidant capacity has been assessed in several in vitro systems [4, 5, 8, 9]. It was reported that blueberries were one of the richest sources of antioxidants in the fresh fruits and vegetables [10].
Assessment of antioxidant capacity has been the subject of extensive studies and arguments [11–13]. Two points may be noteworthy. Firstly, the oxidation of biological molecules in vivo is mediated by multiple oxidants having diverse reactivity and specificity and the antioxidant efficacy depends on the nature of oxidants. Secondly, the capacity of antioxidants to scavenge or quench reactive oxidants does not always correlate linearly with the capacity to inhibit oxidation of biological molecules.
As mentioned above, many kinds of reactive oxidants and related species are involved in the oxidative modification of biological molecules. Some are enzymatic oxidants, while others are free radicals or non-radical oxidants. Considering the physiological concentrations and reactivity of biological molecules and antioxidants, the major oxidants that are target of antioxidants are peroxyl radicals, peroxynitrite, hypochlorite, lipoxygenases, cyclooxygenase, cytochrome P450, singlet oxygen, and ozone [13–16]. Hydroxyl and alkoxyl radicals are too reactive for any antioxidant to scavenge efficiently in vivo before these radicals attack biological molecules, while superoxide and nitric oxide are too unreactive per se to cause oxidative damage. Superoxide is scavenged primarily by superoxide dismutase (SOD) or reacts with nitric oxide to give peroxynitrite in vivo.
It has been shown that peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase (15-LOX), and singlet oxygen oxidize plasma lipids to produce lipid hydroperoxides [17, 18]. Peroxyl radicals play an important role as chain-carrying species in free radical mediated lipid peroxidation [19]. Peroxynitrite is formed by a rapid combination of superoxide and nitric oxide produced physiologically in vivo and acts as one of the major oxidants and nitrating species [20]. Hypochlorite is produced in vivo by myeloperoxidase – hydrogen peroxide – chloride ion and acts as one of the major oxidants in vivo [21]. 15-LOX plays an important role in several inflammatory diseases. Singlet oxygen is a non-radical oxidant which plays an important role especially in photooxidation of skin and eye.
The antioxidant efficacy is determined not only by the capacity to scavenge reactive oxidants but also other factors including concentration, localization, fate ofantioxidant-derived radicals, interaction with other antioxidants, and metabolism [13]. We have recently reported a high throughput method to follow plasma lipid oxidation induced by different oxidants by measuring fluorescence intensity of diphenyl-1-pyrenylphosphine (DPPP) oxide which is formed by the stoichiometric reaction of lipid hydroperoxide and DPPP [17, 18]. Lipid hydroperoxides are produced as major primary products in the oxidation of plasma by many oxidants. This method is useful to assess the antioxidant effects of foods, beverages, supplements, and other natural products in biologically relevant settings using a conventional microplate reader.
The objective of the present study is to assess the capacity of blueberry extracts to scavenge peroxyl radicals and the antioxidant effects against plasma lipid oxidation induced by the aforementioned five kinds of oxidants. The capacity of blueberry to scavenge superoxide, hydrogen peroxide, hydroxyl radical, singlet oxygen, and peroxynitrite was measured previously [7, 22]. To the best of our knowledge, this is the first report on the antioxidant effects of blueberry extracts against plasma lipid oxidation induced by multiple biologically relevant oxidants.
2Methods
2.1Materials
2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) used as radical initiator was obtained from Wako Pure Chemical Ind. Ltd (Osaka, Japan). Peroxynitrite was produced by simultaneous generation of superoxide and nitric oxide from 3-morpholinosydnonimine (SIN-1), which was obtained from DOJINDO Laboratories (Kumamoto, Japan). Sodium hypochlorite was obtained from WAKOPure Chemical Ind. Ltd. (Osaka, Japan). Rabbit reticulocyte 15-LOX was purchased from Enzo Life Science Inc. (NewYork,USA).
3-(1,4-Epidioxy-4-methyl-1,4-dihydro-1-naphthyl) propionic acid (NpEP) used as a singlet oxygen source was obtained from Wakenyaku Co. Ltd. (Kyoto, Japan).
Diphenyl-1-pyrenylphosphine (DPPP) and carboxy-2,5,7,8-tetramethyl-6-hydroxychroman (Trolox) were purchased from Cayman Chemical Company (Michigan, USA). Pyranine (8-hydroxypyrene-1,3,6-trisulfonate trisodium salt) used for the assessment of peroxyl radical scavenging capacity was purchased from Sigma Aldrich (Tokyo, Japan).
Blueberry produced at Gunma prefecture, Japan, was purchased at the market in Kyoto. Fruit and skin were separated from whole blueberry by hand. The extracts were prepared from whole, fruit, and skin of blueberry using either water or DMSO as a solvent.
Plasma was prepared from the blood of wild type male C57BL/6J mouse by centrifugation at 3500 rpm for 15 min at 4°C and frozen on ice immediately and stored until use as reported previously [17, 18]. The animal experiments and care were approved by the Institutional Animal Care and Use Committee of Kyoto Prefectural University of Medicine.
2.2Assessment of peroxyl radical scavenging capacity
The reaction of pyranine with peroxyl radicals was followed in PBS containing 10% mouse plasma by measuring the decay of the absorption at 454 nm with a microplate reader Spectra Max M2 (Molecular Devices, Sunnyvale, CA) equippedwith a thermostatted cell maintained at 37°C under air as reported previously [17, 18]. The reaction was started by the addition of AAPH dissolved in PBS and the absorbance change was recorded continuously over time. The lag phase was determined graphically by extrapolating the slope of maximum decay of pyranine to its intersection with the slope of minimum probe decay at the initial stage.
2.3Effects of blueberry extracts against oxidation of plasma lipids
The oxidation of plasma (10%) was induced by the aforementioned five kinds of oxidants in the presence and absence of blueberry extracts at 37°C in PBS (pH 7.4) under air. The production of lipid hydroperoxides was measured from the fluorescence intensity of DPPP oxide produced by the reaction of lipid hydroperoxides and DPPP, the excitation and emission wave length being 351 and 380 nm respectively, with a microplate reader, Spectra Max M2 (Molecular Devices, Sunnyvale, CA) equipped with a thermostatted cell maintained at 37°C under air. Total volume of the reaction mixture was 200μL.
3Results
3.1Capacity of blueberry extracts for scavenging peroxyl radical
As stated above, peroxyl radical plays an important role as a chain carrying species in free radicalmediated lipid peroxidation. The capacity of blueberry extracts for scavenging peroxyl radical wasmeasured in aqueous plasma solution using pyranine and AAPH as a probe and peroxyl radical sourcerespectively. Pyranine scavenges peroxyl radicals produced by spontaneous decomposition of AAPH and its consumption is measured by a decrease in absorption at 454 nm.Antioxidants scavenge peroxyl radicals preferentially to spare the consumption of pyranine and produce a lag phase. Pyranine acts as a scavenger of peroxyl radicals with modest reactivity and potent antioxidants inhibit pyranine consumption completely, while the antioxidants with low reactivity spare pyranine partially. The capacity of antioxidants for scavenging peroxyl radical is assessed from the lag phase for pyranine consumption.
The water extracts from whole, skin, and fruit of blueberry suppressed the consumption of pyranine induced by AAPH very efficiently and produced a clear lag phase, which was directly proportional to extract concentration (Fig. 1). The lag phase produced by blueberry extracts decreased in the order of skin, whole, and fruit.
The extracts by DMSO exerted similar effects for scavenging peroxyl radicals as those by water. They inhibited the consumption of pyranine induced by AAPH and produced distinct lag phase, which increased linearly with the concentration of extracts (Fig. 2). As observed for water extracts, skin produced the longest lag phase, while fruit produced the shortest lag phase.
The lag phase is given by:
(1)
The lag phase is determined by the concentration of antioxidants ([IH]) andrate of peroxyl radical flux (Ri):
(2)
The amount of radicals that can be scavenged by the antioxidants contained in the test sample, that is, the effective concentration of antioxidants in the extracts, is calculated from eqn (3):
(3)
The rate of peroxyl radical production by 50 mM AAPH in 10% plasma solution was obtained from the lag phase produced by Trolox used as a reference compound as 4.4×10-8 M/s [23]. The concentrations of antioxidants contained in each extract calculated from eqn. (3) with the results shown in Figs. 1 and 2 are summarized in Table 1. It is noted that the slope and antioxidant concentration increased in the order of fruit < whole<skin and that the extracts by DMSO contained more antioxidants than those by water.
3.2Inhibitory effects of blueberry extract against plasma lipid oxidation induced by multiple oxidants
The addition of AAPH, SIN-1, NaOCl, 15-LOX, and NpEP to the plasma solution containing DPPP induced an increase in fluorescence intensity with time, suggesting that peroxyl radical, peroxynitrite, hypochlorite, 15-LOX, and singlet oxygen oxidized plasma lipids to produce lipid hydroperoxides (Figs. 3–5). Little increase in fluorescence intensity was observed in the absence of DPPP (data not shown). Interestingly, lag phase was observed in the oxidation induced by AAPHand SIN-1, but not in the oxidation by hypochlorite, 15-LOX, and singlet oxygen. These results suggest that plasma is protected from free radical mediated lipid oxidation by endogenous antioxidants, but that plasma does not contain efficient antioxidants to protect lipids from oxidation induced by hypochlorite, 15-LOX, and singlet oxygen.
The extracts from blueberry with water and DMSO suppressed the increase in fluorescence intensity (Figs. 3 and 4). The concentration dependence of the blueberry extracts against plasma oxidation induced by SIN-1 is shown in Fig. 5. The extracts from blueberry skin exerted higher antioxidant capacity than those from fruit against every oxidant and the extracts by DMSO were more effective than those by water. The antioxidant effects of blueberry extract by water and DMSO against plasma lipid oxidation induced by AAPH, SIN-1, NaOCl, 15-LOX, and NpEP are summarized in Table 2.
4Discussion
Multiple reactive oxidants are involved in the oxidative modification of biological molecules in vivo. In the present study, peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen were examined as oxidant. These oxidants have been shown to play an important role in the oxidative stress in vivo by oxidizing unsaturated lipids to produce lipid hydroperoxides [19]. As discussed previously [14, 15], hydroxyl radical and alkoxyl radical are too reactive for antioxidants to scavenge efficiently before these radicals attack lipids, while superoxide and nitric oxide are not reactive enough per se to induce lipid oxidation. Other oxidants including cyclooxygenase, cytochrome P450, and ozone oxidize lipidsby different mechanisms and do not produce lipid hydroperoxides.
It has been shown that blueberry contains many kinds of antioxidants [4–7] and its beneficial effects on human health has received much attention [24]. The results of the present study show that blueberry extracts by water and DMSO suppressed plasma lipid oxidation induced by all kinds of oxidants tested, showing that blueberry extracts contain many kinds of antioxidant compounds capable of scavenging multiple biological oxidants. It may be noted that, although vitamin E is a potent radical scavenging antioxidant and inhibits plasma lipid peroxidation quite efficiently in combination with vitamin C, vitamin E does not act as a potent antioxidant against plasma oxidation mediated by hypochlorite and 15-LOX [14].
It was found that DMSO extracts exerted higher antioxidant capacity than water extracts against multiple oxidants. Many phenolic compounds contained in blueberry may present either in free form or as metabolites and their distribution in hydrophilic and lipophilic phase in plasma solution may vary. The relative antioxidant efficacy may depend on the localization of oxidants as well as chemical reactivity toward oxidant. The lipid peroxidation to produce hydroperoxides may proceed predominantly in lipophilic domain, and so lipophilic antioxidants may act as more efficient antioxidant than hydrophilic antioxidant.
The content of radical scavenging antioxidants in natural products is calculated from the lag phase produced by the test sample for the consumption of probe under constant flux of free radicals. Fluorescein and pyranine have often been used as a probe and AAPH as a peroxyl radical source. The results summarized in Table 1 show that blueberry skin contains much more antioxidants than fruit.
Obviously, the antioxidant capacity of blueberry depends on cultivars, climate, processing, and others factors. It may be pointed out that pyranine and fluorescein count weak antioxidants which do not compete well enough with physiological unsaturated lipids and may overestimate antioxidant capacity.
It is also important to understand that the capacity to scavenge free radicals do not always correlate linearly with the capacity to inhibit oxidation of lipids or proteins. The capacity to inhibit lipid oxidation is determined not only by the concentration and reactivity of the antioxidants but also by other factors including fate of antioxidant-derived radical which is formed when the antioxidant scavenges free radicals and interaction with other antioxidants. Therefore, it is important to assess the capacity to inhibit lipid oxidation as well as the capacity to scavenge free radicals. The method used in the present study may be convenient for assessment of antioxidant capacity to inhibit lipid oxidation induced by multiple oxidants in biologically relevant settings. Furthermore, it may be noted that the antioxidant capacity measured in the present study in vitro is useful for understanding characteristics of blueberry as antioxidant, but that the antioxidant effects in vivo are determined by other factors including uptake, distribution, retainment, metabolism, excretion, and interaction with other antioxidants.
Importantly, the efficacy of antioxidants depends on the nature of oxidants and multiple antioxidants with different selectivity are required to cope with oxidative damage in vivo. It is therefore recommended to take antioxidants with foods rather than specific single antioxidant compound or supplement.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Halliwell B , Gutteridge JM , Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 4th ed, (2007) . |
[2] | Niki E . Oxidative stress and antioxidants: Distress or eustress? Arch Biochem Biophys. (2016) ;595: :19–24. |
[3] | Niki E . Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta. (1840) (2):809–17. |
[4] | Pertuzatti PB , Barcia MT , Rodrigues D , da Cruz PN , Hermosín-Gutiérrez I , Smith R , et al. Antioxidant activity of hydrophilic and lipophilic extracts of brazilian blueberries. Food Chem. (2014) ;164: :81–8. |
[5] | Skrovankova S , Sumczynski D , Mlcek J , Jurikova T , Sochor J . Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci. (2015) ;16: (10):24673–706. |
[6] | Forbes-Hernandez TY , Gasparrini M , Afrin S , Bompadre S , Mezzetti B , Quiles JL , et al. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit Rev Food Sci Nutr. (2016) ;56: (Suppl 1):S46–59. |
[7] | Prior RL , Sintara M , Chang T . Multi-radical (ORAC_MR5) antioxidant capacity of selected berries and effects of food processing. J Berry Res. (2016) ;6: :159–73. |
[8] | Parry J , Su L , Luther M , Zhou K , Yurawecz MP , Whittaker P , et al. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J Agric Food Chem. (2005) ;53: (3):566–73. |
[9] | Faria A , Oliveira J , Neves P , Gameiro P , Santos-Buelga C , de Freitas V , et al. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. J Agric Food Chem. (2005) ;53: (17):6896–902. |
[10] | Prior RL , Cao G , Martin A , Sofic E , McEwen J , O’Brien C . et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. (1998) ;46: (7):2686–93. |
[11] | Niki E . Antioxidant capacity: Which capacity and how to assess it? J Berry Res. (2011) ;1: :169–76. |
[12] | Amorati R , Valgimigli L . Advantages and limitations of common testing methods for antioxidants, Free Radical Res. (2015) ;49: :633–49. |
[13] | Niki E . Antioxidant capacity of foods for scavenging reactive oxidants and inhibition of plasma lipid oxidation induced by multiple oxidants. Food Funct. (2016) ;7: (5):2156–68. |
[14] | Niki E . Interaction among vitamin C, vitamin E, and β-carotene, Am J Clin Nutr. (1995) ;62: :322S–6S. |
[15] | Niki E . Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. (2010) ;49: (4):503–15. |
[16] | Niki E . Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic Biol Med. (2014) ;66: :3–12. |
[17] | Morita M , Naito Y , Yoshikawa T , Niki E . Plasma lipid oxidation induced by peroxynitrite, hypochlorite, lipoxygenase and peroxyl radicals and its inhibition by antioxidants as assessed by diphenyl-1-pyrenylphosphine. Redox Biol. (2016) ;8: :127–35. |
[18] | Morita M , Naito Y , Yoshikawa T , Niki E . Inhibition of plasma lipid oxidation induced by peroxyl radicals, peroxynitrite, hypochlorite, 15-lipoxygenase, and singlet oxygen by clinical drugs. Bioorg Med Chem Lett. (2016) ;26: :5411–7. |
[19] | Niki E . Lipid peroxidation: Physiological levels and dual biological effects. Free Radic Biol Med. (2009) ;47: (5):469–84. |
[20] | Carballall S , Bartesaghi S , Radi R , Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim Biophys Acta. (1840) :768–80. |
[21] | Pattison DI , Davies MJ , Hawkins CL , Reactions and reactivity of myeloperoxidase derived oxidants: Differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic Res. (2012) ;46: (8):975–95. |
[22] | Wang SY , Jiao H . Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem. (2000) ;48: (11):5677–84. |
[23] | Morita M , Naito Y , Yoshikawa T , Niki E . Assessment of radical scavenging capacity of antioxidants contained in foods and beverages in plasma solution. Food Funct. (2015) ;6: (5):1591–9. |
[24] | Del Bo’ C , Martini D , Porrini M , Klimis-Zacas D , Riso P . Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. (2015) ;6: (9):2890–917. |
Figures and Tables
Fig.1
Scavenging of peroxyl radicals by blueberry extracts in 10% plasma solution. Effects of water extracts from (A) whole, (B) skin, and (C) fruit of blueberry on the consumption of pyranine induced by AAPH. Plot of lag phase against extract concentration from (D) whole, (E) skin, and (F) fruit. Numbers in the Figures show the concentration of the extract in mg/mL. Initial concentration: [AAPH] = 50 mM; [Pyranine] = 50μM.
![Scavenging of peroxyl radicals by blueberry extracts in 10% plasma solution. Effects of water extracts from (A) whole, (B) skin, and (C) fruit of blueberry on the consumption of pyranine induced by AAPH. Plot of lag phase against extract concentration from (D) whole, (E) skin, and (F) fruit. Numbers in the Figures show the concentration of the extract in mg/mL. Initial concentration: [AAPH] = 50 mM; [Pyranine] = 50μM.](https://content.iospress.com:443/media/jbr/2017/7-1/jbr-7-1-jbr152/jbr-7-jbr152-g001.jpg)
Fig.2
Scavenging of peroxyl radicals by DMSO extract in 10% plasma solution. (A) Effects of extract from whole blueberry on pyranine (50μM) consumption induced by 50 mM AAPH. (B) Plot of lag phase against extract concentration from whole, skin, and fruit of blueberry.
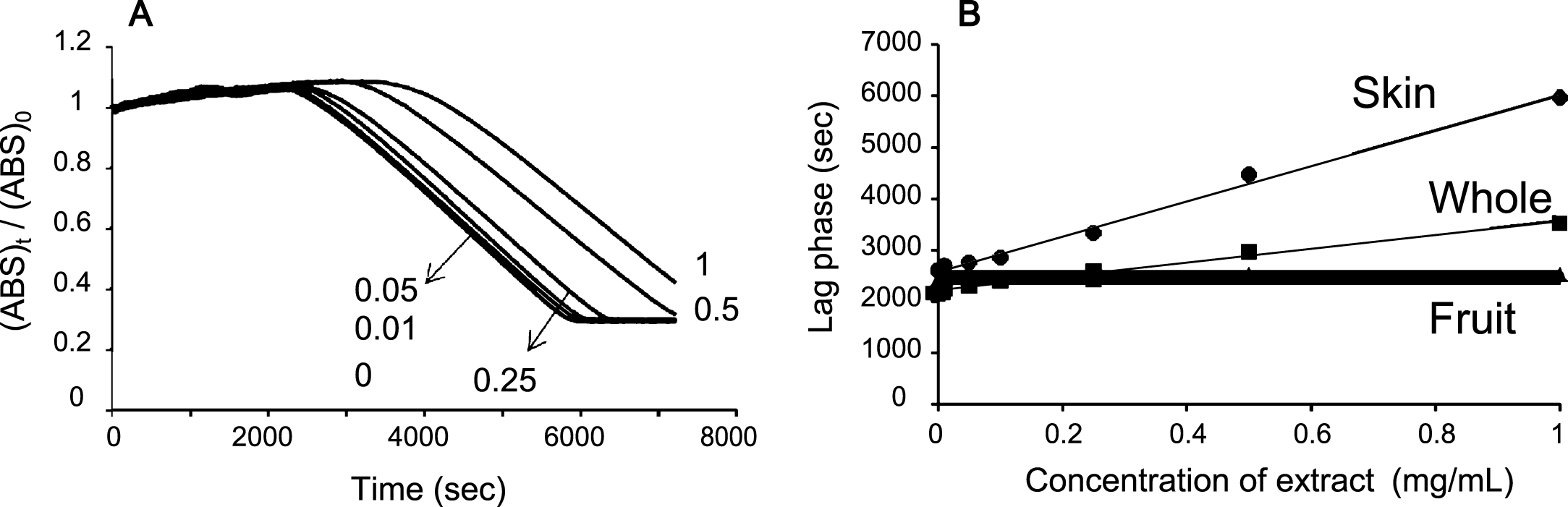
Fig.3
Inhibitory effects of blueberry extracts with water on the plasma oxidation (10%) induced by (A) AAPH (50 mM), (B) SIN-1 (0.5 mM), (C) NaOCl (0.5 mM), (D) 15-LOX (10000 nkat/mL), and (E) NpEP (5 mM). N: without extract; W, F, and S: Whole, fruit, and skin of blueberry respectively.
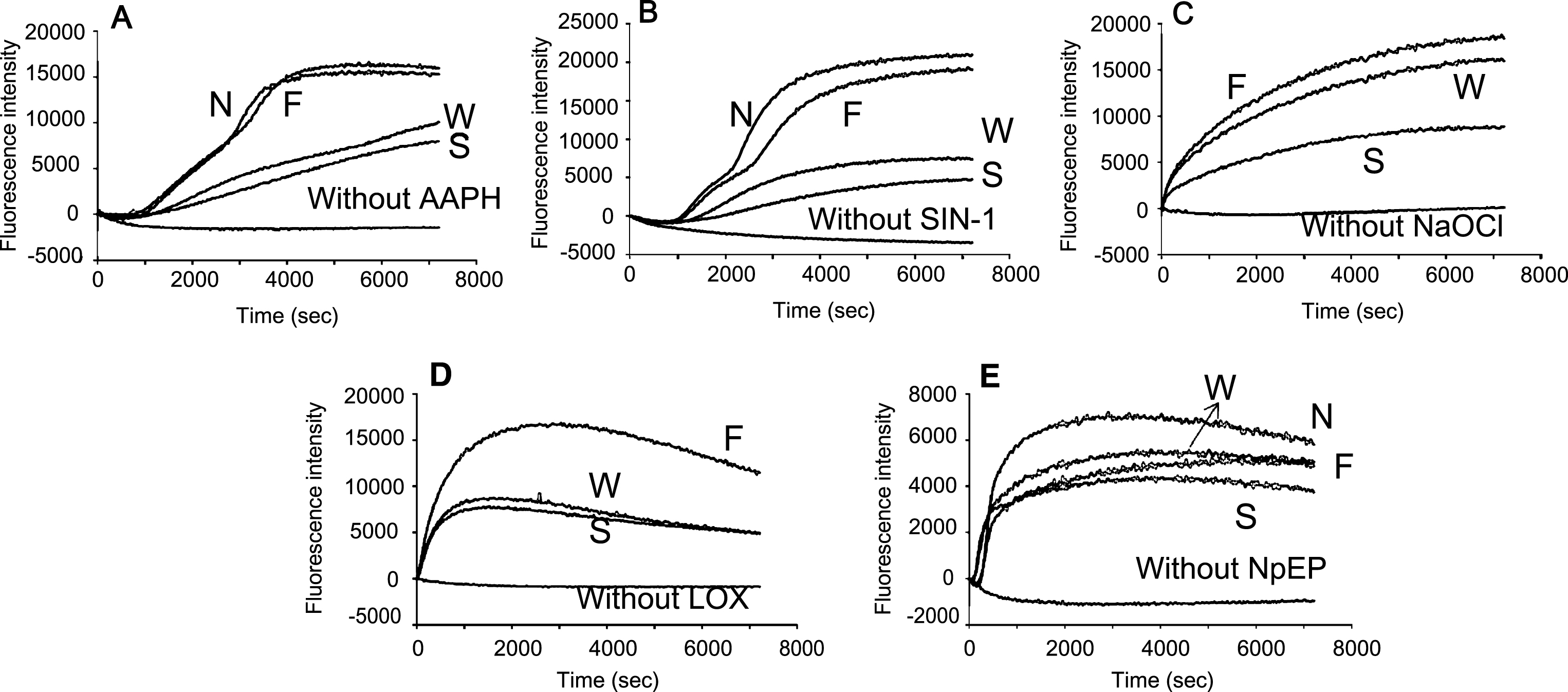
Fig.4
Effects of blueberry extracts with DMSO on the plasma oxidation. The conditions are the same as those in Fig. 3.
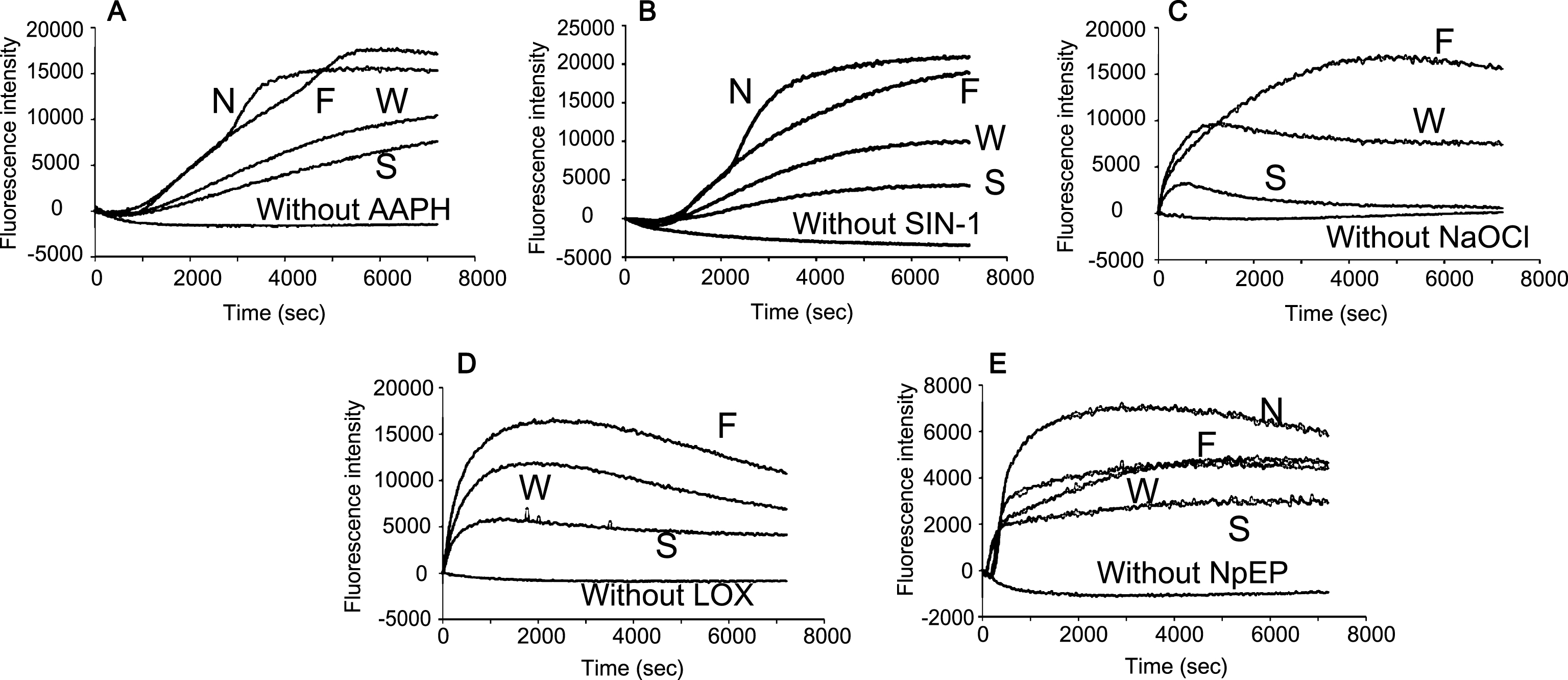
Fig.5
Concentration effects of blueberry extracts by (A) water and (B) DMSO against plasma oxidation induced by SIN-1 (0.5 mM). The numbers in Figure show the concentration in mg/mL.
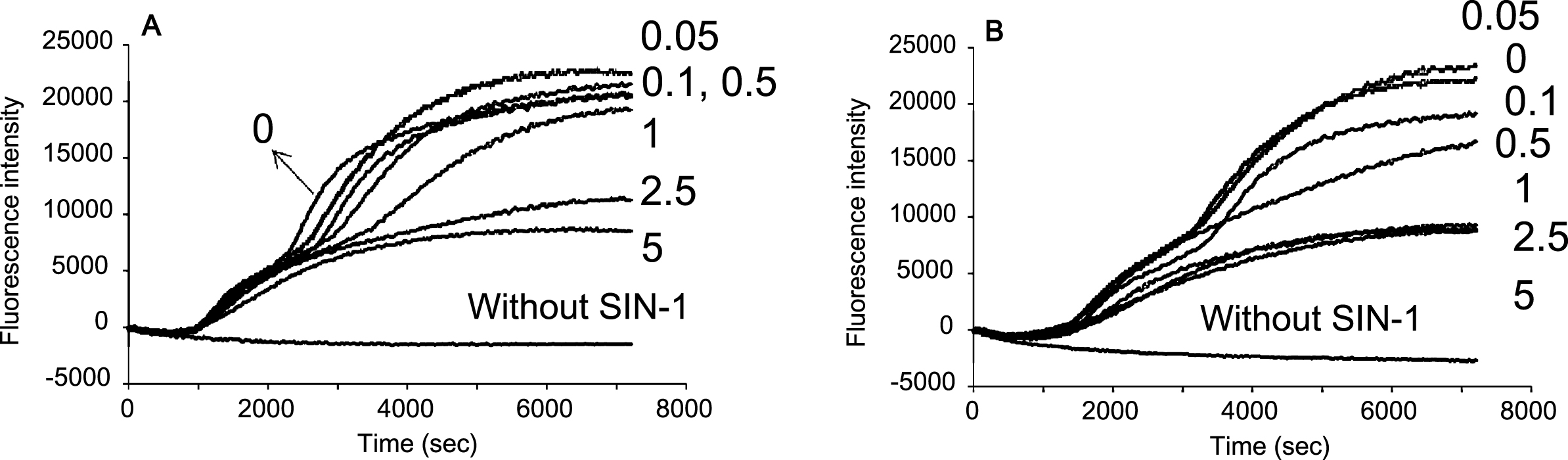
Table 1
Antioxidant concentration in the extracts from whole, skin, and fruit of blueberry by water and DMSO, μmol/ga
| Whole | Skin | Fruit | |
| Water extract | 17.6 + /–0.08 | 126 + /–13 | 1.8 |
| (400 + /–19) | (2860 + /–300) | (42) | |
| DMSO extract | 60 + /–3.6 | 148 + /–18 | 5.7 |
| (1370 + /–81) | (3370 + /–400) | (130) |
aNumbers in the parentheses show the slope of the plot of lag phase (s) against extract concentration (mg/mL).
Table 2
Antioxidant effect of blueberry extracts against plasma oxidation induced by AAPH, SIN-1, NaOCl, 15-LOX, and NpEP
| AAPH | SIN-1 | NaOCl | 15-LOX | NpEP | |
| Water extract | ++ | ++ | + | + | + |
| DMSO extract | + | ++ | ++ | + | + |




