Polyphenol characterization of three varieties of Blackberry fruits (Rubus adenotrichos), cultivated in Costa Rica
Abstract
BACKGROUND:
Blackberries (Rubus spp.) are fruits rich in secondary components (anthocyanins, proanthocyanidins, phenolic acids, carotenoids and others), recognized for their health benefits.
OBJECTIVE:
To evaluate the content of different types of phenolic compounds and their antioxidant activity in several extracts of three varieties of blackberry fruit (Rubus adenotrichos) (red thorned, thornless and sweet), using different blackberry standards.
METHODS:
The varieties of blackberry fruit were analyzed in three stages of maturation (green, red and black). The evaluation of the phenolic compounds was carried out by applying commercial standards and own standards to the same samples, following the Folin-Ciocalteu, differential pH, DMAC, and ORAC procedures.
RESULTS:
The red thorned variety presented the best results with a concentration of polyphenols of 183.0±0.5 mg GAE/g DS, antioxidant capacity of 3322±10 μmol TEAC/g DS, a value of 15.4±0.3 mg of cyanidin-3-glucoside eq/g DS of anthocyanins, and a value of 9.26±0.03 mg 4'-O-methylgallocatechin eq/g DS for of proanthocyanidin content.
CONCLUSIONS:
Our results show the limitation of a currently used standard, gallic acid and 4'-O-methylgallocatechin, for quantification of total polyphenols and proanthocyanidin respectively, and outline the development and validation of a more robust and accurate standard for blackberry fruit analysis.
1Introduction
Plants of the genus Rubus are found worldwide. Hybrid Andean blackberries such as Rubus adenotrichos are native from Mexico to Ecuador and are widely cultivated in the south of Costa Rica for their edible fruits, which are eaten fresh or consumed as juice, jelly and wine. In Costa Rica, the fresh fruit is currently the principal commercial blackberry product [1], with more than 800 Costa Rican families involved in diverse blackberry cultivation tasks such as sowing, care, harvesting, packaging and others. In 2005, approximately 1650 tons of fresh fruit were produced in a cultivated area of about 1550 ha [2], with most of this production (approximately 90%) destined for the domestic market. Blackberries are currently promoted as being a rich source of polyphenols, which are compounds of interest because of their antioxidant activity as radical scavengers and their possible beneficial roles in human health, such as reducing the risk of cancer, cardiovascular disease, antimicrobial activity and other pathologies [3–7]. The results of some studies show the beneficial effect on human health of the consumption of Costa Rican blackberries [8] compared to other fruits and even blackberries from different locations, in the cases of both fresh fruit and processed products. Several classes of phenolic compounds are present in blackberries, including hydroxybenzoic acids, hydroxycinnamic acids and flavonoids. The major phenolic compounds in blackberries are hydrolyzable tannins (gallo and ellagitannins) and anthocyanins, hydroxycinnamic acids, flavonols, and flavan-3-ols, with proanthocyanidins being present in lower amounts [9–11]. A wide variety of analytical methods have been reported for sample extraction and quantification of phenolic content; therefore, valid comparisons between species and varieties is not always possible [12]. To carry out valid comparisons of phenolic levels in fruits and their derived products, it is necessary to use the same method of extraction and a comparative methodology for polyphenols and antioxidant activity quantification [13]. Quantification of total polyphenols and proanthocyanidins in blackberry fruits, leaves, beverages, and nutritional supplements is essential to studies on nutritional and health effects, and resultant product labeling, as well as the determination of potential risks. However, quantification of complex polyphenol mixtures is problematic, since widely used methods such as the Folin-Ciocalteu assay and 4-dimethylaminocinnamaldehyde (DMAC) analysis often give inaccurate results [14]. Application of Folin-Ciocalteu and DMAC assays for the estimation of different types of polyphenolic compounds in fruits, juices, nutritional supplements and botanicals is constrained by the lack of suitable commercial standards. Both the reagents and their respective methods are subject to interference from a wide range of plant compounds, but the most serious problems have to do with the use of gallic acid, catechin or similar molecules as standards for complex polyphenol mixtures. As a result, it is imperative to at least isolate new standards (blackberry polyphenol standards, BB-PS) that can circumvent these limitations and produce accurate results, including the development of standards which are specific to the analysis of blackberry fruits. The objective of this study was to compare the total phenolic, proanthocyanidins, anthocyanins, contents and antioxidant (ORAC) of polyphenol-rich blackberry fruit varieties grown under the same environmental conditions in the south of Costa Rica, using the same extraction and analytical methods with different polyphenol standards.
2Materials and methods
2.1Chemicals
Folin Ciocalteu reagent, TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) and DPPH (2,2-diphenyl-1-picrylhydrazyl radical) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ascorbic acid and gallic acid were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All reagents were either analytical grade or chromatographic grade.
2.2Plant materials
Three varieties of blackberry fruits were used in this experiment; these varieties were identified as red thorned, thornless and sweet. Commercially grown samples were hand-harvested in February 2015 from a farm located in La Trinidad of Copey, Dota-San José, Costa Rica. Fruits in three different stages of maturity were used in this study. The maturity of the berries was mainly judged by their colors. Three independent samples were harvested by hand in three separate plastic containers and taken to the laboratory on the same day in a cooler (6°C). In the laboratory, the samples were frozen, freeze-dried and ground in a Thomas Scientific M4 Wiley mill to pass a 1.0 mm sieve and were stored at –80 °C prior to extraction.
2.3Extract preparations
Prior to sample extraction, a simple experiment to determine the best solvent for the extraction of total polyphenols was performed. A composite fruit sample and six solvents were tested. The solvents used were acetone:methanol (7 : 3), acetone:methanol:water (4 : 4:2), acetone:ethanol (95%):water (5 : 4:1), methanol:water (7 : 3) and acetone:methanol (1 : 1). Fifty mg of sample were extracted four successive times with 2 mL of solvent. During each extraction the sample was sonicated for 5 min at 30 °C and then centrifuged for 10 min at 3000 rpm. The supernatants were collected and standardized to a final volume of 10 mL. Once the best solvent for the extraction of total polyphenol compounds was found, another short experiment was carried out in order to determine the optimal number of extractions needed on the same sample to obtain the highest amount of total polyphenols. To evaluate the results of the extracts rich in polyphenolic compounds in these experiments, the colorimetric method of Folin-Ciocalteu was used. All tests were performed in triplicate and ASSISTAT® statistical software was used to statistically evaluate the results obtained.
2.4Preparation of the Blackberry polyphenol standards (BB-PS)
To ensure purity and repeatability, a method was developed specifically for production of BB-PS [15]. In brief, 75 g of solid phase resin (Waters Preparative C18 125Å, 55–105 μm) were added to a glass column (Kontes, 2.5 cm internal diameter×25 cm L) and conditioned by passing 100 mL of ethanol through the column bed followed by 500 mL of water (1.0 mL L–1 TFA). To prepare the standard, a sample of 10 g of blackberry fruit extract was solubilized in ethanol, and water was then added to dilute the ethanol to less than 10 mL L–1 followed by application of the solution to the column and elution with 2 L of H2O/H+ at a flow rate of 3.5 mL min–1. Ethanol (>300 mL) was eluted and collected until the column bed returned to its original white color. Ethanol was removed by low-temperature (30°C) rotary vacuum evaporation and the residual water was removed by lyophilization to produce the BB-PS.
2.5Folin-Ciocalteu assay for total phenolic compounds
The Folin-Ciocalteu method was used to quantify total phenolic compounds in blackberry samples as described previously [16]. During this study all absorbance readings were made using a Synergy™ HT Multi-Mode Microplate Reader spectrophotometer (Biotek Instruments, Inc., Winooski, VT, USA) with a 96-well polypropylene plate. Water (200 μL), undiluted Folin-Ciocalteu reagent (15 μL, Sigma, St Louis, MO), 30 μL of blackberry extract and 50 μL of 0.2 g mL–1 Na2CO3 were then added to the plate. The plate was introduced into the reader, shaken for 30 s and incubated at 40 °C for 20 min. The absorbance was measured at λ= 755 nm. The results were expressed as mg of gallic acid or BB-PS equivalent per gram of dry sample (mg GAE or BB-PS g–1 DS).
2.6Determinations of total anthocyanin (pH differential method)
Total anthocyanin contents were determined by the pH differential method [17] using a spectrophotometer microplate reader in a 96 well polypropylene plate. The plate was shaken for 15 s, incubated at room temperature for 20 min, and the absorbance of the fruit extract was then measured at 510 and 700 nm in a solution with pH 1.0 and pH 4.5 buffers, respectively, using ASP = (A515 –A700) pH1.0 –(A515 –A700) pH4.5. During this analysis the microplate was divided into two parts; 100 μL of the extract and 200 μL of buffer pH 1 were added to the first part, and 100 μL of sample and 200 μL of buffer pH 4.5 were added to the second part. The results were expressed as milligrams of cyanidin-3-glucoside equivalent per gram of dry sample (mg cy-3-glu g–1 DS) using a standard curve.
2.7Proanthocyanidins (PACS)
The DMAC method [18] was used for the quantification of the total concentration of proanthocyanidins in blackberry samples, with 4'-O-methylgallocatechin (0.04 mg mL–1) and BB-PACS (0.8 mg mL–1) used as standards.
2.7.1Preparation of the blackberry proanthocyanidins standard (BB-PACS)
Ground lyophilized blackberry fruit powder (12 g) was extracted (100 mL×3) with acetone 70% using an ultrasonic bath for 10 min. The extract was centrifuged at 3000 rpm at 15°C for 10 min and the supernatant collected. The supernatant was filtered and the acetone was removed by rotary vacuum evaporation (Güchi R200) at 35°C, and the remaining suspension was solubilized in ethanol. This extract was centrifuged at 10,000 rpm at0 °C for 15 min to eliminate insoluble material. The ethanolic blackberry extract (fruit) was placed in a glass column (2.5 cm i.d.×40 cm length, Kontes, Chromaflex) packed with Sephadex LH-20 that was previously expanded and washed in water and equilibrated with ethanol for 30 min at a flow rate of 5 mL/min. The packed column was sequentially eluted with ethanol, ethanol/methanol (1 : 1), and 80% aqueous acetone (v/v). The 80% acetone fraction that contained the BB-PACS was evaporated under vacuum at 40°C and solubilized in methanol.
2.7.2Sample preparation
Twenty-five (25) mg of the fruit were extracted (2 mL×4) with acetone 70%. For each extraction the sample was sonicated for 5 min at 30 °C and then centrifuged for 10 min at 3000 rpm. The supernatants were collected and standardized to a final volume of 10 mL.
2.7.3DMAC assay
The plate reader protocol was set to read the absorbance (640 nm) of each well in the plate every minute for 60 min, the incubation chamber was set at 25°C, and the system was allowed to equilibrate. Seventy (70) μL of 80% ethanol (blanks), standards, or samples and 210 μL, (0.10 mg mL–1) of DMAC solution were added into all 96 wells, and all samples were analyzed in triplicate.
2.8Antioxidant capacity, using the ORAC assay
ORAC assays [19] were performed with a spectrofluorometer with a 96-well polypropylene plate and a solution of fluorescein (1 : 1000 of the stock solution (4 μM) in 75 mM phosphate buffer pH 7.4) as reagent, made fresh daily, as described previously [20]. The hydrophilic antioxidant value (H-ORAC) was expressed as micromoles of Trolox equivalent antioxidant capacity (TEAC) per gram of fresh weight. Trolox standards were prepared from a 100 μM stock solution in phosphate buffer (pH 7.4).
2.8.1ORAC analysis
The following solution were added to all experimental wells, 150 μL of sodium fluorescein solution, in addition, 25 μL of phosphate buffer (75 mM, pH 7.4) for blanks, 25 μL of Trolox for standards or 25 μL of sample (the same fruit or leaf extract prepared previously). The plate was then allowed to equilibrate by incubating for a minimum of 20 minutes at 37 °C. After incubation, all experimental wells received 25 μL of AAPH solution (153 mM in a phosphate buffer (pH 7.4, 75 mM) made fresh daily) for a final reaction volume of 200 μL. Fluorescence was then monitored kinetically with data taken every minute for 60 minutes. Excitation was performed at 485 nm with a 20 nm bandpass filter and emission was measured at 528 nm with a 20 nm bandpass filter. Reactions were initiated by the addition of 25 μL of AAPH reagent (153 mM), followed by shaking at maximum intensity for 10 seconds.
3Results and discussion
A composite fruit sample and six solvents were tested to determine the best solvent for the extraction of total polyphenols. The solvents used were acetone:methanol (7 : 3), acetone:methanol:water (4 : 4:2), acetone:ethanol(95%):water (5 : 4:1), methanol:water (7 : 3) and acetone:methanol (1 : 1). To evaluate the polyphenolic-rich compounds in the extracts, the colorimetric method of Folin-Ciocalteu was used. The assessment showed that the solvent that generated the best data was ethanol 95% (Table 1). Once the solvent suitable for the extraction of total polyphenols was established, another short experiment was carried out to determine the optimal number of extractions to be carried out on the same sample, and to obtain the highest amount of total polyphenols; once again, the Folin-Ciocalteu method was used to accomplish this objective. The results obtained (Table 2) in this determination show that from the fourth extraction onward there are no statistically significant differences in terms of the amount of extracted phenolic compounds, indicating quantitatively that four extractions from the same sample removed all phenolic compounds, and that additional extractions do not improve the amount of extracted phenolic compounds.
Table 1
Statistical results for the different solvents used for extraction of total phenolic compounds from a composite sample of blackberry fruit
| Solvent or solvent | mg GAE/g DS | Statistic |
| mixture tested | ||
| Acetone:water 7 : 3 (v/v) | 21.7±0.9** | c* |
| Acetone:methanol:water 4 : 4:2 (v/v) | 14.2±0.4 | e |
| Acetone:ethanol:water 5 : 4:1 (v/v) | 17.1±0.6 | d |
| Ethanol 95% | 29.5±0.2 | a |
| Methanol:water 7 : 3 (v/v) | 23.7±0.3 | b |
| Acetone:methanol 1 : 1 (v/v) | 11.7±0.5 | f |
*Letters in the column correspond to statistically significant differences in total polyphenol content in the composite sample, according to the Tukey Test (P < 0.01). **Average of three repetitions.
Table 2
Statistical results for multiple extraction of total phenolic compounds from a composite sample of blackberry fruit
| Extraction number | mg GAE/g DS | Statistic |
| 1 | 9.7±0.3** | d* |
| 2 | 11.2±0.2 | c |
| 3 | 14.1±0.3 | b |
| 4 | 25.5±0.2 | a |
| 5 | 25.7±0.3 | a |
*Letters in the column correspond to statistically significant differences in total polyphenol content in the composite sample, according to the Tukey Test (P < 0.01). **Average of three repetitions.
3.1Total phenolic content
The values obtained in the quantification of total polyphenols using the Folin-Ciocalteu method were reported using two phenolic standards; as equivalent mg of gallic acid (GAE) per gram of dry sample and as equivalent mg of blackberry polyphenols standard (BB-PS) per gram of dry sample. The results are shown in Table 3, and indicate that in general, there are statistically significant differences in the content of the total phenolic compounds among the three varieties of blackberry used in this study (P < 0.01). The red thorned blackberry showed the highest total phenolic content, while the sweet blackberry variety had the lowest. Considering only fruit of all varieties at different stages of maturation, green fruits have the highest total phenolic content. Data in Table 2 show that the use of gallic acid as a standard in the Folin-Ciocalteu assay underestimates the total phenolic content in blackberry samples, and that the use of blackberry polyphenol standard (BB-PS) increases these values by approximately 40 percent. The differences in the concentration of this type of secondary metabolite may be associated with the metabolisms and stages of maturation of each variety, and other biological factors. The three varieties were collected from the same location, but variations in environmental and edaphic factors (sunlight, rainfall, pests), may cause changes in total phenolic content [22]. The values obtained for total polyphenols in our samples are higher than those obtained from other species of blackberry (ripe fruits) collected from temperate climates [23–24]. The values obtained (both those reported in the literature and those obtained in this work) may be unreliable, given that extracts of samples include no phenolic compounds, such as sugars or ascorbic acid and carotenoids, which interfere with the Folin-Ciocalteu reagent [25].
Table 3
Phenolic compounds content of the extract of three varieties of Rubus adenotrichos
| Blackberry | Stages of | mg GAE/ | mg BB-PS/ | Tukey test between standards | |
| varieties | maturation of fruits | g DS | g DS | mg GAE/g DS | mg BB-PS/g DS |
| Sweet | Green fruit | 83±2a,* | 141±4a | b** | a** |
| Pink fruit | 20.3±0.2b | 43.3±0.5b | b | a | |
| Ripe fruit | 13.5±0.3c | 33.5±0.7c | b | a | |
| Thornless | Green fruit | 93±1a | 172±2a | b | a |
| Pink fruit | 25.3±0.2b | 49.8±0.6b | b | a | |
| Ripe fruit | 17.6±0.1c | 42.2±0.3c | b | a | |
| Red thorn | Green fruit | 104±1a | 182±2a | b | a |
| Pink fruit | 31±1b | 60±3b | b | a | |
| Ripe fruit | 20.0±0.5c | 48±1c | b | a | |
*Letters in the columns correspond to statistically significant differences in total polyphenol content in the same variety, according to the Tukey Test (P < 0.01). **Letters in the rows correspond to statistically significant differences in total polyphenol content reported as Gallic Acid or BB-PS (blackberry polyphenol equivalents) in the three varieties according to the Tukey Test (P < 0.01).
3.2Antioxidant activity using the ORAC assay
Various methods have been developed to characterize total antioxidant capacity for biological fluids and natural products. Compared to other methods, the ORAC protocol has received extensive coverage and utilization in the field of antioxidant and oxidative stress. The ORAC assay measures the degree of free radical damage to a fluorescent probe through changes in the intensity of its fluorescence. In the presence of an antioxidant (blackberry extract), the inhibition of free radical damage by the antioxidant, reflected in protection against changes in probe fluorescence in the ORAC assay, is a measure of antioxidant capacity against the free radical. The unique aspect of the ORAC assay is that the reaction is driven to completion and the quantification of the result is achieved by calculating the “area under the curve” (AUC). In particular, the AUC technique allows the ORAC assay to combine both inhibition time and the percentage of inhibition of free radical damage by the antioxidant into a single quantity. Table 4 shows the results of the antioxidant capacity, with the green fruit of the red thorned variety showing the greatest antioxidant activity, with a value of 2805±25 TEAC/DS. On the other hand, the crude extract of ripe fruit (sweet variety) showed the lowest antioxidant capacity with a value of 551±10 TEAC/DS; the difference between these results was statistically significant. Comparing antioxidant capacities between varieties, it may be seen that the red thorned variety shows higher values than the other two varieties, a finding which was corroborated by the Tukey test (p < 0.01).
Table 4
Antioxidant capacity content of extracts of three varieties of Rubus adenotrichos
| Blackberry | Stages of maturation | μmoles Trolox® |
| varieties | of fruits | Eq /g DS |
| Sweetc,** | Green fruit | 1781±28a,* |
| Pink fruit | 866±14b | |
| Ripe fruit | 551±10c | |
| Thornlessb | Green fruit | 2131±15a |
| Pink fruit | 1230±12b | |
| Ripe fruit | 767±5c | |
| Red thorna | Green fruit | 2805±25a |
| Pink fruit | 1543±71b | |
| Ripe fruit | 994±9c |
*Letters in the column correspond to statistically significant differences in antioxidant capacity in the same variety, according to the Tukey Test (P < 0.01). **Letters in the column correspond to statistically significant differences in antioxidant capacity between varieties, according to the Tukey Test (P < 0.01).
Similarly to the results for total phenolic content, the values obtained for the antioxidant capacity in our samples are higher than those reported for other blackberries collected in temperate climates [26]. Phenolic compounds constitute one of the major antioxidant groups of phytochemicals acting as free radical scavengers and antioxidants [27]. The results of the antioxidant assays were correlated with the polyphenolic contents of all blackberry samples, as assessed using linear regression analysis. Interestingly, the results presented in Table 5 show a significant correlation between polyphenolic compounds and the ORAC results (p≥0.01), regardless of the polyphenolic standard used in the Folin-Ciocalteu method, although the correlation coefficient using the BB-PS standard is slightly higher.
Table 5
Correlation coefficients obtained between antioxidant activity (ORAC) and the polyphenolic content of three blackberry varieties extracts
| Variety | Correlation coefficient | Correlation coefficient using |
| using gallic acid as standard (r)* | BB-PS as standard (r) | |
| Sweet | 0.994 | 0.988 |
| Thornless | 0.976 | 0.977 |
| Red Thorn | 0.929 | 0.946 |
(p≥0.01).
3.3Anthocyanins
Anthocyanins are a group of flavonoid derivatives and water soluble natural pigments that give color to flowers and fruits. The primary anthocyanins detected in blackberry is cyanidin-3-O-glucoside. Various other anthocyanins are also detected in blackberry fruit, such as cyanidin-3-O-xyloside, cyanidin-3-O-dioxaloylglucoside and cyanidin-3-O-(malonyl)-glucoside [11]. Animal model studies indicate that anthocyanins have anti-carcinogenic, anti-inflammatory, and anti-obesity qualities in addition to their roles in preventing diabetes mellitus and cardiovascular diseases. The total anthocyanin content of blackberry crude extract, reported as mg equivalent of cyanidin-3-glucoside/g of dry sample, increased as the fruit ripened (Table 6). The red thorn blackberry variety showed the highest values for anthocyanins concentration (Table 5), 15.4±0.3 mg/g of dry sample of ripe fruit, which was equivalent to 159 mg/100 g of fresh blackberry fruit.
Table 6
Anthocyanin content of the extract of three varieties of Rubus adenotrichos using differential pH method
| Variety | Stages of maturation | mg cyanidin-3-glucoside |
| of fruits | Eq/g DS | |
| Sweetc,** | Green | 0.71±0.05c,* |
| Pink | 2.02±0.06b | |
| Ripe | 9.0±0.4a | |
| Thornlessb | Green | 0.92±0.01c |
| Pink | 2.37±0.06b | |
| Ripe | 11.3±0.1a | |
| Red thorna | Green | 1.38±0.06c |
| Pink | 3.3±0.2b | |
| Ripe | 15.4±0.3a |
*Letters in the column correspond to statistically significant differences in anthocyanin content in the same variety, according to the Tukey Test (P < 0.01). **Letters in the column correspond to statistically significant differences in anthocyanin content among varieties, according to the Tukey Test (P < 0.01).
Table 7
PAC content of the extract of three varieties of Rubus adenotrichos using the DMAC method
| Variety | Stages of maturation | mg GE/g DS | mg BB-PRS/g DS |
| of fruits | |||
| Sweetc,** | Green | 7.65±0.03a,* | 37.2±0.2a,* |
| Pink | 2.5±0.1b | 12.1±0.5b | |
| Ripe | 1.81±0.03c | 8.9±0.1c | |
| Thornlessb | Green | 8.38±0.02a | 40.4±0.1a |
| Pink | 3.57±0.07b | 17.6±0.3b | |
| Ripe | 3.14±0.02c | 15.0±0.1c | |
| Red thorna | Green | 9.26±0.03a | 46.0±0.2a |
| Pink | 5.44±0.03b | 27.9±0.2b | |
| Ripe | 4.11±0.09c | 20.2±0.4c |
*Letters in the column correspond to statistically significant differences in concentrations of proanthocyanidins reported as 4'-O-methyl-gallocatechin equivalents (GE) and blackberry proanthocyanidin standard (BBPRS) in the same variety, according to the Tukey Test (P < 0.01). **Letters in the column correspond to statistically significant differences in concentrations of proanthocyanidins among varieties, according to the Tukey Test (P < 0.01).
3.4Proanthocyanidins (PACs)
Proanthocyanidins are a class of biologically active flavonoids found throughout the plant kingdom, and are one of the most potent antioxidants in nature. Fruit samples of blackberry from Sichuan, China in the final ripening stage showed a content of proanthocyanidins (expressed as procyanidin B2 equivalent) of 4.41 mg g–1 fresh weight, although the highest concentration was found in the early unripened stage, reaching 42 mg g–1 fresh weight [28]. In this study we used the 4-dimethylaminocinnamaldehyde (DMAC) colorimetric assay for quantified proanthocyanidin. The DMAC reagent reacts with the end unit of the PAC, thus giving similar molar extinction coefficients for monomers, oligomers, and polymers [29]. In this study, we isolated PAC from blackberry samples for use as a standard in the DMAC assay for quantifying PAC content in all samples. The isolation of standards for use in colorimetric assays has already been accomplished for other food sources such as carob pods [30]. Usually, catechin has been used as the standard of choice for the DMAC reaction; however we used 4'-O-methylgallocatechin because it was the only one available in our laboratory. Additionally, BB-PAC was investigated to determine the reaction kinetics of a standard that represents the structural heterogeneity of PACs found in blackberry. 4'-O-methylgallocatechin showed a much greater slope (y = 0.3276x) than the other standards; however, this figure is lower than that reported for catechin in other recently published research [31]. The other standards showed lower slopes for BB-PACs in green fruit (y = 0.066x), pink fruit (y = 0.0639x), and ripe fruit (y = 0.0667x), as shown in Fig. 1.
Fig.1
Regression lines for 4'-O-methylgallocatechin, BB-PACs in green fruits, BB-PACs in pink fruits, and BB-PACs in ripe fruits after reactions with 4-dimethylaminocinnamaldehyde.
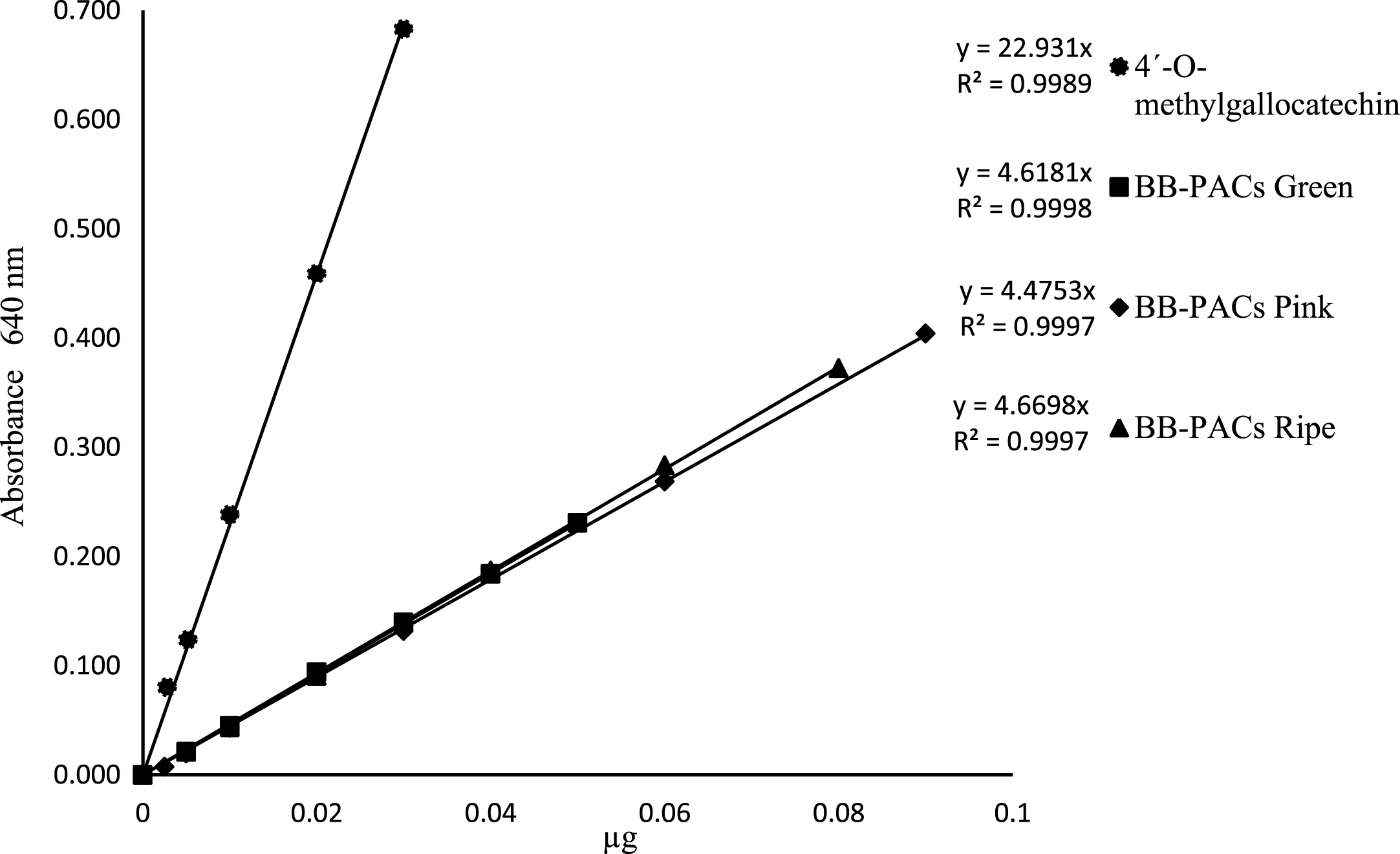
The reduced response of BB-PAC is likely to be due to the proportion of C8 reactive sites on the PAC that are available to participate in the DMAC reaction. If, as previously reported, the C8 terminal unit is the only position available for DMAC reaction, then as the PAC grows linearly in polymer length, each additional monomer adds weight but no additional DMAC reactivity [29]. It is speculated that large polymeric PAC compounds may have a lower response per unit weight than monomeric or other procyanidins [18]. It is therefore necessary to develop better standards, given that most of the natural PACs are natural polymers. The ideal choice is to use PAC as a standard isolated from the sample that is to be analyzed. The accurate determination of PAC content is dependent on the standard used for the DMAC assay. Standard curves were produced for 4'-O-methylgallocatechin and BB-PACs for all blackberry samples (Fig. 1), and the slope of the BB-PACs standard line was 5 times less than the 4'-O-methylgallocatechin, indicating that the content of proanthocyanidins is affected (underestimated) by the standard used (Table 5).
4Conclusion
Results of statistical analysis of three genotypes of Rubus adenotrichos show that the red thorned genotype has the highest concentrations of total polyphenols, anthocyanidins, proanthocyanidins, and antioxidants, while the sweet genotype had the lowest concentrations. Concentration of anthocyanins increases in the black stage of blackberry fruit, while the concentrations of polyphenols and proanthocyanidins decrease in this stage, as does antioxidant capacity. The data obtained showed that the use of gallic acid as a standard in the Folin-Ciocalteu assay underestimates the polyphenol and proanthocyanidin contents of blackberries. There has been increasing interest in investigating polyphenols from berry fruit species because of their potential benefits in the prevention of chronic diseases, but a more realistic and accurate standard should be used during the quantification of these secondary metabolites. The method reported here for producing the different standards may be applied to a wide range of fruits and others natural products.
Acknowledgments
This study was supported by the Universidad Nacional of Costa Rica, and blackberry samples were obtained from the farm of Mr. Víctor Garita.
References
[1] | Orozco R , Flores D , Arguello JF . Efecto de diferentes tipos de propagación en el rendimiento de mora vino (Rubus adenotrichos). (Effect of different types of propagation on the performance of wine blackberries (Rubus adenotrichos). Agron Mesoam. (2011) ;22: (1):91–7. |
[2] | Strik BC , Clark JR , Finn CE , Bañados MP . Worldwide production of blackberries. Horttechnology. (2007) ;17: (2):205–13. |
[3] | Holiman PCH , Hertog MGL , Katan MB . Analysis and health effects of flavonoids. Food Chem. (1996) ;57: :43–6. |
[4] | Bravo L . Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rew. (1998) ;56: :317–33. |
[5] | Sellappan S , Akoh CC , Krewer G . Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem. (2002) ;50: :2432–8. |
[6] | Wang SY , Lin HS . Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage. J Agric Food Chem. (2000) ;48: :140–6. |
[7] | Wada L , Ou B . Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem. (2002) ;50: :3495–500. |
[8] | Rojas G , Fonseca L , Pérez A , Quesada M , Vaillant F . Tropical highland blackberry (Rubus adenotrichos) juice exhibits a protective effect on healthy human subject submitted to a high-fat and high-carbohydrate diet. FAV HEALTH 2009, 3rd International Symposium on Human Health Effects of Fruits and Vegetables, Avignon, France, October 18-21th. |
[9] | Siriwoharn T , Wrolstad RE . Characterization of phenolics in Marion and Evergreen blackberries. J Food Sci. (2004) ;69: :233–40. |
[10] | Häkkinen SH , Kärenlampi SO , Heinonen IM , Mykkänen HM , Törrönen AR . Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. (1999) ;47: :2274–9. |
[11] | Mertz C , Cheynier V , Günata Z , Brat P . Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichos) by high-performance liquid chromatography with diode array detections and electrospray ion trap mass spectroscopy. J Agric Food Chem. (2007) ;55: :8816–24. |
[12] | Jimenez P , Masson L , Barriga A , Chávez J , Robert P . Oxidative stability of oils containing olive leaf extracts obtained by pressure, supercritical and solvent-extraction. European J Lipid Sci Tech. (2011) ;113: (4):497–505. |
[13] | Saiah H , Allem R , Zohra El Kebir F . Antioxidant and antibacterial activities of six Algerian medicinal plants. Int J Pharm Pharm Sci. (2016) ;8: :367–37. |
[14] | Prior RL , Fan E , Ji H , Howell A , Nio C , Payne MJ , Reed J . Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. (2010) ;90: :1473–8. |
[15] | Martin RK , Krueger CG , Rodríguez G , Dreher M , Reed JD . Development of a novel pomegranate standard and new method for the quantitative measurement of pomegranate polyphenols. J Sci Food Agric. (2009) ;89: :157–62. |
[16] | Singleton VL , Orthofer R , Lamuela-Raventos RM . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. (1999) ;299: :152–78. |
[17] | Lee J , Durst W , Wrolstad RE . Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J AOAC Int. (2005) ;88: :1269–78. |
[18] | Krueger C , Reed JD , Feliciano R , Howell A . Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal Bioanal Chem. (2013) ;405: :4385–95. |
[19] | Huang D , Ou B , Hampsch-Woodill M , Flanagan J , Prior RL . Hight-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. (2002) ;50: :4437–44. |
[20] | Suntornsuk L , Gritsanapun W , Nilkamhank S , Poachom A . Quantitation of vitamin C content in herbal juice using direct titration. J Pharm Biomed Anal. (2002) ;28: (5):849–55. |
[21] | Jones RN , Ballow CH , Biedenbach DJ . Multi-laboratory assessment of the linezolid spectrum of activity using the Kirby-Bauer disk diffusion method: Report of the Zyvox® Antimicrobial Potency Study (ZAPS) in the United States. Diagn Microbiol Infect Dis. (2001) ;40: :59–66. |
[22] | Ksouri R , Megdiche W , Falleh H , Trabelsi N , Boulaaba M , Smaoui A , Abdelly C . Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Com Rend Biol. (2008) ;331: :865–73. |
[23] | Reyes-Carmona J , Yousef G , Martínez-Peniche R , Lila M . Antioxidant capacity of fruit extracts of blackberry (Rubus s) produced in different climatic regions. J Food Sci. (2005) ;70: :497–503. |
[24] | Rozema J , Björn LO , Bornman J , Gaberscik A , Hader DP , Trost T , Germ M , Klisch M , Groniger A , Sinha RP , Lebert M , He YY , Buffoni-Hall R , Bakker N , van de Staaij J , Meijkamp BB . The role of UV-B radiation in aquatic and terrestrial ecosystems-an experimental and functional analysis of the evolution of UV-absorbing compounds. J Photoc Photob B. (2002) ;66: :2–12. |
[25] | Binitati J , Elliott WB , Miles PG . Interference by carbohydrate and other substances in the estimation of protein with the Folin-Ciocalteu reagent. Anal Biochem. (1969) ;31: :399–404. |
[26] | Kähkönen MP , Hopia AI , Heinonen M . Berry phenolics and their antioxidant activity. J Agr Food Chem. (2001) ;49: (8):4076–82. |
[27] | Fauconneau B , Waffo-Teguo P , Huguet F , Barrier L , Decendit A , Merillon JM . Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro test. Life Sci. (1997) ;61: :2103–10. |
[28] | Qing C , Xiao-Nan Z , Hao-wei Y , Yan-Wang S , Hao-Ru T . Changes of total anthocyanins and proanthocyanidins in the developing blackberry fruits. Int J ChemTech Res. (2012) ;4: (1):129–37. |
[29] | Pérez-Jiménez J , Arranz S , Saura-Calixto F . Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Res Int. (2009) ;42: (10):1381–8. |
[30] | Feliciano RP , Shea MP , Shanmuganayagam D , Krueger CG , Howell AB , Reed JD . Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as standards in the 4-dimethylaminocinnamaldehyde assay. J Agric Food Chem. (2012) ;60: :4578–85. |
[31] | Wallace TC , Guisti MM . Evaluation of parameters that affect the 4-dimethylaminocinnamaldehyde assay for flavonols and proanthocyanidins. J Food Sci. (2010) ;75: (7):619–25. |




