Phenylalanine ammonia lyase activity, antioxidant properties, fatty acid profile, mineral content and physiochemical analyses of Cissus sicyoides berries
Abstract
BACKGROUND:
Cissus sicyoides berries are currently underutilized and may be a potentially novel source of antioxidants. Extracts from the leaves of this vine are utilized in traditional medicine. The chemical composition and functional properties of C. sicyoides berries were investigated.
OBJECTIVE:
Phenylalanine ammonia lyase (PAL) an essential enzyme in the phenylpropanoid pathway was characterized and the antioxidant properties, fatty acid profile and mineral content of the berries evaluated.
METHODS:
The PAL, free radical scavenging activity and total phenolic content of C. sicyoides berries were determined by use of a spectrophotometric assay. Lipids were extracted, methylated and the fatty acid methyl esters identified by gas chromatography mass spectrometry. The chemical composition of the berries was also evaluated by infra red spectroscopy.
RESULTS:
The PAL enzyme from C. sicyoides exhibits Michaelis Menten kinetics with the substrate phenylalanine (Km, 1.21±0.28 mM) and has a Vmax of 6.24±0.28 μM/min. The enzyme has a temperature optimum of 25°C and a pH optimum of 7.5. C. sicyoides berries possess high free radical scavenging activity (84.4±4.4%) and contain a phenolic content of 3.2±0.4 mg gallic acid/g dry weight. Berry extracts had an ascorbic acid concentration of 2.8±0.1 mg/mL. The major fatty acids identified in extracts of the fruit include palmitic acid (43.6±1.8%), oleic acid (13.8±1.7%) and linoleic acid (13.9±2.0%). The oil has a predicted iodine value of 35. Berry extracts had a refractive index of 17 °Brix. A positive Fehlings test confirmed the presence of reducing sugars. The fruit is low acid with a pH of 6, and a titratable acidity of 0.165 g of tartaric acid per 100 mL of juice. FTIR analysis of ashed samples indicated the presence of the minerals potassium and phosphorus.
CONCLUSION:
C. sicyoides berries possess high levels of free radical scavenging activity and may be a novel source of antioxidants.
1Introduction
Cissus sicyoides L (Cissus verticulata) also known as Princess vine, curtain ivy and anil trepador (Fig. 1) is native to the Dominican Republic but is found abundantly in Brazil especially in the Amazon rainforest and is seen growing throughout the Caribbean. It belongs to the Vitaceae family which is an important source of food for birds and animals. Cissus, the largest genus of Vitaceae, has approximately 350 species which are used as food by the larvae of some Lepidoptera species. C. sicyoides leaves are utilized in traditional medicine exhibiting anti-inflammatory, anti-diuretic, anti-convulsion, anti-influenza and hypoglycemic properties [1–5]. The berries are a rich source of anthocyanins with potential application as a food colorant [6]. The major anthocyanins identified include delphinidin-3-rutinoside, cyanidin-3-rhamnosyl-arabinoside and delphinidin-3-rhamnoside [6]. A coumarin glycoside 5,6,7,8-tetrahydroxy-coumarin-5β-xylopyranoside was isolated from methanolic extracts of the leaves [7].
Fig.1
Cissus sicyoides.

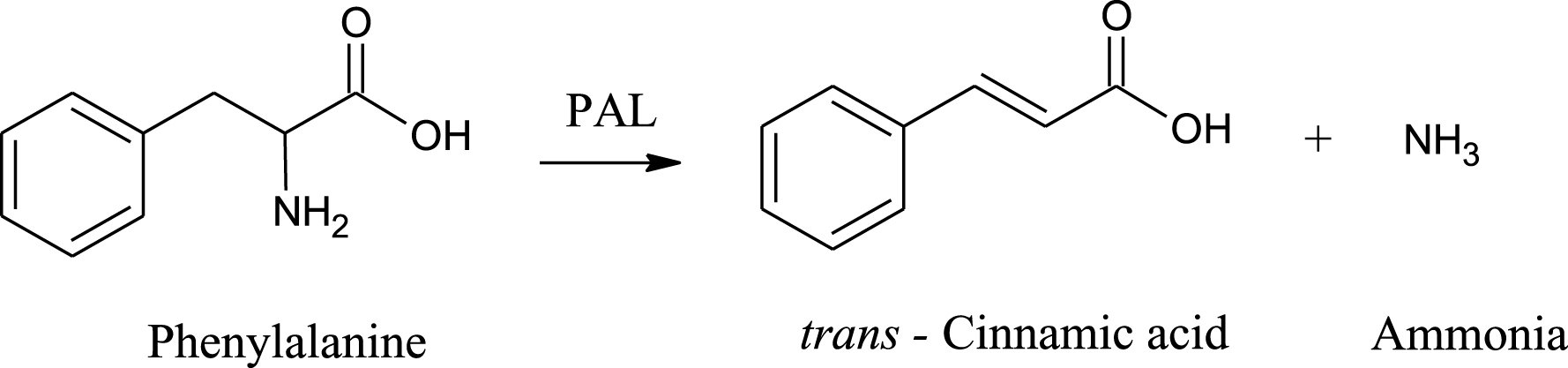
Phenylalanine ammonia lyase (PAL; E.C. 4.3.1.5) catalyzes the non oxidative degradation of L-phenylalanine to trans cinnamic acid and a free ammonium ion [8]. This is the first step of the phenylpropanoid pathway which is used to channel carbon from primary to secondary metabolism (Fig. 2). L-phenylalanine is an essential amino acid which is derived from the shikimic acid pathway and is used in the synthesis of proteins or metabolized via the phenylpropaniod pathway leading to the production of secondary metabolites. Secondary metabolites are essential in the development of the colour and flavor of berries. The characteristic colour of berries is due to the presence of anthocyanins. The flavor of ripe raspberries is due to p-hydroxyphenylbutan-2-one [9].
Fig.2
First step of the phenylpropanoid pathway.
Current literature focuses on the leaves of C. sicyoides with limited information on the berries. It is uncertain as to whether the berries are suitable for human consumption. This study was undertaken as a preliminary investigation into the nutritional and functional properties of C. sicyoides berries. The total phenolics, free radical scavenging activity, fatty acid profile and total ash content of the berries were evaluated. Phenylalanine ammonia lyase, a key enzyme in the synthesis of secondary metabolites of plants was also characterized. The physicochemical parameters pH, total soluble solids (°Brix) and total acidity were investigated as these have an impact on the sensorial properties of the berries.
2Materials and methods
2.1Phenylalanine ammonia lyase extraction and assay
Berries were freshly harvested from vines growing at the University of the West Indies, Kingston, Jamaica. The PAL activity of extracts was measured using an end point spectrophotometric assay [10]. Samples were homogenized in extraction buffer (50 mM Tris-HCl, 1 mM EDTA, 10 mM 2-mercaptoethanol and 2.5% (w/v) polyvinylpolypyrrolidone) in a 1 : 2 ratio (fresh weight basis: buffer). After homogenization, samples were refrigerated for 1 h, and then centrifuged. The resulting supernatant (2 mL) was partitioned with polyethylene glycol 6000 (PEG6000, 11%)/sodium sulphate (Na2SO4, 14%) [11]. Enzyme assays were incubated for 15 min at different temperatures (15C–30C) to determine the temperature optimum. The reaction was terminated by the addition of trichloroacetic acid (25%, 200 μL). Samples were centrifuged and the absorbance of trans cinnamic acid measured at 290 nm. The pH optimum of the enzyme was also determined (pH 7 –8.5). For kinetic analysis, solutions containing phenylalanine (2.5 mM to 40 mM) were utilized.
2.2The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The DPPH assay was performed according to the method of Brand-Williams et al. [12]. Samples (200 mg) were extracted with methanol (2 mL, 80%) containing hydrochloric acid (1%) at room temperature. Extracts were reacted with the stable DPPH radical in an ethanol solution. The reaction mixture consisted of berry extract (0.5 mL), absolute ethanol (3 mL) and DPPH (0.5 mM, 0.3 mL). The reaction was allowed to proceed for 100 min after which the absorbance was measured at 517 nm using a spectrophotometer (Helios Omega, Thermo Fisher Scientific). A mixture of ethanol (3.3 mL) and berry extract (0.5 mL) served as the blank. A control solution was prepared by mixing ethanol (3.5 mL) with the DPPH radical solution (0.3 mL). The data obtained was used to calculate the radical scavenging capacity according to the following formula:
Where
A1 = Absorbance of sample at 517 nm.
A0 = Absorbance of control at 517 nm.
2.3Total phenolic content
Total phenolics was determined using the Folin-Ciocalteu assay with modifications [13]. Dried berry samples (200 mg) were extracted with methanol (2 mL, 80%) containing hydrochloric acid (1%) at room temperature. Extracts (100 μL) were reacted with Folin-Ciocalteu reagent (10%, 750 μL) and mixed for 5 min followed by addition of Na2HCO3 solution (6%, 750 μL). The solution was incubated at 22C (1.5 h) and the absorbance measured at 760 nm using a spectrophotometer (Helios Omega, Thermo Fisher Scientific). A standard calibration curve of gallic acid (0–200 mg/L) was generated and the results expressed as mg gallic acid/g dry weight.
2.4°Brix, Fehlings test, pH, titratable acidity
The °Brix (total soluble solids) of berry extracts was determined utilizing a refractometer (ExTech instruments) and pH with the use a pH meter (Oakton, pH Tutor). The Fehlings test was conducted to determine the presence of reducing sugars. The Fehlings reagent was comprised of Fehlings reagent A (copper sulphate) and Fehlings reagent B (alkaline solution of sodium potassium tartarate) which were mixed together at the time of analysis. Titratable acidity was determined by titrating berry extracts (10 mL) with sodium hydroxide (0.1 M). The end point of the reaction was detected by use of a pH meter and phenolphthalein indicator.
2.5Ascorbic acid determination
The ascorbic acid content of C. sicyoides berry extracts was determined by redox titration with potassium iodate (0.005 M) using starch as indicator. A standard curve was generated and the ascorbic acid content expressed as mg ascorbic acid per mL.
2.6Lipid extraction, fatty acid profile, iodine value determination
Lipids were Soxhlet extracted with n-hexane (reflux) and concentrated in vacuo. Lipid extracts (50 μL) were trans-methylated with methanol/acetyl chloride solution [14] and the resulting fatty acid methyl esters (FAMEs) determined by Gas Chromatography/Mass Spectrometry (GC/MS). The methylated oil in hexane (1.0 μL) was chromatographed on an HP6890 series Gas Chromatograph interfaced with an HP5973 Mass Selective Detector. Constituent FAMEs were eluted with helium carrier gas (flow rate 1 cm3/min) through a DB1-MS column (20 m×0.18 mm i.d.×1.0 μm film thickness, Agilent, Santa Clara, CA) in an oven programmed at 60°C for 3 min and increased at a ramp rate of 10°C/min up to 250°C for 15 min. Samples were injected at 230°C while the detector was maintained at 250°C. Constituents were identified by matching the mass spectra National Institute of Standards and Technology (NIST) library of mass spectra (match quality >90%). Iodine values were calculated based on the FAME content utilizing the formula:
2.7Fourier transform infrared spectroscopy
A Bruker Vector 22 Fourier Transform Infra Red (FTIR) Spectrometer was utilized to record the infrared spectra of dried and ashed samples of C. sicyoides berries. FTIR spectra were recorded between 4000 and 500 cm–1. Each spectrum was obtained by averaging 20 scans recorded at a resolution of 2 cm–1. Spectra were baseline-corrected. OPUS software was used to acquire and manipulate the spectral data.
2.81H NMR and 13C NMR characterization
1H NMR and 13C NMR characterization were performed on a Bruker BioSpin 200 MHz at 200 MHz. Lipid extracts (20 mg) were analyzed in deuterated chloroform (CDCl3) at 25°C, with tetramethylsilane as the internal standard. In reporting 1H NMR the following terms were used; singlet (s), doublet of doublet (dd), triplet (t), multiplet (m).
2.9Data analyses
Samples were analysed in triplicate. Means and standard deviations of the data are presented.
Kinetic data was analyzed using GraphPad Statistical Software (Version 7, California).
3Results and discussion
3.1Physicochemical analysis
C. sicyoides berry extracts had a pH of 6, a titratable acidity of 0.165 g tartaric acid per 100 mL of juice and a refractive index of 17 °Brix. The berries are less acidic (pH 6) and possess a lower titratable acidity compared to various varieties of Vitis vinifera which typically have a pH of 3 and a titratable acidity ranging from of 0.217±0.01% (Victoria) to 0.518±0.02% (Muscat noir) [16]. The berries are a good source of sugars having a total soluble solids content of 17 °Brix which is similar to that observed in the grape varieties Cardinal, Victoria and Muscat blanc [16]. The minimum allowed °Brix for grape juice is 16. It is expected that the berries should be sweet in taste and not tart.
3.2Phenylalanine ammonia lyase activity
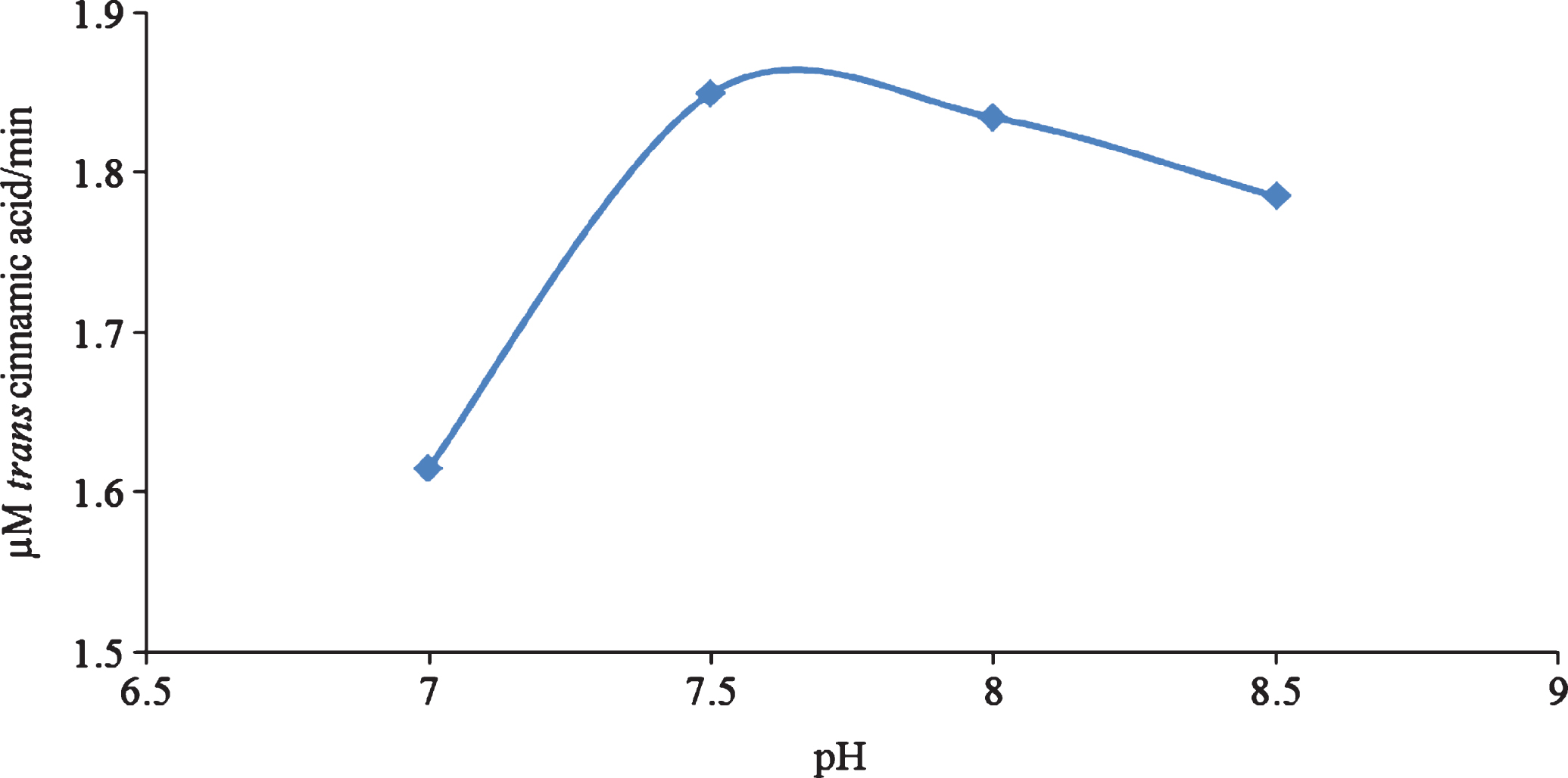
There is currently limited information on the characterization of PAL from fruits. In strawberry, two peaks of PAL activity have been reported, one from the immature fruit and the other from the almost fully mature fruit [17]. The PAL activity of citrus [18] and grapes [19] has also been reported. The PAL enzyme from V. vinifera L. cv. Cardinal was detected in the epidermis of the fruit. Polyvinylpolypyrrolidine (PVPP) or polyethylene glycol (PEG) 4000 was essential in detecting PAL in enzyme extracts of V. vinifera [19]. In the current research the use of PVPP and PEG in the preparation of the enzyme extracts was investigated. An aqueous two phase system (ATPS) containing polyethylene glycol 6000 (PEG6000, 11%)/sodium sulphate (Na2SO4, 14%) was effective in producing an extract in which PAL activity could be easily detected. PAL partitioned in the top layer of the two phase system. The use of an ATPS assists in the removal of interfering substances from the enzyme assay [11]. The PAL activity of C. sicyoides berries has not been previously reported. The enzyme follows Michaelis Menten kinetics having a Km of 1.21±0.28 mM and a Vmax of 6.24±0.28 μM/min. The temperature and pH optimum of the PAL enzyme is 25°C and 7.5 respectively (Figs. 3 and 4).
Fig.3
Temperature dependence studies on the PAL enzyme from C. sicyoides.
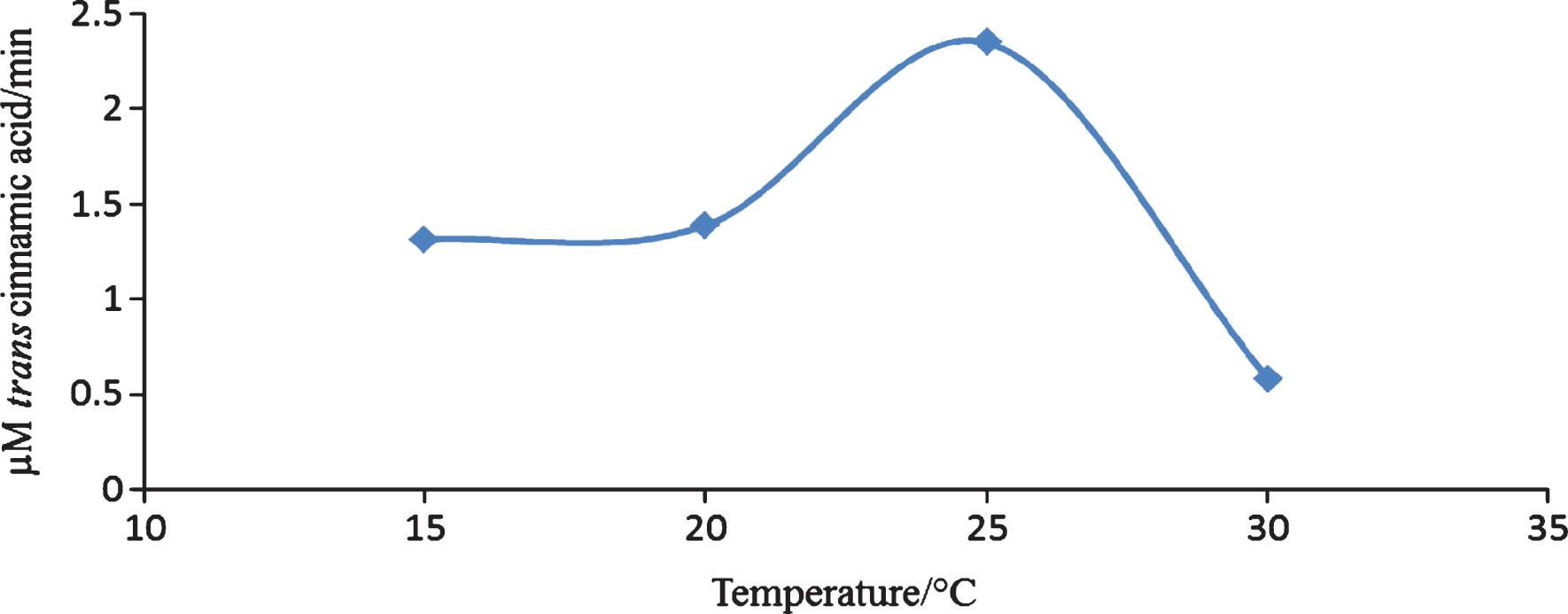
Fig.4
pH dependence studies on the PAL enzyme from C. sicyoides.
3.3Antioxidant, free radical scavenging activity and total phenolic content
Antioxidants are needed for good health. They serve as free radical scavengers, scavenging potentially dangerous reactive oxygen species. Recent studies have shown that regular consumption of antioxidants can reduce the risk of cancer and cardiovascular disease [20, 21]. Antioxidants are also utilized in food preservation to extend the shelf stability of food products [22]. In the quest for healthier foods, consumers and food manufactures are looking to utilize less artificial additives, moving towards the use of natural preservatives and colorants.
C. sicyoides berries were evaluated for their free radical scavenging activity and total phenolic content. Leaves from the plants of the Cissus genus are known to contain sterols, quinones and phenolic compounds [7]. V. vinifera (grapes) are an excellent source of antioxidants (16) with over 17 different phenolic compounds (23). A comparison of the antioxidant capacity of blackberries, strawberries and blueberries revealed that blueberry extracts possess the highest antioxidant capacity (24). At a concentration of 2 mg/mL, blueberry and blackberry extracts have been reported as scavenging 96.96% and 95.37% of the DPPH radical respectively (24). At the same concentration strawberry extracts scavenged 58% of the DPPH radical (24). C. sicyoides berry extracts exhibited a free radical scavenging activity of 84.4±4.4% and are a potentially novel source of antioxidants.
The phenolic content of C. sicyoides berry extracts (3.2±0.4 mg gallic acid/g dry weight) is comparable to that of strawberries. In a study conducted by Huang et al. [24], blueberries had the highest total phenolics (9.4 mg ± 0.2 gallic acid/g dry weight) and strawberries the lowest (2.7±0.2 mg gallic acid/g dry weight).
High anthocyanin content has been reported in gooseberries, blueberries and cranberries [25]. The extracts of these berries exhibited significant lipid peroxidation inhibitory activity [25] suggesting that berries may be beneficial in producing functional foods. Cranberries are also known for cardiovascular health benefits due to their high polyphenol content [26, 27]. Studies have shown that the frequent consumption of fruits and vegetables is cardioprotective [28]. Berries have been associated with improved cardiovascular health and are an excellent source of polyphenols, especially anthocyanins, micronutrients and fiber [27]. Berry extracts have shown antioxidant protection toward lipid and protein oxidation [29]. Human intervention studies with various berries have also shown a decrease in LDL oxidation and peroxidation, a decrease in total cholesterol and increase in total plasma antioxidant capacity [27].
3.4Ascorbic acid determination
C. sicyoides berries are an excellent source of ascorbic acid (vitamin C) compared to traditional citrus sources [30]. The berries possess an ascorbic acid concentration of 2.8±0.1 mg/mL which is tenfold higher than the reported values of 0.12–0.31 mg/mL for various varieties of V. vinifera (16). Blackcurrants are also an excellent source of ascorbic acid with values in the range of 122.4 to 193.2 mg/100 g fresh weight [31].
3.5Fourier transform infrared spectroscopy (FTIR)
The chemical composition of C. sicyoides berries was evaluated by FTIR spectroscopy, a simple and non-destructive method of analysis that requires significantly less time compared to conventional methodologies. Three distinct regions of absorption were observed in the FTIR spectra of the berries (Fig. 5). The fingerprint region, (1000–1200 cm–1) produced characteristic bands which are due to the C-O stretch vibrations (endocyclic and exocyclic) of carbohydrates present. A peak at 1072 cm–1 suggests the presence of glucose or fructose in berry samples [32]. This was confirmed by a positive Fehlings test which gave a characteristic red colour in the presence of reducing sugars.
Fig.5
FTIR analysis of dehydrated C. sicyoides berries.
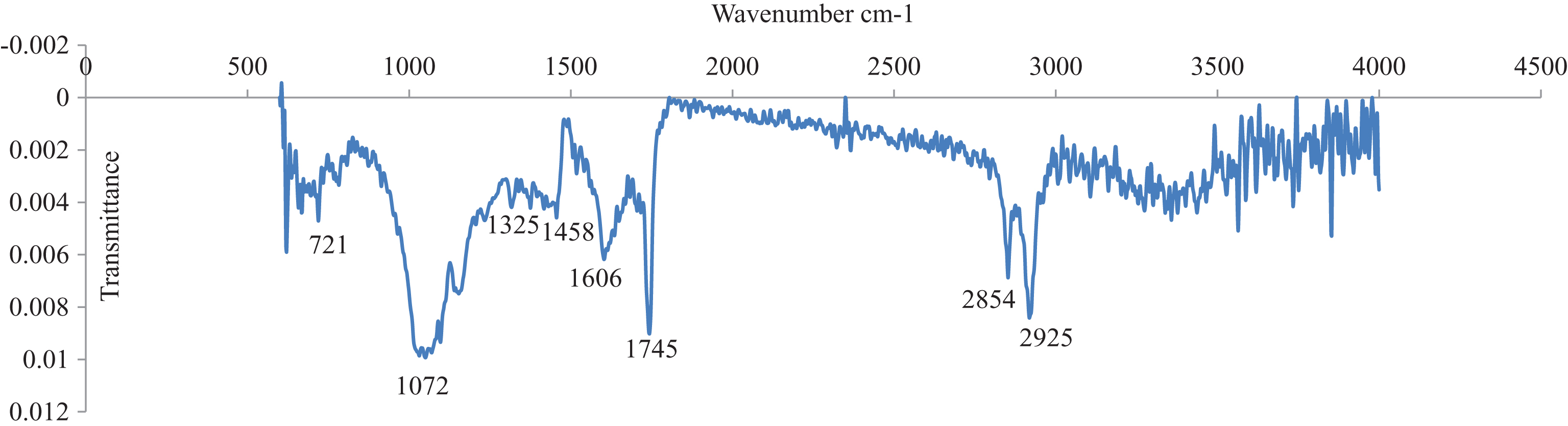
The second region of the spectra (1800–1500 cm–1) corresponds to carbonyl-specific absorptions with sharp bands being observed at 1606 cm–1 and 1745 cm–1. Weaker bands were observed at 1325 cm–1 and 1458 cm–1. Bands at 1325 cm–1 and 1606 cm–1 are due to the amide functionality of proteins present. A sharp band at 1745 cm–1 is due to carboxyl functionalities (ester) of triacylglycerols. Methylene (CH2) bending vibrations from fatty acid side chains present in triacylglycerols was observed at 1458 cm–1 [33]. Neighboring peaks at 2854 cm–1 and 2925 cm–1 correspond to stretching vibrations of CH2 and CH3 which occur mainly in lipid acyl chains. The presence of olefinic carbons was illustrated by a peak occurring at 721 cm–1. A broad band in the region of 3000–3500 cm–1 in the third region of the FTIR spectra confirmed the presence of polyphenolics in extracts of the fruit.
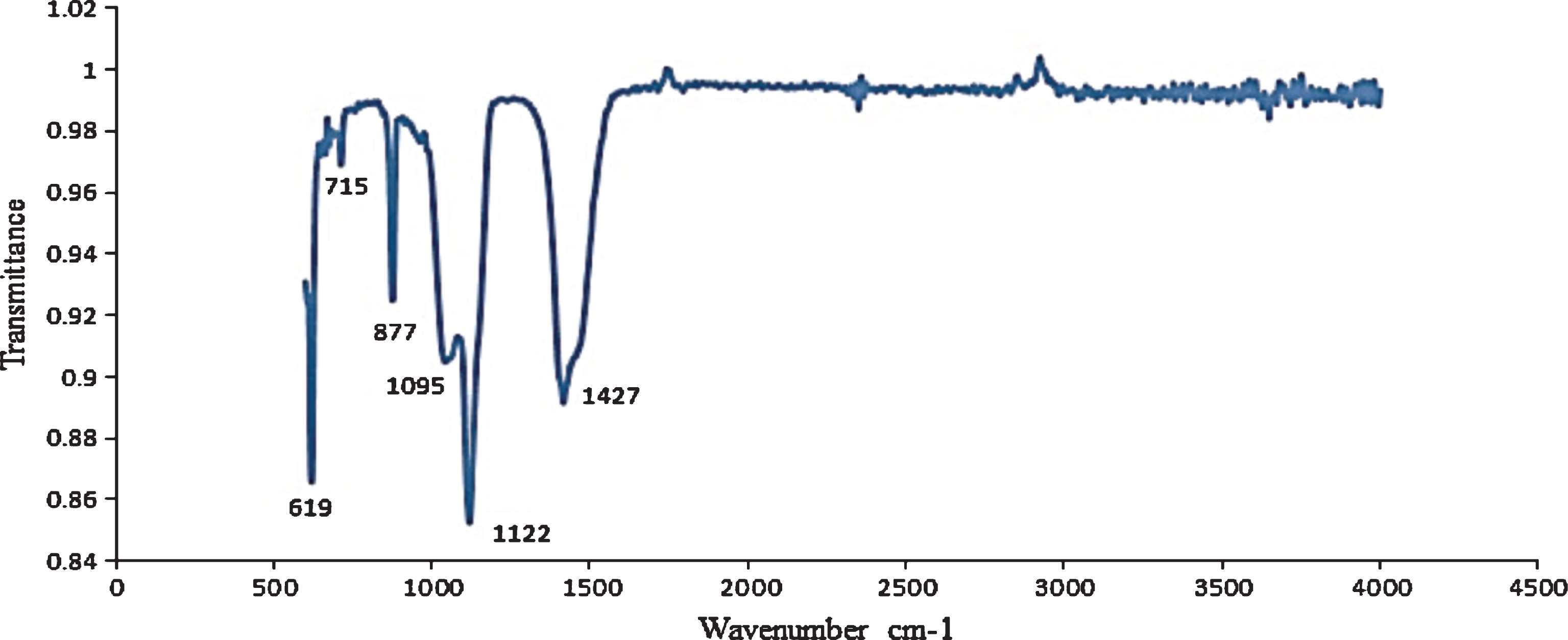
3.6Ashed berry samples
C. sicyoides berries have a total ash content of 10.8±1.2%. FTIR analysis of the ashed berry revealed significant absorption peaks at 619 cm–1, 877 cm–1, 1095 cm–1, 1122 cm–1 and 1427 cm–1 (Fig. 6). The bands at 1095 cm–1 (P–O stretching vibrations) and 619 cm–1 (P–O bending vibrations) are consistent with the presence of phosphates [33]. There is also evidence for the presence of potassium (1427 cm–1) [34] and calcium (877 cm–1) compounds [35, 36]. Minerals that have been identified in V. vinifera L, include potassium (1.11%), phosphorus (0.31%), calcium (0.06%) and magnesium (0.04%) [37].
Fig.6
FTIR analysis of ashed C. sicyoides berries.
3.7Lipid profile of C. sicyoides berries
C. sicyoides berries possess a lipid content of 4.9±0.5%. GC/MS identified palmitic acid (43.6±1.8%) as the major fatty acid present (Table 1). Oleic acid (13.8±1.7%) and linoleic acid (13.9±2.0%) were also detected. The oil has a predicted iodine value of 35. A similar fatty acid profile was reported for lipid extracts of highbush blueberries (Vaccinium corymbosum L.) with palmitic, oleic, linoleic and linolenic acids being reported as the major fatty acids [38]. Palmitoleic acid was not detected in lipid extracts of C. sicyoides berries but has been reported in lipid extracts of Hippophaë rhamnoides L. (sea buckthorn) berries [39].
Table 1
Fatty acid profile of C. sicyoides berries
| Fatty acid | Berries (%) | |
| Palmitic acid | C16 : 0 | 43.64±1.77 |
| Stearic acid | C18 : 0 | 19.99±5.88 |
| Oleic acid (omega 9) | C18 : 1 | 13.78±1.71 |
| Linoleic acid (omega 6) | C18 : 2 | 13.92±2.03 |
3.8Nuclear magnetic resonance spectroscopy
Confirmation of the fatty acid profile was obtained by NMR spectroscopy. The presence of linoleic acid was confirmed by a singlet resonating at δ 2.66 which is due to the protons attached to the bis allyic carbon in linoleic acid [40]. Olefinic protons were observed at δ 5.34 due to the unsaturated fatty acids, oleic acid and linoleic acid (Table 2). Shifts indicative of the presence of oleic acid and linoleic acid were also observed in the carbon NMR (Table 3).
Table 2
1H NMR spectroscopy of C. sicyoides berry lipid extracts
| Proton | Functionality | δ (ppm) |
| CH3 | Terminal methyl | 0.88 |
| CH2 | Methylene | 1.26 |
| CH2-CH2-COO | Acyl chains | 1.61 |
| CH2-CH = CH | All unsaturated fatty acid | 2.02 |
| CH2-COO | All acyl chains | 2.22 |
| C = C-CH2C = C | Protons attached to bis allylic carbon | 2.66 |
| CH2O(α) | Glycerol (triglycerides) | 4.14 |
| CH2O(α) | Glycerol (triglycerides) | 4.29 |
| CHO(β) | Glycerol (triglycerides) | 5.26 |
| CH = CH | Olefinic protons | 5.34 |
Table 3
13C NMR spectroscopy of C. sicyoides berry lipid extracts
| Carbon | Assignment | δ (ppm) |
| α-CH3 | Acyl chains | 14.14 |
| β-CH3 | Acyl chains | 22.70 |
| C3 | Acyl chains | 24.86 |
| C8–11 (oleyl); C8–14 (linoleyl) | Allylic | 27.23 |
| CH2n | Acyl chains | 29.13-29.71 |
| C16 | Linoleyl | 31.94 |
| α- C2 | Acyl chains | 34.06 |
| β-C2 | Acyl chains | 34.11 |
| α-CH2O | Glycerol moiety | 62.08 |
| β-CH2O | Glycerol moiety | 68.91 |
| β-C9 | Oleyl | 129.72 |
| β-C10 | Linoleyl | 130.03 |
| βC1 | Glycerol moiety | 173.37 |
4Conclusion
The PAL enzyme is critical in the development of the colour and flavor of C sicyoides berries. The enzyme has a Km of 1.21±0.28 mM and a Vmax of 6.24±0.28 μM/min. FTIR was used to provide a rapid estimation of the bioactives present in C. sicyoides berries. C. sicyoides berries are a source of antioxidants, polyphenolic compounds, omega fatty acids and minerals which are known to provide many beneficial health effects. The findings suggest that C. sicyoides berries may be an underutilized food resource with potential health benefits.
Acknowledgments
Funding for this project was provided by a Royal Society Science of Chemistry Grant.
The authors confirm that there is no conflict of interest.
References
[1] | Carvajal D , Casaco A , Arruzazabala L , Gonzalez R , Fuentes V . Pharmacological screening of plant detections commonly used in Cuban folk medicine. J Ethnopharmacol. (1991) ;33: (1-2):21–4. doi: 10.1016/0378-8741(91)90155-7 |
[2] | Feng PC , Haynes LJ , Magnus KE , Plimmer JR , Sherratt I . Pharmacological screening of some West Indian medicinal plants. J Pharm Pharmacol. (1962) ;14: (1):556–61. doi: 10.1111/j.2042-7158.1962.tb11139.x |
[3] | Pepato MT , Baviera AM , Vendramini RC , Perez Mda P , Kettelhut Ido C , Brunetti IL . Cissus sicyoides (princess vine) in the long-term treatment of streptozotocin-diabetic rats. Biotechnol Appl Biochem. (2003) ;37: (1):15–20. doi: 10.1042/BA20020065 |
[4] | Salgado JM , Mansi DN , Gagliardi A . Cissus sicyoides: Analysis of glycemic control in diabetic rats through biomarkers. J Med Food. (2009) ;12: (4):722–27. doi: 10.1089/jmf.2008.0157 |
[5] | Viana GS , Medeiros AC , Lacerda AM , Leal LK , Vale TG , Abreu Matos F . Hypoglycemic and anti-lipemic effects of the aqueous extract from Cissus sicyoides . BMC Pharmacol. (2004) ;4: :9. doi: 10.1186/1471-2210-4-9 |
[6] | Toledo MCF , Reyes FGR , Iaderoza M , Francis FJ , Draetta IS . Anthocyanins from Anil Trepador (Cissus sicyoides,L.). J Food Sci. (1983) ;48: (4):1368–369. doi: 10.1111/j.1365-2621.1983.tb09238.x |
[7] | Beltrame F , Ferreira A , Cortez D . Coumarin glycoside from Cissus sicyoides. Nat Prod Lett. (2002) ;16: (4):213–16. doi: 10.1080/10.575630290015736 |
[8] | Koukol J , Conn EE . The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem. (1961) ;236: :2692–98. |
[9] | Borejsza-Wysocki W , Hrazdina G . Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue cultures. Phytochemistry. (1994) ;35: (3):623–28. doi: 10.1016/S0031-9422(00)90575-2 |
[10] | Abell CW , Shen R . Phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis. Method Enzymol. (1987) ;142: :242–48doi: 10.1016/S0076-6879(87)42033-8 |
[11] | Wang WY , Yue HY , Yuan QP , Wang WC . Partitioning of Phenylalanine ammonia-lyase from Rhodotorula glutinis in aqueoustwo-phase systems of PEG/salts. Chem Biochem Eng Q. (2010) ;24: (4):459–65. |
[12] | Brand-Williams W , Cuvelier M , Berset C . Use of a free radical method to evaluate antioxidant activity. LWT Food Sci and Technol. (1995) ;28: (1):25–30. doi: 10.1016/S0023-6438(95)80008-5 |
[13] | Singleton VL , Rossi JA Jr . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. (1965) ;16: (3):144–58. |
[14] | Masood A , Stark KD , Salem N . A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J Lipid Res. (2005) ;46: (10):2299–305. doi: 10.1194/jlr.D500022-JLR200 |
[15] | Kyriakidis NB , Katsiloulis T . Calculation of iodine value from measurements of fatty acid methyl esters of some oils: Comparison with the relevant American Oil Chemists Society method. J Am Oil Chem Soc. (2000) ;77: (12):1235–38. doi: 10.1007/s11746-000-0193-3 |
[16] | Derradji-Benmeziane F , Djamai R , Cadot Y . Antioxidant capacity, total phenolic, carotenoid, and vitamin C contents of five table grape varieties from Algeria and their correlations. J Int Sci Vigne Vin. (2014) ;48: (2):153–162. doi: http://dx.doi.org/10.20870/oeno-one.2014.48.2.1564 |
[17] | Cheng GW , Breen PJ . Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Am Soc Hortic Sci. (1991) ;116: (5):865–69. |
[18] | Dubery IA , Schabort JC . Phenylalanine ammonia-lyase from Citrus sinensis: Purification hydrophobic interaction chromatography and physical characterization. Biochem Intl. (1986) ;13: (4):579–89. |
[19] | Roubelakis-Angelakis KA , Kliewer WM . Phenylalanine ammonia-lyase in berries of Vitis vinifera L.: Extraction and possible sources of error during assay. Am J Enol Vitic. (1985) ;36: :314–15. |
[20] | Adams AK , Wermuth EO , Mcbride PE . Antioxidant vitamins and the prevention of coronary heart disease. Am Fam Physician. (1999) ;60: (3):895–902. |
[21] | Hajhashemi V , Vaseghi G , Pourfarzam M , Abdollahi A . Are antioxidants helpful for disease prevention? Res Pharm Sci (2010) ;5: (1):1–8. |
[22] | Shahidi F , Zhong Y . Novel antioxidants in food quality preservation and health promotion. Eur J Lipid Sci Tech. (2010) ;112: (9):930–940. |
[23] | Revilla E , Ryan JM . Analysis of several phenolic compounds with potential antioxidant properties in grape extracts and wines by high-performance liquid chromatography–photodiode array detection without sample preparation. J Chromatogr A. (2000) ;881: (1-2):461–469. http://dx.doi.org/10.1016/S0021-9673(00)00269-7 |
[24] | Huang WY , Zhang HC , Liu WX , Li CY . Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry. J Zhejiang Univ Sci B. (2012) ;13: (2):94–02. doi: 10.1631/jzus.B1100137 |
[25] | Namiesnik J , Vearasilp K , Kupska M , Ham KS , Kang SG , Park YK , Barasch D , Nemirovski A , Gorinstein S . Antioxidant activities and bioactive components in some berries. Eur Food Res Technol. (2013) ;237: (5):819–29. doi: 10.1007/s00217-013-2041-7 |
[26] | Blumberg JB , Camesano TA , Cassidy A , Kris-Etherton P , Howell A , Manach C , Ostertag LM , Sies H , Skulas-Ray A , Vita JA . Cranberries and their bioactive constituents in human health. Adv Nutr. (2013) ;4: (6):618–32. doi: 10.3945/an.113.004473 |
[27] | Basu A , Rhone M , Lyons TJ . Berries: Emerging impact on cardiovascular health. Nutr Rev. (2010) ;68: (3):168–77. doi: 10.1111/j.1753-4887.2010.00273.x |
[28] | Holt EM , Steffen LM , Moran A , Basu S , Steinberger J , Ross JA , Hong CP , Sinaiko AR . Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. (2009) ;109: (3):414–21. doi: 10.1016/j.jada.2008.11.036 |
[29] | Viljanen K , Kylli P , Kivikari R , Heinonen M . Inhibition of protein and lipid oxidation in liposomes by berry phenolics. J Agricult Food Chem. (2004) ;52: (24):7419–24. doi: 10.1021/jf049198n |
[30] | Pisoschi AM , Pop A , Negulescu GP , Pisoschi A . Determination of ascorbic acid content of some fruit juices and wine by voltammetry performed at Pt and carbon paste electrodes. Molecules. (2011) ;16: :1349–65. doi: 10.3390/molecules16021349 |
[31] | Djordjevic B , Savikin K , Zdunic G , Jankovic T , Vulic T , Pljevljakusic D , Oparnica C . Biochemical properties of the fresh and frozen black currants and juices. J Med Food. (2013) ;16: (1):73–81. doi: 10.1089/jmf.2011.0256 |
[32] | Adina C , Florinela F , Abdelmoumen T , Carmen S . Application of FTIR spectroscopy for a rapid determination of some hydrolytic enzymes activity on sea buckthorn substrate. Rom Biotechnol Lett. (2010) ;15: (6):5738–44. |
[33] | El-Bahy GMS . FTIR and Raman spectroscopic study of Fenugreek (Trigonella foenum graecum L.) seeds. J Appl Spectrosc. (2005) ;72: (1):111–16. doi: 10.1007/s10812-005-0040-6 |
[34] | Miller FA , Wilkins CH . Infrared spectra and characteristic frequencies of inorganic ions. Anal Chem. (1952) ;24: :1253–94. doi: 10.1021/ac60068a007 |
[35] | Berzina-Cimdina L , Borodajenko N . Research of calcium phosphates using Fourier transform infrared spectroscopy. Infrared Spectroscopy: Materials Science, Engineering and Technology. (2012) :123–48. doi: 10.5772/36942 |
[36] | Galván-Ruiz M , Hernández J , Baños L , Noriega-Montes J , Rodríguez-García M . Characterization of calcium carbonate, calciumoxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. J Mater Civ Eng. (2009) ;21: (11):694–98. doi:org/10.1061/(ASCE)0899-1561(2009)21:11(694) |
[37] | Bavaresco L , Vezzulli S , Civardi S , Gatti M , Battilani P , Pietri A , Ferrari F . Effect of lime-induced leaf chlorosis on ochratoxin A, trans-resveratrol, and ∊-viniferin production in grapevine (Vitis vinifera L. ) berries infected by Aspergillus carbonarius. J Agric Food Chem. (2008) ;56: (6):2085–89. doi: 10.1021/jf073456+ |
[38] | Wang L , Peng AC , Proctor A . Varietal difference in lipid content and fatty acid composition of highbush blueberries. J Am Oil Chem Soc. (1990) ;67: (8):499–02. doi: 10.1007/BF02540755 |
[39] | Yang B , Kallio HP . Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J Agric Food Chem. (2001) ;49: (4):1939–47. doi: 10.1021/jf001059s |
[40] | Thoss V , Murphy PJ , Marriott R , Wilson T . Triacylglycerol composition of British bluebell (Hyacinthoides non-scripta) seed oil. RSC Adv. (2012) ;2: :5314–22. doi: 10.1039/C2RA20090B |




