Pharmacologic and Nutritional Interventions for Early Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials
Abstract
Background:
Early intervention is essential for meaningful disease modification in Alzheimer’s disease (AD).
Objective:
We aimed to determine the efficacy and safety of pharmacologic and nutritional interventions for early AD.
Methods:
PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov were searched from database inception until 1 September 2023. We included randomized controlled trials that evaluated the efficacy of interventions in early AD. Only interventions that demonstrated efficacy compared to placebo were included in the network meta-analysis (NMA). Then we performed frequentist fixed-effects NMA to rank the interventions. GRADE criteria were used to evaluate the level of evidence.
Results:
Fifty-eight trials including a total of 33,864 participants and 48 interventions were eligible for inclusion. Among the 48 interventions analyzed, only 6 (12.5%) treatments— ranging from low to high certainty— showed significant improvement in cognitive decline compared to placebo. High certainty evidence indicated that donanemab (standardized mean difference [SMD] –0.239, 95% confidence interval [CI] –0.343 to –0.134) and lecanemab (SMD –0.194, 95% CI –0.279 to –0.108) moderately slowed the clinical progression in patients with amyloid pathology. Additionally, methylphenidate, donepezil, LipiDiDiet, and aducanumab with low certainty showed significant improvement in cognitive decline compared to placebo. However, there was no significant difference in serious adverse events as reported between the six interventions and placebo.
Conclusions:
Only 12.5% of interventions studied demonstrated efficacy in reducing cognitive impairment in early AD. Donanemab and lecanemab have the potential to moderately slow the clinical progression in patients with amyloid pathology. Further evidence is required for early intervention in AD.
INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia and one of the leading causes of morbidity and mortality in the aging population [1]. Globally, it is estimated that there are approximately 32 million individuals with AD dementia, 69 million individuals with prodromal AD, and 315 million individuals with preclinical AD [2]. The risk of developing dementia, including AD, is closely associated with advancing age [3]. The annual number of new cases of AD and other dementias is projected to double by 2050 in the United States because of the increasing number of people age 65 and older [3]. Considering the epidemiological projections, it has become increasingly urgent to explore and develop innovative therapeutics that have the potential to prevent the onset, delay the progression, or improve the management of AD [4].
Billions of dollars have invested in clinical trials to develop novel therapeutics for AD, and there are currently 187 trials assessing 141 drugs for the treatment of AD [4]. Neurotransmitter receptors, amyloid, synaptic function, inflammation, tau biology, oxidation, and proteostasis/proteinopathy are the major targets of drugs [4]. Since 2003, aducanumab and lecanemab are the only drugs to have received accelerated approval for the treatment of AD, and both are monoclonal antibody targeting β-amyloid protein. However, the superiority of new AD drugs over older drugs remains uncertain [5]. On the other hand, some nutritional interventions may slow down the rate of progression of AD, improving cognitive function, and improving the quality of life of these patients [6, 7]. Protective factors for primary prevention of cognitive impairment, dementia, and AD include regular physical activity, higher education, intellectually stimulating work, engaging in stimulating leisure activities, social engagement, and a rich social network [8].
Slowing the progression of AD is considered one of the most significant unmet medical needs of our time [9]. There is a growing consensus that meaningful disease modification in AD requires early therapeutic intervention, ideally during preclinical or prodromal stages of the disease [9]. Previous trials and evidence have primarily focused on mild to moderate AD, often combining mild and moderate stages together [10–12]. Early AD is typically defined as mild dementia due to AD, mild cognitive impairment (MCI) due to AD, and preclinical stage of the disease [13]. However, the effective and optimal treatment for early AD remains unclear. In this study, we conducted a systematic review and network meta-analysis (NMA) of randomized control trials (RCTs) to evaluate the most up-to-date evidence regarding the efficacy and safety of pharmacologic and nutritional interventions for early AD.
METHODS
Data sources and searches
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [14] and the PRISMA statement for NMA [15], and the protocol was registered on international prospective register for systematic reviews (PROSPERO, CRD42023471551). We searched for literature published on PubMed, Embase, Cochrane Library, and grey literature from the ClinicalTrials.gov website from database inception through 1 September 2023. Keywords including “Alzheimer’s disease”, “mild cognitive impairment”, and “randomized control trial” were used to search, and the detailed search strategy was shown in the Supplementary Material. Additionally, we identified references by searching the reference lists of included studies and relevant reviews.
Study selection
We included RCTs evaluating the efficacy of pharmacologic or nutritional interventions in patients with early AD. Early AD was defined as MCI due to AD [16], mild dementia due to AD (or mild AD) [17], the preclinical or asymptomatic stage of AD, or amnestic MCI. Amnestic MCI was included because it has been defined as a precursor to AD [18]. We excluded studies with a follow-up duration of less than 12 weeks and a sample size of less than 50 participants. Additionally, trials that did not report cognitive performance were excluded from our review. We also excluded study protocols, editorials, comments, reviews, conference abstracts, ongoing trials, and studies without sufficient data for analysis. There were no language restrictions for the trials included in our review. The primary outcome was the change in the cognitive scores. Secondary outcomes included any adverse events (AEs), serious adverse events (SAEs), and death.
Data extraction and quality assessment
Two authors reviewed titles and abstracts independently to identify eligible studies that met pre-specified inclusion criteria and extracted data. When consensus was lacking, a third reviewer was consulted. Study characteristics (e.g., journal and year of publication, study design and registration, sample size, and length of follow-up), intervention and comparator characteristics, participant characteristics (e.g., age and sex), and outcomes were extracted. There are various assessment scales and tools used to evaluate AD or MCI, including the Mini-Mental State Examination (MMSE), Clinical Dementia Rating-Sum of Boxes (CDR-SB), and Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). If the primary outcome of a trial was the change in cognitive scores or a combination of cognitive and functional scores, we extracted the primary outcome in our analysis. In cases where change in cognitive scores was secondary outcome, we extracted the relevant data from one of the three scales (CDR-SB, ADAS-Cog, or MMSE). The risk of bias of RCTs was assessed by the Cochrane Collaboration’s tool (RoB 2) [19], which includes the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The quality of evidence for primary outcome in the pairwise meta-analyses was evaluated with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [20]. Two reviewers (BZ and CT) independently assessed the risk of bias and the quality of the evidence. Discrepancies were resolved by discussion.
Data synthesis and analysis
Initially, we performed traditional pairwise meta-analyses for all direct comparisons with at least two studies available. Continuous data (primary outcome) were measured using different rating scales; therefore, we converted outcomes to standardized mean differences (SMD) and 95% confidence intervals (CIs). Different scales used to measure cognitive decline may have varying interpretations. For instance, in the case of MMSE, higher scores indicate better performance, whereas in CDR-SB, lower scores indicate better outcomes. Therefore, when the results favored the intervention, we transformed the SMD to a defined negative value. For dichotomous data (secondary outcomes), we analyzed the outcomes as relative risks (RRs) with 95% CIs. Statistical heterogeneity between the studies was assessed with the χ2 test and the I2 statistics. I2 values of 25%, 50%, and 75% have been suggested to be indicators of low, moderate, and high heterogeneity, respectively [21]. Random effects pairwise meta-analyses were primarily used to synthesize the results. If the I2 values were less than 50%, fixed effects meta-analyses were also conducted to identify potential effective interventions. We then performed a frequentist NMA [22]. There were a limited number of trials available for each intervention and the overall lack of efficacy observed in many interventions compared to placebo, or even their inferiority to placebo. Therefore, only interventions that demonstrated efficacy compared to placebo or showed no inferiority when compared to other interventions with efficacy were included in the NMA. Furthermore, trials rated as high risk by RoB 2 were excluded from the NMA. We performed NMA using a fixed-effects model because heterogeneity estimation in random-effects in the sparse network can generate unreliable results [23]. We used forest plots and league tables of the effects to visualize comparisons of network estimations. We ranked the probability of treatments being the best— associated with increasing efficacy and decreasing SAEs— and ranked each by a surface under the cumulative ranking curve (SUCRA). We used Stata 17.0 for all analyses. We used the metan command for the pairwise meta-analyses, and the network package and network graphs package for the NMA.
RESULTS
Characteristics of included studies
This systematic literature search initially identified 26114 records, after excluding duplicates and irrelevant records, 313 studies were evaluated in full text for eligibility (Fig. 1). Finally, 58 RCTs of 48 pharmacologic or nutritional interventions (including 33,864 patients) were included in the present study (Supplementary Table 1). Among the included studies, 49 were published, and 9 were unpublished data. Three studies were three-arm trials [24–26], and the remaining studies were two-arm trials. Four trials focused on preclinical AD [27–30], 14 trials targeted amnestic MCI, and the remaining 40 trials enrolled participants with MCI due to AD or mild dementia due to AD. The participants enrolled in included trials were 50 years or older. Four trials compared different traditional Chinese medicines (TCMs) with donepezil [31–34], while the remaining trials utilized a placebo as the control group. The follow-up periods ranged from 3 to 36 months. Nearly all trials (49 out of 58) were previously registered and provided registration numbers. The characteristics of individual studies are summarized in Supplementary Table 1. A qualitative assessment was performed by assessing various indicators for each individual trial using RoB 2. Thirty-two of 58 trials were classified as low risk of bias, 17 trials had some concerns, and 9 trials were classified as high risk of bias. Risk of bias assessments in individual studies, including reasons, are listed in the characteristics of included studies in Supplementary Table 2. The quality of evidence for main outcomes were rated following the GRADE framework (Supplementary Table 3).
Fig. 1
Study selection.
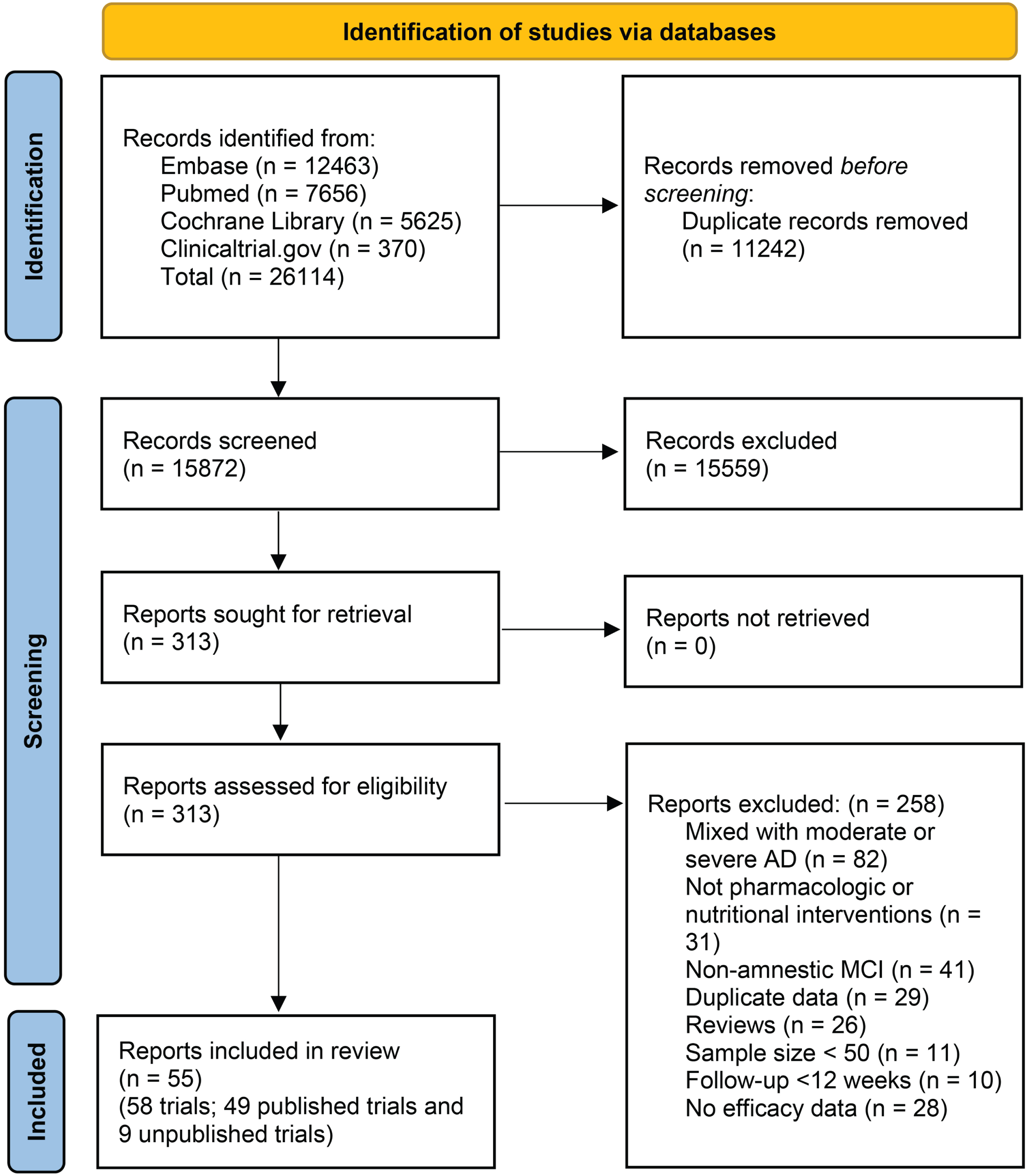
Table 1
Efficacy and safety of pharmacologic and nutritional interventions for early Alzheimer’s disease
| Intervention | Control | N | ROB | Efficacy (SMD with 95% CI) | AE, SAE, and Death (RR with 95% CI, frequency, absolute values) |
| Bushen capsule | Donepezil | 1 | High | –0.698 (–1.219, –0.176), p = 0.009 | AE: 1.75 (0.57, 5.36), 23.3% (7/30) vs. 13.3% (4/30); SAE: NR; Death: NR |
| Chinese herbal medicine | Donepezil | 1 | High | 0.131 (–0.365, 0.626), p = 0.606 | AE: NR; SAE: 0/45 vs. 0/24; Death: 0/45 vs. 0/24 |
| Shenwu capsule | Donepezil | 1 | High | 0.022 (–0.210, 0.254), p = 0.850 | AE: 0.32 (0.23, 0.44), 18.1% (39/216) vs.57.4% (62/108); SAE: NR; Death: NR |
| YishenHuazhuo decoction | Donepezil | 1 | High | –0.403 (–0.733, –0.073), p = 0.017 | AE: 0.83 (0.27, 2.61), 6.9% (5/72) vs. 8.3% (6/72); SAE: NR; Death: NR |
| Aducanumab | Placebo | 2 | Low | Random: –0.078 (–0.173, 0.018), p = 0.111, I2 = 42.1%; Fixed: –0.078 (–0.150, –0.005), p = 0.036 | AE:1.12 (1.02, 1.23), I2 = 42.4%, 56.1% (1234/2198) vs. 20.2% (546/1087); SAE: 0.98 (0.82, 1.18), I2 = 0%, 13.6% (300/2198) vs. 13.9% (151/1087); Death: 1 trial, 1.10 (0.39, 3.16), 0.9% (11/1191) vs. 0.9 (5/547). |
| Atabecestat | Placebo | 1 | Some concern | 0.413 (0.124, 0.702), p = 0.005 | AE: 1.10 (0.98, 1.24), 73.1% (272/372) vs. 66.5% (123/185); SAE: 1.99 (0.94, 4.23), 8.6% (32/372) vs. 4.3% (8/185); Death: 0/372 vs. 0/185 |
| Avagacestat | Placebo | 1 | Low | 0.073 (–0.223, 0.369), p = 0.628 | AE:1.14 (1.05, 1.24), 95.5% (126/132) vs. 84.0% (110/131); SAE: 1.57 (1.07, 2.29),37.1% (49/132) vs. 23.7% (31/131); Death:0/132vs. 0/131 |
| Azeliragon | Placebo | 1 | Some concern | 0.139 (–0.032, 0.310), p = 0.111 | AE: 0.97 (0.90, 1.05), 72.6% (320/441) vs. 74.7% (324/434); SAE: 1.03 (0.76, 1.40), 15.9% (70/441) vs. 15.4% (67/434); Death: 0.79 (0.21, 2.91), 0.9% (4/441) vs. 1.2% (5/434) |
| BI409306 | Placebo | 1 | Some concern | 0.063 (–0.152, 0.278), p = 0.566 | AE: 1.07 (0.87, 1.33), 48.2% (146/303) vs. 45.0% (67/149); SAE: 2.46 (0.29, 20.86), 1.7% (5/303) vs. 0.7% (1/149); Death: 1.48 (0.06, 36.12), 0.3% (1/303) vs. 0.0% (0/149) |
| Benfotiamine | Placebo | 1 | Low | –0.336 (–0.808, 0.137), p = 0.164 | AE: 1.15 (0.84, 1.58), 73.5% (25/34) vs.63.9% (23/36); SAE: 1.06 (0.23, 4.89), 8.8% (3/34) vs. 8.3% (3/36); Death: 0/34 vs.0/36 |
| Benzoate | Placebo | 1 | Some concern | –0.360 (–0.870, 0.151), p = 0.167 | AE: 0.33 (0.01, 7.87), 0.0% (0/30) vs.3.3% (1/30); SAE: NR; Death: NR |
| CAD106 | Placebo | 1 | Some concern | –0.223 (–0.735, 0.289), p = 0.393 | AE: 0.87 (0.75, 1.00), 85.7% (36/42) vs. 100% (21/21); SAE: 0.67 (0.16, 2.71), 9.5% (4/42) vs. 14.3% (3/21); Death: 0/42 vs. 0/21 |
| CNP520 | Placebo | 2 | Some concern | 0.325 (–0.171, 0.822), p = 0.199, I2 = 91.2% | AE: 1 trial, 1.48 (1.19, 1.85), 100% (106/106) vs. 100% (76/76); SAE: 0.98 (0.58, 1.67), I2 = 0%, 7.5% (8/106) vs. 9.2% (7/76); Death: 0/106 vs. 0/76 |
| Copper | Placebo | 1 | High | 0.500 (–0.028, 1.028), p = 0.063 | AE: NR; SAE: 0/29 vs. 0/28; Death: 0/29 vs. 0/28 |
| Crenezumab | Placebo | 2 | Low | –0.257 (–1.044, 0.530), p = 0.522, I2 = 72.6% | AE: 1.02 (0.98, 1.07), I2 = 0%, 79.7% (644/808) vs. 78.2% (628/803); SAE: 0.94 (0.69, 1.28), I2 = 27.0%, 12.4% (100/808) vs. 13.1% (105/803); Death: 0.47 (0.03, 8.84), I2 = 73.5%, 1.0% (8/808) vs. 1.4% (11/803) |
| Davunetide | Placebo | 1 | Low | –0.135 (–0.505, 0.236), p = 0.476 | AE: 1.07 (0.77, 1.49), 54.7% (52/95) vs. 51.0% (25/49); SAE: 0.26 (0.05, 1.36), 2.1% (2/95) vs. 8.2% (4/49); Death: 0/95 vs. 0/49 |
| Donanemab | Placebo | 2 | Low | –0.239 (–0.343, –0.134), p < 0.001, I2 = 0% | AE: 1.05 (0.98, 1.13), I2 = 65.0%, 89.2% (878/984) vs. 83.2% (831/999); SAE: 1.08 (0.89, 1.32), I2 = 0%, 17.4% (171/984) vs. 16.0% (160/999); Death: 1.45 (0.69, 3.06), I2 = 0%, 1.7% (17/984) vs. 1.2% (12/999); |
| Donepezil (6 months) | Placebo | 3 | Some concern | –0.279 (–0.410, –0.149), p < 0.001, I2 = 0% | AE: 2 trials, 1.18 (1.06, 1.31), I2 = 0%, 80.3% (183/228) vs. 70.6% (137/194); SAE: 2 trials, 0.91 (0.37, 2.23),I2 = 0%, 4.4% (10/228) vs. 4.6% (9/194); Death: 1 trial, 1.43 (0.46, 4.46), 3.1% (7/228) vs. 2.6% (5/194) |
| Donepezil (3 years) | Placebo | 1 | Some concern | –0.017 (–0.190, 0.156), p = 0.846 | AE: NR; SAE: NR; Death: NR |
| Elenbecestat | Placebo | 1 | Some concern | –0.038 (–0.123, 0.047), p = 0.377 | AE: 1.35 (1.19, 1.53), 35.6% (391/1099) vs. 26.4% (292/1105); SAE: 1.15 (0.91, 1.45), 12.2% (134/1099) vs.10.6% (117/1105);Death: 0.50 (0.13, 2.01), 0.3% (3/1099) vs.0.5% (6/1105) |
| Gantenerumab | Placebo | 2 | Low | 0.060 (–0.078, 0.199), p = 0.394, I2 = 0% | AE: 1 trial; 0.96 (0.93, 1.01), 91.4% (533/583) vs. 95.5% (339/355); SAE: 1.12 (0.58, 2.16), I2 = 66.8%, 18.2% (106/583) vs. 18.9% (67/355); Death: 1 trial, 0.17 (0.03, 0.82), 0.3% (2/583) vs. 1.7% (6/355) |
| Ginkgo biloba extract | Placebo | 2 | High | –0.765 (–1.427, –0.103), p = 0.024, I2 = 86.1% | AE: 1 trial, 0.97 (0.68, 1.38), 41.3% (43/104) vs. 42.9% (30/70); SAE: 1 trial, 6.09 (0.33, 111.29), 3.8% (4/104) vs. 0.0% (0/70); Death: NR |
| Hydroxychloroquine | Placebo | 1 | Some concern | –0.063 (–0.365, 0.240), p = 0.683 | AE: 1.37 (0.75, 2.48), 24.1% (20/83) vs. 17.6% (15/85); SAE: NR; Death: 2.56 (0.51, 12.83), 6.0% (5/83) vs. 2.4% (2/85) |
| IVIG | Placebo | 1 | Low | 0.191 (–0.370, 0.753), p = 0.504 | AE: 0.52 (0.05, 5.38), 4.2% (1/24) vs. 8.0% (2/25); SAE: 0/24 vs. 2/25; Death: 0.21 (0.01, 4.12), 0.0% (0/24) vs. 8.0% (2/25) |
| Insulin | Placebo | 1 | Low | 0.004 (–0.263, 0.271), p = 0.977 | AE: 1.06 (0.93, 1.21), 81.0% (98/121) vs. 76.5% (91/119); SAE: 1.77 (0.85, 3.68), 14.9% (18/121) vs. 8.4% (10/119); Death: 2.95 (0.12, 71.72), 0.8% (1/121) vs. 0.0% (0/119) |
| LY3202626 | Placebo | 1 | Some concern | –0.496 (–1.109, 0.117), p = 0.113 | AE: 1.15 (1.00, 1.32), 78.6% (143/182) vs. 68.4% (91/133); SAE: 1.32 (0.63, 2.76), 9.9% (18/182) vs. 7.5% (10/133);Death: 0/182 vs. 0/133 |
| LY3303560 | Placebo | 1 | Some concern | 0.149 (–0.138, 0.437), p = 0.308 | AE: 1.11 (0.91, 1.36), 57.4% (139/242) vs. 51.7% (61/118); SAE: 1.43 (0.81, 2.51), 17.0% (41/242) vs. 11.9% (14/118); Death: 0.98 (0.18, 5.25), 1.7% (4/242) vs. 1.7% (2/118) |
| Lanabecestat | Placebo | 2 | Low | 0.069 (–0.020, 0.159), p = 0.129, I2 = 0% | AE: 1.05 (1.01, 1.08),I2 = 0%, 77.0% (2023/2627) vs. 73.5% (952/1296); SAE: 1.10 (0.84, 1.44), I2 = 50.6%, 13.7% (360/2627) vs. 12.2% (158/1296); Death: 0.91 (0.23, 3.64), I2 = 49.7%, 0.5% (13/2627) vs. 0.5% (7/1296) |
| Lecanemab | Placebo | 2 | Low | –0.194 (–0.279, –0.108), p < 0.001, I2 = 0% | AE: 1.04 (0.94, 1.15), I2 = 81.6%, 88.5% (937/1059) vs. 83.3% (951/1142); SAE: 1.11 (0.81, 1.52), I2 = 41.6%, 14.3% (151/1059) vs. 8.8% (101/1142); Death: 0.76 (0.27, 2.12), I2 = 0%, 0.6% (6/1059) vs. 0.8% (9/1142) |
| Lithium | Placebo | 1 | Some concern | –0.125 (–0.669, 0.420), p = 0.653 | AE: NR; SAE: NR; Death: NR |
| LipiDiDiet | Placebo | 1 | Low | –0.365 (–0.589, –0.140), p = 0.001 | AE: 0.99 (0.91, 1.08), 86.8% (132/152) vs. 87.9% (138/157); SAE: 1.17 (0.76, 1.81), 22.4% (34/152) vs. 19.1% (30/157); Death: 4.13 (0.47, 36.55), 2.6% (4/152) vs. 0.6% (1/157) |
| Metformin | Placebo | 1 | Low | 0.220 (–0.220, 0.659), p = 0.328 | AE: 7.00 (0.37, 131.28), 7.5% (3/40) vs. 0.0% (0/40); SAE: 0/40 vs. 0/40; Death: 0/40 vs. 0/40 |
| Methylphenidate | Placebo | 1 | Some concern | –0.938 (–1.477, –0.399), p = 0.001 | AE: 1.40 (0.71, 2.76), 43.3% (13/30) vs. 31.0% (9/29);SAE: 4.83 (0.60, 38.90), 16.7% (5/30) vs. 3.4% (1/29);Death: NR |
| Minocycline | Placebo | 1 | Low | –0.025 (–0.230, 0.180), p = 0.808 | AE: NR; SAE: NR; Death: 0.65 (0.32, 1.35), 4.4% (16/365) vs. 6.7% (12/179) |
| Neflamapimod | Placebo | 1 | Low | 0.042 (–0.268, 0.352), p = 0.792 | AE: 1.06 (0.58, 1.92), 21.7% (18/83) vs. 20.5% (16/78); SAE: 0.63 (0.11, 3.65), 2.4% (2/83) vs. 3.8% (3/78); Death: 0/83 vs. 0/78 |
| PBT2 | Placebo | 1 | Low | –0.146 (–0.606, 0.313), p = 0.533 | AE: 1.18 (0.76, 1.85), 57.1% (28/49) vs. 48.3% (14/29);SAE: 0.12 (0.01, 2.42), 0.0% (0/49) vs. 6.9% (2/29);Death: 0/49 vs. 0/29 |
| Plasmalogen | Placebo | 1 | Low | –0.031 (–0.267, 0.205), p = 0.798 | AE: 1.07 (0.72, 1.61), 22.5% (38/169) vs. 21.0% (35/167); SAE: 0/169 vs. 0/167; Death: 0/169 vs. 0/167 |
| Qinggongshoutao | Placebo | 1 | High | –1.177 (–1.474, –0.881), p < 0.001 | AE: 1.17 (0.86, 1.59), 50.0% (87/174) vs. 42.9% (30/70); SAE: 2.03 (0.10, 41.73), 1.1% (2/174) vs. 0.0% (0/70); Death: NR |
| Renew NCP-5 | Placebo | 1 | Some concern | –0.279 (–0.565, 0.007), p = 0.056 | AE: 1.24 (0.86, 1.80), 41.8% (41/98) vs. 33.7% (31/92);SAE: 0.83 (0.34, 2.07), 8.2% (8/98) vs. 9.8% (9/92);Death: 0.31 (0.01, 7.59), 0.0% (0/98) vs.1.1% (1/92) |
| Saracatinib | Placebo | 1 | Low | 0.151 (–0.197, 0.499), p = 0.395 | AE: 1.14 (1.01, 1.29), 92.4% (73/79) vs. 81.2% (65/80); SAE: 1.74 (0.72, 4.18), 15.2% (12/79) vs. 8.8% (7/80); Death: 3.04 (0.13, 73.46), 1.3% (1/79) vs. 0.0% (0/80) |
| Semorinemab | Placebo | 1 | Low | 0.071 (–0.138, 0.279), p = 0.507 | AE: 0.99 (0.93, 1.05), 92.0% (286/311) vs. 93.1% (121/130); SAE: 1.49 (0.86, 2.60), 16.1% (50/311) vs. 10.8% (14/130); Death: 0.42 (0.06, 2.94), 0.6% (2/311) vs. 1.5% (2/130) |
| Solanezumab | Placebo | 3 | Low | 0.033 (–0.127, 0.194), p = 0.684, I2 = 64.9% | AE: 2 trials; 1.01 (0.99, 1.02), I2 = 0%, 89.6% (1503/1678) vs. 89.1% (1556/1747);SAE: 1.07 (0.81, 1.41), I2 = 69.0%, 21.5% (360/1678) vs. 21.3% (372/1747);Death: 2 trials; 0.64 (0.34, 1.22), I2 = 0%, 0.9% (15/1678) vs. 1.4% (24/1747) |
| Souvenaid | Placebo | 2 | Low | –0.100 (–0.320, 0.120), p = 0.371, I2 = 21.5% | AE: 0.99 (0.73, 1.35), I2 = 67.0%, 51.7% (125/242) vs. 52.7% (127/241); SAE: 1.01 (0.39, 2.61), I2 = 50.5%, 7.0% (17/242) vs. 7.0% (17/241); Death: 0/242 vs. 0/241 |
| Tarenflurbil | Placebo | 1 | Low | 0.019 (–0.081, 0.119), p = 0.708 | AE: 1.03 (0.99, 1.07), 87.2% (750/860) vs. 85.0% (698/821); SAE: 1.14 (0.95, 1.37), 22.7% (195/860) vs. 19.9% (163/821); Death: 1.27 (0.70, 2.33), 2.8% (24/860) vs. 2.2% (18/821) |
| Tilavonemab | Placebo | 1 | Low | –0.067 (–0.312, 0.179), p = 0.594 | AE: 1.01 (0.95, 1.06), 93.6% (306/327) vs. 93.1% (108/116); SAE: 0.79 (0.53, 1.19), 17.7% (58/327) vs. 22.4% (26/116); Death: 1.77 (0.21, 15.02), 1.5% (5/327) vs. 0.9% (1/116) |
| Triflusal | Placebo | 1 | High | –0.164 (–0.409, 0.081), p = 0.190 | AE: NR; SAE: 0/158 vs. 0/156; Death: 0/158 vs. 0/156 |
| Verubecestat | Placebo | 1 | Low | 0.135 (–0.023, 0.293), p = 0.094 | AE: 1.06 (1.01, 1.10), 91.7% (887/967) vs. 87.0% (421/484); SAE: 5.12 (3.35, 7.82), 23.3% (225/967) vs. 4.5% (22/484); Death: 0.67 (0.15, 2.97), 0.4% (4/967) vs. 0.6% (3/484) |
| Vitamin E | Placebo | 1 | Some concern | 0.013 (–0.160, 0.185), p = 0.886 | AE: NR; SAE: NR; Death: 1.01 (0.26, 3.44), 5/NR vs. 5/NR |
| Xanamem | Placebo | 1 | Some concern | 0.038 (–0.256, 0.332), p = 0.799 | AE: 1.03 (0.70, 1.53), 35.1% (33/94) vs. 34.0% (32/94); SAE: 1.00(0.26, 3.89), 4.3% (4/94) vs. 4.3% (4/94); Death: 0/94 vs. 0/94 |
ROB, risk of bias; N, number of trials; SMD, standardized mean difference; RR, relative risk; CI, confidence interval; AE, adverse event; SAE, serious adverse event; NR, not reported; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation.
Pairwise meta-analysis
A total of 44 interventions were directly compared to placebo, with a limited number of trials available for each intervention (Table 1). Specifically, there were three trials each for donepezil [24, 35, 36] and solanezumab [25, 27, 37], two trials each for aducanumab [38], CNP520 [29, 30], crenezumab [39], donanemab [40, 41], gantenerumab [25, 42], ginkgo biloba extract [26, 43], lanabecestat [44], lecanemab [45, 46], and souvenaid [47, 48], and only one trial for each of the remaining interventions. Comparative analysis against placebo revealed significant efficacy for nine interventions. These included aducanumab (2 trials, fixed-effect, SMD –0.078, 95% CI –0.150 to –0.005, p = 0.036, I2 = 42.1%; low certainty), donanemab (2 trials, SMD –0.239, 95% CI –0.343 to –0.134, p < 0.001, I2 = 0%; high certainty), donepezil (3 trials, 6 months, SMD –0.279, 95% CI –0.410 to –0.149, p = 0.036, I2 = 42.1%; low certainty), ginkgo biloba extract (2 trials, SMD –0.765, 95% CI –1.427 to –0.103, p = 0.024, I2 = 86.1%; very low certainty), lecanemab (2 trials, SMD –0.194, 95% CI –0.279 to –0.108, p < 0.001, I2 = 0%; high certainty), LipiDiDiet (1 trial[49], SMD –0.365, 95% CI –0.589 to –0.140, p = 0.001; low certainty), methylphenidate (1 trial[50], SMD –0.938, 95% CI –1.477 to –0.399, p = 0.001; low certainty), and qinggongshoutao (1 trial[26], SMD –1.177, 95% CI –1.474 to –0.881, p < 0.001; very low certainty). The trials evaluating ginkgo biloba extract and qinggongshoutao were identified as having a high risk of bias and were therefore excluded from the NMA. Four trials were conducted to evaluate the efficacy of four different TCMs compared to donepezil (Table 1) [31–34]. Among these trials, two showed significant efficacy: Bushen capsule (1 trial [33], SMD –0.698, 95% CI –1.219 to –0.176, p = 0.017; very low certainty) and Yishen Huazhuo decoction (1 trial [32], SMD –0.403, 95% CI –0.733 to –0.073, p = 0.009; very low certainty), and there was no significant difference in two other trials [31, 34]. All four TCM trials were rated as high risk of bias, which were also excluded from the NMA.
Network meta-analysis
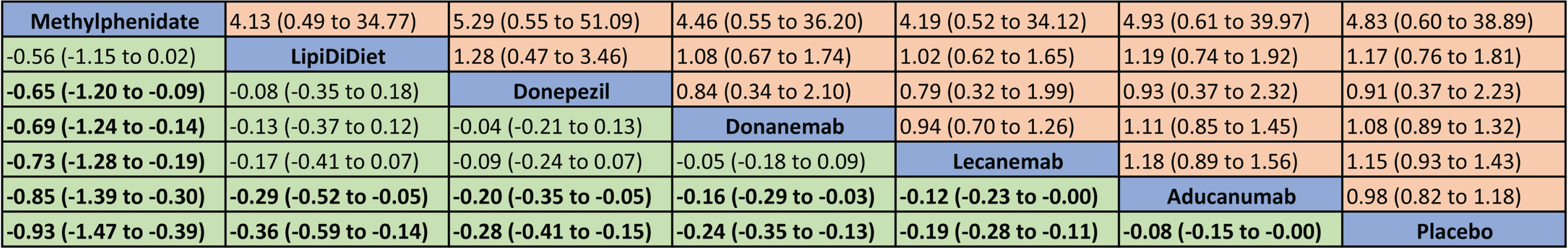
Eleven trials (6 treatments and placebo) including 8137 patients reported the change in the cognitive scores were included in the NMA (Fig. 2) [24, 25, 35, 36, 38, 40, 41, 45, 46, 49, 50]. Treatment ranking probabilities suggested that methylphenidate had the highest probability of being the best treatment, followed by LipiDiDiet, donepezil, donanemab, lecanemab, aducanumab, and placebo (Fig. 3 and Supplementary Figure 1). In the NMA, we found that the top five interventions demonstrated significant cognitive benefit over aducanumab. Ten trials involving 8259 patients reported SAEs. The treatment ranking probabilities indicated that aducanumab was the safest treatment, while methylphenidate had the highest risk (Supplementary Table 4 and Fig. 2). However, no significant difference in SAEs was observed among the seven treatments (Fig. 3).
Fig. 2
Network plot of studies included in network meta-analysis. (Each node indicates a treatment and is sized proportionally to number of trial participants. Each line connecting two nodes indicates a direct comparison between two treatments, and the thickness of each line is proportional to the number of trials directly comparing the two treatments.)
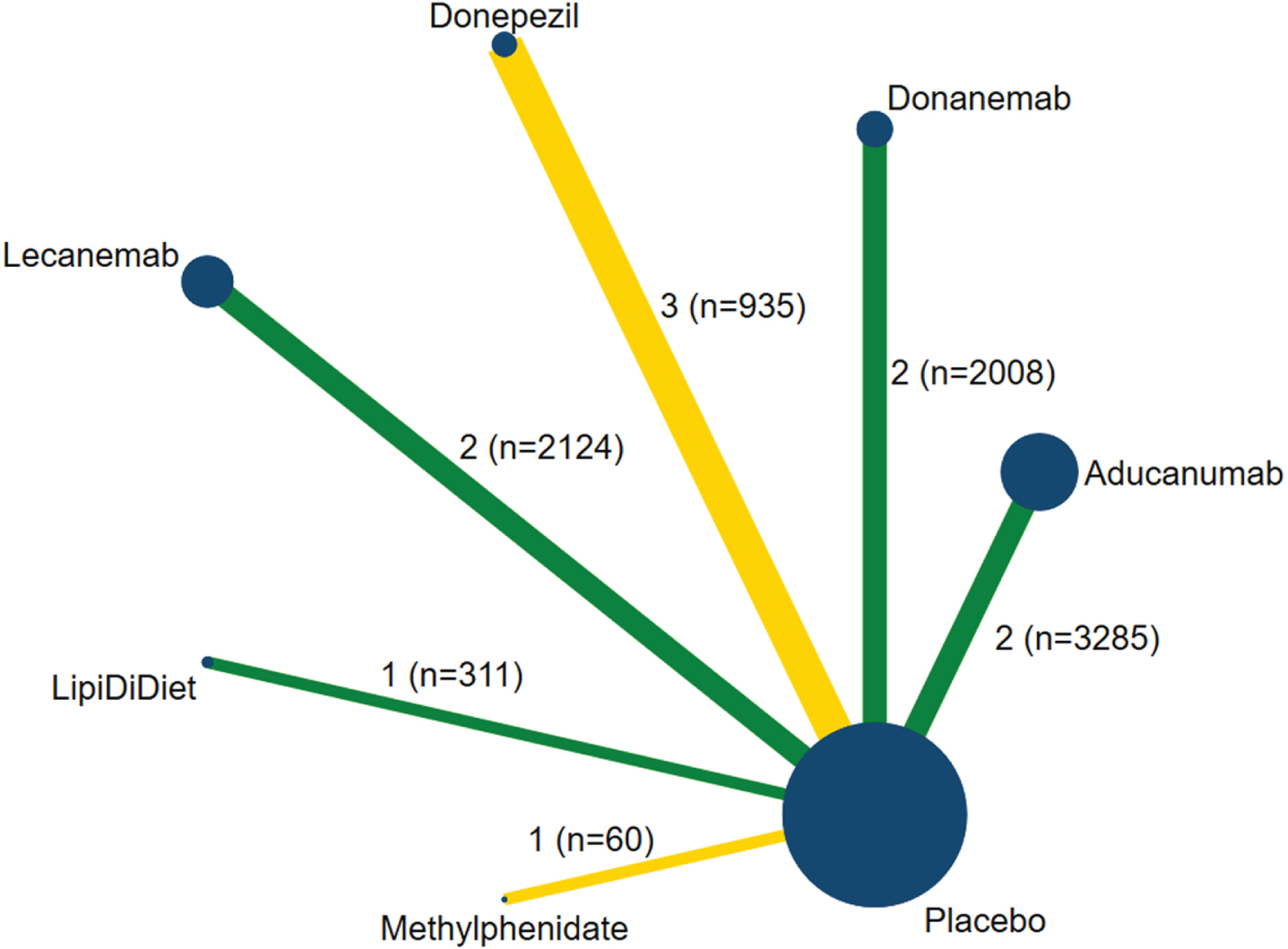
Fig. 3
League tables of the change in the cognitive scores (lower left with green color, standardized mean difference with 95% confidence intervals) and serious adverse events (upper right with yellow color, relative risk with 95% confidence intervals).
DISCUSSION
This systematic review and NMA included 58 RCTs of 48 interventions involving 33,864 older participants with early AD. The primary objective was to provide a comprehensive overview of the efficacy and safety of pharmacologic and nutritional interventions for early AD. Among the 48 interventions analyzed, only six treatments— ranging from low to high certainty— showed significant improvement in cognitive decline. High certainty evidence indicated that donanemab and lecanemab have the potential to moderately slow the clinical progression of early AD when compared to placebo. Additionally, methylphenidate was found to significantly improve cognition, while LipiDiDiet and donepezil moderately reduced cognitive decline, and aducanumab only slightly slowed clinical progression. However, the certainty of results for these four interventions was low. Furthermore, the analysis revealed no significant difference in the occurrence of SAEs between the six effective treatments and placebo. It is worth mentioning that certain TCMs showed moderate or large effects on efficacy; however, the risk of bias associated with these interventions was high.
To the best of our knowledge, our study represents the first comprehensive systematic review and NMA that aims to assess the efficacy and safety of pharmacologic and nutritional interventions for early AD. A previous review published in 2018 concluded that there was insufficient evidence to support the use of studied pharmacologic treatments for cognitive protection in individuals with normal cognition or MCI [51]. However, the trials of newer drugs such as aducanumab, donanemab, and lecanemab have been published in more recent years. A recent NMA, which included only four trials, reported that lithium demonstrated significantly greater efficacy than aducanumab in individuals with MCI or AD [52]. However, lithium did not show efficacy compared to placebo in our review of early AD. Additionally, there was another trial that was not included in our study due to its short 10-week follow-up period, and it did not support the use of lithium treatment in mild AD [53]. Contrary to a previous meta-analysis suggesting positive effects of intranasal insulin on cognition in patients with AD or MCI [54], our study, which included one trial with low risk of bias, revealed no cognitive or functional benefits from intranasal insulin treatment over a 12-month period [55]. Furthermore, our study is consistent with another NMA, as it found that donepezil demonstrated cognitive benefits while memantine did not show cognitive benefits for individuals with mild-to-moderate AD [56].
The minimum clinically important difference (MCID) is fundamental for interpreting clinical trial results, guiding clinical decisions, and designing studies with adequate statistical power to detect meaningful effects. Nevertheless, a consensus or agreement on the MCID for outcomes in AD trials is still lacking [57, 58]. Recent trials of donanemab and lecanemab have shown that the primary outcome met the MCID criteria when assessing change from baseline using a clinical outcome score. However, the drug-placebo difference did not reach the MCID threshold of 0.98 for MCI-AD and 1.63 for mild AD in CDR-SB score as established by Andrews et al [59]. Furthermore, we did not retrieve any MCID information regarding standardized mean differences for AD or dementia.
Although aducanumab became the first approved monoclonal antibody for AD, its efficacy remains a subject of debate [60]. EMERGE and ENGAGE were two phase 3 clinical trials of aducanumab in patients with early AD [38]. The ENGAGE trial did not meet its primary and secondary endpoints. However, in the EMERGE trial, which had the same design as ENGAGE, a statistically significant slowing of clinical decline was observed in the high-dose arm of the study. The findings from the low-dose groups in both trials showed similar magnitude and a numerical preference for aducanumab. In our analysis, we combined the results of both groups and pooled the findings from the two trials. While the random-effect result did not show statistical significance, the fixed-effect meta-analysis indicated significant efficacy (p = 0.036, I2 = 42.1%), although the effect size was small (SMD = –0.078).
Noted that three new drugs, namely aducanumab, donanemab, and lecanemab, are all anti-amyloid monoclonal antibodies that target the underlying biology of AD. These drugs function by eliminating amyloid-β from the brain and mitigating cognitive and functional deterioration in individuals with early AD who exhibit evidence of amyloid-β accumulation in the brain through brain imaging or cerebrospinal fluid analysis [61]. It is crucial to have treatments that address the full scope of Alzheimer’s biology, rather than solely targeting amyloid-β [61]. However, not all anti-amyloid monoclonal antibody treatments have shown favorable effects, such as gantenerumab, solanezumab, and crenezumab. It is worth noting that new drugs designed to lower amyloid levels can lead to amyloid-related imaging abnormalities with edema (ARIA-E) and hemosiderin deposition (ARIA-H). Lecanemab had a 12.6% occurrence of ARIA-E and 17.3% for ARIA-H. Donanemab had rates of 24.0% for ARIA-E and 19.7% for ARIA-H. Aducanumab showed 30.7% for ARIA-E and 17.8% for ARIA-H microhemorrhage. However, in the control groups, the rates of ARIA-E were below 3% and ARIA-H were below 10%. It is important to be cautious when using these medications due to the risk of ARIA.
Only one trial of methylphenidate was included in this meta-analysis [50]. However, the participants in this trial were exclusively men, and the follow-up period was limited to 12 weeks. In the future, it would be beneficial to conduct large-scale trials that encompass a broader range of older adults with early AD and include longer follow-up periods. Noted that while the Petersen study indicated that donepezil was associated with a lower rate of progression to AD in patients with amnestic MCI during the initial 12 months of treatment, this effect was not observed to persist after three years [24]. Therefore, more evidence is needed to evaluate the long-term efficacy of donepezil for early AD.
A total of six TCMs were included in this study. Among them, two treatments demonstrated efficacy when compared to placebo, and two treatments showed efficacy compared to donepezil. There was no significant difference observed between the other two treatments and donepezil. However, the risk of bias in these trials was rated as high. Some research has suggested that traditional Chinese herbs may have the potential to retard amyloid-β deposits and tauopathy, as well as regulate the metabolism of cholinergic neurotransmitters, among other effects [62]. However, due to the complexity of TCMs, there is limited knowledge regarding their safety and efficacy in patients with cognitive impairment [62]. Therefore, it is crucial to conduct well-designed, rigorous, large-scale trials to thoroughly investigate the efficacy and safety of TCMs in AD and MCI.
This review has several strengths. Firstly, the systematic review and NMA followed the PRISMA guidelines and had a protocol registered in PROSPERO. Secondly, risk of bias for included trials was assessed using a valid methodological tool, and the quality of evidence for primary outcome was evaluated using GRADE. Thirdly, we have performed sufficient analysis in the study. A narrative descriptive synthesis and pairwise meta-analysis were conducted for all included interventions to provide a comprehensive overview of their characteristics, efficacy, and safety. Moreover, the NMA was conducted to rank the interventions based on their efficacy or safety.
We acknowledge some limitations. First, there were variations in the characteristics of the patients enrolled in the trials. The definition of early AD varied among studies, including patients with MCI or mild dementia due to AD, preclinical AD, or amnestic MCI. Furthermore, some trials investigating monoclonal antibodies specifically included patients with amyloid pathology confirmed. These differences in patients may introduce heterogeneity and impact the generalizability of the findings. Second, there is a lack of uniformity in the scales used to measure cognitive function across the included trials. For example, the integrated Alzheimer’s Disease Rating Scale (iADRS) is a composite scale that assesses both cognitive and functional domains, while the CDR-SB focuses solely on cognitive function. Additionally, while the change in cognitive scores was reported as the primary outcome in most trials, in some trials it was considered as a secondary outcome. Third, the NMA conducted in this study may be limited due to sparsity in the data. This is because all the trials included in the NMA were directly compared to placebo, and the relative effects between interventions were derived from indirect comparisons. Furthermore, the NMA was performed using a fixed-effect model, which may not provide robust results due to the limited number of trials available for each intervention. Finally, the trials evaluating TCMs were rated as having a high risk of bias. As a result, the efficacy and safety of TCMs in addressing early AD remain uncertain. Further studies are needed to fill this knowledge gap and provide more conclusive evidence.
In conclusion, this systematic review and NMA revealed that only a small portion of the pharmacologic and nutritional interventions studied demonstrated efficacy in reducing cognitive impairment in early AD. High certainty evidence showed donanemab and lecanemab have the potential to moderately slow the clinical progression in patients with amyloid pathology. Methylphenidate significantly improved cognition, LipiDiDiet and donepezil moderately reduced cognitive decline, and aducanumab slightly slowed clinical progression, but the certainty of results for these interventions was low. Further evidence is required to establish the efficacy of methylphenidate, donepezil, LipiDiDiet, and aducanumab in improving cognitive outcomes in early AD.
AUTHOR CONTRIBUTIONS
Baoqi Zeng (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing –original draft; Writing –review & editing); Chunbian Tang (Data curation; Formal analysis; Investigation; Software; Validation; Writing –original draft; Writing –review & editing); Junjian Wang (Investigation; Resources; Validation; Writing –review & editing); Qingqing Yang (Formal analysis; Methodology; Software; Validation; Writing –review & editing); Qingcuo Ren (Resources; Validation; Visualization; Writing –review & editing); Xiaozhi Liu (Conceptualization; Funding acquisition; Project administration; Resources; Writing –review & editing).
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by the Tianjin Science and Technology Plan Project (Grant No. 22ZYQYSY00030), Tianjin Health Technology Project (Grant No. TJWJ2022XK043), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-062B).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-240161.
REFERENCES
[1] | WHO. Dementia., https://www.who.int/news-room/fact-sheets/detail/dementia, Last updated March 15, 2023, Accessed on March 22, 2024. |
[2] | Gustavsson A , Norton N , Fast T , Frolich L , Georges J , Holzapfel D , Kirabali T , Krolak-Salmon P , Rossini PM , Ferretti MT , Lanman L , Chadha AS , van der Flier WM ((2023) ) Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement 19: , 658–670. |
[3] | ((2022) ) 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 18: , 700–789. |
[4] | Cummings J , Zhou Y , Lee G , Zhong K , Fonseca J , Cheng F ((2023) ) Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement (N Y) 9: , e12385. |
[5] | Molchan S , Fugh-Berman A ((2023) ) Are new Alzheimer drugs better than older drugs? JAMA Intern Med 183: , 902–903. |
[6] | Xu Lou I , Ali K , Chen Q ((2023) ) Effect of nutrition in Alzheimer’s disease: A systematic review. Front Neurosci 17: , 1147177. |
[7] | Cesari M , Azzolino D , Arosio B , Canevelli M ((2021) ) Nutritional interventions for early dementia. J Nutr Health Aging 25: , 688–691. |
[8] | Rosenberg A , Mangialasche F , Ngandu T , Solomon A , Kivipelto M ((2020) ) Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis 7: , 29–36. |
[9] | Aisen PS , Jimenez-Maggiora GA , Rafii MS , Walter S , Raman R ((2022) ) Early-stage Alzheimer disease: Getting trial-ready. Nat Rev Neurol 18: , 389–399. |
[10] | Raina P , Santaguida P , Ismaila A , Patterson C , Cowan D , Levine M , Booker L , Oremus M ((2008) ) Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med 148: , 379–397. |
[11] | Birks JS , Chong LY , Grimley Evans J ((2015) ) Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev 9: , CD001191. |
[12] | Trinh NH , Hoblyn J , Mohanty S , Yaffe K ((2003) ) Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: A meta-analysis. JAMA 289: , 210–216. |
[13] | Porsteinsson AP , Isaacson RS , Knox S , Sabbagh MN , Rubino I ((2021) ) Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. J Prev Alzheimers Dis 8: , 371–386. |
[14] | Moher D , Liberati A , Tetzlaff J , Altman DG ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339: , b2535. |
[15] | Hutton B , Salanti G , Caldwell DM , Chaimani A , Schmid CH , Cameron C , Ioannidis JP , Straus S , Thorlund K , Jansen JP , Mulrow C , Catalá-López F , Gøtzsche PC , Dickersin K , Boutron I , Altman DG , Moher D ((2015) ) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med 162: , 777–784. |
[16] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[17] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr. , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 263–269. |
[18] | Griffith HR , Netson KL , Harrell LE , Zamrini EY , Brockington JC , Marson DC ((2006) ) Amnestic mild cognitive impairment: Diagnostic outcomes and clinical prediction over a two-year time period. J Int Neuropsychol Soc 12: , 166–175. |
[19] | Sterne JAC , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , Cates CJ , Cheng HY , Corbett MS , Eldridge SM , Emberson JR , Hernán MA , Hopewell S , Hróbjartsson A , Junqueira DR , Jüni P , Kirkham JJ , Lasserson T , Li T , McAleenan A , Reeves BC , Shepperd S , Shrier I , Stewart LA , Tilling K , White IR , Whiting PF , Higgins JPT ((2019) ) RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366: , l4898. |
[20] | Guyatt GH , Oxman AD , Vist GE , Kunz R , Falck-Ytter Y , Alonso-Coello P , Schünemann HJ ((2008) ) GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: , 924–926. |
[21] | von Hippel PT ((2015) ) The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 15: , 35. |
[22] | Rücker G ((2012) ) Network meta-analysis, electrical networks and graph theory. Res Synth Methods 3: , 312–324. |
[23] | Brignardello-Petersen R , Murad MH , Walter SD , McLeod S , Carrasco-Labra A , Rochwerg B , Schünemann HJ , Tomlinson G , Guyatt GH ((2019) ) GRADE approach to rate the certainty from a network meta-analysis: Avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol 105: , 60–67. |
[24] | Petersen RC , Thomas RG , Grundman M , Bennett D , Doody R , Ferris S , Galasko D , Jin S , Kaye J , Levey A , Pfeiffer E , Sano M , van Dyck CH , Thal LJ ((2005) ) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352: , 2379–2388. |
[25] | Salloway S , Farlow M , McDade E , Clifford DB , Wang G , Llibre-Guerra JJ , Hitchcock JM , Mills SL , Santacruz AM , Aschenbrenner AJ , Hassenstab J , Benzinger TLS , Gordon BA , Fagan AM , Coalier KA , Cruchaga C , Goate AA , Perrin RJ , Xiong C , Li Y , Morris JC , Snider BJ , Mummery C , Surti GM , Hannequin D , Wallon D , Berman SB , Lah JJ , Jimenez-Velazquez IZ , Roberson ED , van Dyck CH , Honig LS , Sánchez-Valle R , Brooks WS , Gauthier S , Galasko DR , Masters CL , Brosch JR , Hsiung GR , Jayadev S , Formaglio M , Masellis M , Clarnette R , Pariente J , Dubois B , Pasquier F , Jack CR Jr. , Koeppe R , Snyder PJ , Aisen PS , Thomas RG , Berry SM , Wendelberger BA , Andersen SW , Holdridge KC , Mintun MA , Yaari R , Sims JR , Baudler M , Delmar P , Doody RS , Fontoura P , Giacobino C , Kerchner GA , Bateman RJ ((2021) ) A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med 27: , 1187–1196. |
[26] | Tian J , Shi J , Wei M , Ni J , Fang Z , Gao J , Wang H , Yao H , Zhang J , Li J , Min M , Su L , Sun X , Wang B , Wang B , Yang F , Zou Y , Hu Y , Lin Y , Xu G , Li K , Li L , Zhen H , Xu J , Chen K , Wang Y ((2019) ) Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment: A 52-week randomized controlled trial. Alzheimers Dement (N Y) 5: , 441–449. |
[27] | Sperling RA , Donohue MC , Raman R , Rafii MS , Johnson K , Masters CL , van Dyck CH , Iwatsubo T , Marshall GA , Yaari R , Mancini M , Holdridge KC , Case M , Sims JR , Aisen PS ((2023) ) Trial of solanezumab in preclinical Alzheimer’s disease. N Engl J Med 389: , 1096–1107. |
[28] | Sperling R , Henley D , Aisen PS , Raman R , Donohue MC , Ernstrom K , Rafii MS , Streffer J , Shi Y , Karcher K , Raghavan N , Tymofyeyev Y , Bogert J , Brashear HR , Novak G , Thipphawong J , Saad ZS , Kolb H , Rofael H , Sanga P , Romano G ((2021) ) Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: A truncated randomized phase 2b/3 clinical trial. JAMA Neurol 78: , 293–301. |
[29] | Novartis Pharmaceuticals ((2017) ) A study of CNP520 versus placebo in participants at risk for the onset of clinical symptoms of Alzheimer’s disease. https://classic.clinicaltrials.gov/show/NCT03131453, Last updated August 5, 2021, Accessed on March 22, 2024. |
[30] | Novartis Pharmaceuticals ((2015) ) A study of CAD106 and CNP520 versus placebo in participants at risk for the onset of clinical symptoms of Alzheimer’s disease. https://classic.clinicaltrials.gov/show/NCT02565511, Last updated July 8, 2021, Accessed on March 22, 2024. |
[31] | Tian J , Shi J , Li T , Li L , Wang Z , Li X , Lv Z , Zheng Q , Wei M , Wang Y ((2017) ) Efficacy and safety of an herbal therapy in patients with amnestic mild cognitive impairment: A 24-week randomized phase III trial. Evid Based Complement Alternat Med 2017: , 4251747. |
[32] | Zhang Y , Lin C , Zhang L , Cui Y , Gu Y , Guo J , Wu D , Li Q , Song W ((2015) ) Cognitive improvement during treatment for mild Alzheimer’s disease with a Chinese herbal formula: A randomized controlled trial. PLoS One 10: , e0130353. |
[33] | Zhang J , Liu Z , Zhang H , Yang C , Li H , Li X , Chen K , Zhang Z ((2016) ) A two-year treatment of amnestic mild cognitive impairment using a compound Chinese medicine: A placebo controlled randomized trial. Sci Rep 6: , 28982. |
[34] | Miao YC , Tian JZ , Shi J , Mao M ((2012) ) Effects of Chinese medicine for tonifying the kidney and resolving phlegm and blood stasis in treating patients with amnestic mild cognitive impairment: A randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 10: , 390–397. |
[35] | Seltzer B , Zolnouni P , Nunez M , Goldman R , Kumar D , Ieni J , Richardson S ((2004) ) Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch Neurol 61: , 1852–1856. |
[36] | Salloway S , Ferris S , Kluger A , Goldman R , Griesing T , Kumar D , Richardson S ((2004) ) Efficacy of donepezil in mild cognitive impairment: A randomized placebo-controlled trial. Neurology 63: , 651–657. |
[37] | Honig LS , Vellas B , Woodward M , Boada M , Bullock R , Borrie M , Hager K , Andreasen N , Scarpini E , Liu-Seifert H , Case M , Dean RA , Hake A , Sundell K , Poole Hoffmann V , Carlson C , Khanna R , Mintun M , DeMattos R , Selzler KJ , Siemers E ((2018) ) Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 378: , 321–330. |
[38] | Budd Haeberlein S , Aisen PS , Barkhof F , Chalkias S , Chen T , Cohen S , Dent G , Hansson O , Harrison K , von Hehn C , Iwatsubo T , Mallinckrodt C , Mummery CJ , Muralidharan KK , Nestorov I , Nisenbaum L , Rajagovindan R , Skordos L , Tian Y , van Dyck CH , Vellas B , Wu S , Zhu Y , Sandrock A ((2022) ) Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis 9: , 197–210. |
[39] | Ostrowitzki S , Bittner T , Sink KM , Mackey H , Rabe C , Honig LS , Cassetta E , Woodward M , Boada M , van Dyck CH , Grimmer T , Selkoe DJ , Schneider A , Blondeau K , Hu N , Quartino A , Clayton D , Dolton M , Dang Y , Ostaszewski B , Sanabria-Bohórquez SM , Rabbia M , Toth B , Eichenlaub U , Smith J , Honigberg LA , Doody RS ((2022) ) Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: Two phase 3 randomized placebo-controlled trials. JAMA Neurol 79: , 1113–1121. |
[40] | Mintun MA , Lo AC , Duggan Evans C , Wessels AM , Ardayfio PA , Andersen SW , Shcherbinin S , Sparks J , Sims JR , Brys M , Apostolova LG , Salloway SP , Skovronsky DM ((2021) ) Donanemab in early Alzheimer’s disease. N Engl J Med 384: , 1691–1704. |
[41] | Sims JR , Zimmer JA , Evans CD , Lu M , Ardayfio P , Sparks J , Wessels AM , Shcherbinin S , Wang H , Monkul Nery ES , Collins EC , Solomon P , Salloway S , Apostolova LG , Hansson O , Ritchie C , Brooks DA , Mintun M , Skovronsky DM ((2023) ) Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330: , 512–527. |
[42] | Ostrowitzki S , Lasser RA , Dorflinger E , Scheltens P , Barkhof F , Nikolcheva T , Ashford E , Retout S , Hofmann C , Delmar P , Klein G , Andjelkovic M , Dubois B , Boada M , Blennow K , Santarelli L , Fontoura P ((2017) ) A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 9: , 95. |
[43] | Zhao MX , Dong ZH , Yu ZH , Xiao SY , Li YM ((2012) ) Effects of Ginkgo biloba extract in improving episodic memory of patients with mild cognitive impairment: A randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 10: , 628–634. |
[44] | Wessels AM , Tariot PN , Zimmer JA , Selzler KJ , Bragg SM , Andersen SW , Landry J , Krull JH , Downing AM , Willis BA , Shcherbinin S , Mullen J , Barker P , Schumi J , Shering C , Matthews BR , Stern RA , Vellas B , Cohen S , MacSweeney E , Boada M , Sims JR ((2020) ) Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: The AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol 77: , 199–209. |
[45] | Berry DA , Dhadda S , Kanekiyo M , Li D , Swanson CJ , Irizarry M , Kramer LD , Berry SM ((2023) ) Lecanemab for patients with early Alzheimer disease: Bayesian analysis of a phase 2b dose-finding randomized clinical trial. JAMA Netw Open 6: , e237230. |
[46] | van Dyck CH , Swanson CJ , Aisen P , Bateman RJ , Chen C , Gee M , Kanekiyo M , Li D , Reyderman L , Cohen S , Froelich L , Katayama S , Sabbagh M , Vellas B , Watson D , Dhadda S , Irizarry M , Kramer LD , Iwatsubo T ((2023) ) Lecanemab in early Alzheimer’s disease. N Engl J Med 388: , 9–21. |
[47] | Scheltens P , Kamphuis PJ , Verhey FR , Olde Rikkert MG , Wurtman RJ , Wilkinson D , Twisk JW , Kurz A ((2010) ) Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement 6: , 1–10.e11. |
[48] | Scheltens P , Twisk JW , Blesa R , Scarpini E , von Arnim CA , Bongers A , Harrison J , Swinkels SH , Stam CJ , de Waal H , Wurtman RJ , Wieggers RL , Vellas B , Kamphuis PJ ((2012) ) Efficacy of Souvenaid in mild Alzheimer’s disease: Results from a randomized, controlled trial. J Alzheimers Dis 31: , 225–236. |
[49] | Soininen H , Solomon A , Visser PJ , Hendrix SB , Blennow K , Kivipelto M , Hartmann T ((2017) ) 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): A randomised, double-blind, controlled trial. Lancet Neurol 16: , 965–975. |
[50] | Padala PR , Padala KP , Lensing SY , Ramirez D , Monga V , Bopp MM , Roberson PK , Dennis RA , Petty F , Sullivan DH , Burke WJ ((2018) ) Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: A double-blind, randomized, placebo-controlled trial. Am J Psychiatry 175: , 159–168. |
[51] | Fink HA , Jutkowitz E , McCarten JR , Hemmy LS , Butler M , Davila H , Ratner E , Calvert C , Barclay TR , Brasure M , Nelson VA , Kane RL ((2018) ) Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: A systematic review. Ann Intern Med 168: , 39–51. |
[52] | Terao I , Honyashiki M , Inoue T ((2022) ) Comparative efficacy of lithium and aducanumab for cognitive decline in patients with mild cognitive impairment or Alzheimer’s disease: A systematic review and network meta-analysis. Ageing Res Rev 81: , 101709. |
[53] | Hampel H , Ewers M , Bürger K , Annas P , Mörtberg A , Bogstedt A , Frölich L , Schröder J , Schönknecht P , Riepe MW , Kraft I , Gasser T , Leyhe T , Möller HJ , Kurz A , Basun H ((2009) ) Lithium trial in Alzheimer’s disease: A randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 70: , 922–931. |
[54] | Cao B , Rosenblat JD , Brietzke E , Park C , Lee Y , Musial N , Pan Z , Mansur RB , McIntyre RS ((2018) ) Comparative efficacy and acceptability of antidiabetic agents for Alzheimer’s disease and mild cognitive impairment: A systematic review and network meta-analysis. Diabetes Obes Metab 20: , 2467–2471. |
[55] | Craft S , Raman R , Chow TW , Rafii MS , Sun CK , Rissman RA , Donohue MC , Brewer JB , Jenkins C , Harless K , Gessert D , Aisen PS ((2020) ) Safety, efficacy, and feasibility of intralnasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: A randomized clinical trial. JAMA Neurol 77: , 1099–1109. |
[56] | Thancharoen O , Limwattananon C , Waleekhachonloet O , Rattanachotphanit T , Limwattananon P , Limpawattana P ((2019) ) Ginkgo biloba extract (EGb761), cholinesterase inhibitors, and memantine for the treatment of mild-to-moderate Alzheimer’s disease: A network meta-analysis. Drugs Aging 36: , 435–452. |
[57] | Liu KY , Schneider LS , Howard R ((2021) ) The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry 8: , 1013–1016. |
[58] | Liu KY , Walsh S , Brayne C , Merrick R , Richard E , Howard R ((2023) ) Evaluation of clinical benefits of treatments for Alzheimer’s disease. Lancet Healthy Longev 4: , e645–e651. |
[59] | Andrews JS , Desai U , Kirson NY , Zichlin ML , Ball DE , Matthews BR ((2019) ) Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement (N Y) 5: , 354–363. |
[60] | Knopman DS , Jones DT , Greicius MD ((2021) ) Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement 17: , 696–701. |
[61] | ((2023) ) 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19: , 1598–1695. |
[62] | Pei H , Ma L , Cao Y , Wang F , Li Z , Liu N , Liu M , Wei Y , Li H ((2020) ) Traditional Chinese medicine for Alzheimer’s disease and other cognitive impairment: A review. Am J Chin Med 48: , 487–511. |



