The 5HT2b Receptor in Alzheimer’s Disease: Increased Levels in Patient Brains and Antagonist Attenuation of Amyloid and Tau Induced Dysfunction
Abstract
BACKGROUND:
Background: Neurodegenerative diseases manifest behavioral dysfunction with disease progression. Intervention with neuropsychiatric drugs is part of most multi-drug treatment paradigms. However, only a fraction of patients responds to the treatments and those responding must deal with drug-drug interactions and tolerance issues generally attributed to off-target activities. Recent efforts have focused on the identification of underexplored targets and exploration of improved outcomes by treatment with selective molecular probes.
Objective:
As part of ongoing efforts to identify and validate additional targets amenable to therapeutic intervention, we examined levels of the serotonin 5-HT2b receptor (5-HT2bR) in Alzheimer’s disease (AD) brains and the potential of a selective 5-HT2bR antagonist to counteract synaptic plasticity and memory damage induced by AD-related proteins, amyloid-β, and tau.
Methods:
This work used a combination of biochemical, chemical biology, electrophysiological, and behavioral techniques. Biochemical methods included analysis of protein levels. Chemical biology methods included the use of an in vivo molecular probe MW071, a selective antagonist for the 5HT2bR. Electrophysiological methods included assessment of long-term potentiation (LTP), a type of synaptic plasticity thought to underlie memory formation. Behavioral studies investigated spatial memory and associative memory.
Results:
5HT2bR levels are increased in brain specimens of AD patients compared to controls. 5HT2bR antagonist treatment rescued amyloid-β and tau oligomer-induced impairment of synaptic plasticity and memory.
Conclusions:
The increased levels of 5HT-2bR in AD patient brains and the attenuation of disease-related synaptic and behavioral dysfunctions by MW071 treatment suggest that the 5HT-2bR is a molecular target worth pursuing as a potential therapeutic target.
INTRODUCTION
Neurodegenerative diseases are characterized by progressive cognitive and behavioral dysfunction that can include memory loss and neuropsychiatric symptoms such as agitation, psychosis, depression, and apathy. Intervention with neuropsychiatric drugs is a component of clinical care [1, 2]. Development of new therapeutics focused on behavioral dysfunction in neurodegenerative diseases, therefore, is a rational complement to parallel drug development efforts targeting molecular pathologies in neurodegeneration, such as in Alzheimer’s disease (AD) and Alzheimer’s disease related dementia (ADRD). Current treatments for behavioral symptoms include a variety of serotonin reuptake inhibitors (SSRI) approved for broad coverage of behavioral disorders including AD [2]. Although such approved drugs have extensive worldwide clinical use for behavioral disorders, it is estimated that only about a third of clinical patients respond. Further, the pharmacological mechanism of action is an ongoing research question in precision medicine [3]. Clearly, there is a need to explore new targets and interventions to attenuate the underlying pathophysiology of synaptic dysfunction that manifests as cognitive dysfunction, anxiety, and depression.
A preponderance of accumulating evidence suggests that the 5HT receptor (5HTR) family, especially the 5HT-2bR, represents potential new druggable targets for treatment of behavioral dysfunctions associated with AD and ADRD [3]. The potential for the 5HT-2bR as a relevant new behavioral therapeutic target is indicated by a wide array of preclinical studies and clinical observations. For example, 5HT-2bR knock-out mice are resistant to existing SSRI drugs [4–6] suggesting a direct or indirect interaction with 5HT-2bR for approved behavioral modification drugs. Relatedly, the 5HT2bR/β-arrestin2 pathway is a molecular mechanism for efficacy of the SSRI drug fluoxetine in a MDD mouse model [7]. Clinically, a trend in cognitive and behavioral improvement was observed in a double-blind, placebo-controlled, multi-center trial of 122 ADRD patients [8] treated with the multi-target 5HT-2bR antagonist drug minaprine [9–13]. Taken in its entirety, the body of evidence raises the hypothesis that the 5HT2bR might be druggable target for treatment of cognitive and behavioral dysfunction in AD patients. However, it is not known if the levels of the 5HT-2bR are increased in AD/ADRD patient brains or whether treatment with a selective 5HT-2bR antagonist could attenuate amyloid- and tau-based dysfunctions in preclinical animal models.
We describe here our findings that the levels of the 5HT-2bR are increased in AD brains compared to age-matched controls and 5HT-2bR inhibition with a highly selective antagonist can prevent amyloid-β and tau-based dysfunction in mouse models. The integration of the clinical pathology results with molecular probe studies in preclinical models suggests that future development of selective and safe 5HT2bR antagonists might provide complementary interventions for the treatment of cognitive and behavioral dysfunctions in AD patients.
METHODS
Western blotting on human sample
Human brain tissue was homogenized in lysis buffer (62.5 mM Tris-HCl pH 6.8, 3% SDS, Halttrademark Protease and Phosphatase Inhibitor Cocktail (100X) and incubated at 4°C for 60 min, then sonicated before centrifugation at 13,000 rpm for 15 min. 5-HT-2B antibodies (Abcam 227722 1:1000, 5% milk in TRIS-buffered saline (TBS) 1X), and GAPDH antibodies (1:5000,CB100 5% milk in TBS) were from MilliporeSigma (St. Louis, MO, USA). For quantitative immunoblot analysis, equal amounts of proteins were loaded into each lane. Blots were re-probed with corresponding pan-antibodies and antibodies for tubulin or GAPDH to confirm equal loading. For quantification, we used a signal in the linear range. Immunoblot data were quantified by measuring the band intensity using imaging software (NIH ImageJ).
Synthesis of MW071
Compound 1 (200 mg, 0.98 mmol) & 4-N-(2-aminoethyl)-N-Boc-piperazine (6 eq, 896 mg, 3.91 mmol) and were reacted in 1-butanol. The temperature was held at 145°C for ∼26 h the reaction was worked up by adding H2O (50 mL), extracted with CH2Cl2 (3×15 mL) and dried with MgSO4. Column purification on silica gel using 5% MeOH/95% CH2Cl2. A yellowish oil compound 2, yield 82% (ESI, 398.25 (MH+) confirmed the boc compound. The intermediate Boc- compound 2 was treated with formic acid in CH2Cl2 for ∼5 h at ambient temperature to yield compound MW071. After workup product was purified on column chromatography, with the product eluting with 5% (v/v) methanol in methylene chloride yielded MW071, 96% pure, yellow powder in 80% yield. 1 H NMR (CD3OD): δ 8.28 (s, 1 H); 7.94 (d, J = 7 Hz, 2 H); 7.67 (m, 3 H); 4.05 (t, J = 5 Hz, 2 H); 3.76 (bs, 4 H); 3.70 (t, J = 5 Hz, 4 H); 3.31 (s, 2 H); 2.60 (s, 3 H); 2.02 (s, 1 H). HPLC (tr/purity): 8.93 min, 97%; ESI m/z (acetonitrile): 298.31 (MH+).
5-HT2b receptor binding affinity
The comparative affinity of MW071 and minaprine for the human 5-HT2B receptor was evaluated in a radioligand binding assay as originally described by Choi et al. (1994) [14]. Briefly, cell membrane containing homogenates (10μg protein) prepared from transfected CHO cells are incubated for 60 min at ambient temperature with 0.2 nM of iodinated (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride, [125I](±)DOI, in the absence or presence of the test compound in a buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 10μM pargyline, and 0.1% ascorbic acid. Nonspecific binding is determined in the presence of 1μM (±)DOI. Following incubation, the samples are filtered rapidly under vacuum through glass fiber filters (GF/B) presoaked with 0.3% polyethyleneimine (PEI) and rinsed several times with ice-cold 50 mM Tris-HCl using a 96-sample cell harvester (Unifilter, Packard). The filters are dried, and radioactivity measured in a scintillation counter (Topcount, Packard) using a scintillation cocktail (Microscint 0, Packard). The results are expressed as a percent inhibition of the control radioligand specific binding. The standard reference compound is (±)DOI, which is tested in each experiment at several concentrations to obtain a competition curve from which its IC50 is calculated.
Screening of 161 GPCRs for agonist/ antagonist activity of MW071
MW071 was screened for agonist and antagonist activity using 161 distinct stably transfected CHO cell lines, each selective for an individual GPCR, and monitoring dose dependent calcium flux using FLIPR technology [15]. Briefly, MW071 was tested in single blind assays by Eurofins Discovery (St Louis, MO) in stably transfected cell lines for each GPCR. MW071 was screened for agonist function at a final concentration of 12,500 nM and screened for antagonist function at a final concentration of 10,000 nM. MW071 was prepared as a DMSO stock and diluted with GPCR Profiler buffer (Hanks Balanced Salt Solution containing 20 mM HEPES and 2.5 mM Probenecid, pH 7.4). Each GPCR was tested in duplicate and sample diluent controls run. Reference agonists for each GPCR was included at Emax (the concentration where the reference agonist elicited a maximal response). All plates were subjected to baseline corrections and maximum fluorescence/luminescence values were used to calculate percentage activation and percentage inhibition according to the equation of (Max RLU)-(Baseline Avg.) / (Positive Avg.)-(Baseline Avg.). MW071 was negative in all the positive-controlled screening activities for agonist function. Similarly, MW071 was negative in all the positive-controlled screening activities for antagonist function except that for the 5HT2b receptor. An IC50 value of MW071 for 5HT2b receptor antagonism was also done by measuring concentration dependent activity using the established 5HT2bR cell line with monitoring of intracellular calcium flux using the FLIPR protocol.
Kinome-wide inhibitor screening
The hierarchal screening was done as previously described [16]. Briefly, the hierarchal Millipore kinase profiler test system was used. It includes 302 kinases representative of all major kinome branches as well as isoforms of individual families. Each optimized kinase mediated reaction mix contains the particular kinase and its cognate substrate in a defined buffer and temperature. The individually optimized conditions for each kinase mediated reaction is used to screen for the effect of an exogenous compound on activity. The initial screen was performed at an inhibitor concentration of 10,000 nM. Preliminary hits from the initial screen are validated as true or false positive hits by a concentration-dependent determination of an IC50 value using a standardized assay protocol. For any kinase for which the inhibitor exhibits IC50 < 1,000 nM, an apparent Ki value is determined.
Acetylcholinesterase (AChE) inhibition
MW071 was tested for AChE inhibition in duplicate at both 10μM and 100μM. Human HEK-293 cells were incubated with compound, 700μM acetylthiocholine substrate in 1% DMSO and 0.1 M sodium phosphate, pH 7.4 for 20 min at 37°C. Inhibition was determined by the spectrophotometric measurement of thiocholine.
MAO-A/MAO-B inhibition
MW071 (0.01–25μM), final DMSO concentration 0.1%, was incubated with recombinant human MAO-A or MAO-B in the presence of the probe substrate kynuramine for 25 min at 37°C. The non-selective MAO inhibitor, tranylcypromine, was screened alongside the test agents as a positive control. The reactions were terminated by addition of methanol containing internal standard for analytical quantification. The quenched samples were incubated at 4°C for 10 min and the supernatant from centrifugation analyzed by LC-MS/MS for the probe metabolite 4-hydroxyquinoline. A decrease in the formation of the metabolite compared to vehicle control is used to calculate an IC50 value.
Serotonin uptake inhibition assay
The dose dependent effects of MW071 on serotonin uptake was done with synaptosomes prepared from rat brain [17] using imipramine as dose dependent reference compound. Study design was single blind where coded samples were provided to a blinded third party for testing. Briefly, the synaptosomes were incubated for 15 min at 37°C with 0.1μCi [3H]serotonin in the absence (control) or presence of MW071 or impramine in a buffer containing 106.2 mM NaCl, 4.5 mM KCl, 2.25 mM MgSO4, 1.08 mM NaH2PO4, 22.5 mM NaHCO3, 9.9 mM glucose, 9μM EGTA, and 45μM ascorbic acid (pH 7.4). Basal control activity is determined by incubating the same mixture for 15 min at 4°C in the presence of 10μM imipramine to block the uptake. Following incubation, the samples are filtered rapidly under vacuum through glass fiber filters (GF/B) and rinsed twice with ice-cold incubation buffer using a 96-sample cell harvester (Unifilter, Packard) to eliminate free [3H]serotonin. The filters are dried and the retained radioactivity is measured in a scintillation counter (MicroBeta2, Packard) using a scintillation cocktail (Microscint 0, Packard). The results are expressed as a percent inhibition of the control uptake of [3H] serotonin.
Other in vitro activity screens related to potential off-target and safety related risks
MW071 was subjected to the Eurofins general off-target and safety related in vitro screening platform. Included were the following receptors, enzymes and ion channels: Receptors A2A, α1A, α2A, β1, β2, CB1, CB2, CCK1 (CCKA), D1, D2L, ETA, H1, H2, kappa (KOP), M1, M2, M3, δ (DOP), μ (MOP), 5-HT1A, 5HT1B, 5-HT2A, 5-HT2B, V1a.; Enzymes COX-1, COX-2, PDE3A, PDE4D2, LCK, and ion channels /uptake mechanisms norepinephrine transporter uptake, dopamine transporter uptake, hNav1.5, hCav1.2, hERG, hKCNQ1/hmink, hnAChR α4β2 agonist Assay, hGABAA α1β2γ2 PAM Assay; and transporter inhibition for OCT2, BSEP, BCRP, MATE1, MATE2-K, MRP2, OAT1, OAT3, OATP1B1, OCT1, OATP1B3, P-gp.
Rodent liver microsome stability
MW071 was screened using previously described approaches [16] for susceptibility to liver microsome preparations as a rapid surrogate for exceptional susceptibility to metabolism, which can impact experimental results due to variances in diet or disease state. Stability screens using microsomes prepared from rat liver samples were done in duplicate using a standard microsome buffer containing 0.5 mg/mL microsomal protein and MW071 at 1μM. The reactions were terminated at time points of 0, 10, 20, 30, and 60 min, samples processed, and analysis done by LC-MS/MS to monitor parent compound. The peak area response ratio (PARR) to internal standard at each time point was compared to the response ratio at time 0 to determine the percent remaining. The assay was linear over 60 min with r2 = 0.969 –1.00. The half-life was calculated based on t1/2 = 0.693/k, where k is the elimination rate constant based on the results of non-linear fitting. When the calculated half-life is longer than the duration of the experiment, the half-life is expressed as > the longest incubation time. The intrinsic clearance (CLint) was calculated based on CLint = k/D, where k is the elimination rate constant and D is protein concentration.
CYP substrate status
MW071 was screened using previously described approaches [16] for susceptibility to common P450 (CYP) first pass metabolism which can impact experimental results due to diet or disease state. MW071 at a final concentration of 0.1μM was test for potential substrate for the human recombinant (hr) CYPs 1A2, 2B6, 2D6, 2C8, 2C9, 2C19, and 3A4. The reactions were terminated at time points of 0, 15, 30, 45, and 60 min, samples processed, and analysis done by LC-MS/MS to monitor parent compound. T1/2 was estimated from the slope of the linear portion of the curve for percentage of remaining parent compound versus time assuming first order kinetics. Substrate stability, expressed as percent of the parent compound remaining, was calculated by comparing the peak area of the compound at the time point relative to that at time 0. A reference compound specific for each CYP was run in parallel. The half-life (T1/2) was estimated from the slope of the initial linear range of the logarithmic curve of compound remaining (%) versus time assuming first-order kinetics.
Animals
The protocol involving animals was approved by Columbia University Institutional Animal Care and Use Committee (IACUC) (# AC-AABG9555). Experiments were performed in accordance with the relevant approved guidelines and regulations. C57BL/6J were obtained from breeding colonies kept in the animal facility of Columbia University. They were 3–4 months of age. Both sexes were used. All mice were maintained on a 12-h light/dark cycle (lights on at 6:00 AM) in temperature and humidity-controlled rooms; food and water were available ad libitum.
Aβ oligomers
Human Aβ42 oligomerization was obtained as described previously [18, 19]. Briefly, a protein film was prepared by dissolving Aβ42 lyophilized powder (AnaSpec, CA, USA) in 1,1,1,3,3,3-Hexafluoro-2-Propanol (HFIP) and subsequent incubation for 2 h at room temperature to allow complete monomerization. The Aβ film was dissolved in dimethylsulfoxide (DMSO), sonicated for 15 min, aliquoted, and stored at –20°C. To oligomerize the peptide, phosphate buffered saline (PBS) was added to an aliquot of DMSO-Aβ to obtain a 5 mM solution that was incubated for 12 h at 4°C. This oligomerized Aβ solution was then diluted to the final concentration of 200 nM in artificial cerebrospinal fluid (ACSF) composed as following: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 2.0 MgCl2 in mM.
Tau oligomers
Tau oligomers were obtained as previously described [20]. Briefly, the tau 4 R/2 N construct was prepared in expression vector pET29a (Bioclone) in the bacterial strain BL21 (DE3) for protein expression. For oligomerization, tau was transferred to protein concentrators and buffer exchanged with oligomerization buffer following incubation with 1 mM H2O2 at room temperature for 20 h for introducing disulfide bonds. Tau protein concentration was determined from the absorption at 280 nm with an extinction coefficient of 7450 cm-1M-1 and oligomers were visualized through western blots.
Electrophysiological recordings
Mice were sacrificed through cervical dislocation and hippocampus was removed immediately after decapitation, as previously described [18]. Transverse hippocampal slices (400μm thickness) were cut on a tissue chopper and transferred to the recording chamber where the physiological conditions in the brain were maintained by perfusion of ACSF. Slices were allowed to recover for at least 90 min before commencing the extracellular field recordings. A bipolar tungsten electrode and a glass electrode filled with ACSF were placed in the Schaeffer collateral fibers and the CA1 stratum radiatum, respectively. After establishing the input-output relationship, a 30 min stable baseline was recorded, and LTP was induced using a theta-burst stimulation (consisting of 4 pulses at 100 Hz, with bursts repeated at 5 Hz and each tetanus including 3 ten-burst trains separated by 15 s). LTP was measured as field excitatory postsynaptic potential (fEPSP) slope expressed as percentage of the baseline and the results were represented as mean±SEM. Hippocampal slices were perfused with recombinant tau (50 nM) or Aβ42 (200 nM) for 20 min prior to LTP induction. For the treatment groups, slices were co-perfused during the last 20 min prior to tetanization with MW071 (10μM).
Behavioral studies
MW071 treatment for behavioral test: For behavioral testing, MW071 was diluted in sterile saline under sterile conditions and administered by i.p. injection at a dose of 5 mg/kg, 30 min prior to the electric shock for fear conditioning experiments in a single injection, or 30 min prior to the 1st and 7th trial of the radial arm water maze (RAWM) assessment in two injections both on day 1 and 2. For the visible platform test, MW071 was given 30 min prior to the first and second session on both days. Similarly, MW071 was given 30 min prior to the open field test on day 1 and 2 and 30 min before sensory threshold assessment.
Stereotaxic surgery and infusion of A β42 and tau: Implant of the cannulas onto the hippocampi was performed as previously described [21]. Briefly, the coordinates were 2.46 mm posteriorly and 1.5 mm laterally from Bregma to a depth of 1.30 mm [22]. After 6–9 days of recovery, awake mice were restrained and gently infused with 200 nM Aβ into dorsal hippocampi bilaterally (one injection, 20 min prior to training for fear conditioning, and 20 min prior to the 1st and 7th trial for RAWM on both days of testing), or 500 nM tau (two injections, 180 min and 20 min prior to fear conditioning, and 180 min and 20 min prior to the 1st trial of the RAWM on both days of testing).
2 Day RAWM and visible platform task: RAWM was performed as previously described [19]. Briefly, during the first day, mice were trained in 15 trials to identify the platform location in a goal arm by alternating between a visible and a hidden platform from trial 1 to 12, and by finding a hidden platform in the last three trials. During the second day, the same procedure was repeated by using only the hidden platform from trial 1 to 15. An entrance into an arm with no platform, or failure to select an arm after 10 s was counted as an error and the mouse was gently pulled back to the start arm. The goal arm did not change among trials, with a different starting arm on successive trials. Data were analyzed and displayed as averages of blocks of 3 trials. A visible platform test was performed to control for possible visual, motor, and motivational deficits. This consisted in a two-day test, with two sessions/day (each consisting of three 1 min trials), in which the time taken to reach a visible platform (randomly positioned in a different place each time), and the speed to reach it, were recorded.
Fear conditioning: Fear conditioning was performed as previously described [19]. Briefly, during the first day, mice were placed in the conditioning chamber for 2 min before the onset of a discrete tone [conditioned stimulus (CS)] (a sound that lasted 30 s at 2800 Hz and 85 dB). In the last 2 s of the CS, mice were given a foot shock [unconditioned stimulus (US)] of 0.80 mA for 2 s through the bars of the floor. After the CS/US pairing, mice were left in the conditioning chamber for 30 s and then returned to their home cages. Freezing behavior, defined as the absence of all movements except for those necessitated by breathing, was automatically scored. During the second day, we evaluated contextual fear learning. Mice were placed in the conditioning chamber and freezing was measured for five consecutive minutes. During the third day, cued fear learning was evaluated by placing mice in a novel context for 2 min (pre-CS test), after which they were exposed to the CS for 3 min (CS test), and freezing was measured.
Open field: The test has been used for assessing exploratory behavior and anxiety levels [21]. Mice were placed in a novel open environment consisting of Plexiglass transparent walls, the arena was divided into sectors (periphery and center). Each mouse started the test in the center of the arena, and was permitted to freely explore the arena for 10 min in two consecutive days. Both percent time spent in the center and number of entries into the center were scored. Their activity was automatically recorded for 10 min on two consecutive days.
Sensory threshold assessment: the test was used for evaluating the animal perception of the shock, as described [19]. Animals were subjected to 1-s foot shocks of increasing intensity from 0.1 to 0.6 mA at 0.1 mA increments every 30 s. The foot shock intensity that elicited the first visible response (flinching), the second motor response (jumping), and the first audible response (vocalization) were noted.
Statistical analyses
For electrophysiological recordings results were analyzed by two-way analysis of variance (ANOVA) for repeated measures comparing traces after tetanic stimulation with treatment condition as main effect. For the behavioral tests, results were analyzed by either two-way ANOVA for repeated measures or one-way ANOVA with Bonferroni post-hoc planned comparisons. Behavioral experiments were designed in a balanced fashion in which the sex of mice was kept equal between the various groups, and for each condition mice were trained and tested in three to four separate sets of experiments in blind. Differences were considered significant at a p value less than 0.05. Results were expressed as Standard Error of the Mean (SEM).
RESULTS
5HT2bR levels are increased in AD patient brains
As shown in Fig. 1, 5HT2bR levels are increased in cortex from post-mortem AD specimens (39 sporadic AD; 22 females and 17 males) compared to 20 age-matched non-demented patients (7 females and 13 males). All specimens were characterized for Braak and Braak stage (VI for the AD sample, 0 for healthy individual), CERAD score (C definite for AD patient, not evaluable for non-demented), and NIA-R (high prob. for the AD patients, not evaluable for non-demented) (Supplementary Table 1). There were no other comorbidities in patient records indicative of alternative explanation of their clinical deficits.
Fig. 1
5HT2bR expression in cortex from postmortem AD specimens. 5HT2bR expression is increased in cortex from post-mortem specimens from AD patients (39 sporadic AD: 22 females and 17 males) and age-matched non-demented controls (20 individuals: 7 females and 13 males). Postmortem interval was recorded. Individuals were characterized for their Braak and Braak stage (VI-0), and CERAD score (C-0). AD patients had no other diseases that might have contributed to the clinical deficits. A) Representative western blotting of 5HT2bR level in human brain samples. B) Cumulative graphs (t-test p = 0.039).
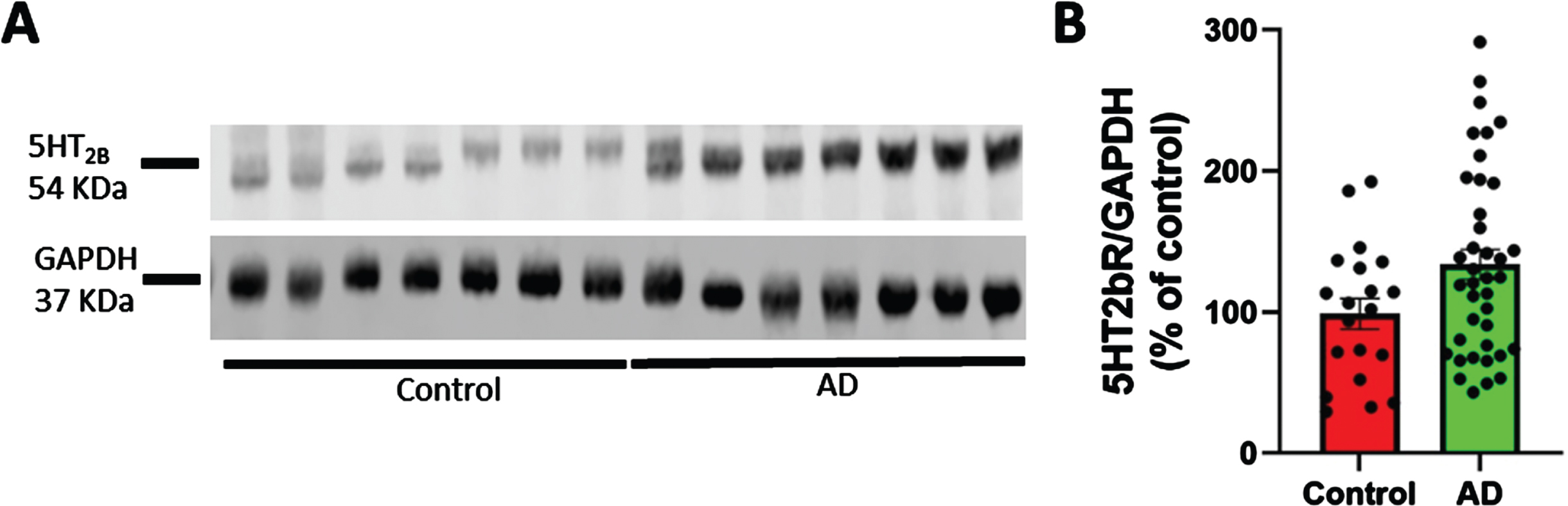
Table 1
Summary of MW071 properties and target selectivity
| Property | |
| MW | 297.4 |
| cLogP | 2.04 |
| Rotatable bonds | 5 |
| H-bond Donor + Acceptor | 7 |
| pKa (potentiometric) | 3.90±0.24, 8.65±0.24 |
| 5HT2bR binding activity | IC50 = 22±9.0 nM |
| Cellular 5HT2bR antagonist activity | IC50 = 54 nM |
| GPCR agonist/antagonist screen (161 GPCRs) | Negative for all agonist screens. Negative for all antagonist screens except for 5HT2bR |
| Other receptors | (α2A, β1, β2, D2L, kappa (KOP), δ (DOP), μ (MOP) |
| Kinome-wide screen (302 kinases) | Negative |
| MAO-A/MAO-B inhibition | Negative |
| Acetylcholinesterase inhibition | Negative |
| Serotonin transporter uptake inhibition | Negative |
| Dopamine transporter uptake inhibition | Negative |
| Norepinephrine transporter uptake inhibition | Negative |
| Transporter inhibition | Negative: OCT1, OCT2, BSEP, BCRP, P-gp, MATE1, MATE2-K, MRP2, OAT1, OAT3, OATP1B1, OATP1B3 |
| COX1/COX2 | Negative |
| PDE3A/PDE4D2 | Negative |
| Rodent microsome stability | T1/2 > 60 min |
| CYP substrate activity | T1/2 > 60 min (negative) for 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4 |
MW071 is a selective molecular probe with 5HT2bR binding affinity and cellular antagonist activity
MW071 [23] differs by one atom from the retired clinical neurotherapeutic minaprine. Table 1 provides a summary of MW071 features. The single oxygen atom in minaprine was changed to a nitrogen atom to generate MW071 [23]. Minaprine exhibits weak 5HT2bR binding activity (IC50 = 990 nM) and is a substrate for CYP2D6, a P450 that exhibits genetic variance in
drug metabolism. CYP2D6 substrate status is a trait that can potentially impact clinical efficacy or toxicology of any drug. In the case of minaprine, CYP2D6 produces a pharmacologically active metabolite that varies among individuals depending on their pharmacogenetics. The single atom change to generate MW071 eliminates the CYP2D6 substrate status and enhances 5HT2bR binding activity (MW071 IC50 = 22nM±9.0 nM). Large scale cell-based screening of agonist and antagonist activity for a diverse array of GPCR targets (Table 2) revealed that MW071 is a highly selective antagonist for 5HT2bR. As shown in Fig. 2, MW071 is an antagonist with IC50 = 54 nM in cell-based activity. In addition to the highly selective GPCR antagonist activity, MW071 exhibits exceptional target selectivity outside of the GPCR class of targets. The biochemical and cellular selectivity profiles of MW071 facilitates its use as a molecular probe to test hypotheses. Especially important for behavioral outcomes in animal models is MW071’s lack of inhibition of MAO, acetylcholinesterase, serotonin, or dopamine transport uptake, COX2, or key PDEs as these are established targets for behavioral modification. MW071 chemical stability is complemented by its metabolic stability in the standard liver microsome assay. Finally, the clean P450 (CPY) profile facilitates interpretation in experimental outcomes in disease models where certain CYPs, especially CYP2D6, increase with disease progression or injury. Overall, the MW071profile allowed its use to probe 5HT2bR involvement in behavior or synaptic dysfunction.
Table 2
List Of GPCRS tested for agonist and antagonist activity
| GPCR | GPCR | GPCR |
| 5-HT1A | CysLT1 | Motilin |
| 5HT1B | CysLT2 | MrgD |
| 5-HT2A | D1 | MRGX1 |
| 5-HT2B | D2 | MRGX2 |
| 5-HT2C | D4 | NK1 |
| 5-HT4B | D5 | NK2 |
| 5-HT6 | DP | NK3 |
| A1 | EP1 | NMU1 |
| A2A | EP2 | NMU2 |
| A2B | EP3 | NOP |
| A3 | EP4 | NPBW1 |
| α1A | ETA | NTR1 |
| ADRA1A | ETB | OPRD1 |
| ADRA1B | FP | OPRK1 |
| ADRA1D (ñ2-79) | FPR1 | OPRM1 |
| ADRA2A | FPR2 | OT |
| ADRB1 | GABAB1b | OX1 |
| ADRB2 | GAL1 | OX2 |
| ADRB3 | GAL2 | P2Y1 |
| APJ | GCGR | P2Y11 |
| AT1 | Ghrelin | P2Y12 |
| BB1 | GIP | P2Y2 |
| BB2 | GLP-1 | P2Y4 |
| BB3 | GLP-2 | PAC1 |
| BDKR2 | GnRH | PAF |
| BLT1 | GPR103 | PK1 |
| C3aR | GPR109A | PK2 |
| C5aR | GPR14 | PRP |
| CaS | GPR39 | PTH1 |
| CB1 | GPR41 | PTH2 |
| CB2 | GPR43 | S1P1 |
| CCK1 | GPR54 | S1P2 |
| CCK2 | GPR68 | S1P3 |
| CCR1 | GPR91 | S1P4 |
| CCR10 | GPR99 | S1P5 |
| CCR2B | H1 | Secretin |
| CCR3 | H2 | sst2 |
| CCR4 | H3 | sst3 |
| CCR5 | IP1 | sst4 |
| CCR6 | LPA1 | sst5 |
| CCR7 | LPA3 | Thrombin-Activated PARs |
| CCR8 | LPA5 | TP |
| CCR9 | M1 | TRH |
| CGRP1 | M2 | Trypsin-Activated PARs |
| ChemR23 | M3 | TSH |
| CRF1 | M4 | V1A |
| CRF2 | M5 | V1B |
| CX3CR1 | MC2 | V2 |
| CXCR1 | MC4 | VPAC1 |
| CXCR2 | MC5 | VPAC2 |
| CXCR3 | MCHR1 | XCR1 |
| CXCR4 | MCHR2 | Y2 |
| CXCR5 | mGlu2 | Y4 |
| CXCR6 | mGlu1 |
Fig. 2
5-HT2bR antagonist activity of MW071. MW071 exhibits a concentration dependent antagonist activity with an IC50 = 54 nM. The assay was done by use of a stably transfected CHO cell line and monitoring dose dependent calcium flux using FLIPR technology [15]. All sample plates were subjected to baseline corrections and maximum fluorescence values were used to calculate percentage activation and percentage inhibition according to the equation of (Max RLU) - (Baseline Avg.) / (Positive Avg.) - (Baseline Avg.). Concentrations of MW071 used in the assay for antagonist activity range was 40,000 nM to 2.4 nM.
![5-HT2bR antagonist activity of MW071. MW071 exhibits a concentration dependent antagonist activity with an IC50 = 54 nM. The assay was done by use of a stably transfected CHO cell line and monitoring dose dependent calcium flux using FLIPR technology [15]. All sample plates were subjected to baseline corrections and maximum fluorescence values were used to calculate percentage activation and percentage inhibition according to the equation of (Max RLU) - (Baseline Avg.) / (Positive Avg.) - (Baseline Avg.). Concentrations of MW071 used in the assay for antagonist activity range was 40,000 nM to 2.4 nM.](https://content.iospress.com:443/media/jad/2024/98-4/jad-98-4-jad240063/jad-98-jad240063-g002.jpg)
The effect of MW071 on synaptic plasticity and memory following Aβ- or tau-oligomer exposure
In these experiments, hippocampal slices are perfused with either MW071 or vehicle alone, concurrently with Aβ or tau for 20 min prior to the ⊖ burst. As shown in Fig. 3A, perfusion with MW071 (10μM) rescues LTP defect in slices perfused with 200 nM Aβ- or 50 nM tau-oligomers. MW071 alone does not affect potentiation.
Fig. 3
Efficacy of MW071 against Aβ- and tau-oligomer induced defects in LTP and memory. A) Perfusion with MW071 (10μM) rescues the LTP defect in hippocampal slices treated with 200 nM Aβ- or 50 nM tau-oligomers. MW071 alone did not affect potentiation. ANOVA for repeated measures between groups: F(1,19) = 27.85, p < 0.0001 vehicle versus Tau; F(1,18) = 24.74, p < 0.0001 vehicle versus Aβ; F(1,22) = 10.37, p = 0.0039 Tau versus Tau + MW071; F(1,19) = 7.222, p = 0.0146 Aβ versus Aβ+ MW071; (F(1, 18) = 0.0082, p = 0.93 vehicle versus MW071.Vehicle: N = 10 (5 males, 5 females), MW071: N = 10 (5 males, 5 females), Aβ: N = 10 (5 males, 5 females), Aβ+ MW071: N = 11 (5 males, 6 females), Tau: N = 11 (6 males, 5 females), Tau + MW071: N = 13 (7 males, 6 females). B, C) MW071 protects mice against the impairment of spatial (B) and associative memory (C) by infusion of 200 nM Aβ- or 500 nM tau-oligomers into dorsal hippocampi bilaterally, while MW071 alone does not affect performance. RAWM: ANOVA for repeated measures among all (day 2): F(5,65) = 6.158, p < 0.0001. One-way ANOVA for block 10: F(5,65) = 8.552, p < 0.0001; Bonferroni’s p < 0.0001 vehicle versus Aβ; p = 0.0006 vehicle versus Tau; p = 0.0013 Aβ versus Aβ + MW071; p = 0.0108 Tau versus Tau + MW071 and p = 1 vehicle versus MW071. Vehicle: N = 10 (5 males, 5 females), MW071: N = 13 (6 males, 7 females), Aβ: N = 12 (6 males, 6 females), Aβ+ MW071: N = 12 (6 males, 6 females), Tau: N = 11 (5 males, 6 females), Tau+ MW071: N = 13 (7males, 6 females). Fear conditioning: Baseline: One-way ANOVA F(5,60) = 1.217, p = 0.3122, 24 h Contextual: One-way ANOVA F(5,60) = 9.391, p < 0.0001; Bonferroni p = 0.0002 vehicle versus Aβ; p < 0.0001 vehicle versus Tau; p = 0.0043 Aβ versus Aβ+ MW071; p = 0.028 Tau versus Tau + MW071; p = 1 vehicle versus MW071. Vehicle: N = 9 (5 males, 4 females), MW071: N = 10 (5 males, 5 females), Aβ: N = 13 (7 males, 6 females), Aβ+ MW071: N = 11 (6 males, 5 females), Tau: N = 11 (5 males, 6 females), Tau+ MW071: N = 12 (6 males, 6 females).
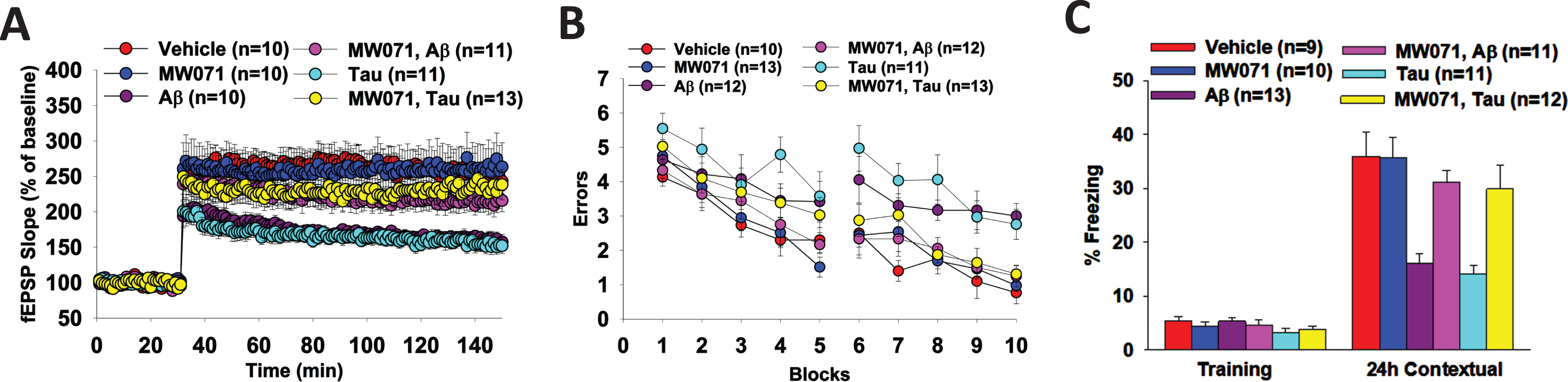
Because LTP is considered an electrophysiological surrogate of memory, we also tested spatial and associative memory, two types of memory that are affected in AD patients [24, 25], and can be assessed through the 2-day RAWM task and the fear conditioning, respectively. As shown in Fig. 3B and 3C, bilateral infusion of 200 nM Aβ oligomers- or 500 nM tau oligomers into dorsal hippocampi dramatically increases the number of errors in the RAWM test (Fig. 3B) and decreases the percent freezing in contextual fear learning (Fig. 3C). However, MW071 protects mice against the impairment of spatial memory (Fig. 3B) and associative memory (Fig. 3C). The RAWM performance and the percent freezing are indistinguishable in Aβ and tau infused animals treated with MW071 from that of mice treated with vehicle. MW071 alone does not affect performance with these tasks in vehicle infused mice.
The same animals performed control behavioral tasks including the visible platform, cued conditioning, open field, and sensory threshold assessment. As shown in Supplementary Figure 1A-B, the visible platform does not show any difference in speed or latency to reach the platform among the six groups, indicating that MW073 induced rescue of Aβ and tau memory defects does not affect mobility, vision and motivation of mice during the RAWM testing. As shown in Supplementary Figure 1C, cued fear learning, a type of learning depending upon amygdala function, is not affected in animals treated with MW071 plus Aβ or Tau. As shown in Supplementary Figure 1D and 1E, open field testing does not reveal any difference among the groups treated with MW071 plus Aβ, tau, indicating that mouse exploratory behavior, which might affect animal performance in the memory task, is not affected by treatment. In addition, as shown in Supplementary Figure 1G, we excluded that the results obtained in fear conditioning is due to rescue of deficits in mice capability to perceive the electric shock, as sensory threshold assessment does not reveal differences among the six groups of mice. Altogether, these control experiments confirm that the effect of MW071 is a genuine effect on memory. Our working assumption is that the MW071 effects are via 5HT2b receptor antagonism and the neuroprotection in ex vivo and in vivo experiments reflect attenuation of synaptic dysfunction induced by Aβ or tau mediated stress.
DISCUSSION
The preponderance of data from clinical observations and animal model studies identifies the 5HT2b receptor (5HT2bR) as a promising but under-explored target for therapeutic development. Our observation that the immunoreactive levels of 5HT2bR were higher in AD brains versus age-matched control brains is consistent with the hypothesis that 5HT2bR is a viable target for pursuit in future neurodegenerative disease research. The biological effects of MW071 in model systems identifies synaptic dysfunction as a cellular mechanism for pathophysiology progression process. The combination of the preclinical model outcomes combined with the human pathology findings for AD brains support the hypothesis that selective 5HT2bR antagonists could be future key components of neuropathophysiology-driven treatment paradigms addressing patient behavioral and cognitive dysfunction.
The NIMH-CH database entry for 5HT2bR [26] documents that numerous approved drugs, psychoactive substances of abuse, and research molecular probes have quantifiable 5HT2bR binding activity. The 5HT2bR engagement by FDA approved drugs, therapeutic candidates and psychoactive substances reflects the polypharmacy of multi-target drugs and unanticipated off-target activity. A subset of the 5HT2bR binding compounds are identified by functional profiling as agonists. A smaller set of 5HT2bR binding molecules have antagonist activity. The relative safety of diverse 5HT2bR engaging drugs adds to the attractiveness of the 5HT2bR hypothesis. However, FDA approved drugs with 5HT2bR agonist activity have been withdrawn due to safety risks, and the regulatory response to those that remain on the market is prominent safety labeling and restricted use. MW071 is a highly selective 5HT2bR antagonist with the absence of 5HT2bR agonist activity and is neuroprotective in animal models. The profile suggests that 5HT2bR antagonism is a tractable goal in future drug development.
MW071 has a single atom difference (a nitrogen atom replacing an oxygen) from minaprine, a retired psychoactive drug with 5HT2bR activity. Minaprine was reported as relatively safe and well-tolerated in AD patients [8–13]. However, MW071 is not a drug. Nevertheless, MW071 provides evidence in support of potential utility of selective 5HT2b antagonists. Further, the cell-based screens of over 150 established GPCRs for agonist or antagonist activity were negative except for 5HT2bR antagonism, and no off-target, safety or drug-drug interaction liabilities were identified in comprehensive screens of the kinome or high-risk enzymes, receptors, transporters, and ion channels (Supplementary Table 2). The MW071 pharmacological profile, therefore, provides a firm foundation for future development of a clinical candidate using the SOSA (systematic optimization of side activity) approach [27]. SOSA is similar to repurposing of an approved drug for new uses in that it leverages the portfolio of the clinical drug, allowing a compressed timeline for a Go or NoGo decision for therapeutic development. However, SOSA can enhance the desired function of an extant drug via conservative chemical changes while retaining the neurotherapeutic features of the parent drug and generating new intellectual property. In contrast, repurposing is restricted to the inherent molecular properties and regulatory controls of the parent drug. SOSA allows improvement of therapeutic potential and removal of clinical liabilities in a novel structure, thereby enhancing the potential for futural clinical approval and commercialization.
The outcomes from our study have important implications. The observation that there are higher levels of 5HT2bRs in the brain of AD patients raises the hypothesis that upregulation of the 5HT2bR is a contributing factor to disease pathophysiology. The demonstration that a highly selective 5HT2bR antagonist devoid of 5HT2bR agonist activity can attenuate synaptic dysfunction, the cellular basis of memory loss and behavioral issues, suggests a tractable approach to future pharmacological interventions. Clearly, there are key limitations to the findings in this initial report. The diversity of the patient population and disease stage of AD, a complex progressive disease, is not fully reflected in the brain bank samples analyzed. Future research needs to extend the coverage of patient samples and relate the pathology to disease progression. While MW071 is a highly selective molecular probe, it is not a drug. Future preclinical drug development using MW071 as a discovery starting point is required before a therapeutic candidate can be identified. Regardless, the congruence of the outcomes from this initial study provides a foundation for developing future diagnostic and neurotherapeutic approaches to the treatment of disease progression and behavioral dysfunction.
AUTHOR CONTRIBUTIONS
Erica Acquarone (Conceptualization; Data curation; Formal analysis; Investigation; Writing – original draft); Elentina Argyrousi (Data curation; Investigation); Ottavio Arancio (Conceptualization; Data curation; Funding acquisition; Investigation; Supervision; Writing – original draft); Martin Watterson (Conceptualization; Data curation; Funding acquisition; Investigation; Supervision; Writing – original draft); Saktimayee M. Roy (Conceptualization; Data curation; Investigation; Supervision; Writing – original draft).
ACKNOWLEDGMENTS
We thank Agnieszka Staniszewski for help with behavioral experiments and tau preparation, as well as Hong Zhang with the electrophysiological experiments.
We thank the Columbia University Alzheimer’s Disease Research Center, funded by NIH grant P30AG066462 to S.A. Small (P.I.), and A. Teich for providing biological samples and associated information.
FUNDING
This work was supported by an NIH award AG066722 to Northwestern University (DMW, SMR) and Columbia University (OA).
CONFLICT OF INTEREST
The investigators are inventors of 5HT2bR related drugs that their employers (Columbia University and Northwestern University) have submitted for USPTO protection.
Ottavio Arancio is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article and its supplementary information file.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-240063.
REFERENCES
[1] | Aboukhatwa M , Dosanjh L , Luo Y ((2010) ) Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Mol Neurodegener 5: , 10. |
[2] | Mdawar B , Ghossoub E , Khoury R ((2020) ) Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen Res 15: , 41–46. |
[3] | Popova NK , Tsybko AS , Naumenko VS ((2022) ) The implication of 5-HT receptor family members in aggression, depression and suicide: Similarity and difference. Int J Mol Sci 23: , 8814. |
[4] | Belmer A , Quentin E , Diaz SL , Guiard BP , Fernandez SP , Doly S , Banas SM , Pitychoutis PM , Moutkine I , Muzerelle A , Tchenio A , Roumier A , Mameli M , Maroteaux L ((2018) ) Positive regulation of raphe serotonin neurons by serotonin 2B receptors. Neuropsychopharmacology 43: , 1623–1632. |
[5] | Diaz SL , Doly S , Narboux-Neme N , Fernandez S , Mazot P , Banas SM , Boutourlinsky K , Moutkine I , Belmer A , Roumier A , Maroteaux L ((2012) ) 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol Psychiatry 17: , 154–163. |
[6] | Quentin E , Belmer A , Maroteaux L ((2018) ) Somato-dendritic regulation of raphe serotonin neurons; a key to antidepressant action. Front Neurosci 12: , 982. |
[7] | Fang Y , Ding X , Zhang Y , Cai L , Ge Y , Ma K , Xu R , Li S , Song M , Zhu H , Liu J , Ding J , Lu M , Hu G ((2022) ) Fluoxetine inhibited the activation of A1 reactive astrocyte in a mouse model of major depressive disorder through astrocytic 5-HT(2B)R/beta-arrestin2 pathway. J Neuroinflammation 19: , 23. |
[8] | Passeri C , Iuliani O , Di Ianni M , Sorrentino C , Giancola R , Abbruzzese L , Dallavalle FM , Gattillo S , Mariano MT , Martino M , Ostuni A , Savignano C , Santoleri L , Tison T , Vacca M , Accorsi P ((2023) ) Comparison between peripheral blood progenitor cell collection on the 4(th) or 5(th) day of granulocyte colony-stimulating factor treatment in allogeneic stem cell donors: Implications for hematopoietic progenitor cell apheresis guidelines. Blood Transfus 21: , 37–41. |
[9] | Amsterdam JD , Dunner DL , Fabre LF , Kiev A , Rush AJ , Goodman LI ((1989) ) Double-blind, placebo-controlled, fixed dose trial of minaprine in patients with major depression. Pharmacopsychiatry 22: , 137–143. |
[10] | Bohacek N , Ravic M , Biziere K ((1987) ) Minaprine and imipramine in the treatment of major depressive disorders. A comparative double-blind study. Drugs Exp Clin Res 13: , 435–442. |
[11] | Del Zompo M , Bernardi F , Burrai C , Bocchetta A ((1990) ) A double-blind study of minaprine versus amitriptyline in major depression. Neuropsychobiology 24: , 79–83. |
[12] | Edwards JG , Dinan TG , Waller DG , Greentree SG ((1996) ) Double-blind comparative study of the antidepressant, unwanted and cardiac effects of minaprine and amitriptyline. Br J Clin Pharmacol 42: , 491–498. |
[13] | Muramatsu M , Tamaki-Ohashi J , Usuki C , Araki H , Chaki S , Aihara H ((1988) ) 5-HT2 antagonists and minaprine block the 5-HT-induced inhibition of dopamine release from rat brain striatal slices. Eur J Pharmacol 153: , 89–95. |
[14] | Choi DS , Birraux G , Launay JM , Maroteaux L ((1994) ) The human serotonin 5-HT2B receptor: Pharmacological link between 5-HT2 and 5-HT1D receptors. FEBS Lett 352: , 393–399. |
[15] | Porter RH , Benwell KR , Lamb H , Malcolm CS , Allen NH , Revell DF , Adams DR , Sheardown MJ ((1999) ) Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128: , 13–20. |
[16] | Roy SM , Minasov G , Arancio O , Chico LW , Van Eldik LJ , Anderson WF , Pelletier JC , Watterson DM ((2019) ) A selective and brain penetrant p38alphaMAPK inhibitor candidate for neurologic and neuropsychiatric disorders that attenuates neuroinflammation and cognitive dysfunction. J Med Chem 62: , 5298–5311. |
[17] | Perovic S , Muller WE ((1995) ) Pharmacological profile of hypericum extract. Effect on serotonin uptake by postsynaptic receptors. Arzneimittelforschung 45: , 1145–1148. |
[18] | Puzzo D , Vitolo O , Trinchese F , Jacob JP , Palmeri A , Arancio O ((2005) ) Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci 25: , 6887–6897. |
[19] | Watterson DM , Grum-Tokars VL , Roy SM , Schavocky JP , Bradaric BD , Bachstetter AD , Xing B , Dimayuga E , Saeed F , Zhang H , Staniszewski A , Pelletier JC , Minasov G , Anderson WF , Arancio O , Van Eldik LJ ((2013) ) Development of novel in vivo chemical probes to address CNS protein kinase involvement in synaptic dysfunction. PLoS One 8: , e66226. |
[20] | Argyrousi EK , Staniszewski A , Nicholls RE , Arancio O ((2018) ) Preparation of tau oligomers after the protein extraction from bacteria and brain cortices. Methods Mol Biol 1779: , 85–97. |
[21] | Acquarone E , Argyrousi EK , van den Berg M , Gulisano W , Fa M , Staniszewski A , Calcagno E , Zuccarello E , D’Adamio L , Deng SX , Puzzo D , Arancio O , Fiorito J ((2019) ) Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade. Mol Neurodegener 14: , 26. |
[22] | Paxinos G , Katritsis DG ((2012) ) Current therapy of non-ST-elevation acute coronary syndromes. Hellenic J Cardiol 53: , 63–71. |
[23] | Chico LK , Behanna HA , Hu W , Zhong G , Roy SM , Watterson DM ((2009) ) Molecular properties and CYP2D6 substrates: Central nervous system therapeutics case study and pattern analysis of a substrate database. Drug Metab Dispos 37: , 2204–2211. |
[24] | Cushman LA , Stein K , Duffy CJ ((2008) ) Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology 71: , 888–895. |
[25] | Swainson R , Hodges JR , Galton CJ , Semple J , Michael A , Dunn BD , Iddon JL , Robbins TW , Sahakian BJ ((2001) ) Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord 12: , 265–280. |
[26] | |
[27] | Wermuth CG ((2006) ) Selective optimization of side activities: The SOSA approach. Drug Discov Today 11: , 160–164. |




