Diagnostic Sensitivity and Symptomatic Relevance of Dopamine Transporter Imaging and Myocardial Sympathetic Scintigraphy in Patients with Dementia with Lewy Bodies
Abstract
Background:
Dementia with Lewy bodies (DLB) presents with various symptoms, posing challenges for early diagnosis challenging. Dopamine transporter (123I-FP-CIT) single-photon emission tomography (SPECT) and 123I-meta-iodobenzylguanidine (123I-MIBG) imaging are crucial diagnostic biomarkers. Hypothesis about body- and brain-first subtypes of DLB indicate that some DLB may show normal 123I-FP-CIT or 123I-MIBG results; but the characteristic expression of these two subtypes remains unclear.
Objective:
This study aimed to evaluate the diagnostic sensitivity of 123I-FP-CIT and 123I-MIBG imaging alone, combined in patients with DLB and explore symptoms associated with the abnormal imaging results.
Methods:
Demographic data, clinical status, and imaging results were retrospectively collected from patients diagnosed with possible DLB. Both images were quantified using semi-automated software, and the sensitivity of each imaging modality and their combination was calculated. Demographic data, cognition, and motor and non-motor symptoms were compared among the subgroups based on the imaging results. Symptoms related to each imaging abnormality were examined using binomial logistic regression analyses.
Results:
Among 114 patients with DLB, 80 underwent 123I-FP-CIT SPECT (sensitivity: 80.3%), 83 underwent 123I-MIBG imaging (68.2%), and 66 both (sensitivity of either abnormal result: 93.9%). Visual hallucinations differed among the four subgroups based on imaging results. Additionally, nocturia and orthostatic hypotension differed between abnormal and normal 123I-MIBG images.
Conclusions:
Overall, 123I-FP-CIT SPECT was slightly higher sensitivity than 123I-MIBG imaging, with combined imaging increasing diagnostic sensitivity. Normal results of a single imaging test may not refute DLB. Autonomic symptoms may lead to abnormal 123I-MIBG scintigraphy findings indicating body-first subtype of patients with DLB.
INTRODUCTION
Dementia with Lewy bodies (DLB) is the second most common form of degenerative dementia after Alzheimer’s disease (AD), accounting for 4.2% of all dementia [1]. The pathological feature of DLB is the presence of α-synuclein aggregates, which comprise Lewy bodies and neurites in the cytoplasm of neurons [2]. Symptomatically, DLB is a diverse disease that involves multiple cognitive domains and behavioral and neurological symptoms, including inattention, visuospatial dysfunction, executive dysfunction, hallucinations, Parkinsonism, cognitive fluctuations, rapid eye movement sleep behavior disorder (RBD), and dysautonomia [3]. DLB is a heterogeneous disease presenting with various combinations of symptoms and different severity [4]. The frequency of clinical diagnosis of DLB varies between reports and the prevalence is much lower than expected, which requires standardized diagnostic practice and a robust diagnostic biomarker [5]. The adequate use of dopamine transporter (DAT) imaging and 123I-meta-iodobenzylguanidine (123I-MIBG) scintigraphy supports the diagnosis of DLB [4].
123I-2β-Carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (123I-FP-CIT), or 123I-ioflupan, a radioligand that binds to presynaptic DAT, is clinically used in single-photon emission tomography (SPECT) for diagnosing Parkinsonian syndrome and DLB. 123I-FP-CIT imaging can serve as a clinical tool for detecting nigrostriatal dopaminergic pathway degeneration [6, 7]. Previous studies have demonstrated that 123I-FP-CIT SPECT significantly improves the accuracy of DLB diagnosis and has higher diagnostic accuracy in differentiating patients with DLB from those without DLB [8–10].
In patients with DLB, Lewy bodies are widely distributed throughout the central and peripheral nervous systems, particularly in the sympathetic cardiac plexus [11, 12]. Therefore, 123I-MIBG cardiac scintigraphy is a potential biomarker for DLB in clinical diagnosis and can detect early derangement of the sympathetic nervous system in DLB [13], which strengthens the diagnostic capacity to distinguish DLB from AD [14].
Quantitative evaluation of imaging modalities can reduce inter-observer discrepancies, enable inter- and intra-individual comparisons, and may further improve the diagnosis of DLB [15]. The striatal specific binding ratio (SBR) is a quantitative indicator of radioactive isotope uptake in the striatum relative to the extrastriatal brain region based on 123I-FP-CIT SPECT images [16, 17]. Age-dependent decline in striatal 123I-FP-CIT binding has been reported in human, primate, and rodent brain histochemical studies [18]. This decline led to the z-score transformation of SBR adjusted based on age [19]. This method is currently commercially available with the advent of the DAT VIEW software. The heart-to-mediastinum uptake ratios (HMR) based on early and delayed images and the cardiac washout rate (WR) are quantitative indicators calculated from the planar image of 123I-MIBG scintigraphy [20, 21]. The WR reflects the release of the initial uptake of sympathetic nerve endings and is influenced by the reuptake and sympathetic nerve turnover rates [22]. The delayed-phase HMR represents sympathetic nerve functions, including distribution, density, and activity. HMR is calculated using the semiautomatic region-of-interest (ROI) setting software Smart MIBG [23]. This software also normalizes the HMR between different SPECT cameras and filters.
Practically, only one imaging was performed, leaving the efficacy of the combination unclear. Therefore, to evaluate the diagnostic utility of the 123I-FP-CIT image and 123I-MIBG scintigraphy in detecting DLB, the role of its combination as a diagnostic approach is of both clinical and pathophysiological interest. Emerging evidence suggests that α-synuclein likely propagates between cells in a prion-like manner [24]. In terms of the originating site of α-synuclein aggregation, two subtypes of DLB were hypothesized, namely, the “brain-first” (top-down) and “body-first” (bottom-up) types of DLB, where the abnormal 123I-FP-CIT image and abnormal 123I-MIBG scintigraphy may correspond to the “brain-first” and “body-first,” respectively [25]. Horsager and colleagues explored de novo Parkinson’s disease (PD) by multimodal imaging tests. They hypothesized that PD with RBD may show as a “body-first” subtype, resulting in lower cardiac 123I-MIBG uptake and lower parasympathetic function demonstrated as dysfunction of the gastrointestinal system, compared to PD without RBD [26]. In this report, because of the diagnosis of PD, the neurodegeneration is at least involved in the nigrostriatal system (i.e., Braak stage 3 or above [27]), and showed that presynaptic dopamine imaging was not different between “brain-first” and “body-first” PD subtype. However, based on this assumption, the characteristic expression of the two subtypes in DLB, remains unclear.
This study aimed to calculate the sensitivity of the two imaging modalities in retrospectively collected data from patients with DLB. Second, we used univariate and multivariate statistical approaches to investigate whether cognitive, motor, autonomic, sleep, and sensory symptoms accounted for the abnormal imaging results.
METHODS
Participants
The Institutional Review Board of the Chiba University Graduate School of Medicine approved this retrospective study. All participants received oral explanations of the study orally and by documents and provided written informed consent. All participants were recruited from the outpatient clinic of the Department of Neurology, Chiba University Hospital, which specializes in patients with movement disorders and dementias. The participants were included based on the fourth report of the DLB consortium criteria for possible DLB, which requires the presence of at least one core clinical feature irrespective of the imaging result [28]. The medical records of all patients were reviewed, and data on sex, age at onset, and disease duration from the onset of dementia symptoms were collected. Cognitive function was measured using the Mini-Mental State Examination (MMSE) [29]. The severity of Parkinsonism was examined using the Unified Parkinson’s Disease Rating Scale (UPDRS) motor subscore [30]. A board-certified neurologist (SH) assessed non-motor symptoms using structured clinical interviews. Constipation according to the Rome III criteria [31], nocturia is defined as two or more urinations per night [32]. Orthostatic hypotension is defined as Q1-3 of the screening test for suspected neurogenic orthostatic hypotension [33], further confirmed through occasional systolic blood pressure < 100 mmHg. Anosmia is defined as reduced subjective olfactory function reconfirmation by the caregiver. Visual hallucinations are defined as seeing aberrant objects and/or people that do not exist. RBD is defined by single-question screening for RBD [34]. No participants were scanned while taking antidepressants which might interfere with the imaging results.
Nuclear imaging protocols
123I-FP-CIT SPECT
Participants were scanned 3 h after the intravenous injection of 167 MBq 123I-FP-CIT using an Infinia+Hawkeye4 (GE Healthcare, Milwaukee, WI, USA) equipped with an extended low-energy general-purpose (ELEGP) collimator. The projection data were acquired over 30 min. The data were reconstructed using the ordered subset expectation maximization method (iterations: 5, subsets: 10). Images were acquired in a 128×128 matrix of 2.95 mm thick axial slices under 4-degree step continuous rotation (8 rotations, 3 min each) and filtered with a Butterworth filter (cutoff frequency 0.5 cycles/cm, order 8) to reconstruct the image. Attenuation was corrected using Chang’s technique (factor: 0.07). The striatal SBR was semi-quantitatively calculated based on Bolt’s method using DAT VIEW software (version 6.1, AZE Ltd., Kanagawa, Japan) [35, 36], and the age-adjusted z-score was subsequently calculated using 250 normal Japanese databases incorporated in the software [19]. A striatal SBR age-adjusted z-score of less than –2.0 on either side was considered abnormal.
123I-MIBG myocardial scintigraphy
Fifteen minutes (early) and 3.5 h (delayed) after intravenous injection of a dose of 111 MBq 123I-MIBG [37], Infinia+Hawkeye4 (GE Healthcare, Milwaukee, WI, USA) equipped with ELEGP collimator was used to capture planar scintigraphy images in the front view. No attenuation or scatter corrections were performed. Standardization was performed using Smart MIBG software (version 3.1.1.0, PD Radiopharma Inc., Tokyo, Japan) and phantom experiments [23]. In the software algorithm, manually pointing to the center of the heart sets the heart ROI as a circle. Simultaneously, a rectangular ROI is determined in the upper mediastinum with a width of 10% of the body and a height of 30% of the mediastinum, which calculates the HMR [38]. To divide the count density of the left ventricular ROI by that of the mediastinal ROI, the HMR and WR were calculated using early and delayed planar images, respectively [39]. Based on a previous report, HMR in delayed images of <2.2 and/or WR > 35% was defined as abnormal [40].
Statistical analysis
The normality of the variables was evaluated using the Kolmogorov–Smirnov test. Categorical and continuous data were compared using the chi-square and Kruskal–Wallis tests, respectively. Subsequently, post hoc analyses were performed using the Mann–Whitney U test to investigate group differences in demographic values. To assess the clinical significance of 123I-FP-CIT SPECT and 123I-MIBG image abnormalities, binominal logistic regression tests were conducted using imaging modalities with imaging results (normal/abnormal) as dependent variables, and non-motor symptoms, MMSE total score, and UPDRS part III score as independent variables. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
The demographic data of patients with DLB who underwent two imaging procedures were classified into four subgroups as follows: abnormal images, 123I-FP-CIT SPECT normal with 123I-MIBG image abnormal (DLBFP–CIT–MIBG +), 123I-FP-CIT SPECT abnormal with 123I-MIBG image normal (DLBFP–CIT +MIBG–), and both normal images. The four subgroups were compared using the chi-square test for sex and clinical symptoms. Differences in age at onset, UPDRS part III score, and MMSE total score among the four patient subgroups were analyzed using the Kruskal–Wallis test. The statistical significance threshold of each test was set at p < 0.05.
RESULTS
The number of core symptoms, number of imaging procedures and their results, as well as the disposition of the patients with DLB are shown in Fig. 1. Overall, 114 patients with DLB were included in this study. Twenty-one patients with DLB had one core clinical feature, including 15 abnormal results of the 19 123I-FP-CIT SPECT and 15 abnormal results of the 21 123I-MIBG images. Ninety-three patients with DLB had two or more core clinical features, including 49 abnormal results of the 61 123I-FP-CIT SPECT performed and 41 abnormal results of the 62 123I-MIBG images. Seventeen patients with DLB did not undergo an examination of either image.
Fig. 1
Imaging assessments and their results: disposition of patients with dementia with Lewy bodies.
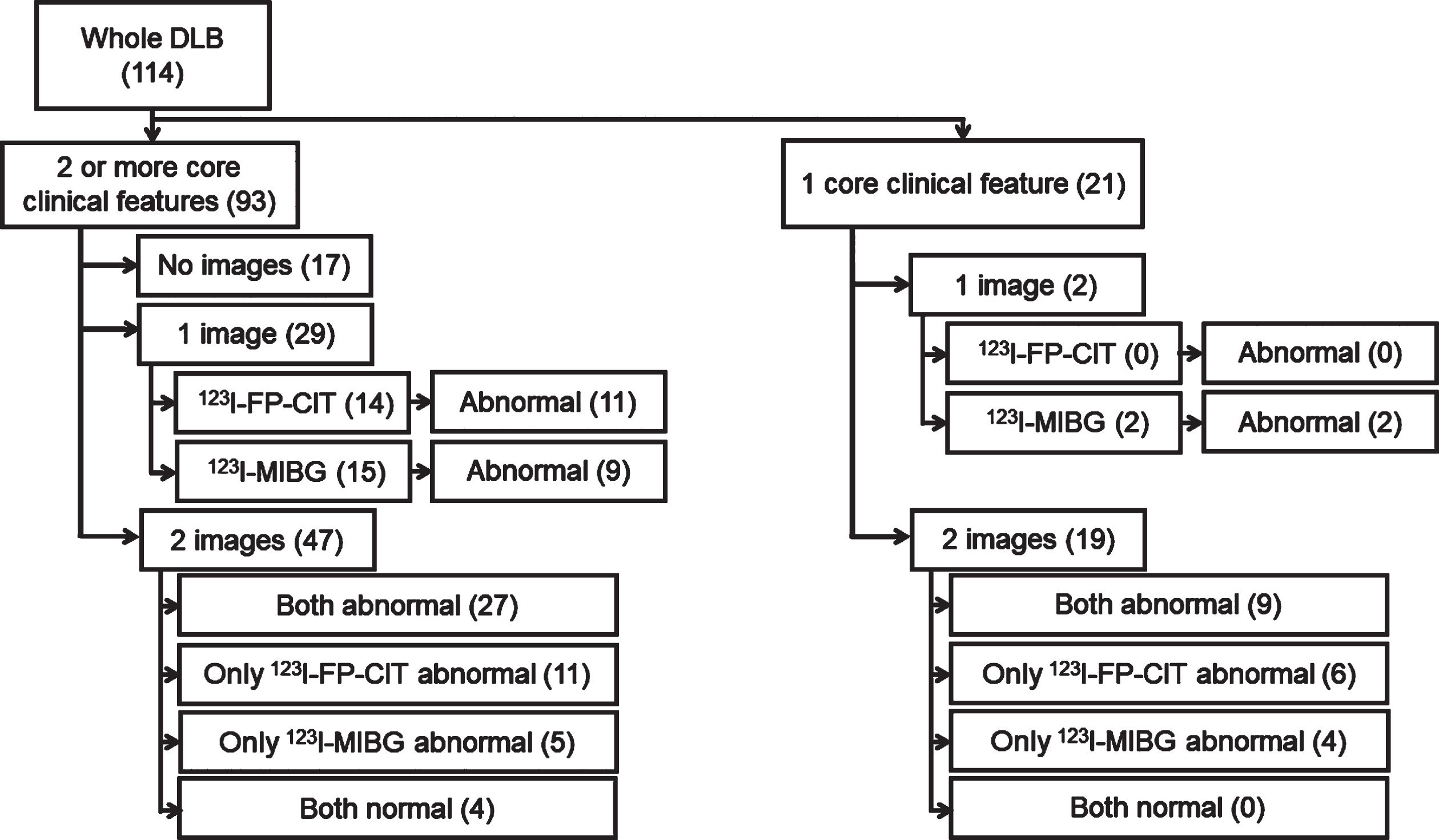
Table 1 summarizes the demographic and clinical data of the 114 patients in the entire DLB group, a subgroup of individuals with DLB who underwent 123I-FP-CIT SPECT (n = 80) and 123I-MIBG scintigraphy (n = 83). In all 114 patients with DLB, the mean age at onset was 74.9 years (standard deviation [SD] = 6.5), slightly predominant in males (58.8%), the mean MMSE total score was 21.4 (SD = 5.6), and the mean UPDRS motor subscore was 19.0 (SD = 16.0). The core clinical symptoms of the 114 patients with DLB were most commonly observed in Parkinsonism (83.3%), followed by cognitive fluctuation (64.0%), visual hallucinations (56.1%), and least frequently in RBD (40.4%). Of the 80 patients who underwent 123I-FP-CIT SPECT, 64 showed abnormal results with a sensitivity of 80.0%. Of the 83 who underwent 123I-MIBG scintigraphy, 56 patients had abnormal results with a sensitivity of 67.5%. The prevalence of core clinical symptoms, MMSE score, and motor severity did not differ between normal and abnormal imaging results on 123I-FP-CIT SPECT or 123I-MIBG scintigraphy.
Table 1
Demographic and clinical data of the patients with dementia with Lewy bodies (DLB) of whole group and subgroup who underwent dopamine transporter (123I-FP-CIT) image and 123I-meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy
| Whole | Patients with DLB who | Patients with DLB who | |||
| DLB group | underwent 123I-FP-CIT | underwent 123I-MIBG | |||
| (n = 114) | SPECT | scintigraphy | |||
| (n = 80) | (n = 83) | ||||
| 123I-FP-CIT | 123I-FP-CIT | 123I-MIBG | 123I-MIBG | ||
| abnormal | normal | abnormal | normal | ||
| (n = 64) | (n = 16) | (n = 56) | (n = 27) | ||
| Age at onset (y) | 74.9±6.5 | 74.2±7.1 | 75.4±5.7 | 74.5±7.1 | 73.9±6.6 |
| Sex, female:male | 47 : 67 | 28 : 36 | 5 : 11 | 18 : 38 | 14 : 13 |
| Disease duration (y) | 2.2±1.9 | 1.8±1.8 | 2.6±2.1 | 2.2±1.8 | 2.1±2.3 |
| MMSE | 21.4±5.6 | 22.2±4.5 | 21.9±7.1 | 21.9±5.0 | 20.2±6.9 |
| UPDRS motor subscore | 19.0±16.0 | 17.0±14.8 | 12.9±10.9 | 15.3±13.5 | 16.2±12.7 |
| Cognitive fluctuation, n (%) | 73 (64.0%) | 36 (56.3%) | 8 (50.0%) | 35 (62.5%) | 14 (51.9%) |
| Parkinsonism, n (%) | 95 (83.3%) | 54 (84.4%) | 12 (75.0%) | 42(75.0%) | 23 (85.2%) |
| Visual hallucination, n (%) | 64 (56.1%) | 34 (53.1%) | 8 (50.0%) | 28 (50.0%) | 14 (51.9%) |
| RBD, n (%) | 46 (40.4%) | 26 (40.6%) | 7 (43.8%) | 23 (41.1%) | 7 (25.9%) |
Continuous variables are presented as mean±SD. The two groups were compared using the Mann–Whitney U and chi-square tests for continuous and categorical variables, respectively. MMSE, Mini-Mental State Examination; RBD, rapid eye movement behavior disorder; SPECT, single photon emission computed tomography; UPDRS, Unified Parkinson’s Disease Rating Scale; SD, standard deviation.
Non-motor symptoms (autonomic symptoms [constipation, nocturia, and orthostatic hypotension], anosmia, RBD, visual hallucination, and MMSE) and motor symptoms (UPDRS part III) were incorporated into a statistical model to examine which factors predicted the imaging abnormality. On 123I-FP-CIT SPECT, no symptomatic differences were found between patients with DLB with abnormal and normal results (Table 2, left). Between abnormal and normal 123I-MIBG image findings in patients with DLB, differences were observed in nocturia (odds ratio = 0.160, 95% confidence interval: 0.042–0.606, p = 0.007) and orthostatic hypotension (odds ratio = 0.165, 95% confidence interval: 0.031–0.879, p = 0.035) (Table 2, right).
Table 2
The statistical results of the symptomatic predictor of imaging abnormality in 66 patients with dementia with Lewy bodies
| Abnormal 123I-FP-CIT image | Abnormal 123I-MIBG scintigraphy | |||||||
| p | Odds ratio | 95% CI for odds ratio | p | Odds ratio | 95% CI for odds ratio | |||
| Lower | Upper | Lower | Upper | |||||
| Constipation | 0.881 | 0.905 | 0.244 | 3.352 | 0.339 | 0.504 | 0.123 | 2.056 |
| Nocturia | 0.495 | 0.647 | 0.185 | 2.264 | 0.007* | 0.160 | 0.042 | 0.606 |
| OH | 0.592 | 1.426 | 0.390 | 5.215 | 0.035* | 0.165 | 0.031 | 0.879 |
| Anosmia | 0.313 | 0.521 | 0.147 | 1.849 | 0.875 | 1.118 | 0.276 | 4.529 |
| RBD | 0.732 | 1.242 | 0.359 | 4.294 | 0.358 | 0.539 | 0.114 | 2.011 |
| VH | 0.562 | 0.692 | 0.199 | 2.402 | 0.750 | 1.239 | 0.332 | 4.628 |
| MMSE | 0.845 | 1.013 | 0.893 | 1.148 | 0.966 | 0.998 | 0.900 | 1.106 |
| UPDRS | 0.536 | 1.016 | 0.966 | 1.069 | 0.330 | 0.975 | 0.928 | 1.026 |
Binomial regression analysis results of 83 and 80 patients with dementia and Lewy bodies in dopamine transporter (left) and 123I-meta-iodobenzylguanidine (right) images, respectively. *p < 0.05 OH, orthostatic hypotension; RBD, rapid eye movement behavior disorder; VH, visual hallucinations; MMSE, Mini-Mental State Examination; UPDRS, Unified Parkinson’s Disease Rating Scale; CI, confidence interval.
In the subset of 66 patients with DLB who underwent both 123I-FP-CIT SPECT and 123I-MIBG image, no differences were observed in age at onset, sex, MMSE, or UPDRS between the four subgroups classified based on imaging results, except for visual hallucinations (p = 0.006) (Table 3). The 17 DLBFP–CIT +MIBG– and 9 DLBFP–CIT–MIBG + subgroups were specifically compared; however, no demographic differences were observed (p > 0.1). Regarding sensitivity, in 66 patients with DLB who underwent two scans, 53 (80.3%) and 45 (68.2%) had abnormal 123I-FP-CIT SPECT and 123I-MIBG images, respectively; the 53 (80.3%) patients who had abnormal 123I-FP-CIT SPECT exhibited higher sensitivity. The combination of 123I-FP-CIT SPECT and 123I-MIBG images, at least one of the images being abnormal, has a sensitivity of 93.9%.
Table 3
Demographic and clinical data of the 66 patients with dementia with Lewy bodies (DLB) who underwent both dopamine transporter (123I-FP-CIT) and myocardial sympathetic imaging and four subgroups classified based on the imaging results
| DLB who underwent both 123I-FP-CIT and 123I-MIBG (n = 66) | 123I-FP-CIT (abnormal) 123I-MIBG (abnormal) (n = 36) | 123I-FP-CIT (abnormal) 123I-MIBG (normal) (n = 17) | 123I-FP-CIT (normal) 123I-MIBG (abnormal) (n = 9) | 123I-FP-CIT (normal) 123I-MIBG (normal) (n = 4) | p | |
| Age at onset (y) | 74.1±7.1 | 73.8±7.4 | 73.5±8.0 | 76.9±5.8 | 73.3±2.6 | 0.695 |
| Sex, female:male | 25;41 | 13 : 23 | 8 : 9 | 1 : 8 | 3 : 1 | 0.125 |
| Disease duration (y) | 2.0±1.9 | 1.7±1.6 | 3.1±2.2 | 2.3±2.3 | 0.67±0.90 | 0.097 |
| MMSE | 21.7±4.8 | 21.9±4.7 | 21.8±4.4 | 22.6±5.0 | 19.8±9.6 | 0.971 |
| UPDRS motor sub-score | 14.9±12.8 | 16.2±13.9 | 14.3±12.1 | 10.9±8.6 | 18.8±14.4 | 0.683 |
| Cognitive fluctuation, n (%) | 33 (50.0%) | 20 (55.6%) | 7 (41.2%) | 3 (33.3%) | 3 (75.0%) | 0.396 |
| Parkinsonism, n (%) | 53 (80.3%) | 28 (77.8%) | 15 (88.2%) | 7 (77.8%) | 3 (75.0%) | 0.929 |
| Visual hallucination, n (%) | 31 (47.0%) | 21 (58.3%) | 4(23.5%) | 2 (22.2%) | 4 (100.0%) | 0.006* |
| RBD, n (%) | 23 (34.9%) | 14 (48.9%) | 5 (29.4%) | 3 (33.3%) | 1 (25.0%) | 0.883 |
| Constipation, n (%) | 41 (62.1%) | 25 (69.4%) | 8 (47.1%) | 5 (55.6%) | 3 (75.0%) | 0.406 |
| Nocturia, n (%) | 34 (51.5%) | 23 (63.9%) | 4 (23.5%) | 5 (55.6%) | 2 (50.0%) | 0.055 |
| Orthostatic hypotension, n (%) | 17 (25.8%) | 13 (36.1%) | 1 (5.9%) | 3 (33.3%) | 0 (0.0%) | 0.066 |
| Anosmia, n (%) | 38 (57.6%) | 24 (66.7%) | 8 (47.1%) | 4 (44.4%) | 2 (50.0%) | 0.437 |
Continuous variables are presented as mean±SD. Four groups were compared using the Kruskal–Wallis one-way analysis of variance; the post hoc test was conducted using the Mann–Whitney U test, adjusted for multiple comparisons for continuous variables, and the chi-square test for categorical variables. *p < 0.05. MMSE, Mini-Mental State Examination; RBD, rapid eye movement behavior disorder; UPDRS, Unified Parkinson’s Disease Rating Scale; SD, standard deviation.
DISCUSSION
This study presents the demographic and clinical data of clinically diagnosed patients with DLB, as well as the sensitivity of 123I-FP-CIT SPECT and 123I-MIBG scintigraphy and their relationship with the symptoms. In DLB, the sensitivity of 123I-FP-CIT SPECT and 123I-MIBG scintigraphy was 80.0% and 67.5%, respectively, and 93.9% when at least one of the images was abnormal by combination. The presence of visual hallucinations differed among the four imaging-based DLB subgroups. Nocturia and orthostatic hypotension are associated with abnormal 123I-MIBG imaging results.
A previous study reported that abnormal DAT images have a sensitivity of 88.8% for detecting clinically probable DLB from AD [41]. However, DAT imaging does not distinguish DLB from other Parkinsonian syndromes [42]. In a meta-analysis, the sensitivity of DAT imaging in clinically diagnosed patients with DLB was 97% (95% confidence interval: 78–100%) [43]. The relatively low sensitivity observed in this study may have resulted from including various patients, the early disease stage examination. A subset of patients has been reported to initially have a normal 123I-FP-CIT SPECT scan, which later converts to abnormal in the follow-up 123I-FP-CIT SPECT scan, indicating that abnormality rates may differ based on disease stage [44]. Abnormal DAT imaging is a prerequisite for diagnosing PD, whereas normal 123I-FP-CIT SPECT findings in patients with DLB do not refute the diagnosis of DLB [45].
No association was found among cognitive impairment, core clinical features (including Parkinsonism), and abnormal DAT. DLB has various clinical presentations that differ between cohorts, particularly in the early or prodromal stages of the disease [46]. The lack of a difference in Parkinsonism between abnormal and normal 123I-FP-CIT SPECT was unexpected, as lower striatal DAT binding may reflect more pronounced Parkinsonism of DLB, as demonstrated in previous studies [47, 48]. However, some studies have failed to identify an association between the severity of Parkinsonism and striatal 123I-FP-CIT uptake in patients with DLB, which is consistent with the results of this study [41, 49, 50]. These results demonstrate that even patients with DLB and normal 123I-FP-CIT images may present with Parkinsonism (Table 1). This result demonstrates that the Parkinsonism observed in patients with DLB is somewhat different from that in those with PD, with DLB deficits extending outside of the nigrostriatal system. These differences may be consistent with the difference in levodopa responsiveness between patients with PD and DLB [51]. Although some studies have demonstrated a relationship between executive dysfunction and striatal DAT binding [47], others have shown no correlation between cognitive function and striatal DAT uptake in patients with DLB [48]. These results indicate that specific cognitive domains, such as executive function, rather than general cognitive function, as well as specific striatal regions, may account for dopaminergic dysfunction in DLB [52]. Cognitive dysfunction in DLB may be multifactorial, including dopaminergic and cholinergic dysfunctions [52]. A previous study showed a significant correlation between reduced DAT uptake in the striatum and visual hallucinations [50]. The degree of visual hallucinations and their presence may differ in pathophysiology; the degree of visual hallucinations may be associated with the striatal dopaminergic system. Another previous study reported that the PD-with-RBD group had more severe striatal dopamine dysfunction than the PD-without-RBD group [53]; however, this was not replicated in this study. To the best of our knowledge, no study has found a correlation between striatal function and cognitive fluctuations, consistent with these results. Most core and non-motor symptoms were examined by asking both patients and caregivers of DLB. In contrast, MMSE and UPDRS were performed only on patients with DLB, which may be influenced by cognitive fluctuation and may vary between the time of examination and scan, leading to no correlation for these scores.
A recent meta-analysis found that the sensitivity of delayed-phase 123I-MIBG image in diagnosing clinical DLB was 93% (95% confidence interval: 81–98%) [43]. Autopsy-confirmed DLB revealed that the sensitivity for delayed HMR of 123I-MIBG scintigraphy was 80.0% (95% confidence interval; 61.4–92.3%) [54]. The sensitivity was slightly lower, possibly resulting from a large number of participants with various initial symptoms. However, it also indicates that DLB can show normal 123I-MIBG image results and normal results may be insufficient to exclude the diagnosis of DLB. A previous neuropathological study reported that DLB with concomitant AD pathology shows relatively preserved cardiac sympathetic function [55], which may account for the lower sensitivity depending on the recruited cohort, where most patients were enrolled from a dementia outpatient clinic.
Similar to the 123I-FP-CIT images, no correlation was observed between 123I-MIBG image results and cognitive impairment, cognitive fluctuation, Parkinsonism, hallucinations, or RBD, which is consistent with a previous report [56]. A previous study showed that the delayed image HMR in the PD-without-RBD group was higher than that in the PD-with-RBD [26], although the situation may differ between PD and DLB. Cardiac autonomic nervous system failure caused by the degeneration of postganglionic sympathetic neurons is also a common feature of DLB [57]. In this study, orthostatic hypotension was associated with abnormal 123I-MIBG image findings. Previous studies have also demonstrated that the average delayed HMR of patients in the DLB group was lower than that of healthy individuals and those in the PD group, corresponding to the high incidence of orthostatic hypotension in the DLB group [58, 59]. To the best of our knowledge, this is the first study to report the relationship between urinary frequency and lower 123I-MIBG HMR in patients with DLB. A hypothesis has been proposed that in the early stages of PD, two different types of neuronal degeneration occur as follows: the dopamine system degenerates first (“brain-first”), and the peripheral autonomic nervous system degenerates first (“body-first”) [26]. The orthostatic hypotension and the urinary frequency may be the symptoms of the “body-first” DLB subtype [60, 61]. Although this result did not show any clinical characteristics in 9 patients with DLBFP–CIT–MIBG +, it may have resulted from the small number of this specific subgroup. A study has proposed the hypothesis that patients with DLB may be more likely to align with the “body-first” subtype [62].
Regarding the sensitivity of the two detection methods, the sensitivity of 123I-FP-CIT SPECT (80.3%) was slightly higher than that of the 123I-MIBG image (68.2%). However, this does not indicate the superiority or inferiority of the test. When first scanned 123I-FP-CIT SPECT were normal, 69.2% (9/13) were abnormal in 123I-MIBG image and first scanned 123I-MIBG image were normal, 81.0% (17/21) had abnormal 123I-FP-CIT image, both of which helped overlooking the diagnosis of DLB by performing two imaging tests. Both tests should be performed, depending on the likelihood, to narrow down the diagnosis, as each test examines different pathological aspects. A previous pathologically proven DLB study showed that 27 of the 30 (90.0%) DLB cases demonstrated both 123I-FP-CIT SPECT and 123I-MIBG images as abnormal, and two (6.7%) showed normal results [63]. The sensitivities for distinguishing clinically diagnosed 76 DLB cases from 57 AD cases using SBR of 123I-FP-CIT SPECT and HMR of 123I-MIBG scintigraphy were 88.2% and 72.4%, respectively, and it was demonstrated that the sensitivity increased to 96.1% compared with using either of these two methods [41]. This finding is consistent with the results of this study. Visual hallucination was more present in patients with DLB with both imaging abnormalities (58.3%) compared to those with only one abnormality. These findings may indicate that visual hallucinations are likely to appear when pathological involvement is diffuse. Although orthostatic hypotension and nocturia were close to statistical significance, no clinical features distinguished between 17 patients with DLBFP–CIT +MIBG– and 9 patients with DLBFP–CIT–MIBG +, probably because of the lack of number of participants. Altogether, 30 out of 66 patients (45.5%) had at least one normal result, probably deviating from Braak’s staging framework [27]. RBD and gastrointestinal symptoms shown in the “body-first” PD subtype in the previous study [26] were not replicated in this DLB study, nor was it related to 123I-MIBG abnormality, indicating that some DLB patients exhibit RBD in normal 123I-MIBG image, implying somewhat different progression between PD and DLB. Lifestyle modification or medications may have improved constipation. Depending on the diagnostic certainty, physicians are less likely to refute the diagnosis of DLB based on a single normal scan, and the other scan is needed to accurately diagnose DLB, which mirrors the fact that a single abnormal scan, may be sufficient for diagnosing DLB. We propose that, when autonomic dysfunction was present in DLB, 123I-MIBG cardiac scintigraphy maybe the initial imaging investigation.
The strength of this study lies in the analysis of imaging using semi-automated quantitative methods; however, the diagnosis may differ from the visual inspection. Additionally, this observation was based on real-world data and did not exclude patients based on imaging results. We made an effort not to exclude DLB by imaging results, emphasizing the value of clinical symptoms in diagnosing DLB. The early detection of DLB is crucial in clinical practice, since DLB deteriorates fast, and has high risk of falling, while early intervention by introducing cholinesterase inhibitors and eliminating redundant medications may induce better outcomes. Nevertheless, this study had some limitations. First, the diagnosis was based on clinical criteria, which may include patients without DLB. However, we believe that this study illustrates a real-world clinical situation. Second, the clinical symptoms collected in this study were primarily based on subjective symptoms, which may differ from objective measurements, and may have included common comorbidities, such as nocturia in prostate hypertrophy with male patients. However, the value of patient-oriented reports is increasing in medical research and does not reduce the value of these findings. Third, this study was conducted at a single tertiary dementia center, which may have introduced bias in the cohort. Multicenter studies are needed since the evaluation methods for SBR and HMR used in this study are comparable between different cameras, which increases the number of patients with DLB. Fourth, the cut-off value for each scan was arbitrarily defined, which may have resulted in the under-recognition of near abnormal results and lower sensitivity. Lastly, the number of subgroups in DLB was small (i.e., 17 patients with DLBFP–CIT +MIBG– and 9 patients with DLBFP–CIT–MIBG +). Together, there are 26 (39.4%) who had normal imaging results in either test, which we believe it is not a small proportion of DLB, worth reporting, but requires further observation from future studies to draw any conclusion regarding the symptomatic difference, such as orthostatic hypotension and nocturia showing near significance (Table 3). Fifth, specificity was unable to calculate due to lack of control group.
Conclusion
Regarding stand-alone diagnostic methods, the sensitivity of 123I-FP-CIT SPECT was slightly higher than that of 123I-MIBG scintigraphy for DLB diagnosis. However, this does not indicate the superiority of the 123I-FP-CIT image, but when autonomic dysfunction was present, 123I-MIBG may be preferable for initial testing. Both tests should be used to increase the accuracy, since a- single normal imaging result does not refute the diagnosis of DLB. Nocturia and orthostatic hypotension are associated with reduced 123I-MIBG cardiac uptake, demonstrating a “body-first” subtype in patients with DLB.
AUTHOR CONTRIBUTIONS
Shigeki Hirano (Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Writing – original draft; Writing – review & editing); Zhihui Tang (Data curation; Formal analysis; Investigation; Writing – original draft); Yume Koizumi (Data curation; Project administration; Writing – review & editing); Michiko Izumi (Data curation; Writing – review & editing); Yoshihisa Kitayama (Data curation; Writing – review & editing); Kosuke Yamagishi (Data curation; Writing – review & editing); Mitsuyoshi Tamura (Data curation; Writing – review & editing); Ai Ishikawa (Data curation; Project administration; Writing – review & editing); Kouichi Kashiwado (Data curation; Project administration; Writing – review & editing); Takashi Iimori (Methodology; Software; Writing – review & editing); Hiroki Mukai (Methodology; Software; Visualization; Writing – review & editing); Hajime Yokota (Software; Visualization; Writing – review & editing); Takuro Horikoshi (Software; Visualization; Writing – review & editing); Takashi Uno (Supervision; Writing – review & editing); Satoshi Kuwabara (Supervision; Writing – review & editing).
ACKNOWLEDGMENTS
We wish to thank the staff of the Department of Radiology, Chiba University Hospital, Chiba, Japan, for their technical assistance with SPECT image acquisition. We would also like to thank the neuropsychologists at the Dementia Medical/Care Center, Chiba University, Japan, supported by Chiba City, for advising on neuropsychological testing.
FUNDING
This work was supported by JSPS KAKENHI (Grant Number 23K06922).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
Numbers in the parenthesis indicate the number of participants.
REFERENCES
[1] | Vann Jones SA , O’Brien JT ((2014) ) The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol Med 44: , 673–683. |
[2] | Pasha T , Zatorska A , Sharipov D , Rogelj B , Hortobágyi T , Hirth F ((2021) ) Karyopherin abnormalities in neurodegenerative proteinopathies. Brain 144: , 2915–2932. |
[3] | Hershey LA , Coleman-Jackson R ((2019) ) Pharmacological management of dementia with Lewy bodies. Drugs Aging 36: , 309–319. |
[4] | McKeith IG , Ferman TJ , Thomas AJ , Blanc F , Boeve BF , Fujishiro H , Kantarci K , Muscio C , O’Brien JT , Postuma RB , Aarsland D , Ballard C , Bonanni L , Donaghy P , Emre M , Galvin JE , Galasko D , Goldman JG , Gomperts SN , Honig LS , Ikeda M , Leverenz JB , Lewis SJG , Marder KS , Masellis M , Salmon DP , Taylor JP , Tsuang DW , Walker Z , Tiraboschi P; prodromal DLB Diagnostic Study Group ((2020) ) Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94: , 743–755. |
[5] | Kane JPM , Surendranathan A , Bentley A , Barker SAH , Taylor JP , Thomas AJ , Allan LM , McNally RJ , James PW , McKeith IG , Burn DJ , O’Brien JT ((2018) ) Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 10: , 19. |
[6] | Benamer TS , Patterson J , Grosset DG , Booij J , de Bruin K , van Royen E , Speelman JD , Horstink MH , Sips HJ , Dierckx RA , Versijpt J , Decoo D , Van Der Linden C , Hadley DM , Doder M , Lees AJ , Costa DC , Gacinovic S , Oertel WH , Pogarell O , Hoeffken H , Joseph K , Tatsch K , Schwarz J , Ries V ((2000) ) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: The [123I]-FP-CIT study group. Mov Disord 15: , 503–510. |
[7] | Booij J , Speelman JD , Horstink MW , Wolters EC ((2001) ) The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 28: , 266–272. |
[8] | McKeith I , O’Brien J , Walker Z , Tatsch K , Booij J , Darcourt J , Padovani A , Giubbini R , Bonuccelli U , Volterrani D , Holmes C , Kemp P , Tabet N , Meyer I , Reininger C; DLB Study Group ((2007) ) Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol 6: , 305–313. |
[9] | Walker Z , Costa DC , Walker RW , Lee L , Livingston G , Jaros E , Perry R , McKeith I , Katona CL ((2004) ) Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: A comparison. Neurology 62: , 1568–1572. |
[10] | Treglia G , Cason E , Cortelli P , Gabellini A , Liguori R , Bagnato A , Giordano A , Fagioli G ((2014) ) Iodine-123 metaiodobenzylguanidine scintigraphy and iodine-123 ioflupane single photon emission computed tomography in Lewy body diseases: Complementary or alternative techniques? J Neuroimaging 24: , 149–154. |
[11] | Tamaki N , Kuge Y , Yoshinaga K ((2013) ) Molecular imaging in heart failure patients. Clin Transl Imaging 1: , 341–351. |
[12] | Tanei ZI , Saito Y , Ito S , Matsubara T , Motoda A , Yamazaki M , Sakashita Y , Kawakami I , Ikemura M , Tanaka S , Sengoku R , Arai T , Murayama S ((2021) ) Lewy pathology of the esophagus correlates with the progression of Lewy body disease: A Japanese cohort study of autopsy cases. Acta Neuropathol 141: , 25–37. |
[13] | Bousiges O , Blanc F ((2022) ) Biomarkers of dementia with Lewy bodies: Differential diagnostic with Alzheimer’s disease. Int J Mol Sci 23: , 6371. |
[14] | Hanyu H , Shimizu S , Hirao K , Kanetaka H , Iwamoto T , Chikamori T , Usui Y , Yamashina A , Koizumi K , Abe K ((2006) ) Comparative value of brain perfusion SPECT and [123I]MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 33: , 248–253. |
[15] | Iwabuchi Y , Kameyama M , Matsusaka Y , Narimatsu H , Hashimoto M , Seki M , Ito D , Tabuchi H , Yamada Y , Jinzaki M ((2021) ) A diagnostic strategy for Parkinsonian syndromes using quantitative indices of DAT SPECT and MIBG scintigraphy: An investigation using the classification and regression tree analysis. Eur J Nucl Med Mol Imaging 48: , 1833–1841. |
[16] | Iwabuchi Y , Nakahara T , Kameyama M , Yamada Y , Hashimoto M , Matsusaka Y , Osada T , Ito D , Tabuchi H , Jinzaki M ((2019) ) Impact of a combination of quantitative indices representing uptake intensity, shape, and asymmetry in DAT SPECT using machine learning: Comparison of different volume of interest settings. EJNMMI Res 9: , 7. |
[17] | Yokoyama K , Imabayashi E , Sumida K , Sone D , Kimura Y , Sato N , Mukai Y , Murata M , Matsuda H ((2017) ) Computed-tomography-guided anatomic standardization for quantitative assessment of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging 44: , 366–372. |
[18] | Hebert MA , Larson GA , Zahniser NR , Gerhardt GA ((1999) ) Age-related reductions in [3H]WIN 35,428 binding to the dopamine transporter in nigrostriatal and mesolimbic brain regions of the fischer 344 rat. J Pharmacol Exp Ther 288: , 1334–1339. |
[19] | Matsuda H , Murata M , Mukai Y , Sako K , Ono H , Toyama H , Inui Y , Taki Y , Shimomura H , Nagayama H , Tateno A , Ono K , Murakami H , Kono A , Hirano S , Kuwabara S , Maikusa N , Ogawa M , Imabayashi E , Sato N , Takano H , Hatazawa J , Takahashi R ((2018) ) Japanese multicenter database of healthy controls for [123I]FP-CIT SPECT. Eur J Nucl Med Mol Imaging 45: , 1405–1416. |
[20] | Kashihara K , Ohno M , Kawada S , Okumura Y ((2006) ) Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson’s disease and dementia with Lewy bodies. J Nucl Med 47: , 1099–1101. |
[21] | Uchiyama Y , Momose M , Kondo C , Kusakabe K , Uchiyama S ((2011) ) Comparison of parameters of (123)I-metaiodobenzylguanidine scintigraphy for differential diagnosis in patients with parkinsonism: Correlation with clinical features. Ann Nucl Med 25: , 478–485. |
[22] | Skowronek C , Zange L , Lipp A ((2019) ) Cardiac 123I-MIBG scintigraphy in neurodegenerative Parkinson syndromes: Performance and pitfalls in clinical practice. Front Neurol 10: , 152. |
[23] | Nakajima K , Okuda K , Yoshimura M , Matsuo S , Wakabayashi H , Imanishi Y , Kinuya S. ((2014) ) Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol 21: , 970–978. |
[24] | Ma J , Gao J , Wang J , Xie A ((2019) ) Prion-like mechanisms in Parkinson’s disease. Front Neurosci 13: , 552. |
[25] | Borghammer P , Horsager J , Andersen K , Van Den Berge N , Raunio A , Murayama S , Parkkinen L , Myllykangas L ((2021) ) Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol Dis 161: , 105557. |
[26] | Horsager J , Andersen KB , Knudsen K , Skjaerbaek C , Fedorova TD , Okkels N , Schaeffer E , Bonkat SK , Geday J , Otto M , Sommerauer M , Danielsen EH , Bech E , Kraft J , Munk OL , Hansen SD , Pavese N , Goder R , Brooks DJ , Berg D , Borghammer P ((2020) ) Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 143: , 3077–3088. |
[27] | Braak H , Del Tredici K , Bratzke H , Hamm-Clement J , Sandmann-Keil D , Rub U ((2002) ) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249: (Suppl 3), III/1-5. |
[28] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor JP , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[29] | Su Y , Dong J , Sun J , Zhang Y , Ma S , Li M , Zhang A , Cheng B , Cai S , Bao Q , Wang S , Zhu P ((2021) ) Cognitive function assessed by Mini-mental state examination and risk of all-cause mortality: A community-based prospective cohort study. BMC Geriatr 21: , 524. |
[30] | Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease ((2003) ) The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord 18: , 738–750. |
[31] | Rome F ((2006) ) Guidelines–Rome III diagnostic criteria for functional gastrointestinal disorders. Liver Dis 15: , 307–312. |
[32] | Denys MA , Cherian J , Rahnama’i MS , O’Connell KA , Singer J , Wein AJ , Dhondt K , Everaert K , Weiss JP ((2018) ) ICI-RS 2015-Is a better understanding of sleep the key in managing nocturia? Neurourol Urodyn 37: , 2048–2052. |
[33] | Gibbons CH , Schmidt P , Biaggioni I , Frazier-Mills C , Freeman R , Isaacson S , Karabin B , Kuritzky L , Lew M , Low P , Mehdirad A , Raj SR , Vernino S , Kaufmann H ((2017) ) The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 264: , 1567–1582. |
[34] | Postuma RB , Arnulf I , Hogl B , Iranzo A , Miyamoto T , Dauvilliers Y , Oertel W , Ju YE , Puligheddu M , Jennum P , Pelletier A , Wolfson C , Leu-Semenescu S , Frauscher B , Miyamoto M , Cochen De Cock V , Unger MM , Stiasny-Kolster K , Fantini ML , Montplaisir JY ((2012) ) A single-question screen for rapid eye movement sleep behavior disorder: A multicenter validation study. Mov Disord 27: , 913–916. |
[35] | Tossici-Bolt L , Hoffmann SM , Kemp PM , Mehta RL , Fleming JS ((2006) ) Quantification of [123I]FP-CIT SPECT brain images: An accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging 33: , 1491–1499. |
[36] | Miyai M , Yamamoto Y , Uchibe T , Yada N , Haramoto M , Katsube T , Kitagaki H ((2015) ) [Comparison of Quantitative Value of Dopamine Transporter Scintigraphy Calculated from Different Analytical Software]. Nihon Hoshasen Gijutsu Gakkai Zasshi 71: , 1209–1214. |
[37] | Travin MI , Matsunari I , Thomas GS , Nakajima K , Yoshinaga K ((2019) ) How do we establish cardiac sympathetic nervous system imaging with 123I-mIBG in clinical practice? Perspectives and lessons from Japan and the US. J Nucl Cardiol 26: , 1434–1451. |
[38] | Okuda K , Nakajima K , Hosoya T , Ishikawa T , Konishi T , Matsubara K , Matsuo S , Kinuya S ((2011) ) Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac Iodine-123 MIBG imaging. J Nucl Cardiol 18: , 82–89. |
[39] | Maruyama Y , Yamada T , Murakami K , Kumano R. ((2015) ) Comparison of the diagnostic performance of H/M ratio between early and delayed phases for Lewy body disease. Nucl Med Commun 36: , 477–480. |
[40] | Nakajima K , Matsumoto N , Kasai T , Matsuo S , Kiso K , Okuda K ((2016) ) Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med 30: , 188–199. |
[41] | Shimizu S , Hirao K , Kanetaka H , Namioka N , Hatanaka H , Hirose D , Fukasawa R , Umahara T , Sakurai H , Hanyu H ((2016) ) Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43: , 184–192. |
[42] | Chang CC , Liu JS , Chang YY , Chang WN , Chen SS , Lee CH ((2008) ) (99m)Tc-ethyl cysteinate dimer brain SPECT findings in early stage of dementia with Lewy bodies and Parkinson’s disease patients: A correlation with neuropsychological tests. Eur J Neurol 15: , 61–65. |
[43] | Nihashi T , Ito K , Terasawa T ((2020) ) Diagnostic accuracy of DAT-SPECT and MIBG scintigraphy for dementia with Lewy bodies: An updated systematic review and Bayesian latent class model meta-analysis. Eur J Nucl Med Mol Imaging 47: , 1984–1997. |
[44] | van der Zande JJ , Booij J , Scheltens P , Raijmakers PG , Lemstra AW ((2016) ) [(123)]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 43: , 1060–1066. |
[45] | Palermo G , Ceravolo R ((2019) ) Molecular imaging of the dopamine transporter. Cells 8: , 872. |
[46] | Blanc F , Bouteloup V , Paquet C , Chupin M , Pasquier F , Gabelle A , Ceccaldi M , de Sousa PL , Krolak-Salmon P , David R , Fischer C , Dartigues JF , Wallon D , Moreaud O , Sauvée M , Belin C , Harston S , Botzung A , Albasser T , Demuynck C , Namer I , Habert MO , Kremer S , Bousiges O , Verny M , Muller C , Philippi N , Chene G , Cretin B , Mangin JF , Dufouil C ((2022) ) Prodromal characteristics of dementia with Lewy bodies: ine results of the MEMENTO memory clinics nationwide cohort. Alzheimers Res Ther 14: , 96–Basel. |
[47] | Siepel FJ , Dalen I , Grüner R , Booij J , Brønnick KS , Buter TC , Aarsland D ((2016) ) Loss of dopamine transporter binding and clinical symptoms in dementia with Lewy bodies. Mov Disord 31: , 118–125. |
[48] | Shimizu S , Hirose D , Namioka N , Kanetaka H , Hirao K , Hatanaka H , Takenoshita N , Kaneko Y , Ogawa Y , Umahara T , Sakurai H , Hanyu H ((2017) ) Correlation between clinical symptoms and striatal DAT uptake in patients with DLB. Ann Nucl Med 31: , 390–398. |
[49] | Ziebell M , Andersen BB , Pinworm LH , Knudsen GM , Stokholm J , Thomsen G , Karlsborg M , Høgh P , Mørk ML , Hasselbalch SG ((2013) ) Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J Nucl Med 54: , 1072–1076. |
[50] | Roselli F , Pisciotta NM , Perneczky R , Pennelli M , Aniello MS , De Caro MF , Ferrannini E , Tartaglione B , Defazio G , Rubini G , Livrea P ((2009) ) Severity of neuropsychiatric symptoms and dopamine transporter levels in dementia with Lewy bodies: A 123I-FP-CIT SPECT study. Mov Disord 24: , 2097–2103. |
[51] | Molloy S , McKeith IG , O’Brien JT , Burn DJ ((2005) ) The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 76: , 1200–1203. |
[52] | Hirano S ((2021) ) Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol Med 27: , 40. |
[53] | Kwak IH , Lee YK , Ma HI , Lee S , Yun M , Kim YJ , Hwang HS , Kim YE ((2023) ) Striatal subregion analysis associated with REM sleep behavior disorder in Parkinson’s disease. J Integr Neurosci 22: , 18. |
[54] | Matsubara T , Kameyama M , Tanaka N , Sengoku R , Orita M , Furuta K , Iwata A , Arai T , Maruyama H , Saito Y , Murayama S ((2022) ) Autopsy validation of the diagnostic accuracy of 123I-Metaiodobenzylguanidine myocardial scintigraphy for Lewy body disease. Neurology 98: , e1648–e1659. |
[55] | Takahashi M , Uchihara T , Yoshida M , Wakabayashi K , Kakita A , Takahashi H , Toru S , Orimo S ((2020) ) Clinical and pathological features affecting cardiac sympathetic denervation in autopsy-confirmed dementia with Lewy bodies. Eur J Neurol 27: , 1155–1163. |
[56] | Kobayashi S , Tateno M , Morii H , Utsumi K , Saito T ((2009) ) Decreased cardiac MIBG uptake, its correlation with clinical symptoms in dementia with Lewy bodies. Psychiatry Res 174: , 76–80. |
[57] | Jeong YJ , Jeong JE , Cheon SM , Yoon BA , Kim JW , Kang DY ((2020) ) Relationship between the washout rate of I-123 MIBG scans and autonomic function in Parkinson’s disease. PLoS One 15: , e0229860. |
[58] | Kim JS , Park HE , Oh YS , Song IU , Yang DW , Park JW , Lee KS ((2015) ) 123I-MIBG myocardial scintigraphy and neurocirculatory abnormalities in patients with dementia with Lewy bodies and Alzheimer’s disease. J Neurol Sci 357: , 173–177. |
[59] | Matsui H , Nishinaka K , Oda M , Komatsu K , Kubori T , Udaka F ((2006) ) Does cardiac metaiodobenzylguanidine (MIBG) uptake in Parkinson’s disease correlate with major autonomic symptoms? Parkinsonism Relat Disord 12: , 284–288. |
[60] | Oka H , Umehara T , Nakahara A , Matsuno H ((2020) ) Comparisons of cardiovascular dysautonomia and cognitive impairment between de novo Parkinson’s disease and de novo dementia with Lewy bodies. BMC Neurol 20: , 350. |
[61] | Borghammer P ((2023) ) The brain-first vs. body-first model of Parkinson’s disease with comparison to alternative models. J Neural Transm 130: , 737–753. |
[62] | Fedorova TD , Knudsen K , Horsager J , Hansen AK , Okkels N , Gottrup H , Vang K , Borghammer P ((2023) ) Dopaminergic dysfunction is more symmetric in dementia with Lewy bodies compared to Parkinson’s disease. J Parkinsons Dis 13: , 515–523. |
[63] | Tiraboschi P , Corso A , Guerra UP , Nobili F , Piccardo A , Calcagni ML , Volterrani D , Cecchin D , Tettamanti M , Antelmi L , Vidale S , Sacco L , Merello M , Stefanini S , Micheli A , Vai P , Capitanio S , Gabanelli SV , Riva R , Pinto P , Biffi AM , Muscio C; SCILLA Working Group ((2016) ) (123) I-2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single photon emission computed tomography and (123) I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with Lewy bodies from other dementias: A comparative study. Ann Neurol 80: , 368–3678. |




