A Mediterranean Diet and Walking Intervention to Reduce Cognitive Decline and Dementia Risk in Independently Living Older Australians: The MedWalk Randomized Controlled Trial Experimental Protocol, Including COVID-19 Related Modifications and Baseline Characteristics
Abstract
Background:
Several clinical trials have examined diet and physical activity lifestyle changes as mitigation strategies for risk factors linked to cognitive decline and dementias such as Alzheimer’s disease. However, the ability to modify these behaviors longer term, to impact cognitive health has remained elusive.
Objective:
The MedWalk trial’s primary aim is to investigate whether longer-term adherence to a Mediterranean-style diet and regular walking, delivered through motivational interviewing and cognitive-behavioral therapy (MI-CBT), can reduce age-associated cognitive decline and other dementia risk factors in older, independently living individuals without cognitive impairment.
Methods:
MedWalk, a one-year cluster-randomized controlled trial across two Australian states, recruited 60–90-year-old people from independent living retirement villages and the wider community. Participants were assigned to either the MedWalk intervention or a control group (maintaining their usual diet and physical activity). The primary outcome is 12-month change in visual memory and learning assessed from errors on the Paired Associates Learning Task of the Cambridge Neuropsychological Test Automated Battery. Secondary outcomes include cognition, mood, cardiovascular function, biomarkers related to nutrient status and cognitive decline, MI-CBT effectiveness, Mediterranean diet adherence, physical activity, quality of life, cost-effectiveness, and health economic evaluation.
Progress and Discussion:
Although COVID-19 impacts over two years necessitated a reduced timeline and sample size, MedWalk retains sufficient power to address its aims and hypotheses. Baseline testing has been completed with 157 participants, who will be followed over 12 months. If successful, MedWalk will inform interventions that could substantially reduce dementia incidence and ameliorate cognitive decline in the community.
Trial registration:
Registered on the Australia New Zealand Clinical Trials Registry ANZCTR 12620000978965 (https://www.anzctr.org.au).
INTRODUCTION
Age-related cognitive decline typically commences in the third decade of life and continues into old age [1], with processes such as episodic memory and spatial working memory declining significantly with age [1]. In some cases, more rapid decline in these cognitive processes is a sign of neuropathological deterioration leading to dementia. Importantly this progression depends on several risk factors which can be positively modified by lifestyle factors, including diet and physical activity (PA). Cardiovascular disease risk measures, such as elevated blood pressure, arterial stiffness [2], impaired glucose regulation and inflammation [3] have an etiological role in cognitive decline and risk of dementia [4], and represent tractable targets for exercise and dietary interventions to reduce cognitive decline and dementia risk.
The World Health Organization [5] and 2020 Lancet Commissions report on Dementia Prevention, Intervention and Care [6] prioritize the need to target known modifiable risk factors, with both PA and Mediterranean diet (MedDiet) being highlighted as interventions with high potential for success in delaying dementia. Nevertheless, evidence to date has been predominately derived from prospective cohort and preclinical studies [7]. The Spanish PREDIMED trial is a notable example of a MedDiet intervention, finding reduced cardiovascular risk in MedDiet groups supplemented with extra-virgin olive oil or nuts relative to those prescribed a low-fat diet [8]. Representing a secondary outcome in a subsample, PREDIMED has also shown MedDiet interventions in older at-risk people are effective for significantly improving memory and other cognitive functions [9]. Similarly, the Australian-based Medley trial [10, 11] showed benefits to cardiovascular risk profile including improved systolic blood pressure and endothelial function, lower triglyceride and F2-isoprostanes (marker of lipid peroxidation) levels over 6 months, but no improvements in cognition [12–14]. In a follow-up, 12 months after trial completion some, but not all, MedDiet principles were maintained, and cardiovascular scores returned to baseline levels [14]. This highlights the importance of not only providing education to encourage diet and PA change, but also providing the tools to support people to sustain lifestyle behavioral changes in the longer term and maintain health benefits.
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial demonstrated the potential to alter cognitive aging trajectories through modification of risk factors by targeting lifestyle activities [15]. FINGER delivered a multimodal intervention to 1,260 participants aged 60–77 years who were at risk of cardiovascular disease and dementia. This intervention, which consisted of nutritional guidance, exercise, cognitive training, social activity, and management of metabolic/vascular risk factors, resulted in reduced cognitive decline over 2 years (measured by the modified Neuropsychological Test Battery). While these findings support the efficacy of multimodal approaches, this complement of interventions would be prohibitively expensive to implement widely [16]. Moreover, it was unclear if the guidance received had led to a change in longer term behavior beyond the duration of the intervention. There are numerous ongoing trials investigating similar approaches, building on the findings of FINGER including the World-Wide FINGERS network [17], using both face to face guidance and WEB-based education and coaching, that is potentially more scalable across populations [16, 18].
The LIILAC randomized controlled trial was a 6 month, 4-arm intervention assessing the effect of: 1) MedDiet, 2) regular walking, 3) combined MedDiet and walking, and 4) habitual lifestyle (control), in people aged 60–90 years who were living independently in retirement villages [19]. Controlling for age, gender, education, and initial cognitive performance, significantly better spatial working memory was observed for the combined diet and exercise group relative to the control group after 6 months. Moreover, in the combined intervention group, as well as the exercise-only group, there was an overall improvement in mood, assessed using the Depression Anxiety Stress Scale [20].
Building on these findings from the LIILAC pilot study, the primary aim of MedWalk is to determine the efficacy of a combined MedDiet and walking intervention (MedWalk), grounded in established psychosocial behavioral change techniques, to attenuate cognitive decline, in independently living older individuals without cognitive impairment. Distinct from LIILAC, MedWalk uses proven, effective behavior strategy, specifically integrated Motivational Interviewing and Cognitive Behavioral Therapy (MI-CBT) [21–23], to improve existing patterns of diet and PA for longer term impact on modifiable dementia risk factors. MI is a goal-orientated technique effective in overcoming ambivalence to elicit personal commitment to change behavior. CBT includes action-orientated treatments to build and support maintenance skills [24].
Importantly, the progression of cognitive decline depends on several risk factors which can be positively modified by lifestyle factors, including diet and exercise. Greater cardiovascular disease risk measures, such as elevated blood pressure, arterial stiffness [2], impaired glucoregulation and inflammation [3], have an etiological role in cognitive decline and risk of dementia and represent tractable targets for exercise and dietary interventions to reduce cognitive decline and dementia risk.
MedWalk secondary aims will investigate effects on mood and quality of life, cardiovascular function and arterial stiffness, and biomarkers associated with cognitive decline, including glucose regulation, inflammation, oxidative stress, and nutrient status. The study also aims to assess the effectiveness of the MedDiet and walking program in promoting and maintaining lifestyle behavior change, cost effectiveness, and health economic impact.
The primary hypothesis is that established and robust measures of cognitive performance will show less decline in participants in the MedWalk intervention program, relative to controls (maintaining habitual lifestyle). Furthermore, it is hypothesized that MedWalk intervention participants would show improvements in mood, cardiovascular function, and blood biomarkers relative to controls.
METHODS
Trial design
MedWalk was designed as a two-year multi-site (across Adelaide, South Australia and Melbourne, Victoria) cluster randomized controlled trial, in older Australian adults residing in retirement villages (each village is a cluster). The recruitment of participants from retirement villages is based on their relatively higher homogeneity, consistency of living conditions within each village compared to the broader community. It also enables greater access to the study as testing and intervention sessions are conducted at the participants’ villages of residence, therefore reducing barriers to participation such as time commitment, travel, and attending a university campus. Finally, recruiting participants at such villages facilitates the fostering of mutual support for the intervention groups, such as group walks and diet sessions.
The study is a diet and walking intervention developed and led by our multidisciplinary team of experts in MI-CBT, dietetics, and exercise physiology. There are two groups: 1) Supported diet and PA change (MedWalk intervention), and 2) Habitual lifestyle (control).
The intervention is delivered over one year, with more intensive delivery of the intervention in the first 6 months, reducing to less frequent diet consultations and group walking sessions in the latter half of the year. Outcomes are assessed at baseline, 6 and 12 months. A second year was originally planned to monitor maintenance of longer-term adherence to, and effects of, the intervention relative to control.
Impacts of COVID-19
The MedWalk trial was approved for funding by the Australian National Health and Medical Research Council (NHMRC) in June 2019. With recruitment set to commence in both Melbourne and Adelaide in the first half of 2020, research staff were contracted, and resources allocated for the commencement of the trial. However, the COVID-19 pandemic impacted human clinical trials in Australia and around the world. Globally, Melbourne was the most ‘locked down’ city [25], with similar impacts in Adelaide, severely restricting and in many cases completely preventing access to test sites for almost two years. Moreover, given the vulnerable group and setting, the pandemic affected subsequent uptake, even in people who were interested in the trial before the pandemic. Due to the impact of COVID-19 related restrictions on the timeline and budget for the study, it was necessary to make several modifications to MedWalk. COVID-19 impacts, trial modifications, as well as the decision process that underlay these modifications, have been documented by applying the CONSERVE protocol [26] and CONSERVE-SPIRIT checklist (Table 1). It is important to note that other than the modification to monitor primary and secondary outcomes over one year rather than two, the experimental aims and hypotheses remain the same. Another key impact of the COVID-19 pandemic was a reduced number of retirement villages that could be accessed. This was mitigated by including recruitment from the wider community (community-dwelling).
Table 1
CONSERVE – spirit checklist
| CONSERVE-SPIRIT Extension: 18/03/2023 | ||||
| Item | Item Title | Description | ||
| I. | Extenuating Circumstances | Following notification of funding received in June 2019, we had mobilized staff and resources to commence testing in the first half of 2020. COVID-19 impacted both of our testing sites over the next two years with Melbourne being the most locked-down city in the world [25] | ||
| We had already contracted several staff with employment agreements in place for commencement of data collection, however we were unable to collect any data for almost two years. This significantly delayed our trial and depleted our finances. | ||||
| II. | Important Modifications | a. Reduction of the trial length from 2 years to 1 year and re-definition of clusters. | ||
| b. As a direct result of the COVID-19 pandemic, trial commencement was significantly delayed, particularly at our Melbourne testing site, depleting our budget. To stay within budget, we reduced the trial length from two years to one year. The monitoring of the effects of the intervention on cognition and other outcomes remained essentially the same over the first year. While the intervention was not intended to be administered in the second year, this extended period was planned to assess maintenance of behavior change over an extended time and the resulting effects on outcomes. | ||||
| As a result of COVID-related restrictions limiting access to retirement communities there were fewer clusters available to access than originally planned. To maximize the numbers of participants to test within a reduced timeline we had to change the definition of a cluster to include a community-based sample. This allowed us to recruit from the community without the same restrictions. | ||||
| These modifications were made from 02/07/2021 – 24/02/2022 | ||||
| III. | Responsible Parties | The budgetary changes and resulting modifications were planned by the Primary Investigator in consultation with the trial Scientific Committee. The modifications were approved by the Scientific Committee and by the Data Safety Monitoring Board and by the funding body, the National Health and Medical Research Council (NHMRC). | ||
| IV. | Interim data | Once baseline testing was complete (October 2022) a power calculation was undertaken by our Statistician. This power calculation indicated that based on the number of clusters and participants per cluster that the trial was still adequately powered (5% significance, 80% power and a 7% rate of attrition). | ||
| SPIRIT Item and Number | For each row, if important modifications occurred, check one or both of “impact” and/or “mitigating strategy” and describe the changes in the protocol. Check “no change” for items that are unaffected in the extenuating circumstance. | |||
| No Change | Impact* | Mitigating Strategy** | ||
| 1 | Title | X | ||
| 2 | Trial registration | X | ANZCTR Trial registration amended to reflect modifications 02/08/2022 | |
| 3 | Protocol version | X | X | |
| 4 | Funding | Funding was significantly impacted due to research staff being contracted but unable to commence data collection. Study timeline was extended by 1 year to facilitate follow-up data collection, further impacting budget | Reduced spending on equipment and devices (instead borrowing from our university laboratories). | |
| Reduced the scope of blood measures so that we have more funds to support staff to complete testing. | ||||
| Reduced travel spending. | ||||
| 5 | Roles and responsibilities | X | ||
| 6 | Background and rationale | X | ||
| 7 | Objectives | X | ||
| 8 | Trial design | Trial length was reduced from 2 years to 1 year. | Follow-up testing at 18 and 24 months was removed, reducing the trial length for each participant to 1 year. This still allows the investigation of the effects of the intervention on cognition and secondary outcomes over 1 year. | |
| Originally the trial was designed as a 1-year tapered intervention followed by a further 1 year of follow-up testing at 18 and 24 months to assess the maintenance of the intervention and the effect of this on the primary outcome – cognition. | ||||
| 9 | Study setting | Ongoing inability to access several previously engaged retirement communities around Melbourne had limited our ability to recruit participants in this setting. | Has largely remained the same with nearly all clusters being in retirement villages around Melbourne and Adelaide. | |
| Given our inability to access several villages around Melbourne, it was decided to also recruit 4 community clusters. The setting is therefore now retirement communities and community based. | ||||
| 10 | Eligibility criteria | See “Study setting” for the same impacts and rationale for change. | Eligibility criteria was changed to accommodate community-based clusters. We recruited based on same inclusion and exclusion criteria however also facilitated those living alone or with a partner in the community. | |
| 11 | Interventions | X | Impacts requiring general precautions and contingencies around COVID-19. | If staff/participants suspected of having COVID - then avoid group activities (walking groups) and in-person diet sessions were cancelled /rescheduled (for a MINIMUM of five days following positive test and until symptom free). Contingencies prepared in the event of further COVID outbreaks – e.g., Telehealth / online intervention which we did not utilize. Alternate qualified staff were used if available. |
| 12 | Outcomes | Length of trial reduced removing outcomes at 18 and 24 months. | Reduced scope of blood testing to mitigate financial impacts. | |
| Impacts on blood analysis. | Additional blood frozen allowing future analysis, subject to additional funding being accessed | |||
| 13 | Participant timeline | See “Outcomes” above | ||
| 14 | Sample size | Impacts on our capacity to recruit within the available timeline has meant that our sample size is significantly reduced. | We have recruited all participants and completed baseline testing. Based on these numbers and the number of clusters (and participants per cluster) and the reduced timeline to observe outcomes, our statistician estimates that we are still powered for our primary outcome | |
| We had lost many people, previously recruited before COVID-19 - who did not wish to (or were unable to) re-engage when COVID lockdowns were over. | ||||
| 15 | Recruitment | Impact on ability to recruit participants due to our reduced timeline and resources as well as restricted access to several retirement communities | Included 4 community-based clusters in Melbourne to offset the loss of independent living facility clusters | |
| 16 | Allocation | X | ||
| 17 | Blinding (masking) | X | ||
| 18 | Data collection methods | X | ||
| 19 | Data management | X | ||
| 20 | Statistical methods | Reduced sample size focusing on outcomes at 12 months | Power re-calculated based on number of clusters, and participants per cluster, at baseline. Still powered for primary outcome | |
| 21 | Data monitoring | X | ||
| 22 | Harms | X | ||
| 23 | Auditing | X | ||
| 24 | Research ethics approval | X | Important modifications have been approved | |
| 25 | Protocol amendments | X | Important modifications have been amended in the Protocol | |
| 26 | Consent or assent | X | ||
| 27 | Confidentiality | X | ||
| 28 | Declaration of interests | X | ||
| 29 | Access to data | X | ||
| 30 | Ancillary and post-trial care | X | ||
| 31 | Dissemination policy | X | ||
| 32 | Informed consent materials | PICF modified: | ||
| •02/07/2021 – to reflect inclusion of community groups. | ||||
| •25/02/2022 - to reflect change in trial length. | ||||
| Previously consented participants were reconsented with the updated documents. | ||||
| 33 | Biological specimens | As a result of impacts on finances we had to reduce the scope of blood testing. | Mitigate by focusing on 0- and 12-month bloods analysis. Reduce number of measures. Store blood at – 80°C and seek funds to allow further analyses in the future. | |
*Aspects of the trial that are directly affected or changed by the extenuating circumstance and are not under the control of investigators, sponsor, or funder. **Aspects of the trial that are modified by the study investigators, sponsor, or funder to respond to the extenuating circumstance or manage the direct impacts on the trial.
Participants
Participants were residing in retirement villages or living alone or with their partner in the wider community. They were 60–90 years of age, free of dementia, and able to walk independently and regularly. The full list of inclusion and exclusion criteria are provided in Table 2. Baseline characteristics are shown in Table 3.
Table 2
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Aged 60–90 years | Cognitive impairment defined as a score <24 on the Mini-Mental State Exam |
| Either residing in independent living supported accommodation (retirement villages) or living alone or with their partner in the community | Score ≥10 on the 15-item Geriatric Depression Scale |
| Able to walk independently and free from major physical ailments that would prevent regular walking | Have been diagnosed with dementia (e.g., Alzheimer’s disease) or other forms of cognitive impairment. |
| Fluent in written and spoken English | Have a history of stroke or other neurological condition that causes significant functional or cognitive issues |
| Vision and hearing adequate (or corrected to adequate) to undertake all aspects of testing and intervention | Have been diagnosed with a mental health condition that is uncontrolled (by medication or intervention) and which has a significant impact on daily life |
| Be willing to provide blood and fecal samples | Use of cholinesterase inhibitors |
| Deemed unable to safely participate in the walking program through an assessment made by an accredited exercise physiologist combined with no written clearance from a physician | |
| Self-report - participating (on average) in >150 min of moderate-to-vigorous leisure time PA per week AND a high MedDiet adherence score (≥10 or above on the 14-item Mediterranean Diet Adherence Screener (MEDAS)) | |
| Diagnosed allergy or intolerance of food groups (e.g., olive oil/nuts/seafood) that would prevent adherence to a Mediterranean diet |
Table 3
Basic baseline characteristics of the MedWalk trial participants
| Characteristic | Group A* | Group B* | All |
| (n = 83) | (n = 74) | (n = 157) | |
| Age (y) | 75.2±6.0 | 74.5±5.7 | 74.9±5.9 |
| Sex | |||
| Female n (%) | 60 (72.3) | 55 (74.3) | 115 (73.3) |
| MMSE | 28.8±1.2 | 28.9±1.1 | 28.9±1.2 |
| Body mass (kg) | 75.9±14.9 | 78.8±15.4 | 77.3±15.2 |
| BMI (kg/m2) | 28.7±5.2 | 29.5±5.5 | 29.1±5.3 |
| Ethnicity | |||
| Caucasian | 83 (100) | 74 (100) | 157 (100) |
| Country of Origin | |||
| Born in Australia | 67 (80.7) | 57 (77.0) | 124 (79.0) |
| Education | |||
| Primary school | 11 (13.3) | 6 (8.1) | 17 (10.8) |
| Secondary school | 15 (18.1) | 26 (35.1) | 41 (26.1) |
| Diploma | 21 (25.3) | 23 (31.1) | 44 (28.0) |
| Undergraduate degree | 22 (26.5) | 11 (14.9) | 33 (21.0) |
| Postgraduate degree | 13 (15.7) | 7 (9.5) | 20 (12.7) |
| Other | 1 (1.2) | 1 (1.4) | 2 (1.3) |
| Marital Status | |||
| Married | 36 (43.4) | 34 (46.0) | 70 (44.6) |
| Widowed | 22 (26.5) | 20 (27.0) | 42 (26.8) |
| Divorced | 14 (16.9) | 11 (14.9) | 25 (15.9) |
| Separated | 3 (3.6) | 4 (5.4) | 7 (4.5) |
| Single | 3 (3.6) | 4 (5.4) | 7 (4.5) |
| Living with someone/de-facto | 5 (6.0) | 1 (1.4) | 6 (3.8) |
| Employment | |||
| Retired | 76 (91.6) | 67 (90.5) | 143 (91.1) |
| Working part-time | 6 (7.2) | 6 (8.1) | 12 (7.6) |
| Working full-time | 1 (1.2) | 1 (1.4) | 2 (1.3) |
Continuous variables expressed as Mean±SD; Categorical variables expressed as n (%); *Groups shown as A and B to maintain blinding.
Recruitment
A rolling recruitment occurred from August 2021 to August 2022 in South Australia and December 2021 to September 2022 in Victoria. Twenty-one clusters were established for recruitment consisting of 11 retirement villages in South Australia and 6 in Victoria, with an additional four community-dwelling clusters in Victoria.
Recruitment of participants: Retirement villages. Recruitment at retirement villages was a two-stage process. Initially contact was made with the management of retirement communities across Victoria and South Australia. Discussions were then held with management as to the willingness and suitability of the community to be involved. Suitability involved having an appropriate retirement village population (as per inclusion/exclusion criteria) and available, suitable space for the researchers to conduct all study testing.
After a relationship and agreement with a retirement village had been established, recruitment of individual participants within that community commenced. This was undertaken primarily through initial within community advertising, followed up by in-person information and recruitment presentations at that site. Participants from each retirement village were combined as a cluster for both statistical and intervention purposes. All in-person interactions for these participants are conducted in their villages, except for blood collection which is undertaken at a local pathology collection center.
Recruitment of participants: Wider community. As a result of the pandemic-related recruitment difficulties, both during and following COVID lockdowns and restrictions, it was decided to recruit several community-based groups, to be run out of Swinburne University of Technology. To this end, the study was advertised on social media.
Forty participants who responded to this advertising were recruited, screened, and underwent baseline assessment. These participants were then clustered into one of four groups. To more closely replicate the similarities found within individual retirement community clusters, these groups were established following baseline assessment, based on statistical analysis of demographic commonalities. The groups were constructed so as to provide matching for the random intervention/control allocations of the four broader community recruited groups in terms of pet ownership, support received at home (e.g., cleaning, home help, gardening, meals, personal care, etc.), sufficient income for needs (always: yes/no) and gender. Additionally, as would be the case in retirement community groups, couples were assigned to the same group. All in-person interactions for these participants are conducted at the university, including blood collection.
Randomization
The 21 groups were cluster randomized within states and within retirement community organizations (if they have more than one site in an organization) and the 4 community clusters. Randomization was carried out by an independent statistician.
Group reveal
Following completion of baseline testing for each group, participants attended a ‘Reveal Presentation,’ in which an envelope was opened to reveal if that group was randomized to intervention or control. If the group was randomized to intervention, study staff gave a 2-3-h presentation on the MedDiet and exercise intervention protocol including resources and support materials. If the group was randomized to ‘control’, study staff gave a 30-min presentation on the importance of a control group in a research study. At the end of 12 months, the control group participants will be invited to attend an ‘Intervention Presentation’. They will receive the same information as the intervention group, as well as samples of the intervention resources.
Blinding
Participants and researchers were blind to the intervention group allocation during baseline testing with randomization occurring after baseline testing was completed. “Blinded cognitive testers” are being maintained for the primary outcome and other cognitive tests for the duration of the trial. These researchers are blind to the intervention group at subsequent 6- and 12-month testing points. Prior to the cognitive assessment visits at these time points, participants are reminded not to reveal their allocated group to the cognitive testers.
The Study Procedure Flowchart is presented in Fig. 1.
Fig. 1
Study procedure flowchart
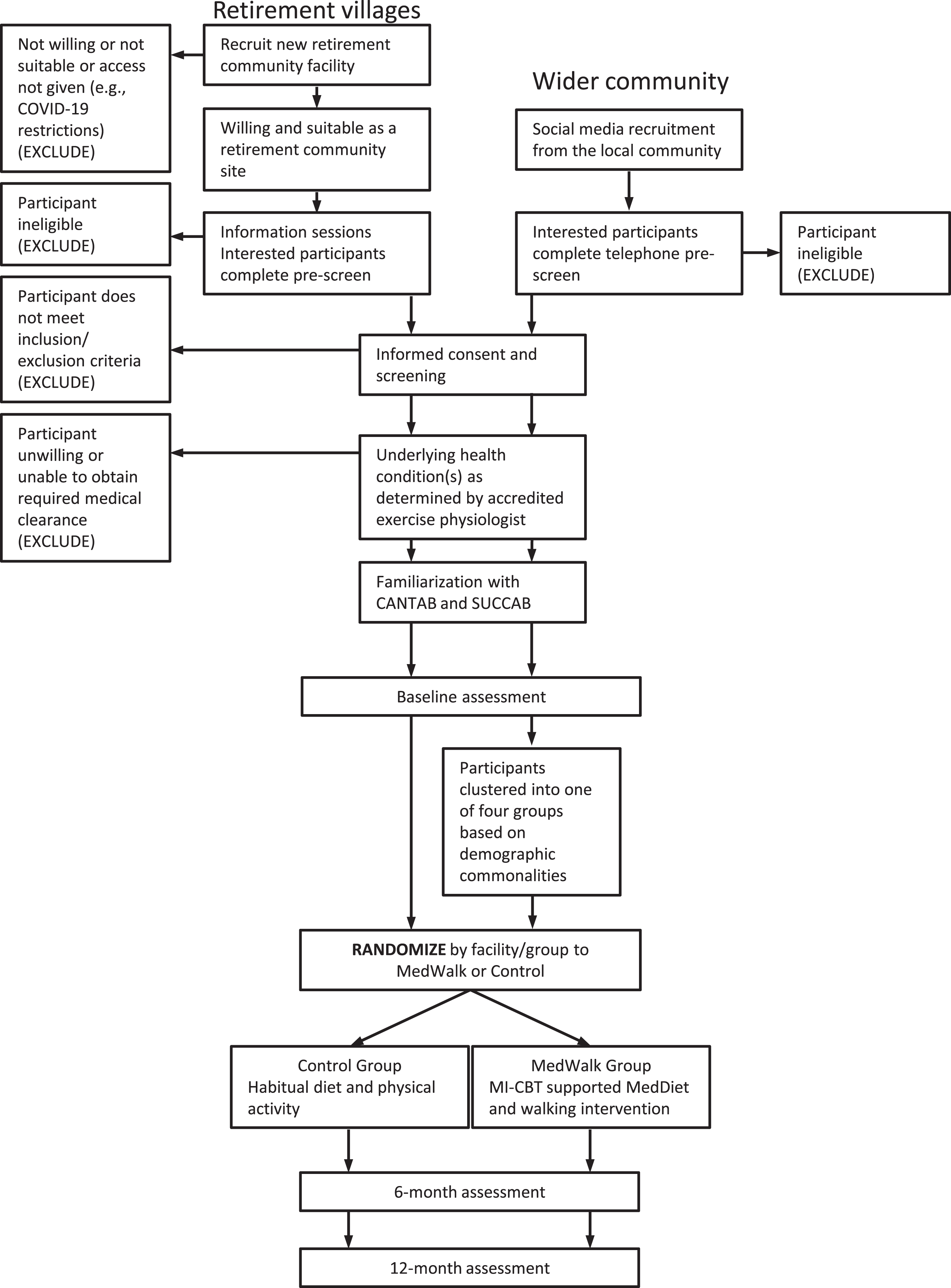
Outcomes
Table 4 provides a summary of outcomes with relevant references and time points.
Table 4
Summary of outcome measures at baseline, 6 and 12-months
| Assessment /data source | Measures | Baseline | 6 m | 12 m |
| CANTAB | Cognitive ability | X | X | X |
| PAL*, MOT, RT, SWM, RVP | ||||
| SUCCAB | Cognitive ability | X | X | X |
| SRT, CRT, SWM | ||||
| Medical history | Illness and injury | X | X | X |
| Concomitant medications | Medication and supplement use | X | X | X |
| Anthropomorphics | Height, weight, hip-waist ratio | X | X | X |
| IPAQ-E | Subjective PA | X | X | X |
| Accelerometrya | Objective PA | X | X | X |
| MW-MEDAS | MedDiet adherence | X | X | X |
| Easy Diet Diaryb | Diet | X | X | X |
| 6MW | Functional aerobic capacity | X | X | X |
| BORG Scale | Perceived exertion | X | X | X |
| Grip strength | Frailty | X | X | X |
| Pain VAS | Knee and back pain | X | X | X |
| Cardiovascularc | Peripheral/central blood pressures and arterial stiffness | X | X | X |
| PSQI | Sleep quality | X | X | X |
| DASS | Depression, anxiety, and stress symptoms | X | X | X |
| POMS | Mood | X | X | X |
| GHQ | Psychosocial wellbeing | X | X | X |
| AQoL-8D v12 | Psychosocial quality of life | X | X | |
| Flourishing Index | Used to undertake a cost utility analysis | X | X | X |
| BSSNI | Social network | X | X | X |
| CEMI | Perception of MI-CBT | X | X | |
| Pure tone audiometry | Hearing acuity | X | X | X |
| Digit Triplet Test | Hearing in background noise | X | X | X |
| APHAB | Hearing in social situations | X | X | X |
| MRI (sub-sample) | ||||
| T1 &T2 weighted imaging | Grey and white matter volumes | X | X | |
| T2 FLAIR | White matter hyperintensities | X | X | |
| Diffusion weighted imaging | White matter integrity | X | X | |
| Arterial spin labeling | Regional cerebral blood flow | X | X | |
| Multi-echo Dixon scan | Hepatic lipid fraction | X | X | |
| MRS | Hepatic lipid fraction | X | X |
CANTAB, Cambridge Neuropsychological Test Automated Battery [27]; PAL, Paired Associates Learning (*Primary Outcome); MOT, Motor Screening Task; RT, Reaction Time; SWM, Spatial Working Memory; RVP, Rapid Visual Information Processing; SUCCAB, Swinburne University Computerised Cognitive Assessment Battery [1]; SRT, Simple Reaction Time; CRT, Choice Reaction Time; SWM, Spatial Working Memory; IPAQ-E, The International Physical Activity Questionnaire modified for the elderly [56]; 6MW; 6-min Walk Test [57]; MW-MEDAS, 14-point MedWalk Mediterranean Diet Adherence Screener; PSQI, Pittsburgh Sleep Quality Index [58]; DASS, Depression Anxiety Stress Scales[59]; POMS, Profile of Mood States [60]; GHQ, General Health Questionnaire [61]; AQoL 8D – v12, Assessment of Quality of Life – 8 dimensions [62]; BSSNI, Berkman-Syme Social Network Index[63]; CEMI, Client Evaluation of Motivational Interviewing [38], DTT, Digit Triple Test [64]; APHAB, Abbreviated Profile of Hearing Aid Benefit [65]. aAccelerometry, tri-axial accelerometer (wGT3X-BT; Actigraph, FL, USA) worn at the right hip for 8 days; bDiary of food and beverage intake (weighed or measured), recorded by participants over 4 days (FoodWorks V10, Xyris Pty Ltd); cCardiovascular assessments using SphygmoCor device (Model XCEL, AtCor Medical, Sydney, Australia) of brachial and aortic blood pressures, as well as peripheral arterial stiffness (augmented pressure) and aortic stiffness (carotid-femoral pulse wave velocity).
The primary outcome is errors on the Paired Associates Learning task (PAL) of the Cambridge Neuropsychological Test Automated Battery (CANTAB) [27], which assesses visual memory and learning. The PAL assessment has been shown to have high sensitivity and specificity for AD (1.0 and 0.92) and mild cognitive impairment (0.83 and 0.82) [28], as well as having substantive predictive value for mild cognitive impairment and AD in older people than that provided by age alone [29]. This assessment has been used in over 700 studies [30] as a sensitive test of cognition and cognitive change, including several recent lifestyle (exercise and/or diet) change intervention trials [31–35].
Secondary outcomes include cognitive performance as assessed by other CANTAB tests - rapid visual information processing, motor learning, reaction time and spatial working memory, assessing three major domains of cognitive function: Attention and Psychomotor Speed; Executive Function; and Memory. Spatial working memory is also measured using the Swinburne University Cognitive Assessment Battery (SUCCAB) that will provide a partial replication of the main finding of the pilot LIILAC trial. SUCCAB is also used to measure simple and choice reaction time. Adherence to MedDiet is measured using the MedWalk Mediterranean Diet Adherence Score (MW-MEDAS) questionnaire, modified from the validated MEDAS 14-item questionnaire to reflect the study requirements. PA is measured using both the International Physical Activity Questionnaire modified for the elderly (IPAQ-E) self-report questionnaire and objectively using the tri-axial accelerometer (wGT3X-BT; Actigraph, FL, USA) worn at the right hip for 8 days. The six-minute walk test is used as a measure of functional aerobic capacity. Mood is assessed using the Depression Anxiety Stress Scales, Profile of Mood states, and General Health Questionnaire. Peripheral and central blood pressure and arterial stiffness, pulse wave velocity, is measured using the SphygmoCor device (Model XCEL, AtCor Medical, Sydney, Australia). The Pittsburgh Sleep Quality Index is used to assess subjective sleep quality, psychosocial aspects of quality of life are assessed using Assessment of Quality of Life – 8 dimensions (AQoL-8D) v12 and the Flourishing Index, and the Berkman-Syme Social Network Index is used to assess the type, size, closeness, and frequency of contacts in a participant’s social network.
Fried frailty phenotype will be determined using the methodology described by Fried et al. [36]. This comprises the measurement of unintentional weight loss over past 12 months, hand grip strength measured using a dynamometer (Jamar Smart Hand Dynamometer, Patterson Medical, Melbourne, Australia), decreased walking speed (over 5 meters), low physical activity (energy expenditure weekly rate calculated from the IPAQ-E) and exhaustion as assessed by two items from the Center for Epidemiological Studies-Depression [37]
Knee and back pain assessments are conducted using visual analogue scales. Outcome measures around hearing impairment include pure tone audiometry, the Abbreviated Profile of Hearing Aid Benefit questionnaire to determine the impacts of hearing loss in social situations and a web-based speech-in-noise hearing test.
Fidelity to the protocol (MI-CBT) by those delivering the intervention, will be assessed using a validated framework and coding tools (i.e., MI Treatment Integrity; MITI) and participant perceptions of MI will be assessed using the Client Evaluation of MI [38]. Economic outcomes measured over the one-year intervention include: 1) health care costs of hospital admissions, ED attendances, allied health services (MBS) and pharmaceuticals (PBS), 2) differential QALY gain/loss over the trial and modelled out to 10 years (combining the AQoL with time). Cost of delivering MedWalk will be measured (staff costs plus consumables) to estimate cost-effectiveness (differential number experiencing significant cognitive decline) and cost/QALY.
Blood-based measures associated with cognitive decline, dementia risk and mechanisms potentially amenable to diet and exercise interventions were assessed at time points according to Table 5. A fasting venous blood sample is used to assess lipid profile (LDL and HDL cholesterol, total cholesterol and triglycerides), liver function (total protein, albumin, ALP, bilirubin, GGT, AST, ALT), kidney function (electrolytes, urea, creatinine), inflammation via high sensitivity C-reactive protein and cytokines (interferon gamma, interleukin 1 beta, 2, 4, 6, 10, 17, and tumor necrosis factor alpha), plasma glucose and long term glycemic control using glycated hemoglobin (HbA1c), homocysteine, lipid peroxidation (F2-isoprostanes), brain derived neurotrophic factor and nutrient markers including erythrocyte and plasma phospholipid fatty acid profiles, carotenoids, vitamins A, C, E, D, B2, B6, B9, B12, and red cell folate. The presence of APOE4 will be assessed as variants in this gene are associated with higher risk of developing AD.
Table 5
Summary of biochemical outcome measures at baseline, 6 and 12-months
| Biochemical assessment | Measures | Baseline | 6 m | 12 m |
| MBA | General health status – renal, liver & lipid profile | X | X | X |
| hsCRP | Inflammation, immunity | X | X | X |
| IFN-γ, IL-1β, 2, 4, 6, 10, 17, TNF-α | X | X | ||
| Glucose | Blood sugar level | X | X | X |
| HbA1c [66] | Long term glycemic control | X | X | |
| Homocysteine | Cardiovascular and dementia risk | X | X | |
| F2-isoprostanes [67] | Oxidative stress | X | X | |
| Apolipoprotein E | Genetic marker – Alzheimer’s disease risk | X | ||
| Brain Derived Neurotrophic Factor | Neuronal survival and growth | X | X | |
| Erythrocyte &fatty acid fatty acid &plasma phospholipid fatty acid profiles, carotenoids, Vitamins A, C, D, E, B2, B6, B9, B12 and [68] | Nutrient status | X | X | X |
| Red cell folate | X | X | ||
| Fecal microbiome | Gut bacterial population | X | X | X |
There has been an ever-increasing interest in the gut-brain axis and its relationship with neurocognition and neurodegenerative diseases such as AD. A recent review of this area found that there is support for the concept that any effects of exercise and diet on cognition may be mediated by the gut microbiome, but that the current evidence is primarily derived from rodent research and that further human intervention trials are highly recommended [39]. To this end, microbial DNA will be isolated from stool samples collected using stool nucleic acid collection and preservation tubes (Norgen Biotek, Canada). The 16S rRNA amplicon sequencing of the V3-V4 hypervariable region will be performed with an Illumina NovaSeq 6000 PE250 [40, 41]. Alpha-diversity will be assessed using both Chao1 and Shannon H diversity index while beta diversity will be assessed using Bray-Curtis. Statistical significance will be determined by Kruskal–Wallis or Permutational Multivariate Analysis of Variance (PERMANOVA). Comparisons at the Phylum, Family and Genus level will be analyzed using classical univariate analysis using Kruskal–Wallis combined with a false discovery rate approach used to correct for multiple testing.
Finally, through access of additional funds, magnetic resonance imaging (MRI), the effects of the intervention on brain and liver are being investigated at 0 and 12 months in a sub-sample of 51 participants in Victoria. TI and T2 weighted MRI sequences are providing data for brain white and grey matter volumetric measurement. A T2 FLAIR scan is being conducted to assess cerebrovascular disease markers (white matter hyperintensities). Diffusion weighted imaging is being used to assess the microstructural integrity of white matter within the brain. Arterial Spin labelling will be used to obtain regional cerebral blood flow. Liver scans in the same participants will investigate intervention effects on liver fat and associations with neurocognition, mood, and inflammation previously reported [42, 43].
Intervention
The intervention combines dietary modification with a supervised progressive walking program, underpinned by established psychosocial behavioral change techniques [44]. The intervention was developed by accredited dietitians, accredited exercise physiologists (AEP), and a psychologist specializing in MI-CBT. It is overseen and delivered by AEPs and dietitians, supported by other qualified staff with specializations in these areas, all of whom have received training in MI-CBT. Participants receive intense support for the first 6 months, and continued support in the last 6 months, empowering them to develop and maintain the intended intervention behaviors.
Mediterranean dietary component
Participants attend sessions of between 0.5 to 1 h, fortnightly for the first 8 weeks, and then monthly for 4 months. Support is then reduced to quarterly visits for the remainder of the 1-year intervention period.
The dietary intervention is based on the PREDIMED dietary guidelines, modified for Australian use [45, 46]. Dietary instructions are shown in Supplementary Table 1 with an overarching goal to increase MedDiet adherence in a way that works best for each participant. The dietitian provides detailed instructions on following a MedDiet, together with meal planning and recipes and utilizing MI-CBT to assist with dietary behavior change and to manage ambivalence toward change. This level of education and support initially, is vital to allow participants to fully comprehend the dietary pattern and how it differs from the Australian diet. As extra-virgin olive oil is an essential component of the MedDiet, extra-virgin olive oil was provided to participants gratis to assist with meal preparation over the intervention period. Similarly other Mediterranean food components (e.g., canned fish, canned vegetables, nuts, wholemeal pasta, and dried herbs) were provided to support this dietary change. Adherence to the dietary component of this study was evaluated using the MW-MEDAS dietary screening tool (modified from the MEDAS-14 [47]), which scores adherence out of 14 points (maximum).
Walking component. An AEP initially assessed baseline aerobic fitness. Group walking sessions, supervised by AEPs or exercise scientists, take place weekly for the first 6 months, and then reduce to monthly sessions for the following 6 months.
The AEP prescribes an individualized, progressive home-based walking program. Participants are provided with a ‘Walking Programme Resources Pack’, which includes information on the benefits of walking, self-tracking progress aids, and warm-up and cool-down information, including stretches (with variations for participants with balance concerns). Depending upon initial fitness, the program starts with 10 to 30 min of walking performed on 2 to 3 days per week and progresses so that participants are asked to walk for at least 30 min on 5 days per week (150 min per week in total, consistent with the current national physical activity guidelines). Intensity is assessed using the Borg 10 Rating of Perceived Exertion, a subjective score of how difficult the walking feels while doing it. Consistent with the American College of Sports Medicine guidelines, training intensity commences at a low-moderate intensity and progressively increases to moderate intensity [48] after participants reach 150 min per week of walking. Each session includes a 5-min warm-up and cool-down period of walking slowly.
Walking groups are organized at each retirement community, or at the university for the community-dwelling groups. The regular supervised group sessions are used to support participants to progressively achieve the desired intensity. Training intensity is progressed through a reduction in rest intervals, increasing walking speed and number of steps per walk, and/or through the prescription of hill walking or interval walk training. All participants in the MedWalk intervention group were provided with a wrist-worn activity tracker (Fitbit Inspire 2) for motivational purposes. Adherence to the walking exercise component will be assessed via attendance at group walking sessions and accelerometer data. The Group Walking Schedule is shown in Supplementary Table 2.
Motivational interviewing: Cognitive behavioral therapy (MI-CBT). MI uses a combination of relational (evocation, compassion, acceptance, collaboration) and technical micro-skills (e.g., open questions, affirmations, reflective listening, summarizing; OARS) to build client autonomy toward change, exploring motives and perceived barriers toward change [49]. The CBT components are action-orientated treatments including social support; identifying change barriers and goals; developing flexible goal setting; action planning; managing relapse; building maintenance strategies and processes for self-monitoring [50]. Our team has recently demonstrated that integrated MI-CBT can increase PA in older insufficiently active secondary care patients [22]. Specific applications for the use of MI in PA [51] and diet modification [52] formed the basis of the training and subsequent MI delivery as well as adaptations required for the use of MI in individual and group settings [53]. The intervention is underpinned with a theoretical framework (self-determination theory) which identifies progression by clients towards autonomous (self-directed) behaviors. This is evaluated for both the delivery and receipt of the intervention. The MI component is delivered by research assistants trained in MI by a chief investigator on the team, who is member of the MI Network of Trainers (MINT), to a level of proficiency recommended by the Motivational Interviewing Treatment Integrity guidelines. Those delivering the intervention who were training in MI were assessed for their competence (treatment fidelity) using the MITI [54].
The program is based on successful MI interventions to promote PA and the 12 session group intervention are: 1) Getting to know each other: ice breaker and team activities (focused around PA and healthy nutrition), 2) psycho-education: the background to PA and diet, group activities and knowledge exchange (elicit-provide-elicit; MI-based strategy), 3) Exploring attitudes and values toward change (PA and diet), 4) Understanding motives and reasons for PA and health nutrition, 5) Getting to know your options: How to take responsibility for your health in your environment, 6) Identifying and managing your barriers to change, 7) Identifying and managing risks of setbacks in yourself and others, 8) Identifying and managing flexible approaches to being more active and eating well, 9) Developing cognitive skills: recognizing thoughts and emotions, 10) Developing cognitive skills: helpful thoughts and emotions, 11) Developing cognitive skills: Staying on track, and 12) Where next and action planning.
Control group: No requirement to change diet and exercise. Participants in clusters randomized to the control group were not asked to change their usual diet and exercise habits. These participants receive AUD$150 in shopping vouchers to provide some comparable reward for the food components that the intervention group receive. At the end of the trial, control group participants will be invited to attend a presentation for education about the intervention. They will also receive a sample hamper of the foods provided, the recipe book, a Fitbit, and MedWalk intervention support documentation.
Statistical power
The original sample size calculations for this cluster-randomized trial with five assessments per participant assumed a significance level of 5%, power of 80%, a moderate effect size (η2 = 0.06) and an attrition rate of 30% (expect <30% based on LIILAC, MedLey, and proposed MI-CBT approach). It was determined that the sample size should equal 364 participants with 13 participants per facility for each of 28 facilities. Ideally there would be 14 facilities in both Victoria and South Australia, with groups randomly assigned to matched facilities in each state.
Due to recruitment difficulties during the pandemic, only 157 participants were recruited, with an average of 7 people per “facility”, and three assessments per participant. The smaller number of participants for each facility meant that, although the smallest effect size that could be detected increased (to η2 = 0.08), the trial was still adequately powered (5% significance, 80% power and 7% attrition).
Analysis plan
The baseline characteristics of the intervention and control group participants will be compared using chi-squared and independent sample t-tests as appropriate. The study hypothesis will be addressed using a 3-level hierarchical linear model analysis with HLM7 software, assuming a Poisson distribution for the total number of PAL errors. The three levels are time, participant, and cluster. With this intention to treat analysis we will test for significant differences between the intervention and control groups over time. This analysis uses all available information from participants. Incomplete data will not be discarded, and missing data will not be replaced with estimated values or observations carried forward. Instead, maximum-likelihood estimation will be applied with available data. Where there are significant group-X-time interactions, planned contrasts will compare changes from baseline under each intervention. Similar tests will be performed for the secondary hypotheses, using appropriate transformations if these measures exhibit non-normal distributions across time points. This analysis will allow us to control for any baseline group differences, for demographic characteristics, cluster characteristics, and to test for moderation effects in terms of these variables over time. Any significant differences between community/retirement village living participants will be investigated.
Assessment of safety and reporting of adverse events
Any unexpected untoward medical event during the trial is considered an adverse event (AE). Serious adverse events (SAEs) are untoward medical events that result in death, are life threatening, result in hospitalization, extend hospitalization, or result in significant incapacity or disruption to normal life functions.
To ensure AEs and changes to concomitant medications are reported, the researchers ask participants at each study visit about any changes to their health, any symptoms they may have developed, and any changes to their current treatment regime. Additionally, if the participant experiences an AE, they are asked to notify the research team immediately. Any medical condition that is present at the time that the subject is screened is considered part of their medical history and not reported as an AE. However, if their condition deteriorates during the study, it is recorded as an AE. Normal physiological responses to exercise or dietary changes, such as mild muscle soreness or digestive changes that do not require medical intervention are not considered as AEs. With their permission, contact is maintained with the participants until the AE is fully resolved.
All AEs occurring during the study are reported and recorded according to legal requirements whether they are non-serious, serious and/or related to the intervention. In addition, the ethics committee is notified by the investigator of SAEs in accordance with the appropriate guidelines. All AEs are tabulated in an Excel spreadsheet and reviewed monthly by the study medical doctor. If the study medical doctor or any other medical staff are concerned for the safety of a participant, the participant is withdrawn.
Data safety monitoring board (DSMB)
The role of the DSMB is to safeguard the interests of trial participants, monitor the main outcome measures including safety and efficacy, and monitor the overall conduct of the trial.
The DSMB receives and reviews information on trial progress and data accrual and provides advice on the conduct of the trial to MedWalk investigators. DSMB members are a group of experts who have no competing interests that could influence decision making. These independent experts include an external chairperson, statistician, and nutritionist, as well as the trial medical doctor.
Ethics
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and the NHMRC National Statement on Ethical Conduct in Human Research [55]. The study was approved by the Human Research Ethics Committee at Swinburne University (Ref: 20201600-3559, 14/02/2020) and The University of South Australia (Ref: 202844, 16/06/2020). Written informed consent was obtained from all participants before commencement.
DISCUSSION
MedWalk is designed to investigate the effects of a MedDiet and walking intervention on cognitive decline in independently living older individuals. Importantly the intervention approach is supported by carefully designed behavioral change and maintenance strategies, embedding healthy diet and exercise behaviors for longer term adoption, to impact risk factors for dementia and cognitive decline.
The original trial was designed to assess cognition over two years, focusing on residents living in retirement villages as a cluster-randomized controlled trial with the intervention delivered in the first year. As a result of COVID-19 impacts on timeline and budget, a reduced number of participants are being followed over a one-year timeframe and recruited largely from retirement villages, but also from the wider community. There is no change in the way that the intervention was planned to be delivered (over 1 year). However, the study is no longer able to determine maintenance of unsupported behavior change in the second year. Importantly, with recruitment and baseline testing now complete, an updated power analysis indicates that the trial is still adequately powered to address our primary hypothesis.
If MedWalk is successful, it has potential to inform future intervention programs that could substantially reduce the incidence of dementia in Australia, associated medical and care costs, and burden on family. There is also potential to adapt MedWalk for use in the broader community to address broader risk factors including chronic disease.
TRIAL STATUS
Trial data collection is ongoing and will be completed by the end of 2023.
ACKNOWLEDGMENTS
We would like to thank the following organizations, their retirement villages, staff, and residents for access to facilities, advice, and assistance with the trial: In South Australia – ECH (Cumberland Park Community Group and Rotary), Karidis (Acacia Park), Lendlease (Vermont), LifeCare (Marion Rose), Retire Australia (Glengowrie, Tea Tree Gardens and Torrens Grove), RSL Care (Sturt) and Southern Cross (Riverpoint and The Pines). In Victoria – Abound Communities (Rushall Park and Leith Park), Australian Unity (Campbell Place and Peninsula Grange) and Villa Maria Catholic Homes (St Joseph Mews and Athelstan).
We acknowledge the contributions of the MedWalk Collaborative Team: Bethany Bartel, Mahima Bedi, Mee Chee Chong, Nicole Echeverria, Gabriella Inguanti, Ashlee Harvey, Kasia Main, Laura Martin, Janis Onuzans, Melissa Rubin, Mahima Shah, Tania Thodis, Nerylee Watson. We would like to acknowledge MedWalk Associate Investigators for their contributions to develop the grant application and to implement the experimental protocol including assistance with recruitment and contributions on the MedWalk Advisory Board: Janet Hiller (Chair), Victoria Cornell, Megan Corlis, Colleen Doyle, Sarah Gray. We would also like to acknowledge the contributions of members on the Data Safety Monitoring Board: Naomi Perry (Chair), Won Sun (Sharon) Chen, Matthew Cook, Edward Ogden.
The authors also acknowledge the facilities and scientific and technical assistance of the National Imaging Facility (NIF), a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at Swinburne Neuroimaging (SNI), Swinburne University. The authors also acknowledge the contributions of Dr. Catherine Mandel and Annalaise Takla in establishing the liver scanning protocols and Dr. Courtney Sullivan in establishing the accelerometer analysis protocol.
FUNDING
The MedWalk trial is funded by a National Health and Medical Research Council (NHMRC) Boosting Dementia Research Initiative grant (GNT1171300). Specsavers funded hearing loss assessments as well as support of a PhD scholarship. A Nutricia Research Foundation grant (2020-67) provided funds for microbiome assessments. Swinburne University funded two PhD scholarships and supported MRI neuroimaging and liver assessments through a Swinburne Neuroimaging Access Grant.
We would also like to acknowledge contributions from the following organizations: Cobram Estate for providing Australian extra virgin olive oil gratis to trial participants; San Remo for providing whole meal pasta; Hoyts for providing herbs and spices and The Almond Board of Australia for providing almonds.
CONFLICT OF INTEREST
Helen Macpherson is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data for this protocol are available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230641.
REFERENCES
[1] | Pipingas A , Harris E , Tournier E , King R , Kras M , Stough CK ((2010) ) Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr Top Nutraceutical Res 8: , 79–88. |
[2] | Pase MP , Pipingas A , Kras M , Nolidin K , Gibbs AL , Wesnes KA , Scholey AB , Stough C ((2010) ) Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens 28: , 1724–1729. |
[3] | Kennedy G , Hardman RJ , MacPherson H , Scholey AB , Pipingas A ((2017) ) How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis 55: , 1–18. |
[4] | Scholey A ((2018) ) Nutrients for neurocognition in health and disease: Measures, methodologies and mechanisms. Proc Nutr Soc 77: , 73–83. |
[5] | World Health Organization ((2019) ) Risk reduction of cognitive decline and dementia: WHO guidelines, Geneva. |
[6] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbaek G , Teri L , Mukadam N , Livingston G , Huntley J , Sommerlad A , Cooper C ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Commissions 6736: , 413–446. |
[7] | Shannon OM , Ranson JM , Gregory S , Macpherson H , Milte C , Lentjes M , Mulligan A , McEvoy C , Griffiths A , Matu J , Hill TR , Adamson A , Siervo M , Minihane AM , Muniz-Tererra G , Ritchie C , Mathers JC , Llewellyn DJ , Stevenson E ((2023) ) Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med 21: , 81. |
[8] | Estruch R , Ros E , Salas-Salvadó J , Covas M-I , Corella D , Arós F , Gómez-Gracia E , Ruiz-Gutiérrez V , Fiol M , Lapetra J , Lamuela-Raventos RM , Serra-Majem L , Pintó X , Basora J , Muñoz MA , Sorlí JV , Martínez JA , Fitó M , Gea A , Hernán MA , Martínez-González MA ((2018) ) Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 378: , e34(1)–e34(14). |
[9] | Valls-Pedret C , Sala-Vila A , Serra-Mir M , Corella D , de la Torre R , Martínez-González MÁ , Martínez-Lapiscina EH , Fitó M , Pérez-Heras A , Salas-Salvadó J , Estruch R , Ros E ((2015) ) Mediterranean diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern Med 175: , 1094–1103. |
[10] | Davis CR , Bryan J , Hodgson JM , Wilson C , Dhillon V , Murphy KJ ((2015) ) A randomised controlled intervention trial evaluating the efficacy of an Australianised Mediterranean diet compared to the habitual Australian diet on cognitive function, psychological wellbeing and cardiovascular health in healthy older adults (MedLey study): Protocol paper. BMC Nutr 1: , 35. |
[11] | Knight A , Bryan J , Wilson C , Hodgson J , Murphy K ((2015) ) A randomised controlled intervention trial evaluating the efficacy of a Mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: The MedLey study. BMC Geriatr 15: , 55. |
[12] | Sarapis K , Thomas CJ , Hoskin J , George ES , Marx W , Mayr HL , Kennedy G , Pipingas A , Willcox JC , Prendergast LA , Itsiopoulos C , Moschonis G ((2020) ) The effect of high polyphenol extra virgin olive oil on blood pressure and arterial stiffness in healthy Australian adults: A randomized, controlled, cross-over study. Nutrients 12: , 2272. |
[13] | Davis CR , Bryan J , Hodgson JM , Woodman R , Murphy KJ ((2017) ) A Mediterranean diet reduces F2-Isoprostanes and triglycerides among older Australian men and women after 6 months. J Nutr 147: , 1348–1355. |
[14] | Murphy KJ , Dyer KA , Hyde B , Davis CR , Bracci EL , Woodman RJ , Hodgson JM ((2022) ) Long-term adherence to a Mediterranean diet 1-year after completion of the MedLey Study. Nutrients 14: , 3098. |
[15] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385: , 2255–2263. |
[16] | Heffernan M , Andrews G , Fiatarone Singh MA , Valenzuela M , Anstey KJ , Maeder AJ , McNeil J , Jorm L , Lautenschlager NT , Sachdev PS , Ginige JA , Hobbs MJ , Boulamatsis C , Chau T , Cobiac L , Cox KL , Daniel K , Flood VM , Guerrero Y , Gunn J , Jain N , Kochan NA , Lampit A , Mavros Y , Meiklejohn J , Noble Y , O’Leary F , Radd-Vagenas S , Walton CC , Brodaty H ((2019) ) Maintain Your Brain: Protocol of a 3-year randomized controlled trial of a personalized multi-modal digital health intervention to prevent cognitive decline among community dwelling 55 to 77 year olds. J Alzheimers Dis 70: , S221–S237. |
[17] | Kivipelto M , Mangialasche F , Snyder HM , Allegri R , Andrieu S , Arai H , Baker L , Belleville S , Brodaty H , Brucki SM , Calandri I , Caramelli P , Chen C , Chertkow H , Chew E , Choi SH , Chowdhary N , Crivelli L , Torre RD La , Du Y , Dua T , Espeland M , Feldman HH , Hartmanis M , Hartmann T , Heffernan M , Henry CJ , Hong CH , Håkansson K , Iwatsubo T , Jeong JH , Jimenez-Maggiora G , Koo EH , Launer LJ , Lehtisalo J , Lopera F , Martínez-Lage P , Martins R , Middleton L , Molinuevo JL , Montero-Odasso M , Moon SY , Morales-Pérez K , Nitrini R , Nygaard HB , Park YK , Peltonen M , Qiu C , Quiroz YT , Raman R , Rao N , Ravindranath V , Rosenberg A , Sakurai T , Salinas RM , Scheltens P , Sevlever G , Soininen H , Sosa AL , Suemoto CK , Tainta-Cuezva M , Velilla L , Wang Y , Whitmer R , Xu X , Bain LJ , Solomon A , Ngandu T , Carrillo MC ((2020) ) World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 16: , 1078–1094. |
[18] | Lim YY , Ayton D , Perin S , Lavale A , Yassi N , Buckley R , Barton C , Bruns L , Morello R , Pirotta S , Rosenich E , Rajaratnam SMW , Sinnott R , Brodtmann A , Bush AI , Maruff P , Churilov L , Barker A , Pase MP ((2021) ) An online, person-centered, risk factor management program to prevent cognitive decline: Protocol for a prospective behavior-modification blinded endpoint randomized controlled trial. J Alzheimers Dis 83: , 1603–1622. |
[19] | Hardman RJ , Kennedy G , Macpherson H , Scholey AB , Pipingas A ((2015) ) A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: The Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628]. Nutr J 14: , 53. |
[20] | Hardman RJ , Meyer D , Kennedy G , Macpherson H , Scholey AB , Pipingas A ((2020) ) Findings of a pilot study investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged-care facilities: The Lifestyle Intervention in Independent Living Aged Care (LIILAC) Study. Curr Dev Nutr 4: , nzaa077. |
[21] | Scott SE , Breckon JD , Copeland RJ ((2019) ) An integrated motivational interviewing and cognitive-behavioural intervention promoting physical activity maintenance for adults with chronic health conditions: A feasibility study. Chronic Illn 15: , 276–292. |
[22] | Barrett S , Begg S , O’Halloran P , Kingsley M ((2018) ) Integrated motivational interviewing and cognitive behaviour therapy can increase physical activity and improve health of adult ambulatory care patients in a regional hospital: The Healthy4U randomised controlled trial. BMC Public Health 18: , 1166. |
[23] | Barrett S , Begg S , O’Halloran P , Kingsley M ((2020) ) A physical activity coaching intervention can improve and maintain physical activity and health-related outcomes in adult ambulatory hospital patients: The Healthy4U-2 randomised controlled trial. Int J Behav Nutr Phys Act 17: , 156. |
[24] | Barrett S , Begg S , O’Halloran P , Kingsley M ((2018) ) Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: A systematic review and meta-analyses. BMC Public Health 18: , 1160. |
[25] | Macreadie I ((2022) ) Reflections from Melbourne, the world’s most locked-down city, through the COVID-19 pandemic and beyond. Microbiol Aust 43: , 3–4. |
[26] | Orkin AM , Gill PJ , Ghersi D , Campbell L , Sugarman J , Emsley R , Steg PG , Weijer C , Simes J , Rombey T , Williams HC , Wittes J , Moher D , Richards DP , Kasamon Y , Getz K , Hopewell S , Dickersin K , Wu T , Ayala AP , Schulz KF , Calleja S , Boutron I , Ross JS , Golub RM , Khan KM , Mulrow C , Siegfried N , Heber J , Lee N , Kearney PR , Wanyenze RK , Hróbjartsson A , Williams R , Bhandari N , Jüni P , Chan A-W , Orkin AM , Gill PJ , Ghersi D , Campbell L , Sugarman J , Emsley R , Steg PG , Weijer C , Simes J , Rombey T , Williams HC , Wittes J , Moher D , Richards DP , Kasamon Y , Getz K , Hopewell S , Dickersin K , Wu T , Ayala AP , Schulz KF , Calleja S , Boutron I , Ross JS , Golub RM , Khan KM , Mulrow C , Siegfried N , Heber J , Lee N , Kearney PR , Wanyenze RK , Hróbjartsson A , Williams R , Bhandari N , Jüni P , Chan A-W , Kiermer V , Corrigan-Curay J , Concato J ((2021) ) Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances. JAMA 326: , 257. |
[27] | Cambridge Cognition ((2019) ) CANTAB® [Cognitive assessment software]. http://www.cantab.com. |
[28] | Chandler JM , Marsico M , Harper-Mozley L , Vogt R , Peng Y , Lesk V , Jager C ((2008) ) P3-111: Cognitive assessment: Discrimination of impairment and detection of decline in Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement 4: , T551–T552. |
[29] | Barnett JH , Blackwell AD , Sahakian BJ , Robbins TW ((2016) ) The paired associates learning (PAL) test: 30 years of CANTAB translational neuroscience from laboratory to bedside in dementia research. Curr Top Behav Neurosci 28: , 449–474. |
[30] | CANTAB Bibliography, http://www.bibliography.cambridgecognition.com, Last updated July 20, 2023, Accessed on July 20, 2023. |
[31] | Hong YJ , Lee J-H , Choi EJ , Han N , Kim JE , Park S-H , Kim H-J , Kang D-W ((2020) ) Efficacies of cognitive interventions in the elderly with subjective cognitive decline: A prospective, three-arm, controlled trial. J Clin Neurol 16: , 304. |
[32] | Jardim NYV , Bento-Torres NVO , Costa VO , Carvalho JPR , Pontes HTS , Tomás AM , Sosthenes MCK , Erickson KI , Bento-Torres J , Diniz CWP ((2021) ) Dual-task exercise to improve cognition and functional capacity of healthy older adults. Front Aging Neurosci 13: , 589299. |
[33] | Gentry AL , Erickson KI , Sereika SM , Casillo FE , Crisafio ME , Donahue PT , Grove GA , Marsland AL , Watt JC , Bender CM ((2018) ) Protocol for Exercise Program in Cancer and Cognition (EPICC): A randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy. Contemp Clin Trials 67: , 109–115. |
[34] | Déry N , Pilgrim M , Gibala M , Gillen J , Martin Wojtowicz J , MacQueen G , Becker S ((2013) ) Adult hippocampal neurogenesis reduces memory interference in humans: Opposing effects of aerobic exercise and depression. Front Neurosci 7: , 66. |
[35] | Bento-Torres NVO , Bento-Torres J , Tomás AM , de Souza LGT , De Freitas JO , Pantoja JA dos S , Picanço-Diniz CW ((2019) ) Water-based exercise and resistance training improve cognition in older adults. Rev Bras Med Esporte 25: , 71–75. |
[36] | Fried LP , Tangen CM , Walston J , Newman AB , Hirsch C , Gottdiener J , Seeman T , Tracy R , Kop WJ , Burke G , Mcburnie MA ((2001) ) Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: , 146–156. |
[37] | Bieniek J , Wilczyński K , Szewieczek J ((2016) ) Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging 11: , 453–459. |
[38] | Madson MB , Mohn RS , Zuckoff A , Schumacher JA , Kogan J , Hutchison S , Magee E , Stein B ((2013) ) Measuring client perceptions of motivational interviewing: Factor analysis of the Client Evaluation of Motivational Interviewing scale. J Subst Abuse Treat 44: , 330–335. |
[39] | Koblinsky ND , Power KA , Middleton L , Ferland G , Anderson ND ((2023) ) The role of the gut microbiome in diet and exercise effects on cognition: A review of the intervention literature. J Gerontol A Biol Sci Med Sci 78: , 195–205. |
[40] | Wang Q , Garrity GM , Tiedje JM , Cole JR ((2007) ) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: , 5261–5267. |
[41] | Quast C , Pruesse E , Yilmaz P , Gerken J , Schweer T , Yarza P , Peplies J , Glöckner FO ((2012) ) The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 41: , D590–D596. |
[42] | D’Mello C , Swain MG ((2011) ) Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol 301: , G749–G761. |
[43] | Savage K , Porter C , Bunnett E , Hana M , Keegan A , Ogden E , Stough C , Pipingas A ((2023) ) Liver and inflammatory biomarker relationships to depression symptoms in healthy older adults. Exp Gerontol 177: , 112186. |
[44] | Hurkmans E ((2013) ) Motivational interviewing and self-regulation to increase physical activity in patients with rheumatoid arthritis. Ann Rheum Dis 71: , 27. |
[45] | Itsiopoulos C , Brazionis L , Kaimakamis M , Cameron M , Best JD , O’Dea K , Rowley K ((2011) ) Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr Metab Cardiovasc Dis 21: , 740–747. |
[46] | Davis C , Bryan J , Hodgson J , Murphy K ((2015) ) Definition of the Mediterranean diet; a literature review. Nutrients 7: , 9139–9153. |
[47] | Schroder H , Fito M , Estruch R , Martinez-Gonzalez MA , Corella D , Salas-Salvado J , Lamuela-Raventos R , Ros E , Salaverria I , Fiol M , Lapetra J , Vinyoles E , Gomez-Gracia E , Lahoz C , Serra-Majem L , Pinto X , Ruiz-Gutierrez V , Covas M-I ((2011) ) A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 141: , 1140–1145. |
[48] | Thompson PD , Arena R , Riebe D , Pescatello LS ((2013) ) ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription. Curr Sports Med Rep 12: , 215–217. |
[49] | Miller WR , Rollnick S ((2013) ), Motivational interviewing: Helping people change (3rd edition), Guilford Press. |
[50] | Naar-King S , Earnshaw P , Breckon J ((2013) ) Toward a universal maintenance intervention: Integrating cognitive-behavioral treatment with motivational interviewing for maintenance of behavior change. J Cogn Psychother 27: , 126–137. |
[51] | Hardcastle S , Taylor A , Bailey M , Castle R ((2008) ) A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns 70: , 31–39. |
[52] | Resnicow K , DiIorio C , Soet JE , Borrelli B , Hecht J , Ernst D ((2002) ) Motivational interviewing in health promotion: It sounds like something is changing. Health Psychol 21: , 444–451. |
[53] | Wagner CC , Ingersoll KS ((2013) ) Motivational interviewing in groups, Guilford Press, New York, NY. |
[54] | Moyers TB , Rowell LN , Manuel JK , Ernst D , Houck JM ((2016) ) The Motivational Interviewing Treatment Integrity Code (MITI 4): Rationale, preliminary reliability and validity. J Subst Abuse Treat 65: , 36–42. |
[55] | The National Health and Medical Research Council ((2007) ) National Statement on Ethical Conduct in Human Research 2007 (Updated 2018), Australian Research Council and Universities, Commonwealth of Australia, Canberra. |
[56] | Hurtig-Wennlf A , Hagstrmer M , Olsson LA ((2010) ) The International Physical Activity Questionnaire modified for the elderly: Aspects of validity and feasibility. Public Health Nutr 13: , 1847–1854. |
[57] | Bohannon RW ((2007) ) Six-Minute Walk Test: A meta-analysis of data from apparently healthy elders. Top Geriatr Rehabil 23: , 155–160. |
[58] | Buysse DJ , Reynolds CF , Monk TH , Berman SR , Kupfer DJ ((1989) ) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: , 193–213. |
[59] | Henry JD , Crawford JR ((2005) ) The short-form version of the Depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br J Clin Psychol 44: , 227–239. |
[60] | McNair DM , Lorr M , Droppleman LF ((1992) ) Profile of Mood States (POMS)–revised manual. Education and Industrial Testing Service, San Diego, CA. |
[61] | Goldberg DP , Williams PA ((2006) ) User’s guide to the General Health Questionnaire: GHQ, GL Assessment, London, England. |
[62] | Richardson J , Iezzi A , Khan MA , Maxwell A ((2014) ) Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 7: , 85–96. |
[63] | Berkman LF , Leonard Syme S ((2017) ) Social networks, host resistance, and mortality: A nine-year follow-up study of Alameda county residents. Am J Epidemiol 185: , 1070–1088. |
[64] | Van den Borre E , Denys S , van Wieringen A , Wouters J ((2021) ) The digit triplet test: A scoping review. Int J Audiol 60: , 946–963. |
[65] | Cox RM , Alexander GC ((1995) ) The abbreviated profile of hearing aid benefit. Ear Hear 16: , 176–186. |
[66] | Messier C ((2004) ) Glucose improvement of memory: A review. Eur J Pharmacol 490: , 33–57. |
[67] | Mariani E , Polidori MC , Cherubini A , Mecocci P ((2005) ) Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromatogr 827: , 65–75. |
[68] | Smith AD , Refsum H ((2016) ) Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36: , 211–239. |
[69] | Bettcher BM , Wilheim R , Rigby T , Green R , Miller JW , Racine CA , Yaffe K , Miller BL , Kramer JH ((2012) ) C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 26: , 103–108. |




