Research Participants’ Perspectives on Precision Diagnostics for Alzheimer’s Disease
Abstract
Background:
Understanding research participants’ responses to learning Alzheimer’s disease (AD) risk information is important to inform clinical implementation of precision diagnostics given rapid advances in disease modifying therapies.
Objective:
We assessed participants’ perspectives on the meaning of their amyloid positron emission tomography (PET) imaging results for their health, self-efficacy to understand their results, psychological impact of learning their results, experience receiving their results from the clinical team, and interest in genetic testing for AD risk.
Methods:
We surveyed individuals who were being clinically evaluated for AD and received PET imaging six weeks after the return of results. We analyzed responses to close-ended survey items by PET result using Fisher’s exact test and qualitatively coded open-ended responses.
Results:
A total of 88 participants completed surveys, most of whom had mild cognitive impairment due to AD (38.6%), AD (28.4%), or were cognitively unimpaired (21.6%). Participants subjectively understood their results (25.3% strongly agreed, 41.8% agreed), which could help them plan (16.5% strongly agreed, 49.4% agreed). Participants with a negative PET result (n = 25) reported feelings of relief (Fisher’s exact p < 0.001) and happiness (p < 0.001) more frequently than those with a positive result. Most participants felt that they were treated respectfully and were comfortable voicing concerns during the disclosure process. Genetic testing was anticipated to be useful for medical care decisions (48.2%) and to inform family members about AD risk (42.9%).
Conclusions:
Participants had high subjective understanding and self-efficacy around their PET results and did not experience negative psychological effects. Interest in genetic testing was high.
INTRODUCTION
Advances in understanding of dementia biomarkers have facilitated more precise diagnosis of Alzheimer’s disease (AD) and related dementias (ADRD) [1–8]. Precision diagnostic approaches, including biomarker identification and genetic testing, can inform an etiologic-specific diagnosis in individuals with clinical symptoms and can identify pathologies and risk factors to guide use of the most effective therapies. Cerebrospinal fluid [7, 8], blood, and neuroimaging biomarkers for amyloid-β and phosphorylated tau [1, 6], as well as exome sequencing, have shown promise for predicting AD/ADRD risk in research settings [9, 10]. Given the recent availability of disease-modifying therapies for AD that act to reduce amyloid-β deposition in the brain [11, 12], use of positron emission tomography (PET) imaging to identify excess amyloid-β in individuals with early AD has become especially clinically relevant [13].
Previous research has examined the impact of returning positive amyloid PET results to individuals who were cognitively unimpaired, had subjective cognitive decline, and had AD on outcomes such as anxiety, depression, test-related distress, recall, health behavior changes, decision-making and motivations or desire for information. Cognitively unimpaired individuals who enroll in AD research generally do not experience significant adverse psychological reactions to learning PET results [14–17]. In a recent European multi-center randomized controlled trial of implementing amyloid PET in clinical practice, return of a positive amyloid PET result to individuals with subjective memory decline was associated with larger but not clinically significant negative psychological impacts compared with a negative result [18]. Participants with negative results generally experience relief, while participants with positive results generally have increased concern about AD. Intention to change behaviors in response to results is commonly reported [15, 16, 19].
Better understanding of the impact on research participants of returning neuroimaging biomarker results requires insight into how the results were communicated. Communicating complex information about AD risk and diagnosis is challenging, and patient-centered communication is critical to meet the needs of patients and families for whom the results might have psychosocial and pragmatic implications [20–22]. To address concerns about communicating AD risk information, there are ongoing efforts to develop standard communication approaches for returning precision diagnostic results, including neuroimaging biomarkers, to families [23, 24]. Communication strategies for uncertain or complex messages, as well as tools for patients’ assessment of the communication experience, have been empirically evaluated in other clinical contexts such as oncology [25, 26]. Yet, to date no data exist on patients’ or participants’ perspectives regarding how well precision diagnostics AD results were communicated during the disclosure session.
The goal of this research was to evaluate participants’ experiences with amyloid-β PET result disclosure within the context of a pilot AD neuroimaging biomarker study. We administered surveys six weeks after return of PET results to assess participants’ understanding of the results returned, self-efficacy to understand their results, psychological impact of results, experience of how results were communicated, and interest in genetic testing for AD/ADRD. Our findings can inform the design of future research studies and clinical implementation of biologically based approaches to early AD/ADRD diagnosis and treatment.
METHODS
Participants
We recruited individuals who were undergoing clinical evaluation and amyloid PET imaging for AD at the Nantz National Alzheimer Center of Houston Methodist Hospital between 2020 and 2021. Clinical services were disrupted by the COVID-19 pandemic for approximately two weeks during the study period. English-speaking individuals 50 years of age or older were included if they were: cognitively normal, had subjective memory complaints, or met a screening diagnostic category of pre-symptomatic AD dementia, mild cognitive impairment (MCI) due to AD dementia, or AD dementia [1]; able to consent to research or had a legally authorized representative; and, if cognitively impaired, had a study partner. This research was approved by the Houston Methodist Research Institute Institutional Review Board (HMRI IRB, protocol PRO00025999).
Imaging and return of results
All participants with subjective memory complaints and cognitive impairment were under the routine care of a neurologist. As part of this study, the neurologist who cared for each participant explained the reasons for obtaining PET imaging and implications of the possible results regarding the diagnosis of AD or other diseases and arranged for the participant to have an amyloid PET scan. Amyloid scans were performed with one of three amyloid PET tracers: 11C-PiB, 18F-florbetapiir, or 18F-florbetaben. Scans were considered positive when by visual read there was increased signal in at least two different cortical regions. Other than imaging, all testing was performed in a clinical unit in which cognitively impaired individuals receive care.
At a subsequent clinic visit, the neurologist showed the participant, and their study partner if applicable, the PET images and explained the test results and their clinical implications. Specifically, for participants with cognitive impairment, the neurologist explained that a positive PET result indicated that the participant’s cognitive impairment was caused by AD. For participants without cognitive impairment, the neurologist explained that a positive amyloid PET scan increased the likelihood of becoming cognitively impaired, particularly in those younger than 80 years of age. The process of returning results resembled that of any clinical test, with the neurologist addressing any questions and trying to ensure understanding but not using a formal disclosure protocol. When neurologists were asked questions regarding the amount of amyloid in the participant’s brain, the neurologist indicated that based on current scientific knowledge, the test result was considered either positive (amyloid present) or negative (amyloid absent), rather than as a gradient based on the amount of protein present.
Surveys and measures
We surveyed participants approximately six weeks after they received their results to allow them time to process what they were told. A study coordinator contacted each participant via phone to ask them to complete a survey which would be emailed to them. The study team then emailed the participant a link to an online survey administered via REDCap [27] and, if not completed, up to three email reminders were sent. The survey was designed to take about 10 minutes to complete. In some cases, participants received assistance from a study partner to complete the survey or a study partner entered survey responses on behalf of the participant. No compensation was provided for completing the survey. Survey data were collected between March 2021 and November 2021.
The surveys were designed to evaluate participants’ experience of receiving PET results in several domains. We assessed participants’ subjective understanding of their PET imaging results using one item adapted from the Psychological Adaptation to Genetic Information Scale [28], “How well do you understand your imaging results?”, with a five-point response scale of Not at all (=1) to Very well (=5). We also asked an open-ended question, “What do you think your imaging results mean for your current and future health?” We assessed participants’ self-efficacy to understand how their results affect their health using five items adapted from Kaphingst et al. [29].
We assessed the psychological impact of learning PET results using nine items adapted from the REVEAL Impact of Genetic Testing in Alzheimer’s Disease (IGT-AD) Scale [30]. To evaluate participants’ experience with the result communication process, surveys included 14 questions that were either novel or modified from the Patient Assessment of cancer Communication Experience (PACE) [31] and other communication evaluation surveys [32, 33]. Given that genetic test results can be interpreted alongside biomarker and clinical information to inform care and thus may be incorporated into future research protocols that build upon the current pilot study, the survey included items about genetic testing even though it was not part of the clinical evaluation that participants received. We assessed participants’ interest in genetic testing for AD risk using an item adapted from Kaphingst et al. [29] and how they anticipated that genetic testing for AD/ADRD might be useful using eight items adapted from the MedSeq Project [34]. The survey also included questions about sociodemographic information and self-reported general health.
Data analysis
Based on an initial review of responses to the open-ended question about what imaging results meant for the participant’s current and future health, we developed three categories of outlooks: positive, ambivalent, and negative. Positive was defined as expressing appreciation for having information regardless of test results and perceiving the results as having positive implications for the participant’s current and future health. It included finding the test results useful for future health, being hopeful, being able to enroll in clinical trials, or knowing they are at low risk for AD. Ambivalent included responses that had no affect and included describing disease risk as “not now” or “so far ok,” or wanting to help others or advance research, as well as having neutral or unclear implications for current and future health; this included responses about health behaviors in response to their test results. Negative was defined as expressing devastation or unhappiness with results or their future health outlook, such as the participant stating that they have an AD diagnosis or a similar diagnosis or the disease being fatal. We then applied these codes to the responses. One author (JOR) coded all responses in Excel, which a second author (AL) independently reviewed. Discrepancies in coding were resolved by consensus. Counts of categories were calculated to describe frequency of outlooks.
We calculated descriptive statistics for survey items that used quantitative response scales. We also compared the frequency of survey responses in each category by amyloid PET result (positive versus negative) as determined by the clinical team using Fisher’s exact test. We included all available responses to each survey item, and we did not impute missing responses. We analyzed data using Stata 17 (College Station, TX).
RESULTS
Participant characteristics
A total of 145 survey invitations were sent, and 88 participants completed a survey (response rate: 61%). Participants had a mean (SD) age of 71.6 (7.9) years, and the majority were male (54.5%), married (86.4%), and White or European American (92.0%). Responses were entered by participants on their own (35.2%), by participants with the assistance of a study partner (18.2%), or by study partners who responded on the participant’s behalf (45.5%).
Participant imaging results and diagnoses
All participants had amyloid PET imaging, and 63 (71.6%) had a positive result. The most frequent consensus diagnoses of survey participants were MCI due to AD (n = 34, 38.6%) and AD (n = 25, 28.4%), both of which were associated with a positive amyloid PET scan. Some participants had subjective memory complaints (n = 10, 11.4%), two of whom had a positive PET result. Others were cognitively unimpaired but had a family history of AD (n = 9, 10.2%), two of whom had a positive PET result. A small group had cognitive impairment and a negative PET (n = 5, 5.7%) and were diagnosed with diseases other than AD, including depression, Lewy-body dementia, corticobasal degeneration, and behavioral variant frontotemporal degeneration.
Participant-reported results and subjective understanding
Most participants reported having had PET imaging (n = 69, 78.4%). Of 63 participants with a positive amyloid PET result, 41 (65.1%) correctly reported being told that they had abnormal build-up of amyloid plaque in their brain, while others responded that they were told they did not have abnormal build-up of amyloid (3.2%), or were not told anything about amyloid plaques (3.2%), or they were not sure (4.8%), or left the question blank (23.8%). In response to the survey item to assess subjective understanding, participants reported that they understood their imaging results not at all (8.0%), a little (13.6%), somewhat well (30.7%), well (28.4%), or very well (14.8%), or left the question blank (4.5%). In response to the survey question which asked what they had been told about their current diagnosis, participants most frequently reported that they had AD (42.0%), MCI (30.7%), or no cognitive symptoms (11.4%). Among participants with a clinical diagnosis of AD or of MCI with AD pathophysiology, 89.8% (n = 53/59) recalled being told that they had AD or MCI. Among participants with a clinical diagnosis of MCI without AD pathophysiology, three out of five recalled being told that they had MCI.
Perceived meaning of results for health
Forty-two participants who received positive amyloid results provided responses to the open-ended question about what their imaging results meant for their health. Five of those participants had positive outlooks about what their imaging results meant for their health after receiving their results. The results provided “awareness of how to plan for the future” (ID 110) and hope that the results will help them enroll in clinical trials. Twenty-three participants had more negative outlooks, described as “scary, devastating” (ID 156) and a “terrible future” (ID 108). Nine participants who received positive amyloid results were more ambivalent, focusing on next steps. For example, one participant noted they “focus on diet and exercise now in order to be as health[y] as possible” (ID 163). Sixteen participants who received negative amyloid results provided responses to the open-ended question about what their imaging results meant for their health. Thirteen of those participants reported a negative (n = 3) or ambivalent (n = 10) outlook. Two participants, who received negative amyloid results and reported a positive outlook, generally understood that to mean they were at lower risk for AD. Some participants worried about other forms of dementia or mentioned continuing to search for answers (Table 2).
Table 1
Participant characteristics (n = 88)
| Characteristic | n (%) |
| Agea | |
| Mean (SD) | 71.6 (7.9) |
| Gender | |
| Male | 48 (54.5%) |
| Female | 39 (44.3%) |
| Missing | 1 (1.1%) |
| Race and ethnicity | |
| Black or African American | 1 (1.1%) |
| Hispanic or Latino | 4 (4.5%) |
| White or European American | 81 (92.0%) |
| More than one race | 1 (1.1%) |
| Prefer not to answer | 1 (1.1%) |
| Marital status | |
| Married | 76 (86.4%) |
| Widowed | 5 (5.7%) |
| Divorced | 6 (6.8%) |
| Living with partner | 1 (1.1%) |
| Number of children | |
| None | 7 (8.0%) |
| One or more | 79 (89.8%) |
| Missing | 2 (2.3%) |
| Education | |
| High school graduate | 5 (5.7%) |
| Some college | 11 (12.5%) |
| Associate (2-year) college degree | 6 (6.8%) |
| Bachelor’s degree | 36 (40.9%) |
| Graduate or professional degree | 29 (33.0%) |
| Missing | 1 (1.1%) |
| Self-reported general health | |
| Excellent | 12 (13.6%) |
| Very Good | 23 (26.1%) |
| Good | 34 (38.6%) |
| Fair | 14 (15.9%) |
| Poor | 5 (5.7%) |
| Household income | |
| $0 to $99,999 | 30 (34.1%) |
| $100,000 or more | 51 (58.0%) |
| Missing | 7 (8.0%) |
| Survey respondent | |
| Participant | 31 (35.2%) |
| Participant with help from study partner | 16 (18.2%) |
| Study partner | 40 (45.5%) |
| Missing | 1 (1.1%) |
| Participant-reported current diagnosis | |
| No cognitive symptoms | 10 (11.4%) |
| Mild cognitive impairment | 27 (30.7%) |
| Alzheimer’s disease | 37 (42.0%) |
| Another disease that causes memory or thinking problems | 6 (6.8%) |
| Results were unclear | 3 (3.4%) |
| Unsure | 3 (3.4%) |
| Missing | 2 (2.3%) |
| Clinical consensus diagnosis | |
| Alzheimer’s disease | 25 (28.4%) |
| Mild cognitive impairment due to Alzheimer’s disease | 34 (38.6%) |
| Mild cognitive impairment without Alzheimer’s disease | 5 (5.6%) |
| Cognitively unimpaired with family history of Alzheimer’s disease | 9 (10.2%) |
| Subjective memory complaints | 11 (11.4%) |
| Other diagnosisb | 5 (5.6%) |
aCalculated based on 74 responses; bOther diagnoses included depression, Lewy-body dementia, corticobasal degeneration, and behavioral variant frontotemporal degeneration.
Table 2
Exemplar quotes of positive, ambivalent, and negative outlooks according to amyloid PET resultsa,b
| Amyloid positive | Amyloid negative | |
| Positive outlook (n = 7) | “I am hopeful. I have strong family support and I am enrolled in a clinical trial.” (ID 129, Alzheimer’s disease) | “Cognitive impairment will advance more slowly than if I had amyloid” (ID 155, mild cognitive impairment) |
| “Awareness of how to plan for the future.” (ID 110, Alzheimer’s disease) | “Good” (ID 103, no report) | |
| “At first I thought Alzheimer’s was in my future. Now I think I may not develop it.” (ID 161, mild cognitive impairment) | N/Ac | |
| Ambivalent outlook (n = 19) | “helps with diagnosis” (ID 119, Alzheimer’s disease) | “So far, I’m okay” (ID 109, unclear results) |
| “I focus on diet and exercise now in order to be as health as possible” (ID 163, Alzheimer’s disease) | “No detectable presence of Alzheimer’s disease now” (ID 127, mild cognitive impairment) | |
| “MRI can be compared to future MRI’s for understanding disease progression. PET scan was a research procedure.” (ID 104, mild cognitive impairment) | “ . . . we continued further testing” (ID 184, other disease that causes memory or thinking problems) | |
| Negative outlook (n = 26) | “scary, devastating” (ID 156, Alzheimer’s disease) | “I have MCI and probably dementia with Lewy bodies. These conditions will probably progress and ultimately become fatal.” (ID 111, other disease that causes memory or thinking problems) |
| “terrible future” (ID 108, Alzheimer’s disease) | “Cognitive issues will increase” (ID 118, suspected Lewy Body dementia) | |
| “They confirmed I have ALZ so my brain will continue to shut down affecting my abilities and my body’s functions until ultimately I no longer breath[e]. There is currently no cure and minimal helps.” (ID 126, Alzheimer’s disease) | “Still have cognitive impairment” (ID 130, mild cognitive impairment) |
aThe question asked was: “What do you think your imaging results mean for your current and future health?”. bSelf-reported PET result matched clinical PET result for all results presented. cN/A, not applicable; Only two participants who received negative amyloid results provided responses to the open-ended question that were categorized as positive outlooks.
Participants’ perspectives on communication
Overall, participants reported positive perceptions of the result communication experience. Most participants trusted the clinical team, felt that they were treated with respect and listened to, and were comfortable voicing concerns (Table 3). Although most participants agreed that they had the necessary support to cope with any uncertainty or unknowns (n = 23, 26.1% strongly agree; n = 25, 28.4% agree) and felt comfortable asking questions (n = 27, 42.0% strongly agree; n = 37, 42.0% agree), some still reported that they had questions they were unable to ask (n = 2, 2.3% strongly agree; n = 8, 9.1% agree). Participants with a negative PET result more frequently strongly agreed that they were treated with sensitivity and respect (n = 19, 76.0% strongly agree; n = 2, 8.0% agree) compared to those who received a positive result (n = 35, 55.6% strongly agree; n = 21, 33.3% agree; p = 0.023). There were no differences in responses to other items that assessed perspectives on communication depending on whether the participant had received positive versus negative PET results.
Table 3
Perceptions of communication assessed using items modified from the Patient Assessment of cancer Communication Experience (PACE) [31] and other communication evaluation surveys [32, 33]
| Survey question | Total n (%) | PET Positive n (%) | PET negative n (%) | Fisher’s exact test p |
| n = 88 | n = 63 | n = 25 | ||
| I was treated with sensitivity and respect. | 0.023 | |||
| Strongly Disagree | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Disagree | ||||
| Neither Agree nor Disagree | 4 (4.5%) | 4 (6.3%) | 0 (0.0%) | |
| Agree | 23 (26.1%) | 21 (33.3%) | 2 (8.0%) | |
| Strongly Agree | 54 (61.4%) | 35 (55.6%) | 19 (76.0%) | |
| Missing | 7 (8.0%) | 3 (4.8%) | 4 (16.0%) | |
| I trust the clinical team. | 0.581 | |||
| Strongly Disagree | 1 (1.1%) | 1 (1.6%) | 0 (0.0%) | |
| Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 3 (3.4%) | 3 (4.8%) | 0 (0.0%) | |
| Agree | 31 (35.2%) | 25 (39.7%) | 6 (24.0%) | |
| Strongly Agree | 46 (52.3%) | 31 (49.2%) | 15 (60.0%) | |
| Missing | 5 (5.7%) | 1 (1.6%) | 4 (16.0%) | |
| I felt comfortable asking questions and voicing my concerns. | 0.272 | |||
| Strongly Disagree | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 5 (5.7%) | 5 (7.9%) | 0 (0.0%) | |
| Agree | 37 (42.0%) | 29 (46.0%) | 8 (32.0%) | |
| Strongly Agree | 37 (42.0%) | 24 (38.1%) | 13 (52.0%) | |
| Missing | 7 (8.0%) | 3 (4.8%) | 4 (16.0%) | |
| I felt listened to. | 0.093 | |||
| Strongly Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Disagree | 0 (0.0%) | 7 (11.1%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 7 (8.0%) | 21 (33.3%) | 0 (0.0%) | |
| Agree | 25 (28.4%) | 31 (49.2%) | 4 (16.0%) | |
| Strongly Agree | 48 (54.5%) | 2 (3.2%) | 17 (68.0%) | |
| Missing | 6 (6.8%) | 7 (11.1%) | 4 (16.0%) | |
| The clinical team checked to make sure I understood the information. | 0.105 | |||
| Strongly Disagree | 2 (2.3%) | 1 (1.6%) | 1 (4.0%) | |
| Neither Agree nor Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Disagree | 9 (10.2%) | 9 (14.3%) | 0 (0.0%) | |
| Agree | 28 (31.8%) | 23 (36.5%) | 5 (20.0%) | |
| Strongly Agree | 41 (46.6%) | 27 (42.9%) | 14 (56.0%) | |
| Missing | 6 (6.8%) | 1 (1.6%) | 5 (20.0%) | |
| The clinical team explained complicated topics well. | 0.307 | |||
| Strongly Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Disagree | 1 (1.1%) | 1 (1.6%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 11 (12.5%) | 7 (11.1%) | 4 (16.0%) | |
| Agree | 37 (42.0%) | 31 (49.2%) | 6 (24.0%) | |
| Strongly Agree | 32 (36.4%) | 21 (33.3%) | 11 (44.0%) | |
| Missing | 5 (5.7%) | 1 (1.6%) | 4 (16.0%) | |
| I got clear, understandable information. | 0.224 | |||
| Strongly Disagree | 3 (3.4%) | 3 (4.8%) | 0 (0.0%) | |
| Disagree | 1 (1.1%) | 1 (1.6%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 11 (12.5%) | 8 (12.7%) | 3 (12.0%) | |
| Agree | 38 (43.2%) | 32 (50.8%) | 6 (24.0%) | |
| Strongly Agree | 29 (33.0%) | 18 (28.6%) | 11 (44.0%) | |
| Missing | 6 (6.8%) | 1 (1.6%) | 5 (20.0%) | |
| I felt comfortable talking about sensitive issues or embarrassing subjects with the clinical team. | 0.520 | |||
| Strongly Disagree | 0 (0.0%) | 1 (1.6%) | 1 (1.1%) | |
| Disagree | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 2 (8.0%) | 13 (20.6%) | 15 (17.0%) | |
| Agree | 10 (40.0%) | 29 (46.0%) | 39 (44.3%) | |
| Strongly Agree | 9 (36.0%) | 17 (27.0%) | 26 (29.5%) | |
| Missing | 4 (16.0%) | 3 (4.8%) | 7 (8.0%) | |
| I felt I had the information and support available to me to answer any questions I had after receiving my test results. | 0.600 | |||
| Strongly Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Disagree | 9 (10.2%) | 8 (12.7%) | 1 (4.0%) | |
| Neither Agree nor Disagree | 14 (15.9%) | 10 (15.9%) | 4 (16.0%) | |
| Agree | 34 (38.6%) | 26 (41.3%) | 8 (32.0%) | |
| Strongly Agree | 22 (25.0%) | 14 (22.2%) | 8 (32.0%) | |
| Missing | 7 (8.0%) | 3 (4.8%) | 4 (16.0%) | |
| The clinical team helped me cope with any uncertainty or unknowns. | 0.461 | |||
| Strongly Disagree | 4 (4.5%) | 4 (6.3%) | 0 (0.0%) | |
| Disagree | 3 (3.4%) | 3 (4.8%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 24 (27.3%) | 16 (25.4%) | 8 (32.0%) | |
| Agree | 25 (28.4%) | 20 (31.7%) | 5 (20.0%) | |
| Strongly Agree | 23 (26.1%) | 15 (23.8%) | 8 (32.0%) | |
| Missing | 9 (10.2%) | 5 (7.9%) | 4 (16.0%) | |
| The clinical team noticed when I had problems understanding. | 0.975 | |||
| Strongly Disagree | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Disagree | 3 (3.4%) | 3 (4.8%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 31 (35.2%) | 23 (36.5%) | 8 (32.0%) | |
| Agree | 29 (33.0%) | 21 (33.3%) | 8 (32.0%) | |
| Strongly Agree | 17 (19.3%) | 13 (20.6%) | 4 (16.0%) | |
| Missing | 8 (9.1%) | 3 (4.8%) | 5 (20.0%) | |
| It was hard to make sense out of the information. | 0.120 | |||
| Strongly Disagree | 16 (18.2%) | 9 (14.3%) | 7 (28.0%) | |
| Disagree | 29 (33.0%) | 21 (33.3%) | 8 (32.0%) | |
| Neither Agree nor Disagree | 22 (25.0%) | 20 (31.7%) | 2 (8.0%) | |
| Agree | 7 (8.0%) | 5 (7.9%) | 2 (8.0%) | |
| Strongly Agree | 5 (5.7%) | 3 (4.8%) | 2 (8.0%) | |
| Missing | 9 (10.2%) | 5 (7.9%) | 4 (16.0%) | |
| It was hard to ask questions about this information. | 0.137 | |||
| Strongly Disagree | 18 (20.5%) | 9 (14.3%) | 9 (36.0%) | |
| Disagree | 34 (38.6%) | 27 (42.9%) | 7 (28.0%) | |
| Neither Agree nor Disagree | 16 (18.2%) | 13 (20.6%) | 3 (12.0%) | |
| Agree | 8 (9.1%) | 7 (11.1%) | 1 (4.0%) | |
| Strongly Agree | 3 (3.4%) | 2 (3.2%) | 1 (4.0%) | |
| Missing | 9 (10.2%) | 5 (7.9%) | 4 (16.0%) | |
| I had questions about this information that I was unable to ask. | 0.597 | |||
| Strongly Disagree | 22 (25.0%) | 16 (25.4%) | 6 (24.0%) | |
| Disagree | 37 (42.0%) | 26 (41.3%) | 11 (44.0%) | |
| Neither Agree nor Disagree | 11 (12.5%) | 10 (15.9%) | 1 (4.0%) | |
| Agree | 8 (9.1%) | 6 (9.5%) | 2 (8.0%) | |
| Strongly Agree | 2 (2.3%) | 1 (1.6%) | 1 (4.0%) | |
| Missing | 8 (9.1%) | 4 (6.3%) | 4 (16.0%) | |
| I received too much information to understand. | 0.363 | |||
| Strongly Disagree | 24 (27.3%) | 15 (23.8%) | 9 (36.0%) | |
| Disagree | 33 (37.5%) | 28 (44.4%) | 5 (20.0%) | |
| Neither Agree nor Disagree | 14 (15.9%) | 10 (15.9%) | 4 (16.0%) | |
| Agree | 6 (6.8%) | 5 (7.9%) | 1 (4.0%) | |
| Strongly Agree | 4 (4.5%) | 3 (4.8%) | 1 (4.0%) | |
| Missing | 7 (8.0%) | 2 (3.2%) | 5 (20.0%) | |
Self-efficacy and psychological impact of receiving PET biomarker results
Participants’ responses to questions related to self-efficacy to understand results did not differ according to whether their PET result was positive or negative (Table 4). Overall, most participants reported feeling confident in their ability to understand their results (n = 20, 22.7%; strongly agree; n = 33, 37.5% agree) and that they felt it would be easy to get information about what their results mean (n = 15, 17.0% strongly agree; n = 34, 38.6% agree). Participants also reported that they understood how their results could affect their health (n = 19, 21.6% strongly agree; n = 48, 54.5% agree), that they had a good idea about how their results may influence their risk for disease generally (n = 17, 19.3% strongly agree; n = 36, 40.9% agree), and that they could explain to others how their results might affect their own health (n = 15, 17.0% strongly agree; n = 31, 35.2% agree).
Table 4
Participant-reported self-efficacy to understand neuroimaging results for Alzheimer’s disease
| Survey question | Total n (%) | PET Positive n (%) | PET negative n (%) | Fisher’s exact test p |
| n = 88 | n = 63 | n = 25 | ||
| I am confident in my ability to understand my results. | 0.553 | |||
| Strongly Disagree | 2 (2.3%) | 1 (1.6%) | 1 (4.0%) | |
| Disagree | 9 (10.2%) | 8 (12.7%) | 1 (4.0%) | |
| Neither Agree nor Disagree | 15 (17.0%) | 10 (15.9%) | 5 (20.0%) | |
| Agree | 33 (37.5%) | 23 (36.5%) | 10 (40.0%) | |
| Strongly Agree | 20 (22.7%) | 16 (25.4%) | 4 (16.0%) | |
| Missing | 9 (10.2%) | 5 (7.9%) | 4 (16.0%) | |
| It is easy for me to get information about what my results mean. | 0.818 | |||
| Strongly Disagree | 9 (10.2%) | 7 (11.1%) | 2 (8.0%) | |
| Disagree | 9 (10.2%) | 8 (12.7%) | 1 (4.0%) | |
| Neither Agree nor Disagree | 14 (15.9%) | 9 (14.3%) | 5 (20.0%) | |
| Agree | 34 (38.6%) | 24 (38.1%) | 10 (40.0%) | |
| Strongly Agree | 15 (17.0%) | 11 (17.5%) | 4 (16.0%) | |
| Missing | 7 (8.0%) | 4 (6.3%) | 3 (12.0%) | |
| I understand how my results can affect my health. | 0.299 | |||
| Strongly Disagree | 1 (1.1%) | 0 (0.0%) | 1 (4.0%) | |
| Disagree | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | |
| Neither Agree nor Disagree | 12 (13.6%) | 7 (11.1%) | 5 (20.0%) | |
| Agree | 48 (54.5%) | 37 (58.7%) | 11 (44.0%) | |
| Strongly Agree | 19 (21.6%) | 14 (22.2%) | 5 (20.0%) | |
| Missing | 6 (6.8%) | 3 (4.8%) | 3 (12.0%) | |
| I have a good idea about how my results may influence my risk for disease generally. | 0.501 | |||
| Strongly Disagree | 4 (4.5%) | 3 (4.8%) | 1 (4.0%) | |
| Disagree | 11 (12.5%) | 9 (14.3%) | 2 (8.0%) | |
| Neither Agree nor Disagree | 13 (14.8%) | 7 (11.1%) | 6 (24.0%) | |
| Agree | 36 (40.9%) | 28 (44.4%) | 8 (32.0%) | |
| Strongly Agree | 17 (19.3%) | 12 (19.0%) | 5 (20.0%) | |
| Missing | 7 (8.0%) | 4 (6.3%) | 3 (12.0%) | |
| I can explain to others how my results may affect my health. | 0.993 | |||
| Strongly Disagree | 5 (5.7%) | 4 (6.3%) | 1 (4.0%) | |
| Disagree | 15 (17.0%) | 11 (17.5%) | 4 (16.0%) | |
| Neither Agree nor Disagree | 15 (17.0%) | 11 (17.5%) | 4 (16.0%) | |
| Agree | 31 (35.2%) | 23 (36.5%) | 8 (32.0%) | |
| Strongly Agree | 15 (17.0%) | 10 (15.9%) | 5 (20.0%) | |
| Missing | 7 (8.0%) | 4 (6.3%) | 3 (12.0%) | |
Figure 1 displays findings on the psychological impact of receiving PET imaging results. Overall, most participants strongly disagreed (n = 19, 21.6%) or disagreed (n = 18, 30.7%) that they were concerned it would be difficult to talk to family members about their results. Most participants reported feeling confident (n = 13, 14.8% strongly agreed; n = 39, 44.3% agreed) that their results would help them plan better for the future. Most participants did not report feeling regretful about having testing (n = 49, 55.7% strongly disagree; n = 15, 17.0% disagree). However, most participants reported feeling frustrated that there was no good prevention or cure for AD/ADRD, with participants who received a positive PET imaging result more frequently agreeing that they felt frustrated (n = 33, 52.4% strongly agree; n = 14, 22.2% agree) than those who received a negative result (n = 7, 28% strongly agree; n = 11, 44.0% agree; p = 0.05). Similarly, participants with a positive PET result more frequently reported feeling concerned about how their insurance status might be affected (n = 6, 9.5% strongly agree; n = 8, 12.7% agree) than those who received a negative result (none strongly agree, n = 2, 8.0% agree; p = 0.03).
Fig. 1
Psychological impact of receiving imaging results related to risk for Alzheimer’s disease and related dementias.
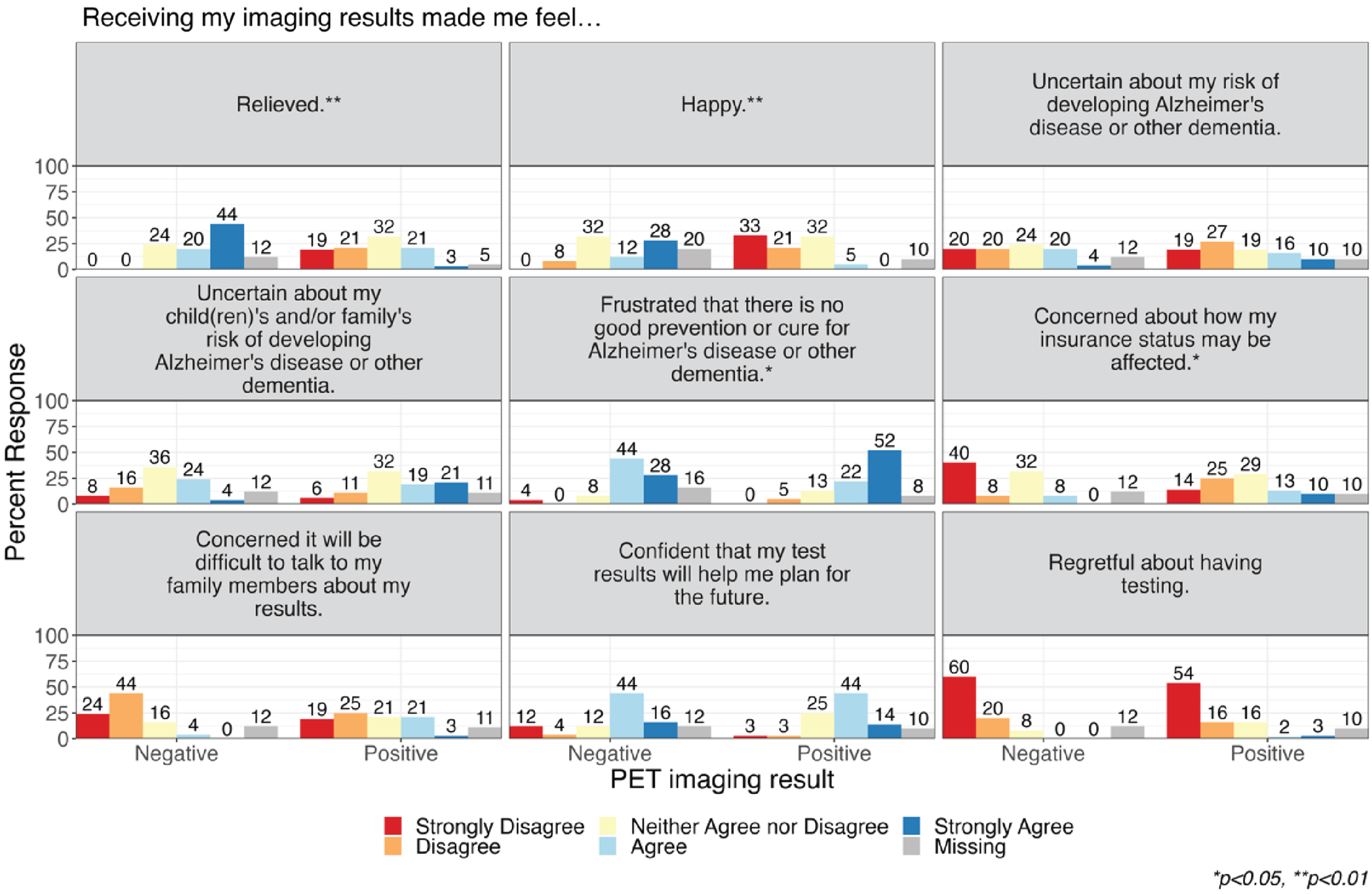
While overall approximately a third of participants neither agreed nor disagreed that the imaging results made them feel relieved (n = 26, 29.5%) and happy (n = 28, 31.8%), participants who received a negative PET imaging result more frequently agreed that their imaging results made them feel relieved (n = 11, 44.0% strongly agree; n = 5, 20.0% agree) compared to those who received a positive result (n = 2, 3.2% strongly agree; n = 13, 20.6% agree; p < 0.001). Similarly, participants who received a negative PET imaging result more frequently agreed that their imaging results made them feel happy (n = 7, 28.0% strongly agree; n = 3, 12.0% agree) compared to those who received a positive result (none strongly agree, n = 3, 4.8% agree; p < 0.001). In subgroup analyses of participants with MCI and with normal cognition (family history of AD or subjective memory complaints), we observed qualitatively similar response patterns by PET imaging result, yet small sample sizes prevented statistical comparison (Supplementary Material).
Genetic testing for AD/ADRD: Interest and potential utility
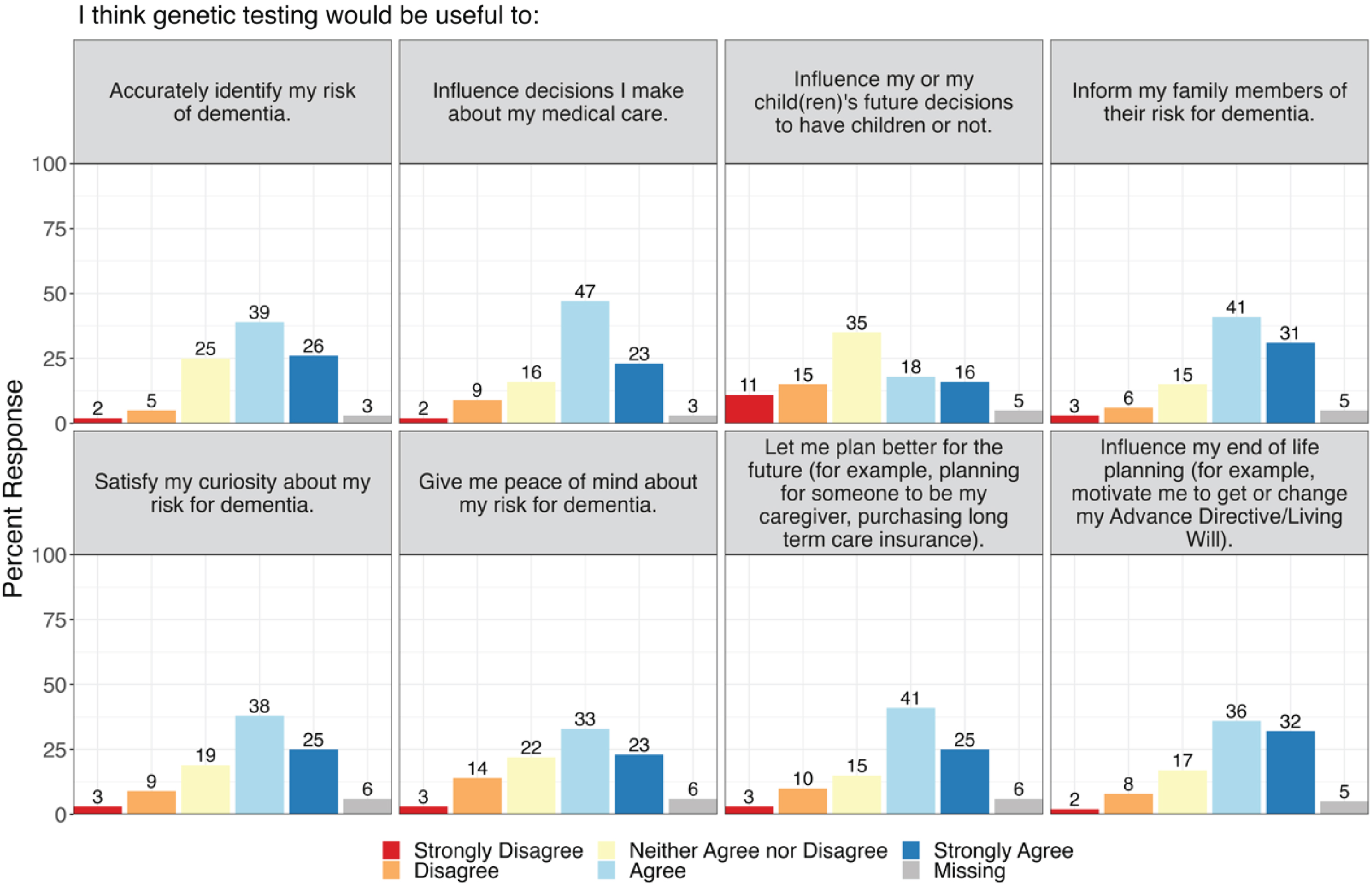
The majority (85.2%) of participants reported that they had not previously had any genetic testing related to AD/ADRD. However, most participants were interested (n = 24, 27.3%) or very interested (n = 33, 37.5%) in receiving genetic testing to learn more about their risk for AD/ADRD. Participants reported thinking that genetic testing would be useful to accurately identify their risk for dementia (n = 23, 26.1% strongly agree; n = 34, 38.6% agree), influence decisions they would make about their medical care (n = 20, 22.7% strongly agree; n = 41, 46.6% agree), inform family members of their risk for dementia (n = 27, 30.7% strongly agree; n = 36, 40.9% agree), and influence their end-of-life planning (n = 28, 31.8% strongly agree; n = 32, 36.4% agree). Participants also thought that genetic testing for AD/ADRD risk would help satisfy their curiosity (n = 22, 25.0% strongly agree; n = 33, 37.5% agree) and give them peace of mind (n = 20, 22.7% strongly agree; n = 29, 33.0% agree) about their risk for dementia (Fig. 2). Perceptions of potential utility of genetic testing did not differ by PET imaging outcome in pooled analyses or in analyses by PET result (Supplementary Material).
Fig. 2
Perceived utility of genetic testing for Alzheimer’s disease and related dementias.
DISCUSSION
In this survey study of participants who received results of neuroimaging biomarkers of AD, the majority of participants reported high self-efficacy around their results. While frustration about lack of AD/ADRD treatment was common, disease-modifying therapies have become available since our survey data were collected. Similar to research studies that have evaluated the psychological impacts of receiving genetic testing and neuroimaging testing for AD/ADRD risk, most participants in our study did not endorse negative feelings in response to testing [35–37]. Our results thus further support the disclosure of AD biomarker results in research and clinical care [38, 39]. However, we found that a sizable minority of survey participants did not remember their imaging results and expressed negative feelings, suggesting that further research is needed to understand how to communicate results and best support patients and families who receive biomarker results. Although our study was performed in the context of a research protocol, the process by which results were returned by the physicians clinically treating the participants resembled usual clinical care, where test results are routinely returned and their clinical implications are discussed.
Given that results disclosure was unscripted, our study provides novel insights regarding participants’ perspectives of the PET imaging result communication experience in an environment reflective of real-world clinical care. Our findings suggest that most participants had high trust in their clinical team, felt listened to, and felt comfortable voicing concerns. Notably, not all participants felt that they had the necessary support to answer their questions; some reported that it was hard to ask questions or that they had questions that they were unable to ask. These findings highlight the importance of assessing patients’ and research participants’ perceptions of the communication experience to inform approaches to returning complex risk information. Tools like checking for understanding, teach-back, and emotional support framing may be useful when communicating this information [23, 40–45].
While participants in our study did not receive genetic testing as part of the AD/ADRD evaluation, most expressed interest in receiving genetic testing to learn more about their AD/ADRD risk. They predicted that genetic testing would be useful for decision making about clinical care, future planning, and family members. These responses are aligned with findings from qualitative research with geriatricians, who also recognized the potential personal utility of genetic testing for AD/ADRD risk [46]. However, geriatricians may be less optimistic about the clinical utility of testing without availability of disease-modifying therapies and remain concerned about the potential of genetic test results to cause patients anxiety, especially if learned through a direct-to-consumer test [46]. Communication with participants and patients will need to include appropriate expectation setting about the certainty and utility of results. As more individuals without cognitive symptoms might want to undergo testing, additional research should focus on the development of standard protocols for disclosing a pre-clinical AD/ADRD diagnosis, training of clinical team members to communicate risk disclosure, and the clinical utility of early diagnosis [39, 47]. Participants in our study did not receive any education regarding genetic testing for AD/ADRD, and future research should explore the impact of education on perceptions of the utility of genetic testing.
The psychological impact of learning amyloid results and interest in genetic testing for AD/ADRD may vary based on whether individuals already have a clinical diagnosis of AD, MCI, or are cognitively unimpaired [19, 48, 49]. Our analysis is limited by small sample sizes of participants who had MCI without AD and who had no cognitive impairment, which prevented statistical comparison of survey responses across groups. Moreover, because we surveyed participants at a single time point, we are unable to determine whether outcomes such as psychological response might have shifted over time.
We did not directly compare participants’ self-reported diagnosis with their consensus diagnosis because the survey response categories did not map cleanly onto the consensus diagnosis categories. Our study population was not representative of all neurology clinic patients in terms of sociodemographic characteristics, and study participants were mostly highly educated, White or European American, and higher income individuals who may have more access to health care and resources for navigating a dementia diagnosis. Given disparities within AD research overall, [50] future research should ensure that the perspectives of individuals who reflect the AD/ADRD patient population are represented [51, 52].
Improved understanding of the communication process and validated instruments that are appropriate to assess the communication of precision diagnostic results in AD/ADRD are important to guide return of results. Additionally, given that there is a requirement for study partners to enroll alongside participants in AD/ADRD research, future studies should explore the perspectives of study partners on topics such as genetic testing that may have implications for their caregiving roles and, if biologically related to the study participant, their own health. Better understanding of whether and to what extent objective understanding of results and access to resources affect self-efficacy to understand and act upon AD/ADRD risk information is needed. Our findings can inform future clinical implementation of AD/ADRD through improved understanding of participants’ experience with return of results and interest in genetic testing.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This study was jointly sponsored by funds from Houston Methodist Hospital and Baylor College of Medicine.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data are available from the corresponding author upon request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230609.
REFERENCES
[1] | Jack CR , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Contributors ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[2] | Bruni AC , Bernardi L , Maletta R ((2021) ) Evolution of genetic testing supports precision medicine for caring Alzheimer’s disease patients. Curr Opin Pharmacol 60: , 275–280. |
[3] | Bellenguez C , Grenier-Boley B , Lambert J-C ((2020) ) Genetics of Alzheimer’s disease: Where we are, and where we are going. Curr Opin Neurobiol 61: , 40–48. |
[4] | Bellenguez C , Kücükali F , Jansen IE , Kleineidam L , Moreno-Grau S , Amin N , Naj AC , Campos-Martin R , Grenier-Boley B , Andrade V , et al. ((2022) ) New insights into the genetic etiology ofAlzheimer’s disease and related dementias. Nat Genet 54: , 412–436. |
[5] | Goldman JS , Van Deerlin VM ((2018) ) Alzheimer’s disease and frontotemporal dementia: The current state of genetics and genetic testing since the advent of next-generation sequencing. Mol Diagn Ther 22: , 505–513. |
[6] | Sperling RA , Mormino EC , Schultz AP , Betensky RA , Papp KV , Amariglio RE , Hanseeuw BJ , Buckley R , Chhatwal J , Hedden T , Marshall GA , Quiroz YT , Donovan NJ , Jackson J , Gatchel JR , Rabin JS , Jacobs H , Yang H-S , Properzi M , Kirn DR , Rentz DM , Johnson KA ((2019) ) The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol 85: , 181–193. |
[7] | Sutphen CL , Jasielec MS , Shah AR , Macy EM , Xiong C , Vlassenko AG , Benzinger TLS , Stoops EEJ , Vanderstichele HMJ , Brix B , Darby HD , Vandijck MLJ , Ladenson JH , Morris JC , Holtzman DM , Fagan AM ((2015) ) Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol 72: , 1029–1042. |
[8] | Toledo JB , Zetterberg H , van Harten AC , Glodzik L , Martinez-Lage P , Bocchio-Chiavetto L , Rami L , Hansson O , Sperling R , Engelborghs S , Osorio RS , Vanderstichele H , Vandijck M , Hampel H , Teipl S , Moghekar A , Albert M , Hu WT , Monge Argiles JA , Gorostidi A , Teunissen CE , De Deyn PP , Hyman BT , Molinuevo JL , Frisoni GB , Linazasoro G , de Leon MJ , van der Flier WM , Scheltens P , Blennow K , Shaw LM , Trojanowski JQ , Alzheimer’s Disease Neuroimaging Initiative ((2015) ) Alzheimer’s disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain 138: , 2701–2715. |
[9] | Khan S , Barve KH , Kumar MS ((2020) ) Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr Neuropharmacol 18: , 1106–1125. |
[10] | Lashley T , Schott JM , Weston P , Murray CE , Wellington H , Keshavan A , Foti SC , Foiani M , Toombs J , Rohrer JD , Heslegrave A , Zetterberg H ((2018) ) Molecular biomarkers of Alzheimer’s disease: Progress and prospects. Dis Model Mech 11: , dmm031781. |
[11] | Sims JR , Zimmer JA , Evans CD , Lu M , Ardayfio P , Sparks J , Wessels AM , Shcherbinin S , Wang H , Monkul Nery ES , Collins EC , Solomon P , Salloway S , Apostolova LG , Hansson O , Ritchie C , Brooks DA , Mintun M , Skovronsky DM , TRAILBLAZER-ALZ 2 Investigators ((2023) ) Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330: , 512–527. |
[12] | van Dyck CH , Swanson CJ , Aisen P , Bateman RJ , Chen C , Gee M , Kanekiyo M , Li D , Reyderman L , Cohen S , Froelich L , Katayama S , Sabbagh M , Vellas B , Watson D , Dhadda S , Irizarry M , Kramer LD , Iwatsubo T ((2023) ) Lecanemab in early Alzheimer’s disease. N Engl J Med 388: , 9–21. |
[13] | Rabinovici GD , Gatsonis C , Apgar C , Chaudhary K , Gareen I , Hanna L , Hendrix J , Hillner BE , Olson C , Lesman-Segev OH , Romanoff J , Siegel BA , Whitmer RA , Carrillo MC ((2019) ) Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321: , 1286–1294. |
[14] | Mozersky J , Hartz S , Linnenbringer E , Levin L , Streitz M , Stock K , Moulder K , Morris JC ((2021) ) Communicating 5-year risk of Alzheimer’s disease dementia: Development and evaluation of materials that incorporate multiple genetic and biomarker research results. J Alzheimers Dis 79: , 559–572. |
[15] | Grill JD , Cox CG , Harkins K , Karlawish J ((2018) ) Reactions to learning a “not elevated” amyloid PET result in a preclinical Alzheimer’s disease trial. Alzheimers Res Ther 10: , 125. |
[16] | Grill JD , Raman R , Ernstrom K , Sultzer DL , Burns JM , Donohue MC , Johnson KA , Aisen PS , Sperling RA , Karlawish J ((2020) ) Short-term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol 77: , 1–10. |
[17] | Milne R , Bunnik E , Diaz A , Richard E , Badger S , Gove D , Georges J , Fauria K , Molinuevo J-L , Wells K , Ritchie C , Brayne C ((2018) ) Perspectives on communicating biomarker-based assessments of Alzheimer’s disease to cognitively healthy individuals. J Alzheimers Dis 62: , 487–498. |
[18] | Caprioglio C , Ribaldi F , Visser LNC , Minguillon C , Collij LE , Grau-Rivera O , Zeyen P , Molinuevo JL , Gispert JD , Garibotto V ,et al. ((2023) ) Analysis of psychological symptoms following disclosure of amyloid-positron emission tomography imaging results to adults with subjective cognitive decline. JAMA Netw Open 6: , e2250921. |
[19] | Largent EA , Harkins K , van Dyck CH , Hachey S , Sankar P , Karlawish J ((2020) ) Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS One 15: , e0229137. |
[20] | Goldfarb D , Sheard S , Shaughnessy L , Atri A ((2019) ) Disclosure of Alzheimer’s disease and dementia: Patient- and care partner-centric decision-making and communication. J Clin Psychiatry 80: , MS18002BR1C. |
[21] | Institute of Medicine (US) Committee on Quality of Health Care in America (2001) Crossing the Quality Chasm: A New Health System for the 21st Century, National Academies Press (US), Washington (DC). |
[22] | Largent EA , Karlawish J ((2019) ) Preclinical Alzheimer’s disease and the dawn of the “pre-caregiver.”. JAMA Neurol 76: , 631–632. |
[23] | Fruijtier AD , van der Schaar J , van Maurik IS , Zwan MD , Scheltens P , Bouwman F , Pijnenburg YAL , van Berckel BNM , Ebenau J , van der Flier WM , Smets EMA , Visser LNC ((2023) ) Identifying best practices for disclosure of amyloid imaging results: A randomized controlled trial. Alzheimers Dement 19: , 285–295. |
[24] | Lingler JH , Butters MA , Gentry AL , Hu L , Hunsaker AE , Klunk WE , Mattos MK , Parker LS , Roberts JS , Schulz R ((2016) ) Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis 52: , 17–24. |
[25] | Street RL , Mazor KM , Arora NK ((2016) ) Assessing patient-centered communication in cancer care: Measures for surveillance of communication outcomes. J Oncol Pract 12: , 1198–1202. |
[26] | Arora NK , Street RL , Epstein RM , Butow PN ((2009) ) Facilitating patient-centered cancer communication: A road map. Patient Educ Couns 77: , 319–321. |
[27] | Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG ((2009) ) Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: , 377–381. |
[28] | Read CY , Perry DJ , Duffy ME ((2005) ) Design and psychometric evaluation of the Psychological Adaptation to Genetic Information Scale. J Nurs Scholarsh 37: , 203–208. |
[29] | Kaphingst KA , McBride CM , Wade C , Alford SH , Reid R , Larson E , Baxevanis AD , Brody LC ((2012) ) Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genet Med 14: , 681–687. |
[30] | Chung WW , Chen CA , Cupples LA , Roberts JS , Hiraki SC , Nair AK , Green RC , Stern RA ((2009) ) A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord 23: , 50–56. |
[31] | Mazor KM , Street RL , Sue VM , Williams AE , Rabin BA , Arora NK ((2016) ) Assessing patients’ experiences with communication across the cancer care continuum. Patient Educ Couns 99: , 1343–1348. |
[32] | Locatis C , Williamson D , Gould-Kabler C , Zone-Smith L , Detzler I , Roberson J , Maisiak R , Ackerman M ((2010) ) Comparing in-person, video, and telephonic medical interpretation. J Gen Intern Med 25: , 345–350. |
[33] | Kuo D , Fagan MJ ((1999) ) Satisfaction with methods of Spanish interpretation in an ambulatory care clinic. J Gen Intern Med 14: , 547–550. |
[34] | Lupo PJ , Robinson JO , Diamond PM , Jamal L , Danysh HE , Blumenthal-Barby J , Lehmann LS , Vassy JL , Christensen KD , Green RC , McGuire AL , MedSeq Project team ((2016) ) Patients’ perceived utility of whole-genome sequencing for their healthcare: Findings from the MedSeq project. Per Med 13: , 13–20. |
[35] | Guan Y , Roter DL , Erby LH , Wolff JL , Gitlin LN , Roberts JS , Green RC , Christensen KD ((2017) ) Disclosing genetic risk of Alzheimer’s disease to cognitively impaired patients and visit companions: Findings from the REVEAL Study. Patient Educ Couns 100: , 927–935. |
[36] | Guan Y , Roter DL , Wolff JL , Gitlin LN , Christensen KD , Roberts JS , Green RC , Erby LH ((2018) ) The impact of genetic counselors’ use of facilitative strategies on cognitive and emotional processing of genetic risk disclosure for Alzheimer’s disease. Patient Educ Couns 101: , 817–823. |
[37] | Guan Y , Roter DL , Erby LH , Wolff JL , Gitlin LN , Roberts JS , Green RC , Christensen KD ((2018) ) Communication predictors of patient and companion satisfaction with Alzheimer’s genetic risk disclosure. J Health Commun 23: , 807–814. |
[38] | Largent EA , Grill JD , O’Brien K , Wolk D , Harkins K , Karlawish J ((2023) ) Testing for Alzheimer disease biomarkers and disclosing results across the disease continuum. Neurology 100: , 1010–1019. |
[39] | Erickson CM , Clark LR , Ketchum FB , Chin NA , Gleason CE , Largent EA ((2022) ) Implications of preclinical Alzheimer’s disease biomarker disclosure for US policy and society. Alzheimers Dement (Amst) 14: , e12339. |
[40] | Mozersky J , Sankar P , Harkins K , Hachey S , Karlawish J ((2018) ) Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol 75: , 44–50. |
[41] | Grill JD , Apostolova LG , Bullain S , Burns JM , Cox CG , Dick M , Hartley D , Kawas C , Kremen S , Lingler J , Lopez OL , Mapstone M , Pierce A , Rabinovici G , Roberts JS , Sajjadi SA , Teng E , Karlawish J ((2017) ) Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther 9: , 35. |
[42] | Albert MS , DeKosky ST , Dickson D , Dubois B , Feldman HH , Fox NC , Gamst A , Holtzman DM , Jagust WJ , Petersen RC , Snyder PJ , Carrillo MC , Thies B , Phelps CH ((2011) ) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: , 270–279. |
[43] | Bodenheimer T ((2018) ) Teach-back: A simple technique to enhance patients’ understanding. Fam Pract Manag 25: , 20–22. |
[44] | Centrella-Nigro AM , Alexander C ((2017) ) Using the teach-back method in patient education to improve patient satisfaction. J Contin Educ Nurs 48: , 47–52. |
[45] | Ha Dinh TT , Bonner A , Clark R , Ramsbotham J , Hines S ((2016) ) The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: A systematic review. JBI Database System Rev Implement Rep 14: , 210–247. |
[46] | Arias JJ , Lin GA , Tyler AM , Douglas MP , Phillips KA ((2022) ) Geriatricians’ perspectives on the multiple dimensions of utility of genetic testing for Alzheimer’s disease: A qualitative study. J Alzheimers Dis 90: , 1011–1019. |
[47] | Caselli RJ , Langbaum J , Marchant GE , Lindor RA , Hunt KS , Henslin BR , Dueck AC , Robert JS ((2014) ) Public perceptions of presymptomatic testing for Alzheimer disease. Mayo Clin Proc 89: , 1389–1396. |
[48] | Grill JD , Cox CG , Kremen S , Mendez MF , Teng E , Shapira J , Ringman JM , Apostolova LG ((2017) ) Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement 13: , 924–932. |
[49] | Grill JD , Raman R , Ernstrom K , Sultzer DL , Burns JM , Donohue MC , Johnson KA , Aisen PS , Sperling RA , Karlawish J , A4 Study Team ((2020) ) Short-term psychological outcomes of disclosing amyloid imaging results to research participants who do not have cognitive impairment. JAMA Neurol 77: , 1504. |
[50] | Raman R , Quiroz YT , Langford O , Choi J , Ritchie M , Baumgartner M , Rentz D , Aggarwal NT , Aisen P , Sperling R , Grill JD ((2021) ) Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Netw Open 4: , e2114364. |
[51] | Gaugler JE , McCarron HR , Mitchell LL ((2019) ) Perceptions of precision medicine among diverse dementia caregivers and professional providers. Alzheimers Dement (N Y) 5: , 468–474. |
[52] | Rosas LG , Nasrallah C , Park VT , Vasquez JJ , Duron Y , Garrick O , Hattin R , Cho M , David SP , Evans J , McClinton-Brown R , Martin C ((2020) ) Perspectives on precision health among racial/ethnic minority communities and the physicians that serve them. Ethn Dis 30: , 137–148. |




