Palliative Care in Nursing Home Residents with Young-Onset Dementia: Professional and Family Caregiver Perspectives
Abstract
Background:
The evidence underpinning palliative care in dementia is mostly based on research in older populations. Little is known about the palliative care needs of people with young-onset dementia (YOD).
Objective:
To describe palliative care practices including advance care planning (ACP) in people with YOD residing in Dutch nursing homes.
Methods:
The study presents baseline questionnaire data from an observational cohort study. Physicians, family caregivers, and nursing staff completed questionnaires about 185 residents with YOD. The questionnaires included items on sociodemographics, quality of life measured with the quality of life in late-stage dementia (QUALID) scale, dementia-related somatic health problems, symptoms, pain medication, psychotropic drugs, and ACP.
Results:
The mean age was 63.9 (SD 5.8) years. Half (50.3%) of them were female. Alzheimer’s disease dementia (42.2%) was the most prevalent subtype. The mean QUALID score was 24.0 (SD 7.9) as assessed by family caregivers, and 25.3 (SD 8.6) as assessed by the nursing staff. Swallowing problems were the most prevalent dementia-related health problem (11.4%). Agitation was often reported by physicians (42.0%) and nursing staff (40.5%). Psychotropics were prescribed frequently (72.3%). A minority had written advance directives (5.4%) or documentation on treatment preferences by the former general practitioner (27.2%). Global care goals most often focused on comfort (73.9%). Proportions of do-not-treat orders were higher than do-treat orders for all interventions except for hospitalization and antibiotics.
Conclusions:
ACP must be initiated earlier, before nursing home admission. A palliative approach seems appropriate even though residents are relatively young and experience few dementia-related health problems.
INTRODUCTION
Dementia-specific palliative care strategies are mainly based on research on persons with late-onset dementia (LOD) [1, 2]. It is unclear whether these strategies should also apply to people with young-onset dementia (YOD) [3], who develop symptoms before the age of 65 [4]. However, those with YOD could benefit from palliative care as it is aimed at improving the quality of life (QOL) of those facing problems associated with life-threatening illnesses [5].
Good palliative care for people with dementia comprises multiple domains according to the white paper by the European Association for Palliative Care [2]. The baseline is a palliative care approach that refers to all treatment and care, including adequate treatment of behavioral and psychological symptoms of dementia, comorbid diseases, and health problems. Palliative care also entails support for family members as may be needed in their role as proxy decision-makers and in dealing with the caregiving situation. Advance care planning (ACP) is one of the key components of palliative care in dementia. A recent umbrella review showed that ACP is associated with improved end-of-life outcomes [6]. ACP involves involves reflection and dialogue about preferences for future care between the patient and the healthcare team, but also with family caregivers who may continue the dialogue with the healthcare team if the patient can no longer be involved. It may include the completion of advance directives and discussion of treatment orders to anticipate future scenarios [7]. ACP continues to evolve and undergoes reconceptualization as a holistic, ongoing process throughout the life course, encompassing both tailored in-the-moment decisions and advance choices at every life stage [8].
It can be expected that the palliative care needs of people with YOD differ from people with LOD due to distinct characteristics of YOD including fewer somatic comorbidities [9], more neuropsychiatric symptoms [10], higher disease awareness [11], and larger caregiver burden [12]. However, palliative care needs remain underinvestigated despite a growing number of studies on people with YOD. The German EPYLOGE-study is the only study on this topic so far [13]. Its cohort consisted of people with advanced YOD and LOD living at home or in long-term care (LTC) facilities. Those younger than 65 years experienced symptoms that were significantly more distressing than older patients. Patients with YOD had fewer somatic comorbidities and were less frequently admitted to a hospital in the last three months of life [14]. There are no known reports on palliative care needs and provision in LTC settings that are specialized in the care of residents with YOD.
YOD special care units are available across the Netherlands [15]. The Young-Onset Dementia Knowledge Center, founded in 2013, supports healthcare organizations in providing age-appropriate and high-quality care for patients with YOD according to a National Care Program [16]. Therefore, the Dutch setting offers the opportunity to study palliative care practices in the unique context of infrastructure established for specialized nursing home care for young people living with dementia. The aim of this study is to describe health problems, symptoms, symptom treatment, and ACP, focusing on medical treatments, in a sample of residents with YOD living in special care units.
METHODS
This paper reports baseline data of the observational Care4Youngdem-study (WHO International Clinical Trials Registry Platform (ICTRP) ID NTR5989, registration date 2016-09-08) which aims to map the practice of palliative care in YOD from three perspectives: physician or advanced nurse practitioner, family caregiver, and nursing staff. Questionnaires were administered after inclusion (baseline) and after death. Data collection started in 2016 and ended in 2022.
Context
After admission to a LTC facility in the Netherlands, physicians or advanced nurse practitioners, employed by the LTC organization, provide medical care. Certified elderly care physicians who have specialized as primary care experts in geriatric medicine serve as attending physicians [17]. Daily nursing care is delivered by nurse assistants with three years of vocational training (European Qualification Framework (EQF) 3), nurse aides (EQF 2), and registered nurses (EQF 4–6) [18].
Recruitment
Patient enrolment and collection of baseline data took place from 2016 to 2020. We approached 34 LTC organizations and recruited 16 (response 47%) with a total of 18 YOD special care units. All participating LTC organizations were affiliated with the Young-Onset Dementia Knowledge Center [16].
Members of the nursing home staff approached family caregivers and provided them with an information letter. All residents, regardless of their length of stay, were eligible for inclusion if 1) there was a physician diagnosis of dementia with first symptom onset before the age of 65 years; 2) family caregivers provided informed consent to participate and to transfer data about the resident to the researchers.
Data collection
After receiving informed consent, the resident’s physician or advanced nurse practitioner, family caregiver, and nurse assistants or registered nurses were asked to complete the questionnaires (Table 1). Validated and translated assessment instruments were available mostly through research on palliative care in LOD [19].
Table 1
Overview of instruments and measures used including type of respondent.
| Topic | Measurement instrument or question | Range or options | Respondent |
| Demographics | Physician | ||
| Relationship with the resident Date of diagnosis | Family caregiver Physician | ||
| Date of diagnosis | |||
| Dementia type | Alzheimer’s disease Frontotemporal dementia Vascular dementia Lewy Body Dementia or Parkinson dementia Alcohol-related dementia Mixed dementia of Alzheimer’s disease and vascular dementia Other dementiaa aOther dementia was recoded if possible | Physician | |
| Dementia severity | Global Deterioration Scale [20] | 7 categories with elaborate verbal descriptions scored 1 - 7 | Physician |
| Quality of life | The quality of life in late-stage dementia scale | 1–5 per item with item-specific response options 11–55 total score with lower scores representing better quality of life | Family caregiver and nursing staff |
| Weight loss | Resident Assessment Instrument-Minimum Data Set 2.0 [56] | Present (body weight reduction 5% over last month or 10% over last six months) Absent | Physician |
| Nutritional status | Very cachecticb Cachectic Normal Obese Very obesec bVery cachectic was recoded into cachectic cVery obese was recoded into obese | Physician | |
| Hydration status | Dehydrated Mildly dehydrated Normal | Physician | |
| Swallowing problems | Present or absent | Nursing staff | |
| Life expectancy | Surprise question: “Would I be surprised if the patient died in the next year?” [23] | Yes or no | Physician |
| Symptoms (pain and agitation) | Frequency in the last week | Yes, no and do not know Yes (2-3 days, 4-5 days, and 6-7 days) or no (<1 day or never) | Physician Nursing staff |
| Symptoms (various) | End-of-Life in Dementia Scale - Symptom Management [25] | Frequency categories scored 0 – 5 per item 0–45 total score | Family caregiver |
| Symptom treatment | Anatomical Therapeutic Chemical classification system [21] | Physician | |
| Written advance directives | Present, absent or unknown | Physician | |
| Documentation of the former general practitioner on treatment preferences Medical decision-making capacity of resident | Present, absent or unknown Yes Partly No | Physician Physician | |
| Prioritized global care goal | 1. Palliative, i.e., aimed at well-being and quality of life, irrespective of shortening or prolonging of lifed 2. Symptomatic, i.e., aimed at well-being and quality of life, additional prolonging of life undesirabled 3. Maintaining or improving function 4. Life prolongation 5. Not yet determined 6. Other dPalliative and symptomatic together constitute a comfort care goal | Physician | |
| Treatment order | Resuscitation Hospitalization Admission to an intensive care unit Tube feeding Intravenous therapy Hypodermoclysis Antibiotics | Do-treat Do-not-treat Discussed but no order Not discussed and no order | Physician |
The questionnaire included items on demographics, date of admission, type and severity of dementia, and date of diagnosis. The type of dementia was asked as stated in the medical record. The type was categorized into Alzheimer’s disease, vascular dementia, frontotemporal dementia, mixed dementia of Alzheimer’s disease dementia and vascular dementia, alcohol-related dementia, dementia by multiple causes, other causes of dementia, and dementia not otherwise specified. Dementia severity was assessed with the Global Deterioration Scale (GDS) [20]. This scale describes seven different stages of dementia and has been validated against behavioral, neuroanatomic, and neuropsychological measures. Medication overviews were provided to determine pain medication and psychotropics including pro re nata prescriptions. These were classified according to the Anatomical Therapeutic Chemical (ATC) classification [21].
Pre-structured items from the Resident Assessment Instrument-Minimum Data Set 2.0 [22] on nutritional status and hydration status were completed. Weight loss was defined as 5% over the last month or 10% over the last six months. Swallowing problems were assessed as either being present or absent. Physicians were asked the surprise question (“Would you be surprised if this patient died within the next 12 months?”) which is a frequently used screening tool to create awareness around identifying people nearing the end of life within various patient groups [23, 24].
The frequency of pain and agitation over the last week was assessed by both the physician and nursing staff. Symptoms during the previous month were assessed by family caregivers with the Dutch version of the End-of-Life in Dementia Scale - Symptom Management (EOLD-SM) [25]. It assesses the frequency of nine items: pain, shortness of breath, depression, fear, anxiety, agitation, calm, skin breakdown, and resistance to care. Higher scores (range 0 to 45) indicate better symptom control. The scale showed acceptable Cronbach’s α coefficients and approximately normal distributions in a Dutch nursing home cohort [26].
The Quality of Life in Late-Stage Dementia (QUALID) [27] was used as a proxy rating of QOL by the family caregiver and nurse (assistant). It comprises 11 items that assess observed well-being through the intensity of a resident’s behavior. The scale was initially developed for nurses. However, it can be administered by family caregivers who are familiar with the resident’s behavior [28]. Lower scores (range 11 to 55) indicate better QOL. The validated Dutch version of the QUALID showed good test-retest reliability and moderate interrater reliability [29].
Questions related to ACP included the presence of written advance directives, the existence of documents drawn up before admission by the former general practitioner (GP) on (future) treatment preferences of the person with YOD, the medical decision-making making capacity of the resident as assessed by the physician, and questions regarding treatment orders anticipating future scenarios. Care goals and treatment orders could be agreed upon by physicians and residents or family caregivers depending on the medical decision-making capacity of a resident. The prioritized care goal was presented with five possible pre-structured options and a sixth open-ended option. Treatment orders referred to resuscitation, hospitalization, admission to an intensive care unit, tube feeding, intravenous therapy, hypodermoclysis, and antibiotics. Most of these treatments are delineated within a Dutch primary care physicians guideline on ACP and also part of ACP-discussions in daily nursing home practice [30, 31]. Response options included do-treat, do-not-treat, discussed but no order, and not discussed and no order.
Some previous studies in the field of palliative care either exclusively enrolled individuals with advanced dementia or conducted analyses in a subgroup of those with advanced dementia [32, 33]. Therefore, we performed an additional analysis which included the QUALID scale and all characteristics related to palliative care and ACP of residents with advanced dementia, which was defined as a GDS score of 7.
Statistical analysis
The statistical software SPSS version 25.0 was used to conduct statistical analysis (SPSS Inc, 2017, IBM, USA). Continuous variables were presented as mean with standard deviation and range. Categorical variables were represented as frequencies and corresponding percentages. In cases of no more than one-third of missing QUALID or EOLD-SM items, the total score was generated by imputing the mean patient item score.
Ethical considerations
The study protocol (number 2016-2505) was reviewed and declared exempt from the Medical Research Involving Human Subjects Act (WMO) by the designated Medical Research Ethics Committee (Commissie Mensgebonden Onderzoek Regio Arnhem/Nijmegen) on 9 June 2016. The research project was performed in accordance with the principles of the Declaration of Helsinki and the Dutch Medical Treatment Contracts Act.
RESULTS
A total of 215 family caregivers agreed to participate and collect data about the resident (Supplementary Figure 1). We report on 185 residents (86.0%) for which all three respondents (physicians, family caregivers, and nursing staff) completed the questionnaire.
Patient and dementia-related characteristics
The mean age of residents with YOD was 63.9 years, ranging from 45 to 76 years (Table 2). Women comprised 50.3% of the residents. Most of the family caregivers were partners (55.7%). The average length of stay in long-term care was 2.3 years with a range from 10 days to 13 years.
Table 2
Patient and dementia-related characteristics of residents (N = 185)
| Number | % | |
| Age, y | ||
| Mean (SD) | 63.9 (5.8) | |
| Range | 45–76 | |
| Sex | ||
| Female | 93 | 50.3 |
| Male | 92 | 49.7 |
| Family caregiver relationship | ||
| Partner | 103 | 55.7 |
| Sibling | 35 | 18.9 |
| Child | 29 | 15.7 |
| Parent | 2 | 1.1 |
| Professional legal guardian | 2 | 1.1 |
| Other | 14 | 7.6 |
| Length of stay in long-term care facility | ||
| Mean (SD) | 2.3 (2.4) y | |
| Range | 10 days-13.0 y | |
| Type, number (%) | ||
| Alzheimer’s disease dementia | 78 | 42.2 |
| Frontotemporal dementia | 37 | 20.0 |
| Vascular dementia | 13 | 7.0 |
| Lewy Body Dementia or Parkinson dementia | 10 | 5.4 |
| Mixed dementia of Alzheimer’s and vascular dementia | 8 | 4.3 |
| Alcohol-related dementia | 7 | 3.8 |
| Dementia due to multiple causesa | 7 | 3.8 |
| Other causes of dementiab | 15 | 8.1 |
| Dementia not otherwise specified | 10 | 5.4 |
| Time since diagnosisc | ||
| Mean (SD) | 4.8 (3.1) y | |
| Range | 5 mo–22.7 y | |
| GDSd | ||
| ≤4, No to moderate cognitive decline | 21 | 11.4 |
| 5, Moderately severe cognitive decline | 47 | 25.5 |
| 6, Severe cognitive decline | 67 | 36.4 |
| 7, Very severe cognitive decline | 49 | 26.6 |
| QUALIDe | ||
| Score assessed by family caregiversf | ||
| Mean (SD) | 24.0 (7.9) | |
| Range | 11–47 | |
| Score assessed by nursing staff | ||
| Mean (SD) | 25.3 (8.6) | |
| Range | 11–48 |
aCombination of vascular and frontotemporal dementia (N = 3), Alzheimer’s disease dementia and frontotemporal dementia (N = 2), Alzheimer’s disease dementia and Lewy body dementia (N = 1), and vascular dementia and alcohol-related dementia (N = 1). bPosterior cortical atrophy (N = 2) and various other types of dementia (all N = 1). cN = 179. dGlobal deterioration Scale, N = 184. eQuality of life in late-stage dementia scale; lower scores indicate better quality of life. fN = 183; N = 2 imputed values.
Alzheimer’s disease dementia had the highest prevalence (42.2%) followed by frontotemporal dementia (20.0%) and vascular dementia (7.0%). The majority of residents experienced severe or very severe cognitive decline (36.4% and 26.6%, respectively). However, moderate (8.7%) and moderately severe cognitive decline (25.5%) were not rare. Family caregivers rated QOL slightly higher than nursing staff as indicated by lower QUALID scores on average: 24.0 (SD 7.9) versus 25.3 (SD 8.6). In case of advanced dementia, mean QUALID scores were 24.8 (SD 8.7) and 28.6 (SD 9.2), respectively.
Palliative care-related characteristics
Only 8.1% of residents experienced weight loss (Table 3). Cachexia was present in 8.7% of residents. The prevalence of obesity was 15.8%. Dehydration was uncommon (1.1%). Difficulties with swallowing occurred in 11.4%. In over half (56.5%) of cases the physician would be surprised if the resident were to die within a year.
Table 3
Palliative care-related characteristics of residents
| All residents (N = 185) | Residents with advanced dementia (N = 49) | |||
| Number | % | Number | % | |
| Dementia-related health problems | ||||
| Weight loss | ||||
| Present | 15 | 8.1 | 10 | 20.4 |
| Absent | 160 | 86.5 | 46 | 73.5 |
| Unknown | 10 | 5.4 | 3 | 6.1 |
| Nutritional statusa | ||||
| Cachectic | 16 | 8.7 | 11 | 22.4 |
| Normal | 139 | 75.5 | 33 | 67.3 |
| Obese | 29 | 15.8 | 5 | 10.2 |
| Hydration statusa | ||||
| Dehydrated | 2 | 1.1 | 2 | 4.1 |
| Mildly dehydrated | 5 | 2.7 | 4 | 8.2 |
| Normal | 177 | 96.2 | 43 | 87.8 |
| Swallowing problems | ||||
| Present | 21 | 11.4 | 9 | 18.4 |
| Absent | 164 | 88.6 | 40 | 81.6 |
| Surprise questiona,b | ||||
| Yes | 104 | 56.5 | 13 | 26.5 |
| No | 80 | 43.5 | 36 | 73.5 |
| Symptoms in the previous week | ||||
| Pain | ||||
| Yes, according to physicianc | 33 | 18.6 | 7 | 15.6 |
| Yes, according to nurse | 56 | 30.2 | 13 | 26.5 |
| Agitation | ||||
| Yes, according to physiciand | 73 | 42.0 | 26 | 53.1 |
| Yes, according to nurse | 75 | 40.5 | 24 | 49.0 |
| EOLD - Symptom Managemente | ||||
| Mean (SD) | 31.9 (8.5) | 31.5 (9.0) | ||
| Range | 10–45 | 11–45 | ||
| Pain medication (total of M01A, N02A, and N02BE01)f | 51 | 29.5 | 16 | 34.8 |
| NSAIDs (M01A) | 5 | 2.7 | 1 | 2.2 |
| Opioids (N02A) | 13 | 7.0 | 4 | 8.7 |
| Paracetamol (N02BE01) | 42 | 24.3 | 13 | 28.3 |
| Psychoactive medication (total of N03A, N05, N06A, and N06D) f,g,h | 125 | 72.3 | 34 | 73.9 |
| Antipsychotics (N05A) | 72 | 41.6 | 18 | 39.1 |
| Anxiolytics (N05B) | 33 | 19.1 | 10 | 21.7 |
| Antidepressants (N06A) | 69 | 39.9 | 19 | 41.3 |
aN = 184. bAnswer to the question: “Would I be surprised if the patient died within one year?”. cN = 177 and 45. dN = 174 and 49. eEnd-of-Life in Dementia scales, N = 182; detailed information can be found in Supplementary Table 1. fN = 173 and 46 (12 missing medication overviews). gN03A comprises antiepileptics. hN06D comprises antidementia drugs.
Pain was frequently reported by the nursing staff and physician (30.2% and 18.6%, respectively). Agitation was observed in almost half of cases (40.2% and 42.0%, respectively). The mean EOLD-SM total score as assessed by family caregivers was 31.9 (SD 8.5). The family caregivers had observed agitation (34.6%) and pain (25.2%) at least once a week over the previous month (Supplementary Table 1). Skin breakdown (4.8) and shortness of breath (4.5) had the highest mean values of the EOLD-SM of all items indicating that in most cases these symptoms had not occurred in the last month (91.2% and 82.0%, respectively). Anxiety had the highest mean score (2.7), 41.5% of residents experienced this symptom at least once a week. Pain medication was administered to 29.5% of residents. Almost three-quarters (72.3%) of the residents were prescribed at least one psychotropic drug, mostly antipsychotics (41.6%) and antidepressants (39.9%). Residents with advanced dementia experienced more often agitation and a higher symptom burden as shown by lower EOLD-SM scores. Malnutrition, cachexia, and dehydration were more prevalent.
ACP-related characteristics
Written advance directives were present in 5.4% of residents (Table 4). Documentation of the former GP specifically on treatment preferences was available in 27.2% of cases. In around a third of cases (34.2%), the physician deemed the resident at least partly able to make decisions regarding medical treatments.
Table 4
ACP-related characteristics of residents
| All residents (N = 184) | Residents with advanced dementia (N = 49) | |||
| Number | % | Number | % | |
| Written advance directive (living will) | ||||
| Present | 10 | 5.4 | 3 | 6.1 |
| Absent | 160 | 87.0 | 44 | 89.8 |
| Unknown | 14 | 7.6 | 2 | 4.1 |
| Documentation of the former general practitioner on treatment preferences | ||||
| Present | 50 | 27.2 | 14 | 28.6 |
| Absent | 119 | 64.7 | 28 | 57.1 |
| Unknown | 15 | 8.2 | 7 | 14.3 |
| Medical decision-making capacity of resident | ||||
| Yes | 10 | 5.4 | 2 | 4.1 |
| Partly | 53 | 28.8 | 1 | 2.0 |
| No | 121 | 65.8 | 46 | 93.9 |
| Care goal | ||||
| Palliativea,c | 114 | 62.0 | 34 | 69.4 |
| Symptomaticb,c | 22 | 12.0 | 9 | 18.4 |
| Maintaining or improving function | 26 | 14.1 | 2 | 4.1 |
| Life prolongation | 16 | 8.7 | 3 | 6.1 |
| Other | 4 | 2.2 | 1 | 2.0 |
| Undecided | 2 | 1.1 | 0 | 0.0 |
aDefinition: aimed at well-being and quality of life, irrespective of shortening or prolonging of life. bDefinition: aimed at well-being and quality of life, additional prolonging of life undesirable. cPalliative and symptomatic together constitute a comfort care goal.
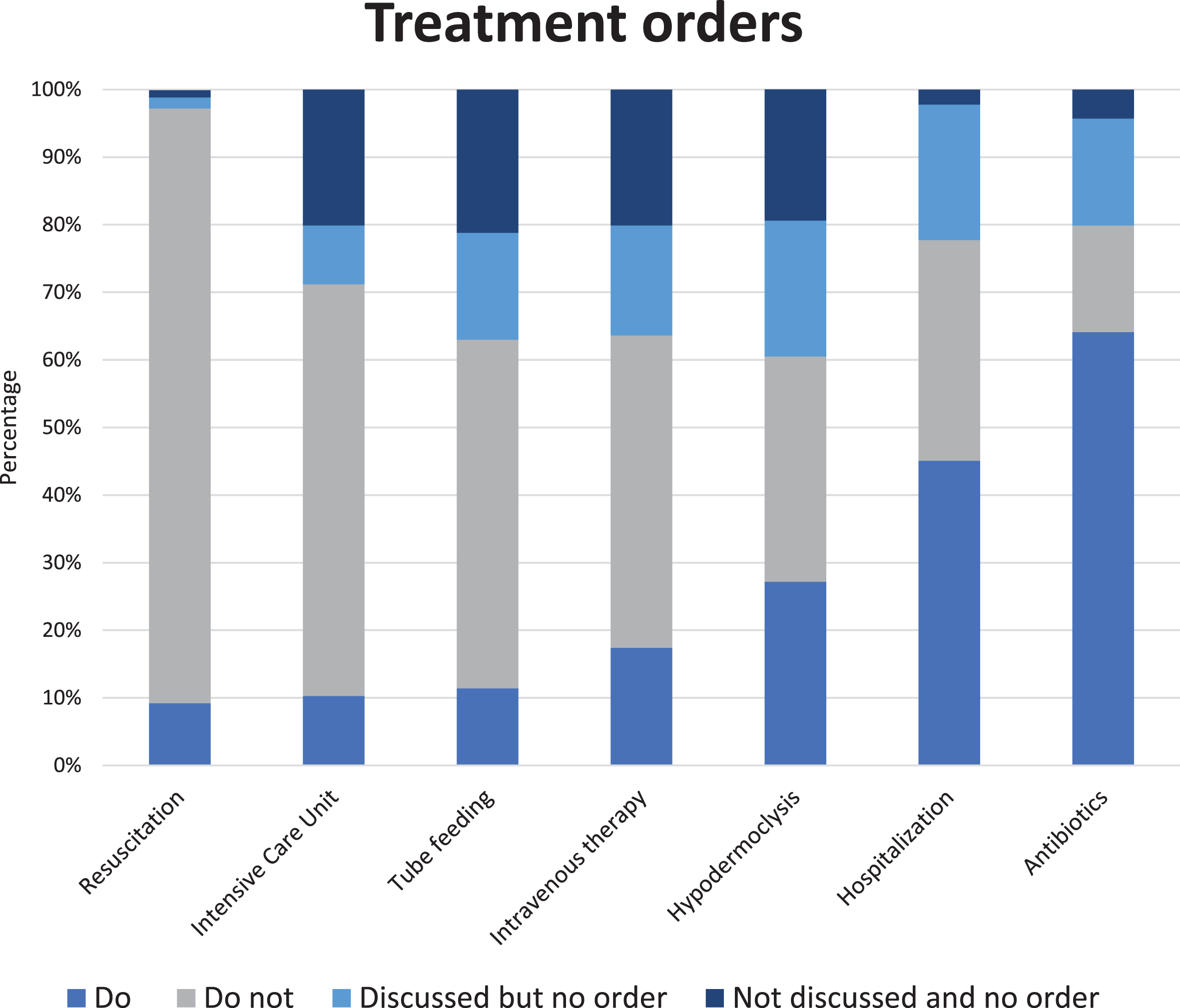
A global care goal was established for 98.9% of residents. For the majority of residents (73.9%), the prioritized care goal was comfort. Maintaining or improving function was the main goal for 14.1% and life prolongations for 8.7% of residents. Do-not-treat orders (Fig. 1 and Supplementary Table 2) were most common with regard to resuscitation (88.0%) and admission to an intensive care unit (60.9%). Lower percentages were found for tube feeding (51.6%), intravenous therapy (46.2%), hypodermoclysis (33.3%), hospital admission (32.4%), and use of antibiotics (15.8%). Resuscitation had the lowest percentage of ‘no order’ (2.7%) in which a decision had not yet been made and tube feeding advance orders were set least often (37.0%). The physician and family caregiver had almost always discussed resuscitation (98.9%), hospitalization (97.8%), and antibiotics (95.7%), in contrast to the other types of treatment. Residents with advanced dementia (Table 4 and Supplementary Table 2) more often had a comfort care goal (87.8%). The proportion of do-no-treat orders was higher for all types of treatment as compared the whole cohort.
Fig. 1
Percentages (N = 184) of specific advance treatment orders. Percentage ‘do-not-treat’ order per type of treatment in descending order: resuscitation 88.0%, intensive care unit 60.9%, tube feeding 51.6%, intravenous therapy 46.2%, hypodermoclysis 33.3%, hospitalization 32.6%, and use of antibiotics 15.8%; we refer to Supplementary Table 2 for percentages of all answer options, including from residents with advanced dementia, presented separately.
DISCUSSION
This is the first study on palliative care-related characteristics of a group of residents living in YOD special care units in the Netherlands from three perspectives. Dementia severity, dementia type, and quality of life varied. Health problems, of which swallowing problems were most frequently reported, were relatively uncommon. However, neuropsychiatric symptoms were reported often. Psychotropic drugs, antipsychotics in particular, were prescribed frequently. Advance directives and ACP agreements before admission were uncommon, yet for the large majority of residents, comfort care goals and do-not-treat orders were established afteradmission.
QOL can be considered one of the main objectives of palliative care. It has been studied in YOD with the use of different proxy ratings including the QUALID scale [13]. In our population, mean QUALID scores (24.0) as assessed by family caregivers were higher than in the EPYLOGE cohort (21.0), pointing to lower observed QOL in our sample. The higher QUALID scores are consistent with more frequent antipsychotic drug treatment and neuropsychiatric symptoms such as fear, agitation, and lack of calmness in our cohort as compared to EYPLOGE, in which these factors were found to be negatively associated with QOL. High neuropsychiatric symptom burden was observed by family caregivers as indicated by low mean values of EOLD-SM items referring to psychological and behavioral symptoms. Agitation was common according to physicians and nursing staff. High prevalence rates of neuropsychiatric symptoms in our study, despite the frequent use of psychotropic drugs, is in line with previous research on nursing home resident with YOD [34]. Psychosocial interventions must also be considered since positive effects have been shown in dementia care [35]. An international panel agreed that non-pharmacological interventions are often the most appropriate way of treating behavioral symptoms in YOD [36]. However, it is unclear which YOD-tailored interventions are effective.
One of the domains of palliative care is providing prognostic information [2]. Recognition of dementia-related health problems is relevant as these may affect prognosis [33, 37, 38]. It can guide the physician in initiating ACP discussions [39]. Previous studies have also indicated that persons with YOD and caregivers wish to have a better understanding of symptom progression and prognosis [40]. Malnutrition, indicated by cachexia or weight loss, occurred less frequently than in the general Dutch LTC population in which numbers have remained relatively stable over the years [41]. The prevalence rates of dehydration and swallowing problems were also lower than those found in older nursing home populations [42, 43]. Prevalence of swallowing problems was highest among dementia-related health problems, which may be relevant as swallowing difficulty was the most frequently observed symptom at the end of life in EPYLOGE [14]. The relatively low prevalence rates of dementia-related health problems could point to better physical fitness of residents with YOD compared to other nursing home residents. Despite low frequencies of these somatic health problems, in almost half of cases the physician would not be surprised if the person with YOD were to die within 12 months. Therefore, physicians may be taking other scenarios into account, such as cognitive deterioration or the possibility of acquiring pneumonia, when estimating life expectancy.
Advance directives, written by people with YOD themselves, were not common in our study (5.4%). The lack of advance directives can be considered problematic as a strong association has been found between having a written advance directive and the quality of dying for nursing home residents with dementia [44]. This low number is also in contrast to the hypothesis that people with YOD have different attitudes compared to those with LOD toward their disease and prognosis, asking for autonomy in the decision-making process, and playing an active role in ACP [3, 40]. Advance directives were more common in studies conducted in Germany (70%) and the United States (17%) for both YOD and LOD indicating that cultural differences play a role [14, 45]. Cultural differences concerning ACP, partly because of different legal frameworks, have been described in qualitative YOD research [46]. American caregivers relied on legal professionals from nonmedical domains for guidance in correctly completing living wills whereas Belgian patients and caregivers turned to healthcare professionals, such as GPs.
No document of the former GP was available on treatment preferences in more than half of cases (64.7%). Relatedly, in Dutch primary care ACP conversations between GPs and people with dementia are rare [47]. There are fewer end-of-life and ACP discussions compared to cases of people with cancer or organ failure [48]. Important barriers found in a systematic review were [49]: uncertainty about the timing, lack of knowledge about dementia, difficulties assessing decisional capacities of people with dementia, and changing preferences.
Uncertainty about timing due to a better health status could also play a role in YOD [9, 39]. A gap of knowledge hindered ACP engagement in YOD according to qualitative research from Flanders as well [50]. In this study, doubt regarding cognitive ability was also identified as a reason not to initiate ACP. In our cohort, around two thirds of residents were assessed as being unable to make decisions regarding medical treatments. Another possible explanation for low prevalence of ACP conversations and documentation in the YOD population was the the unpredictability of the disease trajectory which can make couples feel unprepared [50, 51].
A Belgian interview study illustrated that patients with YOD and caregivers nevertheless recognize the potential benefits of ACP [52]. One frequently emerging motivation for engaging in ACP was that persons with YOD would be enabled to participate in decision-making. A second reason was found in the relief that planning in advance would bring to the caregivers. Our study focuses on future medical care as part of ACP. However, it was found that an overly medicalized approach might impede people’s engagement. Therefore, implementation as a holistic, flexible, and relational communication process is suggested [52].
In most cases family caregivers and physicians established a comfort care goal (73.9%). A lower percentage (64.7%) was found in the DEOLD study which included almost exclusively older nursing home residents with dementia [53]. The prevalence of do-not-resuscitate orders (88.0%) is in line with previous Dutch long-term care studies [54]. In our study, do-not-treat orders were more common than do-treat orders, except for hospitalization and antibiotics. In case of advanced dementia do-not-treat orders were even more common. The high numbers of comfort care goals and do-not-treat orders may seem counterintuitive given the relatively young age of the YOD population. Admission to a long-term care unit may be the appropriate time for caregivers to discuss ACP and to choose a palliative approach.
Methodological considerations
One of the strengths of our study is the use of validated instruments that allow for comparison with other populations. Further, we report on the perspectives of physicians, family caregivers, and nursing staff, all of which are relevant to palliative care research. Different scores such as QUALID were found for different respondents, which could be due to differences in reference period or level of involvement with the resident.
Our findings may reflect characteristics specific to the Dutch context in which LTC organizations are stimulated to improve dedicated services for YOD, for example by adapting a multidisciplinary approach and improving team expertise through staff training [16]. Different practices, not tailored to YOD, could be common elsewhere perhaps related to different palliative care outcomes.
Physicians were asked to report the dementia type as stated in the medical record without detail on diagnostic work-up. Atypical combinations of dementia types were seen. Therefore, the diagnostic accurateness could be questioned. However, most residents probably had received adequate work-up to determine the type of dementia as was found in a Dutch study with a similar nursing cohort [15]. Moreover, the Dutch dementia guideline also recommends to refer younger patients to a memory clinic [55].
Conclusion and implications
Our study describes a sample of people with YOD residing in YOD special care units. Dementia-related health problems were less common than in the general nursing home population. A palliative care approach should focus on neuropsychiatric symptoms; particularly fear, agitation, and resistiveness to care are relevant as these are more prevalent than physical symptoms. Advance directives and ACP agreements before admission to long-term care were low despite the necessity of timely discussion of care preferences between people with YOD, caregivers, and professionals. After admission, on the other hand, ACP practices were common in the form of care goals and treatment orders. Despite their relatively young age and the low number of dementia-related health problems, most YOD residents had comfort care goals and do-not-treat orders. Therefore, a palliative approach can be considered appropriate for most people with YOD. This realization could help GPs and other professionals in timely discussion of ACP. They should fulfill an active role in initiating ACP conversations to ensure that future palliative care is in accordance with the person’s wishes. Future studies may examine the impact of timely implementation of ACP on quality of care and life.
ACKNOWLEDGMENTS
We would like to thank all the respondents and participating nursing homes for their invaluable contribution to this study.
FUNDING
The study was supported by ZonMw (project number 839120003).
CONFLICT OF INTEREST
Jenny T. van der Steen is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available upon reasonable request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230486.
REFERENCES
[1] | Iliffe S , Davies N , Vernooij-Dassen M , Van Riet Paap J , Sommerbakk R , Mariani E , Jaspers B , Radbruch L , Manthorpe J , Maio L , Haugen D , Engels Y ((2013) ) Modelling the landscape of palliative care for people with dementia: A European mixed methods study, BMC Palliat Care 12: , 30. |
[2] | van der Steen JT , Radbruch L , Hertogh CM , de Boer ME , Hughes JC , Larkin P , Francke AL , Junger S , Gove D , Firth P , Koopmans RT , Volicer L , European Association for Palliative Care ((2014) ) White paper defining optimal palliative care in older people with dementia: A Delphi study and recommendations from the European Association for Palliative Care, Palliat Med 28: , 197–209. |
[3] | Koopmans RT , van der Steen JT , Bakker C ((2015) ) Palliative care in people with young-onset dementia (YOD): An undiscovered area!, J Am Med Dir Assoc 16: , 1008–1009. |
[4] | van de Veen D , Bakker C , Peetoom K , Pijnenburg Y , Papma J , PRECODE study group, de Vugt M , Koopmans R ((2022) ) Provisional consensus on the nomenclature and operational definition of dementia at a young age, a Delphi study, Int J Geriatr Psychiatry 37: , doi: 10.1002/gps.5691. |
[5] | Sepúlveda C , Marlin A , Yoshida T , Ullrich A ((2002) ) Palliative care: The World Health Organization’s global perspective, J Pain Symptom Manage 24: , 91–96. |
[6] | Wendrich-van Dael A , Bunn F , Lynch J , Pivodic L , Van den Block L , Goodman C ((2020) ) Advance care planning for people living with dementia: An umbrella review of effectiveness and experiences, Int J Nurs Stud 107: , 103576. |
[7] | Rietjens JAC , Sudore RL , Connolly M , van Delden JJ , Drickamer MA , Droger M , van der Heide A , Heyland DK , Houttekier D , Janssen DJA , Orsi L , Payne S , Seymour J , Jox RJ , Korfage IJ , European Association for Palliative Care ((2017) ) Definition and recommendations for advance care planning: An international consensus supported by the European Association for Palliative Care, Lancet Oncol 18: , e543–e551. |
[8] | Hickman SE , Lum HD , Walling AM , Savoy A , Sudore RL ((2023) ) The care planning umbrella: The evolution of advance care planning, J Am Geriatr Soc 71: , 2350–2356. |
[9] | Gerritsen AAJ , Bakker C , Verhey FR , de Vugt ME , Melis RJ , Koopmans RT ((2016) ) Prevalence of comorbidity in patients with young-onset Alzheimer disease compared with late-onset: A comparative cohort study, J Am Med Dir Assoc 17: , 318–323. |
[10] | Appelhof B , Bakker C , Van Duinen-van Den IJCL , Zwijsen SA , Smalbrugge M , Verhey FRJ , de Vugt ME , Zuidema SU , Koopmans R ((2019) ) Differences in neuropsychiatric symptoms between nursing home residents with young-onset dementia and late-onset dementia, Aging Ment Health 23: , 581–586. |
[11] | van Vliet D , de Vugt ME , Kohler S , Aalten P , Bakker C , Pijnenburg YA , Vernooij-Dassen MJ , Koopmans RT , Verhey FR ((2013) ) Awareness and its association with affective symptoms in young-onset and late-onset Alzheimer disease: A prospective study, Alzheimer Dis Assoc Disord 27: , 265–271. |
[12] | Kimura NRS , Simoes JP , Santos RL , Baptista MAT , Portugal MDG , Johannessen A , Barca ML , Engedal K , Laks J , Rodrigues VM , Dourado MCN ((2021) ) Young- and late-onset dementia: A comparative study of quality of life, burden, and depressive symptoms in caregivers, J Geriatr Psychiatry Neurol 34: , 434–444. |
[13] | Hartmann J , Rossmeier C , Riedl L , Dorn B , Fischer J , Slawik T , Fleischhaker M , Hartmann F , Egert-Schwender S , Kehl V , Haller B , Schneider-Schelte H , Dinkel A , Jox RJ , Diehl-Schmid J ((2021) ) Quality of life in advanced dementia with late onset, young onset, and very young onset, J Alzheimers Dis 80: , 283–297. |
[14] | Roβmeier C , Hartmann J , Riedl L , Dorn B , Fischer J , Hartmann F , Egert-Schwender S , Kehl V , Schneider-Schelte H , Jox RJ , Dinkel A , Diehl-Schmid J ((2021) ) How do persons with young and late onset dementia die? J Alzheimers Dis 81: , 843–852. |
[15] | Mulders AJ , Zuidema SU , Verhey FR , Koopmans RT ((2014) ) Characteristics of institutionalized young onset dementia patients–the BEYOnD study, Int Psychogeriatr 26: , 1973–1981. |
[16] | Bakker C , Verboom M , Koopmans R ((2022) ) Reimagining postdiagnostic care and support in young-onset dementia, J Am Med Dir Assoc 23: , 261–265. |
[17] | Koopmans R , Pellegrom M , van der Geer ER ((2017) ) The Dutch move beyond the concept of nursing home physician specialists, J Am Med Dir Assoc 18: , 746–749. |
[18] | European Commission ((2008) ) The European Qualification Framework for lifelong learning (EQF). Office for Official Publications of the European Communities, Luxembourg. |
[19] | van der Steen JT , Ribbe MW , Deliens L , Gutschow G , Onwuteaka-Philipsen BD ((2014) ) Retrospective and prospective data collection compared in the Dutch End Of Life in Dementia (DEOLD) study, Alzheimer Dis Assoc Disord 28: , 88–94. |
[20] | Reisberg B , Ferris SH , de Leon MJ , Crook T ((1982) ) The Global Deterioration Scale for assessment of primary degenerative dementia, Am J Psychiatry 139: , 1136–1139. |
[21] | WHO Collaborating Centre for Drug Statistics Methodology ((2021) ) ATC classification index with DDDs. https://www.whocc.no/atc_ddd_index/. Accessed December 1, 2021. |
[22] | Morris JN , Nonemaker S , Murphy K , Hawes C , Fries BE , Mor V , Phillips C ((1997) ) A commitment to change: Revision of HCFA’s RAI, J Am Geriatr Soc 45: , 1011–1016. |
[23] | Moss AH , Ganjoo J , Sharma S , Gansor J , Senft S , Weaner B , Dalton C , MacKay K , Pellegrino B , Anantharaman P , Schmidt R ((2008) ) Utility of the “surprise” question to identify dialysis patients with high mortality, Clin J Am Soc Nephrol 3: , 1379–1384. |
[24] | van Lummel EVTJ , Ietswaard L , Zuithoff NPA , Tjan DHT , van Delden JJM ((2022) ) The utility of the surprise question: A useful tool for identifying patients nearing the last phase of life? A systematic review and meta-analysis, Palliat Med 36: , 1023–1046. |
[25] | Volicer L , Hurley AC , Blasi ZV ((2001) ) Scales for evaluation of end-of-life care in dementia, Alzheimer Dis Assoc Disord 15: , 194–200. |
[26] | van der Steen JT , Gijsberts MJ , Knol DL , Deliens L , Muller MT ((2009) ) Ratings of symptoms and comfort in dementia patients at the end of life: Comparison of nurses and families, Palliat Med 23: , 317–324. |
[27] | Weiner MF , Martin-Cook K , Svetlik DA , Saine K , Foster B , Fontaine CS ((2000) ) The quality of life in late-stage dementia (QUALID) scale, J Am Med Dir Assoc 1: , 114–116. |
[28] | Clare L , Quinn C , Hoare Z , Whitaker R , Woods RT ((2014) ) Care staff and family member perspectives on quality of life in people with very severe dementia in long-term care: A cross-sectional study, Health Qual Life Outcomes 12: , 175. |
[29] | van Schalkwijk DB , Verlare LR , Muller MT , Knol DL , Van der Steen J ((2009) ) Het meten van kwaliteit van leven bij ernstig demente verpleeghuisbewoners: Psychometrische eigenschappen van de QUALID schaal, Tijdschr Gerontol Geriatr 40: , 184–192. |
[30] | NHG, Richtlijn Proactieve Zorgplanning (Guideline Advance Care Planning), https://palliaweb.nl/richtlijnen-palliatieve-zorg/richtlijn/proactieve-zorgplanning, Accessed September 16, 2023. |
[31] | Bavelaar L , Visser M , Schlicksupp P , Tilburgs B , van der Maaden T , Achterberg WP , van der Steen JT ((2022) ) Change in advance care plans of nursing home residents with dementia and pneumonia: Secondary analysis of randomized controlled trial data, J Am Med Direct Assoc 23: , 1741.e19–1741.e26. |
[32] | Mitchell SL , Teno J , Kiely DK , Shaffer ML , Jones RN , Prigerson HG , Volicer L , Givens JL , Hamel MB ((2009) ) The clinical course of advanced dementia, N Engl J Med 361: , 1529–1531. |
[33] | H Hendriks SA , Smalbrugge M , van Gageldonk-Lafeber AB , Galindo-Garre F , Schipper M , Hertogh CMPM , van der Steen JT ((2017) ) Pneumonia, intake problems, and survival among nursing home residents with variable stages of dementia in the Netherlands: Results from a prospective observational study, Alzheimer Dis Assoc Disord 31: , 200–208. |
[34] | Mulders AJ , Fick IW , Bor H , Verhey FR , Zuidema SU , Koopmans RT ((2016) ) Prevalence and correlates of neuropsychiatric symptoms in nursing home patients with young-onset dementia: The BEYOnD Study, J Am Med Dir Assoc 17: , 495–500. |
[35] | Koch J , Amos JG , Beattie E , Lautenschlager NT , Doyle C , Anstey KJ , Mortby ME ((2022) ) Non-pharmacological interventions for neuropsychiatric symptoms of dementia in residential aged care settings: An umbrella review, Int J Nurs Stud 128: , 104187. |
[36] | Couzner L , Day S , Draper B , Withall A , Laver KE , Eccleston C , Elliott KE , McInerney F , Cations M ((2022) ) What do health professionals need to know about young onset dementia? An international Delphi consensus study, BMC Health Serv Res 22: , 14. |
[37] | Vandervoort A , Van den Block L , van der Steen JT , Volicer L , Stichele RV , Houttekier D , Deliens L ((2013) ) Nursing home residents dying with dementia in Flanders, Belgium: A nationwide postmortem study on clinical characteristics and quality of dying, J Am Med Direct Assoc 14: , 485–492. |
[38] | Sanders CL , Wengreen HJ , Schwartz S , Behrens SJ , Corcoran C , Lyketsos CG , Tschanz JT ((2018) ) Nutritional status is associated with severe dementia and mortality: The Cache County Dementia Progression Study, Alzheimer Dis Assoc Disord 32: , 298–304. |
[39] | van der Steen JT , van Soest-Poortvliet MC , Hallie-Heierman M , Onwuteaka-Philipsen BD , Deliens L , de Boer ME , Van den Block L , van Uden N , Hertogh CM , de Vet HC ((2014) ) Factors associated with initiation of advance care planning in dementia: A systematic review, J Alzheimers Dis 40: , 743–757. |
[40] | Bannon SM , Reichman MR , Wang K , Uppal S , Grunberg VA , Vranceanu A-M ((2022) ) A qualitative meta-synthesis of common and unique preferences for supportive services among persons with young onset dementia and their caregivers, Dementia 21: , 519–539. |
[41] | Everink IHJ , van Haastregt JCM , Manders M , de van der Schueren MAE , Schols JMGA ((2021) ) Malnutrition prevalence rates among Dutch nursing home residents: What has changed over one decade? A comparison of the years 2009, 2013 and 2018, J Nutr Health Aging 25: , 999–1005. |
[42] | Paulis SJC , Everink IHJ , Halfens RJG , Lohrmann C , Schols J ((2018) ) Prevalence and risk factors of dehydration among nursing home residents: A systematic review, J Am Med Dir Assoc 19: , 646–657. |
[43] | Hägglund P , Gustafsson M , Lövheim H ((2022) ) Oropharyngeal dysphagia and associated factors among individuals living in nursing homes in northern Sweden in 2007 and 2013, BMC Geriatrics 22: , 421. |
[44] | Vandervoort A , Houttekier D , Vander Stichele R , van der Steen JT , Van den Block L ((2014) ) Quality of dying in nursing home residents dying with dementia: Does advanced care planning matter? A nationwide postmortem study, PLoS One 9: , e91130. |
[45] | Tjia J , Dharmawardene M , Givens JL ((2018) ) Advance directives among nursing home residents with mild, moderate, and advanced dementia, J Palliat Med 21: , 16–21. |
[46] | Van Rickstal R , De Vleminck A , Morrison SR , Koopmans RT , van der Steen JT , Engelborghs S , Neugroschl J , Aldridge MD , Sano M , Van den Block L ((2020) ) Comparing advance care planning in young-onset dementia in the USA vs Belgium: Challenges partly related to societal context, J Am Med Dir Assoc 21: , 851–857. |
[47] | Azizi B , Tilburgs B , van Hout HPJ , van der Heide I , Verheij RA , Achterberg WP , van der Steen JT , Joling KJ ((2022) ) Occurrence and timing of advance care planning in persons with dementia in general practice: Analysis of linked electronic health records and administrative data, Front Public Health 10: , 653174. |
[48] | Evans N , Pasman HR , Donker GA , Deliens L , Van den Block L , Onwuteaka-Philipsen B , on behalf of E ((2014) ) End-of-life care in general practice: A cross-sectional, retrospective survey of ‘cancer’, ‘organ failure’ and ‘old-age/dementia’ patients, Palliat Med 28: , 965–975. |
[49] | Tilburgs B , Vernooij-Dassen M , Koopmans R , van Gennip H , Engels Y , Perry M ((2018) ) Barriers and facilitators for GPs in dementia advance care planning: A systematic integrative review, PLoS One 13: , e0198535. |
[50] | Van Rickstal R , De Vleminck A , Aldridge MD , Morrison SR , Koopmans RT , van der Steen JT , Engelborghs S , Van den Block L ((2019) ) Limited engagement in, yet clear preferences for advance care planning in young-onset dementia: An exploratory interview-study with family caregivers, Palliat Med 33: , 1166–1175. |
[51] | Bannon SM , Grunberg VA , Reichman M , Popok PJ , Traeger L , Dickerson BC , Vranceanu AM ((2021) ) Thematic analysis of dyadic coping in couples with young-onset dementia, JAMA Netw Open 4: , e216111. |
[52] | Van Rickstal R , Vleminck A , Engelborghs S , Versijpt J , Van den Block L ((2022) ) A qualitative study with people with young-onset dementia and their family caregivers on advance care planning: A holistic, flexible, and relational approach is recommended, Palliat Med 36: , 964–975. |
[53] | Hendriks SA , Smalbrugge M , Hertogh CM , van der Steen JT ((2017) ) Changes in care goals and treatment orders around the occurrence of health problems and hospital transfers in dementia: A prospective study, J Am Geriatr Soc 65: , 769–776. |
[54] | Bouwstra H , Smalbrugge M , Hertogh CM ((2015) ) Physician treatment orders in Dutch nursing homes, J Am Med Dir Assoc 16: , 715 e711–715. |
[55] | NHG, Richtlijn dementie (Guideline dementia), https://richtlijnen.nhg.org/standaarden/dementie#samenvatting-consultatie-en-verwijzing. Accessed September 16, 2023. |
[56] | Saliba D , Buchanan J ((2012) ) Making the investment count: Revision of the Minimum Data Set for nursing homes, MDS 3.0, J Am Med Dir Assoc 13: , 602–610. |




