Effect of Nordic Sensi® Chair on Behavioral and Psychological Symptoms of Dementia in Nursing Homes Residents: A Randomized Controlled Trial1
Abstract
Background:
Behavioral and psychological symptoms of dementia (BPSD) are present in most people with dementia (PwD), including Alzheimer’s disease. There is consensus that non-pharmacological therapies represent the first line of treatment to address BPSD.
Objective:
We explore the efficacy of the use of a rocking chair (Nordic Sensi® Chair, NSC) in the treatment of BPSD in nursing home residents with moderate and severe dementia.
Methods:
We carried out a 16-week randomized, single-blind, controlled, clinical trial with PwD admitted to nursing homes. Participants were assigned to a treatment group (n = 40) that received three times a week one session per day of 20 minutes in the NSC and a control group (n = 37). The Neuropsychiatric Inventory-Nursing Home (NPI-NH) was used as primary efficacy outcome. Occupational distress for the staff was evaluated using the NPI-NH Occupational Disruptiveness subscale (NPI-NH-OD). Statistical analyses were conducted by means of a Mixed Effects Model Analysis.
Results:
Treatment with the NSC was associated with a beneficial effect in most of BPSD, as reflected by differences between the treatment and control group on the NPI-NH total score (mean change score –18.87±5.56 versus –1.74±0.67, p = 0.004), agitation (mean change score –2.32±2.02 versus –0.78±1.44, p = 0.003) and irritability (mean change score –3.35±2.93 versus –1.42±1.31, p = 0.004). The NPI-NH-OD total score also improved the most in the treatment group (mean change score –9.67±7.67 versus –7.66±6.08, p = 0.003).
Conclusions:
The reduction in overall BPSD along with decreased caregiver occupational disruptiveness represent encouraging findings, adding to the potential of nonpharmacological interventions for nursing home residents living with dementia.
Trial registration: This study is registered on ClinicalTrials.gov with the identifier NCT05706792 on January 31, 2023.
INTRODUCTION
Dementia is a syndrome characterized by a progressive impairment of the cognitive and functional abilities with important implications for individuals and society. The number of people with dementia (PwD) is expected to increase to 82 million by 2030 and almost double by 2050 [1].
In addition to the cognitive and functional deficits, behavioral and psychological symptoms of dementia (BPSD) are one of the most important challenges that both PwD and their caregivers face throughout the course of the disease [2]. BPSD consist of a heterogeneous group of symptoms such as depression, delusions, hallucinations, irritability, disinhibition, agitation, apathy, or sleep and eating problems [3]. BPSD results in decreased PwD well-being, impaired quality of life, and cause a heavy burden on caregivers, often leading caregivers to make the decision to institutionalize [4, 5]. In nursing-homes BPSD can be a major stress for both the care staff and the residents themselves [6].
BPSD management includes both pharmacological and non-pharmacological therapies [7].
Medication is often used and many PwD are treated with psychotropic drugs, although in many cases achieve only modest benefits in controlling symptoms while exposing patients to the risk of possible adverse events [8]. On the contrary, non-pharmacological interventions are considered to have fewer undesirable effects making them safer options, with at least the same efficacy as medication, in most cases [9, 10]. In fact, currently there is a consensus to consider non-pharmacological therapies as the first line treatment of BPSD with the exception of emergency situations [11].
A wide range of non-pharmacological approaches have shown positive results for the management of BPSD including physical exercise, music therapy, multisensory stimulation, psycho-educational interventions for caregivers or care staff training [12]. However, the need for the development and application of new non-pharmacological therapies is present [11, 13].
Within this context, modern rocking chairs may be suitable for long-term care because rocking, a rhythmically repeated movement, can contribute to psychosocial wellbeing [14]. However, only a few studies have evaluated the use of rocking chairs for PwD. A 6-week study in nursing homes showed that the use of a rocking chair produced improvements in anxiety and depression as well as reductions in pain medication [14]. The results of a repeated-measures study revealed that the use of a glider significantly improved emotions and relaxation in people with severe dementia admitted to nursing homes [15]. In the same line, findings from a study using a rocking chair showed a decrease in BPSD and an increase in quality of life in PWD in a nursing home [16]. In a multicenter survey of long-term care facilities staff reported the use of a rocking chair improved quality of care and contributed to a calmer environment for PwD [17].
In this regard, it is of interest to consider the therapeutic role of the Nordic Sensi® Chair (NSC) in the treatment of BPSD based on its ability to offer PwD a sensory experience that brings the benefits of music, therapeutic tactile stimulation, vestibular stimulation, and relaxation in an integrated way, especially those in nursing homes.
Music-based interventions were originally developed with the aim of accomplishing individualized goals and offer a promising option if targeted and evaluated effectively [18]. The use of music in PwD is based on the ancestral link between sounds and the human being and its potential to evoke emotions experienced throughout their lives. Music can become a way of expressing their emotions in daily life, thus preventing the onset of anxious or agitated behaviors [19, 20].
If PwD is hyperaroused, tactile stimulation and vestibular stimulation is a powerful tool to help regulate arousal levels to enable self-calming and focused attention, especially when PwD is agitated [21]. Linear movement activities (e.g., forward–back rocking and swinging) coupled with low-frequency sounds are calming and serve to inhibit the reticular activating system via the vestibular system [21].
The main objective of this study was to evaluate the effectiveness of the NSC in the management of BPSD in real clinical practice in PwD admitted to nursing homes. The secondary objective was to assess the benefits of the NSC on cognitive functioning and quality of life of PwD as well as its potential benefits on the occupational disruptiveness of care staff.
METHODS
Participants
Study participants had a diagnosis of dementia according to the criteria of the 11th edition of the International Classification of Diseases of the World Health Organization [22] and/or probable Alzheimer’s disease (AD) according to the criteria of the National Institute on Aging Alzheimer’s Association workgroups (NIA/AA) [23]. PwD were recruited from two nursing homes specialized in dementia care: Centro Residencial Almudena (Rincón de la Victoria, Málaga, Spain) and Residencia DomusVi Fuentesol (Alhaurín de la Torre, Málaga, Spain). Dementia severity was assessed with the Reisberg Global Deterioration Scale (GDS) [24] by the clinician in charge. PwD included in the study were clinically defined in stages 4 to 7 of the GDS.
Centro Residencial Almudena has a capacity for 50 users and offers specialized services for Alzheimer’s and other dementias. The healthcare team is made up of internists and psychologists, in addition to medical advisors in each specialty. DomusVi Fuentesol Residence has a total of 146 beds and center has an interdisciplinary team who offers specialized services for dementia and neurocognitive disorders.
Exclusion criteria included PwD who had any evidence of focal vascular lesions (such as hematomas), stroke, normal pressure hydrocephalus; those with serious systemic diseases such as hypothyroidism or chronic renal failure; those with a chronic sensory disorder (e.g., severe vision and hearing impairment) or severe psychiatric disorder.
Considering the variability reported in the literature of the clinical assessment instruments used in the present study, it was anticipated that a sample of 70 PwD (35 in each of the two groups) would allow detection, with 80% power and an anticipated effect size of 0.5, for the primary efficacy variable NPI-NH total score of a statistically significant difference in the mean of 2.5 points or more between the two study groups, assuming a standard deviation of 1.5 points [25].
The study included the evaluation of care staff from both nursing homes who participated in the direct provision of care to the participating PwD. The degree to which the presence of BPSD disturbed the normal development of their professional activities was assessed.
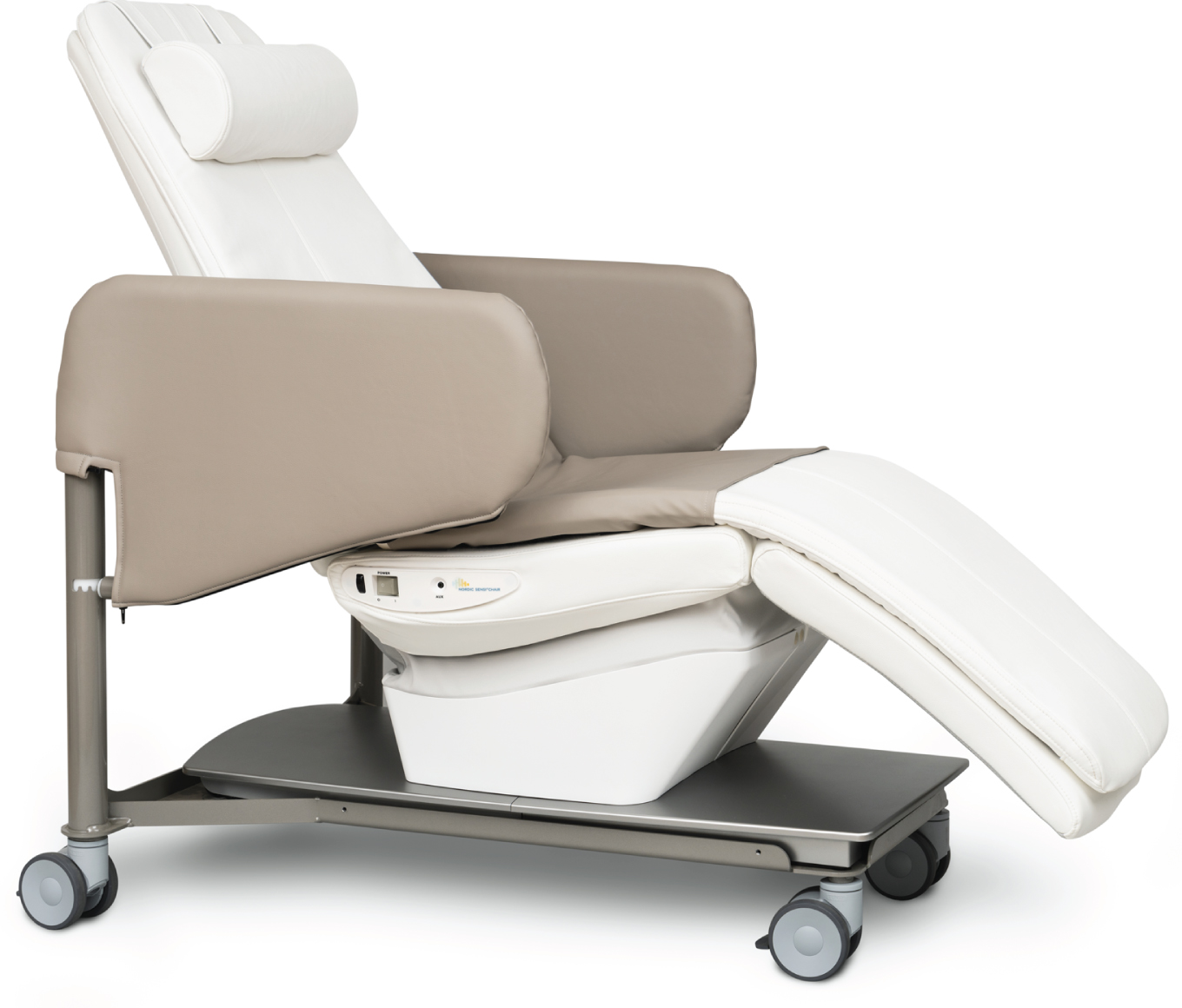
The Nordic Sensi® Chair
The NSC (Wellness Nordic A/S, Espergaerde, Denmark, Fig. 1) is an electrically operated rocking chair with built-in music MusiCure® composed by Niels Eje [26]. It is equipped with an integrated audio system with music recording. MusiCure® is used in a wide variety of different types of treatment and research projects such as cardiac patients, surgery and recovery or psychiatric patients suffering from anxiety, depression, delirium, or sleep problems [26]. Recently MusiCure® has also been used for the treatment of PwD [16].
Fig. 1
The Nordic Sensi® Chair.
This framework requires consideration of a person-centered approach to focus to interventions that have a greater likelihood of effecting a positive influence on their quality of life. As research demonstrates, person-centered interventions can be effective in reducing BPSD in PwD and healthcare service providers should be encouraged to use person-centered care as an essential part of treatment when attempting to reduce BPSD [27].
The NSC has three different programs: Relax for deep relaxation, Refresh for recovery and Comfort for gentle relaxation. A 3.7 kg fiber blanket increases the feeling of security and relaxation, while helping users to perceive their own body. In addition to musical programming, the NSC provides predefined tactile stimulation and rocking motion, for a relaxing multi-sensory experience. This approach could facilitate the achievement of a balance between stimulation and sensory calm that would contribute to the effective management of BPSD. All settings can be easily customized at the touch of a button.
For the purposes of this study, the NSC Relax for deep relaxation program (Relax Program) was used. The Relax Program lasts 20 min, during which the backrest descends to a semi-reclined position that is maintained throughout the program. The chair has also a footrest that can be raised and lowered. At the end of the program, the backrest returns to the sitting position. At the same time that the chair is rocking in a linear direction, the PwD perceives the automatic relaxation music along with tactile stimulation on the back.
Study design
This was a 16-week randomized, parallel, single-blind, controlled, clinical trial (RCT). After assessments for eligibility PwD were randomly assigned to two groups of equal size: a treatment group that received three times a week one session per day of 20 min in the Relax Program of the NSC and a control group that did not participate in the activity mentioned for the treatment group, but received, at the same time and duration, the care and activities that were part of the daily routines of the center, including group sessions of cognitive stimulation, training in activities of daily living or communication training.
Based on the methodology used in previous studies, we consider that a frequency of three times per week would be adequate to study the effect of the NSC on BPSD [14–17]. A research team from the Instituto Andaluz de Neurociencia (four neuropsychologists and one psychiatrist) filled the outcomes measures of the study results. They were blinded to the group assigned to the patients. An anonymized data base was generated. The safety of the intervention was closely monitored by relying on continuous supervision of the intervention by skilled nurse assistants. During the treatment session, the nurse assistant remained next to the PwD, ensuring that the user was safe, relaxed, and comfortable while seated in the NSC.
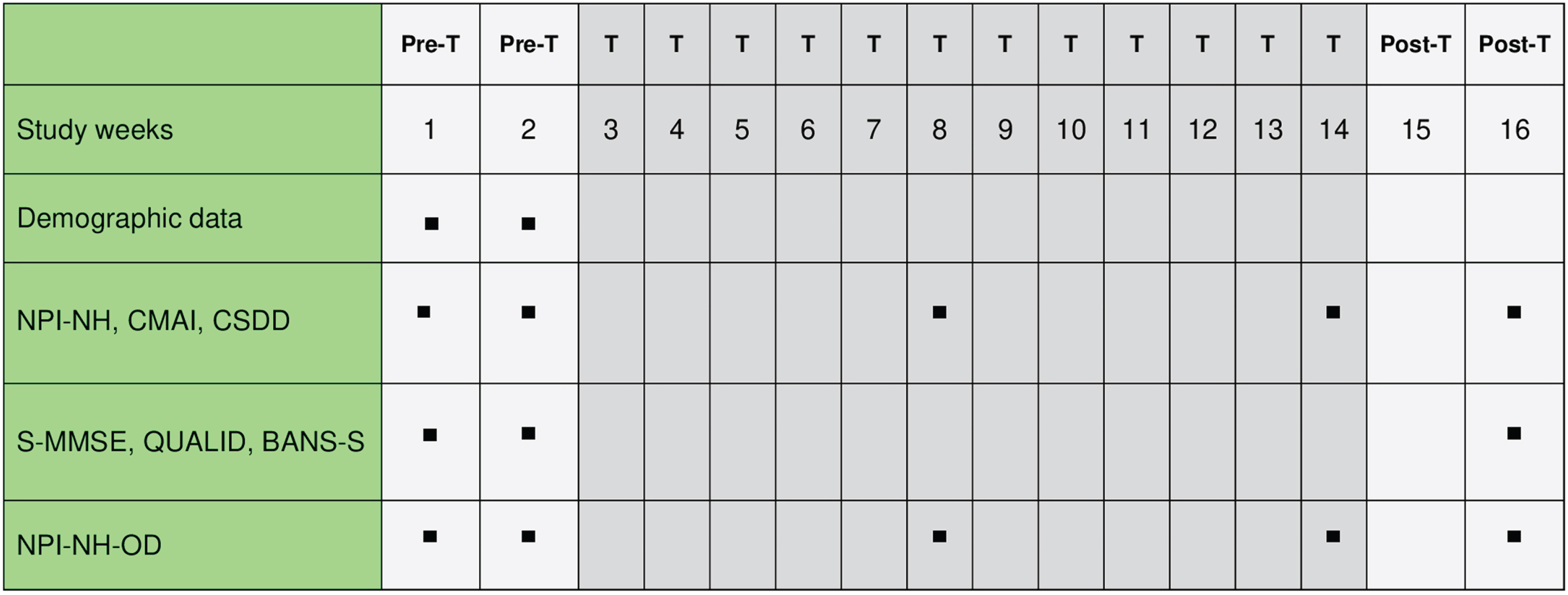
The 16-week study extension included a first 2-week pre-intervention phase followed by a second 12-week intervention phase with the use of the NSC and a third 2-week post-intervention phase without receiving NSC. Given the duration of the study we chose time points for assessment according to a reasonable sequence: at pre-intervention phase (baseline, Time 0), at mid-intervention phase (week 8, Time 1), at the end of the intervention phase (week 14, Time 2), and two weeks after completion of the intervention phase to check if the NSC effect continued (week 16, Time 3). A schematic chart of the assessment schedule is shown in Fig. 2.
Fig. 2
NSC Study Assessments Schedule. NSC, Nordic Sensi® Chair; PreT, pre-treatment phase; T, treatment phase; PosT, post-treatment phase; NPI-NH, Neuropsychiatric Inventory-Nursing Home; CMAI, Cohen Mansfield Agitation Inventory; CSDD, Cornell Scale for Depression in Dementia; S-MMSE, Severe Mini Mental State Examination; QUALID, Quality of Life in Dementia Scale; BANS-S, Bedford Alzheimer Nursing-Severity Scale; NPI-NH-OD, Neuropsychiatric Inventory-Nursing Home Occupational Disruptiveness.
Upon entry into the study, PwD who met the inclusion criteria were randomized to the treatment group or the control group. Randomization was carried out by blocks generating random numbers with repetition, one per block. Randomization numbers were assigned sequentially for all study participants.
Intervention
In both nursing homes, the treatment was carried out on weekdays, during the day shift. The chairs were placed in a room intended exclusively for the treatment sessions. To facilitate confidence and adherence with intervention, PwD were always introduced to the chair by the same nurse assistant. To facilitate the adaptation to the therapeutic process, participants were carefully introduced to the chair, e.g., just suggesting them sit down the first time, carefully rock the second time, start of the full program session the third time. Each PwD had their own schedule of rocking chair use throughout the study.
Ethics approval and consent to participate
The study protocol was approved by the Málaga Research Ethics Committee (Approval number: 03/2022ICPS3). This study is registered on ClinicalTrials.gov with the identifier NCT05706792 on January 31, 2023. A written informed consent was signed by PwD who were able to give or their legal representative. Informed consent was also requested from care staff who participated in the study. The study followed the ethical standards adopted by the Declaration of Helsinki in its latest version (Fortaleza, Brasil, 2013) and was conducted in accordance with the standards of Good Clinical Practice, as described in the Tripartite Harmonized Standards of the International Conference on Harmonisation for Good Clinical Practice 1996.
Outcomes
The primary efficacy measure was the Neuropsychiatric Inventory-Nursing Home (NPI-NH) [28] that is an instrument to be used by the nursing staff to evaluate neuropsychiatric symptoms in PwD in the nursing home setting. The NPI-NH is composed of 12 domains that rate the most frequent BPSD in dementia patients (delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep disturbances, and appetite changes). If a symptom was present during the previous month, each item was scored for frequency (range 0–4) and severity (range 0–3) and transformed to a total composite score (frequency x severity, range 0–12). We calculated the total NPI-NH score as the sum of total composite scores (range 0–144). Higher scores indicate more severe BPSD. For the purposes of this study, total score on the NPI-NH and the 12 domains were considered as primary efficacy measures. In this study the NPI-NH had an internal consistency of Cronbach’s α of 0.67. The internal consistency of NPI-NH domains were between 0.87 and 0.41. The agitation and apathy domains had the highest scores of internal consistency, with 0.87. Sleep disturbances obtained the lowest score with 0.41.
Secondary efficacy measures were Cohen-Mansfield Agitation Inventory (CMAI) [29] and Cornell Scale for Depression in Dementia (CSDD) [30]. The CMAI is composed of 30 items that form four subscales: psychically aggressive behaviors, non-psychically aggressive behaviors, verbally aggressive behaviors, and non-verbally aggressive behaviors. The CMAI also includes the frequency and the severity of the agitation-correlated behaviors and allows to quantify the agitated behaviors in a continuous measure, which is sensitive to the changes. Cronbach’s α for the CMAI was found to be 0.86 for this study.
The CSDD is a 19-item semi structured interview designed to assess depression in PwD with scores above 10 indicating a possible depression and scores above 18 suggesting a definitive depression. In this study the CSDD had high internal consistency of 0.84.
Likewise, an assessment of cognitive functions, functional capacity, and quality of life (QoL) of PwD was carried out using the Severe Mini-Mental State Examination (S-MMSE) [31], the Bedford Alzheimer Nursing-Severity Scale (BANS-S) [32], and the Quality of Life in Late-stage Dementia (QUALID) [33]. The S-MMSE assesses the cognitive deterioration in advanced dementia. It is composed of 10 items and the score can reach 30 points. The S-MMSE had a high reliability according with a Cronbach’s α= 0.88 for this study. The BANS-S consists of 7 items with 4 categories that enables to discriminate changes in advanced phases of dementia. The score ranges from 7 (no impairment) to 28 (total impairment). It assesses the PwD ability to perform three daily activities (dressing, eating and mobility), their ability to speak, their ability to maintain visual contact, the regularity of their sleep-wake cycle and the state of their muscles. The BANS-S had a Cronbach’s α= 0.81.
The QUALID is rated by the care staff who has had significant contact with the patient over the previous week and consists of 11 items and evaluates three domains: affective state, comfort, and basic activities of life. Score ranges from 11 to 55, with lower scores being the highest quality of life. The scale had high internal consistency with a coefficient alpha of 0.80 for this study.
Finally, the assessment of occupational distress for the care staff was carried out by means of the Occupational Disruptiveness subscale of the Neuropsychiatric Inventory-Nursing Home (NPI-NH-OD). It assesses the grade of self-reported professional care staff burden. Care staff rates the extent to which each of the 12 behaviors disrupts them and/or generate more work. Score ranges from 0 to 5 points (from not at all to very severely). We calculated the total NPI-NH-OD score (range 0–60). The NPI-NH-OD subscale had an internal consistency of α= 0.68.
Statistical analysis
Demographic variables were reported using the mean, standard deviation in the case of quantitative variables; and number and percentage for qualitative variables. Baseline differences between the two treatment groups were assessed by an analysis of variance (ANOVA) or nonparametric tests, as appropriate.
A Mixed Effects Model Analysis for Repeated Measurements (MMRM) was carried out in order to evaluate changes in neuropsychiatric, cognitive, and functional scores and to handle missing values in some of the follow-up assessments. The effect of time (between the mean baseline measurements and each time point), treatment and interaction between time and treatment were evaluated. The change scores at Time 1 and at Time 2 and the mean change scores differences within and between groups were calculated from the MMRM. All analyses were controlled for demographic and clinical characteristics that approached significance on univariate analysis. Post hoc analyses for multiple comparisons were conducted using Bonferroni's correction. Cohen’s d standardized effect sizes were calculated and defined as small d = 0.20, medium d = 0.50 and large d = 0.80 [34]. The main efficacy analysis was based on the Modified Intent-to-Treat (mITT) population using a Last Observation Carried Forward (LOCF) imputation. This mITT-LOCF population was pre-defined as all randomized PwD who received at least one week of the Nordic Sensi® Chair treatment and had a baseline and at least one post-baseline assessment for the primary efficacy variable on treatment.
MMRM analysis did not include the two weeks post-intervention data. After completion of the 12 weeks of intervention period, Student’s t-test was used to compare within-group mean score differences at Time 2 and Time 3.
Statistical analyses were carried out using the Statistical Package for the Social Sciences Software (SPSS 25.0, IBM Corporation, Armonk, NY, USA) and the significance level was set at 0.05. For MMRM analyses of the primary efficacy measure a significance level of p≤0.004 was considered significant.
RESULTS
Demographic and baseline scores
Eighty-eight PwD were entered into the study and were randomized. In the first week of the intervention phase, 2 participants dropped out because of occasional dizziness that worsened when sitting in the chair and 3 refused to continue with the study because they did not enjoy sitting in the NSC. Six other participants died before the first post-baseline assessment. The mITT-LOCF population comprised 77 PwD, all of whom completed the 12-week intervention phase (Fig. 3). Of these 77 PwD (65 female, 12 male), 40 (52%) were in the treatment group (37 female, 92.5%), and 37 (48%) were in the control group (28 female, 75.7%). PwD had a mean age of 81.77±8.69 years (range 47–102) with median of 82 years and mean years of education of 7.31±3.24 (range 6–18). All participants were Caucasian. The main efficacy analysis did not include the 2-week post-intervention period.
Fig. 3
Flowchart of patients participating in the study.
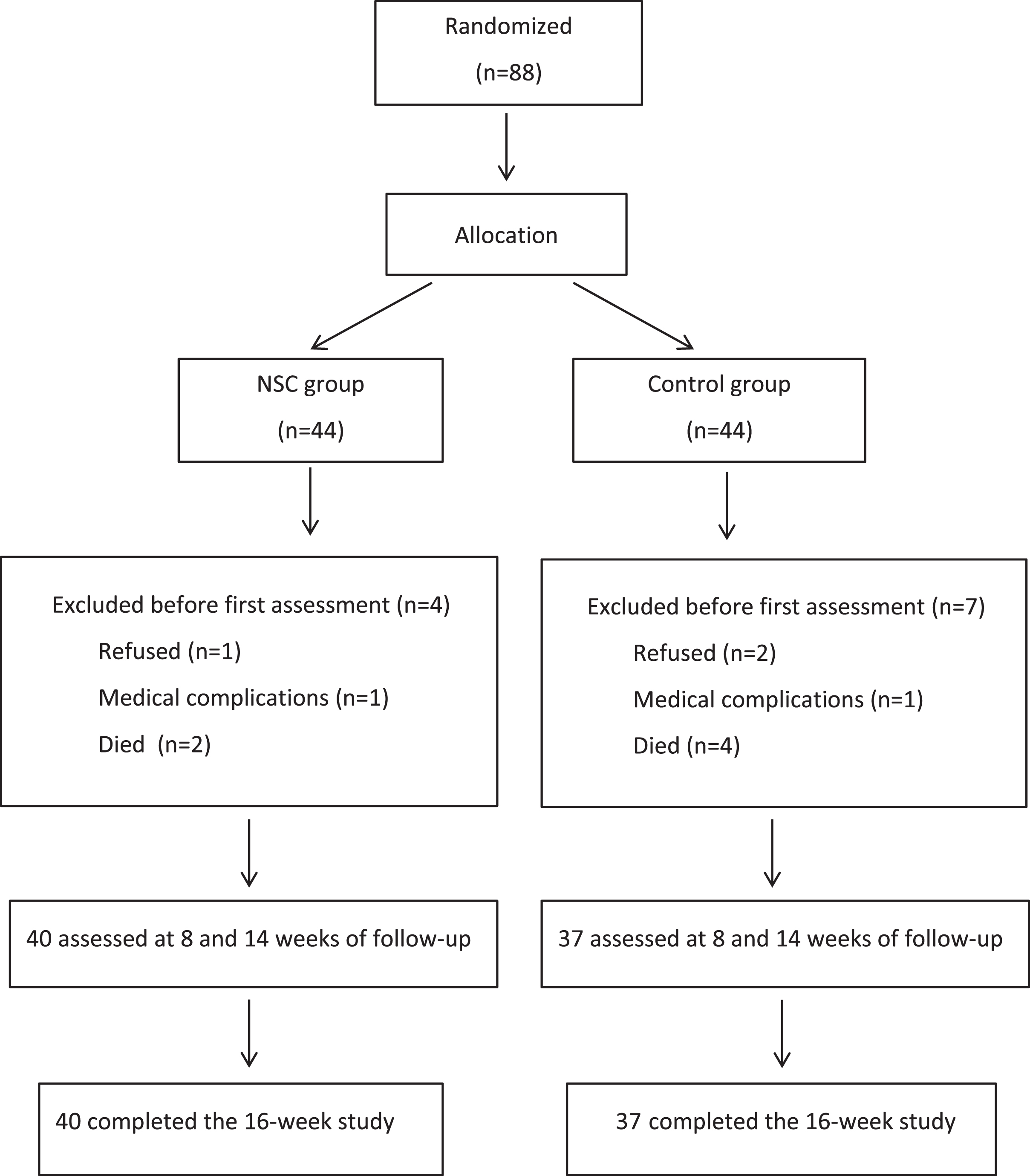
At baseline, there were no statistically significant differences between the two groups for the demographic and clinical variables except for sex (women, 84.4%, p = 0.042) and anxiolytic use (benzodiazepines; higher in the control group, 67.6% than in the treatment group, 32.5%, p = 0.002) (Table 1). No deaths or serious adverse events occurred during the study. Care staff informed that NSC was well accepted and tolerated with no differences through different stages of GDS.
Table 1
Demographic and clinical characteristics of PwD at baseline
| Variable | Overall (n = 77) | NSC group (n = 40) | Control group (n = 37) | p |
| Age | 81.77±8.69 | 82.58±8.10 | 80.89±9.32 | 0.402 |
| Sex | ||||
| Female | 65 (84.4) | 37 (92.5) | 28 (75.7) | |
| Male | 12 (15.6) | 3 (7.5) | 9 (24.3) | 0.042 |
| Education, y | 7.31±3.24 | 7.50±3.46 | 7.11±3.02 | 0.599 |
| Dementia duration, months | 105.80±93. | 96.89±40.88 | 115.40±129.20 | 0.499 |
| Marital status | ||||
| Married | 12 (15.6) | 6 (15) | 6 (16.2) | |
| Single/divorced | 14 (18.2) | 6 (15) | 8 (21.6) | |
| Widowed | 51 (66.2%) | 28 (70) | 23 (62.2) | 0.832 |
| GDS | ||||
| 4 | 8 (10.4) | 4 (10) | 4 (10.8) | |
| 5 | 15 (19.5) | 6 (15) | 9 (24.3) | |
| 6 | 35 (45.5) | 17 (42.5) | 18 (48.6) | |
| 7 | 19 (24.7) | 13 (32.5) | 6 (16.2) | 0.375 |
| Hypertension | 48 (62.3) | 23 (57.5) | 25 (67.6) | 0.362 |
| Diabetes mellitus | 10 (13) | 3 (7.5) | 7 (18.9) | 0.136 |
| Dyslipidemia | 18 (23.4) | 10 (25.0) | 8 (21.6) | 0.946 |
| Obesity | 3 (3.9) | 3 (7.5) | 0 (0.0) | 0.089 |
| Smoking | 1 (1.3) | 1 (2.5) | 0 (0.0) | 0.333 |
| Psychiatric history | 30 (39) | 18 (45) | 12 (32.4) | 0.379 |
| Family history of dementia | 5 (6.5) | 4 (10) | 1 (2.7) | 0.389 |
| Ongoing medication | ||||
| AChEI | 14 (18.2) | 6 (15) | 8 (21.6) | 0.404 |
| Memantine | 21 (27.3) | 13 (32.5) | 8 (21.6) | 0.456 |
| AChEI+Memantine | 18 (23.4) | 11 (27.5) | 7 (18.9) | 0.462 |
| Antidepressants | 51 (66.2) | 25 (62.5) | 26 (70.3) | 0.441 |
| Benzodiazepines | 38 (49.40) | 13 (32.5) | 25 (67.6) | 0.002 |
| Neuroleptics | 41 (53.2) | 20 (50) | 21 (56.8) | 0.553 |
| Hypnotics | 28 (36.4) | 14 (35) | 14 (37.8) | 0.796 |
Values are mean±SD or number (%). Independent samples t-test were used for continuous data and χ2 on categorical data. PwD, people with dementia; NSC, Nordic Sensi® Chair; GDS, Global Deterioration Scale; AChEI, acetylcholinesterase inhibitors.
The most common cause of dementia was AD (46 participants, 59.7%) followed by vascular dementia (7 participants, 9.1%), frontotemporal dementia (3 participants, 3.9%), Lewy body dementia (2 participants, 2.6%), and other types of dementia (19 participants, 24.7%). There were no statistically significant differences between the treatment group and the control group for dementia diagnoses (χ2 = 3.560, p = 0.469) or severity of dementia (χ2 = 4.240, p = 0.375). Neuropsychological, neuropsychiatric, and functional performance of patients at baseline is shown in Table 2.
Table 2
Neuropsychological, neuropsychiatric, and functional performance of PwD at baseline
| Variable | Overall (n = 77) | NSC group (n = 40) | Control group (n = 37) | p |
| NPI-NH total score (0–144) | 23.81±22.19 | 24.70±25.23 | 22.84±18.65 | 0.712 |
| NPI-NH subscores (0–12) | ||||
| Delusions | 1.70±2.89 | 1.69±2.76 | 1.70±3.06 | 0.988 |
| Hallucinations | 0.96±2.36 | 1.20±2.94 | 0.70±1.48 | 0.348 |
| Agitation | 3.18±3.74 | 2.88±3.78 | 3.51±3.71 | 0.458 |
| Depression | 1.37±2.43 | 1.36±2.62 | 1.38±2.24 | 0.972 |
| Anxiety | 2.12±2.83 | 2.30±3.20 | 1.92±2.38 | 0.554 |
| Euphoria | 1.12±2.41 | 1.48±2.88 | 0.73±1.72 | 0.170 |
| Apathy | 3.58±4.16 | 4.18±4.26 | 2.95±4.02 | 0.198 |
| Disinhibition | 2.14±3.45 | 2.43±3.89 | 1.84±2.92 | 0.454 |
| Irritability | 3.04±3.30 | 3.53±3.67 | 2.51±2.80 | 0.177 |
| Aberrant motor behavior | 1.68±3.35 | 1.48±3.35 | 1.89±3.39 | 0.589 |
| Sleep disturbances | 1.62±3.00 | 1.13±2.41 | 2.17±3.50 | 0.140 |
| Appetite changes | 1.30±2.87 | 1.05±2.16 | 1.57±3.50 | 0.442 |
| NPI-NH-OD | 11.76±12.10 | 11.53±13.61 | 12.05±10.43 | 0.867 |
| CSDD | 9.77±6.59 | 9.89±7.29 | 9.65±5.88 | 0.873 |
| CMAI | 51.08±25.17 | 51.64±26.06 | 50.49±24.54 | 0.843 |
| BANS-S | 17.44±4.68 | 17.97±4.63 | 16.86±4.72 | 0.307 |
| QUALID | 25.37±8.14 | 25.03±8.02 | 25.73±8.35 | 0.709 |
| S-MMSE | 9.52±8.74 | 8.58±9.07 | 10.61±8.36 | 0.331 |
Values are mean±SD, Independent samples t-test were used for continuous data. NSC, Nordic Sensi® Chair; NPI-NH, Neuropsychiatric Inventory-Nursing Home; NPI-NH-OD, Neuropsychiatric Inventory-Nursing Home Occupational Disruptiveness; CSDD, Cornell Scale for Depression in Dementia; CMAI, Cohen Mansfield Agitation Inventory; BANS-S, Bedford Alzheimer Nursing-Severity Scale; QUALID, Quality of Life in Dementia Scale; S-MMSE, Severe Mini-Mental State Examination; PwD, People with Dementia.
Primary efficacy measure
Results from the MMRM for both groups are presented in Table 3. The MMRM analysis showed a statistically significant time by treatment effect for NPI-NH total score (F(1, 75) = 5.523, p = 0.022), agitation (F(1, 75) = 0.817, p = 0.002), apathy (F(1, 75) = 3.931, p = 0.013), disinhibition (F(1, 75) = 3.704, p = 0.021), irritability (F(1, 75) = 5.115, p = 0.027), aberrant motor behavior (F(1, 75) = 2.431, p = 0.039), and euphoria (F(1, 75) = 3.817, p = 0.026). The MMRM displayed the same significant results with and without sex and benzodiazepines adjustment.
Table 3
Results from the mixed effects model analysis for repeated measurements
| NSC group (n = 40) | Control group (n = 37) | Between-group differences in mean change score | ||||||||||||
| Variable | Mean change score from baseline | Mean change score from baseline | ||||||||||||
| at T1 | at T2 | p | #d | at T1 | at T2 | p | #d | at T1 | p | #d | at T2 | p | d | |
| NPI-NH | –16.89 (9.65) | –18.87 (5.56) | 0.003 | 0.25 | 0.25 (0.12) | –1.74 (0.67) | 0.457 | –17.14 | 0.001 | 0.56 | –17.13 | 0.004 | 0.61 | |
| Delusions | –0.96 (1.51) | –1.63 (1.52) | 0.004 | 0.44 | 0.02 (0.21) | –1.08 (0.99) | 0.351 | –0.98 | 0.672 | –0.55 | 0.398 | |||
| Hallucinations | –0.82 (1.33) | –1.15 (1.42) | 0.021 | 0.23 | –0.08 (1.22) | –0.54 (1.11) | 0.860 | –0.74 | 0.214 | –0.61 | 0.662 | |||
| Agitation | –1.47 (1.41) | –2.32 (2.02) | 0.002 | 0.48 | –0.45 (1.32) | –0.78 (1.44) | 0.452 | –0.02 | 0.003 | 0.29 | –1.54 | 0.003 | 0.37 | |
| Depression | –0.87 (1.24) | –0.95 (1.78) | 0.399 | –0.12 (0.89) | –0.58 (1.42) | 0.783 | –0.75 | 0.429 | –0.37 | 0.771 | ||||
| Anxiety | –1.49 (1.67) | –2.16 (2.68) | 0.001 | 0.30 | –0.38 (0.63) | –0.86 (0.91) | 0.429 | –1.11 | 0.321 | –1.30 | 0.449 | |||
| Euphoria | –1.43 (2.18) | –1.40 (1.99) | 0.002 | 0.10 | 0.23 (0.89) | –0.56 (1.12) | 0.785 | –1.66 | 0.004 | 0.39 | –0.84 | 0.126 | ||
| Apathy | –2.75 (1.89) | –3.04 (2.78) | 0.001 | 0.12 | 0.43 (0.82) | –1.74 (1.96) | 0.294 | –3.18 | 0.004 | 0.43 | –1.30 | 0.441 | ||
| Disinhibition | –2.12 (2.01) | –2.03 (1.93) | 0.01 | 0.05 | 0.19 (0.91) | –0.94 (1.56) | 0.632 | –2.31 | 0.003 | 0.51 | –1.09 | 0.167 | ||
| Irritability | –2.99 (2.11) | –3.35 (2.93) | 0.001 | 0.16 | 0.19 (0.22) | –1.42 (1.31) | 0.098 | –3.18 | 0.001 | 0.45 | –1.93 | 0.004 | 0.49 | |
| Aberrant motor behavior | –1.14 (1.99) | –0.80 (1.87) | 0.477 | 0.61 (1.76) | –0.68 (1.98) | 0.611 | –1.75 | 0.003 | 0.20 | –0.12 | 0.662 | |||
| Sleep disturbances | –1.05 (2.02) | –1.09 (2.24) | 0.221 | –0.61 (1.96) | –1.44 (2.22) | 0.057 | –0.44 | 0.558 | 0.35 | 0.621 | ||||
| Appetite changes | –0.78 (1.89) | –0.16 (1.12) | 0.679 | 0.46 (1.67) | 0.03 (1.01) | 0.669 | –1.24 | 0.556 | –0.19 | 0.278 | ||||
| CSDD | –2.47 (2.49) | –5.74 (5.22) | 0.022 | 0.46 | 0.38 (0.22) | –4.40 (3.34) | 0.080 | –2.85 | 0.662 | –10.14 | 0.941 | |||
| CMAI | –12.79 (6.11) | –17.72 (8.23) | 0.001 | 0.68 | 5.54 (3.31) | –6.70 (3.02) | 0.571 | –18.33 | 0.001 | 0.46 | –11.02 | 0.002 | 0.44 | |
| NPI-NH-OD | –6.45 (4.21) | –9.67 (7.67) | 0.001 | 0.52 | –0.56 (0.48) | –7.66 (6.08) | 0.055 | –5.89 | 0.001 | 0.18 | –2.01 | 0.003 | 0.21 | |
The results displayed are adjusted for sex and benzodiazepines use. Values are mean (standard deviation); d, Cohen’s d effect size; #d, effect size from T0 to T2. NSC, Nordic Sensi® Chair; NPI-NH, Neuropsychiatric Inventory-Nursing Home; CSDD, Cornell Scale for Depression in Dementia; CMAI, Cohen Mansfield Agitation Inventory; NPI-NH-OD, Neuropsychiatric Inventory-Nursing Home Occupational Disruptiveness.
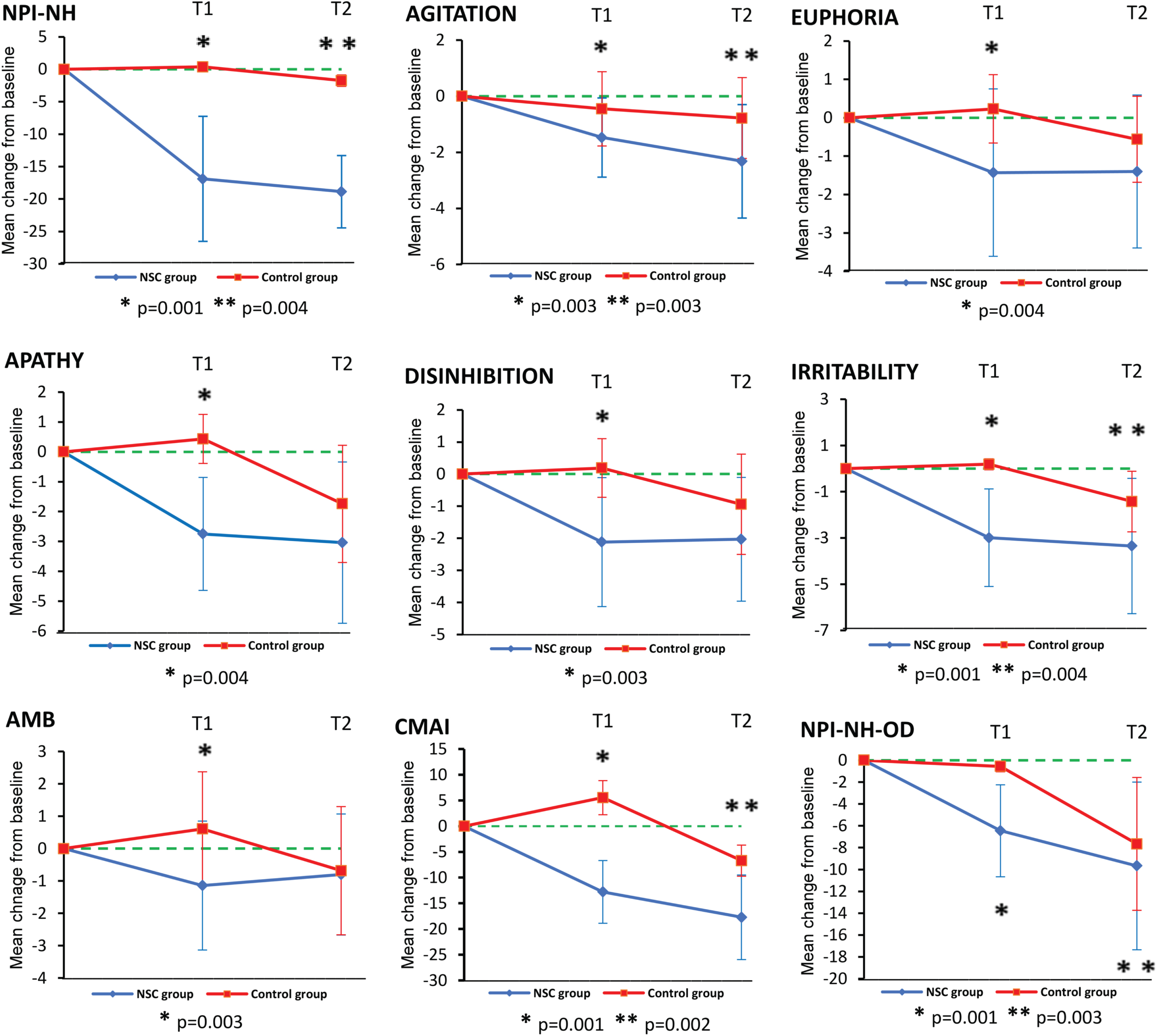
The NSC group performed better than the control group at Time 2 in the NPI-NH total score (mean change score –18.87±5.56 versus –1.74±0.67, p = 0.004), agitation (mean change score –2.32±2.02 versus –0.78±1.44, p = 0.003), and irritability (mean change score –3.35±2.93 versus –1.42±1.31, p = 0.004) and showed an improvement already at Time 1 in all of them. The NSC group performed better than the control group at Time 1 in euphoria (mean change score –1.43±2.18 versus 0.23±0.89, p = 0.004), apathy (mean change score –2.75±1.89 versus 0.43±0.82, p = 0.004), disinhibition (mean change score 2.12±2.01 versus 0.19±0.91, p = 0.003), and aberrant motor behavior (mean change score –1.14±1.99 versus 0.61±1.76, p = 0.003) (Fig. 4).
Fig. 4
Mean changes from baseline as estimated by the mixed model. NSC, Nordic Sensi® Chair; NPI-NH, Neuropsychiatric Inventory-Nursing Home; AMB, Aberrant motor behavior; CMAI, Cohen Mansfield Agitation Inventory; NPI-NH-OD, Neuropsychiatric Inventory-Nursing Home Occupational Disruptiveness.
Concerning the within-group changes, NPI-NH mean change score and mean change scores for delusions, hallucinations, agitation, anxiety, euphoria, apathy, disinhibition, and irritability showed statistically significant differences at the end of the intervention period (Time 2) from baseline. There were no statistically significant differences in each of these variables when comparing mean scores at Time 2 and Time 3 (Table 3).
Secondary efficacy measures
Regarding the CMAI, the MMRM analysis showed a statistically significant interaction effect between time and treatment (p = 0.021). The NSC group performed better than the control group at Time 2 (mean change score –17.72±8.23 versus –6.70±3.02, p = 0.002) and showed an improvement already at Time 1 (Fig. 4). The NSC group showed a statistically significant difference at the end of the intervention period (Time 2) from baseline. There was no significant difference in CMAI in the NSC group when comparing mean scores at Time 2 and Time 3 (Table 3).
The MMRM analysis showed no statistically significant interaction effect between time and treatment for the CSDD (F = 1.071, p = 0.304). There were no significant differences between-groups. The NSC group showed a statistically significant difference at the end of the intervention period from baseline. There was no significant difference in CSDD in the NSC group when comparing mean scores at Time 2 and Time 3 (Table 3).
Cognitive performance, functional status, and quality of life
With regard to the S-MMSE neither the NSC group (mean change score 5.59±5.66 versus 7.43±8.17, p = 0.812) nor the control group (mean change score 6.67±9.28 versus 10.61±7.34, p = 0.443) showed statistically significant differences at the end of the treatment period from baseline. Concerning the BANS-S the NSC group showed statistically significant improvement at the end of the intervention period from baseline (mean change score 15.62±6.01 versus 17.97±4.92, p = 0.05). There was no significant difference in BANS-S in the NSC group when comparing mean scores at Time 2 and Time 3 (p = 0.263). In regard to the QUALID the NSC group showed a statistically significant improvement at the end of the treatment period from baseline (mean change score 19.59±8.77 versus 25.83±8.12, p = 0.003). There was no significant difference in QUALID in the NSC group when comparing mean scores at Time 2 and Time 3 (p = 0.467).
Occupational disruptiveness
Concerning the NPI-NH-OD, the MMRM analysis showed a statistically significant interaction effect between time and treatment (p = 0.042). The NSC group performed better than the control group at Time 2 (mean change score –9.67±7.67 versus –7.66±6.08, p = 0.003) and showed an improvement already at Time 1 (Fig. 4). The NSC group showed a statistically significant difference at the end of the intervention period from baseline (Table 3). There was no significant difference in NPI-NH-OD in the NSC group when comparing mean scores at Time 2 and Time 3 (Table 3).
DISCUSSION
This study was performed to explore the efficacy of the NSC in the treatment of BPSD in nursing home residents with moderate and severe dementia, primarily of the Alzheimer’s type. The treatment with the NSC was well tolerated and was associated with a beneficial effect on overall BPSD. PwD treated with the NSC showed statistically significant superiority on the NPI-NH over PwD in the control group.
The NSC showed benefits in most of BPSD. Notably, its use yielded significant benefit regarding agitation, apathy, irritability, disinhibition, aberrant motor activity, and euphoria over the 12-week of treatment. Consistent with the significant reduction in the NPI-NH agitation domain score, we also found a significant decrease in the CMAI score. Importantly, the NPI-NH-OD total score improved significantly in the treatment group. In addition, our findings also showed significant improvement in the residents' functional status and quality of life over 12 weeks.
Previous studies have highlighted that the use of a rocking chair has potential benefits in the treatment of BPSD, while demonstrating a very low risk of harm [14–16]. The efficacy in improving the psychological well-being and balance using a platform rocking chair was examined in 25 PwD admitted to nursing homes for 6 weeks [14]. PwD showed small improvements in depression, anxiety and pain medication use but found no improvement in agitation. In a quasi-experimental, repeated-measures design study [15] the effects of a glider swing on emotions, relaxation, and aggressive behaviors in a group of 30 nursing home residents with dementia were evaluated for 10 days. The glider intervention significantly improved emotional state and aggressive symptoms were observed to decrease from the beginning of treatment until after the end of treatment. More recently, in a single-case study [16], performed using a mixed-methods approach, six PwD in a nursing home setting used the NSC for a mean number of five times per week, for eight weeks in total. The results indicated a decrease in BPSD symptoms and increased quality of life upon using the NSC.
Therefore, based on our findings and those provided by early studies, a potential therapeutic role of the NSC in the treatment of dementia BPSD based on its ability to offer patients, in an integrated way, a sensory experience that brings the benefits of music therapy, therapeutic tactile stimulation, vestibular stimulation, multi-sensory stimulation and relaxation should be considered. Although less and less frequently, sometimes PwD, especially those who are institutionalized, may be in a situation of sensory deprivation or, conversely, exposed to excessive environmental stimulation, which may contribute to their experiencing a sense of intrapsychic discomfort, thus favoring the onset of BPSD such as agitation, anxiety or irritability [35, 36]. Therefore, interventions with residents should facilitate the achievement of a balance between stimulation and sensory calm [12]. In this sense, procedures that favor multisensory stimulation such as the NSC are a very appropriate option in these patients and can contribute to achieve a relaxing and stimulating environment at the same time [37, 38].
As care staff stated the NSC was considered an affordable, easy to use, nonlabour intensive intervention in the care of PwD. When care staff was asked to what extent the use of the NSC helped them, most believed that it improved the quality of care, freed up staff time and contributed to a calmer and more pleasant environment for everyone. Care staff in the treatment group benefited from the behavioral improvement that patients experienced and reported less occupational disruptiveness on NPI-NH-OD scores than staff in the control group. This is consistent with research showing the relevant role that the presence of BPSD has on the burden of nursing home staff [39, 40].
In the same line, to evaluate caregiver opinion, a multicenter survey was conducted among long term care facilities in several European countries. Care staff reported their opinion of the utility of the NSC in the management of BPSD. Most respondents believed that the quality of care provided improved, they had more time for care and helped create a calmer environment and patient friendly [17].
Interestingly, PwD in the NSC group showed significant improvement in functional capacity and quality of life as measured by the BANS-S and QUALID at 14-week follow-up. Quality of life is difficult to measure in PwD, but it is believed to be influenced, among others factors, by the presence of BPSD [41]. These benefits could be related to the long-term intervention and person-centered therapeutic process developed in this study, which better matching specific interventions to specific individuals. Applying this multisensory approach based on the specific stimuli offered by the NSC and accompanying support from the dedicated nurse assistant enhances the personalization of interventions for participants and increases the chance of efficacious findings. The specific stimuli offered by the NSC and developed through individualized care in feasible and structured sessions were well accepted, with no negative effects. This is in line with other results showing that person-centered interventions show effects on reducing BPSD and improving quality of life in dementia care [42, 43].
When considering the results of an intervention, it is important to consider not only statistically significant improvements but also clinically significant efficacy. For this purpose, it is necessary to compare the observed changes with differences that are considered clinically relevant. For clinical trials in dementia of the Alzheimer’s type, it has been assumed that differences as small as 4.5 points on the NPI-NH [44] are clinically relevant. In our study, average differences that we observed on NPI-NH over the 12 weeks study period exceeded this clinically relevant difference (mean difference within group = –19.92 points and mean difference between groups = –16.28 points). In addition, the magnitude of the observed effect on BPSD could be contextualized using standardized effect sizes. In line with this, the clinical relevance of the significant effect of the NSC on behavioral test scores could be supported by the magnitude of the between group effect sizes with a median Cohen’s d of 0.51.
A strength of this work compared to previous studies was the higher number of PwD evaluated and its design as a RCT. In addition, the participants in the study underwent a comprehensive behavioral and functional evaluation with widely used outcome measures focused on reducing observation bias. However, here are also some limitations that should be considered. This study was carried out in two sites and, therefore the generalization of its findings to other settings and PwD should be interpreted with caution. Although the research team was blinded to the group assigned to the PwD, the outcome measures were obtained from care staff who were not completely blinded to the intervention. As a result, although much effort was put into mitigating bias, it is necessary to consider the possible bias from the care staff when report BPSD. It was not feasible for the staff members to be completely unaware of the experimental condition of the PwD, and this could have biased the ratings on the evaluation instruments. Although care staff was not present during the treatment delivery they could have received anecdotal information from other staff members.
Another potential source of bias comes from the assessment of all outcome measures that were answered by the care staff. A critical point about person-centered approaches is that interventions targeting BPSD should be tailored to each individual and circumstances, which change and possibly reflect different unmet needs over time. In fact, tailoring music to individual preferences seems to be more effective at reducing BPSD (e.g., agitation) than applying generic classical or relaxation music. It should also be noted that the application of this approach may provide more specific, but less generalizable, results. Finally, we acknowledge the potential for assessment bias, as PwD (and even staff members) got more attention to themselves throughout the intervention period, i.e., Hawthorne Effect.
Conclusions
These results may have clinical significance in choosing non-pharmacological therapies for BPSD in PwD. The reduction in overall BPSD along with decreased in caregiver occupational disruptiveness and improved quality of life for residents suggest that the NSC represents an encouraging new non-pharmacological approach to improving BPSD of nursing home residents with dementia. The results suggest that NSC is an intervention with potential interest for use in nursing homes as part of the PwD care plan. This study should inspire the design of future long-term randomized controlled trials that contribute to supporting the use of the NSC as a non-pharmacological person-centered intervention for improving BPSD in PwD in nursing homes.
ACKNOWLEDGMENTS
The authors wish to thank ARJO for providing the NSC for this study. We wish to thank patients and caregivers for their participation in the study. There was no financial compensation for either the patients or the staff of the two residences that participated in the study. The NSC was provided for the study purposes only and was removed from the nursing homes by ARJO at the end of the study.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
[1] | World Health Organization (2020) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed on December 15, 2022. |
[2] | García-Alberca JM , Lara P , González-Barón S , BarbanchoMA , Porta D , Berthier M ((2008) ) Prevalencia y comorbilidad desíntomas neuropsiquiátricos en la enfermedad de Alzheimer, Act Esp Psiquiatria 36: , 265–270. |
[3] | van der Linde R , Dening T , Stephan B , Prina A , Evans E , Brayne C ((2016) ) Longitudinal course of behavioural and psychological symptomsof dementia: Systematic review. B J Psychiatry 209: , 366–377. |
[4] | Rocca P , Leotta D , Liffredo C , Mingrone C , Sigaudo M , Capellero B , Rocca G , Simoncini M , Pirfo E , Bogetto F ((2010) ) Neuropsychiatric symptoms underlying caregiver stress and insight in Alzheimer’s disease. Dement Geriatr Cogn Disord 30: , 57–63. |
[5] | Tatsumi H , Nakaaki S , Torii K , Shinagawa Y , Watanabe N , Murata Y , Sato J , Mimura M , Furukawa TA ((2009) ) Neuropsychiatric symptomspredict change in quality of life of Alzheimer disease patients: Atwo-year follow-up study. Psychiatry Clin Neurosci 63: , 374–384. |
[6] | Wetzels RB , Zuidema SU , de Jonghe JF , Verhey FRJ , Koopmans RTCM ((2010) ) Determinants of quality of life in nursing home residentswith dementia. Dement Geriatr Cogn Disord 29: , 189–197. |
[7] | Dyer SM , Harrison SL , Laver K , Whitehead C , Crotty M ((2018) ) An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr 30: , 295–309. |
[8] | Bessey LJ , Walaszek A ((2019) ) Management of behavioral andpsychological symptoms of dementia., Curr Psychiatry Rep 21: , 66. |
[9] | Gill S , Bronskill E , Normand SLT , Anderson GM , Sykora K , Lam K , Bell CM , Lee PE , Fischer HD , Herrmann N , Gurwitz JH , Rochon PA ((2007) ) Antipsychotic drug use and mortality in older adults with dementia. Ann Int Med 146: , 775–786. |
[10] | McDermott O , Charlesworth G , Hogervorst E , Stoner C , Moniz-Cook E , Spector A , Csipke E , Orrell M ((2019) ) Psychosocial interventions for people with dementia: A synthesis of systematic reviews. Aging Ment Health 23: , 393–403. |
[11] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Coope Cr , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementiaprevention, intervention, and care: 2020 report of theCommission. Lancet 396: , 413–446. |
[12] | Seitz DP , Brisbin S , Herrmann N , Saks K , Soto-Martin M , Meyer H , Rapoport MJ , Wilson K , Gill SS , Rines J , Le Clair K , Conn D ((2012) ) Efficacy and feasibility of nonpharmacological interventions forneuropsychiatric symptoms of dementia in long term care: Asystematic review. J Am Med Dir Assoc 13: , 503–506. |
[13] | Meyer C , O’Keefe F ((2020) ) Non-pharmacological interventions for people with dementia: A review of reviews. Dementia (London) 19: , 1927–1954. |
[14] | Watson NM , Wells TJ , Cox C ((1998) ) Rocking chair therapy for dementiapatients: Its effects on psychosocial well-being and balance. Am J Alzheimers Dis 13: , 296–308. |
[15] | Snyder M , Tseng Y , Brandt C ((2001) ) A glider-swing intervention forpeople with dementia. Geriatr Nurs 22: , 86–90. |
[16] | Gusdal AK , Gustafsson C ((2020) ) Using a rocking chair in the care ofpeople with dementia: A single-case research study. OBMGeriatrics 4: , 86–90. |
[17] | Janof K , Cooper P , Rozario D (2020) Novel therapeutic chair benefits residents with dementia. New international study advances understanding of a non-pharmacological intervention with dementia populations. White Paper 2020. http://www.arjo.com. Accessed on November 15, 2022. |
[18] | American Music Therapy Association (2005) What is music therapy? Retrieved from www.musictherapy.org/faqs.html. |
[19] | Zhang Y , Cai J , An L , Hui F , Ren T , Ma H , Zhao Q ((2017) ) Does music therapy enhance behavioral and cognitive function in elderly dementia patients? A systematic review and meta-analysis. Ageing Res Rev 35: , 1–11. |
[20] | Moreno-Morales C , Calero R , Moreno-Morales P , Pintado C ((2020) ) Musictherapy in the treatment of dementia: A systematic review andmeta-analysis. Front Med (Lausanne) 7: , 160. |
[21] | Campbell EA , Kantor J , Kantorová L , Svobodová Z , Wosch T ((2022) ) Tactile low frequency vibration in dementia management: Ascoping review. Front Psychol 13: , 854794. |
[22] | World Health Organization. ICD-11 revision. https://icd.who.int/en. Accessed on December 1, 2022. |
[23] | McKhann GM , Knopman DS , Chertkow H , Hyman BT , Jack CR Jr , Kawas CH , Klunk WE , Koroshetz WJ , Manly JJ , Mayeux R , Mohs RC , Morris JC , Rossor MN , Scheltens P , Carrillo MC , Thies B , Weintraub S , Phelps CH ((2011) ) The diagnosis of dementia due to Alzheimer’s disease:Recommendations from the National Institute on Aging Alzheimer’sassociation workgroups on diagnostic guidelines for Alzheimer’sdisease. Alzheimers Dement 7: , 263–269. |
[24] | Reisberg B , Ferris SH , De Leon MD , Crook T ((1982) ) The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry 139: , 1136–1139. |
[25] | Lu X , Ye R , Wu J , Rao D , Liao X ((2022) ) Comparing behavioral and psychological symptoms of dementia and caregiver distress caused between older adults with dementia living in the community and in nursing home, Front Psychiatry 13: , 881215. |
[26] | MusiCure. Copenhagen and Denmark: Gefion Records (2009) http://www.musicure.com/. |
[27] | Hacketta K , Sabatb SR , Giovannetti T ((2022) ) A person-centeredframework for designing music-based therapeutic studies in dementia:Current barriers and a path forward., Aging Ment Health 26: , 940–949. |
[28] | Wood S , Cummings JL , Hsu M-A , Barclay T , Wheatley MV , Yarema KT , Schnelle JF ((2000) ) The use of the Neuropsychiatric Inventory in nursing home residents, characterization and measurement. Am J Geriatr Psychiatry 8: , 75–83. |
[29] | Cohen-Mansfield J , Marx MS , Rosenthal AS ((1989) ) A description of agitation in a nursing home. , M, J Gerontol 44: , 77–84. |
[30] | Alexopoulos GS , Abrams RC , Young RC , Shamoian CA ((1988) ) CornellScale for Depression in Dementia. Biol Psychiatry 23: , 271–284. |
[31] | Harrell LE , Marson D , Chatterjee A , Parrish JA ((2000) ) The SevereMini-Mental State Examination: A new neuropsychologic instrument forthe bedside assessment of severely impaired patients with Alzheimerdisease. Alzheimer Dis Assoc Disord 14: , 168–175. |
[32] | Volicer L , Hurley AC , Lathi DC , Kowall NW ((1994) ) Measurement ofseverity in advanced Alzheimer’s disease, J Gerontol Med Sci 49: , 223–226. |
[33] | Weiner MF , Martin-Cook K , Svetlik D , Saine K , Foster B , Fontaine CS ((2000) ) The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc 1: , 114–116. |
[34] | Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences, Routledge Academic, New York. |
[35] | Deborah Koder GEH ((2014) ) Staff’s views on managing symptoms ofdementia in nursing home residents., Nurs Older People 26: , 31–36. |
[36] | Gruber-Baldini AL , Boustani M , Sloane PD , Zimmerman S ((2004) ) Behavioral symptoms in residential care/assisted living facilities:Prevalence, risk factors, and medication management. J AmGeriatr Soc 52: , 1610–1617. |
[37] | Kales HC , Gitlin LN , Lyketsos CG ((2015) ) Assessment and management of behavioral and psychological symptoms of dementia, BMJ 350: , h369. |
[38] | Zucchella C , Sinforiani E , Tamburin S , Federico A , Mantovani E , Bernini S , Casale R , Bartolo M ((2018) ) The multidisciplinary approach to Alzheimer’s disease and dementia. A narrative review of non-pharmacological treatment. Front Neurol 9: , 1058. |
[39] | Krutter S , Schaffler-Schaden D , Essl-Maurer R , Wurm L , Seymer A , Kriechmayr C , Mann E , Osterbrink J , Famm M ((2020) ) Comparingperspectives of family caregivers and healthcare professionalsregarding caregiver burden in dementia care: Results of a mixedmethods study in a rural setting. Age Ageing 49: , 199–207. |
[40] | Sprangers S , Dijkstra K , Romijn-Luijten A ((2015) ) Communicationskills training in a nursing home: Effects of a brief interventionon residents and nursing aides. Clin Interv Aging 10: , 311–319. |
[41] | Burks HB , des Bordesb JKA , Chadhaa R , Holmes HM , Rianon HJ ((2021) ) Quality of life assessment in older adults with dementia: Asystematic review. Dement Geriatr Cogn Disord 50: , 103–110. |
[42] | Kim SK , Park M ((2017) ) Effectiveness of person-centered care onpeople with dementia: A systematic review and meta-analysis. Clin Interv Aging 12: , 381–397. |
[43] | Lee KH , Lee JY , Kim B ((2022) ) Person-centered care in persons livingwith dementia: A systematic review and meta-analysis. , , Gerontologist 62: , e253–e264. |
[44] | Tariot PN , Cummings JL , Katz IR , Mintzer J , Perdomo CA , Schwam EM , Whalen E ((2001) ) A randomized, double-blind, placebo-controlled studyof the efficacy and safety of Donepezil in patients with Alzheimer’sdisease in the nursing home setting, J Am Geriatr Soc 49: , 1590–1599. |



