Vitreous Humor Biomarkers Reflect Pathological Changes in the Brain for Alzheimer’s Disease and Chronic Traumatic Encephalopathy
Abstract
Background:
Patients with eye disease have an increased risk for developing neurodegenerative disease. Neurodegenerative proteins can be measured in the eye; however, correlations between biomarker levels in eye fluid and neuropathological diagnoses have not been established.
Objective:
This exploratory, retrospective study examined vitreous humor from 41 postmortem eyes and brain tissue with neuropathological diagnoses of Alzheimer’s disease (AD, n = 7), chronic traumatic encephalopathy (CTE, n = 15), both AD + CTE (n = 10), and without significant neuropathology (controls, n = 9).
Methods:
Protein biomarkers i.e., amyloid-β (Aβ40,42), total tau (tTau), phosphorylated tau (pTau181,231), neurofilament light chain (NfL), and eotaxin-1 were quantitatively measured by immunoassay. Non-parametric tests were used to compare vitreous biomarker levels between groups. Spearman’s rank correlation tests were used to correlate biomarker levels in vitreous and cortical tissue. The level of significance was set to α= 0.10.
Results:
In pairwise comparisons, tTau levels were significantly increased in AD and CTE groups versus controls (p = 0.08 for both) as well as AD versus AD+CTE group and CTE versus AD+CTE group (p = 0.049 for both). Vitreous NfL levels were significantly increased in low CTE (Stage I/II) versus no CTE (p = 0.096) and in low CTE versus high CTE stage (p = 0.03). Vitreous and cortical tissue levels of pTau 231 (p = 0.02, r = 0.38) and t-Tau (p = 0.04, r = –0.34) were significantly correlated.
Conclusion:
The postmortem vitreous humor biomarker levels significantly correlate with AD and CTE pathology in corresponding brains, while vitreous NfL was correlated with the CTE staging. This exploratory study indicates that biomarkers in the vitreous humor may serve as a proxy for neuropathological disease.
INTRODUCTION
The incidence of neurodegenerative disorders like Alzheimer’s disease (AD) and related dementias continues to rise. As of 2021, 6.2 million North Americans above 65 years of age are suffering from AD; however, this number is projected to reach 13.2 million by 2060 [1]. AD is the most frequent cause of dementia in Western countries [2]. Currently, the definitive diagnosis of AD is made by postmortem neuropathological examination [3]. In 2018, the framework of diagnosing AD shifted to defining it by its underlying pathophysiological processes using biomarkers such as amyloid-β (Aβ) and tau on imaging and cerebrospinal fluid (CSF) concentrations [4]. In AD, neuropathological changes occur decades before symptom onset, so by the time a patient is diagnosed the therapeutic effect is often limited. Similar to AD, chronic traumatic encephalopathy (CTE) is a tauopathy that shares homologous neuropathological characteristics such as neurofibrillary tangles and hyperphosphorylated tau protein aggregates [5] and post-mortem analysis is needed for definitive diagnosis [6]. Given the difficulty in diagnosis, novel sources of biomarkers that can aid in earlier diagnosis of these neurodegenerative diseases are of particular interest.
Interest in finding noninvasive diagnostic criteria of neurodegenerative diseases has led to studies on possible markers in the eye. Thinning of the retinal and choroidal layers have been previously demonstrated via noninvasive imaging with optical coherence tomography (OCT) in patients with AD compared to controls [7–16]. However, the use of OCT as a diagnostic tool for AD is limited by common comorbidities such as aging, diabetes, hypertension, and glaucoma, most of which are associated with AD and contribute to retinal and choroidal thinning [10, 17, 18]. Even though patients with eye morbidities can commonly develop AD, they are excluded from studies investigating OCT and OCT angiography as a diagnostic device. Alternately, the amyloid, tau, and neurodegeneration (AT(N)) biomarker index in the eye may be of potential diagnostic value in neurodegenerative diseases [4]. The shared embryologic origin of the eye and the brain can improve our understanding of both neurological and ophthalmic disorders [19–23]. Several studies have established a link between neurodegenerative diseases like AD and ophthalmic conditions like glaucoma, diabetic retinopathy, age-related macular degeneration, and cataracts [24–27]. Therefore, patients with eye disease represent a population at increased risk for the development of AD, and the investigation of biomarkers in this target population will be important for early diagnosis and proper management. Our study group has previously reported biomarkers amyloid-β40 (Aβ40), Aβ42, total tau, and neurofilament light chain (NfL) in the vitreous humor of the eye was significantly correlated with impaired cognitive function but not associated with presence of an eye disease [28, 29].
In this novel exploratory study, we aim to investigate the association of these neurodegenerative biomarkers in postmortem vitreous humor with postmortem pathological brain diagnosis, and cortical tissue in the corresponding brains.
METHODS
Autopsy participants were drawn from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) brain bank [30]. Inclusion criteria requires a history of repetitive head injury (RHI), including those from contact sport play, military service, physical violence, and other sources. The criteria were recently expanded to include a history of moderate to severe traumatic brain injury [30]. Donations with postmortem intervals greater than 72 hours were excluded. Brain donations are made by the next-of-kin who contacted the brain bank at the time of death. Other donations came through referrals from medical examiners and/or the Concussion Legacy Foundation. Consent for the use of tissue for research purposes was obtained from the legal healthcare proxy and the study was approved by the Boston University Medical Center Institutional Review Board (IRB H-37370). The study was performed in accordance with the ethical standards of the Committee on Human Experimentation of our institution and the Declaration of Helsinki. Herein, we conduct an exploratory retrospective clinical study examining 41 donated postmortem eyes and corresponding brains, which underwent neuropathologic examination during autopsy as described below. Vitreous samples were collected at the time of postmortem autopsy and were frozen at –80°C.
Neuropathological analysis
Neuropathological processing included comprehensive screening for neurodegenerative conditions following procedures established for the VA-BU-CLF brain bank [30, 31]. Briefly, all cases were evaluated based on paraffin-embedded tissue sections taken from standardized regions for histochemical and immunohistochemical staining. They were assessed for the extent of involvement/spread of cerebral Aβ, neurofibrillary tangles, and neuritic amyloid plaques. Those with intermediate/high AD neuropathologic change as per National Institute on Aging and Reagan Institute (NIA-RI) criteria were classified as having AD [32]. CTE was neuropathologically diagnosed using the NIH/NINDS consensus criteria, which include abnormal perivascular accumulations of hyperphosphorylated tau (p-tau) in neurons, with or without astrocytes, and cell processes concentrated at the sulcal depths [33, 34]. CTE is staged based on regional p-tau involvement [33, 35]. Controls were defined as those participants with a history of RHI, but without significant neurodegenerative disease (e.g., no CTE and no to low AD neuropathological changes).
Immunoassay measurement for amyloid, tau, NfL, and Eotaxin-1/CCL11 proteins
Protein biomarker levels were measured both in the vitreous humor of postmortem eyes and cortical tissue of the brain by Meso Scale Discovery (MSD, Rockville, MD, USA) quantitative immunoassays. Biomarkers measured include Aβ40 and Aβ42, total and phosphorylated tau (tTau, pTau181, and pTau231 respectively), NfL, and Eotaxin-1/CCL11. These markers were specifically chosen as they have been previously detected in antemortem human vitreous samples at significant levels in prior reports by our group [28, 29]. Vitreous samples were centrifuged for 15 min at 12,000 rpm to separate the cellular contents, aliquoted at 100μL, and frozen at –80°C. For all assays vitreous fluid was diluted 1:1 with 1% Blocker A (MSD #R3BA 4) in wash buffer for a total volume of 200μL. Samples were subsequently spun down at 17,000 g and 4°C for 15 min, and the supernatant was applied to the immunoassay. Brain tissue was diluted with five-fold volume of ice cold 5 M Guanidine Hydrochloride /50 mM Tris-HCL, pH 8.0, with protease inhibitors (Thermo Scientific, 78439) and phosphatase inhibitors (Sigma, P5726 and P0044). Tissue was homogenized using TissueLyser LT (Qiagen 85600) at 50 Hz speed for 5 min. The homogenates were shaken at room temperature overnight and then diluted with ice cold 1% Blocker A (MSD, #R93BA-4) in wash buffer by 1:300 and centrifuged at 17,000× g, for 15 min at 4°C. The supernatant was carefully removed and stored at –80°C until use. Immunoassay was performed using MSD platform following the manufacturer’ instructions and in duplicate (Meso Scale Discovery, Gaithersburg, MD). Assay kits we utilized for Aβ40, and Aβ42 (MSD #K15200E) and pTau231 (K15121D). Tau antibodies BT2, AT270 and HT7 (Thermo Fisher Scientific, #MN1010, MN1050 and MN1000B) were used to capture/detect tTau and pTau181. Measurements were performed using the multi-detection SPECTOR 6000 Imager (MSD).
Statistical methods
The level and spread of vitreous biomarker within each group were summarized by reporting medians and interquartile range (IQR). Non-parametric ANOVA - Kruskal-Wallis Rank sum test was performed to determine any significant differences in vitreous biomarker levels (Aβ40, Aβ42, tTau, pTau181, pTau231, NfL, Eotaxin-1/CCL11) by the pathology group. After finding significant differences using the Kruskal-Wallis test, post hoc analysis was done using Wilcoxon Rank Sum test to assess significant pairwise differences in vitreous biomarker levels between groups. Significant differences in vitreous biomarker levels among different stages of CTE, i.e., low (stage I and II) and high (stage III and IV) were also tested using non-parametric ANOVA - Kruskal-Wallis Rank sum test. Wilcoxon rank sum tests was performed for pairwise comparisons, with Benjamini & Hochberg method to adjust for multiple comparisons. Spearman’s rank correlation was used to compare biomarker levels in vitreous humor and cortical tissue. All p-values were adjusted for multiple comparisons using the Benjamini & Hochberg method [36], but we provide both unadjusted and adjusted p values in this study. Two-sided p values of less than 0.10 were considered statistically significant because of the exploratory nature and limited sample size of this study. Outlier data, defined as data beyond three times the IQR below and above the first and third quartile value, were removed for the results presented here. The results are not age adjusted because of the exploratory nature of the study with a small sample size with large variability.
RESULTS
A total of 41 brain and corresponding eye postmortem specimens were examined. The median age at death was 72 (65.0 to 81.0) years and 39 (95.1%) specimens were from male patients (Table 1). Out of the 41 specimens, 9 (21.95%) were controls, 7 (17.07%) had AD pathology only, 15 (36.59%) had CTE pathology only, and 10 (24.39%) were diagnosed with combined AD and CTE pathology (Table 1). Also, out of 41 specimens, 16 (39%) had no evidence of CTE, 5 (12.2%) had low CTE i.e., CTE stage I or II and 20 (48.8%) had high CTE i.e., CTE Stage III or IV (Table 1).
Table 1
Demographic characteristics of postmortem study specimens, by individual study groups and CTE staging
| Study parameter | Entire | Controls, | AD only, | CTE only, | AD + CTE, |
| [Median (IQR) | cohort, | n = 9 | n = 7 | n = 15 | n = 10 |
| or n (%)] | n = 41 | ||||
| Age, y | 72 (65–81) | 63 (46–69) | 81 (69.5–82.5) | 69 (46.5–74.5) | 81.5 (77.5–83.8) |
| Sex, Male | 39 (95.1) | 8 (88.9) | 7 (100) | 14 (93.3) | 10 (100) |
| Braak Stage | |||||
| 0 | 10 (25.6) | 5 (55.6) | 0 (0) | 5 (38.5) | 0 (0) |
| I-II | 4 (10.3) | 3 (33.3) | 0 (0) | 1 (7.7) | 0 (0) |
| III-IV | 11 (28.2) | 1 (11.1) | 1 (14.3) | 6 (46.2) | 3 (30) |
| V-VI | 14 (35.9) | 0 (0) | 6 (85.7) | 1 (7.7) | 7 (70) |
| CERAD Score | |||||
| 0 | 20 (48.8) | 8 (88.9) | 0 (0) | 12 (80) | 0 (0) |
| 1 | 10 (24.4) | 1 (11.1) | 1 (14.3) | 3 (20) | 5 (50) |
| 2 | 7 (17.1) | 0 (0) | 4 (57.1) | 0 (0) | 3 (30) |
| 3 | 4 (9.8) | 0 (0) | 2 (28.6) | 0 (0) | 2 (20) |
| Study parameter | Entire | No CTE, | Low stage | High stage CTE | |
| [Median (IQR) | cohort, | n = 16 | CTE (Stage I | (Stage III or | |
| or n (%)] | n = 41 | or II), n = 5 | IV), n = 20 | ||
| Age, y | 72 (65–81) | 69 (58.8–75.8) | 32 (27–46) | 77.5 (69.8–83) | |
| Sex, Male | 39 (95.1) | 15 (93.8) | 5 (100) | 19 (95) | |
| Braak Stage | |||||
| 0 | 10 (25.6) | 5 (31.2) | 4 (80) | 1 (5.6) | |
| I-II | 4 (10.3) | 3 (18.8) | 0 (0) | 1 (5.6) | |
| III-IV | 11 (28.2) | 2 (12.5) | 0 (0) | 9 (50) | |
| V-VI | 14 (35.9) | 6 (37.5) | 1 (20) | 7 (38.9) | |
| CERAD Score | |||||
| 0 | 20 (48.8) | 8 (50) | 4 (80) | 8 (40) | |
| 1 | 10 (24.4) | 2 (12.5) | 0 (0) | 8 (40) | |
| 2 | 7 (17.1) | 4 (25) | 1 (20) | 2 (10) | |
| 3 | 4 (9.8) | 2 (12.5) | 0 (0) | 2 (10) | |
IQR, Interquartile range; AD, Alzheimer’s disease; CTE, chronic traumatic encephalopathy.
Relationship between different vitreous biomarkers and pathological diagnosis
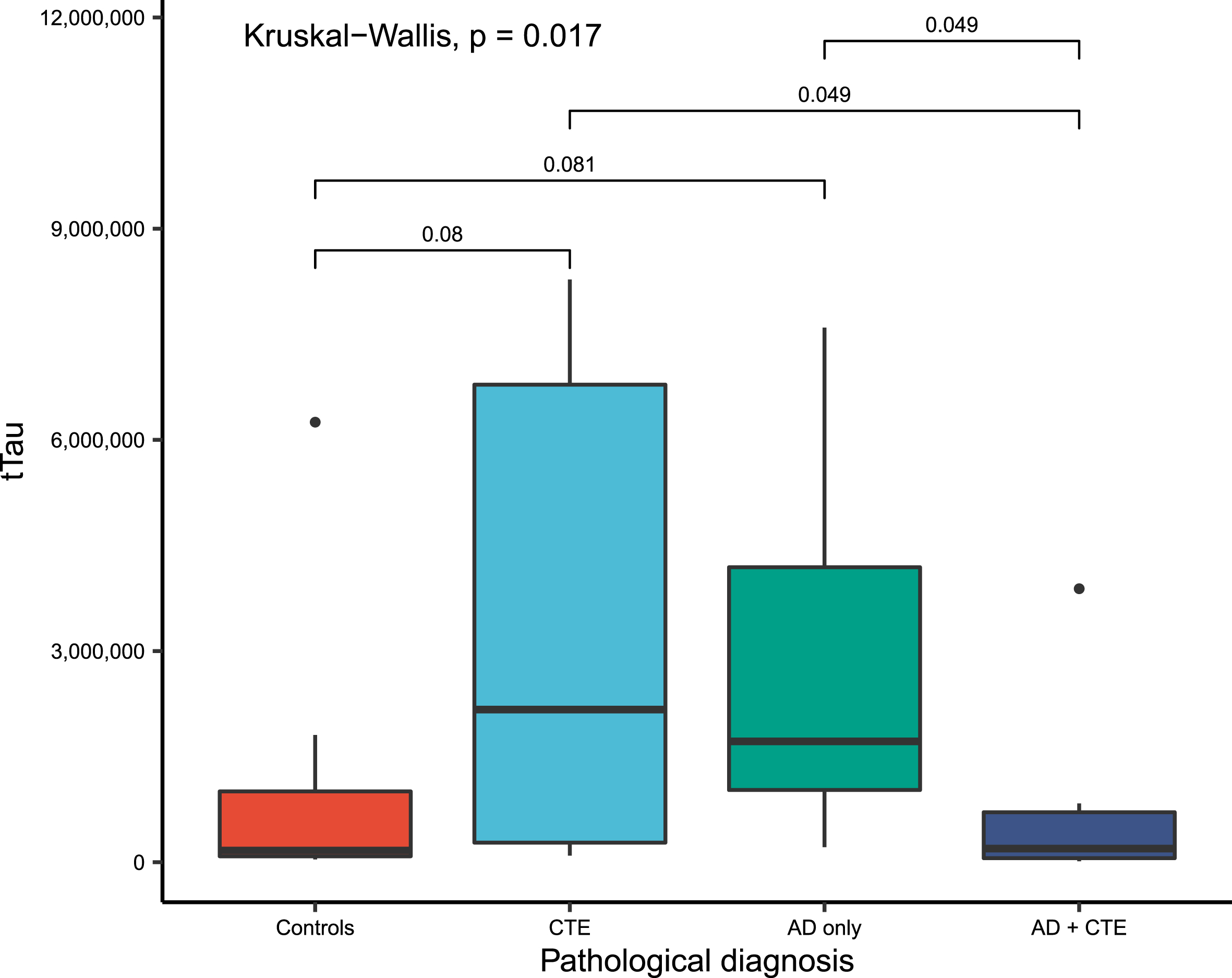
On the Kruskal-Wallis rank sum test, vitreous biomarker levels were significantly different between the neuropathological diagnoses for vitreous tTau (p = 0.02) and pTau231 (p = 0.09). Further pairwise comparisons with adjustment for multiple comparisons revealed that the vitreous tTau was significantly increased in AD only (p = 0.08) and CTE (p = 0.08) groups compared to controls, and similarly increased in AD only (p = 0.049) and CTE (p = 0.049) groups compared to the AD+CTE group (Table 2, Fig. 1). There were statistically significant increases in levels of vitreous pTau181 in AD only and CTE only versus controls; vitreous pTau231 levels in AD only and CTE only versus AD+CTE; and vitreous eotaxin-1 levels in controls and AD only versuss AD+CTE–all of which lost significance after adjustment for multiple comparisons (Table 2).
Table 2
Wilcox-Test results for pairwise comparisons between all study groups, unadjusted for multiple comparison and adjusted for multiple comparisons (Benjamini & Hochberg)
| Vitreous | Control | Versus AD | Versus | Versus | AD only | Versus AD+CTE | Versus AD+CTE | CTE only | Versus AD+CTE | AD + CTE | ||||||
| Biomarker | only | CTE | AD+CTE | |||||||||||||
| Median | p | p# | p | p# | p | p# | Median | p | p# | p | p# | Median | p | p# | Median | |
| (IQR) | (IQR) | (IQR) | (IQR) | |||||||||||||
| tTau, pg/mL | 167000 | 0.054* | 0.08* | 0.04** | 0.08* | 0.82 | 0.98 | 1720000 | >0.99 | >0.99 | 0.016** | 0.049** | 2170000 | 0.015** | 0.049** | 194000 |
| (84400–1010000) | (1030000–4190000) | (27900–6780000) | (57700–710000) | |||||||||||||
| n = 8 | n = 7 | n = 15 | n = 9 | |||||||||||||
| pTau181, U/mL | 102 (55–256) | 0.09* | 0.28 | 0.056* | 0.28 | 0.96 | 0.96 | 364 (284–446) | 0.49 | 0.59 | 0.46 | 0.59 | 273 (175–435) | 0.31 | 0.59 | 120 (39.6–410) |
| n = 8 | n = 7 | n = 15 | n = 9 | |||||||||||||
| pTau231, U/mL | 2510 (836–9480) | 0.28 | 0.42 | 0.17 | 0.33 | 0.48 | 0.58 | 23000 (5610–29000) | 0.97 | 0.97 | 0.04** | 0.13 | 17800 (1840–36400) | 0.04** | 0.13 | 1180 (198–5190) |
| n = 8 | n = 7 | n = 14 | n = 9 | |||||||||||||
| Aβ40, pg/mL | 484 (363–700) | 0.61 | 0.74 | 0.15 | 0.71 | 0.54 | 0.74 | 482 (382–546) | 0.24 | 0.71 | 0.84 | 0.84 | 358 (176–482) | 0.52 | 0.74 | 458 (245–563) |
| n = 8 | n = 7 | n = 15 | n = 9 | |||||||||||||
| Aβ42, pg/mL | 29.6 (21.6–43) | 0.54 | 0.64 | 0.10 | 0.60 | 0.32 | 0.64 | 30.0 (20.5–31.8) | 0.24 | 0.64 | >0.99 | >0.99 | 18.1 (6.02–25.7) | 0.48 | 0.64 | 30.2 (10.1–33.1) |
| n = 8 | n = 7 | n = 15 | n = 9 | |||||||||||||
| NfL, pg/mL | 811 (577–9700) | 0.53 | 0.96 | 0.80 | 0.96 | 0.52 | 0.96 | 2640 (1050–2650) | 0.96 | 0.96 | 0.65 | 0.96 | 1730 (714–4750) | 0.34 | 0.96 | 1130 (99.6–3480) |
| n = 7 | n = 5 | n = 14 | n = 6 | |||||||||||||
| Eotaxin-1, pg/mL | 49.8 (39.5–92.1) | 0.96 | >0.99 | 0.87 | >0.99 | 0.09* | 0.28 | 66.5 (20.9–115) | >0.99 | >0.99 | 0.07* | 0.28 | 54.1 (22.4–162) | 0.14 | 0.28 | 19.5 (13.5–46.4) |
| n = 8 | n = 7 | n = 14 | n = 9 | |||||||||||||
p, unadjusted p value; p#, p value after adjusting for multiple comparisons testing. *p < 0.10; **p < 0.05. IQR, interquartile range; AD, Alzheimer’s disease; CTE, chronic traumatic encephalopathy; Aβ, amyloid-β; tTau, total tau; pTau, phosphorylated tau; NfL, neurofilament; pg/mL, picograms per milliliter; U/mL, units per milliliter.
Fig. 1
Boxplots of vitreous fluid biomarkers levels of total Tau, by study groups, compared using Kruskal-Wallis rank sum test with p values adjusted for multiple comparisons and only statistically significant values (p < 0.10) mentioned. tTau, total tau proteins; CTE, chronic traumatic encephalopathy; AD, Alzheimer’s disease.
Relationship between various vitreous biomarker levels and pathological staging
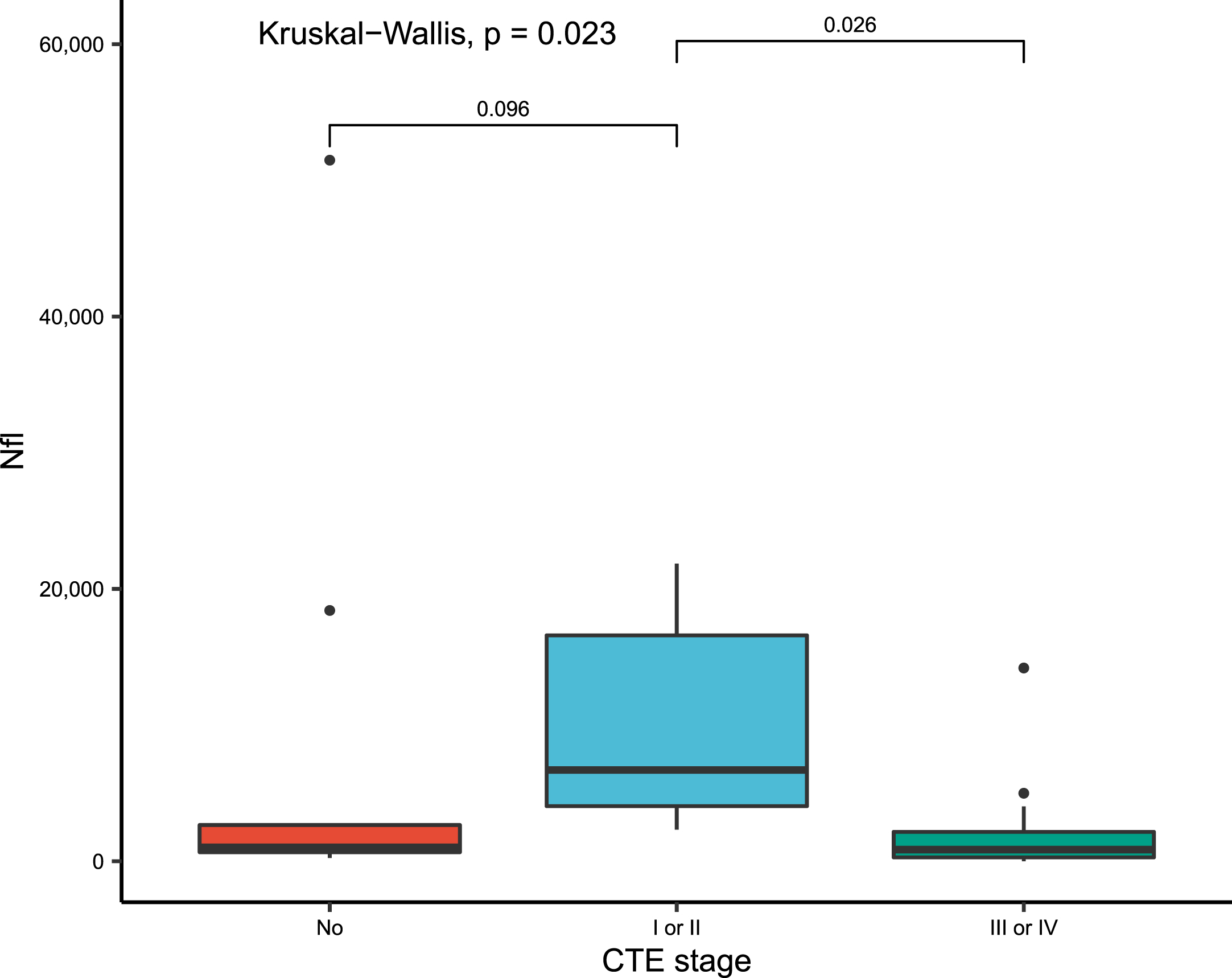
Vitreous biomarkers were compared between groups having no CTE, low CTE (Stage I or II) and high CTE (Stage III or IV). In pairwise comparison adjusting for multiple comparisons, NfL was significantly increased in low stage CTE versus no CTE (p = 0.096) and versus high stage CTE (p = 0.03) (Table 3, Fig. 2). In pairwise comparison, Aβ42 vitreous levels were significantly decreased in low CTE versus no CTE (p = 0.07); however, this association was lost after adjustment for multiple comparisons.
Table 3
Wilcox-Test results for pairwise comparisons between CTE stages, unadjusted for multiple comparison and adjusted for multiple comparisons (Benjamini & Hochberg)
| Vitreous | No CTE | Versus CTE | Versus CTE | CTE Stage I/II | Versus CTE | CTE Stage III/IV | |||
| Biomarker | Stage I/II | Stage III/IV | (low stage) | Stage III/IV | (high stage) | ||||
| Median (IQR) | p | p# | p | p# | Median (IQR) | p | p# | Median (IQR) | |
| tTau, pg/mL | 743000 | 0.20 | 0.29 | 0.78 | 0.78 | 5340000 | 0.18 | 0.30 | 710000 |
| (167000– 2080000) | (661000– 7770000) | (112000– 3030000) | |||||||
| n = 15 | n = 5 | n = 19 | |||||||
| pTau181, U/mL | 260 (102– 424) | >0.99 | >0.99 | 0.70 | >0.99 | 196 (154– 207) | 0.72 | >0.99 | 290 (126– 466) |
| n = 15 | n = 5 | n = 19 | |||||||
| pTau231, U/mL | 7390 (1090– 24600) | 0.40 | 0.58 | 0.58 | 0.58 | 15500 (5190– 20100) | 0.29 | 0.58 | 3620 (410– 22200) |
| n = 15 | n = 5 | n = 18 | |||||||
| Aβ40, pg/mL | 482 (361– 612) | 0.27 | 0.40 | 0.22 | 0.40 | 458 (98.4– 466) | 0.95 | 0.95 | 417 (250– 535) |
| n = 15 | n = 5 | n = 19 | |||||||
| Aβ42, pg/mL | 30 (19– 34.8) | 0.07* | 0.20 | 0.26 | 0.26 | 10.1 (2.07– 15.6) | 0.21 | 0.26 | 21.9 (10.7– 32.3) |
| n = 15 | n = 5 | n = 19 | |||||||
| NfL, pg/mL | 1020 (665– 2660) | 0.06* | 0.096* | 0.27 | 0.27 | 6700 (4040– 16600) | 0.01** | 0.03** | 848 (282– 2160) |
| n = 12 | n = 5 | n = 15 | |||||||
| Eotaxin-1, pg/mL | 56.1 (25.9– 115) | >0.99 | >0.99 | 0.17 | 0.50 | 51.7 (36– 120) | 0.49 | 0.74 | 31.4 (14.8– 71.4) |
| n = 15 | n = 5 | n = 18 | |||||||
p = unadjusted p value and p# = p value after adjusting for multiple comparisons testing. *p < 0.10 and **p < 0.05. IQR, interquartile range; AD, Alzheimer’s disease; CTE, chronic traumatic encephalopathy; Aβ, amyloid-β; tTau, total tau; pTau, phosphorylated tau; NfL, neurofilament light; pg/mL, picograms per milliliter; U/mL, units per milliliter.
Fig. 2
Boxplots of vitreous fluid biomarkers levels of NfL, by CTE staging, compared using Kruskal-Wallis rank sum test with p values adjusted for multiple comparisons and only statistically significant values (p < 0.10) mentioned. NfL, neurofilament light chain; CTE, chronic traumatic encephalopathy.
Relationship between vitreous and cortical tissue biomarker levels
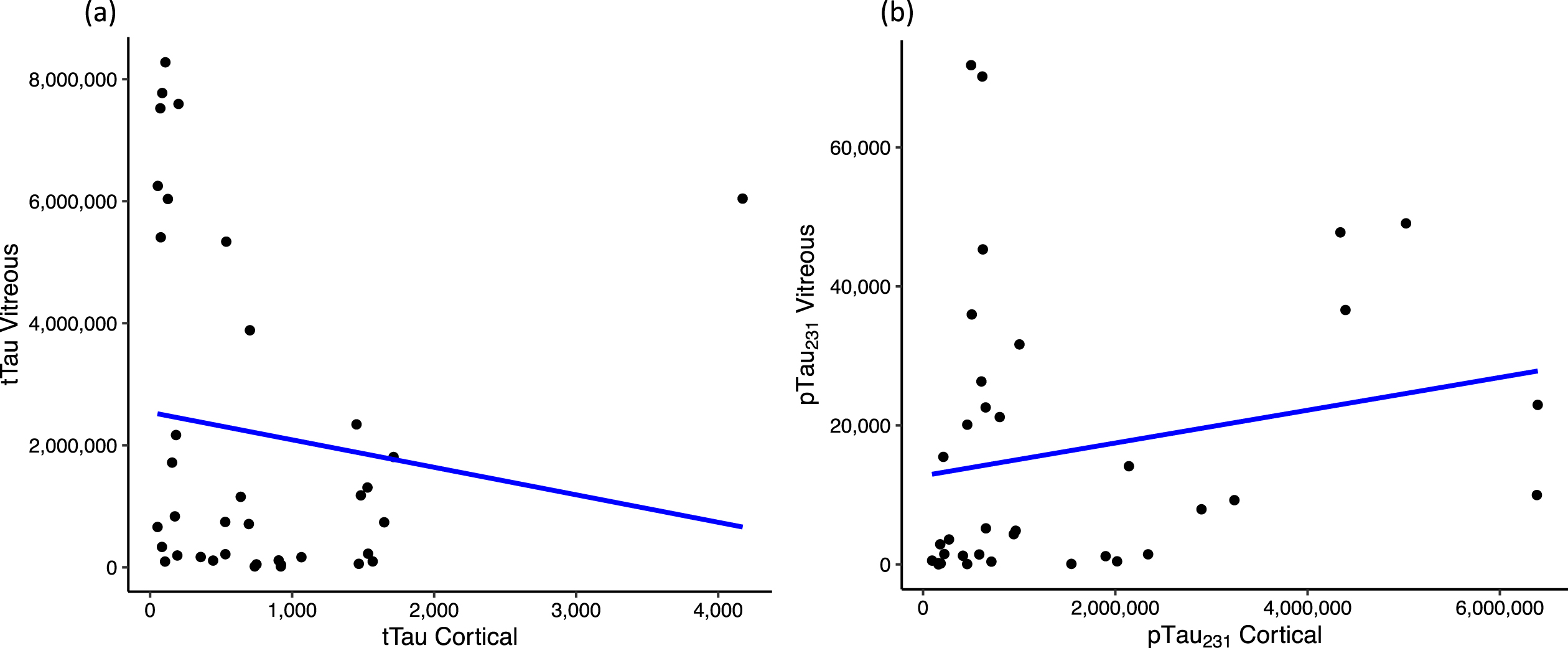
When assessing correlation between vitreous biomarker levels and corresponding cortical brain tissue biomarker levels, tTau (r = –0.34, p = 0.04, n = 37) and pTau231 (r = 0.38, p = 0.02, n = 36) were significantly correlated (Fig. 3). tTau had a weak to moderate negative correlation whereas pTau231 had a weak to moderate positive correlation between the vitreous and cortical levels.
Fig. 3
Scatter plot showing correlation between vitreous and cortical biomarker levels for (a) tTau and (b) pTau231; assessed using Spearman’s correlation test and only statistically significant values (p < 0.10) mentioned. tTau, total tau proteins; pTau231, phosphorylated tau 231.
DISCUSSION
Recent studies have investigated different ocular biomarkers to diagnose neurodegenerative disease such as neurodegenerative proteins in the retina [37] as well as the vitreous, aqueous, and tear fluids of the eye [38, 39]. A previous study from our group found an association of elevated Aβ40, Aβ42, and tTau in the vitreous humor of patients with lower MMSE scores, but no formal diagnosis of dementia at the time of participation [28]. In our current study we found that the postmortem vitreous humor levels of tTau were significantly associated with the neuropathological diagnoses of AD and CTE in postmortem brains. Vitreous levels of tTau were significantly elevated in AD and CTE groups when comparing to the AD+CTE group and controls. We additionally found that NfL levels were found to have a significant association with CTE stage. NfL was significantly increased in low stage CTE versus no CTE as well as low stage CTE versus high stage CTE. Finally, we found that vitreous levels of pTau231 and tTau were significantly correlated to cortical brain tissue levels of the same biomarkers. This is the first study to our knowledge to study the role of vitreous biomarkers in prognostication of neurodegenerative diseases and relationship with cortical biomarker levels. Our findings provide further evidence to support the potential role of vitreous biomarkers in early diagnosis and prognostication of neurodegenerative diseases like AD and CTE.
In CSF, tTau and pTau levels have been proven to be highly concordant with AD and helpful in making a diagnosis [40, 41]. Additionally, our group has recently demonstrated pTau231 levels in CSF to be significantly higher in both low stage and high stage CTE groups compared to the controls or AD only group. Conversely Aβ1 - 42 was significantly decreased in CTE, suggesting that the relationship between cortical and CSF Aβ levels is altered in CTE compared to AD [42]. Hence, the combination of these biomarkers could be useful in distinguishing CTE from AD. Previously, Olczak et. al. investigated biofluids including vitreous humor and tested levels of microtubule associated protein tau in patients with severe TBI at death, but only found elevated levels in urine and saliva [43]. The lack of change in the vitreous in that study may be due to the acute nature of the injury or due to the relatively small sample size. Hart de Ruyter et al. recently demonstrated that the pathological tau load in the retina was increased in patients with AD and correlated with the Braak stage for neurofibrillary tangles, hence correlating with the tau pathology in the brain [37]. In this study, we see that tTau levels in vitreous humor were significantly increased in both the AD only and CTE groups relative to controls, concordant with the prior literature on CSF changes in AD and CTE [5, 42, 44–46]. Interestingly, similar to results previously reported in postmortem CSF [42], vitreous levels of Aβ42 were decreased in low stage CTE relative to no CTE, although significance was lost after adjustment for multiple comparisons. Similarly, low Aβ42 CSF levels were also reported in a group of professional athletes who have experienced repetitive concussive traumatic brain injury, when compared to healthy controls [47]. Hence, these eye-based protein biomarkers could be a more accessible and cost-effective adjunct for diagnosis or prognostication in neuropathological disease diagnosis.
Also, this study reports a significant increase in vitreous tTau in both the AD only and CTE only groups compared to the AD+CTE group. Additionally, pTau231 in vitreous humor was significantly increased in both the AD only and CTE only groups relative to the AD+CTE group on initial analysis. Eotaxin-1/CCL11 has previously been shown to be increased in frontal cortex and CSF in CTE, but not in AD [48]. We did not see this trend in vitreous fluid although preliminary analyses showed that levels of eotaxin-1/CCL11 were decreased in AD+CTE compared to controls and the AD only group. The relationship between various protein biomarkers in coexistent AD+CTE are not fully understood but these results indicate a decreasing trend of these biomarkers in groups with both AD+CTE compared to AD only or CTE only, which may be a result of greater disease burden with combined AD and CTE. These results could serve as a starting point to investigate the relationship between these two neuropathological entities and the resultant changes in vitreous humor.
NfL has been widely studied in both blood and CSF as a marker of traumatic brain injury (TBI) [49–52]. Less is known about the association between NfL levels and CTE, a neurodegenerative disease linked with repetitive mild TBIs. However, preliminary studies also suggest that blood NfL levels increase with the severity of concussion as measured by decreased white matter integrity [49, 52] and in those with CTE [53]. Our group previously reported that NfL levels in the vitreous of cognitively normal patients with underlying eye disease are associated with AD markers such as Aβ42, Aβ40, and t-tau as well as other inflammatory and vascular cytokines [29]. This study did not find an association of vitreous NfL with the neuropathological diagnosis, but it was associated with CTE staging, consistent with prior studies in CSF. We saw statistically significant increase in NfL in low stage CTE versus controls as well as versus high stage CTE, which was retained on further adjustment for multiple comparisons. Further larger sample sized studies could be helpful to elucidate its role as a biomarker for CTE staging.
In this study, we additionally found a significant correlation between the vitreous and brain cortical levels of tTau (negative correlation) and pTau231 (positive correlation). Tau proteins play a similar role in the brain and the eye – they regulate axonal and cytoskeletal transport in both organs and impact Aβ accumulation and cell-survival signaling in the retina and brain [54]. Although release of tTau from the brain is a possible source of this protein in the vitreous, several studies have found tau aggregates in the retina correlated to ophthalmic diseases such as age-related macular degeneration, retinitis pigmentosa, macular hole, diabetic retinopathy, and glaucoma [55, 56]. Further studies are needed to demonstrate correlation of vitreous and cortical levels with clinical diagnosis and pathology.
The potential utility of procuring eye fluid for immunoassay analyses, as a diagnostic marker for AD or CTE, is still being explored. Compared to CSF analysis, a sampling eye fluid may be potentially safer, quicker, and more accessible to obtain with a potentially less risk of adverse events. It may be done as an outpatient office procedure via aspiration with a needle tap and, depending on the type of eye fluid, it may not require surgical tools such as a mechanized biopsy with a vitrector. Ophthalmologic data on this procedure has demonstrated a high success rate for the diagnostic yield as well as similar safety profile compared to surgical vitreous biopsy [57, 58]. Furthermore, study of other ocular specimens such as aqueous humor or tear film as potential biomarkers of neurodegenerative diseases would further mitigate safety risks, especially tear fluid which can be obtained noninvasively.
Our findings build upon our previous work that vitreous fluid reflects pathological changes in the brain. In addition, this is the first study to find a link between vitreous biomarkers and CTE. These findings are foundational for future studies that may investigate the role of vitreous biomarkers and other eye fluid biomarkers in the diagnosis, prognostication, and management of diseases such as AD and CTE.
There were limitations to our study. First, we had a small sample size due to the reliance on donation of post-mortem brain and ocular tissue samples for our study methodology. Given this limitation, subdividing the samples into pathologic groups resulted in several mixed cases of both AD and CTE and the overlap in the pathology of these diseases could be potentially confounding. Future studies with larger numbers will also allow for consideration of vascular and white matter pathologies. Secondly, the median age of our control group was younger which might affect the levels of tTau, which is known to increase with aging. We were unable to adjust for age in our statistical analysis because of the small sample size and variability in age. As the AD only and AD+CTE groups are older than other groups, we should be cautious on interpreting the results when comparing these groups to others. Thirdly, our study uses controls with a history of RHI, and not true controls with no RHI or pathology, which potentially underestimates our effect and may bias towards null. Also, this study was retrospective by design, hence there is a possibility for selection bias; nearly all patients were male; thus, further longitudinal studies with larger sample sizes are needed to yield generalizable results to the patient population. Lastly, we did not have clinical information on any eye diseases which may have affected biomarker levels.
To summarize, this exploratory study found a link of several vitreous biomarkers with pathologically confirmed AD and CTE. Alterations of neurodegenerative proteins in the eye may reflect neuropathological changes in the brain and further support investigations into the eye’s potential role in the diagnosis of neurodegenerative diseases.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by R03, National Institute of Health/National Institute of Aging/Extramural Research Program (NIH/NIA/ERP,1R03AG063255-01); United States (U.S.) Department of Veterans Affairs, Veterans Health Administration, Veterans Affairs Biorepository (BX002466); BLRD Merit Award (I01BX005933, I01BX005161); National Institute of Neurological Disorders and Stroke (U54NS115266, K23NS102399); National Institute of Aging Boston University AD Research Center (P30AG072978); and the Concussion Legacy Foundation. This work was also supported by unrestricted gifts from the Andlinger Foundation and WWE. This material is the result of work supported with resources and the use of facilities at the VA Bedford Healthcare System. The sponsor or funding organization had no role in the design or conduct of this research.
CONFLICT OF INTEREST
Weiming Xia, Michael L. Alosco, and Manju Subramanian are Editorial Board Members of this journal but were not involved in the peer-review process nor had access to any information regarding its peer-review.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
[1] | ((2021) ) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17: , 327–406. |
[2] | Mayeux R , Stern Y ((2012) ) Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2: , a006239. |
[3] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34: , 939–944. |
[4] | Jack CRJ , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[5] | Turner RC , Lucke-Wold BP , Robson MJ , Lee JM , Bailes JE ((2016) ) Alzheimer’s disease and chronic traumatic encephalopathy: Distinct but possibly overlapping disease entities. Brain Inj 30: , 1279–1292. |
[6] | Alosco ML , Mariani ML , Adler CH , Balcer LJ , Bernick C , Au R , Banks SJ , Barr WB , Bouix S , Cantu RC , Coleman MJ , Dodick DW , Farrer LA , Geda YE , Katz DI , Koerte IK , Kowall NW , Lin AP , Marcus DS , Marek KL , McClean MD , McKee AC , Mez J , Palmisano JN , Peskind ER , Tripodis Y , Turner RW 2nd, Wethe J V , Cummings JL , Reiman EM , Shenton ME , Stern RA ((2021) ) Developing methods to detect and diagnose chronic traumatic encephalopathy during life: Rationale, design, and methodology for the DIAGNOSE CTE Research Project. Alzheimers Res Ther 13: , 136. |
[7] | Zhang Y , Wang Y , Shi C , Shen M , Lu F ((2021) ) Advances in retina imaging as potential biomarkers for early diagnosis of Alzheimer’s disease. Transl Neurodegener 10: , 6. |
[8] | Cheung CY , Mok V , Foster PJ , Trucco E , Chen C , Wong TY ((2021) ) Retinal imaging in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 92: , 983–994. |
[9] | Snyder PJ , Alber J , Alt C , Bain LJ , Bouma BE , Bouwman FH , DeBuc DC , Campbell MCW , Carrillo MC , Chew EY , Cordeiro MF , Dueñas MR , Fernández BM , Koronyo-Hamaoui M , La Morgia C , Carare RO , Sadda SR , van Wijngaarden P , Snyder HM ((2021) ) Retinal imaging inAlzheimer’s and neurodegenerative diseases.. Alzheimers Dement 17: , 103–111. |
[10] | López-Cuenca I , Salobrar-García E , Elvira-Hurtado L , Fernández-Albarral JA , Sánchez-Puebla L , Salazar JJ , Ramírez JM , Ramírez AI , de Hoz R ((2021) ) The value of OCT andOCTA as potential biomarkers for preclinical Alzheimer’s disease: Areview study. Life (Basel) 11: , 712. |
[11] | Colligris P , Perez de Lara MJ , Colligris B , Pintor J ((2018) ) Ocular manifestations of Alzheimer’s and other neurodegenerative diseases: The prospect of the eye as a tool for the early diagnosis of Alzheimer’s disease. J Ophthalmol 2018: , 8538573. |
[12] | Childs C , Barker LA , Gage AM , Loosemore M ((2018) ) Investigating possible retinal biomarkers of head trauma in Olympic boxers using optical coherence tomography. Eye Brain 10: , 101–110. |
[13] | Gao L , Liu Y , Li X , Bai Q , Liu P ((2015) ) Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch Gerontol Geriatr 60: , 162–167. |
[14] | Liu D , Zhang L , Li Z , Zhang X , Wu Y , Yang H , Min B , Zhang X , Ma D , Lu Y ((2015) ) Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol 15: , 14. |
[15] | Chan VTT , Sun Z , Tang S , Chen LJ , Wong A , Tham CC , Wong TY , Chen C , Ikram MK , Whitson HE , Lad EM , Mok VCT , Cheung CY ((2019) ) Spectral-domain OCT measurements inAlzheimer’s disease: A systematic review and meta-analysis. Ophthalmology 126: , 497–510. |
[16] | Armstrong GW , Kim LA , Vingopoulos F , Park JY , Garg I , Kasetty M , Silverman RF , Zeng R , Douglas VP , Lopera F , Baena A , Giraldo M , Norton D , Cronin-Golomb A , Arboleda-Velasquez JF , Quiroz YT , Miller JB ((2021) ) Retinal imaging findings in carriers with PSEN1 -associated early-onset familial Alzheimer disease before onset of cognitive symptoms. JAMA Ophthalmol 139: , 49–56. |
[17] | Lee CS , Apte RS ((2020) ) Retinal biomarkers of Alzheimer disease. Am J Ophthalmol 218: , 337–341. |
[18] | Miki A , Medeiros FA , Weinreb RN , Jain S , He F , Sharpsten L , Khachatryan N , Hammel N , Liebmann JM , Girkin CA , Sample PA , Zangwill LM ((2014) ) Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 121: , 1350–1358. |
[19] | Richardson R , Tracey-White D , Webster A , Moosajee M ((2017) ) The zebrafish eye-a paradigm for investigating human ocular genetics. Eye (Lond) 31: , 68–86. |
[20] | Sernagor E , Eglen SJ , Wong ROL ((2001) ) Development of retinal ganglion cell structure and function. Prog Retin Eye Res 20: , 139–174. |
[21] | Cooper LS , Wong TY , Klein R , Sharrett AR , Bryan RN , Hubbard LD , Couper DJ , Heiss G , Sorlie PD ((2006) ) Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: The Atherosclerosis Risk in Communities Study. Stroke 37: , 82–86. |
[22] | Hirai T , Ando Y , Yamura M , Kitajima M , Hayashida Y , Korogi Y , Yamashita T , Yamashita Y ((2005) ) Transthyretin-related familial amyloid polyneuropathy: Evaluation of CSF enhancement on serial T1-weighted and fluid-attenuated inversion recovery images following intravenous contrast administration. AJNR Am J Neuroradiol 26: , 2043–2048. |
[23] | Patton N , Aslam T , Macgillivray T , Pattie A , Deary IJ , Dhillon B ((2005) ) Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J Anat 206: , 319–348. |
[24] | Mancino R , Martucci A , Cesareo M , Giannini C , Corasaniti MT , Bagetta G , Nucci C ((2018) ) Glaucoma and Alzheimer disease: One age-related neurodegenerative disease of the brain. Curr Neuropharmacol 16: , 971–977. |
[25] | Wen L-Y , Wan L , Lai J-N , Chen CS , Chen JJ-Y , Wu M-Y , Hu K-C , Chiu L-T , Tien P-T , Lin H-J ((2021) ) Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: A nationwide population-based study. PLoS One 16: , e0250440. |
[26] | Lee CS , Gibbons LE , Lee AY , Yanagihara RT , Blazes MS , Lee ML , McCurry SM , Bowen JD , McCormick WC , Crane PK , Larson EB ((2022) ) Association between cataract extraction and development of dementia. JAMA Intern Med 182: , 134–141. |
[27] | Lee CS , Larson EB , Gibbons LE , Lee AY , McCurry SM , Bowen JD , McCormick WC , Crane PK ((2019) ) Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement 15: , 34–41. |
[28] | Wright LM , Stein TD , Jun G , Chung J , McConnell K , Fiorello M , Siegel N , Ness S , Xia W , Turner KL , Subramanian ML ((2019) ) Association of cognitive function with amyloid-β and tau proteins in the vitreous humor. J Alzheimers Dis 68: , 1429–1438. |
[29] | Subramanian ML , Vig V , Chung J , Fiorello MG , Xia W , Zetterberg H , Blennow K , Zetterberg M , Shareef F , Siegel NH , Ness S , Jun GR , Stein TD ((2020) ) Neurofilament light chain in thevitreous humor of the eye. Alzheimers Res Ther 12: , 111. |
[30] | Mez J , Solomon TM , Daneshvar DH , Murphy L , Kiernan PT , Montenigro PH , Kriegel J , Abdolmohammadi B , Fry B , Babcock KJ , Adams JW , Bourlas AP , Papadopoulos Z , McHale L , Ardaugh BM , Martin BR , Dixon D , Nowinski CJ , Chaisson C , Alvarez VE , Tripodis Y , Stein TD , Goldstein LE , Katz DI , Kowall NW , Cantu RC , Stern RA , McKee AC ((2015) ) Assessing clinicopathological correlation in chronic traumatic encephalopathy: Rationale and methods for the UNITE study. Alzheimers Res Ther 7: , 62. |
[31] | Vonsattel JPG , del Amaya MP , Keller CE ((2008) ) Twenty-first century brain banking. Processing brains for research: The Columbia University methods. Acta Neuropathol 115: , 509–532. |
[32] | Newell KL , Hyman BT , Growdon JH , Hedley-Whyte ET ((1999) ) Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 58: , 1147–1155. |
[33] | McKee AC , Cairns NJ , Dickson DW , Folkerth RD , Dirk Keene C , Litvan I , Perl DP , Stein TD , Vonsattel J-P , Stewart W , Tripodis Y , Crary JF , Bieniek KF , Dams-O’Connor K , Alvarez VE , Gordon WA , TBI/CTE group ((2016) ) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131: , 75–86. |
[34] | Bieniek KF , Cairns NJ , Crary JF , Dickson DW , Folkerth RD , Keene CD , Litvan I , Perl DP , Stein TD , Vonsattel J-P , Stewart W , Dams-O’Connor K , Gordon WA , Tripodis Y , Alvarez VE , Mez J , Alosco ML , McKee AC ((2021) ) The second NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. J Neuropathol Exp Neurol 80: , 210–219. |
[35] | Alosco ML , Cherry JD , Huber BR , Tripodis Y , Baucom Z , Kowall NW , Saltiel N , Goldstein LE , Katz DI , Dwyer B , Daneshvar DH , Palmisano JN , Martin B , Cantu RC , Stern RA , Alvarez VE , Mez J , Stein TD , McKee AC ((2020) ) Characterizing tau deposition in chronic traumatic encephalopathy (CTE): Utility of the McKee CTE staging scheme. Acta Neuropathol 140: , 495–512. |
[36] | Benjamini Y , Hochberg Y ((1995) ) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: , 289–300. |
[37] | Hart de Ruyter FJ , Morrema THJ , den Haan J , Twisk JWR , de Boer JF , Scheltens P , Boon BDC , Thal DR , Rozemuller AJ , Verbraak FD , Bouwman FH , Hoozemans JJM ((2023) ) Phosphorylated tau in the retina correlates with tau pathology in the brain in Alzheimer’s disease and primary tauopathies. Acta Neuropathol 145: , 197–218. |
[38] | Dehghani C , Frost S , Jayasena R , Masters CL , Kanagasingam Y ((2018) ) Ocular biomarkers of Alzheimer’s disease: The role of anterior eye and potential future directions. Invest Ophthalmol Vis Sci 59: , 3554–3563. |
[39] | Gijs M , Ramakers IHGB , Visser PJ , Verhey FRJ , van de Waarenburg MPH , Schalkwijk CG , Nuijts RMMA , Webers CAB ((2021) ) Association of tear fluid amyloid and tau levels with disease severity and neurodegeneration. Sci Rep 11: , 22675. |
[40] | Zetterberg H , Blennow K ((2021) ) Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol Neurodegener 16: , 10. |
[41] | McGrowder DA , Miller F , Vaz K , Nwokocha C , Wilson-Clarke C , Anderson-Cross M , Brown J , Anderson-Jackson L , Williams L , Latore L , Thompson R , Alexander-Lindo R ((2021) ) Cerebrospinal fluid biomarkers of Alzheimer’s disease: Current evidence and future perspectives. Brain Sci 11: , 215. |
[42] | Turk KW , Geada A , Alvarez VE , Xia W , Cherry JD , Nicks R , Meng G , Daley S , Tripodis Y , Huber BR , Budson AE , Dwyer B , Kowall NW , Cantu RC , Goldstein LE , Katz DI , Stern RA , Alosco ML , Mez J , McKee AC , Stein TD ((2022) ) A comparison between tau and amyloid-β cerebrospinal fluid biomarkers in chronic traumatic encephalopathy and Alzheimer disease. Alzheimers Res Ther 14: , 28. |
[43] | Olczak M , Poniatowski ŁA , Niderla-Bielińska J , Kwiatkowska M , Chutorański D , Tarka S , Wierzba-Bobrowicz T ((2019) ) Concentrationof microtubule associated protein tau (MAPT) in urine and saliva asa potential biomarker of traumatic brain injury in relationship withblood-brain barrier disruption in postmortem examination. Forensic Sci Int 301: , 28–36. |
[44] | Blennow K , Zetterberg H ((2018) ) Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J Intern Med 284: , 643–663. |
[45] | Katsumoto A , Takeuchi H , Tanaka F ((2019) ) Tau pathology in chronic traumatic encephalopathy and Alzheimer’s disease: Similarities and differences. Front Neurol 10: , 980. |
[46] | Taghdiri F , Multani N , Tarazi A , Naeimi SA , Khodadadi M , Esopenko C , Green R , Colella B , Wennberg R , Mikulis D , Davis KD , Goswami R , Tator C , Levine B , Tartaglia MC ((2019) ) Elevated cerebrospinal fluid total tau in former professional athletes with multiple concussions. Neurology 92: , e2717–e2726. |
[47] | Shahim P , Tegner Y , Marklund N , Höglund K , Portelius E , Brody DL , Blennow K , Zetterberg H ((2017) ) Astroglial activation and altered amyloid metabolism in human repetitive concussion. Neurology 88: , 1400–1407. |
[48] | Cherry JD , Stein TD , Tripodis Y , Alvarez VE , Huber BR , Au R , Kiernan PT , Daneshvar DH , Mez J , Solomon TM , Alosco ML , McKee AC ((2017) ) CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer’s disease. PLoS One 12: , e0185541. |
[49] | Karantali E , Kazis D , McKenna J , Chatzikonstantinou S , Petridis F , Mavroudis I ((2022) ) Neurofilament light chain in patients with a concussion or head impacts: A systematic review and meta-analysis. Eur J Trauma Emerg Surg 48: , 1555–1567. |
[50] | Shahim P , Politis A , van der Merwe A , Moore B , Chou Y-Y , Pham DL , Butman JA , Diaz-Arrastia R , Gill JM , Brody DL , Zetterberg H , Blennow K , Chan L ((2020) ) Neurofilament light as a biomarker in traumatic brain injury. Neurology 95: , e610–e622. |
[51] | Gao W , Zhang Z , Lv X , Wu Q , Yan J , Mao G , Xing W ((2020) ) Neurofilament light chain level in traumatic brain injury: A system review and meta-analysis. Medicine (Baltimore) 99: , e22363. |
[52] | Taghdiri F , Multani N , Ozzoude M , Tarazi A , Khodadadi M , Wennberg R , Mikulis D , Green R , Colella B , Davis KD , Blennow K , Zetterberg H , Tator C , Tartaglia MC ((2020) ) Neurofilament-light in former athletes: A potential biomarker of neurodegeneration and progression. Eur J Neurol 27: , 1170–1177. |
[53] | Asken BM , Tanner JA , VandeVrede L , Casaletto KB , Staffaroni AM , Mundada N , Fonseca C , Iaccarino L , La Joie R , Tsuei T , Mladinov M , Grant H , Shankar R , Wang KKW , Xu H , Cobigo Y , Rosen H , Gardner RC , Perry DC , Miller BL , Spina S , Seeley WW , Kramer JH , Grinberg LT , Rabinovici GD ((2022) ) Multi-modal biomarkers of repetitive head impacts and traumatic encephalopathy syndrome: A clinicopathological case series. J Neurotrauma 39: , 1195–1213. |
[54] | Ho W-L , Leung Y , Tsang AW-T , So K-F , Chiu K , Chang RC-C ((2012) ) Review: Tauopathy in the retina and optic nerve: Does it shadow pathological changes in the brain? Mol Vis 18: , 2700–2710. |
[55] | Löffler KU , Edward DP , Tso MO ((1995) ) Immunoreactivity against tau, amyloid precursor protein, and beta-amyloid in the human retina. Invest Ophthalmol Vis Sci 36: , 24–31. |
[56] | Yoneda S , Hara H , Hirata A , Fukushima M , Inomata Y , Tanihara H ((2005) ) Vitreous fluid levels ofbeta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol 49: , 106–108. |
[57] | Lobo A , Lightman S ((2003) ) Vitreous aspiration needle tap in the diagnosis of intraocular inflammation. Ophthalmology 110: , 595–599. |
[58] | Han DP , Wisniewski SR , Kelsey SF , Doft BH , Barza M , Pavan PR ((1999) ) Microbiologic yields and complication rates of vitreous needle aspiration versus mechanized vitreous biopsy in the Endophthalmitis Vitrectomy Study. Retina 19: , 98–102. |




