Case Identification and Characterization of Migrants with Dementia in the Lazio Region Using Health Administrative Data
Abstract
Background:
A crucial step for planning effective public health policies for migrants with dementia is the collection of data on the local dimensions of the phenomenon and patients’ characteristics.
Objective:
This study aimed to identify and characterize migrants with dementia in the Lazio region using health administrative databases.
Methods:
Residents with dementia aged 50 years or older, living in the Lazio region as of December 31, 2018, were identified using a validated algorithm based on hospital discharge(s), claims for antidementia drugs, and co-payment exemption for dementia. Migrants were defined as people born abroad and grouped in migrants from High Migratory Pressure Countries (HMPCs) and Highly Developed Countries (HDCs). Overall and age-specific prevalence rates were estimated in native- and foreign-born patients.
Results:
Dementia was ascertained in 38,460 residents. Among them, 37,280 (96.9%) were born in Italy, 337 (0.9%) were migrants from HDCs, and 843 (2.2%) from HMPCs. Dementia prevalence was higher among natives (1.15%, 95% CI 1.14–1.16) relative to migrants from HDCs (0.60%, 95% CI 0.54–0.67) and HMPCs (0.29%, 95% CI 0.27–0.31). The prevalence of comorbidities did not differ between groups. Migrants with dementia had a lower likelihood of receiving antidementia treatments compared with natives (51.6% in migrants from HDCs, 49.3% in migrants from HMPCs, and 53.5% among Italians).
Conclusion:
Routinely collected data in healthcare administrative databases can support the identification of migrants with dementia. Migrants exhibited a lower age-standardized prevalence of registered dementia and lower access to dedicated treatments than Italians. These findings are suggestive of underdiagnosis and undertreatment of dementia in migrants.
INTRODUCTION
Dementia is one of the leading causes of mortality, disability, and dependency among older people globally [1, 2]. As advocated in the Global Action Plan on the Public Health Response to Dementia by the World Health Organization, there is a need to develop plans and policies ensuring equitable access to prevention, diagnosis, and treatment of dementia for all population groups, especially the most vulnerable [3, 4]. In this regard, a growing interest is addressed in dementia in migrants (i.e., people living in a country but born abroad). Indeed, due to population aging and ongoing international migrations, the number of older migrants, thus at higher risk of developing dementia, has grown rapidly in many World regions, especially in Europe [5]. More than one million dementia and mild cognitive impairment cases can be estimated among migrants living in Europe by applying age-standardized prevalence rates [6, 7]. An increase in the number of migrants with cognitive disturbances referred to dedicated healthcare facilities (e.g., memory clinics) has been documented in some European countries [8–10]. However, recent surveys have shown that these services are still only partially prepared to manage dementia in patients with a history of migration and that several gaps and barriers (e.g., low health literacy of migrants, poor adoption of cross-cultural cognitive assessment tools, lack of specific training for healthcare professionals, absence of dedicated referral pathways) still challenge a diversity-sensitive provision of care and support [11–13].
A crucial step for the design of effective public health policies for migrants with cognitive disorders is the collection of “real world” data on the local dimensions of the phenomenon and the sociodemographic and clinical characteristics of affected individuals. This information is crucial to understanding their health needs and risk profile and for implementing tailored healthcare strategies. Health information systems could be very useful for this purpose [14]. Accurate algorithms have been developed and validated to identify patients with dementia based on data from hospital admissions/discharges, drug prescriptions, and co-payment exemptions [15, 16]. This information can be easily linked with records on the country of birth or other proxies of the migration status that are routinely collected in administrative databases. Such record linkage procedures have already been used in several European countries to estimate the prevalence of dementia in migrants [17–19]. To date, no similar study has been conducted in Italy, one of the oldest countries in the world and one of the main destinations for international migration in Europe.
The present population-based, cohort study aimed to 1) identify migrants living with dementia in the Lazio region based on data collected in health administrative databases, and 2) investigate possible differences in prevalence, clinical characteristics, and treatment of dementia between migrant and Italian-born patients.
METHODS
Study setting
The Lazio region is located in the central part of Italy and is the third most populated region of the country. A total of nearly 5,900,000 residents (2,848,727 men and 3,047,966 women) lived in the region on the enrollment day December 31, 2018 (source: https://demo.istat.it/). Lazio is divided into 10 local health units representing autonomous bodies of the National Health Service and 56 health districts nested within them. The regional capital is Rome, the largest Italian city and the first at the national level for the number of residing migrants (more than 340,000 at the beginning of 2019) (source: https://demo.istat.it/).
Data sources
A population-based, retrospective cohort study was conducted using linked healthcare and administrative databases of the Lazio region. The primary data source was the Regional Health Assistance file, which includes all individuals registered with a general practitioner or who have ever had contact with the regional health system. The following registries were used to identify and characterize the study population: 1) the Hospital Discharge Registry, which gathers demographic and clinical data from all regional hospitals; 2) the Drug Claims Registry, which collects individual records for each drug prescription dispensed from public and private pharmacies, and reimbursed by the healthcare system; and 3) the Ticket Exemption Registry, which contains information on all residents who are entitled to co-pay fee exemptions for chronic diseases, low income, or old age.
Definition of dementia
People aged 50 years or older living with dementia in the Lazio region as of December 31, 2018 (enrollment date), were identified using a validated case-finding algorithm based on the three above mentioned registries [20]. Specifically, at least one of the following criteria had to be met to operationally define the presence of dementia: 1) hospital discharge(s) in the previous five years with a primary or secondary diagnosis of dementia according to well-established codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (Supplementary Material); 2) at least one prescription claim for cholinesterase inhibitors (i.e., donepezil, rivastigmine, and galantamine) or memantine in the previous five years (Anatomical Therapeutic Chemical code N06D); 3) previous exemption for dementia or Alzheimer’s disease (codes 011 and 029).
Definition and classification of migrants
Migrants were operationally defined as people living in Italy but born abroad and were further classified according to their country of birth as follows: 1) migrants from High Migratory Pressure Countries (HMPCs: Central-Eastern Europe, Africa, Asia -except for Israel and Japan-, and Latin America); and 2) migrants from Highly Developed Countries (HDCs: North America, Europe— except for Central and Eastern Europe, Oceania, Israel, and Japan) [21, 22].
Data analysis
The prevalence of dementia on December 31, 2018, was calculated as the ratio between the number of registered residents with dementia living in the Lazio region and the total number of registered residents (multiplied by 100). This analysis was restricted to residents older than 50 years. Age-specific prevalence rates were calculated considering 5-year age groups, from ages 50–54 to 90–94, and then age 95 or more. Age-standardized prevalence rates were calculated separately for men and women, using the direct standardization method with all residents in Italy (December 31, 2018) as the standard population. For all prevalence rates, 95% confidence intervals (95% CI) were estimated.
Information on demographics (i.e., age and gender), comorbidities, and drug consumption of all residents aged 50 or more were retrieved. The following comorbidities were considered if present in the two years preceding the enrollment date and grouped based on ICD-9-CM codes: infectious diseases, cancer, cardiovascular diseases, respiratory diseases, gastrointestinal diseases, genitourinary diseases, psychiatric disorders, and endocrine/metabolic disorders. The following drugs, prescribed in the same 2-year period, were considered and categorized according to the Anatomical Therapeutic Chemical (ATC) codes: antidementia drugs (i.e., cholinesterase inhibitors and memantine), antipsychotics drugs, cardiac therapies, antihypertensive agents, lipid-modifying agents, statins, antiplatelet agents, insulin and analogs, blood glucose-lowering drugs, antidepressants, anxiolytics, hypnotics and sedative drugs, and analgesic drugs.
Data were presented separately for Italians, migrants from HDCs, and migrants from HMPCs as absolute numbers and percentages. The demographic and clinical characteristics of these three groups of participants were compared using the Chi-square test. The level of statistical significance was set at 5% (p < 0.05). All analyses were performed using SAS Version 9.2.
Ethical considerations
The study was conducted in accordance with the World Medical Association Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Italian National Institute of Health (Protocol 10749; 5 April 2018). The Department of Epidemiology of the Lazio Regional Health Service has full access to anonymized health administrative databases. Data used in the present study were analyzed anonymously, using a standardized methodology according to the national privacy law, and reported in aggregate form (i.e., individuals cannot be identified directly or through identifiers). The collection of informed consent from participants was therefore not required.
RESULTS
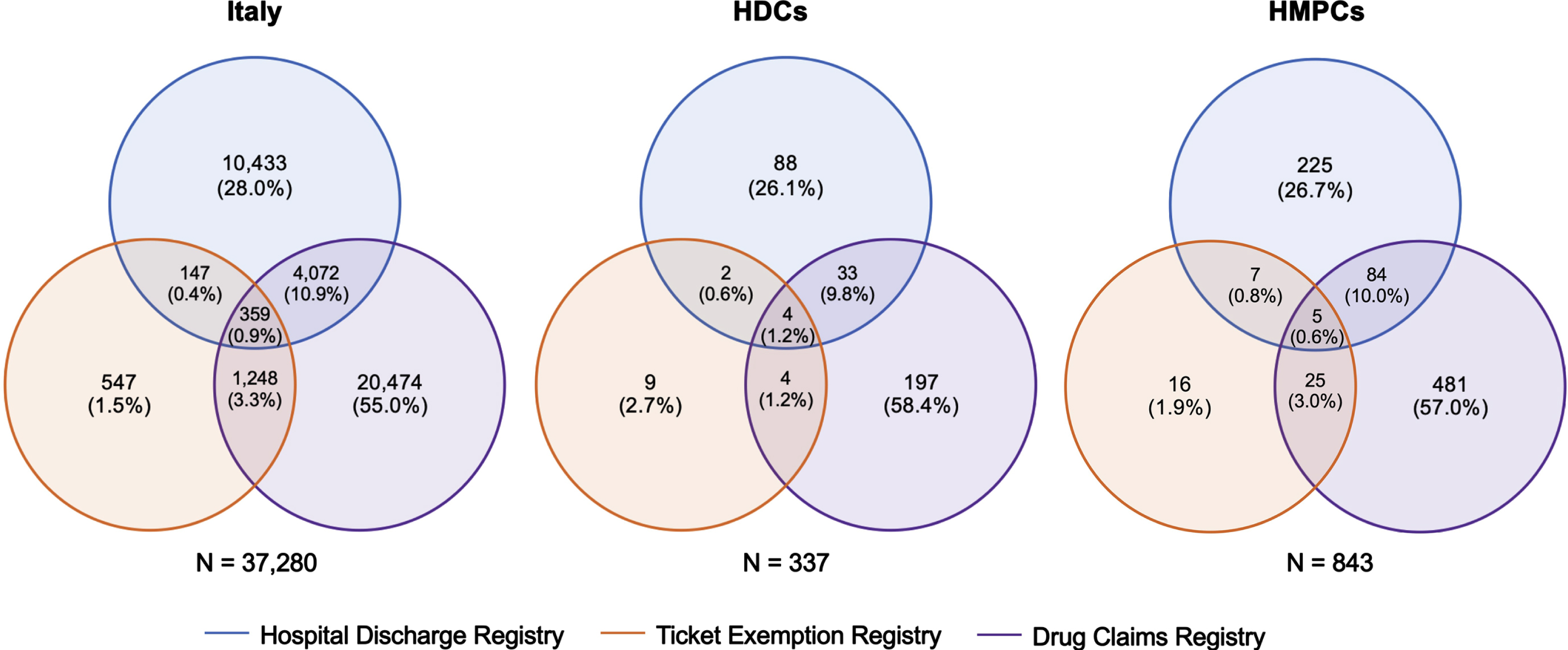
A total of 3,601,809 residents older than 50 years lived in the Lazio region as of December 31, 2018. Based on the adopted case-finding algorithm, 38,460 dementia cases were identified, with a prevalence of 1.07% (95% CI 1.06–1.08). Among people with dementia, 37,280 (96.9%) were born in Italy, 337 (0.9%) were migrants from HDCs, and 843 (2.2%) were migrants from HMPCs (Fig. 1). The number of dementia cases identified in each of the three healthcare and administrative datasets by migrant status and the intersects of these three data sources are shown in Fig. 2. The Drug Claim Registry accounted for most dementia cases in all groups, with an overall unique contribution of more than 50%.
Fig. 1
Study population. HDCs, highly developed countries; HMPCs, high migratory pressure countries.
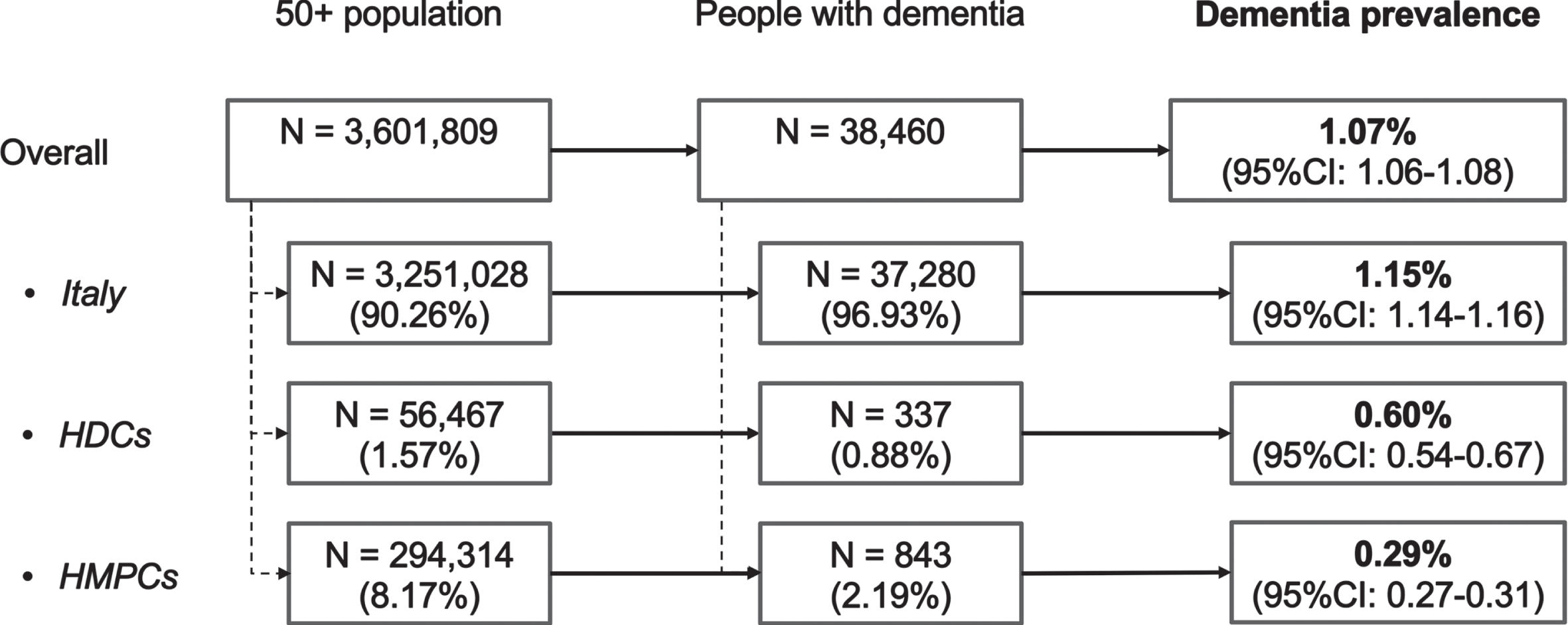
Fig. 2
Venn diagrams showing the number of dementia cases identified in each of the three healthcare and administrative datasets by migrant status and the intersects of these three data sources. HDCs, highly developed countries; HMPCs, high migratory pressurecountries.
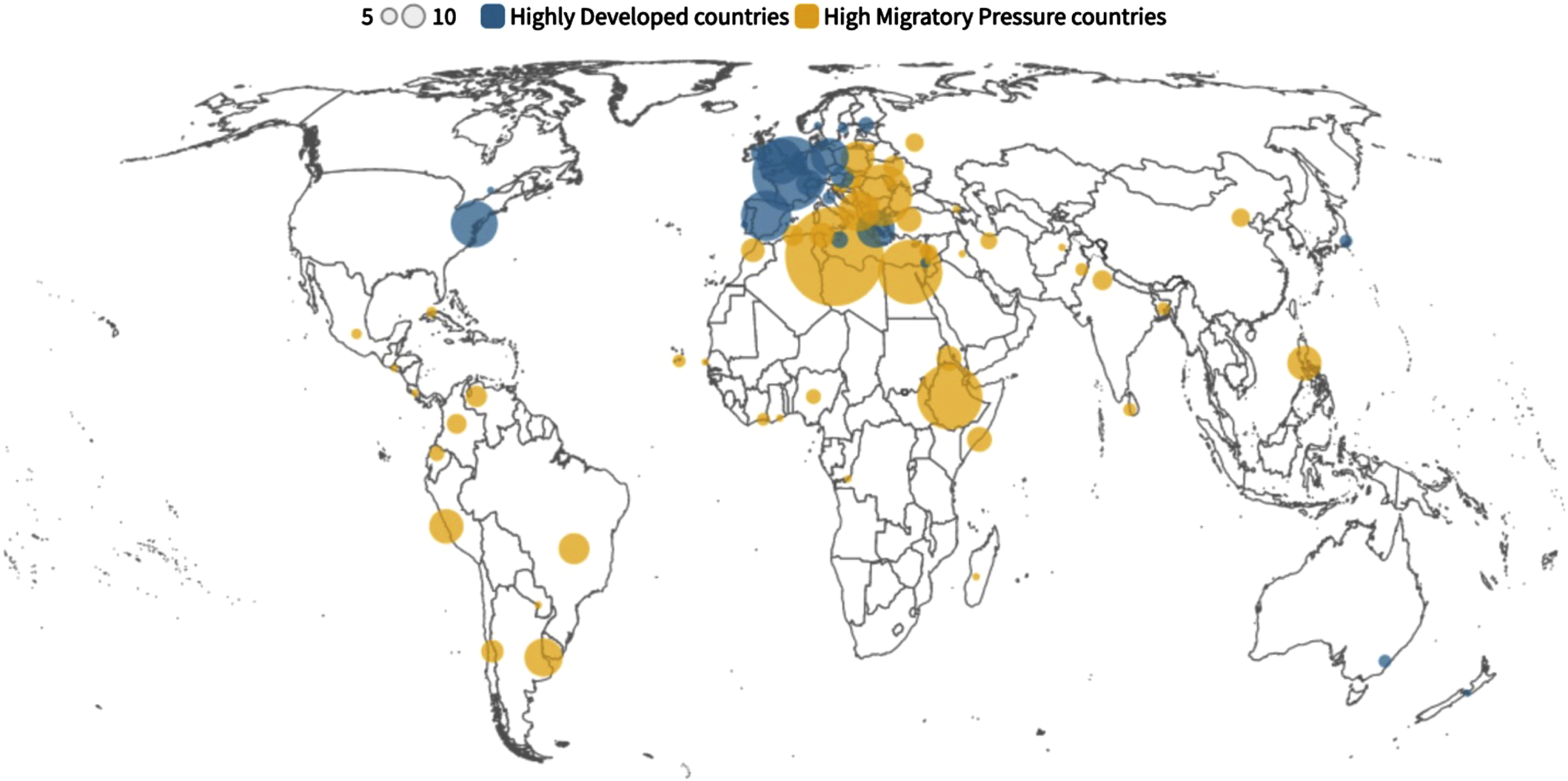
The most common countries of birth of migrants were Libya (n = 171), Tunisia (n = 105), France (n = 98), Ethiopia (n = 75), Egypt (n = 73), Romania (n = 65), and Spain (n = 45) (Fig. 3). Most migrants from HDCs (85.5%) were born in neighboring European countries whereas most migrants from HMPCs (42.3%) came from Northern Africa. Eighty-one different countries of birth were represented in the cohort of migrants with dementia living in the Lazio region (Fig. 3).
Fig. 3
Countries of birth of migrants with dementia living in the Lazio region.
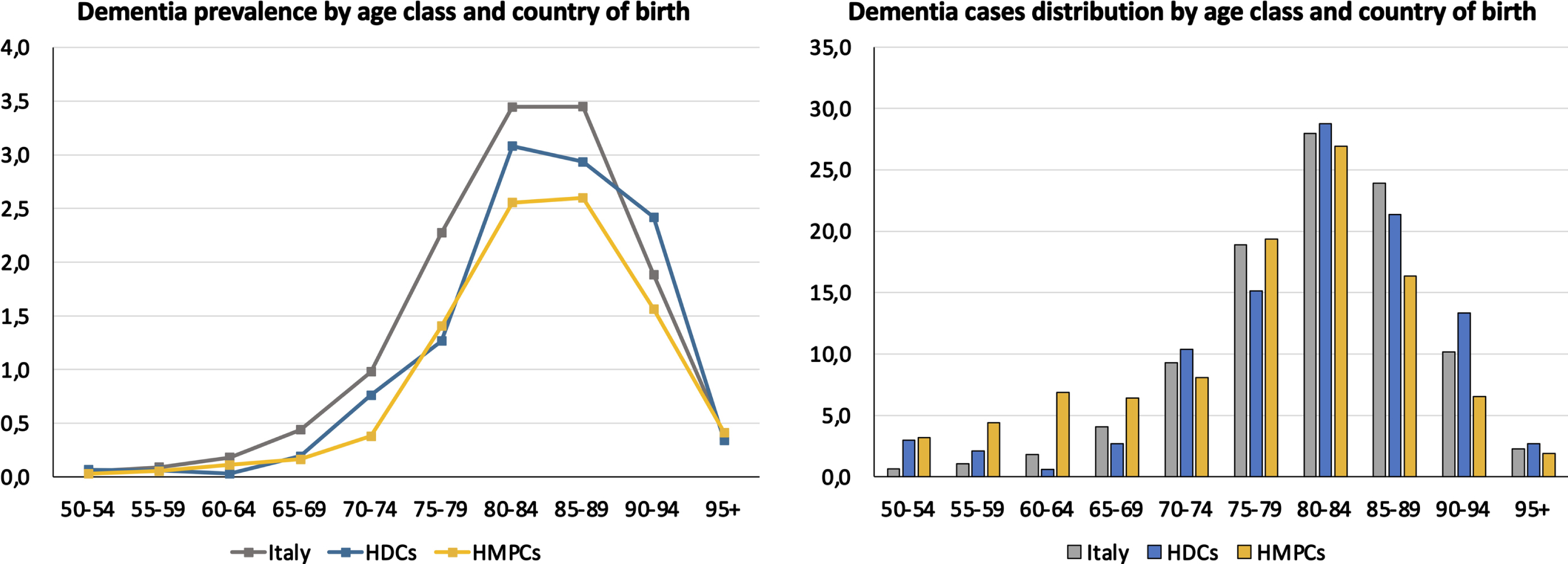
Overall, the crude prevalence estimated through health administrative databases was significantly higher among Italian natives (1.15%, 95% CI 1.14–1.16) as compared with that documented among migrants from HDCs (0.60%, 95% CI 0.54–0.67) and HMPCs (0.29%, 95% CI 0.27–0.31) (Fig. 1). The same pattern was observed for age-standardized prevalence rates that were higher among Italians than among both migrant groups, in both women and men (Table 1). Accordingly, higher age-specific prevalence rates were observed in Italians relative to the two migrant groups in almost all age classes (Fig. 4A). The distribution of cases by age class did not show relevant differences in the three groups except for a higher proportion of cases in younger people among migrants from HMPCs (Fig. 4B). Indeed, the share of participants younger than 65 years was 14.5% in migrants born in HMPCs, 5.6% in migrants from HDCs, and 3.5% in Italian natives.
Table 1
Age-standardized dementia prevalence (per 100 population) by country of birth and gender
| Italians | HDCs | HMPCs | |||||||
| std rate | 95% IC | std rate | 95% IC | std rate | 95% IC | ||||
| Men | 0.75 | 0.73 | 0.76 | 0.63 | 0.50 | 0.80 | 0.48 | 0.42 | 0.54 |
| Women | 1.07 | 1.06 | 1.09 | 0.73 | 0.64 | 0.83 | 0.67 | 0.61 | 0.73 |
| Total | 0.92 | 0.91 | 0.93 | 0.70 | 0.63 | 0.78 | 0.59 | 0.55 | 0.64 |
HDCs, highly developed countries; HMPCs, high migratory pressure countries.
Fig. 4
Dementia prevalence (A) and case distribution (B) by age class in among Italians and migrants from highly developed countries (HDCs) and high migratory pressure countries (HMPCs).
As shown in Table 2, the three groups differed in terms of gender distribution with a higher proportion of women in migrants (HDCs: 77.2%; HMPCs: 69.2%) relative to Italians (64.8%) (p < 0.001). Migrants from HMPCs were significantly younger relative to the other two groups (p < 0.001). No significant between-group differences were found in terms of comorbidities, except for a higher prevalence of gastrointestinal diseases in the HMPCs group (p = 0.04). Migrants with dementia had a lower likelihood of receiving antidementia treatments in contrast with their native counterparts (p = 0.05). Conversely, a similar prescription rate of antipsychotics was documented according to the migrant status. These results remained substantially unchanged when the comparison was restricted to Italians and migrants from HMPCs. Overall, Italians exhibited a greater consumption of most of the considered drugs (especially antihypertensive and lipid-modifying agents, antiplatelet agents, and antidepressants) (Table 3).
Table 2
Sociodemographic and clinical characteristics of people with dementia living in the Lazio region, by country of birth. Data are updated to December 31, 2018
| Italy | HDCs | HMPCs | p | ||||
| (N = 37,280) | (N = 337) | (N = 843) | |||||
| N | % | N | % | N | % | ||
| Gender | < 0.001 | ||||||
| Men | 13,132 | 35.2 | 77 | 22.8 | 260 | 30.8 | |
| Women | 24,148 | 64.8 | 260 | 77.2 | 583 | 69.2 | |
| Age classes | < 0.001 | ||||||
| 50–74 | 6,275 | 16.8 | 63 | 18.7 | 244 | 28.9 | |
| 75–84 | 17,460 | 46.8 | 148 | 43.9 | 390 | 46.3 | |
| 85+ | 13,545 | 36.3 | 126 | 37.4 | 209 | 24.8 | |
| Comorbidities (last 2 years) | |||||||
| Infectious diseases | 1,197 | 3.2 | 8 | 2.4 | 32 | 3.8 | 0.43 |
| Cancer | 1,646 | 4.4 | 15 | 4.5 | 39 | 4.6 | 0.96 |
| Cardiovascular diseases | 10,257 | 27.5 | 88 | 26.1 | 221 | 26.2 | 0.60 |
| Respiratory diseases | 4,035 | 10.8 | 32 | 9.5 | 92 | 10.9 | 0.73 |
| Gastrointestinal diseases | 2,711 | 7.3 | 16 | 4.7 | 75 | 8.9 | 0.04 |
| Genitourinary diseases | 3,661 | 9.8 | 33 | 9.8 | 72 | 8.5 | 0.47 |
| Psychiatric disorders | 6,517 | 17.5 | 61 | 18.1 | 150 | 17.8 | 0.93 |
| Endocrine/metabolic disorders | 5,141 | 13.8 | 42 | 12.5 | 119 | 14.1 | 0.75 |
| Musculoskeletal disorders | 2,012 | 5.4 | 19 | 5.6 | 33 | 3.9 | 0.16 |
| Drugs (last 2 years) | |||||||
| Antidementia drugs | 19,950 | 53.5 | 174 | 51.6 | 416 | 49.3 | 0.05 |
| Antipsychotics | 11,770 | 31.6 | 99 | 29.4 | 245 | 29.1 | 0.21 |
| Antidementia and antipsychotics | 5,728 | 15.4 | 47 | 13.9 | 117 | 13.9 | 0.39 |
HDCs, highly developed countries; HMPCs, high migratory pressure countries.
Table 3
Drug prescriptions for people with dementia living in the Lazio region, by country of birth. Data refer to 2019
| Italy | HDCs | HMPCs | p | ||||
| (N = 37,280) | (N = 337) | (N = 843) | |||||
| N | % | N | % | N | % | ||
| Cardiac therapies | 5,242 | 14.0 | 1 | 0.3 | 104 | 12.2 | < 0.001 |
| Antihypertensive agents | 27,923 | 74.8 | 214 | 63.5 | 532 | 62.7 | < 0.001 |
| Lipid-modifying agents | 13,352 | 35.8 | 101 | 30.0 | 271 | 31.9 | < 0.01 |
| Statins | 11,670 | 31.3 | 88 | 26.1 | 238 | 28.0 | 0.02 |
| Antiplatelet agents | 21,982 | 58.9 | 176 | 52.2 | 413 | 48.6 | < 0.001 |
| Insulin and analogs | 2,472 | 6.6 | 18 | 5.3 | 59 | 6.9 | 0.58 |
| Blood glucose-lowering drugs | 5,991 | 16.0 | 43 | 12.8 | 131 | 15.4 | 0.24 |
| Antidepressants drugs | 14,533 | 38.9 | 117 | 34.7 | 299 | 35.2 | 0.03 |
| Anxiolytics | 72 | 0.2 | 0 | 0.0 | 1 | 0.1 | 0.81 |
| Hypnotics and sedatives | 80 | 0.2 | 0 | 0.0 | 3 | 0.4 | 0.65 |
| Analgesic drugs | 3,725 | 10.0 | 38 | 11.3 | 72 | 8.5 | 0.28 |
HDCs, highly developed countries; HMPCs, high migratory pressure countries.
4DISCUSSION
The main objective of this study was to identify migrants with dementia living in the Lazio region from administrative databases and describe their health characteristics compared to Italian-born residents. The study represents the first attempt to compare the prevalence of dementia and its clinical correlates between natives and migrants living in an Italian region. Based on the data collected in healthcare administrative databases, migrants currently account for nearly 3% of overall people with dementia living in the region. Migrants, especially those from low- and middle-income countries, exhibited a lower age-standardized prevalence of registered dementia and lower access to dementia treatment than Italians.
These findings align with those from previous registry-based studies conducted in Scandinavian countries (i.e., Norway, Denmark, and Sweden) documenting lower prevalence of dementia and use of antidementia therapies in migrants relative to their native counterparts [17–19]. These results are likely suggestive of underdiagnosis and undertreatment of dementia in migrants rather than of a more favorable risk profile among individuals with a migration background. Indeed, higher prevalence rates have repeatedly been observed in migrants and minority groups relative to natives in population-based studies adopting culture-sensitive cognitive tools and/or procedures to ascertain the presence of dementia at the community level [23–25]. Such discrepancy between registry-based and population-based studies implies that a relevant proportion of dementia cases remain underdiagnosed in migrants and, thus, not captured by healthcare and administrative records [26]. Accordingly, there is growing evidence that migrants with cognitive impairment are less likely to access healthcare services due to cultural preferences, reluctance to seek medical help, poor health literacy, and logistic and structural barriers [27–29]. Therefore, they are still poorly represented in those care settings where dementia is diagnosed and managed [11–13]. For instance, based on the findings of a recent survey of Italian memory clinics in which 39 of the 52 facilities located in the Lazio region participated, only 358 migrants (i.e., less than one-third of those captured by regional healthcare registries) were referred to these services in 2019 [13]. As in previous studies, the underestimation of dementia seems particularly relevant in migrants from low- and middle-income countries rather than among migrants from neighboring highly developed ones. A lower level of education and health literacy, a higher degree of cultural diversity, and a greater difficulty in \nobreak navigating the health system in the host country may contribute to such discrepancy.
Other possible explanations, often used to interpret the evidence of a lower prevalence of disease in migrants, seem less plausible for our results. The similar morbidity pattern documented in migrants and natives does not support the hypothesis of a “healthy migrant effect” (i.e., an empirically observed advantage in terms of mortality and health status among migrants relative to the majority population in host countries), that has never been clearly documented for dementia [30]. In particular, native and foreign-born participants had a similar prevalence of vascular risk factors that have sometimes been considered responsible for an unbalanced prevalence of dementia in some ethnic groups [24]. Moreover, health vulnerability and resilience change over time and the theoretical migrants’ health advantage may deteriorate with time in the host country due to multiple socio-economic factors and individual characteristics (e.g., educational level, working conditions, language skills, and legal status). In addition, no data was available to pursue the hypothesis of a “salmon bias” (i.e., the observed tendency of migrants to return to their origin destinations when they are in poor health or before death) [31].
The facts and figures emerging from our analysis should be relevant for healthcare professionals and policymakers. The clinical approach and public health response to dementia must be inclusive for a relevant and growing number of patients with a history of migration living in our societies. The cultural diversity of people living with dementia requires using cross-cultural cognitive assessment tools, resorting to dedicated professional figures (e.g., cultural mediators and professional interpreters), and providing culturally adapted information to patients and their caregivers [32–34]. Clinicians should be trained in how to provide culturally competent care and support. At the local level, it may be important to establish relationships with migrant communities (at least the most represented ones) to raise awareness about dementia, how to use dedicated resources, and the importance of prevention.
The present study has some limitations and leaves some unanswered questions. Longitudinal studies are needed to explore the incidence of dementia in migrants, the role of putative risk factors, and the health outcomes of affected individuals (e.g., mortality, hospitalization, access to emergency departments, use and discontinuation of antidementia therapies and antipsychotics). This is crucial to achieving a better understanding of their risk profile and health needs and designing tailored healthcare pathways. Our analysis is probably not representative of the entire population of migrants with cognitive impairment living in the Lazio region. Indeed, some migrant groups (e.g., refugees, asylum-seekers, and irregular migrants) have limited access to the healthcare system and, thus, may not be captured by healthcare and administrative registries. Moreover, even if residing in the region, some individuals may suffer from unequal access to health care services and may not be fully tracked in administrative databases. Therefore, the number of migrants with dementia at the regional level is likely higher than that presently documented. In addition, different figures could have emerged in other Italian regions due to the different structure and composition of the migrant population at the local level [13]. Finally, the definition of participants’ migrant status was entirely based on their place of birth (as commonly reported in the literature). However, this approach ignores that some foreign-born people may have Italian language, culture, and attitudes (for instance, those born in neighboring European countries and former Italian colonies like Libya and Ethiopia). In this regard, our analysis failed to consider some determinants (e.g., education, socioeconomic status, social network, living condition, lifestyle) that have a well-established influence on the individual’s risk for dementia and the prevalence of this condition [35, 36]. For instance, these factors may partially explain the observed high prevalence of dementia in younger migrants from low- and middle-income countries.
In conclusion, this study confirms how data routinely collected in regional healthcare and administrative databases can support the identification of migrants living with dementia. However, it is likely that studies based on health administrative databases provide an underestimation of the phenomenon due to high rates of underdiagnosis in migrants. The present findings extend previous observations from Scandinavian countries, suggesting that the underdiagnosis and undertreatment of dementia in migrants is a general, rather than localized, issue across European countries. It is therefore imperative to identify the barriers that preclude migrants’ access to the diagnosis and treatment of dementia. It is also necessary to investigate the risk of dementia in diverse populations to implement targeted and culturally situated preventive policies.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The present study was supported by a research grant of the Italian Ministry of Health for the project “Dementia in immigrants and ethnic minorities living in Italy: clinical-epidemiological aspects and public health perspectives” (ImmiDem) (GR-2016-02364975).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Marco Canevelli is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-221146.
REFERENCES
[1] | GBD 2019 Collaborators ((2021) ) Global mortality from dementia: Application of a new method and results from the Global Burden of Disease Study 2019, Alzheimers Dement (N Y) 7: , e12200. |
[2] | GBD 2016 Dementia Collaborators ((2019) ) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurol 18: , 88–106. |
[3] | World Health Organization (2017) Global action plan on the public health response to dementia 2017-2025. http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/. Accessed on March 24, 2021. |
[4] | World Health Organization (2021) Global status report on the public health response to dementia. 2021. https://www.who.int/publications/i/item/9789240033245. Accessed on March 24, 2021. |
[5] | United Nations Department of Economic and Social Affairs, Population Division (2020) International Migrant Stock 2020. https://www.un.org/development/desa/pd/content/international-migrant-stock. Accessed on April 10, 2021. |
[6] | Canevelli M , Lacorte E , Cova I , Zaccaria V , Valletta M , Raganato R , Bruno G , Bargagli AM , Pomati S , Pantoni L , Vanacore N ((2019) ) Estimating dementia cases amongst migrants living in Europe, Eur J Neurol 26: , 1191–1199. |
[7] | Canevelli M , Zaccaria V , Lacorte E , Cova I , Remoli G , Bacigalupo I , Cascini S , Bargagli AM , Pomati S , Pantoni L , Vanacore N ((2020) ) Mild cognitive impairment in the migrant population living in Europe: An epidemiological estimation of the phenomenon, J Alzheimers Dis 73: , 715–721. |
[8] | Cova I , Del Tedesco F , Maggiore L , Pantoni L , Pomati S ((2020) ) Cognitive disorders in migrants: Retrospective analysis in a Center for Cognitive Disorders and Dementia in Milan, Aging Clin Exp Res 32: , 535–538. |
[9] | Chen Y , Lebouvier T , Skrobala E , Volpe-Gillot L , Huvent-Grelle D , Jourdan N , Leroy M , Richard F , Pasquier F and the Meotis network((2020) ) Twenty-year trends in patient referrals throughout the creation and development of a regional memory clinic network, Alzheimers Dement (N Y) 6: , e12048. |
[10] | Chen Y , Caramelli P ((2022) ) Dementia among international migrants: An urgent call for better care, Eur J Neurol 29: , 1865–1866. |
[11] | Nielsen TR , Vogel A , Riepe MW , de Mendonça A , Rodriguez G , Nobili F , Gade A , Waldemar G ((2011) ) Assessment of dementia in ethnic minority patients in Europe: A European Alzheimer’s Disease Consortium survey, Int Psychogeriatr 23: , 86–95. |
[12] | Brown S , Livingston G , Mukadam N ((2021) ) A national memory clinic survey to assess provision for people from diverse ethnic backgrounds in England and Wales, Int J Environ Res Public Health 18: , 1456. |
[13] | Canevelli M , Cova I , Remoli G , Bacigalupo I , Salvi E , Maestri G , Nicotra A , Valletta M , Ancidoni A , Sciancalepore F , Cascini S , Bargagli AM , Pomati S , Pantoni L , Vanacore N ImmiDem Study Network; ImmiDem Study Group((2022) ) A nationwide survey of Italian Centers for Cognitive Disorders and Dementia on the provision of care for international migrants, Eur J Neurol 29: , 1892–1902. |
[14] | WHO Regional Office for Europe (2020) Collection and integration of data on refugee and migrant health in the WHO European Region. https://apps.who.int/iris/handle/0665/337694. Accessed on April 21, 2022. |
[15] | Francesconi P , Gini R , Roti L , Bartolacci S , Corsi A , Buiatti E ((2007) ) The Tuscany experimental registry for Alzheimer’s disease and other dementias: How many demented people does it capture? , Aging Clin Exp Res 19: , 390–393. |
[16] | Jaakkimainen RL , Bronskill SE , Tierney MC , Herrmann N , Green D , Young J , Ivers N , Butt D , Widdifield J , Tu K ((2016) ) Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: A validation study using family physicians’ electronic medical records, J Alzheimers Dis 54: , 337–349. |
[17] | Diaz E , Kumar BN , Engedal K ((2015) ) Immigrant patients with dementia and memory impairment in primary health care in Norway: A national registry study, Dement Geriatr Cogn Disord 39: , 321–331. |
[18] | Stevnsborg L , Jensen-Dahm C , Nielsen TR , Gasse C , Waldemar G ((2016) ) Inequalities in access to treatment and care for patients with dementia and immigrant background: A Danish Nationwide Study, J Alzheimers Dis 54: , 505–514. |
[19] | Wändell P , Carlsson AC , Li X , Gasevic D , Sundquist J , Sundquist K ((2019) ) Dementia in immigrant groups: A cohort study of all adults 45 years of age and older in Sweden, Arch Gerontol Geriatr 82: , 251–258. |
[20] | Canevelli M , Lacorte E , Cova I , Cascini S , Bargagli AM , Angelici L , Giusti A , Pomati S , Pantoni L , Vanacore N ImmiDem Study Group ((2020) ) Dementia among migrants and ethnic minorities in Italy: Rationale and study protocol of the ImmiDem project, BMJ Open 10: , e032765. |
[21] | Trappolini E , Marino C , Agabiti N , Giudici C , Davoli M , Cacciani L ((2021) ) Mortality differences between migrants and Italians residing in Rome before, during, and in the aftermath of the great recession. A longitudinal cohort study from 2001 to 2015, BMC Public Health 21: , 2112. |
[22] | Pacelli B , Zengarini N , Broccoli S , Caranci N , Spadea T , Di Girolamo C , Cacciani L , Petrelli A , Ballotari P , Cestari L , Grisotto L , Giorgi Rossi P IN-LiMeS Group ((2016) ) Differences in mortality by immigrant status in Italy. Results of the Italian Network of Longitudinal Metropolitan Studies, Eur J Epidemiol 31: , 691–701. |
[23] | Livingston G , Leavey G , Kitchen G , Manela M , Sembhi S , Katona C ((2001) ) Mental health of migrant elders–the Islington study, Br J Psychiatry 179: , 361–366. |
[24] | Adelman S , Blanchard M , Rait G , Leavey G , Livingston G ((2011) ) Prevalence of dementia in African–Caribbean compared with UK-born White older people: Two-stage cross-sectional study, Br J Psychiatry 199: , 119–125. |
[25] | Parlevliet JL , Uysal-Bozkir Ö , Goudsmit M , van Campen JP , Kok RM , Ter Riet G , Schmand B , de Rooij SE ((2016) ) Prevalence of mild cognitive impairment and dementia in older non-western immigrants in the Netherlands: A cross-sectional study, Int J Geriatr Psychiatry 31: , 1040–1049. |
[26] | Selten JP , Termorshuizen F , van Sonsbeek M , Bogers J , Schmand B ((2021) ) Migration and dementia: A meta-analysis of epidemiological studies in Europe, Psychol Med 51: , 1838–1845. |
[27] | Mukadam N , Cooper C , Livingston G ((2011) ) A systematic review of ethnicity and pathways to care in dementia, Int J Geriatr Psychiatry 26: , 12–20. |
[28] | Sagbakken M , Spilker RS , Nielsen TR ((2018) ) Dementia and immigrant groups: A qualitative study of challenges related to identifying, assessing, and diagnosing dementia, BMC Health Serv Res 18: , 910. |
[29] | Cooper C , Tandy AR , Balamurali TBS , Livingston G ((2010) ) A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research, Am J Geriatr Psychiatry 18: , 193–203. |
[30] | Di Napoli A , Rossi A , Alicandro G , Ventura M , Frova L , Petrelli A ((2021) ) Salmon bias effect as hypothesis of the lower mortality rates among immigrants in Italy, Sci Rep 11: , 8033. |
[32] | Alzheimer Europe (2018) The development of intercultural care and support for people with dementia from minority ethnic groups. https://www.alzheimer-europe.org/resources/publications/2018-alzheimer-europe-reportdevelopment-intercultural-care-and-support. Accessed on March 22, 2021. |
[33] | Gove D , Nielsen TR , Smits C , Plejert C , Rauf MA , Parveen S , Jaakson S , Golan-Shemesh D , Lahav D , Kaur R , Herz MK , Monsees J , Thyrian JR , Georges J ((2021) ) The challenges of achieving timely diagnosis and culturally appropriate care of people with dementia from minority ethnic groups in Europe, Int J Geriatr Psychiatry 36: , 1823–1828. |
[34] | Franzen S , European Consortium on Cross-Cultural Neuropsychology (ECCroN) Watermeyer TJ , Pomati S , Papma JM , Nielsen TR , Narme P , Mukadam N , Lozano-Ruiz Á , Ibanez-Casas I , Goudsmit M , Fasfous A , Daugherty JC , Canevelli M , Calia C , van den Berg E , Bekkhus-Wetterberg P ((2022) ) Cross-cultural neuropsychological assessment in Europe: Position statement of the European Consortium on Cross-Cultural Neuropsychology (ECCroN), Clin Neuropsychol 36: , 546–557. |
[35] | Yaffe K , Falvey C , Harris TB , Newman A , Satterfield S , Koster A , Ayonayon H , Simonsick E Health ABC Study ((2013) ) Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. , f, BMJ 347: , 7051. |
[36] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission, Lancet 396: , 413–446. |



