Is Dementia Associated with COVID-19 Mortality? A Multicenter Retrospective Cohort Study Conducted in 50 Hospitals in Germany
Abstract
Background:
Dementia has been identified as a major predictor of mortality associated with COVID-19.
Objective:
The objective of this study was to investigate the association between dementia and mortality in COVID-19 inpatients in Germany across a longer interval during the pandemic.
Methods:
This retrospective study was based on anonymized data from 50 hospitals in Germany and included patients with a confirmed COVID-19 diagnosis hospitalized between March 11, 2020 and July, 20, 2022. The main outcome of the study was the association of mortality during inpatient stays with dementia diagnosis, which was studied using multivariable logistic regression adjusted for age, sex, and comorbidities as well as univariate logistic regression for matched pairs.
Results:
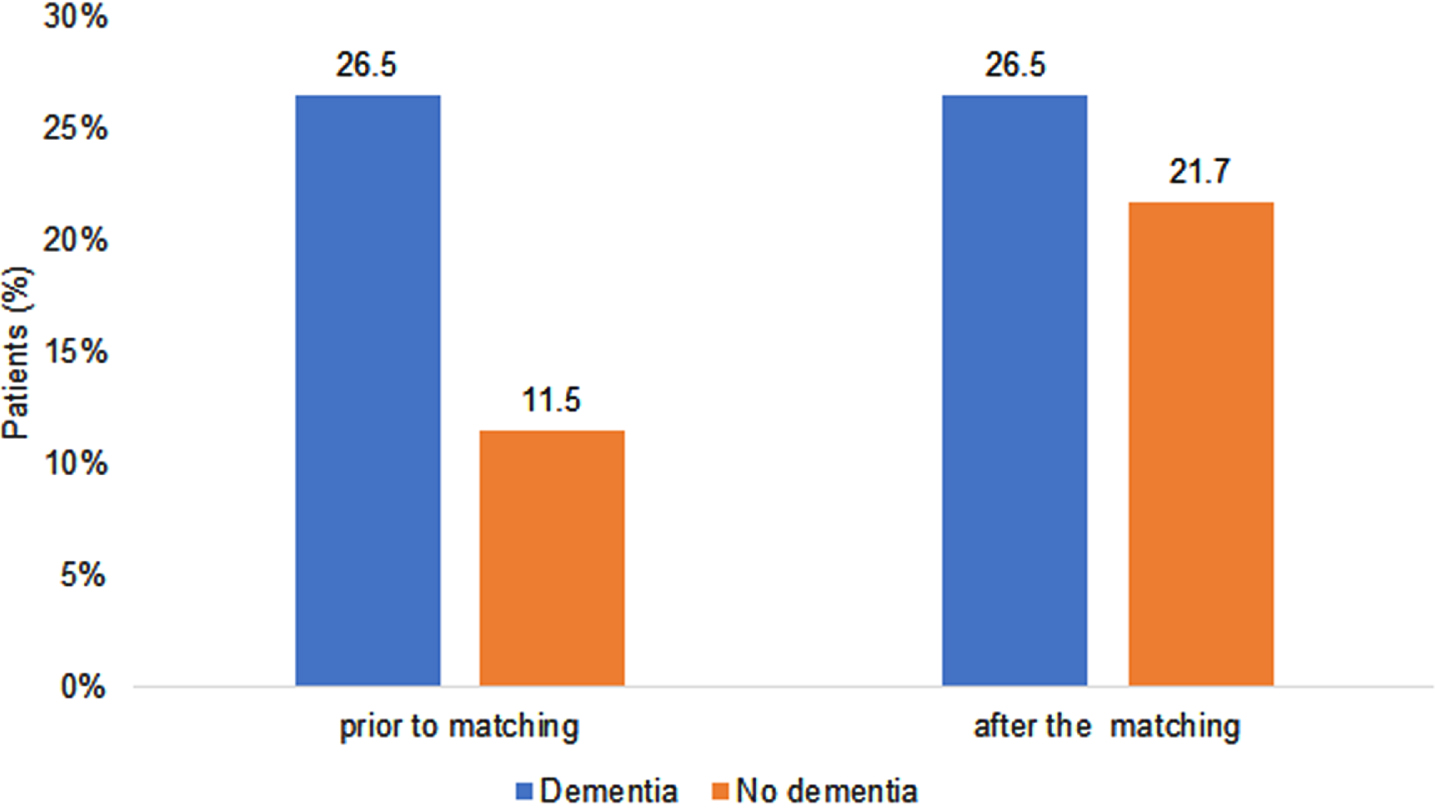
Of 28,311 patients diagnosed with COVID-19, 11.3% had a diagnosis of dementia. Prior to matching, 26.5% of dementia patients and 11.5% of non-dementia patients died; the difference decreased to 26.5% of dementia versus 21.7% of non-dementia patients within the matched pairs (n = 3,317). This corresponded to an increase in the risk of death associated with dementia (OR = 1.33; 95% CI: 1.16–1.46) in the univariate regression conducted for matched pairs.
Conclusion:
Although dementia was associated with COVID-19 mortality, the association was weaker than in previously published studies. Further studies are needed to better understand whether and how pre-existing neuropsychiatric conditions such as dementia may impact the course and outcome of COVID-19.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in December 2019 and has spread rapidly across the world [1]. Since December 2019, around 597 million people have been diagnosed with COVID-19 worldwide, while approximately six million individuals have died as a result of the disease [2]. With the succession of SARS-CoV-2 variants and the progression of the vaccination campaign, the proportion of COVID-19 patients with hospital admissions and COVID-19 related mortality has fluctuated strongly over the course of the pandemic and has also varied widely between countries [3]. Hospital treatment was required in about 10–20% of COVID-19 patients, with this proportion decreasing overtime [4].
Kloka et al. analyzed 561,379 hospitalized COVID-19 patients in Germany, 30% of whom were in the age group 80–85 years [3]. Previous research has shown that a diagnosis of pre-existing dementia is a major risk factor for COVID-19-associated mortality [5–13]. For example, one study of adults aged 65 years or older living in the United States found that dementia patients had a 13% greater risk of death than non-dementia patients, and this difference was even greater among nursing home residents [14]. In another study from Italy using data from 627 hospitalized patients with COVID-19 pneumonia, the mortality rate was 62% among patients with dementia compared to 26% in patients without dementia. Even after adjusting for age, dementia was strongly (HR: 1.84) associated with an increased mortality risk [6]. In a study conducted in South Korea, Alzheimer’s disease (AD) was one of the main predictors of COVID-19 mortality [7]. A large study from the United Kingdom (UK) including 269,070 COVID-19 patients found that dementia diagnosis was associated with a 3.5 times higher risk of hospitalization and a 7.3 times higher risk of death [8]. In a study from New York, dementia was associated with a 2.0 times higher risk of death but not with the increased application of mechanical ventilation [10]. Meanwhile, a study from Turkey including 16,942 hospitalized adults with COVID-19 found that dementia was associated with mortality (Odds Ratio (OR) = 1.63 in the age group 60–79 and OR = 1.47 in the age group 80+) [11]. In the study of Dutch nursing home residents with COVID-19 by Rutten et al., dementia was associated with a 1.3 times higher risk of 30-day mortality [15].
Several meta-analyses have confirmed the impact of dementia on mortality in COVID-19 patients. A meta-analysis of 9 studies published in 2020 showed that the mortality rate of individuals with COVID-19 and dementia was 5.1 times higher than that of individuals with COVID-19 and no dementia [16]. In another meta-analysis based on data from 10 studies, dementia was associated with a 1.8 times higher mortality in COVID-19 patients [17].
Although the above studies have unanimously identified dementia as a risk factor for a fatal course of COVID-19, they are subject to several limitations that need to be taken into account. One major limitation is that most of the studies mentioned above were conducted during the first months of the COVID-19 pandemic. Hence, it is possible that the effects of dementia on COVID-19 mortality may have changed over time, especially after vaccines became available and were broadly administered and as different variants of SARS-CoV-2 evolved.
In this retrospective study including a large number of patients treated in several large hospitals in Germany over a long interval during the pandemic, we verify the association of dementia diagnosis with the risk of mortality as a result of COVID-19.
METHODS
Study population
This retrospective study based on anonymized electronic medical data from public healthcare service hospitals across Germany, all belonging to the same hospital group, included 28,311 patients with a confirmed COVID-19 diagnosis (ICD-10 U07.1) hospitalized between March 11, 2020 and July 20, 2022.
Initially, data were collected as part of the “CORONA Germany”—Clinical Outcome and Risk in hospitalized COVID-19 patients—study, a multicenter observational, prospective, epidemiological cohort study. All data collected from the data repository were validated using the hospital network’s quality management database. The initial results of the prospective study have been published previously [18, 19]. The study was approved by the ethics committee of the General Medical Council (Aerztekammer) for the City of Hamburg and the ethics committee of the General Medical Council (Aerztekammer) for the City of Munich.
For the present study, demographic data (age, sex), COVID-19 relevant data (ventilation, mortality), time of COVID-19 diagnosis, and co-diagnosis data were used.
Study outcome
The main outcome of the study was the association of dementia diagnosis with an increased risk of death during the hospital stay.
Statistical analyses
First, baseline characteristics of study patients were shown as proportions (sex, co-morbidities, probable COVID-19 variant) or mean (SD) (age) separately for patients with and without dementia. These co-diagnoses included cancer (ICD-10: C00–C97), diabetes mellitus (ICD-10: E10–E14), lipid metabolism disorder (ICD-10: E78), obesity (ICD-10: E66), heart failure (ICD-10: I50), ischemic heart disease (ICD-10: I20–I25), cerebrovascular disease (ICD-10: I60–I69), and cirrhosis of the liver (ICD-10: K70.3, K74) as these diagnoses are known to be common causes of mortality.
In the next step, the association between dementia and death was studied using multivariable logistic regression adjusted for age, sex, cancer, diabetes mellitus, lipid metabolism disorder, obesity, heart failure, ischemic heart disease, cerebrovascular disease, cirrhosis of the liver, and probable COVID-19 variant. Based on the dominance of the respective variants, the latter was defined as non-omicron (all cases from March 2020 to December 2021) or omicron (all cases from January 2022 to July 2022) [20, 21]. The effects of AD, vascular dementia (VaD), and undefined dementia were also tested in a sub-analysis. The results of the logistic regression analyses are presented as odds ratios (ORs) with 95% confidence intervals (CIs).
Finally, individuals without dementia were matched to those with dementia using propensity scores based on sex, age, the aforementioned comorbidities, and probable COVID-19 variant. Univariate conditional logistic regression was conducted for matched pairs to study the association between dementia and death.
In sensitivity analyses, we retrospectively followed dementia patients and matched non-dementia patients till death or end of hospital stay and displayed cumulative mortality using Kaplan-Meier curves. Finally, a Cox regression model was used to estimate the association between dementia and time to death.
P values < 0.05 were considered statistically significant. All analyses were done using R version 4.2.0 (2022-04-22)
RESULTS
Of 28,311 patients diagnosed with COVID-19, 3,317 (11.3%) had a dementia diagnosis and 24,994 (88.3%) had no dementia diagnosis. Among dementia patients, 67% had undefined dementia, 18% AD, and 15% VaD. As expected, patients with dementia were much older (mean (standard deviation) age: 83 (7) years versus 63 (21) years), more often female (53.9% versus 48.0%), and had a higher prevalence of comorbidities (Table 1). After propensity score matching, 3,317 patients with dementia and 3,317 patients without dementia with the same age, sex, comorbidity structure, and probable COVID-19 variant were available for analysis.
Table 1
Baseline characteristics of study patients with and without dementia diagnosis
| Variable | Patients without | Patients with | p** |
| dementia | dementia | ||
| (N = 24,994)* | (N = 3,317)* | ||
| Male | 12,995 (52.0) | 1,529 (46.1) | <0001 |
| Female | 11,969 (48.0) | 1,788 (53.9) | |
| Unknown | 30 | 0 | |
| Age (Mean, SD) | 63 (21) | 83 (7) | <0001 |
| Cancer | 1,421 (5.7) | 100 (3.0) | <0001 |
| Diabetes mellitus | 5,505 (22.0) | 881 (26.6) | <0001 |
| Lipid metabolism disorder | 3,724 (14.9) | 612 (18.5) | <0001 |
| Obesity | 1,432 (5.7) | 75 (2.3) | <0001 |
| Heart failure | 3,448 (13.8) | 629 (19.0) | <0001 |
| Ischemic heart disease, | 3,378 (13.5) | 526 (15.9) | <0001 |
| Cerebrovascular disease | 1,671 (6.7) | 483 (14.6) | <0001 |
| Cirrhosis of the liver | 236 (0.9) | 19 (0.6) | 0.033 |
| Omicron variant | 10,295 (41.2) | 1,367 (41.2) | 0.980 |
| No omicron variant | 14,699 (58,8) | 1,950 (58.8) |
*Data are presented as absolute numbers and percentages unless otherwise specified. **Welch Two Sample t-test; two-sample test for equality of proportions.
Figure 1 shows the prevalence of death in patients with and without dementia. Prior to matching, 26.5% of dementia patients and 11.5% of non-dementia patients died; the difference was smaller within the matched pairs, with 26.5% of dementia versus 21.3% of non-dementia patients dying (Fig. 1).
Fig. 1
Proportion of patients with COVID-19-related mortality depending on dementia diagnosis.
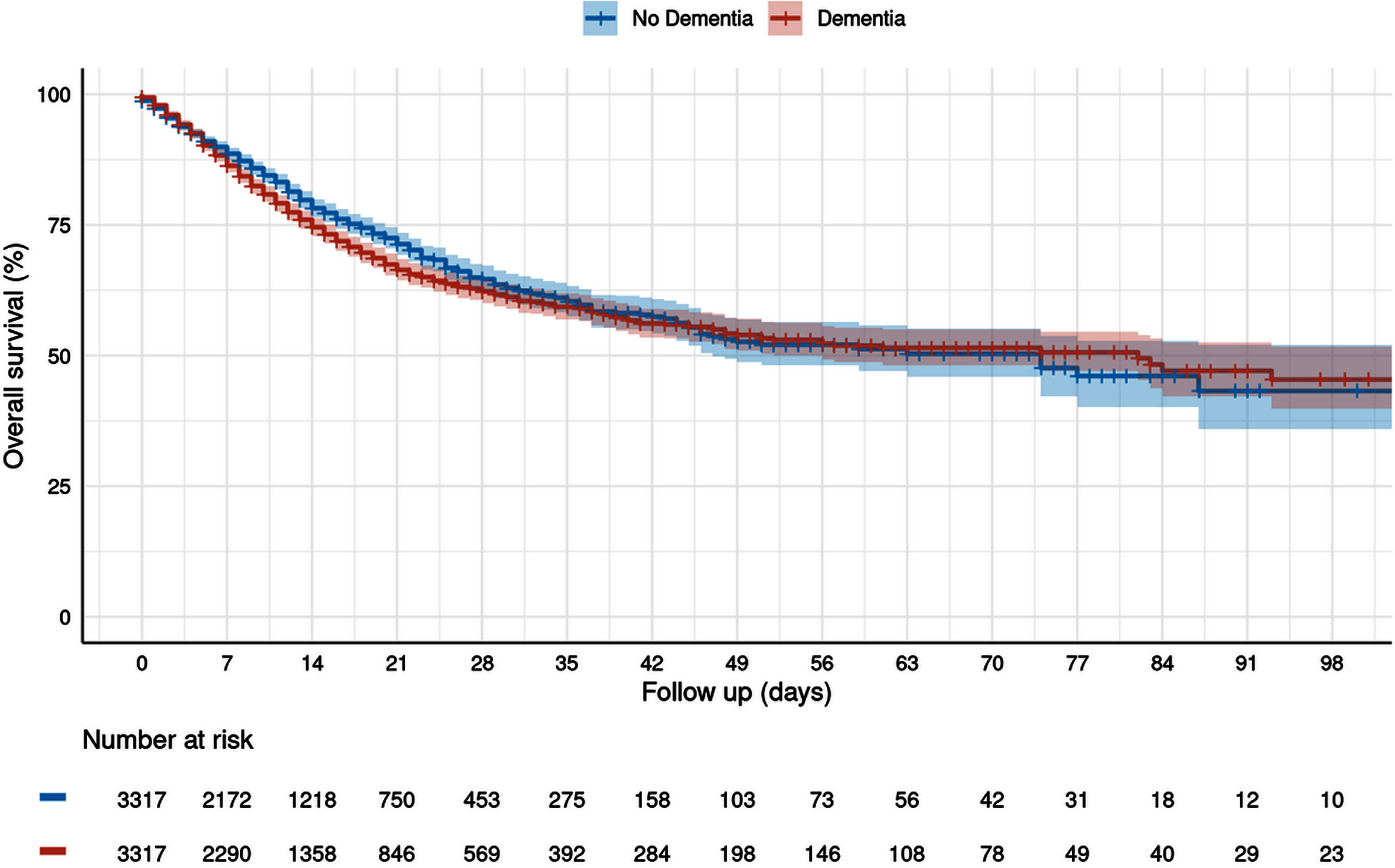
The Kaplan Meier curves are shown in Fig. 2. There were no significant differences in the overall survival between dementia and non-dementia patients both 30 and 90 days after hospital admission.
Fig. 2
Cumulative mortality during the hospital stay depending on dementia diagnosis (Kaplan-Meier curves).
There was a strong relationship between death and the application of ventilation. In total, 3,074 patients (10.9%) received ventilation, of whom 40.0% died; of the 25,237 patients without ventilation, 10.0% died. In addition, dementia patients were rarely ventilated (127/3,317; 3.8%) and the proportion of dementia patients who died despite ventilation was much higher (79/127; 62.2%) than in the total population.
In the multivariable logistic regression, dementia was associated with an 84% (OR = 1.84; 95% CI: 1.52–2.24) increase in the risk of death. In the univariate logistic regression conducted for matched pairs, dementia was associated with a 33% (OR = 1.33; 95% CI: 1.16–1.53) increase in the risk of death (Table 2). Finally, in the univariate Cox regression, dementia was associated with a 12% (HR: 1.12; 95% CI: 1.01–1.23) increase in the risk of death (Table 2).
Table 2
Association between dementia and death due to COVID-19 in hospitalized patients
| Model | Design | Number of | Odds Ratio (95% CI) | p |
| patients | or Hazard | |||
| Ratio (95% CI) | ||||
| Multivariable logistic regression model adjusted for age, sex, cancer, diabetes mellitus, lipid metabolism disorder, obesity, heart failure, ischemic heart disease, cerebrovascular disease, cirrhosis of the liver, and COVID-19 variant | Without matching | 28,311 | OR: 1.84 (1.52–2.24) | <0.001 |
| Univariate logistic regression | Matched pairs | 6,634 | OR: 1.30 (1.16–1.46) | <0.001 |
| Univariate Cox regression | Matched pairs | 6,634 | HR: 1.12 (1.01–1.23) | 0.031 |
In the multivariable logistic regression, there was a significant association between undefined dementia and mortality (OR: 2.07; 95% CI: 1.61–2.65), as well as VaD and mortality (OR: 1.67; 95% CI: 1.14–2.47). The association between AD and mortality was positive but not significant (OR: 1.44; 95% CI: 0.91–2.29).
The multivariable logistic regression model indicated that other variables were strongly associated with COVID-19 mortality. Age (OR = 6.96 per year), non-omicron variant (OR = 2.55), obesity (OR = 2.07), cirrhosis of the liver (OR = 2.66), and cancer (OR = 2.13), were variables which had a stronger association with mortality than dementia.
DISCUSSION
This retrospective study including more than 28,000 COVID-19 patients treated in 50 Asklepios hospitals in Germany between March 2020 and July 2022 showed that mortality was higher in those diagnosed with dementia than in those without dementia. Dementia was associated with an increased mortality risk, but the association was weaker than that reported in the majority of previous publications. To the best of the authors’ knowledge, this is one of the first studies to have investigated the association between dementia and COVID-19 mortality using data collected for more than two years and applying two different statistical methods in parallel.
Recently, a substantial body of research has focused on the impact of dementia on COVID-19-related mortality. Rutten et al. reported a risk increase for 30-day mortality for dementia patients [15] that was similar to our findings for the association between dementia and mortality in matched pairs. In the study by Zhang et al., patients with AD had significantly (20%) (OR: 1.20) higher odds of dying from COVID-19 than patients without AD [22]. However, in most published studies, dementia was associated with an increased mortality risk, whereby, depending on the study setting, the risk increase was up to five times [16].
There are several hypotheses that may explain the discrepancy between the findings of the published literature and the present study. First, COVID-19 mortality has decreased since the beginning of the pandemic [23, 24]. Second, pre-existing conditions associated with an impaired functional state and a poor rehabilitative outlook, such as dementia, may elevate the threshold for the indication of ventilation or the living will of the respective patient may explicitly exclude intensive care measures in such a situation [25]. Accordingly, the proportion of patients treated with ventilation was much lower among dementia patients.
Nevertheless, there is a lack of data on the effects of different dementia types on COVID-19 mortality. In the study by Yu et al., a diagnosis of frontotemporal dementia (OR 16.0) and AD (OR 4.2) but not VaD was associated with a higher risk of death from COVID-19 [26]. In the study by Matias-Guiu et al., a diagnosis of AD was independently associated with a higher risk of death, but a diagnosis of frontotemporal dementia was not [27]. In our study, the association between VaD and COVID-19 mortality was stronger than the association between AD and COVID-19, although dementia type was not known for the majority of dementia patients (undefined dementia). Interestingly, it has been demonstrated that the genes associated with Alzheimer’s disease (APOE and BIN1) in patients who are at risk of developing the condition or who already suffer from it are responsible for COVID-19 severity and even death in these patients [28, 29].
Two major strengths of this study are the large sample size (n = 28,311) and the inclusion of patients diagnosed with COVID-19 across a long period of the pandemic from March 2020 to July 2022. A further strength is the use of propensity score matching which eliminates the effects of confounding due to baseline variable differences. Indeed, it is often difficult to determine the degree to which regression-based adjustments minimize differences between groups [30], especially when the age differences among participants are as big as in this study.
However, our study is also subject to a number of limitations. Although different chronic conditions were used for adjustment in regression models, other diseases which were not included could have an impact on the study outcome. Such diseases include anemia, chronic obstructive pulmonary disease, chronic kidney disease, and vitamin D deficiency, which are associated with COVID-19 mortality in the literature [31, 32]. No detailed information is available on the causes of death in those patients who died. Most but not all mortality cases listed COVID-19 as the main cause of death. In addition, no medications used for COVID-19 therapy and no other medications were analyzed. Information on the vaccination status of the patients included in the study was missing. Although Germany actually has a very high COVID-19 vaccination rate among the elderly population, the period of this study also included the year 2020 when no vaccinations were available. Gomes et al. demonstrated the very high effectiveness of the BNT162b2 COVID-19 vaccine in elderly populations, finding that two doses of the vaccine significantly lowered the risk of hospitalization as well as mortality [33]. Furthermore, given that our study only included patients treated in hospitals, the association between dementia and a fatal course of COVID-19 cannot be generalized to indicate an association between dementia and COVID-19 severity in patients treated outside of hospitals in Germany. Other authors have conducted studies on dementia patients with COVID-19 in other settings and countries [34, 35]. For example, in Madrid (Spain), the mortality of the residents living in nursing homes with COVID-19 was almost 45% [34]. Another study conducted in the USA found that the weekly mortality rate in 2020 was 68% higher among assisted living residents with dementia than among residents without dementia [35]. From a data perspective, one further limitation of our study is our use of the ICD-10 coding system, which might lead to misclassification and undercoding of certain diagnoses. Furthermore, data on socioeconomic status (e.g., education and income) and lifestyle-related risk factors (e.g., smoking, alcohol consumption, and physical activity) are also lacking. These possible confounders could not be matched in our analysis, which would have beendesirable.
Finally, viral variants were not determined individually for patients. Assignment of variants was based on the predominant variant at the time the patient was diagnosed with COVID-19 and a distinction was only made based on whether patients were diagnosed before or since the omicron variant emerged (1/1/2022).
Conclusions
This study including approximately 28,000 patients treated in 50 Aklepios hospitals in Germany between 2020 and 2022 found that a diagnosis of dementia was only slightly associated with COVID-19 mortality. Although SARS-CoV-2 has changed over time and vaccination has greatly improved the prognosis of those who contract COVID-19 in general, further studies are needed to identify, prevent, and treat risk factors for mortality of this disease.
ACKNOWLEDGMENTS
The authors would like to thank our study team Claudia Kalkowski, Susanne Scholz, Charlotte Arms, Hanna Nugent, Francis Maren Konermann, Philipp Anders, Tobias Gethmann, Aaron Wilhelm Sievering, and Victor Rechl for data entry and especially Kathrin Heitmann, Ina Koch, Sara Oldfield, and Kai Jaquet for management and organization.
We would also like to express our sincere thanks to Christoph Jermann, Monika Grimm, and the team of the Asklepios Campus Hamburg, Semmelweis University for providing us with the infrastructure and support we needed to complete this study. In addition, we warmly thank the IT team, especially Claudio Forte and Thomas Koschmieder, for their assistance.
We would also like to thank all of the other members of the steering committee: Melanie Gunawardene, Tino Schnitgerhans, Sebastian Wirtz, Martin Bergmann, Martin Bachmann, Axel Stang, Sebastian Wirtz, Ulrich-Frank Pape, Christian Gloeckner, Thomas Hoelting, Rüdiger Schreiber, Juergen Behr, and Stephan Willems.
Finally, we thank all the physicians and nurses from the COVID-19 wards of all participating centers for the patient care they have provided during the ongoing pandemic.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0918r2).
REFERENCES
[1] | Sanyaolu A , Okorie C , Hosein Z , Patidar R , Desai P , Prakash S , Jaferi U , Mangat J , Marinkovic A ((2021) ) Global pandemicity of COVID-19: Situation report as of June 9, 2020. Infect Dis (Auckl) 14: , 1178633721991260. |
[2] | World Health Organization. Coronavirus (COVID-19) Dashboard https://covid19.who.int/. Accessed on August 14, 2022. |
[3] | Kloka JA , Blum LV , Old O , Zacharowski K , Friedrichson B ((2022) ) Characteristics and mortality of 561,379 hospitalized COVID-19 patients in Germany until December 2021 based on real-life data. Sci Rep 12: , 11116. |
[4] | European Centre for Disease Prevention and Control. Data on Hospital and ICU Admission Rates and Current Occupancy for COVID-19 https://www.ecdc.europa.eu/en/publications-data/download-data-hospital-and-icu-admission-rates-and-current-occupancy-covid-19. Accessed August 27, 2022. |
[5] | Wan Y , Wu J , Ni L , Luo Q , Yuan C , Fan F , Liu H , Zhang C , Xiang Y , Xie Q ((2020) ) Prognosis analysis of patients with mental disorders with COVID-19: A single-center retrospective study. Aging (Albany NY) 12: , 11238–11244. |
[6] | Bianchetti A , Rozzini R , Guerini F , Boffelli S , Ranieri P , Minelli G , Bianchetti L , Trabucchi M ((2020) ) Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging 24: , 560–562. |
[7] | Hwang JM , Kim JH , Park JS , Chang MC , Park D ((2020) ) Neurological diseases as mortality predictive factors for patients with COVID-19: A retrospective cohort study. Neurol Sci 41: , 2317–2324. |
[8] | Atkins JL , Masoli JAH , Delgado J , Pilling LC , Kuo CL , Kuchel GA , Melzer D ((2020) ) Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci 75: , 2224–2230. |
[9] | Harrison SL , Fazio-Eynullayeva E , Lane DA , Underhill P , Lip GYH ((2020) ) Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med 17: , e1003321. |
[10] | van Gerwen M , Alsen M , Little C , Barlow J , Genden E , Naymagon L , Tremblay D ((2021) ) Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol 93: , 907–915. |
[11] | Esme M , Koca M , Dikmeer A , Balci C , Ata N , Dogu BB , Cankurtaran M , Yilmaz M , Celik O , Unal GG , Ulgu MM , Birinci S ((2021) ) Older adults with coronavirus disease 2019: A nationwide study in Turkey. J Gerontol A Biol Sci Med Sci 76: , e68–e75. |
[12] | Hariyanto TI , Putri C , Situmeang RFV , Kurniawan A ((2021) ) Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur Arch Psychiatry Clin Neurosci 271: , 393–395. |
[13] | Becerra-Muñoz VM , Núñez-Gil IJ , Eid CM , GarcíaAguado M , Romero R , Huang J , Mulet A , Ugo F , Rametta F , Liebetrau C , Aparisi A , Fernández-Rozas I , Viana-Llamas MC , Feltes G , Pepe M , Moreno-Rondón LA , Cerrato E , Raposeiras-Roubín S , Alfonso E , Carrero-Fernández A , Buzón-Martín L , Abumayyaleh M , Gonzalez A , Fernández Ortiz A , Macaya C , Estrada V , Fernández-Pérez C , Gómez-Doblas JJ ((2021) ) Clinicalprofile and predictors of in-hospital mortality among older patientshospitalised for COVID-19. Age Ageing 50: , 326–334. |
[14] | Gilstrap L , Zhou W , Alsan M , Nanda A , Skinner JS ((2022) ) Trends in mortality rates among Medicare enrollees with Alzheimer disease and related dementias before and during the early phase of the COVID-19 pandemic. JAMA Neurol 79: , 342–348. |
[15] | Rutten JJS , van Kooten J , van Loon AM , van Buul LW , Joling KJ , Smalbrugge M , Hertogh CMPM ((2021) ) Dementia and Parkinson’s disease: Risk factors for 30-day mortality in nursing home residents with COVID-19. J Alzheimers Dis 84: , 1173–1181. |
[16] | Liu N , Sun J , Wang X , Zhao M , Huang Q , Li H ((2020) ) The impact of dementia on the clinical outcome of COVID-19: A systematic review and meta-analysis. J Alzheimers Dis 78: , 1775–1782. |
[17] | July J , Pranata R ((2021) ) Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: A systematic review and meta-analysis. Geriatr Gerontol Int 21: , 172–177. |
[18] | Gessler N , Gunawardene MA , Wohlmuth P , Arnold D , Behr J , Gloeckner C , Herrlinger K , Hoelting T , Pape UF , Schreiber R , Stang A , Wesseler C , Willems S , Arms C , Herborn CU ((2021) ) Clinical outcome, risk assessment, and seasonal variation in hospitalized COVID-19 patients-Results from the CORONA Germany study. PLoS One 16: , e0252867. |
[19] | Gunawardene MA , Gessler N , Wohlmuth P , Heitmann K , Anders P , Jaquet K , Herborn CU , Arnold D , Bein B , Bergmann MW , Herrlinger KR , Stang A , Schreiber R , Wesseler C , Willems S ((2021) ) Prognostic impact of acute cardiovascular events in COVID-19 hospitalized patients-results from the CORONA Germany Study. J Clin Med 10: , 3982. |
[20] | Robert Koch Institut. Wochenberichte zu COVID-19. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenberichte_Tab.html;jsessionid=29FCD78C0A4B497DB83308D010AC3430.internet051?nn=2444038. Accessed on September 9, 2022. |
[21] | Schilling J , Tolksdorf K , Marquis A , Faber M , Pfoch T , Buda S , Haas W , Schuler E , Altmann D , Grote U , Diercke M ; RKI COVID-19 Study Group ((2021) ) Die verschiedenen Phasen der COVID-19-Pandemie in Deutschland: Eine deskriptive Analyse von Januar 2020 bis Februar 2021. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 64: , 1093–1106. |
[22] | Zhang Q , Schultz JL , Aldridge GM , Simmering JE , Kim Y , Ogilvie AC , Narayanan NS ((2021) ) COVID-19 case fatality and Alzheimer’s disease. J Alzheimers Dis 84: , 1447–1452. |
[23] | Fan G , Yang Z , Lin Q , Zhao S , Yang L , He D ((2021) ) Decreased case fatality rate of COVID-19 in the second wave: A study in 53 countries or regions. Transbound Emerg Dis 68: , 213–215. |
[24] | Jones S , Mason N , Palser T , Swift S , Petrilli CM , Horwitz LI ((2021) ) Trends in risk-adjusted 28-day mortality rates for patients hospitalized with COVID-19 in England. J Hosp Med 16: , 290–293. |
[25] | Zeeh J , Memm K , Heppner HJ , Kwetkat A ((2020) ) Covid-19 pandemic. Mechanical ventilation in geriatric patients – an ethical dilemma? MMW Fortschr Med 162: , 40–45. |
[26] | Yu Y , Travaglio M , Popovic R , Leal NS , Martins LM ((2021) Alzheimer’s and Parkinson’s diseases predict different COVID-19 outcomes: A UK Biobank Study. Geriatrics (Basel) 26: , 10. |
[27] | Matias-Guiu JA , Pytel V , Matías-Guiu J ((2020) ) Death rate due to COVID-19 in Alzheimer’s disease and frontotemporal dementia. J Alzheimers Dis 78: , 537–541. |
[28] | Magusali N , Graham AC , Piers TM , Panichnantakul P , Yaman U , Shoai M , Reynolds RH , Botia JA , Brookes KJ , Guetta-Baranes T , Bellou E , Bayram S , Sokolova D , Ryten M , Sala Frigerio C , Escott-Price V , Morgan K , Pocock JM , Hardy J , Salih DA ((2021) ) A genetic link between risk for Alzheimer’s disease and severe COVID-19 outcomes via the OAS1 gene. Brain 144: , 3727–3741. |
[29] | Sirin S , Nigdelioglu Dolanbay S , Aslim B ((2022) ) The relationship of early- and late-onset Alzheimer’s disease genes with COVID-19. J Neural Transm (Vienna) 129: , 847–859. |
[30] | Austin PC , Xin Yu AY , Vyas MV , Kapral MK ((2021) ) Applying propensity score methods in clinical research in neurology. Neurology 97: , 856–863. |
[31] | Panagiotou OA , Kosar CM , White EM , Bantis LE , Yang X , Santostefano CM , Feifer RA , Blackman C , Rudolph JL , Gravenstein S , Mor V ((2021) ) Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med 181: , 439–448. |
[32] | AbuRuz S , Al-Azayzih A , ZainAlAbdin S , Beiram R , Al Hajjar M ((2022) ) Clinical characteristics and risk factors for mortality among COVID-19 hospitalized patients in UAE: Does ethnic origin have an impact. PLoS One 17: , e0264547. |
[33] | Gomes D , Beyerlein A , Katz K , Hoelscher G , Nennstiel U , Liebl B , Überla K , von Kries R ((2021) ) Is the BNT162b2 COVID-19 vaccine effective in elderly populations? Results from population data from Bavaria, Germany. PLoS One 5: , e0259370. |
[34] | Cascini S , Agabiti N , Marino C , Acampora A , Balducci M , Calandrini E , Davoli M , Bargagli AM ((2022) ) Incidence and outcomes of SARS-CoV-2 infection in older adults living with dementia: A population-based cohort study. J Alzheimers Dis 89: , 681–693. |
[35] | Hua CL , Cornell PY , Zimmerman S , Carder P , Thomas KS ((2022) ) Excess mortality among assisted living residents with dementia during the COVID-19 pandemic. J Am Med Dir Assoc 23: , 1743–1749.e6. |




