The Association Between Temporal Atrophy and Episodic Memory Is Moderated by Education in a Multi-Center Memory Clinic Sample
Abstract
Background:
Cognitive reserve (CR) is hypothesized to partially explain the discrepancy between Alzheimer’s disease related brain pathology and cognitive performance. Educational attainment is often used as a proxy for CR.
Objective:
To examine the association of years of education and the relationship between atrophy in the medial temporal lobe and episodic memory, in a cross-sectional ecological multi-center memory clinic cohort.
Methods:
Included patients (n = 702) had undergone memory clinic examination and were diagnosed with subjective cognitive impairment (n = 99), mild cognitive impairment (n = 471), or dementia (n = 132). Total years of education were used as a moderating variable and neuropathology was operationalized as visual ratings of medial temporal lobe atrophy (MTA) on magnetic resonance imaging and computer tomography images. Weighted least squares regression and multiple regression were used to analyze moderation and the effect of education separately by diagnostic group. A composite score of two episodic memory tests constituted the dependent variable.
Results:
After controlling for age and gender the interaction term between MTA and years of education was significant indicating moderation. In particular, the regression model showed that at low levels of MTA, high education individuals had better episodic memory performance. However, at higher MTA levels, high education individuals had the lowest episodic memory performance. Education had a significant positive effect on episodic memory in SCI and MCI, but not dementia.
Conclusion:
These results extend the findings of education moderating the effect of MTA on cognition to a naturalistic memory clinic setting. Implications of the findings for theories on CR are discussed.
INTRODUCTION
In aging and Alzheimer’s disease (AD), comparable degrees of brain pathology exhibit varying clinical impact across individuals [1], which could be partly explained by cognitive reserve (CR). The Collaboratory on Research Definitions for Reserve and Resilience recently suggested a framework for terms used in reserve related research where CR is defined as “… a property of the brain that allows for cognitive performance that is better than expected given the degree of life-course related brain changes and brain injury or disease” [2]. Functional neural underpinnings for reserve have been theorized, such as an ability to employ task-related and alternative compensatory neural networks more efficiently [3]. Although not a direct measure of CR, sociobehavioral proxy measures (such as education) are often used in reserve-related research. This is done with the assumption that more cognitively stimulating activities throughout the lifespan build greater reserve [4]. In the present study, we investigate whether years of education moderates the relationship between brain atrophy and episodic memory in a clinical aging population with heterogenous cognitive status. We also investigate how the effect of education on episodic memory potentially differs by clinical diagnosis.
In AD brain atrophy typically begins in the medial temporal lobe [5]. Therefore, a visual rating scale for medial temporal lobe atrophy (MTA) is routinely used in clinical settings to aid the diagnosis and classification of AD [6, 7]. The rating scale assesses atrophy of the hippocampus and parahippocampal region, which are integral structures for the encoding and retrieval of episodic memory [8]. Consequently, episodic memory is a cognitive domain usually affected in AD, and MTA as well as other measures of regional atrophy in the temporal lobes have been found to be linked to episodic memory deficits [9, 10]. Similar memory deficits are linked to MTA in patients with mild cognitive impairment (MCI) [11], the clinical stage that often progresses to AD dementia. Interestingly, there are also findings of reduced volume in the medial temporal lobe and related structures in patients with subjective cognitive impairment (SCI) [12, 13], which is thought to be a preclinical stage of AD preceding MCI, in some individuals [14, 15].
Vascular dementia (VaD) is the second most common form of dementia, caused by cerebrovascular disease. There is partial clinical and pathophysiological overlap between AD and VaD regarding, for example, subcortical vascular lesions and specific cognitive domain deficits [16, 17]. AD and VaD also share several risk factors including hypertension and smoking [18, 19]. Moreover, dementia can be caused by concurrent AD-related pathology and cerebrovascular disease, then labeled mixed dementia (MD). While the role of CR has been investigated in vascular conditions such as white matter lesions, stroke, and vascular risk factors [20–22] with some studies lending support to the CR hypothesis, CR is understudied among patients with MD.
Recent years have seen an increased interest in (potentially modifiable) risk factors for AD, the seemingly multifactorial genesis of the disease, and how these interact throughout the entire lifespan [23–25]. In parallel, interventions targeting lifestyle-related modifiable risk factors that may reduce the risk of dementia or improve symptoms have been experimentally tried, with some promising results [26]. Similar efforts are currently being adapted globally, with geographical and cultural adaptions [27]. With a cure for AD not being available at this time and since the worldwide prevalence of dementia is predicted to rapidly increase [28] one could argue for the need of structured preventive interventions targeting large portions of the population. In such efforts, concepts such as CR are of interest since it is hypothesized to add to resilience against AD symptoms. This, in turn, requires a better understanding of which lifestyle-related factors contribute to CR, and how these interact with cognition and functioning in clinical populations.
The clinical impact of MTA on cognition has been demonstrated, and level of education relates to the risk of developing dementia [29]. Moreover, it is suggested that in healthy older adults, education moderates the relationship between hippocampal volume and memory performance on neuropsychological tests [30]. In AD patients, education has been shown to moderate the effect of MTA on cognitive performance [31]. To further the understanding of how years of education relates to memory in AD and increase generalizability, studying larger more unselected clinical samples is of interest. Because of the role of MTA in AD and its association with episodic memory, which is a core clinical symptom in typical AD, we investigated the relationship between MTA and education in that context. AD dementia is preceded by MCI and, in some cases, SCI and since CR is conceptualized as a dynamic construct, we decided to study patients in all clinical stages including patients diagnosed with MD where AD-pathology is present. This is performed to expand findings of how years of education potentially moderates cognition in the entire theoretical AD-continuum, and how the effect of education on episodic memory may differ across different clinical stages. Based on previous findings and theories of CR we hypothesized that years of education would moderate the association between MTA and episodic memory.
MATERIALS AND METHODS
Participants
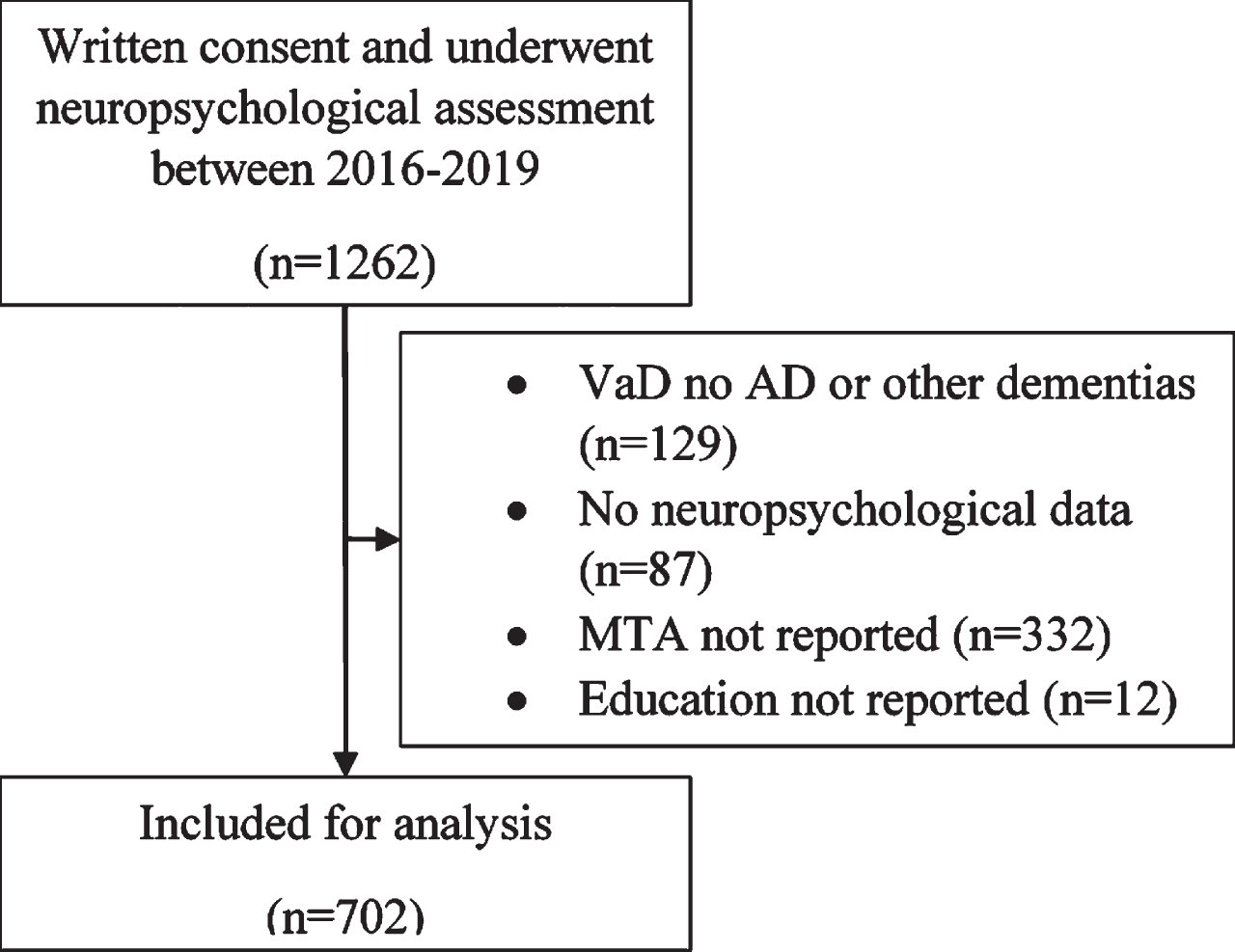
All patients in the present study were part of the MemClin-cohort [32]. In short, MemClin is a Sweden-based initiative to collect memory clinic patient data from nine out of ten memory clinics in the Stockholm area. The cohort consisted of patients who underwent an extended dementia examination at one of the (at the time six) MemClin associated memory clinics, from the onset of data collection during 2016 through early 2019 and who had given their written consent to be involved in the project. At the point of data collection only clinics that evaluates individuals over the age of 65 had contributed with data. MemClin follows the Helsinki declaration and has ethical approval from the regional ethics committee in Stockholm (Dnr: 2016/29–31/1). The included patients provided signed written consent. The different clinical procedures included in the dementia examinations and diagnostic procedures have been previously described [32]. In short, all patients receive a clinical consensus diagnosis through interdisciplinary rounds at the respective clinic in accordance with the International Classification of Diseases (ICD-10). Patients diagnosed with ICD codes R418A, Z032A, Z033, and Z038, where no objective cognitive impairment had been verified, were classified as SCI. Furthermore, patients with MCI (ICD code F067), AD (ICD code F001), or mixed AD and vascular dementia (AD/VaD, ICD code F002) were included. In the MD group VaD is clinically characterized as subcortical, mixed subcortical/cortical, multi-infarct, or unspecified based on neuroradiological findings. Included patients were divided into three groups, one SCI group, one MCI group, and one dementia group including patients with both “pure” AD and MD with AD-pathology. Patients with vascular dementia without concomitant AD or patients with other types of dementia were excluded. In those cases where a patient had been evaluated at the memory clinic more than once, data from the first evaluation where neuropsychological tests were administered was used. Lastly all patients who did not have an MTA score, years of education reported, or any neuropsychological test data registered were excluded (Fig. 1).
Fig. 1
Sample inclusion flowchart MTA, medial temporal lobe atrophy; AD, Alzheimer’s disease.
Brain imaging and medial temporal atrophy
In the present sample, 160 patients had undergone a 1.5T/3T magnetic resonance imaging scan and 542 patients a computed tomography scan at one of the local centers as part of their clinical examination. Radiologists at the respective centers then examined and analyzed the images based on the Scheltens scale for MTA [6]. The MTA scale includes scores from 0 to 4, with a higher value indicating more brain atrophy. The patients MTA scores were extracted from their patient journals. Since separate scores for left and right temporal lobe are not always routinely reported, in some cases an average score for the MTA range reported was used.
Years of education
Self-reported total years of education was analyzed as a moderating independent variable. This was ascertained in clinical interviews with an assessing neuropsychologist at one of the MemClin centers and extracted to the database from the patient journals.
Episodic memory
Neuropsychological test scores from the following tests were used
Rey Auditory Verbal Learning Test (RAVLT) delayed recall considered a measure of verbal episodic memory [33]. Some individuals had been tested with an alternative version of RAVLT that also contained a distraction task in the form of a separate wordlist being presented once after the learning trial of the task. In order to examine if there was any difference in the performance of delayed recall between the group tested with distraction (n = 202) and without (n = 392), an independent samples t-test was conducted. No significant difference was found and the results from both groups were therefore treated as a single variable (see the Supplementary Material).
Rey-Osterieth Complex Figure Test (RCFT) delayed recall considered a measure of visual episodic memory [34].
Standardized Z-scores for the two tests were calculated from the raw scores of each individual using the mean and standard deviation (SD) of the SCI group in the present sample. The two scores were then averaged together to obtain a composite memory domain score for each participant.
Statistical analyses
SPSS version 26.0 was used for all statistical analyses. Differences in the independent variables between genders (female versus male) and diagnostic groups (SCI versus MCI versus Dementia) were assessed with one-way ANOVA or independent samples t-test, where appropriate.
Missing values analysis revealed 10.8% missingness in the RAVLT delayed recall variable and 33.3% missingness in the RCFT delayed recall variable and the result of Little’s MCAR [35] was significant (p < 0.05). Therefore, multiple imputation by chained equations (MICE) was used to create 25 datasets, with different values multiply imputed, using the fully conditional Markov chain Monte Carlo (MCMC) method provided in SPSS. Where appropriate pooled results were analyzed and are presented.
During analysis of the full sample, visual inspection of the residual plot revealed unequal variance thought to be caused by disproportionate number of patients having received a score of zero on one or two of the neuropsychological tests. Because of the resulting distribution of the neuropsychological test data weighted least squares (WLS) regression was used to assess the moderating effect of education on the relationship between MTA and episodic memory. In WLS, “weights” are assigned to each observation to account for the fact that error variance is not constant. In theory these weights should be known a priori but in practice, as is the case in the current study, the weights are unknown and must be estimated. As such a regular ordinary least squares regression was performed, and the absolute residuals was regressed on the predicted values (standard deviation function) to estimate the weights and run the WLS. The model was hierarchically built in two steps: 1) The episodic memory composite score was used as the outcome and MTA and years of education were added as predictors. Age and gender were also added as predictors to control for any effects. 2) An interaction term was created by centering the general MTA and years of education variables (
RESULTS
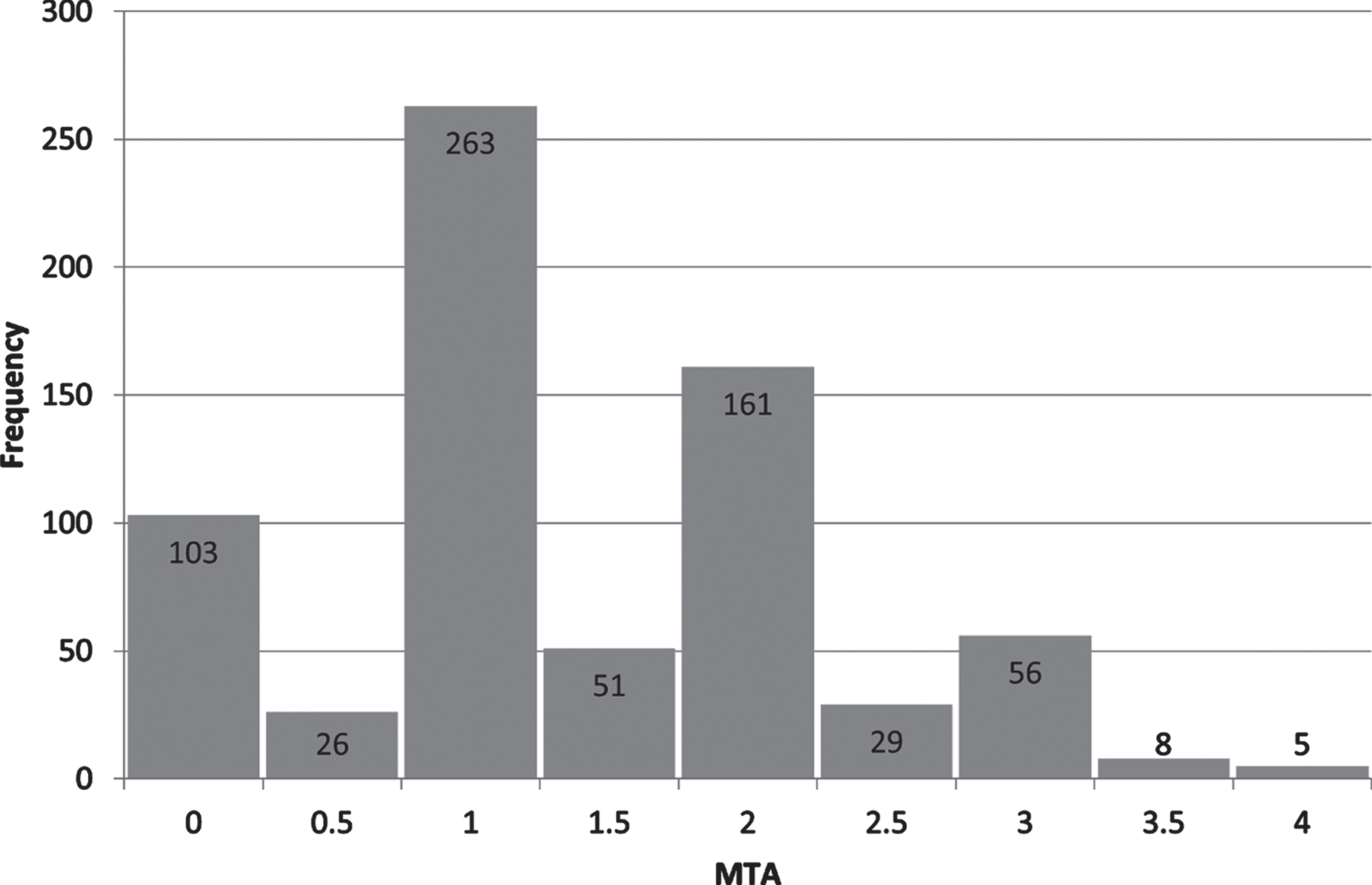
Descriptive statistics, including information on the neuropsychological test data after imputation, are reported in Table 1. Distribution of MTA scores in the sample are presented in Fig. 2. Total years of education ranged from 6 to 26 years in the sample.
Table 1
Sample descriptive statistics
| Total | SCI | MCI | Dementia (n = 132), | |
| (n = 702) | (n = 99) | (n = 471) | (AD = 66; AD/VaD = 66) | |
| Age y | 77.8 (6.15)a | 75.4 (6.08) | 77.8 (6.07) | 79.3 (5.98)c |
| Sex % female | 50.3 | 59.6 | 49.3 | 47 |
| Years of education | 12.62 (3.67) | 13.72 (3.93)b | 12.6 (3.57) | 11.85 (3.65) |
| MTA | 1.37 (.90)a | 0.81 (0.73) | 1.36 (.88) | 1.83 (0.85)c |
| Before imputation: | ||||
| Verbal memory Z* | –1.40 (1.21) | – | –1.46 (1.07) | –2.27 (0.76) |
| Visual memory Z | –1.16 (1.15) | – | –1.25 (1.01) | –2.15 (0.71) |
| Memory composite | –1.21 (1.05) | –0.00 (0.83) | –1.34 (0.86) | –2.24 (0.58) |
| After imputation: | ||||
| Verbal memory Z | –1.43 | –0.03 | –1.49 | –2.24 |
| Visual memory Z | –1.27 | –0.12 | –1.31 | –2.00 |
| Memory composite | –1.35 | –0.07 | –1.40 | –2.12 |
MTA, medial temporal lobe atrophy; SCI, subjective cognitive impairment; MCI, mild cognitive impairment; AD, Alzheimer’s disease; VaD, vascular dementia. aFemales > males. bSCI > MCI > Dementia. cDementia > MCI > SCI * Z values calculated using the mean and standard deviation of the SCI group.
Fig. 2
Distribution of MTA scores in the sample. MTA, medial temporal lobe atrophy; X-axis, a higher value indicating more atrophy; Y-axis, number of patients.
Moderation analysis
Correlations between the variables used in regression and the neuropsychological test variables are presented in Table 2. All variables were significantly correlated with each other except for the interaction term (MTA *Education). The interaction term was significantly correlated with the neuropsychological tests and memory composite.
Table 2
Correlation matrix of variables used in regression
| Age | Education | MTA | Verbal memory | Visual memory | Memory composite | |
| Age | – | |||||
| Education | –0.212** | – | ||||
| MTA | 0.304** | –0.100** | – | |||
| Verbal memory | –0.277** | 0.165** | –0.277** | – | ||
| Visual memory | –0.280** | 0.252** | –0.258** | 0.498** | – | |
| Memory composite | –0.321** | 0.239** | –0.310** | 0.875** | 0.855** | – |
| MTA*Education | 0.058 | –0.040 | –0.016 | –0.101** | –0.085* | –0.108** |
MTA, medial temporal lobe atrophy. **Significant effect at p < 0.001; *Significant effect at p < 0.05.
The results from the hierarchical WLS regression are presented in Table 3. Age, MTA, and education all had a significant effect on memory composite scores and gender did not. In the final model (model 2) when controlling for age, years of education and level of MTA, the interaction term had a significant effect (pooled p = 0.003) indicating moderation. Pooled R2 increased from 0.162 in model 2 to 0.173 in model 3 (ΔR2 = 0.011, p < 0.01) and the pooled standard error (SE) of the estimate was reduced indicating a better model fit when accounting for interaction.
Table 3
B-values, standard errors (SE), and pooled R2 from the regression model
| Model 1 | Model 2 | |||
| R2 = 0.162 | R2 = 0.173 | |||
| B | SE | B | SE | |
| Age | –0.204** | 0.037 | –0.196** | 0.037 |
| Gender | 0.092 | 0.072 | 0.092 | 0.071 |
| Education | 0.134** | 0.036 | 0.155** | 0.037 |
| MTA | –0.215** | 0.036 | –0.228** | 0.036 |
| Education*MTA | –0.097** | 0.032 | ||
MTA, medial temporal lobe atrophy. **Significant effect at p < 0.001; *Significant effect at p < 0.05.
Simple slope analysis
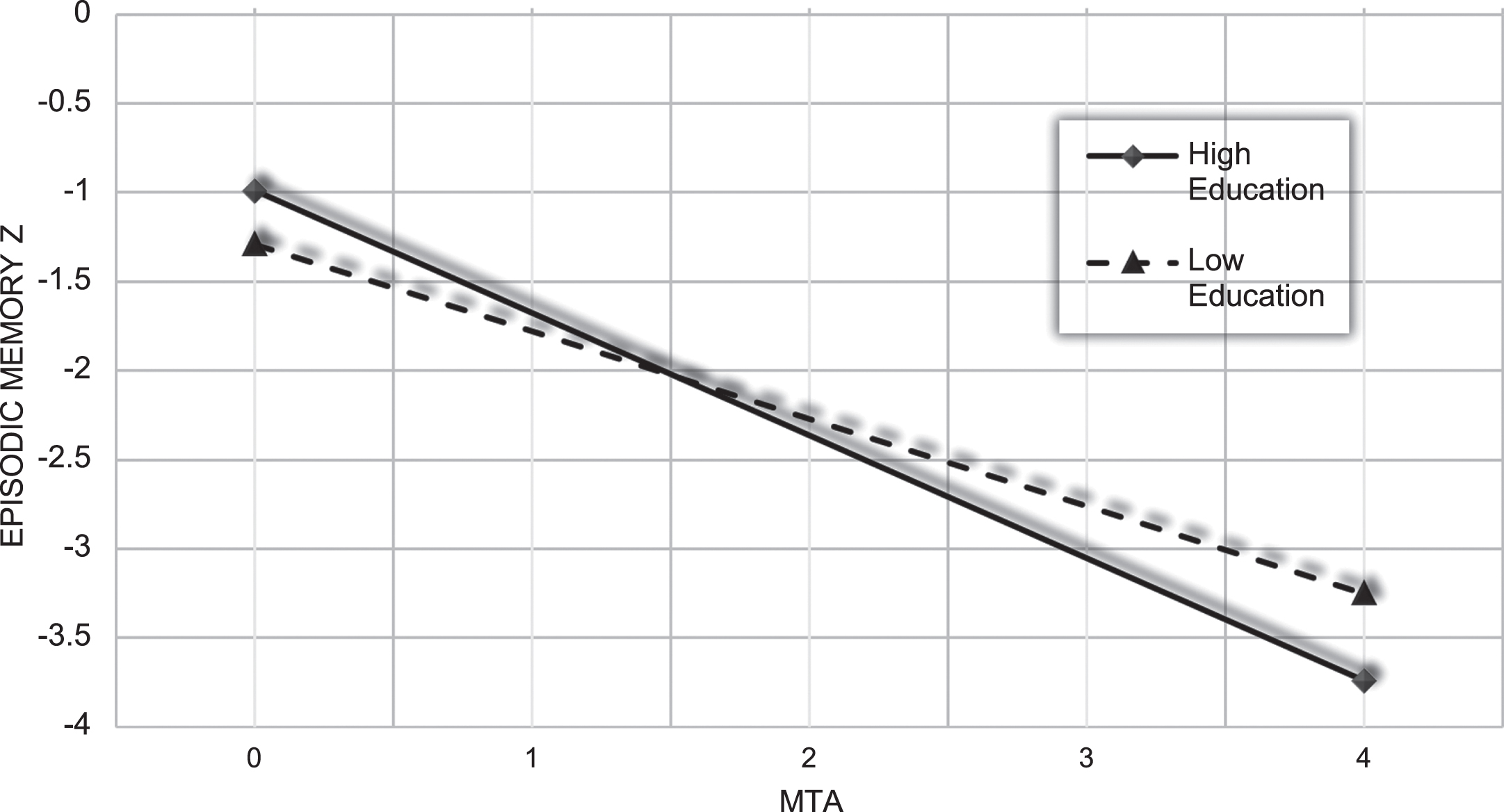
The quantitative moderation effect is illustrated with a simple slope analysis (Fig. 3) where the effect of MTA on episodic memory is shown for those in the sample one SD above and below the mean for years of education (which in the present sample roughly represents individuals who have completed elementary schooling or less versus individuals with a tertiary education level).
Fig. 3
Predicted memory composite Z score from the regression model for individuals with high (≥16.29 years of education) and low education (≤8.95 years of education). MTA, medial temporal lobe atrophy.
Effect of education on episodic memory by diagnosis
Standardized beta weights from episodic memory being regressed on years of education, while controlling for age, gender, and MTA, are presented for each diagnostic group in Table 4. In SCI and MCI, but not dementia, education had a significant positive effect on episodic memory performance. In dementia male gender had a positive effect on episodic memory.
Table 4
B-values and standard errors from episodic memory being regressed on years of education in all diagnostic groups
| SCI | MCI | Dementia | ||||
| B | SE | B | SE | B | SE | |
| Age | –0.296** | 0.089 | –0.204** | 0.040 | 0.060 | 0.058 |
| Gender | 0.103 | 0.162 | 0.082 | 0.077 | 0.308* | 0.111 |
| MTA | 0.037 | 0.107 | –0.085* | 0.041 | –0.113 | 0.060 |
| Education | 0.167* | 0.080 | 0.144** | 0.039 | –0.075 | 0.056 |
SCI, subjective cognitive impairment; MCI, mild cognitive impairment; MTA, medial temporal lobe atrophy **Significant effect at p < 0.001; *Significant effect at p < 0.05.
DISCUSSION
In the present cross-sectional study, years of education had a significant moderating effect on the relationship between MTA and episodic memory. In addition, when separately analyzing the clinical stages, years of education was found to have a significant effect on episodic memory performance in SCI and MCI, but not dementia. This was hypothesized based on the concept of CR and previous findings on the effect of education on the relationship between brain imaging indices in the medial temporal lobe and cognition, in clinical and non-clinical settings [30, 31]. The regression model indicated better memory performance at lower levels of MTA for patients with more years of education. Moreover, when visualizing the interaction effect between years of education and MTA the predicted level of cognitive performance is lower in the high education group, at higher levels of MTA. By studying data from the MemClin cohort, which involves multiple memory clinics, we were able to extend the findings of education as a moderator to a cognitively heterogenous group with differing diagnoses (including mixed AD/VaD) and a wide distribution of education.
Our findings of a significant moderation effect for education corresponds to some previous research [36–38] but the findings have differed regarding the direction of the interaction effect. Also, when comparing the effect of education on cognition stratified by diagnosis, some findings have been in line with to ours (that is education affects episodic memory in SCI and MCI, but not dementia) [37], while others have found education to have the strongest effect on cognition in dementia [36]. Methodological differences such as choice of neuropathological markers, neuropsychological tests used and how education is operationalized most likely contributes to these somewhat differing results. Moreover, there are preliminary findings that education and other measures of CR impart differing effects depending on the cognitive domain being investigated [39, 40]. In the present study, preliminary analyses revealed similar effects of years of education on both visual and verbal episodic memory and for conceptual clarity the choice was made to combine the measures, in order to achieve a more domain-general episodic memory composite. It should also be noted that the findings from the current sample could have been influenced by MCI and dementia patients having somewhat lower levels of MTA, which could in part be because of the inclusion of MD in the dementia group.
The fact that the slopes for high, and low education intersect in our regression model, does not necessarily mean that individuals with more years of education in a memory clinic setting eventually will develop poorer memory performance than individuals with less years of education at a group level. Such an interaction effect could instead be indicative of a nonlinear relationship between cognition and brain pathology in the presence of level of education or CR [41], which has been implicated in the CR literature [42]. The predicted steeper slope for individuals with more years of education in the present study, and education having a significant effect on cognition in SCI and MCI but not dementia, could be said to somewhat correspond to findings of high CR individuals showing a faster cognitive decline, at certain clinical stages [43–45]. Taken together this could also be argued in accordance with the original hypothetical model CR, where in high CR cognition is maintained longer but the decline is faster once an inflection point is reached. The theoretical explanation for this is that once cognitive decline begins in individuals with high CR, they are closer to a level of brain pathology where cognitive functioning cannot be maintained, despite CR. This would also correspond to our finding of education no longer having a positive effect on episodic memory in the dementia group.
CR remains a theoretical construct and neurobiological correlates of reserve are still lacking. However, repeated findings of different measures of education moderating the effect of neuropathology on cognition highlights the need to consider variance in educational levels in memory clinic patients as it can impact the association of biomarkers with clinical expression and motivates further investigation. That being said, differences in the nature of the interactions being found and in what clinical stages education affects cognition also calls for robust methodology to untangle these effects. Trajectories of separate cognitive domains, multiple measures of brain pathology and how these interact with different lifestyle related sociobehavioral variables should be longitudinally investigated. Such studies could be used to construe demographically adjusted predictions of prognosis in a clinical setting and definitions of disease thresholds, which are urgently needed in the clinic. Furthermore, with a clearer understanding of how lifestyle related factors may promote healthy cognitive aging, and how these factors potentially relate to CR, preventive interventions that combine such factors could be tailored to reduce cognitive decline.
Strengths and limitations
MemClin being an ecological multi-center memory clinic sample constitutes a major strength of the current study. The cognitive and demographic heterogeneity in the sample reduces the risk of some sampling-related bias. Furthermore, the ecological validity of the results facilitates generalization to memory clinic patients and the aging population at large.
There were also some limitations to the present study. We observed a proportion of missing data in the neuropsychological test results. Following Madley-Dowd et al. [46], we decided to impute the missing values to minimize potential bias. Years of education is not by itself an optimal proxy for CR as it might not accurately correspond to the level or quality of individual educational attainment. Total years of education was used since many of the demographic variables in the MemClin-cohort are self-reported and extracted from the clinical interviews at the memory clinics, and highest level of education is therefore not routinely reported. This is also why other sociobehavioral variables, such as occupational attainment and leisure activities, were not included in the present study. Unfortunately, we did not have reliable information on MCI-subtypes and APOE status which potentially could have given additional explanatory value. In this study, both magnetic resonance and computed tomography imaging was used to assess MTA. Although having all the MTA scores done on the same imaging modality would be preferable, Wattjes et al. [47] demonstrated that MTA scores from magnetic resonance and computer tomography imaging can be combined. This motivated the combined use of images from both modalities in our current study, achieving greater statistical power. The present study is a multi-center study with somewhat different practices and clinicians at the centers in respect to cognitive testing, CSF sampling, additional CT/MRI evaluations, and other health measures. This potential inconsistency between centers is common in large-scale clinical studies, and we cannot exclude that this, at least to some degree, have affected the variability in the present study.
Conclusion
In a multi-center memory clinic sample, we showed that years of education was a moderator of the association between MTA and episodic memory. At lower levels of MTA high education patients had better memory performance, but as MTA goes above 1.5 its predicted effect on episodic memory is also greater in this group. Education had a significant positive effect on episodic memory in SCI and MCI, but not dementia. Longitudinal studies are needed to investigate how education interacts with neuropathology to affect cognition and disease progression over time, and what the neurobiological correlates of these interactions are.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
ACKNOWLEDGMENTS
We would like to thank the included participants as well as the clinicians in the MemClin cohort, as they made this study possible.
FUNDING
The Swedish Foundation for Strategic Research (SSF), the Swedish Research Council (VR), the Swedish Research Council for Health, Working Life and Welfare (FORTE), the Center for Innovative Medicine (CIMED), the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro), Swedish Brain Power, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the joint research funds of KTH Royal Institute of Technology and Stockholm County Council (HMT), Loo och Hans Ostermans stiftelse för medicinsk forskning, Hjärnfonden, Alzheimerfonden, the Åke Wiberg Foundation, and Birgitta och Sten Westerberg for additional financial support. The funding sources had no role in the study design, data collection, analysis, interpretation, or the writing of the manuscript.
CONFLICT OF INTEREST
The authors declared no conflicts of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220741.
REFERENCES
[1] | Snowdon DA ((1997) ) Aging and Alzheimer’s disease: Lessons from the nun study. Gerontologist 37: , 150–156. |
[2] | The Collaboratory on Research Definitions for Reserve and Resilience, Consensus Definitions., Last updated February 8, 2021, Accessed on June 5, 2021–https://reserveandresilience.com/definitions. |
[3] | Stern Y ((2009) ) Cognitive reserve. Neuropsychologia 47: , 2015–2028. |
[4] | Harrison SL , Sajjad A , Bramer WM , Ikram MA , Tiemeier H , Stephan BC ((2015) ) Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J Clin Exp Neuropsychol 37: , 253–264. |
[5] | Whitwell JL ((2010) ) Progression of atrophy in Alzheimer’s disease and related disorders. Neurotox Res 18: , 339–346. |
[6] | Scheltens P , Leys D , Barkhof F , Huglo D , Weinstein HC , Vermersch P , Kuiper M , Steinling M , Wolters EC , Valk J ((1992) ) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55: , 967–972. |
[7] | Wahlund LO , Westman E , van Westen D , Wallin A , Shams S , Cavallin L , Larsson EM ; From the Imaging Cognitive Impairment Network (ICINET) ((2017) ) Imaging biomarkers of dementia: Recommended visual rating scales with teaching cases. Insights Imaging 8: , 79–90. |
[8] | Dickerson BC , Eichenbaum H ((2010) ) The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology 35: , 86–104. |
[9] | Di Paola M , Macaluso E , Carlesimo GA , Tomaiuolo F , Worsley KJ , Fadda L , Caltagirone C ((2007) ) Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy: A voxel-based morphometry study. J Neurol 254: , 774–781. |
[10] | Smits LL , Tijms BM. , Benedictus MR , Koedam ELE , Koene T , Reuling IE , Barkhof F , Scheltens P , Pijneburg YA , Wattjes MP , van der Flier WM ((2014) ) Regional atrophy is associated with impairment in distinct cognitive domains in Alzheimer’s disease. Alzheimers Dement 10: , 299–305. |
[11] | Loewenstein DA , Acevedo A , Potter E , Schinka JA , Raj A , Greig MT , Agron J , Barker WW , Wu Y , Small B , Schofield E , Duara R ((2009) ) Severity of medial temporal atrophy and amnestic mild cognitive impairment: Selecting type and number of memory tests. Am J Geriatr Psychiatry 17: , 1050–1058. |
[12] | Striepens N , Scheef L , Wind A , Popp J , Spottke A , Cooper-Mahkorn D , Suliman H , Wagner M , Schild HH , Jessen F ((2010) ) Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord 29: , 75–81. |
[13] | Hu X , Teunissen CE , Spottke A , Heneka MT , Düzel E , Peters O , Li S , Priller J , Buerger K , Teipel S , Laske C , Verfaillie SCJ , Barkhof F , Coll-Padrós N , Rami L , Molinuevo JL , van der Flier WM , Jessen F ((2019) ) Smaller medial temporal lobe volumes in individuals with subjective cognitive decline and biomarker evidence of Alzheimer’s disease: Data from three memory clinic studies. Alzheimers Dement 15: , 185–193. |
[14] | Jessen F , Amariglio RE , van Boxtel M , Breteler M , Ceccaldi M , Chételat G , Dubois B , Dufouil C , Ellis KA , van der Flier WM , Glodzik L , van Harten AC , de Leon MJ , McHugh P , Mielke MM , Molinuevo JL , Mosconi L , Osorio RS , Perrotin A , Petersen RC , Rabin LA , Rami L , Reisberg B , Rentz DM , Sachdev PS , de la Sayette V , Saykin AJ , Scheltens P , Shulman MB , Slavin MJ , Sperling RA , Stewart R , Uspenskaya O , Vellas B , Visser PJ , Wagner M ; Subjective Cognitive Decline Initiative (SCD-I) Working Group ((2014) ) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10: , 844–852. |
[15] | Diaz-Galvan P , Ferreira D , Cedres N , Falahati F , Hernández-Cabrera JA , Ames D , Barroso J , Westman E ((2021) ) Comparing different approaches for operationalizing subjective cognitive decline: Impact on syndromic and biomarker profiles. Sci Rep 11: , 4356. |
[16] | Attems J , Jellinger KA ((2014) ) The overlap between vascular disease and Alzheimer’s disease: Lessons from pathology. BMC Med 12: , 206. |
[17] | Kapasi A , Schneider JA ((2016) ) Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta 1862: , 878–886. |
[18] | O’Brien JT , Thomas A ((2015) ) Vascular dementia. Lancet 386: , 1698–1706. |
[19] | Santos CY , Snyder PJ , Wu WC , Zhang M , Echeverria A , Alber J ((2017) ) Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst) 7: , 69–87. |
[20] | Mortamais M , Portet F , Brickman AM , Provenzano FA , Muraskin J , Akbaraly TN , Berr C , Touchon J , Bonafé A , le Bars E , Menjot de Champfleur N , Maller JJ , Meslin C , Sabatier R , Ritchie K , Artero S ((2014) ) Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 22: , 1336–1345. |
[21] | Shin M , Sohn MK , Lee J , Kim DY , Lee SG , Shin YI , Oh GJ , Lee YS , Joo MC , Han EY , Han J , Ahn J , Chang WH , Shin MA , Choi JY , Kang SH , Kim Y , Kim YH ((2020) ) Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke: The KOSCO study. Stroke 51: , 99–107. |
[22] | Soldan A , Pettigrew C , Zhu Y , Wang MC , Gottesman RF , DeCarli C , Albert M ; BIOCARD Research Team ((2020) ) Cognitive reserve and midlife vascular risk: Cognitive and clinical outcomes. Ann Clin Transl Neurol 7: , 1307–1317. |
[23] | Norton S , Matthews FE , Barnes DE , Yaffe K , Brayne C ((2014) ) Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol 13: , 788–794. |
[24] | de Bruijn RF , Bos MJ , Portegies ML , Hofman A , Franco OH , Koudstaal PJ , Ikram MA ((2015) ) The potential for prevention of dementia across two decades: The prospective, population-based Rotterdam study. BMC Med 13: , 132. |
[25] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimäki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbæk G , Teri L , Mukadam N ((2020) ) Dementia prevention, intervention, and care: 2020 Report of the Lancet Commission. Lancet 396: , 413–446. |
[26] | Ngandu T , Lehtisalo J , Solomon A , Levälahti E , Ahtiluoto S , Antikainen R , Bäckman L , Hänninen T , Jula A , Laatikainen T , Lindström J , Mangialasche F , Paajanen T , Pajala S , Peltonen M , Rauramaa R , Stigsdotter-Neely A , Strandberg T , Tuomilehto J , Soininen H , Kivipelto M ((2015) ) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly People (FINGER): A randomised controlled trial. Lancet 385: , 2244–2263. |
[27] | Kivipelto M , Mangialasche F , Snyder HM , Allegri R , Andrieu S , Arai H , Baker L , Belleville S , Brodaty H , Brucki SM , Calandri I , Caramelli P , Chen C , Chertkow H , Chew E , Choi SH , Chowdhary N , Crivelli L , Torre R , Du Y , Dua T , Espeland M , Feldman HH , Hartmanis M , Hartmann T , Heffernan M , Henry CJ , Hong CH , Håkansson K , Iwatsubo T , Jeong JH , Jimenez-Maggiora G , Koo EH , Launer LJ , Lehtisalo J , Lopera F , Martínez-Lage P , Martins R , Middleton L , Molinuevo JL , Montero-Odasso M , Moon SY , Morales-Pérez K , Nitrini R , Nygaard HB , Park YK , Peltonen M , Qiu C , Quiroz YT , Raman R , Rao N , Ravindranath V , Rosenberg A , Sakurai T , Salinas RM , Scheltens P , Sevlever G , Soininen H , Sosa AL , Suemoto CK , Tainta-Cuezva M , Velilla L , Wang Y , Whitmer R , Xu X , Bain LJ , Solomon A , Ngandu T , Carrillo MC ((2020) ) World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 16: , 1078–1094. |
[28] | Prince MJ , Wimo A , Guerchet MM , Ali GC , Wu YT , Prina M ((2015) ) World Alzheimer Report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London. |
[29] | Meng X , D’arcy C ((2012) ) Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 7: , e38268. |
[30] | O’Shea DM , Langer K , Woods AJ , Porges EC , Williamson JB , O’Shea A , Cohen RA ((2018) ) Educational attainment moderates the association between hippocampal volumes and memory performances in healthy older adults.361. Front Aging Neurosci 10: . |
[31] | Perneczky R , Wagenpfeil S , Lunetta KL , Cupples LA , Green RC , DeCarli C , Farrer LA , Kurz A ((2009) ) Education attenuates the effect of medial temporal lobe atrophy on cognitive function in Alzheimer’s disease: The MIRAGE study. J Alzheimers Dis 17: , 855–862. |
[32] | Ekman U , Ferreira D , Muehlboeck JS , Wallert J , Rennie A , Eriksdotter M , Wahlund LO , Westman E ((2020) ) The MemClin Project: A prospective multi memory clinics study targeting early stages of cognitive impairment. BMC Geriatr 20: , 93. |
[33] | Rey A ((1958) ) L’examen clinique en psychologie. Presses Universitaries De France, Paris. |
[34] | Rey A ((1941) ) L’examen psychologique dans les cas d’encéphalopathie traumatique. (Les problems.). Arch Psychol 28: , 215–285. |
[35] | Little RJA ((1988) ) A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 83: , 1198–1202. |
[36] | Staekenborg SS , Kelly N , Schuur J , Koster P , Scherder E , Tielkes CEM , Scheltens P , Claus JJ ((2020) ) Education as proxy for cognitive reserve in a large elderly memory clinic: “Window of benefit”. J Alzheimers Dis 76: , 671–679. |
[37] | Jansen MG , Geerligs L , Claassen JAHR , Overdorp EJ , Brazil IA , Kessels RPC , Oosterman JM ((2021) ) Positive effects of education on cognitive functioning depend on clinical status and neuropathological severity. Front Hum Neurosci 15: , 723–728. |
[38] | Mungas D , Fletcher E , Gavett BE , Widaman K , Zahodne LB , Hohman TJ , Mayeda ER , Dowling NM , Johnson DK , Tomaszewski Farias S ((2021) ) Comon of education and episodic memory as modifiers of brain atrophy effects on cognitive decline: Implications for measuring cognitive reserve. J Int Neuropsychol Soc 27: , 401–411. |
[39] | Lavrencic LM , Churches OF , Keage HAD ((2017) ) Cognitive reserve is not associated with improved performance in all cognitive domains. Appl Neuropsychol Adult 25: , 472–485. |
[40] | Li X , Song R , Qi X , Xu H , Yang W , Kivipelto M , Bennett DA , Xu W ((2021) ) Influence of cognitive reserve on cognitive trajectories: Role of brain pathologies. Neurology 97: , e1695–e1706. |
[41] | Belzak WCM , Bauer DJ ((2019) ) Interaction effects may actually be nonlinear effects in disguise: A review of the problem and potential solutions. Addict Behav 94: , 99–108. |
[42] | McQuail JA , Dunn AR , Stern Y , Barnes CA , Kempermann G , Rapp PR , Kaczorowski CC , Foster TC ((2021) ) Cognitive reserve in model systems for mechanistic discovery: The importance of longitudinal studies. Front Aging Neurosci 12: , 607685. |
[43] | Andel R , Vigen C , Mack WJ , Clark LJ , Gatz M ((2006) ) The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc 12: , 147–152. |
[44] | Soldan A , Pettigrew C , Cai Q , Wang J , Wang MC , Moghekar A , Miller MI , Albert M ; BIOCARD Research Team ((2017) ) Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging 60: , 164–172. |
[45] | Zahodne LB , Mayeda ER , Hohman TJ , Fletcher E , Racine AM , Gavett B , Manly JJ , Schupf N , Mayeux R , Brickman AM , Mungas D ((2019) ) The role of education in a vascular pathway to episodic memory: Brain maintenance or cognitive reserve? Neurobiol Aging 84: , 109–118. |
[46] | Madley-Dowd P , Hughes R , Tilling K , Heron J ((2019) ) The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 110: , 63–73. |
[47] | Wattjes MP , Henneman WJ , van der Flier WM , de Vries O , Träber F , Geurts JJ , Scheltens P , Vrenken H , Barkhof F ((2009) ) diagnostic imaging of patients in a memory clinic: Comon of MR imaging and 64-detector row CT. Radiology 253: , 174–183. |




