Communicating and Using Dementia Risk Evidence
Abstract
Advances in biomarkers, genetics, and other data used as dementia risk evidence (DRE) are increasingly informing clinical diagnosis and management. The purpose of this Mini-Forum is to provide a solutions-based discussion of the ethical and legal gaps and practical questions about how to use and communicate these data. Investigators often use DRE in research. When participants ask for their personal results, investigators have concerns. Will data that was intended to study groups be valid for individuals? Will sharing data cause distress? Debates around sharing DRE became heated when blood-based amyloid tests and amyloid reducing drugs appeared poised to enable clinicians easily to identify people with elevated brain amyloid and reduce it with a drug. Such an approach would transform the traditional role of DRE from investigational to foundational; however, then the high costs, uncertain clinical benefits and risks of the therapy led to an urgent need for education to support clinical decision making. Further complicating DRE use are direct to consumer genetic testing and increasingly available biomarker testing. Withholding DRE becomes less feasible and public education around responsible use and understanding become vital. A critical answer to these legal and ethical issues is supporting education that clearly delineates known risks, benefits, and gaps in knowledge, and communication to promote understanding among researchers, clinicians, patients, and all stakeholders. This paper provides an overview and identifies general concepts and resource documents that support more informed discussions for individuals and interdisciplinary groups.
DEMENTIA RISK EVIDENCE (DRE) YESTERDAY AND TODAY
Dementia researchers are developing technologies that estimate risk for Alzheimer’s disease (AD) and other forms of dementia with increasing ease and precision. For example, the process of detecting brain changes contributing to AD is being transformed. Researchers used to restrict definitive AD diagnosis until after death when the abnormal forms of the proteins amyloid and tau could be identified in the brains of patients. Biomarkers make possible detection of AD during life. A biomarker (short for biological marker) is an objective measure that detects what is happening in a cell or an organism at a given moment (for further explanation, see [1, 2]). Biomarkers for amyloid and tau, derived from cerebrospinal fluid (CSF) obtained by lumbar puncture or spinal tap, or measured by positron emission tomography (PET) brain scans in living people, revealed that this brain pathology builds up decades before Alzheimer’s symptoms develop [3]. Many patients are reluctant to undergo spinal taps, and PET scans are not easily available outside of specialized dementia research and clinical settings, hence access to these tests was limited. But now there are blood test-based biomarkers for amyloid which could easily be used outside of specialty dementia clinics, and other blood-based biomarkers are emerging. Just as people can obtain information about a genetic risk for AD from companies that sell their services direct to consumers, it is technically feasible that a blood-based amyloid biomarker could similarly be made available. Dementia experts know that an Alzheimer’s diagnosis is complex and should never be based exclusively on a biomarker alone [4]. Experts also advise caution in using blood-based biomarkers while they are new, since sometimes these blood-based amyloid biomarkers yield subtly different results from more well studied biomarkers [5]. There are now drugs that can reduce brain amyloid levels (e.g., aducanumb, lecanumab, gantenerumab). Although these drugs have risks and the clinical meaningfulness of their effects may be small, the potential for slowing progression or preventing AD could increase pressure on clinicians to perform blood tests and prescribe these medications.
The story of amyloid biomarkers and therapies is just one example of why the field needs to examine how we use and share DRE to minimize risk, maximize benefits, and respect the individual needs of people living with and at risk for dementia. This process is evolving. Different forms of DRE are beginning to provide different types of potentially clinically actionable information. Some of the data researchers hold thus may be clinically meaningful to their participants. Research participants are asking for their results [6–8] and researchers are increasingly sharing these data [9, 10].
The use of DRE is also becoming more complex and nuanced. Traditionally genetics was used to identify vulnerability to dementia. Biomarkers provided additional information around the current disease state. Now, pharmacogenomics is using genetics to predict drug response (e.g., [11]). The US Food and Drug Administration (FDA) identifies multiple, diverse roles for biomarkers [12]. Because the controversial FDA accelerated approval of aducanumab for the treatment of AD is based heavily on biomarker evidence, educating patients about the risks, benefits, and limitations of the research becomes critical for informed decisions [13]. Aside from drug therapies, the predictive power of DRE also has the potential to guide clinical practice (e.g., [14]) and social policy [15] as large datasets from populations (e.g., [16–18]) offer new opportunities to learn about real world risks and protective factors.
The debates around these issues have been ongoing for many years; however, recent scientific advances raise the urgency of developing ongoing, effective, balanced, dementia risk communication tools and guidance. For example, in this Mini-Forum we discuss how a blood test predicting brain amyloid now makes this biomarker easily available but requires judicious use [19], and more of these tests are under development. This introduction to the Mini-Forum has a dual purpose: 1) to define commonly used concepts, acronyms, and to identify key foundational references and 2) to provide a context for the papers in the Mini-Forum on Communicating and Using Dementia Risk Evidence. The most effective guidance will likely come from multidisciplinary perspectives and open, and fluid, consultation among researchers, clinicians, ethical, and legal scholars, and informed consumers and stakeholders. Contributions to this Mini-Forum and many of the ideas presented here come from members and subgroups of the Advisory Group on Risk Evidence Education for Dementia (AGREEDementia.org), an open working group that discusses and identifies issues related to this topic [20], and papers submitted to Ethics Review that were relevant. There is a strong emphasis here on US guidelines. Models from outside the US come from excellent guidance [21, 22] and examples of clinical trial registries that handle DRE effectively [23, 24]. There was also a great example of a survey on the use of CSF in mild cognitive impairment in Germany [25]. But more work is needed. In general, this Mini-Forum focuses on the perspectives of the research and clinical community, but it is important to consider the perspectives of the research participants, their support partners and families, and the community receiving care. We begin this Mini-Forum with a proposal from the AGREEDementia stakeholder group [8]. Finally, in this overview we often use the term DRE rather than restricting discussion to biomarkers and genetics, the classical indicators of dementia risk. The US FDA biomarker guidance indicates biomarkers do not include measures of “how an individual feels, functions, or survives” [12]. We believe it is important to not exclude what Au and colleagues have termed “digital biomarkers” of behavior (e.g., actigraphy to derive sleep or activity, audiometric analyses of speech, or video gait analyses) which likely have parallels to classical biomarkers and their own, additional, ethical issues [26].
A RESEARCH PARTICIPANT’S BILL OF RIGHTS
This Mini-Forum begins with a strong statement from a group of stakeholders (including people living with and at risk for dementia and advocacy organizations that support them) that researchers should increase sharing of individual level data with their participants [8]. The importance of planning for communicating unexpected, incidental findings that reveal urgent care needs has long been recognized (e.g., [27–29]). This paper revisits the issue of the return of individual-level research results in the context of current innovations in Alzheimer’s research. In general, sharing information that identifies urgent treatment needs is mandatory. Sharing non-urgent information that is clinically useful for guiding therapy is always encouraged. The authors suggest that for many participants, clinically valid (i.e., having scientific evidence of validity and reliability) results of increased DRE are personally valuable and inform life decisions even if there is no established treatment. This group also advises that interested participants could be more meaningfully engaged as partners in research if they received their own, “cutting edge” data, that do not yet have evidence of clinical validity, if there are appropriate safeguards and education.
GENERAL ETHICAL PRINCIPLES APPLIED TO DRE
Ethical principles (autonomy, beneficence, maleficence, justice) provide a powerful framework for the effective use of DRE. Whereas there are more extensive reviews of ethics applied to DRE (e.g., [30]), here we provide examples and these themes occur throughout the Mini-Forum.
Autonomy is supported through promoting 1) knowledge and 2) personal control over healthcare to generate informed decisions. Knowledge is enhanced through education around the risks and benefits of different choices and understanding personal relevance such as through full access to personal medical records [31, 32]. Many individuals with elevated risk for development of dementia in the future have demonstrated a high interest in learning their individual results; however, the rapid advances in DRE discovery make it challenging stay informed and communicate knowledge effectively to those individuals. In this Mini-Forum, Walter et al. [33, 34] show a powerful method of building partnership by including people living with dementia in scientific conferences, where both those naïve and experienced in research demonstrated high levels of engagement and satisfaction. Personal control, the second critical component of autonomy, is supported when clinicians collaborate with patients and families around the decisions to collect and use DRE. In direct to consumer (DTC) testing the public obtains DRE such as magnetic resonance imaging (MRI) [35] or genetic testing [36] independently and outside the healthcare system. Whereas DTC enables independence, consumers miss the expert discussions from genetic counsellors to be fully informed around the choice to pursue testing and often seek professional guidance after they receive their results [37].
Clinicians and society also have a duty to support beneficence, using DRE for good. Ultimately DRE should be useful, providing either clinical utility (e.g., helping diagnosis) or personal utility (e.g., informing life planning) or the supporting the needs of society (e.g., through research and public health decisions). Clinical use of genetics is supported through published guidance and genetic counselling (e.g., [38–40]). Direct to consumer genomics has some US FDA approved tests, but many tests are loosely regulated [41]. Clinical use of biomarkers are validated and applied according to appropriate context of use guidance from the US Food and Drug Administration [12] and through appropriate use criteria (AUC) (e.g., [5, 42–44]).
Malfeasance, avoiding willful harm, is often a concern which makes clinicians avoid sharing DRE. There is a rich literature on individuals’ reactions to learning genetic and biomarker status, sharing of this information, and dimensions of stigma related to diagnosis (e.g., [45, 46–48]). In general, learning personal DRE that reflect mildly/moderately elevated dementia risk (e.g., APOE or amyloid status) does not cause long term emotional harm; however, most evidence supporting this claim has been performed in carefully selected research cohorts. Naturalistic populations are beginning to be explored [49]. There is a danger of over-interpreting the importance of a selective source of DRE and missing treatable conditions; hence, DRE are best interpreted in the context of a comprehensive clinical evaluation [22, 25]. A more detailed description of how fluid biomarkers support dementia diagnosis is available elsewhere [4]. In this Mini-Forum, Galasko et al. [19] discuss issues around communicating blood-test based Alzheimer’s biomarkers. The ease of obtaining these blood tests raises the likelihood of a DTC product option. As with the genetic counselors having to explain the DTC genetic test results, dementia clinicians may then be faced with patients believing falsely they have AD based solely on DTC biomarker results.
Justice in the context of DRE is supported through identifying protections for discrimination such as laws related to genetic status (Genetic Information Nondiscrimination Act, GINA). It is important to warn people that knowing they are positive for a biomarker, such as by being amyloid positive, puts them at risk for various forms of discrimination such as exclusion from long term care insurance [50]. Furthermore, keeping this information out of their medical record may not protect them because under certain contexts, having this knowledge may compel them to disclose this information if asked (Arias, personal communication).
ETHICAL TOOLS/APPROACHES FOR DRE COMMUNICATION
Ethical principles often conflict, and autonomous choices depend critically on deeply personal values related to risks and benefits, both anticipated and unanticipated. These discussions take time and expertise. Specialists such as genetic counselors and specialty dementia clinics are ideal for supporting these discussions, but there may be insufficient numbers of clinicians to fulfil the need. In this Mini-Forum, Arias et al. [51] interviewed alternative front line care providers, geriatricians, about APOE genetic testing. Their main concern was the clinical utility of these tests. Also in this Mini-Forum, we describe a structure for an inclusive, multidisciplinary, ongoing, discussion forum, AGREEDementia that is helpful in developing education and other solutions to meet the need [20].
Decision aids/tools
One way of efficiently guiding informed choice is through educational support materials for patients and stakeholders before and after tests. A decision aid occurs before DRE testing to help people decide whether they want to collect or learn DRE. These decision aids pose questions and provide information. Another aid is provided after DRE are collected and interpreted to describe the meaning of results. Both of these components support autonomy in the right to know or not know, and to understand the limitations and implications of that DRE for other decisions. Examples have been published [52–54] and are available from NIA and on the AGREEDementia website [55]. Supporting these decisions becomes complicated in the context of people living with dementia, particularly in longitudinal studies where decision making capacity is expected to decline. In this Mini-Forum, Largent et al. [56] explain why identifying a study partner to support such discussions as learning DRE is helpful as cognition declines in participants.
Control over digital privacy and the medical record
The US 21st Century Cures Act enables full access to the medical record and thus introduces both the opportunity to use DRE clinically, but also the risk for misinterpretation and misuse of DRE [31, 32, 57]. In this Mini-Forum, Lerner [58] cautions that clinicians should consider carefully why they are collecting DRE and they also have a duty to protect patients who have biomarker or genetic data in their medical record (e.g., when participating in a research study) from the false conclusion that this information is sufficient to constitute a diagnosis. Patients may access biomarker results in their medical record before a clinician can explain these results. One solution some centers have used (e.g., Washington University) is to delay access to this information until the clinician discusses the results with the patient (for a discussion on this practice, see [57]). People sometimes choose to undergo direct to consumer genetic testing to learn results. In this Mini-Forum, Zallen has also found that they also choose to obtain the tests outside of the healthcare settings to keep them out of their medical record [59]. Digital biomarkers can also have particular privacy risks, such as with geolocation or proximity indicators (i.e., when the individual is near another person identified by their unique phone). For information-rich datasets such as MRI, there is the potential for identification with sufficiently powerful algorithms [60], hence there are often additional protections such as warnings during informed consent or anonymization software.
CRITICAL CONCEPTS AROUND DRE
The issues in this section are relevant for deciding to undergo testing and understanding test results. Part of what causes conflict around communicating and sharing DRE is that, with evolving science, there are levels of uncertainty. Measures used for clinical decision making must be accurate. Specifically, they must be reliable (e.g., if measured on two consecutive days results will be comparable) and valid (i.e., show evidence that they measure what was intended or predict an outcome). Even if a measure is accurate, there are other reasons for uncertainty in conclusions which we describe below.
Accuracy of results
It is important to discuss with stakeholders and patients the accuracy and level of uncertainty of DRE. This information is particularly important for genetic testing where direct to consumer products are available [41, 61]. Clinical Laboratory Improvement Amendments (CLIA) certified test results adhere to FDA governed clinical standards. In contrast Laboratory Developed Tests (LDT) do not adhere to these standards. The FDA discusses when LDT are appropriate and when there are risks for relying on them [62]. FDA approval may only hold for specific genetic tests but deriving additional data may not be within the approval. For example, 23 and Me has FDA approval for APOE genetic testing. Among other requirements, this approval mandates that related education and information on support be provided. However, this company also enables consumers to take their genetic data to less well validated, online services (e.g., Promethease) to explore other genetic mutations and these results do not have the same rigorous validation. Serious and unnecessary distress could result if, for example, an individual receives erroneous information that they carry a penetrant mutation for a serious illness (Jill Goldman, personal communication).
Genetic penetrance versus susceptibility
People considering genetic testing must understand that mutations vary in how consistently they lead to a disease. The classic example is Huntington’s disease, in which an expansion in the HTT gene guarantees developing the illness. The term “pathogenic variant” is preferred when referring to the penetrant mutations associated with frontotemporal dementia [63]. For highly penetrant pathogenic variants, genetic counsellors are critical to help people decide whether they wish to know, with high certainty, that they and their family members will inherit the disease [64, 65]. In contrast, for genetic variants that convey mild to moderately elevated risk and only increase susceptibility to dementia, the general guidance is that sharing this information with people without symptoms is not useful since predictive certainty is much lower [39, 46], and elevated genetic risk for dementia can be stigmatizing (but see [8] in this issue regarding personal utility). For example, the ɛ4 allele of APOE, the gene encoding apolipoprotein E, is associated with higher risk of AD dementia, but many APOE ɛ4 carriers never develop dementia. The calculus becomes different when there is clinical utility. APOE ɛ4 carriers have a higher risk of amyloid-related imaging abnormalities (ARIA) related to aducanumab and other amyloid immunotherapies (including gantenerumab, lecanamab, and donanemab), so that APOE ɛ4 carrier status may be relevant to clinical decision making regarding these treatments [42]. The US National Human Genome Research Institute (NHGRI) has educational resources and the American College of Medical Genetics and the National Society of Genetic Counselors produce guidelines regarding disclosure of genetic results which identify important concepts [39, 63]. There are standard conventions for referring to genes and the proteins they encode which we provide for a gene strongly associated with risk for AD, APOE, in Table 1 (courtesy of Suzanne Schindler).
Table 1
| The protein, apolipoprotein E, apoE |
| •When written in lowercase, apoE refers to a protein, apolipoprotein E |
| •ApoE4 is the protein isoform that is encoded by an individual with an APOE ɛ4 allele |
| The gene that encodes apolipoprotein E, APOE |
| •When capitalized and italicized, APOE is the symbol for the gene that encodes the apolipoprotein E (apoE) protein |
| •APOE has three common forms or polymorphisms, called alleles: ɛ2, ɛ3, and ɛ4. Note these alleles are designated with the Greek symbol epsilon and not E |
| •APOE genotype typically refers to both alleles that an individual carries, e.g., ɛ3/ɛ4 |
| •APOE ɛ4 is the high-risk polymorphism associated with Alzheimer’s disease |
| •APOE ɛ4 carrier status is often used to refer to whether or not individuals carry the high risk ɛ4 allele |
Biomarkers: Use and interpretation
Whereas it may be tempting to assume biomarker tests will always yield simple, dichotomous (positive/negative) answers, with new biomarker tests, interpretations may need to be nuanced as their relative strengths and weaknesses are uncovered. For example, different biomarkers for AD (amyloid, tau, and neurodegeneration/hippocampal atrophy) reveal pathology at different disease stages and the A/T/N framework that leverages these multiple indicators of disease progression may ultimately serve as a method of describing these stages [66]. At the same time, different modalities for measuring these biomarkers (e.g., CSF, blood, PET, MRI) and types of measures (e.g., p-tau181, p-tau217) must be validated across different disease stages (e.g., [67]). A Roadmap and glossary of terms for validation of biomarkers has been addressing these issues [67, 68] built upon the approach of oncology [69].
Biomarker context of use and appropriate use criteria
Clinicians typically use AD biomarkers in accordance with the recommendations of expert panels that define AUC (e.g., [5, 42–44]). The FDA oversees formal approval of biomarker tests. This approval may increase the likelihood of insurance reimbursement for AD biomarker testing. Although several amyloid PET tracers are FDA approved, amyloid PET is not reimbursed outside of research studies. The FDA also approves the purpose for which they are used (although tests are sometimes used “off-label”, i.e., outside of FDA approved indications). The term describing approved indications is context of use (https://www.fda.gov/drugs/biomarker-qualification-program/context-use) (Fig. 1) [12]. For example, biomarkers might be used in guiding treatment selection, monitoring treatment response, supporting early detection, enabling early intervention and prevention, and as surrogate endpoints [12]. Biomarkers indicating the presence of amyloid in the brain can be helpful in dementia diagnosis. For example, if a patient with memory impairment has biomarker evidence of amyloid in their brain, this finding may support AD as the etiology of memory impairment. A prognostic biomarker needs to be predictive. For example, an amyloid PET scan or blood test may not be an appropriate prognostic test in a person without symptoms since not everyone with a positive brain amyloid scan develops dementia and hence in patients without clear evidence of cognitive dysfunction there is a reluctance to use this information clinically.
Fig. 1
FDA Guidance on context of use (https://www.fda.gov/drugs/biomarker-qualification-program/context-use).
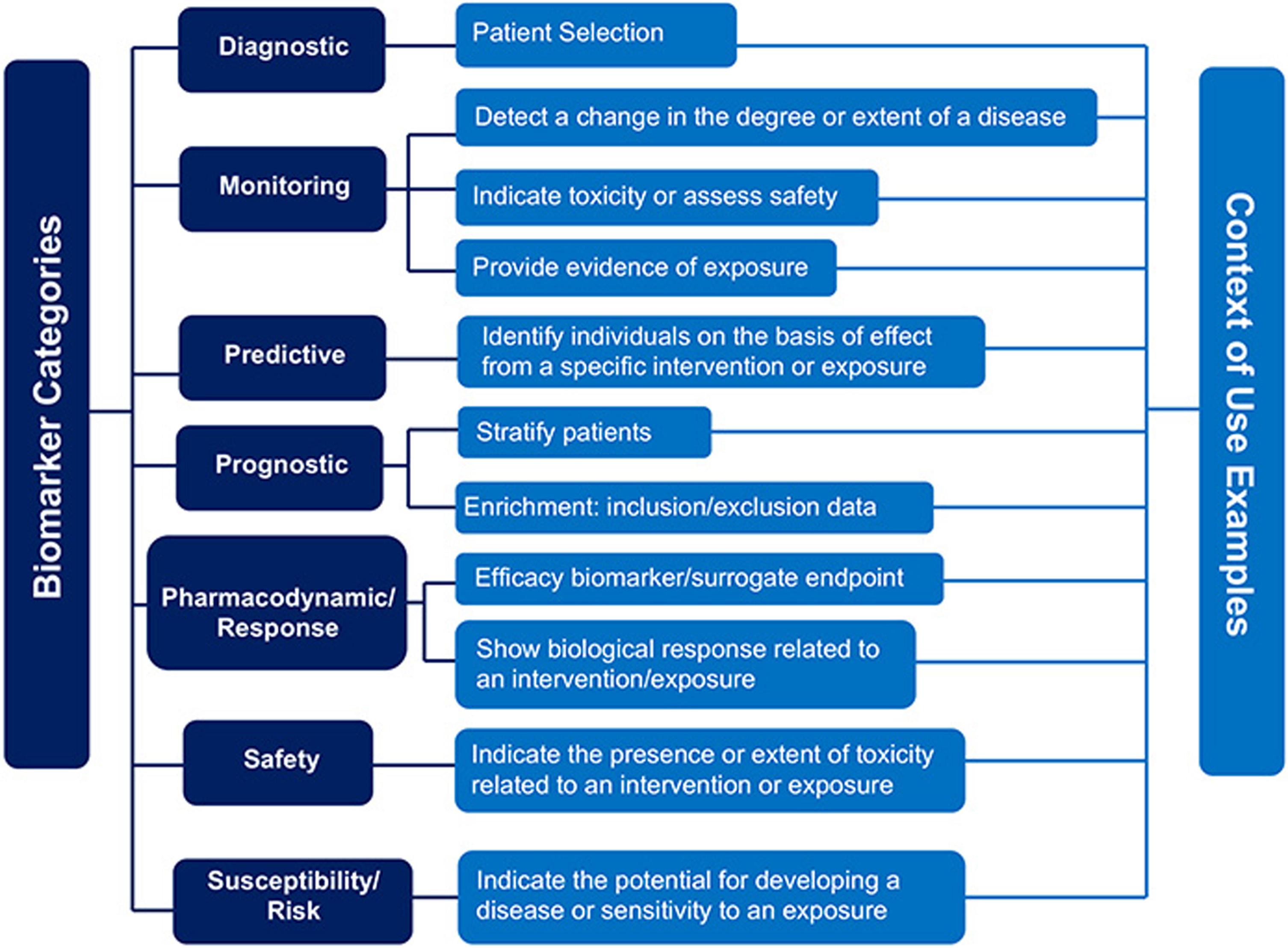
The surrogate endpoint use of biomarkers is critical to understand for Alzheimer’s-related medications that target amyloid or tau reduction. Whereas clinical trials ideally target clinical outcomes such as making patients feel better, slowing cognitive decline, or living longer, these outcomes may take too long to be feasible in a trial. A biomarker could serve as a surrogate endpoint, a substitute for a clinical endpoint, but should be mechanistically related to the disease and should correlate with a direct measure of clinical endpoint. For example, a reduction in brain amyloid as measured by PET is claimed as evidence that a drug has affected AD [70]. However, the evidence supporting a clinical benefit associated with amyloid reduction is inconsistent (e.g., [13, 71, 72]). In this Mini-Forum, Schindler suggests that one potential reason for the difficulty in finding relationships between amyloid levels and cognition is that the relationship is nonlinear [73]. She also suggests that understanding this relationship could someday give patients an answer to an often-asked question of how long they have before their cognition declines. Also in this Mini-Forum, Parra suggests that leveraging subtle and refined brain-behavior relationships will support more sensitive associations between measures of brain pathology and cognition [74]. A previous Mini-Forum on digital cognitive testing further demonstrates examples of approaches to improve sensitivity to cognitive decline that could support more sensitive associations to measures of brain integrity [75].
SOME CURRENT CONTROVERSIES
Biomarkers in cognitively normal individuals
Clinical biomarkers such as measures of elevated amyloid are typically only collected clinically in people with cognitive dysfunction because this information has benefits such as supporting differential diagnosis or indicating they may be appropriate candidates for amyloid lowering treatments. For people with no symptoms, biomarkers may not provide clear information at an individual-level about risk for dementia. Because of the insidious onset of most dementias, the distinction between normal and symptomatic is not binary. Whereas neuropsychological tests often have appropriate normative data, the majority of biomarker studies are derived from highly educated, Caucasian individuals. Still more problematic is that individuals with subjective cognitive decline are at high risk for subsequent cognitive decline [76]. These individuals are not satisfied with being told the disclosure of biomarker results is not relevant for them. The AGREEDementia stakeholder working group states that people, regardless of cognitive status, should be able to receive these data and that for people without symptoms these data are personally valuable in helping them make plans [8]. In this Mini- Forum, the AGREEDementia working group that focuses on applications to people without symptoms provided a thoughtful discussion on issues to consider and language to use in applying biomarkers to people without symptoms [77].
Diversity, social determinants of health, comorbidities
Ethical concerns loom large as DRE is used to guide policy. In this Mini-Forum, Karikari [78] explains that with the advent of blood-based biomarkers, there is great potential for expanding access to minoritized populations and developing countries. However, even for established laboratories, there may be uncertainty in classifying level of risk in the context of various individual differences due in great part to the failure of dementia research as a whole to recruit cohorts that reflect the racial and ethnic and socioeconomic diversity, particularly in the US. For example, biomarker levels sometimes show differences across cohorts that differ in racial ethnic status, comorbidities, and social determinants of health, hence predicting dementia may need adjustments that are not yet well worked out [79–83]. In this Mini-Forum, Daly et al. [15] urges that since treatments that target brain pathology are not yet demonstrated to be therapeutically valuable, it is important to allocate resources toward risk reduction that maximizes the overall societal good. Intervening in populations where social determinants of health are disproportionately contributing to dementia would thus be an opportunity.
How to implement biomarker-based criteria for therapies is also problematic in light of the lack of experience with diverse cohorts. For example, if biomarker levels erroneously suggest greater brain health in a certain race (e.g., lower amyloid levels), the cut point for access to therapies may be higher, and there may thus be diminished access to the biomarker-related treatments [84]. Conversely, a lower bar may lead to over treatment. Recent studies suggest that some but not all dementia biomarkers may be resilient against these effects [85]. Regardless, there is a great need for clinical trials in diverse cohorts to ensure therapeutic results generalize [86].
OVERVIEW OF MINI-FORUM
These basic concepts provide a framework for appreciating the papers in the Mini-Forum on the communication and use of DRE in light of recent advances. The Mini-Forum begins with Walter and Taylor et al. [8] who put forward a proposed Research Participant’s Bill of Rights advocating that participants in dementia research should have increased access to their individual results. Karikari [78] describes how ease of use in blood-based biomarkers makes DRE more widely available in under-resourced settings, thus supporting international justice through biomarker supported diagnosis. Daly et al. [15] advise to be strategic about how resources are deployed to at-risk populations to provide the most societal good, maximizing beneficence.
With rapid advances there must be careful attention to the quality of evidence surrounding DRE as it accumulates and outreach supporting appropriate use. Galasko et al. [19] describe how novel blood-based biomarkers must be used carefully to ensure their results are valid in the settings they are used. Effective analyses enhance the power of DRE and Schindler shows how it is possible to use these data from biomarkers to answer meaningful questions such as “How long do I have” before developing dementia [73]. Effective cognitive measures are also crucial for validating biomarkers and Parra [74] also points to the importance leveraging cutting edge neuroscience to improve the sensitivity of cognitive measures to brain dysfunction.
Educating, engaging, and understanding the perspectives of diverse clinicians, people living with and at risk for dementia and their advocates, and the public is also vital for supporting effective use of DRE. Rosen et al. [20] describe a group (AGREEDementia) that facilitates ongoing discussion, education, and dissemination. Arias et al. [51] describe an example of how to survey the perspective of frontline clinicians, geriatricians, about DRE. Walter et al. [34] describe a method of engaging people living with dementia in attending scientific conferences. Largent [56] suggests including study partners of people with increased risk of dementia before cognitive decline progresses to ensure their wishes are protected in longitudinal studies.
Finally, understanding the context surrounding how and where DRE is represented is important for representing its significance. Lerner [58] cautions that failure to provide context for DRE, such as when biomarker data from research participants are in the medical record, can lead care staff to infer dementia diagnosis from an individual biomarkers. Zallen [59] observed that keeping DRE out of the medical record while learning results is a motivator for people pursuing direct to consumer testing. Finally, Mozersky et al. [77] caution that FDA approvals of novel amyloid reducing medications to treat mild AD could alter the disclosure of amyloid positive biomarkers in research on people without symptoms. They provide examples of language that could be used to facilitate this communication.
CONCLUSIONS
This discussion provided a basic explanation and context for the Mini-Forum on ethical, legal, and practical issues on communicating and using DRE. The increased availability of DRE such as through blood-based biomarkers and direct to consumer genetic tests could transform diagnosis and care; however, use must be judicious. DRE varies in predictive ability and utility for individuals. We discussed how a general ethical framework can be related to these decisions and, in our experience, respect for autonomy and differences in values facilitates consensus. Decision tools are efficient methods for helping improve informed choices in patients and other stakeholders, particularly when expert clinician time is limited. Presently, the most effective use of DRE is where it can contribute to diagnosis and care in people with symptoms; however, we provide information on off label use in people without symptoms [77]. Once DRE have been collected, it is vital that they not be used as short-cut for a comprehensive and careful evaluation of contributors to symptoms, particularly treatable conditions. With advances in the predictive ability of DRE and therapies we hope someday, DRE can enable presymptomatic detection and prevention of dementia. In the face of rapidly evolving innovation, making decisions around appropriate context of use, assuring that effective communication leads to understanding, and informed decision making will be challenging. Above all, there is a need for avoiding paralysis of indecision in the face of uncertainty since the degenerative process of dementia waits for no one, especially not the people living with dementia.
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0722r1).
REFERENCES
[1] | FDA, About Biomarkers and Qualification, https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification. |
[2] | NIEHS, Biomarker Definition, https://www.niehs.nih.gov/health/topics/science/biomarkers/index.cfm. |
[3] | Bateman RJ , Xiong C , Benzinger TLS , Fagan AM , Goate A , Fox NC , Marcus DS , Cairns NJ , Xie X , Blazey TM , Holtzman DM , Santacruz A , Buckles V , Oliver A , Moulder K , Aisen PS , Ghetti B , Klunk WE , McDade E , Martins RN , Masters CL , Mayeux R , Ringman JM , Rossor MN , Schofield PR , Sperling RA , Salloway S , Morris JC ((2012) ) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367: , 795–804. |
[4] | Schindler S ((2022) ) Fluid biomarkers in dementia diagnosis. Continuum 28: , 822–833. |
[5] | Hansson O , Edelmayer RM , Boxer AL , Carrillo MC , Mielke MM , Rabinovici GD , Salloway S , Sperling R , Zetterberg H , Teunissen CE (2022) The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dementia. doi: 10.1002/alz.12756. |
[6] | Frank L , Wesson Ashford J , Bayley PJ , Borson S , Buschke H , Cohen D , Cummings JL , Davies P , Dean M , Finkel SI ((2018) ) Genetic risk of Alzheimer’s disease: Three wishes now that the genie is out of the bottle. J Alzheimers Dis 66: , 421–423. |
[7] | Gooblar J , Roe CM , Selsor NJ , Gabel MJ , Morris JC ((2015) ) Attitudes of research participants and the general public regarding disclosure of Alzheimer disease research results. JAMA Neurol 72: , 1484–1490. |
[8] | Walter S , Taylor A , Tyrone J , Langer S , Pagan JR , Huling-Hummel C , Wheaton B , Zallen DT , Rosen AC ((2022) ) Disclosing individual results in dementia research: A proposed study participant’s bill of rights. J Alzheimers Dis 90: , 945–952. |
[9] | Roberts JS , Ferber R , Blacker D , Rumbaugh M , Grill JD , Advisory Group on Risk Evidence Education for Dementia (AGREED) ((2021) ) Disclosure of individual research results at federally funded Alzheimer’s Disease Research Centers. Alzheimers Dement (N Y) 7: , e12213. |
[10] | National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on the Return of Individual-Specific Research Results Generated in Research Laboratories (2018) Returning individual research results to participants: Guidance for a new research paradigm. |
[11] | Cacabelos R , Meyyazhagan A , Carril JC , Cacabelos P , Teijido Ó ((2018) ) Pharmacogenetics of vascular risk factors in Alzheimer’sdisease. J Pers Med 8: , 3. |
[12] | FDA-NIH Biomarker Working Group (2016) BEST (Biomarkers, EndpointS, and other Tools) resource. |
[13] | Alexander GC , Emerson S , Kesselheim AS ((2021) ) Evaluation of Aducanumab for Alzheimer disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 325: , 1717–1718. |
[14] | Rabinovici GD , Gatsonis C , Apgar C , Chaudhary K , Gareen I , Hanna L , Hendrix J , Hillner BE , Olson C , Lesman-Segev OH , Romanoff J , Siegel BA , Whitmer RA , Carrillo MC ((2019) ) Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321: , 1286–1294. |
[15] | Daly T , Mastroleo I , Migliaccio R ((2022) ) Avoiding over-reliance on multi-domain interventions for dementia prevention. J Alzheimers Dis 90: , 989–993. |
[16] | Weiner MW , Veitch DP , Aisen PS , Beckett LA , Cairns NJ , Green RC , Harvey D , Jack CR Jr , Jagust W , Morris JC , Petersen RC , Salazar J , Saykin AJ , Shaw LM , Toga AW , Trojanowski JQ ,Alzheimer’s Disease Neuroimaging Initiative ((2017) ) The Alzheimer’s Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimers Dement 13: , 561–571. |
[17] | Bycroft C , Freeman C , Petkova D , Band G , Elliott LT , Sharp K , Motyer A , Vukcevic D , Delaneau O , O’Connell J , Cortes A , Welsh S , Young A , Effingham M , McVean G , Leslie S , Allen N , Donnelly P , Marchini J ((2018) ) The UK Biobank resource with deep phenotyping and genomic data. Nature 562: , 203–209. |
[18] | Kornblith E , Bahorik A , Boscardin WJ , Xia F , Barnes DE , Yaffe K ((2022) ) Association of race and ethnicity with incidence of dementia among older adults. JAMA 327: , 1488–1495. |
[19] | Galasko DR , Grill JD , Lingler JH , Heidebrink JL , and for the Symptomatic Subcommittee of the Advisory Group on Risk Evidence Education for Dementia (AGREEDementia) ((2022) ) A blood test for Alzheimer’s disease: It’s about time or not ready for prime time? J Alzheimers Dis 90: , 963–966. |
[20] | Rosen AC , Arrias JJ , Ashford JW , Blacker D , Chhatwal JP , Chin NA , Clark L , Denny SS , Goldman J , Gleason CE , Grill JD , Heidebrink JL , Henderson VW , Lavacot JA , Lingler JH , Menon M , Nosheny RL , Oliveira FF , Parker MW , Rahman-Filipiak A , Revoori A , Rumbaugh M , Sanchez D , Schindler S , Schwartz C , Toy L , Tyrone J , Walter S , Wang LS , Wijsman EM , Zallen DT , Aggarwal NT ; members of AGREEDementia ((2022) ) The Advisory Group on Risk Evidence Education for Dementia: Multidisciplinary and open to all. J Alzheimers Dis 90: , 953–962. |
[21] | Frederiksen KS , Nielsen TR , Appollonio I , Andersen BB , Riverol M , Boada M , Ceccaldi M , Dubois B , Engelborghs S , Frölich L ((2021) ) Biomarker counseling, disclosure of diagnosis and follow-up in patients with mild cognitive impairment: A European Alzheimer’s disease consortium survey. Int J Geriatr Psychiatry 36: , 324–333. |
[22] | Frederiksen K , Nielsen T , Winblad B , Schmidt R , Kramberger M , Jones R , Hort J , Grimmer T , Georges J , Frölich L , Engelborghs S , Dubois B , Waldemar G ((2021) ) European Academy of Neurology/European Alzheimer’s Disease Consortium position statement on diagnostic disclosure, biomarker counseling, and management of patients with mild cognitive impairment. Eur J Neurol 28: , 2147–2155. |
[23] | Rostamzadeh A , Schwegler C , Gil-Navarro S , Rosende-Roca M , Romotzky V , Ortega G , Canabate P , Moreno M , Schmitz-Luhn B , Boada M , Jessen F , Woopen C ((2021) ) Biomarker-based risk prediction of Alzheimer’s disease dementia in mild cognitive impairment: Psychosocial, ethical, and legal aspects. J Alzheimers Dis 80: , 601–617. |
[24] | Milne R , Bunnik E , Tromp K , Bemelmans S , Badger S , Gove D , Maman M , Schermer M , Truyen L , Brayne C , Richard E ((2017) ) Ethical issues in the development of readiness cohorts in Alzheimer’s disease research. J Prev Alzheimers Dis 4: , 125–131. |
[25] | Bartels C , Kogel A , Schweda M , Wiltfang J , Pentzek M , Schicktanz S , Schneider A ((2020) ) Use of cerebrospinal fluid biomarkers of Alzheimer’s disease risk in mild cognitive impairment and subjective cognitive decline in routine clinical care in Germany. J Alzheimers Dis 78: , 1137–1148. |
[26] | Au R , Kolachalama VB , Paschalidis IC ((2021) ) Redefining and validating digital biomarkers as fluid, dynamic multi-dimensional digital signal patterns. Front Digit Health 3: , 751629. |
[27] | Illes J , Desmond JE , Huang LF , Raffin TA , Atlas SW ((2002) ) Ethical and practical considerations in managing incidental findings in functional magnetic resonance imaging. Brain Cogn 50: , 358–365. |
[28] | Wolf SM , Crock BN , Van NB , Lawrenz F , Kahn JP , Beskow LM , Cho MK , Christman MF , Green RC , Hall R , Illes J , Keane M , Knoppers BM , Koenig BA , Kohane IS , Leroy B , Maschke KJ , McGeveran W , Ossorio P , Parker LS , Petersen GM , Richardson HS , Scott JA , Terry SF , Wilfond BS , Wolf WA ((2012) ) Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med 14: , 361–384. |
[29] | Illes J , Rosen AC , Huang L , Goldstein RA , Raffin TA , Swan G , Atlas SW ((2004) ) Ethical consideration of incidental findings on adult brain MRI in research. Neurology 62: , 888–890. |
[30] | van der Schaar J , Visser LNC , Bouwman FH , Ket JCF , Scheltens P , Bredenoord AL , van der Flier WM ((2022) ) Considerations regarding a diagnosis of Alzheimer’s disease before dementia: A systematic review. Alzheimers Res Ther 14: , 31. |
[31] | Lye CT , Forman HP , Daniel JG , Krumholz HM ((2018) ) The 21st Century Cures Act and electronic health records one year later: Will patients see the benefits? . J Am Med Inform Assoc 25: , 1218–1220. |
[32] | Office of the National Coordinator for Health Information Technology (ONC), Department of Health and Human Services (HHS). 21st Century Cures Act: Interoperability, information blocking, and the ONC Health IT Certification Program. |
[33] | Walter S , Wheaton B , Huling Hummel C , Tyrone J , Ziolkowski J , Shaffer E , Aggarwal N ((2021) ) Can virtual scientific conferences facilitate two-way learning between dementia researchers and participants? . J Prev Alzheimers Dis 8: , 387–388. |
[34] | Walter S , Kim AB , Flores M , Ziolkowski J , Shaffer E , Aggarwal NT ((2022) ) Including general audiences in a virtual scientific dementia conference: Will they get anything from it? J Alzheimers Dis 90: , 1003–1011. |
[35] | Hommes D , Klatte D , Otten W , Beltman M , Klass G , Zand A , Sprangers R ((2020) ) Health outcomes and experiences of direct-to-consumer high-intensity screening using both whole-body magnetic resonance imaging and cardiological examination . PLoS One 15: , e0242066. |
[36] | Roberts JS , Gornick MC , Carere DA , Uhlmann WR , Ruffin MT , Green RC ((2017) ) Direct-to-consumer genetic testing: User motivations, decision making, and perceived utility of results. Public Health Genomics 20: , 36–45. |
[37] | Middleton A , Mendes Á , Benjamin CM , Howard HC ((2017) ) Direct-to-consumer genetic testing: Where and how does geneticcounseling fit? . Per Med 14: , 249–257. |
[38] | Goldman J , Xie S , Green D , Naini A , Mansukhani MM , Marder K ((2021) ) Predictive testing for neurodegenerative diseases in the age of next-generation sequencing. J Genet Counsel 30: , 553–562. |
[39] | Goldman JS , Hahn SE , Catania JW , LaRusse-Eckert S , Butson MB , Rumbaugh M , Strecker MN , Roberts JS , Burke W , Mayeux R , Bird T , American College of Medical Genetics and the National Society of Genetic Counselors ((2011) ) Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med 13: , 597–605. |
[40] | Koriath CA , Kenny J , Ryan NS , Rohrer JD , Schott JM , Houlden H , Fox NC , Tabrizi SJ , Mead S ((2021) ) Genetic testing in dementia— Utility and clinical strategies. Nat Rev Neurol 17: , 23–36. |
[41] | National Academies of Sciences Engineering, Medicine, Health (2020) The National Academies Collection: Reports funded by National Institutes of Health. In Exploring the Current Landscape of Consumer Genomics: Proceedings of a Workshop, Beachy SH, Alper J, Addie S, Hackmann M, eds. National Academies Press (US),Washington (DC). |
[42] | Cummings J , Aisen P , Apostolova LG , Atri A , Salloway S , Weiner M ((2021) ) Aducanumab: Appropriate use recommendations. J Prev Alzheimers Dis 8: , 398–410. |
[43] | Johnson KA , Minoshima S , Bohnen NI , Donohoe KJ , Foster NL , Herscovitch P , Karlawish JH , Rowe CC , Carrillo MC , Hartley DM , Hedrick S , Pappas V , Thies WH ((2013) ) Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 9: , E1–E16. |
[44] | Shaw LM , Arias J , Blennow K , Galasko D , Molinuevo JL , Salloway S , Schindler S , Carrillo MC , Hendrix JA , Ross A , Illes J , Ramus C , Fifer S ((2018) ) Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement 14: , 1505–1521. |
[45] | Largent EA , Harkins K , van Dyck CH , Hachey S , Sankar P , Karlawish J ((2020) ) Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS One 15: , e0229137. |
[46] | Zallen DT ((2018) ) “Well, good luck with that": Reactions to learning of increased genetic risk for Alzheimer disease. Genet Med 20: , 1462–1467. |
[47] | Largent EA , Stites SD , Harkins K , Karlawish J ((2021) ) ‘That would be dreadful’: The ethical, legal, and social challenges of sharing your Alzheimer’s disease biomarker and genetic testing results with others. J Law Biosci 8: , lsab004. |
[48] | Stites SD , Rubright JD , Karlawish J ((2018) ) What features of stigma do the public most commonly attribute to Alzheimer’s disease dementia? Results of a survey of the U.S. general public. Alzheimers Dement 14: , 925–932. |
[49] | Hallquist ML , Tricou EP , Ormond KE , Savatt JM , Coughlin CR , Faucett WA , Hercher L , Levy HP , O’Daniel JM , Peay HL ((2021) ) Application of a framework to guide genetic testing communication across clinical indications. Genome Med 13: , 1–12. |
[50] | Arias JJ , Tyler AM , Oster BJ , Karlawish J ((2021) ) The proactive patient: Long-term care insurance discrimination risks of Alzheimer’s disease biomarkers. J Law Med Ethics 46: , 485–498. |
[51] | Arias JJ , Lin GA , Tyler AM , Douglas MP , Phillips KA ((2022) ) Geriatricians’ perspectives on the multiple dimensions of utility of genetic testing for Alzheimer’s disease: A qualitative study. J Alzheimers Dis 90: , 1013–1021. |
[52] | Lingler JH , Butters MA , Gentry AL , Hu L , Hunsaker AE , Klunk WE , Mattos MK , Parker LS , Roberts JS , Schulz R ((2016) ) Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis 52: , 17–24. |
[53] | Zallen DT Genetestornot Decision Aid, https://www.genetestornot.org, Accessed June, 2022. |
[54] | Zallen DT (2008) To Test or Not to Test: A Guide to Genetic Screening and Risk, Rutgers University Press, New Jersey. |
[55] | NIA, Alzheimer’s & Dementia Outreach, Recruitment & Engagement Resources (ADORE), https://www.nia.nih.gov/research/alzheimers-dementia-outreach-recruitment-engagement-resources. |
[56] | Largent EA , Bhardwaj T , Clapp JT , Sykes OS , Harkins K , Grill JD ((2022) ) You’ve got a friend in me: How cognitively unimpaired older adults select a study partner to participate with them in Alzheimer’s disease research. J Alzheimers Dis 90: , 1023–1035. |
[57] | Largent EA , Bradbury AR ((2022) ) Bringing Alzheimer disease testing and results disclosure into the 21st Century Cures Act. JAMA Neurol 79: , 219–220. |
[58] | Lerner A ((2022) ) Biomarkers and mindfulness: A way forward. J Alzheimers Dis 90: , 995–998. |
[59] | Zallen DT ((2022) ) Alzheimer disease: Risk testing and the medical record. J Alzheimers Dis 90: , 999–1001. |
[60] | Schwarz CG , Kremers WK , Therneau TM , Sharp RR , Gunter JL , Vemuri P , Arani A , Spychalla AJ , Kantarci K , Knopman DS , Petersen RC , Jack CR ((2019) ) Identification of anonymous MRI research participants with face-recognition software. N Engl J Med 381: , 1684–1686. |
[61] | ACMG Board of Directors ((2016) ) Direct-to-consumer genetic testing: A revised position statement of the American College of Medical Genetics and Genomics. Genet Med 18: , 207–208. |
[62] | FDA, FDA Guidance on Laboratory Developed Tests, https://www.fda.gov/media/102367/download. |
[63] | Goldman JS , Hahn SE , Catania JW , LaRusse-Eckert S , Butson MB , Rumbaugh M , Strecker MN , Roberts JS , Burke W , Mayeux R , Bird T ((2019) ) ADDENDUM: Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med 21: , 2404. |
[64] | Greaves CV , Rohrer JD ((2019) ) An update on genetic frontotemporal dementia. J Neurol 266: , 2075–2086. |
[65] | Goldman JS ((2015) ) Genetic testing and counseling in the diagnosisand management of young-onset dementias. Psychiatr Clin NorthAm 38: , 295–308. |
[66] | Jack CR Jr. , Bennett DA , Blennow K , Carrillo MC , Dunn B , Haeberlein SB , Holtzman DM , Jagust W , Jessen F , Karlawish J , Liu E , Molinuevo JL , Montine T , Phelps C , Rankin KP , Rowe CC , Scheltens P , Siemers E , Snyder HM , Sperling R , Contributors, Elliott C , Masliah E , Ryan L , Silverberg N ((2018) ) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: , 535–562. |
[67] | Ashton NJ , Leuzy A , Karikari TK , Mattsson-Carlgren N , Dodich A , Boccardi M , Corre J , Drzezga A , Nordberg A , Ossenkoppele R , Zetterberg H , Blennow K , Frisoni GB , Garibotto V , Hansson O ((2021) ) The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur J Nucl Med Mol Imaging 48: , 2140–2156. |
[68] | Boccardi M , Gallo V , Yasui Y , Vineis P , Padovani A , Mosimann U , Giannakopoulos P , Gold G , Dubois B , Jack CR Jr. , Winblad B , Frisoni GB , Albanese E ((2017) ) The biomarker-based diagnosis of Alzheimer’s disease. 2-lessons from oncology. Neurobiol Aging 52: , 141–152. |
[69] | Pepe MS , Etzioni R , Feng Z , Potter JD , Thompson ML , Thornquist M , Winget M , Yasui Y ((2001) ) Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 93: , 1054–1061. |
[70] | Sevigny J , Chiao P , Bussière T , Weinreb PH , Williams L , Maier M , Dunstan R , Salloway S , Chen T , Ling Y ((2016) ) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537: , 50–56. |
[71] | Grill JD , Karlawish J ((2021) ) Implications of FDA approval of a first disease-modifying therapy for a neurodegenerative disease on the design of subsequent clinical trials. Neurology 97: , 496–500. |
[72] | Knopman DS , Jones DT , Greicius MD ((2021) ) Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement 17: , 696–701. |
[73] | Schindler SE ((2022) ) Predicting symptom onset in sporadic Alzheimer’s disease: “How long do i have?” J Alzheimers Dis 90: , 975–979. |
[74] | Parra MA ((2022) ) Barriers to effective memory assessments for Alzheimer’s disease. J Alzheimers Dis 90: , 981–988. |
[75] | Libon DJ , Baliga G , Swenson R , Au R ((2021) ) Digital neuropsychological assessment: New technology for measuring subtle neuropsychological behavior. Alzheimers Dis 82: , 1–4. |
[76] | Jessen F , Amariglio RE , Buckley RF , Flier WM , Han Y , Molinuevo JL , Rabin L , Rentz DM , Rodriguez-Gomez O , Saykin AJ ((2020) ) The characterisation of subjective cognitive decline. Lancet Neurol 19: , 271–278. |
[77] | Mozersky J , Roberts JS , Rumbaugh M , Chhatwal J , Wijsman EM , Galasko D , Blacker D ((2022) ) Spillover: The approval of new medications for Alzheimer’s disease dementia will impact biomarker disclosure among asymptomatic individuals. Alzheimers Dis 90: , 1037–1045. |
[78] | Karikari T ((2022) ) Blood tests for Alzheimer’s disease: Increasing efforts to expand and diversify research participation is critical for widespread validation and acceptance. J Alzheimers Dis 90: , 967–974. |
[79] | Morris JC , Schindler SE , McCue LM , Moulder KL , Benzinger TL , Cruchaga C , Fagan AM , Grant E , Gordon BA , Holtzman DM ((2019) ) Assessment of racial disparities in biomarkers for Alzheimer disease. }. JAMA Neurol 76: , 264–273. |
[80] | Schindler SE , Cruchaga C , Joseph A , McCue L , Farias FHG , Wilkins CH , Deming Y , Henson RL , Mikesell RJ , Piccio L , Llibre-Guerra JJ , Moulder KL , Fagan AM , Ances BM , Benzinger TLS , Xiong C , Holtzman DM , Morris JC ((2021) ) African Americans have differences in CSF soluble TREM2 and associated genetic variants. Neurol Genet 7: , e571. |
[81] | Blue EE , Horimoto A , Mukherjee S , Wijsman EM , Thornton TA ((2019) ) Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement 15: , 1524–1532. |
[82] | Brickman AM , Manly JJ , Honig LS , Sanchez D , Reyes-Dumeyer D , Lantigua RA , Lao PJ , Stern Y , Vonsattel JP , Teich AF , Airey DC , Proctor NK , Dage JL , Mayeux R ((2021) ) Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 17: , 1353–1364. |
[83] | Schindler SE , Karikari TK ((2022) ) Comorbidities confound Alzheimer’s blood tests. Nat Med 28: , 1349–1351. |
[84] | Vyas DA , Eisenstein LG , Jones DS ((2020) ) Hidden in plain sight - reconsidering the use of race correction in clinical algorithms.. N Engl J Med 383: , 874–882. |
[85] | Schindler SE , Karikari TK , Ashton NJ , Henson RL , Yarasheski KE , West T , Meyer MR , Kirmess KM , Li Y , Saef B , Moulder KL , Bradford D , Fagan AM , Gordon BA , Benzinger TLS , Balls-Berry J , Bateman RJ , Xiong C , Zetterberg H , Blennow K , Morris JC ((2022) ) Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology 99: , e245–e257. |
[86] | Padala SP , Yarns BC ((2022) ) Under-represented populations left out of Alzheimer’s disease treatment with aducanumab: Commentary on ethics. J Alzheimers Dis Rep 6: , 345–348. |




